Abstract
Objective:
Total endovascular repair of aortic arch aneurysms is feasible in select patients. This study aims to evaluate the feasibility and early outcomes of total endovascular arch repair using 3-vessel company-manufactured devices (CMDs) and physician-modified endo grafts (PMEGs).
Methods:
Patients unfit for open repair who underwent 3-vessel total arch repair at a single institution from 2018 to 2021 were reviewed. Patients received either 3-vessel inner-branch CMDs or PMEGs. Three-vessel designs were used to incorporate the innominate, left common carotid, and left subclavian arteries. The antegrade inner branches in both devices were accessed via right brachial or carotid approach. The left carotid was accessed via carotid cutdown or femoral approach. The left subclavian artery was accessed via transfemoral approach. The study endpoints included procedural technical success, patient survival, neurologic events, cardiac complications, reinterventions, and target artery patency.
Results:
Nine patients underwent treatment. Four patients were treated with PMEGs, and 5 with CMDs. Procedural technical success was 100%. There were no in-hospital deaths. There were no strokes, transient ischemic attacks, myocardial infarction, or spinal ischemia in the perioperative period. Major adverse events occurred in 3 patients (33%). Two (22%) vascular access complications and one (11%) acute kidney injury occurred. One (11%) patient required early reintervention for an access complication. The median follow-up period was 358 days (CMD, 392 days; PMEG, 198 days). There was a late reintervention and conversion to open repair at 142 days of follow-up in a patient with a PMEG that developed an aortic infection, leading to death on postoperative day 239. The mean length of stay was 7±4 days. Computed tomography imaging obtained during the immediate postoperative period revealed endoleak in 6 (66%) patients, out of which 5 resolved spontaneously and 1 required reintervention via left subclavian artery stenting. Target artery patency was 100% at the end of the follow-up period.
Conclusions:
Three-vessel total endovascular aortic arch repair using a CMD or PMEG is feasible with optimal early outcomes. Physician-modified stent-grafts are a feasible option for patients who do not meet anatomic criteria for CMDs.
Clinical Impact
Management of aortic arch disease remains a significant challenge in vascular surgery. This study showcases the feasibility and safety of using a total endovascular approach to repair the aortic arch, which could potentially reduce morbidity and mortality associated with traditional surgical approaches. The results suggest that this minimally invasive technique could be an alternative treatment option for high-risk patients and could significantly improve outcomes for those requiring aortic arch repair. Overall, this study represents a promising development in the field of endovascular surgery and highlights the potential to improve patient outcomes.
Keywords: aortic arch, aortic arch aneurysm, aortic arch dissection, thoracic endovascular aortic repair, endovascular aneurysm repair
Introduction
Total endovascular aortic arch repair remains the final frontier of endovascular aortic surgery. Currently, open surgical repair is the reference standard for the treatment of the aortic arch. Outcomes of open and hybrid repair of the aortic arch have improved over the years, but substantial short-term mortality rates of 5% to 25.6% are still reported.1–5 Moreover, other devastating complications such as paraplegia and stroke rates range from 5% to 15%. 4
Elderly patients and those with severe comorbidities are frequently unfit for open or hybrid arch repair. Global multicenter studies have reported the feasibility of total endovascular arch repair using patient-specific 3-vessel inner-branch stent grafts.3,6 However, these custom-made devices are currently available only for a select number of centers and patients who meet the manufacturer’s criteria for use. Therefore, several patients unfit for open and endovascular repair with company-made devices need alternative therapies, including the use of modified devices (Figures 1 and 2). At our center and based on our previous experience with thoracoabdominal modified devices, the multidisciplinary vascular and cardiac surgery team has developed and adopted the use of physician-modified 3-vessel arch branch devices for the total endovascular treatment of aortic arch diseases of patients unfit for open repair and unsuitable for company-made devices. The present study explores a single-center experience in the treatment of aortic arch diseases using both company-manufactured devices (CMDs) and back-table 3-vessel physician-modified endografts (PMEGs).
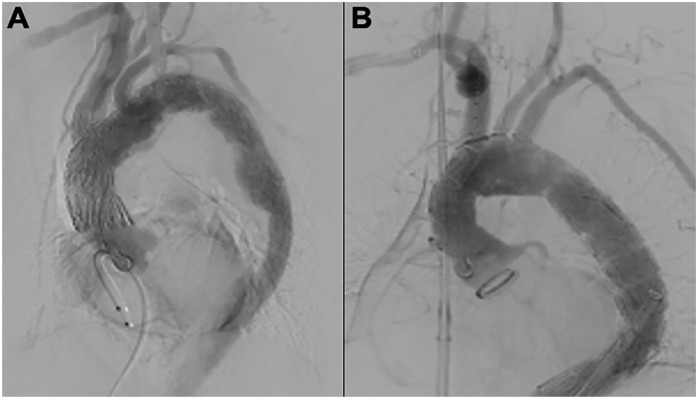
Figure 1.
(A) Total endovascular 3-vessel aortic arch repair using a custom-made endograft. (B) Total endovascular 3-vessel aortic arch repair using a physician-modified endograft.
