Abstract
Background
Heart disease patients necessitate precise monitoring to ensure the safety and efficacy of their physical activities when managing conditions such as hypertension or heart failure. This study, therefore, aimed to evaluate the accuracy of photoplethysmography (PPG)-based monitoring of pulse rate (PR), interbeat-intervals (IB-I) and oxygen saturation (SpO2) during high-intensity interval training (HIIT).
Methods
Between January and March 2024, healthy volunteers were subjected to a cycling HIIT workout with bike resistance increments to evaluate performance within different heart rate ranges. To determine the accuracy of PPG-based measurements for PR, IB-I, and SpO2 using the CardioWatch 287–2 (Corsano Health, the Netherlands), measurements throughout these ranges were compared to paired reference values from the Covidien Nellcor pulse oximeter (PM10N) and Vivalink’s wearable ECG patch monitor. Subgroups were defined for Fitzpatrick skin type and gender.
Results
In total, 35 healthy individuals participated, resulting in 7183 paired measurements for PR, 22,713 for IB-I, and 41,817 for SpO2. The PR algorithm showed an average root mean square (Arms) of 2.51 beats per minute (bpm), bias at 0.05 bpm, and limits of agreement (LoA) from −4.87 to 4.97 bpm. The IB-I algorithm achieved an Arms of 23.00 ms, a bias of 1.00 ms, and LoA from −43.82 to 46.21 ms. Finally, the SpO2 algorithm showed an Arms of 1.28%, a bias of 0.13%, and LoA from −2.37% to 2.62%. The results were consistent across different demographic subgroups.
Conclusions
This study demonstrates that the PPG-based CardioWatch 287–2 can accurately monitor PR, IB-I, and SpO2 during HIIT. However, further research is recommended to evaluate the algorithm's performance in heart disease patients during demanding exercise.
Keywords: Pulse rate, Oxygen saturation, Interbeat intervals, Photoplethysmography, Continuous monitoring, High-intensity interval training
Background
Heart disease remains a leading cause of mortality and morbidity worldwide, necessitating improved and early monitoring strategies to mitigate its impact [1, 2]. This is especially critical for cardiac patients who engage in high-intensity activities, such as high-intensity interval training (HIIT), which can place additional stress on the cardiovascular system. These patients, often managing conditions such as hypertension or heart failure, require precise and continuous monitoring to ensure the safety and efficacy of their exercise routines [3].
As the benefits of HIIT for cardiac patients gain recognition, there is a growing focus on the role of wearable devices in monitoring key physiological parameters such as oxygen saturation (SpO2), pulse rate (PR), and interbeat intervals (IB-I) [4]. Real-time monitoring is essential for these individuals, providing immediate insights into their physiological responses and enabling timely interventions and personalized care plans.
Recent advancements in wearable health technology have revolutionized patient care, offering new ways to continuously monitor vital signs [5–7]. Among these innovations, photoplethysmography (PPG) has emerged as a promising tool for real-time physiological measurements [5–8]. This non-invasive technology operates on the principle of light absorption and reflection by blood vessels, and provides an indirect but reliable means of measuring blood flow and oxygenation, making it ideal for integration into wearable devices [9–11].
PPG technology has since been incorporated into various wearable health devices, offering continuous and convenient monitoring without the need for invasive procedures [5, 6]. Vital parameters such as SpO2, PR, and IB-I are crucial indicators of a patient’s clinical status and are integral components of the Early Warning Score (EWS) system for predicting clinical deterioration [12, 13]. Accurate measurement of these parameters using PPG in wearable devices can simplify patient monitoring and enhance safety during exercise.
Despite the promise of wearable technology, few devices have demonstrated the reliability, accuracy, and usefulness required for monitoring cardiac patients, particularly during physical activity. This study aims to evaluate the performance of a PPG-based wristband, the Corsano CardioWatch 287–2, in recording PR, IB-I, and SpO2 during HIIT. By assessing the device's accuracy in real-world conditions, the study seeks to provide valuable insights that can inform healthcare strategies for cardiovascular patients engaged in high-intensity exercise.
Results
In total, 35 healthy volunteers participated in the study and performed the HIIT. The demographic characteristics of participants in the HIIT study are presented in Table 1.
Table 1.
