Abstract
Background
HTLV-1 is a worldwide distribution retrovirus with 10–20 million infected individuals. ATLL is an Adult T-cell leukaemia lymphoma caused by aggressive T-cell proliferation that is infected by HTLV-1 and is associated with an inferior prognosis. The exact molecular pathogenesis has yet to be fully understood. CREB, a transcription factor, acts as a molecular switch that controls the expression of numerous genes in response to various extracellular signals. Its activation is primarily mediated through phosphorylation by multiple kinases, including MAPKs. MAPKs, a family of serine/threonine kinases, serve as crucial mediators of intracellular signaling cascades.
Method and material
This study investigated, 38 HTLV-I-infected individuals, including 18 HTLV-1 asymptomatic carriers (ACs) and 20 ATLL subjects. mRNA was extracted and converted to cDNA from Peripheral blood mononuclear cells (PBMCs), and then the expression of TAX, HBZ, CREB, and MAPK was analyzed by TaqMan qPCR. The genomic HTLV-1 Proviral loads were examined among the study group.
Results
The data analysis showed a significant difference in the mean of CREB expression amongst study groups (ATLL and carriers, (p = 0.002). There is no statistical difference between the MAPK gene expression (p = 0.35). HBZ, TAX, and HTLV-1 proviral load weree significantly higher in ATLL subjects compared to ACs (p = 0.002, 0.000, and 0.000), respectively. Moreover, our results, demonstrated a direct positive correlation among HBZ, CREB, and TAX gene expression in ATLL patients (p = 0.001), whilst between the ACs, TAX gene expression had a positive significant correlation with HBZ and HTLV-1 proviral load (p = 0.007 and p = 0.004, respectively).
Conclusion
The present study demonstrated that CREB gene expression was higher in the ATLL group than ACs, while there was no difference for MAPK. Therefore, this pathway may not strongly involve in the activation of CREB. The CREB may be a prognostic factor for the development of HTLV-I-associated diseases and can be used as a monitoring marker for the efficiency of the therapeutic regime and prognosis.
Keywords: HTLV-1, ATLLATLLL, HTLV-1-aymptomatic carriers, CREB, MAPK, TAX, HBZ
Introduction
HTLV-1 belongs to the Retroviridae family and is transmitted primarily through sexual contact, blood transfusion, or from mother to child via breastfeeding [1]. The virus primarily targets CD4 + T cells and integrates its genetic material into the host genome [2]. HTLV-1 has a long latency period, with symptoms typically manifesting after several decades of infection [3]. This retrovirus is estimated to infect more than 10 million people worldwide [2, 3]. While the majority of individuals infected with HTLV-1 remain asymptomatic carriers, a small percentage can develop into severe complications, including adult T-cell leukemia/lymphoma (ATLL), HTLV-1 associate uveitis, and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) [3, 4]. Adult T-cell Leukemia/Lymphoma (ATLL): ATLL is a rare and aggressive form of T-cell lymphoma. It is characterized by uncontrolled proliferation of infected CD4 + T cells and can affect various organs, including the skin, lymph nodes, liver, and spleen. ATLL is associated with a poor prognosis, and treatment options are often limited. [5, 6].
HTLV-1 is the retrovirus that encoded several regulatory proteins which interfere with cellular processes, leading to uncontrolled cell proliferation and evasion of immune surveillance [7, 8]. These viral proteins, including The HTLV-1 transactivator protein Tax and HTLV-1 bZIP factor (HBZ), play crucial roles in modulating cellular signaling pathways and altering gene expression, contributing to the development of ATLL [6, 9].
The Mitogen-Activated Protein Kinase (MAPK) and cAMP response element-binding protein (CREB) signaling pathways play crucial roles in various cellular processes, including cell growth, survival, differentiation, and gene expression [10, 11]. These pathways serve as critical regulators of physiological and pathological conditions, making them subjects of extensive research [12, 13].
The MAPK pathway consists of a cascade of protein kinases that transmit signals from cell surface receptors to the nucleus, where they regulate gene expression. The three major MAPK subfamilies are extracellular signal-regulated kinases (ERK), c-Jun N-terminal kinases (JNK), and p38 MAPK [14, 15].
Various extracellular stimuli, including growth factors, cytokines, and stress signals, activate this pathway. Upon activation, MAPKs phosphorylate downstream targets, such as transcription factors, leading to alterations in gene expression and cellular responses [10, 16]. The ERK pathway is primarily associated with cell growth and proliferation, while JNK and p38 MAPK pathways are involved in stress responses, inflammation, and apoptosis [10, 17].
CREB is a transcription factor that regulates gene expression in response to a diverse range of extracellular signals. It is involved in numerous cellular processes, including neuronal plasticity, metabolism, cell survival, and immune responses [18, 19].
CREB is activated by various kinases, including protein kinase A (PKA) and MAPKs, phosphorylating a specific site (Ser133) [20, 21]. Upon activation, phosphorylated CREB binds to cAMP response elements (CREs) in the promoter regions of target genes, modulating their transcription [20, 21]. CREB regulates the expression of genes involved in cell survival, synaptic plasticity, and memory formation [19, 21].
The MAPK pathway can activate CREB through the phosphorylation of CREB by ERK or other MAPKs [15, 17]. Conversely, CREB can influence MAPK signaling by regulating the expression of MAPK pathway components or interacting with MAPK signaling intermediates. This crosstalk allows for the integration and coordination of signaling events, leading to precise cellular responses.
Dysregulation of MAPK and CREB pathways plays a significant role in cancer development [15, 17]. Aberrant activation of MAPKs, particularly ERK, is commonly observed in various cancers, promoting cell proliferation, survival, and metastasis [15, 17]. CREB is also implicated in cancer progression, as it regulates the expression of genes involved in tumor growth and angiogenesis [21, 22].