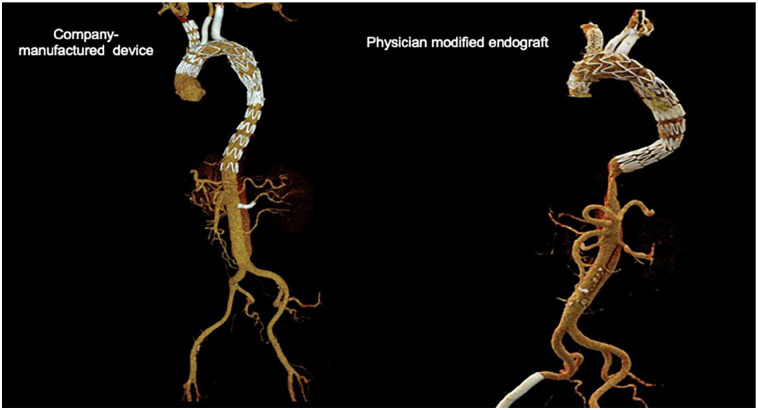
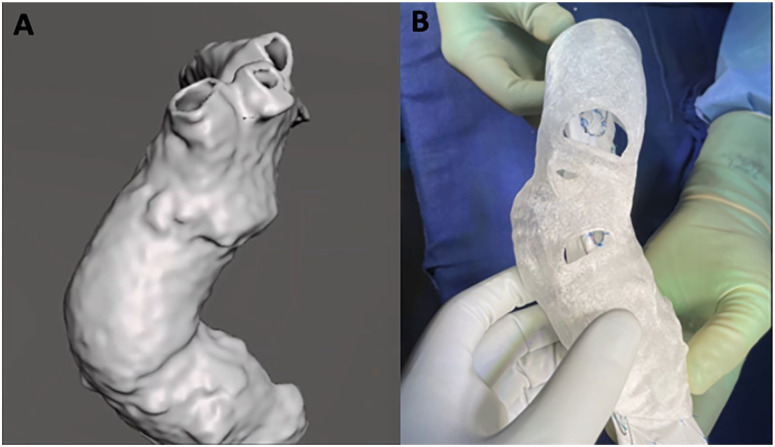
Figure 2.
Company-manufactured custom-made endograft and physician-modified endograft, 3-dimensional reconstruction.
Methods
Study Design
A retrospective review of an institutional database was performed. All patients included in the study were treated at a single multispecialty academic center. Patients were evaluated by a multidisciplinary team consisting of members from vascular surgery, cardiothoracic surgery, and cardiology. All patients were deemed unfit for traditional open arch repair based on comorbidities and anatomic factors (see Supplemental Material for exclusion criteria for open arch repair). Patients undergoing procedures with company-made devices were enrolled in a physician-sponsored investigational device exemption (IDE) study protocol (IDE #G140108, National Clinical Trial [NCT] #02266719). Informed consent was obtained prior to their repair. Institutional review board approval was obtained for this study.
Demographics and clinical data were assessed for the group, including procedural data and imaging. Aneurysm characteristics, aortic coverage zones, procedure adjuncts, and complication variables were defined according to the Society for Vascular Surgery reporting standards.7,8 Procedural technical success was defined as successful deployment and implantation of the aortic arch endograft with all target branch vessel stents deployed and patent at the time of the completion angiography.
Follow-up assessments took place at 2 weeks, 1 month, 3 months, 6 months, 1 year, and yearly thereafter. Computed tomography angiography (CTA) was performed prior to discharge and prior to the above-listed postoperative visits starting at 1 month. Early outcomes were defined as 30 day outcomes or the outcomes measured in initial hospital stay if the length of stay was greater than 30 days. Long-term follow-up data were collected regarding mortality, reintervention, target vessel patency, and the presence of endoleaks.
Anatomic Suitability
Among patients unfit for open repair, those with ascending aortic size ≤38 mm for the proximal seal were considered for company-made devices. The proximal seal zone had to be at least 4 cm for the native aorta and greater than 2 cm for patients with prior ascending aortic repair with a surgical graft. The length of the sinotubular junction to the innominate artery (IA) needed to be greater than 5 cm. The iliofemoral access site had to be able to accommodate 18 Fr to 24 Fr delivery cannulas. 9 Patients with excessively “shaggy” aortas were not considered candidates.
Four patients who underwent treatment with PMEGs were previously considered for CMDs but were excluded based on the manufacturer’s instructions for use. The anatomic reasons for the use of PMEGs included angulation >60°, presence of graft kinks, dissection of the target vessels, and small access (<7 mm in diameter). There were also medical reasons why patients received PMEGs, including the presence of symptomatic or large thoracic or abdominal components (>6.5 cm) that required urgent repair. Among the patients included in the study, 2 patients had severe angulations: 1 patient with a short proximal landing zone and the other with a small access.
Device Design and Planning
Aneurysm morphology was analyzed via high-resolution CTA. The analysis process was aided by a 3-dimensional (3D) reconstruction software program (TeraRecon, Durham, NC, USA). Company-manufactured patient-specific devices were designed with 3-vessel inner branches (Figure 4, William Cook Europe, Bjaeverskov, Denmark). Physician-modified devices were designed in the back table with Cook Zenith Alpha thoracic stent grafts (Figure 5, Cook Medical Inc, Bloomington, IN, USA). The internal branches were created using Gore Viabahn-covered self-expanding stents (W. L. Gore & Associates, Flagstaff, AZ, USA). The nose cone of the Alpha device is the same component as the standard thoracic device. The custom-made device for the arch has a shorter nose cone, which is not yet commercially available. Gooseneck snares consisting of a gold-plated tungsten loop with a nitinol shaft were used for visualization and reinforcement of the external opening of the branches and fenestration. The device is constrained using 3-0 Prolene sutures to allow for the cannulation of inner branches while maintaining antegrade cerebral perfusion. A 3D model was designed and printed based on preoperative CTA for guidance on the location of the branches and the design of the modified devices.