Demographics of enrolled and analyzed participants (n = 35)
| Mean (± SD) | |
|---|---|
| Age, years | 30 (± 10) |
| Male, N (%) | 22 (63%) |
| Weight, kg | 73 (± 11) |
| Height, cm | 176 (± 9) |
|
Skin color (Fitzpatrick), N (%) [23] Class I-II Class III-IV Class V-VI |
11 (31%) 13 (37%) 11 (31%) |
SD standard deviation, N number of patients
No adverse or unexpected events occurred during the study, and no participants (prematurely) discontinued the study. Furthermore, no participants were excluded based on poor data quality or technical errors.
Pulse rate
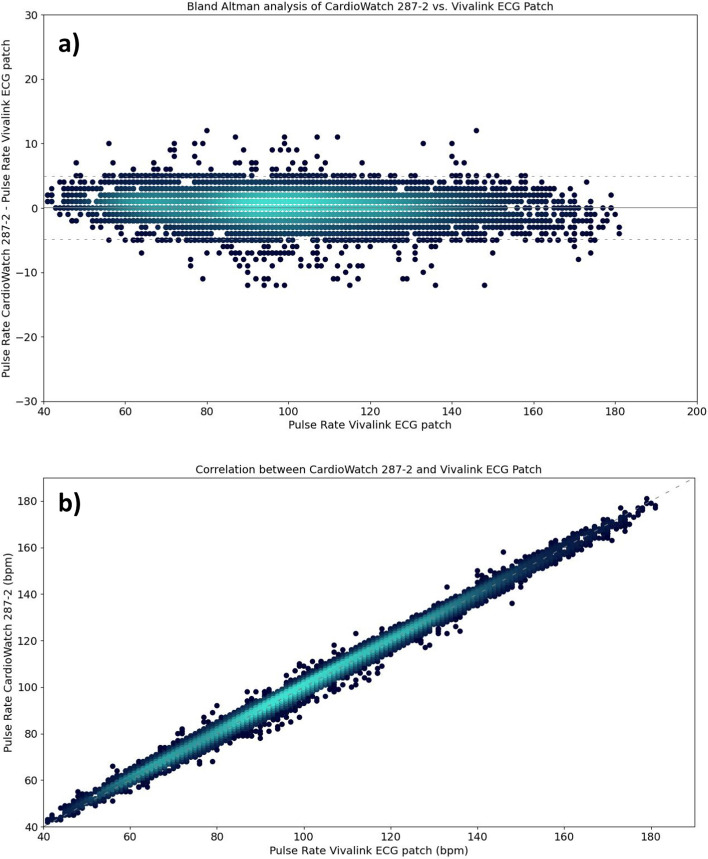
The accuracy of the pulse rate algorithm implemented in the Corsano CardioWatch 287–2 bracelet was compared with the Vivalink ECG patch as a reference and is given in Table 2 and Fig. 1. The analysis was conducted on a pooled dataset of 7,183 data points, as well as stratified by gender and Fitzpatrick skin type. The Arms across all data points was 2.51 bpm. The overall bias indicated a near-zero mean percentage difference (0.05 bpm) with a 95% CI ranging from −0.01 bpm to 0.11 bpm. Bland–Altman analysis for the LoA showed the lower LoA at −4.87 bpm (95% CI −4.93 bpm, −4.81 bpm) and the upper LoA at 4.97 bpm (95% CI 4.91 bpm, 5.02 bpm). Subgroup analyses revealed similar results.
Table 2.
Accuracy of pulse rate of Corsano CardioWatch 287–2 compared to Vivalink ECG patch
| Pooled (n = 7183) | Female (n = 2593) | Male (n = 4590) | Fitzpatrick I-IV (n = 4993) | Fitzpatrick V-VI (n = 2190) | |
|---|---|---|---|---|---|
| Arms [bpm] | 2.51 | 2.59 | 2.46 | 2.54 | 2.45 |
| Bias (+ 95% CI) [%] | 0.05 (−0.01, 0.11) | −0.10 (−0.21, 0.00) | 0.14 (0.07, 0.21) | 0.00 (−0.07, 0.07) | 0.16 (0.06, 0.26) |
| 95% LoA (+ 95% CI) [%] Lower | −4.87 (−4.93, −4.81) | −5.19 (−5.28, −5.09) | −4.68 (−4.75, −4.61) | −4.98 (−5.05, −4.91) | −4.62 (−4.72, −4.52) |
| 95% LoA (+ 95% CI) [%] Upper | 4.97 (4.91, 5.02) | 4.97 (4.87, 5.07) | 4.96 (4.88, 5.02) | 4.98 (4.91, 5.05) | 4.94 (4.84, 5.05) |
n number of samples, Arms Average root mean square, CI confidence interval, LoA Limits of Agreement
Fig. 1.