Altered MAPK and CREB signaling have been associated with neurodegenerative diseases, including Alzheimer's disease, Parkinson's disease, neuroblastoma, brain tumor, and Huntington's disease [19, 23, 24]. Dysregulated MAPK signaling contributes to neuronal death and impaired synaptic plasticity, while altered CREB activity affects memory formation and cognitive functions.
Ongoing research efforts aim to unravel the mechanisms underlying HTLV-1 pathogenesis, improve diagnostic tools, and develop targeted therapies. Understanding the intricate interaction between HTLV-1 and the host immune system is crucial for developing effective treatment strategies, vaccines, and preventive measures. Additionally, efforts to raise awareness about HTLV-1 transmission and promote safe practices are essential to prevent new infections. Therefore, this study aims to investigate the involvement and significance of the MAPK and CREB signaling pathways in the pathogenesis of Adult T-Cell Leukemia/Lymphoma (ATLL) patients.
Method and material
Collection of samples
The study included 20 new cases of ATLL patients who had not received any prior therapeutic regimen at the time of the investigation and 18 HTLV-1 healthy carriers who were referred to the hematology-oncology department of Ghaem and Imam Reza hospitals in Mashhad, Iran, between January 2018 and December 2021. All included patients were matched by age and gender.
PBMCs and RNA extraction
Blood samples were collected from all participants before any treatment or chemotherapy. The peripheral blood mononuclear cells (PBMCs) were isolated from the blood using the ficoll gradient method, and RNA was extracted from the PBMCs using Trizol reagents (Lymphodex INNO-TRAIN, company Cat Num 002 041 600).
cDNA synthesis
To prevent contamination, the extracted RNA was treated with DNase before converting it to cDNA. The integrity of the RNA was confirmed using spectrophotometry and electrophoresis. Complementary DNA (cDNA) was synthesized using random primers and reverse transcriptase (Cat Num: K-2046.Bioneer). According to the manufacturer’s protocol, the reaction conditions were 30 s at 24 °C, 4 min at 44 °C, and 30 s at 55 °C. These conditions were repeated 12 times, and the reaction stopped at 5 min 95 °C. The synthesis procedure was confirmed using GAPDH primers.
HTLV-1 proviral load and gene expression measurement
Specific primers for the genes of interest (TAX, HBZ, CREB, and MAPK) were designed using Allele ID software (Table 1). Real-time PCR (TaqMan method) was performed by Real-Time PCR Thermocycler Qiagen Q using the AccuPower® Plus DualStar™Master Mix (2X, Cat Num: K-6603) and the designed primer sets. All samples were analyzed in duplicate, and the expression of the β2 Micro globulin gene was used as a reference.
Table 1.
Primer and probe of TAX, HBZ, CREB, MAPK, and β2 Micro globulin genes
| Name of Gene’s interest | Forward Primer (5′–3′) | Reveres Primer (5′–3′) | Probe (5′–3′) Fam-BHQ1 |
|---|---|---|---|
| TAX | ATCCCGTGGAGACTCCTCAA | CCTGGGAAGTGGGCCATG | CATGCCCAAGACCCGTCGGAGG |
| HBZ | CTCGACCTGAGCTTTAAACTTACC | CATGACACAGGCAAGCATCG | CGGACGCAGTTCAGGAGGCACCAC |
| CREB | ACTCCAAAAGTAAAGTCCCGTTAC | TCCAAAGAAACAGGAAGCAGATTG | TTCTCCTCCCACCGCCCTTGTCCC |
| MAPK | GAGCAGTATTACGACCCGAGTG | TCCTTAGGCAAGTCATCCAATTCC | AGCCCATCGCCGAAGCACCATTCA |
| β2 Micro globulin | TTGTCTTTCAGCAAGGACTGG | CCACTTAACTATCTTGGGCTGTG | TCACATGGTTCACACGGCAGGCAT |
The PCR reaction involved heating the sample at 95 °C for 4 min, followed by 45 cycles of denaturation at 94 °C for 15 s, annealing at the optimal temperature for 20 s, and extension at 72 °C for 20 s.
To measure the proviral load of HTLV-I, PBMCs were isolated from blood samples treated with EDTA. Real-time PCR was performed using a commercial kit (Novin Gene, Iran) to quantify the HTLV-I proviral load. Specific primers and a fluorogenic probe were used, and the HTLV-I copy number was determined by comparing it to the albumin gene as a reference. The HTLV-I proviral load was expressed as the number of HTLV-I proviruses per 104 PBMCs.
Statically analysis
Statistical analysis was conducted using SPSS version 18. The mean ± SD values were presented, and statistical methods such as Mann–Whitney and Spearman analysis were used to compare the differences between the ATLL group and the healthy control group. A p-value of less than 0.05 was considered a statistically significant difference.
Ethical statement
The sampling protocols mentioned above were approved by the Research Ethics Committee of Mashhad University of Medical Sciences in Iran (ethical code: 910,679). All patients provided written informed consent.
Results
Clinical manifestation and demographic data
The average age of the ATLL patients was fifty-three years, while for the healthy carriers, it was forty-nine years.
Among the ATLL subjects, seventeen cases presented lymphoid gland involvement, five individuals had skin lesions, and six patients had immunodeficiency situations with a body temperature higher than 38.5 degrees centigrade. Only two patients exhibited both lymphadenopathy and skin lesions, and only one patient had both immunodeficiency and skin lesions at the same time. None of the patients in this study exhibited all three clinical symptoms concurrently.
Gene expression results
Viral pathogen markers
The qRT-PCR TaqMan method was applied to investigate viral pathogen markers such as HBZ and TAX gene expression. The expressions of these transcription factors were normalized by dividing them by the reference gene (B2M). This normalization process allowed for the calculation of a Normalized Index (NI) for each gene of interest in each sample.