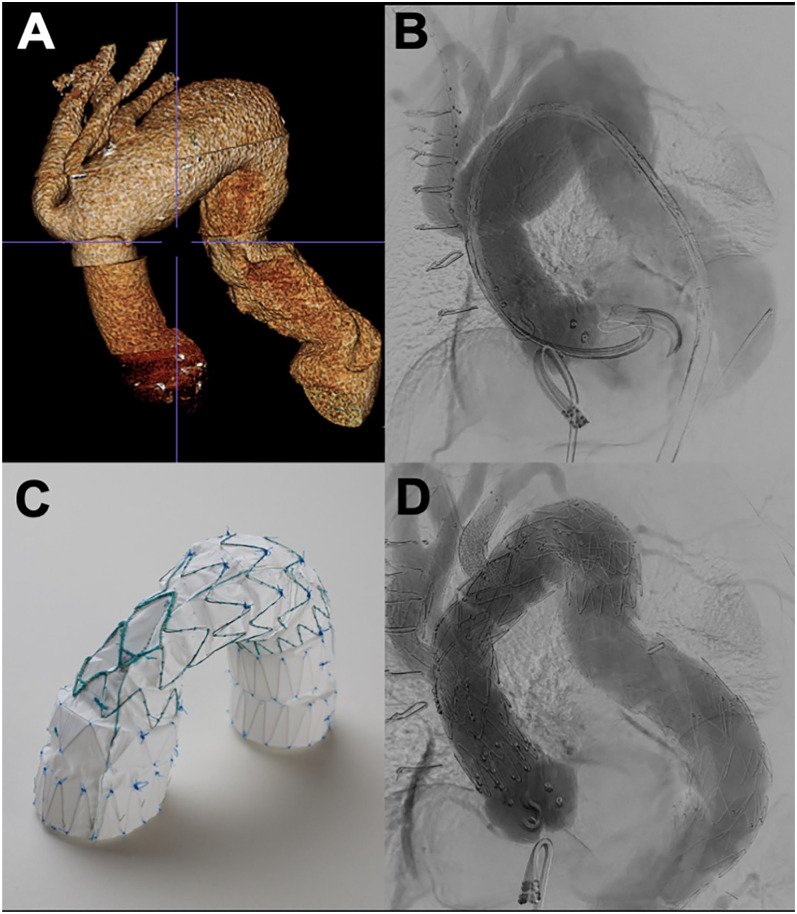
Figure 4.
(A) Three-dimensional reconstruction of computed tomography imaging. (B) Intraoperative aortogram prior to repair. (C) Custom-made endograft utilized for the repair. (D) Completion angiogram showing successful exclusion of the arch aneurysm.
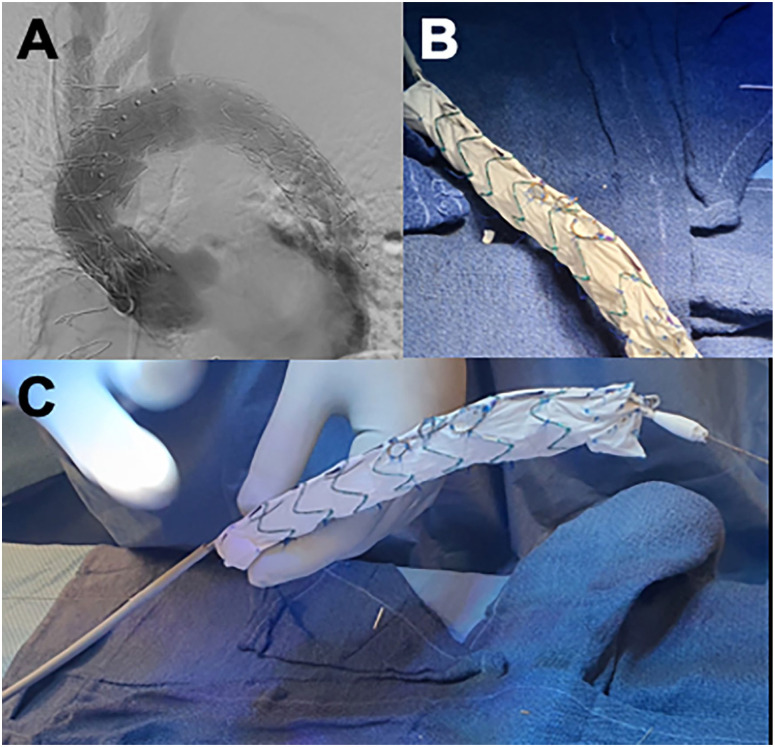
Figure 5.
(A) Completion angiogram after physician-modified endograft placement. (B) Fenestrations are reinforced with gooseneck snare, and inner branches are constructed using self-expanding covered stents. (C) Spine-stabilization wire is interwoven onto the inner cannula using an 0.014 nitinol wire.
Procedural Details
All procedures were performed under general anesthesia in hybrid operating rooms. Fusion imaging guidance, intravascular ultrasound (IVUS), and cone-beam computed tomographies were used. Patients had continuous transcranial Doppler or electroencephalogram for neuromonitoring. Spinal drains were not routinely placed, and instead, we used near-infrared spectroscopy to assess spinal perfusion.
Any device modifications occurred simultaneously as the patient underwent general anesthesia with appropriate lines and monitor placement. Percutaneous femoral access and bilateral cervical incisions were used to access the common carotid arteries when premanufactured devices were used. Systemic heparinization with continuous infusion was used to maintain the activated clotting time level at or around 300 seconds. The delivery device was flushed with carbon dioxide for 2 minutes and then with heparinized saline. The main device was delivered via femoral access and deployed under rapid pacing via a transvenous pacer.