Comparison of CardioWatch 287–2 Pulse Rate and Vivalink ECG patch reference Pulse Rate by means of a) Bland–Altman, and b) correlation analyses, visualized through density plots (high to low density = light to dark blue)
Interbeat-intervals
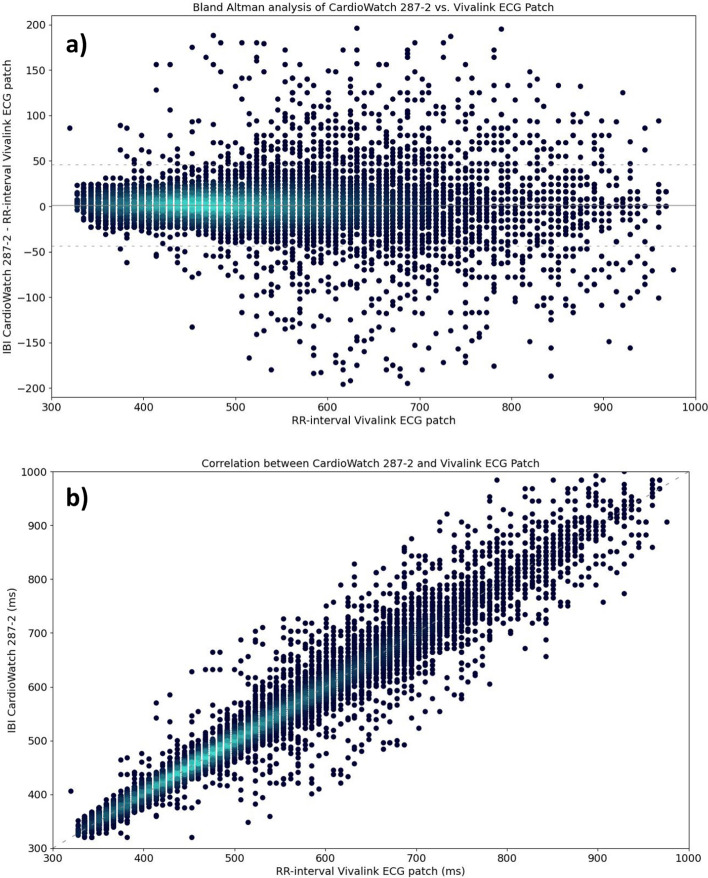
The accuracy of the IB-I algorithm was compared with the Vivalink ECG patch, analyzing 22,713 data points, and given in Table 3 and Fig. 2. The Arms for the pooled data were 23.00 ms. The bias showed a positive deviation of 1.00 ms (95% CI 0.89 ms, 1.49 ms) for the pooled analysis. The Bland–Altman LoA shows the lower LoA at −43.82 ms (95% CI −44.12 ms, −43.52 ms) and the upper LoA at 46.21 ms (95% CI 45.91 ms, 46.50 ms). The subgroup analyses revealed similar results.
Table 3.
Accuracy of interbeat-intervals of Corsano CardioWatch 287–2 compared to Vivalink ECG patch
| Pooled (n = 22713) | Female (n = 8605) | Male (n = 14180) | Fitzpatrick I-IV (n = 16141) | Fitzpatrick V-VI (n = 6411) | |
|---|---|---|---|---|---|
| Bias (+ 95% CI) [%] | 1.19 (0.89, 1.49) | 1.58 (1.10, 2.06) | 0.84 (0.56, 1.33) | 1.16 (0.80, 1.52) | 1.30 (0.76, 1.84) |
| 95% LoA (+ 95% CI) [%] Lower | −43.82 (−44.12, −43.52) | −42.89 (−43.37, −42.41) | −44.38 (−44.75, −44.00) | −44.48 (−44.83, −44.12) | −42.13 (−42.68, −41.59) |
| 95% LoA (+ 95% CI) [%] Upper | 46.21 (45.91, 46.50) | 46.06 (45.58, 46.54) | 46.27 (45.88, 46.65) | 46.80 (46.44, 47.15) | 44.74 (44.19, 45.28) |
n number of samples, Arms Average root mean square, CI confidence interval, LoA Limits of Agreement
Fig. 2.