Higher mRNA expression levels of the TAX gene were observed between the ATLL and healthy carrier groups, as shown in Fig. 1. The mean expression ratio of TAX to β2 microglobulin was 1.9 ± 1.6 NI in the ATLL group and 0.007 ± 0.007 NI in the control group. Statistical analysis using the Mann–Whitney test revealed a significant difference in TAX gene expression between the two groups (CI 95%-p = 0.002). The TAX mRNA expression was only observed in 40 percent of ATLL patients, while minimal gene expression was identified in fifty percent of asymptomatic carriers.
Fig. 1.
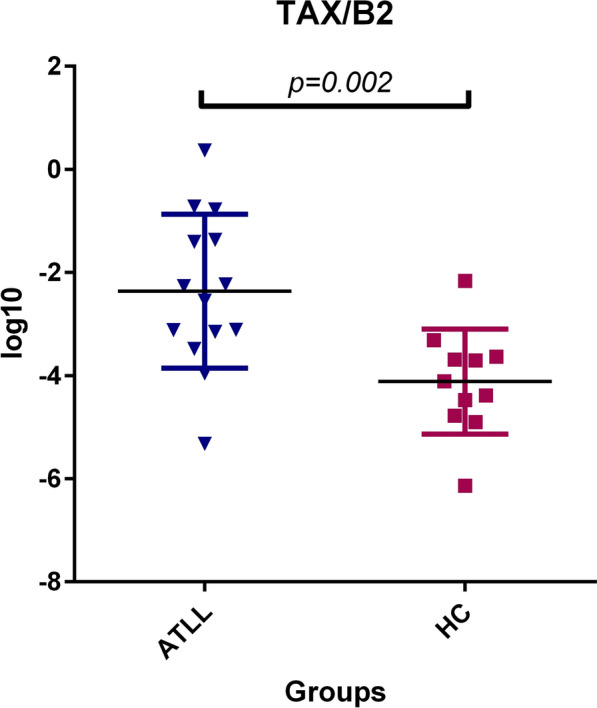
Gene expression of the Tax gene was found to be significantly higher in patients with ATLL compared to asymptomatic carriers (ACs) (p = 0.002, Mann–Whitney U test). Tax is a transcriptional activator of the PX region and is associated with Adult T cell Leukemia lymphoma (ATLL). HC: Healthy carrier
In addition, mRNA expression levels of the HBZ gene were increased among the ATLL compared to healthy carrier groups, as shown in Fig. 2. The mean expression ratio of HBZ to β2 microglobulin was 1.01 ± 0.6 in the ATLL group and 0.001 ± 0.0005 in the control group. Furthermore, statistical analysis revealed a highly significant difference in HBZ gene expression among the study population (CI 95%-p = 0.000).
Fig. 2.
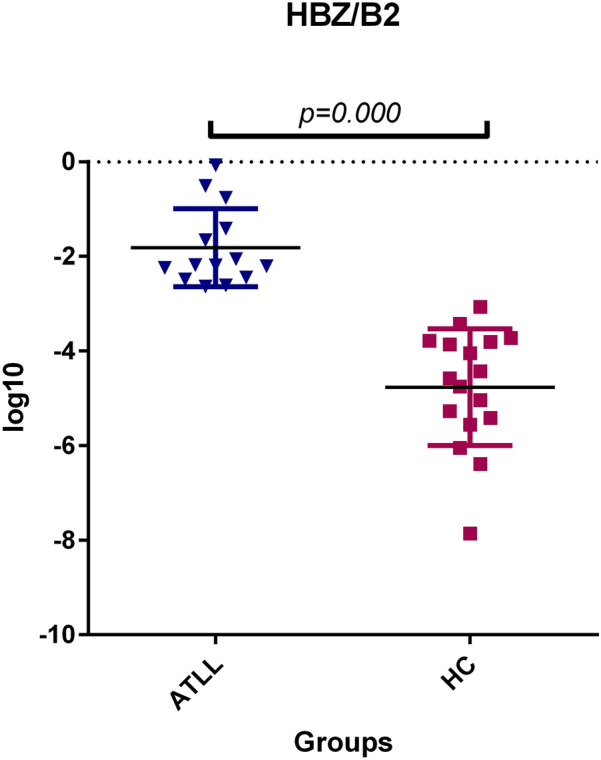
Gene expression of the HBZ gene was found to be significantly higher in patients with ATLL compared to asymptomatic carriers (ACs) (p = 0.000, Mann–Whitney U test). HBZ is a transcriptional activator of the PX region and is associated with Adult T cell Leukemia lymphoma (ATLL). HC: Healthy carrier
HTLV-1 proviral load
The average HTLV-1 proviral load (PVL) in ATLL patients was 114.4 ± 37.7 copies/10 4 PBMCs (95% CI: 0.53–590). This data suggests that some of the infected T cells have more than one copy of the HTLV-1genome.
In comparison, the average HTLV-I proviral load among Healthy Carriers was 5.3 ± 1.2 copies/10 4PBMCs (95% CI: 0–13.3) (Fig. 3). These values indicate a significantly higher PVL and a significant difference in proviral load between the study population, as determined by Mann–Whitney analysis (p-value = 0.000).
Fig. 3.