For physician-modified devices, the Cook Zenith Alpha thoracic endograft was first removed from the delivery system. The bare-metal stents from the Alpha proximal component can be inverted to provide better proximal fixation, apposition, and improved radial force, functioning as a nesting stent. Because precise landing in the ascending aorta is required, and given the proximity to the aortic valve, the bare-metal stent is inverted or occasionally removed. Proximal barbs were removed from the Zenith Alpha endograft if landing into the native aorta. Any potential for trauma to the ascending aorta that may lead to retrograde dissection is thus minimized. When the ascending aorta is already replaced with a graft, the barbs are left intact. Next, based on the preoperative CTA, the branch sites were marked according to centerline and multiplanar reconstruction (MPR) measurements. Further confirmation of the sites was performed using 3D arch model prints. After applying saline to the graft material, the openings were created with a hand-held fine-tip high-temperature cautery. Then, using the gold ring of a gooseneck snare, the fenestrations were reinforced with continuous 5-0 polypropylene sutures. Afterward, the inner branches were built using a self-expandable Viabahn stent sewn to the gold ring. Of note, gold markers were placed at the free distal edge of the inner branch stent to assist in the visualization of the branch during implantation. The inner edge of the internal branches is fastened on the endograft stents for fixation. Next, a “spine”-stabilizing wire is incorporated into the device using a 0.018 Roadrunner wire (Cook Medical Europe, Bjaeverskov, Denmark). Adding the stabilizing spine wire is a crucial step in providing support and secure alignment of the device and the branch openings along the outer curve of the aortic arch (Figure 3). With a 21 gauge needle, an opening is created on the delivery system where the wire is delivered, and the wire exits at the proximal end of the sheath. Then, the stabilizing wire is used to secure the inner cannula of the device at the 12 o’clock position, which is crucial to maintain the alignment of the branches and fenestrations toward the greater curve of the aortic arch. With the 3D model, we check if the branches are aligned with the target vessel origins (Figure 5). Temporary diameter constraint was achieved using 3-0 polypropylene sutures, which were placed around the stents and 1 of the nitinol wires running between the stent and the fabric at the posterior location. The constraining sutures are released, and the device is returned to its original diameter when the nitinol wires are withdrawn during the deployment sequence. Next, the device is returned to the delivery sheath with the help of an umbilical tape and a peel-away sheath.
Figure 3.
(A) A 3-dimensional model of aortic arch is created from high-resolution computed tomography. (B) Reconstructed 3D images are then printed using a 3D printer with flexible thermoplastic polymer.
The proximal end of the aortic arch device is advanced into the ascending aorta. All devices were deployed under rapid transvenous pacing. Sequential catheterization of the target arteries is performed through brachial, carotid, and femoral access. Live 3D-fusion and IVUS guidance were used in all cases.
Statistical Analysis
Categorical variables are presented as frequencies with percentages. Continuous variables were presented as means with standard deviations or median with interquartile ranges (IQRs). A statistical analysis was performed using Microsoft Excel (Redmond, WA, USA).
Results
Patient Demographics
Nine patients were treated during the study period; 4 of which were treated using PMEGs, and 5 with CMDs. Seven patients (78%) were men. Five (56%) patients had a prior ascending aortic surgery. All patients were considered unfit for open repair. The mean maximum diameter of the aortic aneurysm was 65±9 mm. Demographics and clinical characteristics are listed in Table 1.
Table 1.
Demographics and Clinical and Anatomic Characteristics.
| Variable | PMEG (n=4) | CMD (n=5) | All (n=9) |
|---|---|---|---|
| Age, years | 62±7 | 78±13 | 70±7 |
| Male, gender | 3 (75) | 4 (80) | 7 (78) |
| Hypertension | 4 (100) | 5 (100) | 99 (100) |
| Hypercholesterolemia | 4 (100) | 3 (60) | 7 (78) |
| Smoking | 2 (50) | 1 (20) | 3 (33) |
| Coronary artery disease | 3 (75) | 1 (20) | 4 (44) |
| COPD | 2 (25) | 2 (40) | 4 (44) |
| CHF | 0 | 0 | 0 |
| PAD | 0 | 2 (40) | 2 |
| Stroke/TIA | 0 | 1 (20) | 1 |
| CKD | 6 (67) | ||
| Stage IIIa | 0 | 2 (40) | 2 (22) |
| Stage IIIb | 2 (50) | 1 (20) | 3 (33) |
| Stage IV | 0 | 1 (20) | 1 (11) |
| Previous open aortic surgery ascending | 3 (75) | 2 (40) | 5 (56) |
| Previous aortic type A dissection | 3 (75) | 2 (40) | 5 (56) |
| Diabetes | 0 | 0 | 0 |
| Maximum aortic diameter, mm | 66 | 51 | 58 |
| Serum creatinine, mg/dL | 1.55±0.78 | 1.54±0.29 | 1.55±0.5 |
| eGFR, mL/min/1.73 m2 | 53±34 | 48±16 | 50±24 |
| Body mass index, kg/m2 | 27±3 | 25±3.4 | 26±3.1 |
| ASA class | |||
| Class III | 2 (50) | 3 (60) | 5 (56) |
| Class IV | 2 (50) | 2 (40) | 4 (44) |
Values are provided as mean (SD), median (IQR), or No. (%).