Comparison of CardioWatch 287–2 Interbeat-Intervals and Vivalink ECG patch reference Pulse Rate by means of a) Bland–Altman, and b) correlation analyses, visualized through density plots (high to low density = light to dark blue)
Oxygen saturation
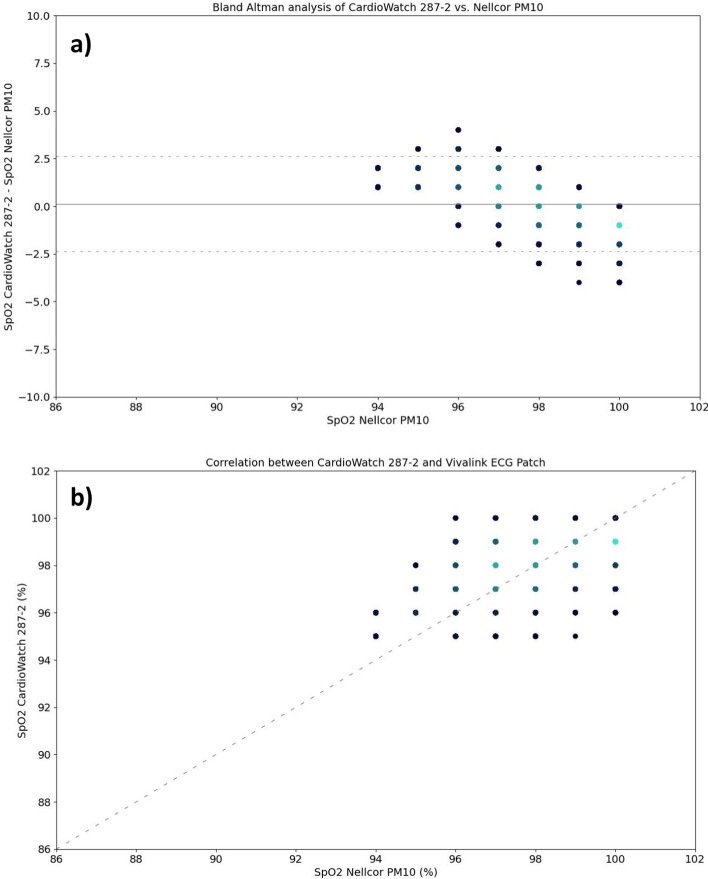
In the evaluation of SpO2 measurement accuracy, the Corsano CardioWatch 287–2's performance was assessed against the Nellcor PM10 and given in Table 4 and Fig. 3. The dataset comprised 41,817 data points, showing an Arms of 1.28%. The bias was slightly positive at 0.13% (95% CI 0.11%, 0.14%) for the pooled data. The 95% LoA ranged from −2.37% (95% CI −2.38%, −2.36%) in the lower bound to 2.62% (95% CI 2.61%, 2.63%) in the upper bound. Subgroup analyses revealed similar results.
Table 4.
Accuracy of SpO2 of Corsano CardioWatch 287–2 compared to Nellcor PM10
| Pooled (n = 41817) | Female (n = 14809) | Male (n = 27008) | Fitzpatrick I-IV (n = 30139) | Fitzpatrick V-VI (n = 11678) | |
|---|---|---|---|---|---|
| Arms [%] | 1.28 | 1.20 | 1.32 | 1.26 | 1.33 |
| Bias (+ 95% CI) [%] | 0.13 (0.11, 0.14) | −0.23 (−0.25, −0.21) | 0.32 (0.31, 0.34) | 0.08 (0.06, 0.09) | 0.25 (0.23, 0.28) |
| 95% LoA (+ 95% CI) [%] Lower | −2.37 (−2.38, −2.36) | −2.54 (−2.56, −2.52) | −2.19 (−2.20, −2.17) | −2.38 (−2.39, −2.36) | −2.32 (−2.34, −2.29) |
| 95% LoA (+ 95% CI) [%] Upper | 2.62 (2.61, 2.63) | 2.07 (2.06, 2.09) | 2.83 (2.81, 2.85) | 2.53 (2.52, 2.55) | 2.82 (2.79, 2.84) |
n number of samples, Arms Average root mean square, CI confidence interval, LoA Limits of Agreement
Fig. 3.