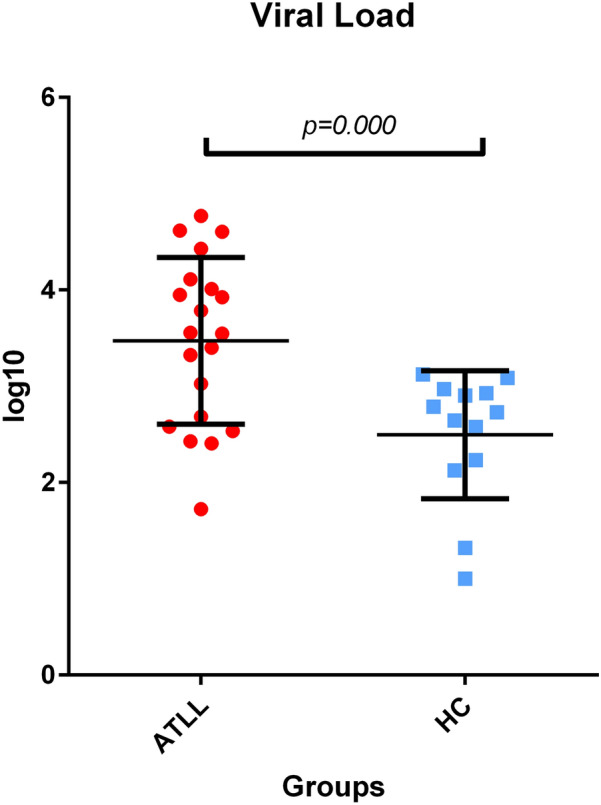
HTLV-1 proviral load was significantly higher in ATLL compared to healthy carriers (p = 0.000, Mann–Whitney U test). ATLL is Adult T-cell Leukemia lymphoma, and HC is a healthy carrier
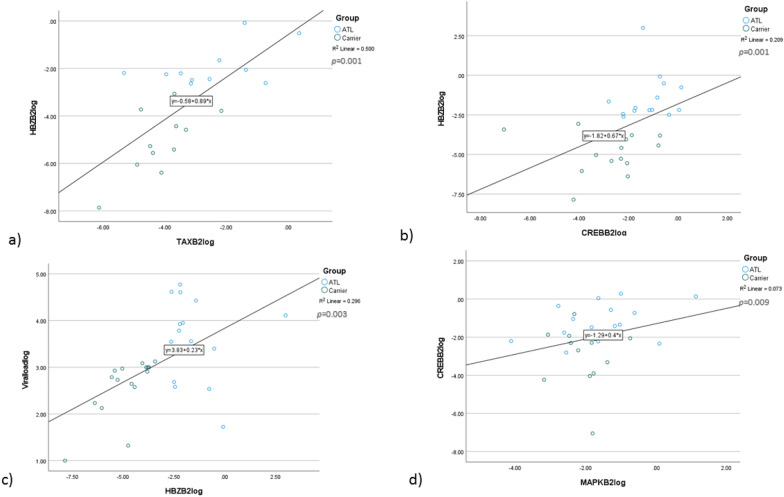
Among all the measured viral markers, the TAX and HBZ have a direct positive correlation on their mRNA expression (R = 0.63, p = 0.001), while only HBZ demonstrated a positive correlation with proviral load (R = 0.5, p = 0.003) (Fig. 4a–c) (Table 2).
Fig. 4.
Gene Correlation Analysis of TAX, HBZ, CREB, and MAPK in Relation to HTLV-1 Proviral Load. a Direct Positive Correlation Between TAX and HBZ in ATLL Patients. b Significant Positive Correlations Among HBZ, and CREB, among ATLL Study Group. c Positive Correlations of HBZ Gene Expression with and HTLV-1 Proviral Load in ATLL subjects. d Significant Positive Correlation Between MAPK and CREB in ATLL patients. To better demonstrate we considered log10 index for gene expression values
Table 2.
The gene expression correlations are demonstrated in the table
| Gene Expression Correlations | TAX/B2 | HBZ/B2 | Viral Load | CREB/B2 | MAPK/B2 | ||
|---|---|---|---|---|---|---|---|
| Spearman's rho | TAX/B2 | Correlation Coefficient | 1.000 | 0.636** | 0.203 | 0.264 | 0.144 |
| Sig. (2-tailed) | . | 0.001 | 0.354 | 0.224 | 0.533 | ||
| N | 25 | 22 | 23 | 23 | 21 | ||
| HBZ/B2 | Correlation Coefficient | 0.636** | 1.000 | 0.507** | 0.585** | 0.187 | |
| Sig. (2-tailed) | 0.001 | . | 0.003 | 0.001 | 0.394 | ||
| N | 22 | 38 | 33 | 27 | 23 | ||
| Viral Load | Correlation Coefficient | 0.203 | 0.507** | 1.000 | 0.294 | -0.248 | |
| Sig. (2-tailed) | 0.354 | 0.003 | . | 0.109 | 0.211 | ||
| N | 23 | 33 | 41 | 31 | 27 | ||
| CREB/B2 | Correlation Coefficient | 0.264 | 0.585** | 0.294 | 1.000 | 0.484** | |
| Sig. (2-tailed) | 0.224 | 0.001 | 0.109 | . | 0.009 | ||
| N | 23 | 27 | 31 | 33 | 28 | ||
| MAPK/B2 | Correlation Coefficient | 0.144 | 0.187 | -0.248 | 0.484** | 1.000 | |
| Sig. (2-tailed) | 0.533 | 0.394 | 0.211 | 0.009 | . | ||
| N | 21 | 23 | 27 | 28 | 29 | ||
The significant correlations are bolded to better identification. **Correlation is significant at the 0.01 level (2-tailed)
MAPK and CREB gene expression level
The mRNA expression levels of MAPK indicate non-significant differences between ATLL and healthy carriers (Fig. 5). The mean of MAPK / β2 Micro globulin was 9.6 ± 8.3 NI and 0.25 ± 0.13 NI in ATLL and Healthy carriers, respectively, which indicates a higher gene expression level in the ATLL patients' group. However, this value does not prove a statistically significant difference (CI 95% p = 0.351).
Fig. 5.