Abbreviations: ASA, American Society of Anesthesiologists; CHF, congestive heart failure; CKD, chronic kidney disease; CMD, custom-made device; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; IQR, interquartile range; PAD, peripheral artery disease; PMEG, physician-modified endograft; SD, standard deviation; TIA, transient ischemic attack.
Anatomic Measurements and Stent-Graft Design
The mean diameter of the proximal sealing zone was 36 mm (IQR, 33–38 mm) in the CMD group and 37 mm (IQR 32–40 mm) in the PMEG group. The mean length from the sinotubular junction to the IA was 83 mm (median, 83 mm; IQR, 74–101 mm).
Perioperative Details
All surgeries were performed under general anesthesia. The mean volume of contrast used was 147±50 mL. Physician-modified endografts had longer mean operating time (385±121 minutes vs 319±39 minutes) and longer fluoroscopy time (85±13 vs 54±20) than CMDs. The mean length of stay was 7±4 days. Procedure details are included in Table 2.
Table 2.
Procedure Details.
| Variable | PMEG (n=4) | CMD (n=5) | All (n=9) |
|---|---|---|---|
| General anesthesia | 4 (100) | 5 (100) | 9 (100) |
| CSF drainage | 0 | 0 | 0 |
| Neuromonitoring | |||
| EEG | 1 (20) | 3 (60) | 4 (44) |
| TCD | 1 (20) | 3 (60) | 4 (44) |
| SSEP | 1 (20) | 2 (40) | 3 (33) |
| NIRS | 4 (100) | 3 (60) | 7 (77) |
| Rapid paced | 2 (50) | 4 (100) | 6 (67) |
| Fusion imaging | 4 (100) | 5 (100) | 9 (100) |
| Amount of contrast used, mL | 159 | 147±50 | 253±150 |
| Total operating time, minutes | 385±121 | 319±39 | 348±150 |
| Total fluoroscopy time, minutes | 85±13 | 54±20 | 68±23 |
| Total air kerma, Gy | 1071±407 | 714±140 | 873±327 |
| Estimated blood loss, mL | 775±822 | 310±340 | 517±609 |
| ICU stay, days | 2.8±2 | ||
| Hospital stay, days | 7±2 | 7±5 | 7.2±3.5 |
| Bilateral carotid access | 1 (25) | 5 (100) | 6 (67) |
| Technical success | 4 (100) | 5 (100) | 9 (100) |
Values are provided as mean (SD), median (IQR), or No. (%).
Abbreviations: CMD, custom-made device; CSF, cerebrospinal fluid; EEG, electroencephalography; ICU, intensive care unit; IQR, interquartile range; NIRS, near-infrared spectroscopy; PMEG, physician-modified endograft; SSEP, somatosensory evoked potential; TCD, transcranial Doppler.
Major Adverse Outcomes
Mortality and major adverse events during follow-up are summarized in Table 3. There was no in-hospital mortality. There were no strokes, transient ischemic attacks, myocardial infarction, paraplegia, or ruptures during the perioperative period. Four (33%) early major adverse events occurred in 3 patients. Two were vascular access complications, and 2 (11%) were acute kidney injuries. The median follow-up period was 358 days (company-made devices, 392 days; PMEGs, 198 days). One patient had a stroke 6 months after the surgery without significant functional impairment. One late mortality and multiple major adverse events occurred in a single patient. This patient developed severe malnutrition, renal insufficiency requiring dialysis, pneumonia, urinary tract infection, and sepsis after his endovascular arch repair. He subsequently developed enterococcus graft infection with associated aorto-esophageal fistula, leading to an open explanation of the endograft, open arch reconstruction, and pedicle omental flap on postoperative day 142. The patient was eventually discharged to a rehabilitation facility and later died on postoperative day 239. Another patient had an ascending aorta pseudoaneurysm of the previous open repair near the sinotubular junction that was percutaneously embolized at 259 days from the index procedure.
Table 3.
Perioperative Outcomes.
| Variable | PMEG (n=4) | CMD (n=5) | All (n=9) |
|---|---|---|---|
| Death | 1 | 0 | 1 |
| Myocardial infarction | 0 | 0 | 0 |
| Respiratory failure | 0 | 0 | 0 |
| Stroke | 0 | 0 | 0 |
| TIA | 0 | 0 | 0 |
| Bowel ischemia | 0 | 0 | 0 |
| Spinal cord ischemia | 0 | 0 | 0 |
| Acute kidney injury | 1 | 1 | 2 |
| New-onset dialysis | 1 | 1 | 2 |
| Access complication | 0 | 1 | 1 |
| Estimated blood loss >1 L | 1 | 0 | 1 |
| Severe malnutrition | 1 | 1 | 2 |
| Ruptures | 0 | 0 | 0 |
| Graft infection | 1 | 0 | 1 |
| Follow-up, mean days | 198 | 392 | 358 |
Abbreviations: CMD, custom-made device; PMEG, physician-modified endograft; TIA, transient ischemic attack.
Secondary Interventions and Target Vessel Patency and Instability
A total of 40 bridging stents were placed (23 in CMDs, 17 in PMEGs; Table 4). Custom-made or Gore iliac branch endoprosthesis limbs (W. L. Gore & Associates, Flagstaff, AZ, USA) were used for the IA. The most commonly-utilized stents for carotid arteries were Gore Viabahn balloon-expandable (VBX) stents (W. L. Gore & Associates, Flagstaff, AZ, USA). Left subclavian arteries were stented with Gore Viabahn self-expanding stents (W. L. Gore & Associates, Flagstaff, AZ, USA). The left subclavian artery retrograde inner branch was reinforced with VBX in most cases or with bare-metal stents.