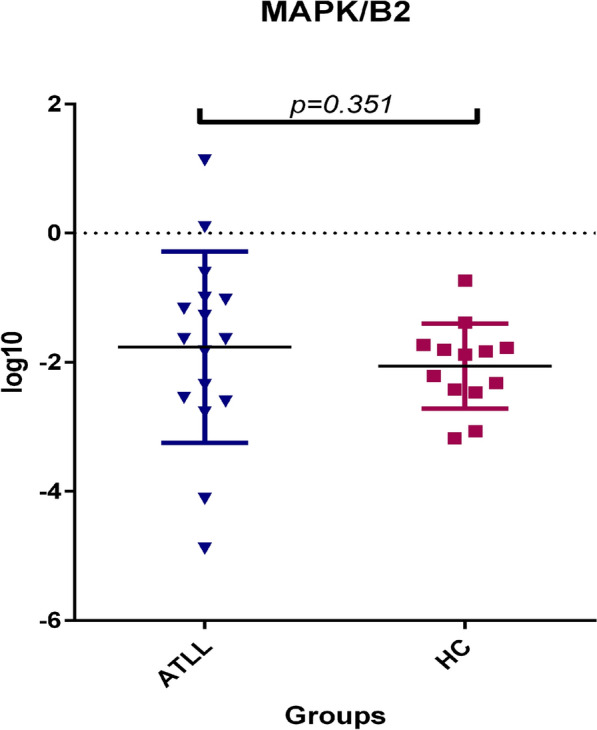
Gene expression of the MAPK gene was found to be higher in patients with ATLL but is not statically significant in comparison to asymptomatic carriers (ACs) (p = 0.351, Mann–Whitney U test). Mitogen-activated protein kinases are serine and threonine protein kinases. ATLL: Adult T cell Leukemia lymphoma. HC: healthy carrier
The CREB gene expression level was significantly higher in the ATLL group than in the asymptomatic carrier group (CI 95% p = 0.002) (Fig. 6). The mean of CREB/ β2 Micro globulin was 3.2 ± 1.3 and 0.27 ± 0.15 in the ATLL and healthy carrier groups, respectively.
Fig. 6.
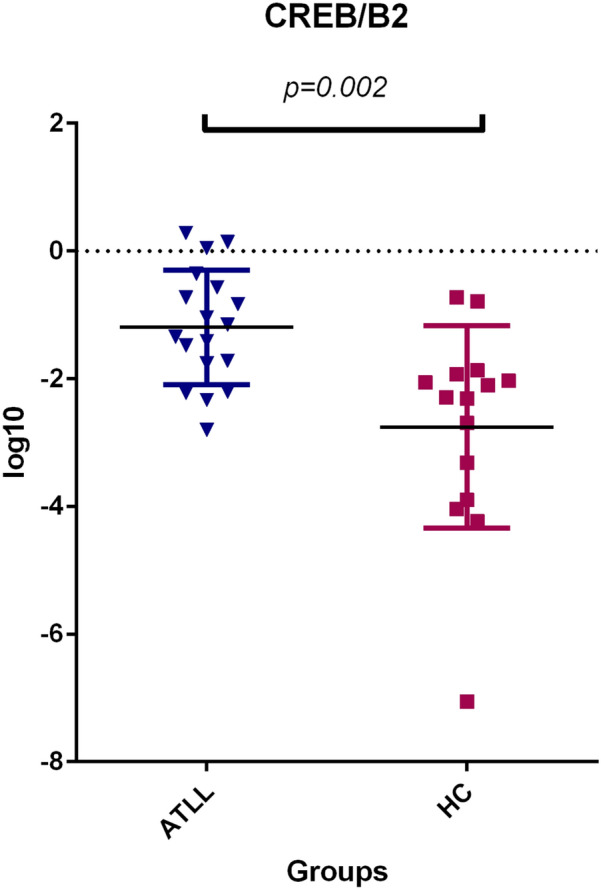
Gene expression of the CREB gene was found to be significantly higher in patients with ATLL compared to asymptomatic carriers (ACs) (p = 0.002, Mann–Whitney U test). CREB or cAMP response element-binding protein is a cellular transcription factor and is associated with ATLL. HC: healthy carrier. ATLL: Adult T cell Leukemia lymphoma
MAPK gene expression is directly correlated with CREB to increase gene activation and produce more mRNA synthetizes among all study groups (R = 0.48, p = 0.009) (Fig. 4d). In addition, the Spearman correlation analysis for determining virus-host interaction was done and demonstrated that only HBZ has a positive direct correlation with CREB gene expression, which indicates the acceleration of mRNA expression (R = 0.58, p = 0.001) (Fig. 4b).
Discussion
Adult T-cell leukemia/lymphoma (ATLL) is a rare and aggressive form of T-cell malignancy caused by human T-cell lymphotropic virus type 1 (HTLV-1) infection. The exact molecular aspects of HTLV-1 pathogenesis are not fully understood, but the role of two main viral proteins, TAX and HBZ, are demonstrated [6, 25]. These proteins can manipulate several signaling pathways, which can change the regular cell cycles and induce the malignant condition [26, 27]. Our findings demonstrated that both TAX and HBZ significantly increased in ATLL patients compared to asymptomatic patients. In addition, our results demonstrated that the TAX protein can only be expressed in less than half of patients, which may result from its excellent immune dominant properties. Furthermore, the Spearman correlation examination proved that only HBZ gene expression has a direct relation with increasing HTLV-1 proviral load, while the TAX gene expression has a significant positive correlation with the HBZ gene. Therefore, the HTLV-1 can multiply its genome to produce the HBZ protein or mRNA then it led to less increase in TAX gene expression among ATLL patients.
The pathogenesis of ATLL also may involve dysregulation of various signaling pathways, including the cAMP response element-binding protein (CREB) and mitogen-activated protein kinase (MAPK) pathways. Our findings demonstrated that the CREB mRNA level is significantly increased within the ATLL patients and is often overexpressed, leading to the upregulation of target genes involved in cell cycle progression, anti-apoptotic pathways, and immune evasion [19, 28].