Table 4.
Target Vessel Stents (n=40).
| Variable | IA (n=9) | LCCA (n=12) | LSA (n=19) |
|---|---|---|---|
| CMD/IBE limb | 9 | 0 | 0 |
| VBX balloon-expandable | 0 | 8 | 3 |
| Viabahn self-expanding | 0 | 1 | 7 |
| iCAST | 0 | 1 | 0 |
| Adjunctive self-expanding BMS | 0 | 1 | 3 |
| Bridging stent | 0 | 1 | 6 |
Abbreviations: BMS, bare-metal stent; CMD, custom-made device; IA, innominate artery; IBE, iliac branch endoprosthesis; LCCA, left common carotid artery; LSA, left subclavian artery; VBX, Gore Viabahn balloon-expandable stent.
Computed tomography angiography images obtained during the immediate postoperative period revealed endoleak in 6 (66%) patients. One patient required a late reintervention via left subclavian artery stenting for type IIIc endoleak secondary to undersizing the bridging stent because of incorrect graft plan labeling of the inner branch diameter by the manufacturer (Table 5). Three patients had type II endoleaks. One instance of type Ia and type Ib endoleaks occurred on the follow-up CTA. The decision was made to observe the type Ia endoleak found at 1 month follow-up as the leak was seen at the overlap zone between the arch endograft and the ascending aortic graft from prior type A repair, and the contrast did not propagate to the aneurysm sac. The type Ia endoleak resolved on the subsequent 6 month CTA. Type Ib endoleak was an expected finding as the patient had an extension of his chronic aortic dissection through his abdominal aorta, and there was a plan in place for a staged repair of the thoracoabdominal aorta in the future. No kinks, occlusions, or stenosis were seen during the follow-up period.
Table 5.
Secondary Interventions.
| Type of aortic repair | Secondary interventions | ||
|---|---|---|---|
| Days after procedure | Reason | Description | |
| Early secondary intervention, <30 days | |||
| CMD | 11 | Femoral pseudoaneurysm | Femoral pseudoaneurysm repair |
| Late secondary intervention, >30 days | |||
| CMD | 34 | Type IIIc endoleak from LSA | Revision with VBX stent 11×29 mm |
| PMEG | 254 | Ascending pseudoaneurysm of previous open repair | Embolization of ascending pseudoaneurysm with coils and Onyx |
| PMEG | 142 | Enterococcus aortic graft infection, aortoenteric fistula | Explantation of grafts, ascending graft to infrarenal aorta repair, bypass to distal innominate, ligation of LCCA and LSA, pedicle omental flap |
Abbreviations: CMD, custom-made device; LCCA, left common carotid artery; LSA, left subclavian artery; PMEG, physician-modified endograft; VBX, Gore Viabahn balloon-expandable stent.
Discussion
The early results of this study suggest that total endovascular repair of the aortic arch may be an option for patients unfit for open repair. Both company-manufactured and physician-modified devices may be used. Extensive experience with endovascular repair of complex aortic aneurysms, meticulous planning, advanced imaging capabilities, and a dedicated multispecialty team needs to be available to successfully implant these endografts. Our early data also suggest that physician-modified stent grafts are a feasible option for patients who do not meet anatomic criteria for CMDs.
Open arch repair is the standard treatment for patients with aortic arch diseases, but it is plagued with significant challenges, particularly among elderly patients or those with severe comorbidities. An increasing number of patients at our institution are considered unfit for open repair. The most frequent factors for exclusion from open repair include patient age ≥70 years, prior ascending aortic or aortic arch repair, multiple (≥2) prior sternotomies, ischemic cardiomyopathy with a multi-vessel disease and/or positive stress test, chronic pulmonary diseases with forced expiratory volume ≤1500 mL, chronic kidney disease with an eGFR ≤60 mL/kg/h, large aneurysm abutting the sternum with a risk of disruption during sternotomy, prior cervical or chest irradiation, and severe deconditioning and immobility. For these patients, who are not candidates for open repair, 2 different types of endovascular treatment options have been implemented at our institution as long as they still demonstrate reasonable life expectancy and may benefit from repair.
The present study contributes to the current treatment of aortic arch diseases as no significant case series of patients undergoing physician-modified 3-vessel endovascular arch repair have been reported. Although the current sample size for PMEGs is small, our early experience and device design technique, as outlined above, indicate that modified devices may be used with acceptable results, comparable to premanufactured devices. 10 The feasibility of 3-vessel endovascular arch repair has been demonstrated in a study by Haulon et al. 11 However, company-made devices are only accessible in select centers, limited by manufacturing delays, and with strict anatomic requirements. 12 Physician-modified endografts offer an alternative for patients unfit for open or endovascular repair with off-the-shelf devices and can achieve comparable early outcomes regarding major adverse events and procedural technical success. As reported here, our early outcomes compare favorably against open arch repair and previously-published endovascular arch repair.