Tax has been shown to contribute to the development of aneuploidy in HTLV-I-transformed cells. Aneuploidy refers to an abnormal number of chromosomes in a cell, which can have detrimental effects on cell division and function [29]. In a study conducted on CTLL cells stably expressing Tax, it was observed that these cells exhibited aneuploidy compared to a Tax clone deficient for CREB transactivation. These findings suggest that Tax transactivation through the CREB/ATF pathway plays a role in the aneuploid phenotype [29]. Further analysis of altered genes in Tax-expressing cells revealed the presence of CREB/ATF consensus sequences. These genes had diverse functions, with subsets involved in G2/M phase regulation, particularly kinetochore assembly [29, 30]. Chromatin immunoprecipitation confirmed the presence of CREB, Tax, and RNA Polymerase II at specific gene promoters in Tax-expressing cells, further supporting the role of Tax in altering the transcription profile and contributing to aneuploidy. The Spearman correlation results do not demonstrate the direct association between TAX and CREB expression. This may be because most of our patients did not present flower-like cells in their peripheral blood smears.
Tax is known to recruit the human transcriptional coactivator and histone acetyltransferase p300/CBP to the HTLV-I promoter, aiding in viral replication and pathogenesis [29]. The interaction between Tax and the KIX domain of p300/CBP has been extensively studied [31]. Circular dichroism spectroscopy, nuclear magnetic resonance chemical shift perturbation mapping, and sedimentation equilibrium analysis have shed light on the structural features of this interaction [29, 31]. The Tax-binding surface of the KIX domain is distinct from that utilized by cellular transcription factors, demonstrating the specific interaction between Tax and p300/CBP. Moreover, it has been shown that Tax and the phosphorylated KID domain of CREB can simultaneously bind to the KIX domain, forming a ternary complex [31]. These findings provide a molecular understanding of the recruitment of p300/CBP by Tax and phosphorylated CREB, which is crucial for Tax-mediated gene expression.
We found that CREB mRNA has a direct accumulative correlation with HBZ gene expression statutes. Therefore, the HBZ can induce the CREB mRNA transcription to manipulate the cell signaling pathway and activate its related transcription factors [32, 33]. Previous studies investigated the interaction between HTLV-1 viral protein and CREB signaling pathway. Their research revealed that HBZ and the CREB have an interaction both in vivo and in vitro, and this interaction is facilitated through the bZIP domain present in each protein [34]. Additionally, they observed that HBZ-bZIP also interacts with CREM-Ia and ATF-1, which possess significant similarities in their bZIP domains compared to CREB [34]. This interaction between HBZ and CREB hinders the binding of CREB to the viral CRE elements, both in vitro and in vivo. These findings indicate that HBZ displaces CREB from a cellular CRE and suggest that HBZ may disrupt the regulation of CREB-dependent gene expression in cells.
Ma et al. in their study investigated the role of cAMP response element-binding protein (CREB) and mitogen-activated protein kinase (MAPK) in the pathogenesis of adult T-cell leukemia/lymphoma ATLL [35]. This study revealed that CREB is overexpressed in ATLL patients, leading to the upregulation of genes involved in cell cycle progression, anti-apoptotic pathways, and immune evasion [35]. Additionally, the constitutive activation of the MAPK pathway, including ERK, JNK, and p38 MAPK, is observed in ATLL cells, promoting uncontrolled cell proliferation, survival, and evasion of apoptosis. While our study data suggest the higher expression of MAPK in ATLL patients, it seems this higher expression is not significantly different with control group and did not have any positive correlation with viral markers, therefore, this observed association between high MAPK expression and ATL requires further validation in larger, prospective studies.
Our findings demonstrated that CREB and MAPK had an accumulative significant correlation with each other (p = 0.009). Despite the total mean MAPK gene expression level being higher in ATLL patients, it is not confirmed as a significant statistical difference (p = 0.351). Fukushima et al. in investigated the relationship between interferon-gamma (IFN-gamma) expression, HTLV-I p19 antigen, and the activation of p38 mitogen-activated protein kinase (p38 MAPK) in T cell lines derived from patients with HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP) and adult T cell leukemia (ATLL) [36]. The researchers observed that the expression of phosphorylated (activated)-p38 MAPK was significantly increased in the HAM/TSP-derived T cell lines (HCT-1 and HCT-4), along with high levels of both IFN-gamma and HTLV-I p19 antigen expression [36]. In contrast, the ATLL-derived T cell lines did not exhibit such high levels of activated p38 MAPK [36]. Treatment with a specific inhibitor of p38 MAPK resulted in the suppression of IFN-gamma and HTLV-I p19 antigen expression in HAM/TSP-derived cell lines and peripheral blood CD4 (+) T cells of HAM/TSP patients. These findings suggest that the activation of the p38 MAPK signaling pathway plays a role in the up-regulation of IFN-gamma expression in HAM/TSP patients with a high HTLV-I proviral load.
Based on the results and findings, it seems that the HTLV-1 genome initiates its replication, leading to an overexpression of Tax. This protein manipulates the cell signaling pathways and drives the infected cell to induce malignancy. Since the Tax protein is a complex antigen, the host immune response attempts to eliminate the infected cells. As a result, the virus downregulates TAX expression to a minimum level and activates transcription from the HBZ gene, thereby promoting cell proliferation signaling pathways. The attachment of HBZ to the CREB promoter induces the overexpression of CREB mRNA, ultimately activating other related transcriptions such as MAPK. All of these activities may will prepare the infected cell to transform malignant T cell stages.
Conclusion
HTLV-1 infection represents a significant global health concern, with a small proportion of infected individuals developing severe complications such as ATLL and HAM/TSP. Understanding the transmission, clinical manifestations, and pathogenesis of HTLV-1 is crucial for early diagnosis, appropriate management, and ongoing research efforts. Continued research and collaboration are essential to improve patient outcomes, develop targeted therapies, and ultimately eradicate HTLV-1-related diseases. The CREB and MAPK signaling pathways may play crucial roles in HTLV-1 infection and pathogenesis. Based on our findings it seems that Tax-mediated activation of CREB and MAPK pathways may promote cell growth, survival, and immune evasion, contributing to the development of ATLL and HAM/TSP. Understanding the intricate interplay between HTLV-1 and these signaling pathways will provide insights into the mechanisms of viral persistence and cellular transformation and need for further prospective studies with larger sample size. Furthermore, we suggested that targeting MAPK-CREB pathways may offer potential therapeutic avenues for the management and treatment of HTLV-1-associated diseases.
Acknowledgements
In memorial of Mrs. Maryam Karimi for her invaluable contributions to this manuscript which relies on her thesis work as its foundation and would be inconceivable without her talent and effort.
Author contributions
All authors contributed to the study conception, and this review was designed by [HR] and [MMA]. Data collection and analysis were performed by [HR], [SAR], [ZF] and [MMA]. The samples were provided by [HR]. The first draft of the manuscript was written by [ZF], [HR] and [MMA], and all authors commented on previous versions of the manuscript. The first draft was edited by [MMA] and [HR]. All authors read and approved the final manuscript.
Funding
This study is financially supported by Mashhad university of Medical sciences (Grant Number: P910679).
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All the samples and methods mentioned above were approved by the Research Ethics Committee of Mashhad University of Medical Sciences in Iran (ethical code: 910679). All patients provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barr RS, Drysdale SB, Boullier M, Lyall H, Cook L, Collins GP, et al. A review of the prevention of mother-to-child transmission of human T-cell lymphotrophic virus type 1 (HTLV-1) with a proposed management algorithm. Front Med (Lausanne). 2022;9: 941647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stufano A, Jahantigh HR, Cagnazzo F, Centrone F, Loconsole D, Chironna M, et al. Work-related human t-lymphotropic virus 1 and 2 (HTLV-1/2) infection: a systematic review. Viruses. 2021;13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramezani S, Rezaee SA, Farjami Z, Ebrahimi N, Abdullabass HK, Ibrahim Jebur MI, et al. HTLV, a multi organ oncovirus. Microb Pathog. 2022;169:105622. [DOI] [PubMed] [Google Scholar]
- 4.Tamaki K, Mishima T, Tateishi Y, Mera H, Ogura H, Tsugawa J, et al. HTLV-1-associated demyelinating neuropathy: a case report and review of the literature. NeurologicalSci. 2023;31:100453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosa BL, Silva TS, Dias MA, Araujo I, Bittencourt AL. Progression of infective dermatitis associated with HTLV-1 to adult T-cell leukemia/lymphoma-case report and literature review. Am J Dermatopathol. 2022;44(5):368–71. [DOI] [PubMed] [Google Scholar]
- 6.Akbarin MM, Shirdel A, Bari A, Mohaddes ST, Rafatpanah H, Karimani EG, et al. Evaluation of the role of TAX, HBZ, and HTLV-1 proviral load on the survival of ATLLATLLL patients. Blood Res. 2017;52(2):106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramezani S, Shirdel A, Rafatpanah H, Akbarin MM, Tarokhian H, Rahimi H, et al. Assessment of HTLV-1 proviral load, LAT, BIM, c-FOS and RAD51 gene expression in adult T cell leukemia/lymphoma. Med Microbiol Immunol. 2017;206(4):327–35. [DOI] [PubMed] [Google Scholar]
- 8.Torshizi R, Ghayour Karimani E, Etminani K, Akbarin MM, Jamialahmadi K, Shirdel A, et al. Altered expression of cell cycle regulators in adult T-cell leukemia/ lymphoma patients. Rep Biochem Mol Biol. 2017;6(1):88–94. [PMC free article] [PubMed] [Google Scholar]
- 9.Jafarian M, Mozhgani SH, Patrad E, Vaziri H, Rezaee SA, Akbarin MM, et al. Evaluation of INOS, ICAM-1, and VCAM-1 gene expression: a study of adult T cell leukemia malignancy associated with HTLV-1. Arch Virol. 2017;162(4):1009–15. [DOI] [PubMed] [Google Scholar]
- 10.Buhler S, Laufer SA. p38 MAPK inhibitors: a patent review (2012–2013). Expert Opin Ther Pat. 2014;24(5):535–54. [DOI] [PubMed] [Google Scholar]
- 11.Wydra VR, Ditzinger RB, Seidler NJ, Hacker FW, Laufer SA. A patent review of MAPK inhibitors (2018 - present). Expert Opin Ther Pat. 2023. [DOI] [PubMed]
- 12.Muthusamy V, Piva TJ. The UV response of the skin: a review of the MAPK, NFkappaB and TNFalpha signal transduction pathways. Arch Dermatol Res. 2010;302(1):5–17. [DOI] [PubMed] [Google Scholar]
- 13.Peter AT, Dhanasekaran N. Apoptosis of granulosa cells: a review on the role of MAPK-signalling modules. Reprod Domest Anim. 2003;38(3):209–13. [DOI] [PubMed] [Google Scholar]