Although other endovascular therapies for arch diseases have been reported, the 3-vessel endovascular arch repair described here offers a superior proximal seal compared to scallops, parallel chimney grafts, or in-situ fenestrations. Achieving an adequate proximal seal zone remains a significant challenge in most endovascular arch repairs. A recent review of physician-modified arch repair showed endoleak rates up to 52%, with type I endoleak being the most prevalent endoleak type at 77.8%. 11 Experience with arch PMEGs in the past with a short seal zone distal to the left common carotid artery (mean length 11 mm, [5–15 mm]) resulted in a 32.4% type Ia endoleak rate at the time of discharge and 16.2% aneurysm enlargement at follow-up. 2 In patients who underwent chimney graft arch repairs, the main issues are the rate of type Ia endoleaks and gutters, which were 13% in single-/double-chimney repairs and 42% in triple-chimney repairs.13,14 The long-term durability of this type of repair is in serious jeopardy based on these early outcomes. The 3-vessel arch branch repair allows for the proximal extension of the endograft and complete anatomic sealing of the bridging stents and inner branches, improving the proximal seal without the concern for gutter leaks that may be seen in chimney grafts. The importance of a good proximal seal has been demonstrated in another study by Canaud et al., 15 who described a total endovascular aortic repair with double-fenestrated physician-modified endovascular grafts for zone 0, using large fenestration for the IA and left common carotid artery without stent placement along with left subclavian artery fenestration and stent. 16 In their cohort of 50 patients, they achieved 0% type Ia endoleak and 8% reintervention rates at 16 months. 16 The lack of complete sealing around the origin of the great vessels and the inevitable progression of disease are critical limitations for the long-term success and durability of these repairs.
Our design for the physician-modified endograft had an IA retrograde or antegrade inner branch and 2 inner branches or fenestrations for the left common carotid artery and left subclavian artery. The innominate inner branch configuration is required to attain complete sealing in the target artery, which, given its size and anatomic configuration, requires larger self-expanding bridging stents, like those used for iliac arteries. Fenestrations and flaring of large balloon-expandable covered stents would not provide adequate proximal and distal fixation and sealing at this level. Based on the patient's anatomy, the innominate inner branches bridging stents can be designed in a retrograde or antegrade fashion. This alleviates difficulties in aortic arch navigation, especially in patients with acute angulation of the aortic arch. Even though inner branches were initially used for the common carotid and left subclavian arteries, fenestrations have been used more recently as these appear to provide adequate sealing, and their alignment is usually not an issue. The other critical aspect of the PMEG design is incorporating the spiral attachment wire using an interwoven 0.018 wire. This additional “spine”-stabilizing wire helps to prevent misalignments of the external openings of the inner branches and fenestrations at the time of deployment. During the early trials of PMEGs, we had difficulty aligning the fenestrations to the target arch vessels. A better method to secure the graft with the precurved inner cannula along the outer curve of the arch was necessary to facilitate the lineup of the graft to the 12 o’clock position. When the CMD became available at our institution, we noted that the first trigger wire of the deployment sequence was the spiral “spine” wire. We adapted this concept to the PMEG by adding a 0.018 in nitinol wire that secures the precurved cannula to the 12 o’clock position of the graft, which is removed following vessel cannulation. Preloaded catheters, as seen in CMDs, were not incorporated in our device modification. However, this did not impair the ability for branch cannulation in the authors’ experience. Endovascular aortic arch repair is still a technology in evolution, and long-term follow-up will bring better device design to achieve more durable repairs.
Our center currently uses 3D models to aid with the placement of the inner branches and fenestrations. This technique has been previously described by Baron and Guevara as an additional guide to achieving more accurate placement of fenestrations at the time of device modification. 17 The 3D model is created based on high-resolution CTA and printed using a 3D printer to a flexible thermoplastic polymer (Figure 5). In addition to individual measurements based on the imaging software, the 3D model allows the operator to confirm the location of the fenestrations and branches and assists in resolving discrepancies between MPR and centerline measurements.18,19 We also print the entire ascending aorta and aortic arch in select cases for bench-testing modified devices. This is particularly helpful in more challenging anatomies to observe the ex-vivo device behavior and to simulate any pitfalls such as misalignment or inability to achieve seal.
Our major adverse outcome from this study was the mortality following the endograft infection requiring eventual explantation. Based on the current literature, thoracic endograft infection rates range from 1.5% to 4.7%.3,4 Although endograft infection rates are low, such devastating complications are associated with an exceedingly-high mortality rate up to 70%.3,4 As mentioned earlier, the source of endograft infection in our series was likely related to the patient’s postoperative pneumonia, urinary tract infection, and sepsis.
There are several limitations to our study. This is a retrospective, single-center case series. Further investigation with a larger sample size is warranted. All operations were performed by operators with a robust experience in all types of thoracoabdominal aortic procedures in a center where fenestrated/branched endovascular aortic repair is performed regularly. This limits the generalizability of our findings, and similar outcomes may not be reproducible.
Conclusion
Our early case series of total endovascular aortic arch repair using 3-vessel stent-grafts with either premanufactured or physician-modified endografts suggests that this approach may be an acceptable option for patients unfit for open repair. Early mortality and major complication rates are acceptable and comparable to those in previous reports of both open and endovascular arch repairs.