- 14.Gruszka R, Zakrzewski K, Liberski PP, Zakrzewska M. microRNA interaction with MAPK and AKT pathways in paediatric brain tumours - preliminary results and review of the literature. Folia Neuropathol. 2020;58(2):123–32. [DOI] [PubMed] [Google Scholar]
- 15.Hendrikse CSE, Theelen PMM, van der Ploeg P, Westgeest HM, Boere IA, Thijs AMJ, et al. The potential of RAS/RAF/MEK/ERK (MAPK) signaling pathway inhibitors in ovarian cancer: A systematic review and meta-analysis. Gynecol Oncol. 2023;171:83–94. [DOI] [PubMed] [Google Scholar]
- 16.Braicu C, Buse M, Busuioc C, Drula R, Gulei D, Raduly L, et al. A comprehensive review on MAPK: a promising therapeutic target in cancer. Cancers (Basel). 2019;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Safa A, Abak A, Shoorei H, Taheri M, Ghafouri-Fard S. MicroRNAs as regulators of ERK/MAPK pathway: a comprehensive review. Biomed Pharmacother. 2020;132: 110853. [DOI] [PubMed] [Google Scholar]
- 18.Vecchi C, Montosi G, Garuti C, Canali S, Sabelli M, Bergamini E, et al. CREB-H is a stress-regulator of hepcidin gene expression during early postnatal development. J Mol Med (Berl). 2023. [DOI] [PubMed]
- 19.Wang Z, Lu Z, Chen Y, Wang C, Gong P, Jiang R, et al. Targeting the AKT-P53/CREB pathway with epicatechin for improved prognosis of traumatic brain injury. CNS Neurosci Ther. 2023. [DOI] [PMC free article] [PubMed]
- 20.Rodriguez Esquivel M, Hayes E, Lakomy O, Hassan M, Foretz M, Stocco C. Salt-inducible kinases regulate androgen synthesis in theca cells by enhancing CREB signaling. Mol Cell Endocrinol. 2023;1:112030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang S, Jia Y, Wu Z, Fu B, Zhou S, Pires LV, et al. Activation of G protein-coupled estrogen receptor stimulates placental human chorionic gonadotropin expression through PKA-CREB signaling. Mol Cell Endocrinol. 2023;1:112033. [DOI] [PubMed] [Google Scholar]
- 22.Kandezi N, Mohammadi M, Ghaffari M, Gholami M, Motaghinejad M, Safari S. Novel insight to neuroprotective potential of curcumin: a mechanistic review of possible involvement of mitochondrial biogenesis and PI3/Akt/ GSK3 or PI3/Akt/CREB/BDNF signaling pathways. Int J Mol Cell Med. 2020;9(1):1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mlakar V, Morel E, Mlakar SJ, Ansari M, Gumy-Pause F. A review of the biological and clinical implications of RAS-MAPK pathway alterations in neuroblastoma. J Exp Clin Cancer Res. 2021;40(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Mello SR. When good kinases go rogue: GSK3, p38 MAPK and CDKs as therapeutic targets for alzheimer’s and huntington’s disease. Int J Mol Sci. 2021;22:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martella C, Waast L, Pique C. Tax, the puppet master of HTLV-1 transcription. Med Sci (Paris). 2022;38(4):359–65. [DOI] [PubMed] [Google Scholar]
- 26.Vandermeulen C, O’Grady T, Wayet J, Galvan B, Maseko S, Cherkaoui M, et al. The HTLV-1 viral oncoproteins Tax and HBZ reprogram the cellular mRNA splicing landscape. PLoS Pathog. 2021;17(9): e1009919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiang J, Rauch DA, Huey DD, Panfil AR, Cheng X, Esser AK, et al. HTLV-1 viral oncogene HBZ drives bone destruction in adult T cell leukemia. JCI Insight. 2019;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao J, Yu W, Wei W, Wang S, Zheng Z, Li L, et al. MAPK-ERK-CREB signaling pathway upregulates Nav16 in oxaliplatin-induced neuropathic pain in the rat. Toxicol Lett. 2023;1:1. [DOI] [PubMed] [Google Scholar]
- 29.de la Fuente C, Gupta MV, Klase Z, Strouss K, Cahan P, McCaffery T, et al. Involvement of HTLV-I Tax and CREB in aneuploidy: a bioinformatics approach. Retrovirology. 2006;3:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuzaki Y, Fujisawa J, Yoshida M. Identification of transcriptional activation domain of TREB5, a CREB/ATF family protein that binds to HTLV-1 enhancer. J Biochem. 1995;117(2):303–8. [DOI] [PubMed] [Google Scholar]
- 31.Vendel AC, McBryant SJ, Lumb KJ. KIX-mediated assembly of the CBP-CREB-HTLV-1 tax coactivator-activator complex. Biochemistry. 2003;42(43):12481–7. [DOI] [PubMed] [Google Scholar]
- 32.Jiang S, Inada T, Tanaka M, Furuta RA, Shingu K, Fujisawa J. Involvement of TORC2, a CREB co-activator, in the in vivo-specific transcriptional control of HTLV-1. Retrovirology. 2009;6:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim YM, Geiger TR, Egan DI, Sharma N, Nyborg JK. The HTLV-1 tax protein cooperates with phosphorylated CREB, TORC2 and p300 to activate CRE-dependent cyclin D1 transcription. Oncogene. 2010;29(14):2142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemasson I, Lewis MR, Polakowski N, Hivin P, Cavanagh MH, Thebault S, et al. Human T-cell leukemia virus type 1 (HTLV-1) bZIP protein interacts with the cellular transcription factor CREB to inhibit HTLV-1 transcription. J Virol. 2007;81(4):1543–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma Y, Zheng S, Wang Y, Zang W, Li M, Wang N, et al. The HTLV-1 HBZ protein inhibits cyclin D1 expression through interacting with the cellular transcription factor CREB. Mol Biol Rep. 2013;40(10):5967–75. [DOI] [PubMed] [Google Scholar]
- 36.Fukushima N, Nishiura Y, Nakamura T, Yamada Y, Kohno S, Eguchi K. Involvement of p38 MAPK signaling pathway in IFN-gamma and HTLV-I expression in patients with HTLV-I-associated myelopathy/tropical spastic paraparesis. J Neuroimmunol. 2005;159(1–2):196–202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.