Supplemental Material
Supplemental material, sj-png-1-jet-10.1177_15266028231163069 for Early Results and Feasibility of Total Endovascular Aortic Arch Repair Using 3-Vessel Company-Manufactured and Physician-Modified Stent-Grafts by K. Benjamin Lee, Jesus Porras-Colon, Carla K. Scott, Khalil Chamseddin, Mirza S. Baig and Carlos H. Timaran in Journal of Endovascular Therapy
Footnotes
Authors’ Note: This study was presented at the 45th Annual Meeting of the Southern Association for Vascular Surgery, Manalapan, FL, USA, January 19–22, 2021.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C.H.T. has been a consultant for and received research support from Cook Medical Inc, W. L. Gore & Associates, Inc, and Phillips Healthcare. The other editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: K. Benjamin Lee  https://orcid.org/0000-0003-2938-9227
https://orcid.org/0000-0003-2938-9227
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Estrera AL, Sandhu HK, Afifi RO, et al. Early and late outcomes after complete aortic replacement. Ann Thorac Surg. 2015;100(2):528–534. [DOI] [PubMed] [Google Scholar]
- 2. Kurimoto Y, Maruyama R, Ujihira K, et al. Thoracic endovascular aortic repair for challenging aortic arch diseases using fenestrated stent grafts from zone 0. Ann Thorac Surg. 2015;100(1):24–32; discussion 32–33. [DOI] [PubMed] [Google Scholar]
- 3. Lyons OT, Patel AS, Saha P, et al. A 14-year experience with aortic endograft infection: management and results. Eur J Vasc Endovasc Surg. 2013;46(3):306–313. [DOI] [PubMed] [Google Scholar]
- 4. Moulakakis KG, Mylonas SN, Antonopoulos CN, et al. Comparison of treatment strategies for thoracic endograft infection. J Vasc Surg. 2014;60(4):1061–1071. [DOI] [PubMed] [Google Scholar]
- 5. Urbanski PP, Luehr M, Di Bartolomeo R, et al. Multicentre analysis of current strategies and outcomes in open aortic arch surgery: heterogeneity is still an issue. Eur J Cardiothorac Surg. 2016;50(2):249–255. [DOI] [PubMed] [Google Scholar]
- 6. Spear R, Haulon S, Ohki T, et al. Editor’s choice—subsequent results for arch aneurysm repair with inner branched endografts. Eur J Vasc Endovasc Surg. 2016;51(3):380–385. [DOI] [PubMed] [Google Scholar]
- 7. Oderich GS, Forbes TL, Chaer R, et al. Reporting standards for endovascular aortic repair of aneurysms involving the renal-mesenteric arteries. J Vasc Surg. 2021;73(1S):4S–52S. [DOI] [PubMed] [Google Scholar]
- 8. Fillinger MF, Greenberg RK, McKinsey JF, et al. Reporting standards for thoracic endovascular aortic repair (TEVAR). J Vasc Surg. 2010;52(4):1022–1033, 1033.e15. [DOI] [PubMed] [Google Scholar]
- 9. Safi HJ, Miller CC, III, Estrera AL, et al. Optimization of aortic arch replacement: two-stage approach. Ann Thorac Surg. 2007;83(2):S815–S818; discussion S824–S831. [DOI] [PubMed] [Google Scholar]
- 10. Tenorio ER, Oderich GS, Kölbel T, et al. Multicenter global early feasibility study to evaluate total endovascular arch repair using three-vessel inner branch stent-grafts for aneurysms and dissections. J Vasc Surg. 2021;74(4):1055–1065.e4. [DOI] [PubMed] [Google Scholar]
- 11. Mougin J, Azogui R, Guihaire J, Tyrrell MR, Oderich GS, Fabre D, Haulon S. “First in Man” Total Percutaneous Aortic Arch Repair With 3-Inner-branch Endografts: A Report of Two Cases. Ann Surg. 2021. Dec 1;274(6):e652-e657. [DOI] [PubMed] [Google Scholar]
- 12. Blanco Amil CL, Mestres Alomar G, Guarnaccia G, et al. The initial experience on branched and fenestrated endografts in the aortic arch. Ann Vasc Surg. 2021;75:29–44. [DOI] [PubMed] [Google Scholar]
- 13. Wang T, Shu C, Li M, et al. Thoracic endovascular aortic repair with single/double chimney technique for aortic arch pathologies. J Endovasc Ther. 2017;24(3):383–393. [DOI] [PubMed] [Google Scholar]
- 14. Wang L, Huang Y, Guo D, et al. Application of triple-chimney technique using C-TAG and Viabahn or Excluder iliac extension in TEVAR treatment of aortic arch dilation diseases. J Thorac Dis. 2018;10(6):3783–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Canaud L, Chassin-Trubert L, Abouliatim I, Hireche K, Bacri C, Alric P, Gandet T. Total Arch Thoracic Endovascular Aortic Repair Using Double Fenestrated Physician-Modified Stent-Grafts: 100 Patients. J Endovasc Ther. 2022. Aug 4. [DOI] [PubMed] [Google Scholar]
- 16. Chassin-Trubert L, Gandet T, Lounes Y, et al. Double fenestrated physician-modified stent-grafts for total aortic arch repair in 50 patients. J Vasc Surg. 2021;73(6):1898–1905.e1. [DOI] [PubMed] [Google Scholar]
- 17. Barón V, Guevara R. Three-dimensional printing-guided fenestrated endovascular aortic aneurysm repair using open source software and physician-modified devices. J Vasc Surg Cases Innov Tech. 2019;5(4):566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Müller-Eschner M, Rengier F, Partovi S, et al. Accuracy and variability of semiautomatic centerline analysis versus manual aortic measurement techniques for TEVAR. Eur J Vasc Endovasc Surg. 2013;45(3):241–247. [DOI] [PubMed] [Google Scholar]
- 19. Rengier F, Weber TF, Partovi S, et al. Reliability of semiautomatic centerline analysis versus manual aortic measurement techniques for TEVAR among non-experts. Eur J Vasc Endovasc Surg. 2011;42(3):324–331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-png-1-jet-10.1177_15266028231163069 for Early Results and Feasibility of Total Endovascular Aortic Arch Repair Using 3-Vessel Company-Manufactured and Physician-Modified Stent-Grafts by K. Benjamin Lee, Jesus Porras-Colon, Carla K. Scott, Khalil Chamseddin, Mirza S. Baig and Carlos H. Timaran in Journal of Endovascular Therapy