Comparison of CardioWatch 287–2 Oxygen Saturation and Covidien Nellcor pulse oximeter reference by means of a) Bland–Altman, and b) correlation analyses, visualized through density plots (high to low density = light to dark blue)
Discussion
This study analyzed the accuracy of Corsano’s CardioWatch 287–2 for monitoring PR, IB-I, and SpO2 during HIIT compared to references from gold standard devices. The Arms across all paired measurements for the PR, IB-I and SpO2 were, 2.51 bpm, 23 ms, and 1.28%, respectively. Similar results were obtained for all subgroup analyses, demonstrating compliance with the ISO Universal Standard [18]. The bias, which indicates systematic error, was minimal, with an overall mean difference approaching zero. This suggests that the CardioWatch 287–2 provides highly accurate measurements, demonstrating only a slight and mostly consistent overestimation. The Bland–Altman LoA further supports the accuracy by defining the range within which most differences between the CardioWatch 287–2 and the reference measurements lie. These findings indicate the accuracy of PR, SpO2 and IB-I by the PPG-based CardioWatch 287–2 during HIIT. Adding to the evidence regarding the capabilities of PPG-based vital parameter monitoring during demanding activities.
In clinical practice, multiple vital parameters are often combined to interpret a patient’s clinical status, commonly through tools such as the EWS. PPG-based measurements not only offer the advantage of continuously recording parameters such as SpO2, PR and IB-I, but also allow the monitoring of other critical parameters, such as blood pressure, core body temperature, and atrial fibrillation. This capability holds great promise for improving patient outcomes and reducing the healthcare burden through continuous at-home and in-clinic patient monitoring [24–26]. However, for this application medical-grade accuracy in measuring each vital parameter is crucial. While many commercial devices offer the ability to record various vital parameters, most are not medically certified and are thus unsuitable for in-clinic or remote patient monitoring.
Although this study yielded significant findings, it is important to recognize its limitations. First, the study concerned a single-center study with a limited number of participants. Moreover, this study included healthy adults in the age range of 20 to 60 years, this could potentially limit the applicability of the CardioWatch 287–2 in patients or elderly. Secondly, during the performance of the HIIT on the LifeSpan R3i stationary bike, participants were asked to reach an elevated heart rate within a period of 20 min. Consequently, intensive exercise was simulated in a relatively short time frame. This could limit the correspondence with the actual activity performed by patients in their day-to-day life.
However, S. Blok et al. (2021) concluded that the algorithms of the CardioWatch 287 can monitor PR and IB-I in CVD patients with high accuracy [27]. The current study provides evidence for the high accuracy of the algorithms for monitoring PR, IB-I, and SpO2 during exercise and an elevated heart rate. These findings combined could lead to the conclusion that Corsano’s wearable yields the possibility of monitoring cardiac patients during their activities to manage conditions such as hypertension or heart failure.
Future research should involve larger, multi-center cohorts of patients with cardiological diseases, among other health conditions and physical states. This is recommended to validate the findings of this study across diverse and suitable populations and healthcare settings. Moreover, the current study only focused on performing HIIT on a stationary bike. Further studies, are therefore, advised to also investigate different types of exercise to evaluate the performance of the algorithms in different ambulatory settings.
Conclusion
The PPG-based CardioWatch 287–2 can accurately monitor PR, IB-I, and SpO2 during intense exercise. Due to the non-intrusiveness and continuous monitoring capabilities, PPG-based monitoring holds great promise for remote monitoring of patients during demanding activities.
Methods
This study constituted a single-center, non-randomized interventional study involving healthy volunteers equipped with the Corsano CardioWatch 287–2 during HIIT. The study consisted of a single visit per participant and took place from January 2024 till March 2024 at the Hague Tech center (The Hague, the Netherlands).
The evaluation of PR, IB-I, and SpO2 recorded by the Corsano CardioWatch 287–2 during HIIT, is a component of a comprehensive study (validating PR, IB-I, SpO2, respiration rate and non-invasive blood pressure) registered under NL.85330.058.23 (ToetsingOnline.nl). The study was approved by the regional Dutch Medical Ethical Committee (METC-LDD), and the study adhered to the principles of the Declaration of Helsinki.
Study population
Healthy volunteers aged 18 and older were included in this study. The participants were recruited through engagement with various media platforms associated with the Hague Tech center. Prior to participation, written informed consent was obtained from all participants. To achieve a representative demographic sample, we aimed for at least 30% representation from both males and females. Additionally, we targeted the inclusion of 30% of participants with a Fitzpatrick skin type score of V or VI, which corresponds to individuals with darker skin tones. This focus on diverse skin types is critical, as it allows for a more comprehensive understanding of how various skin characteristics may influence the accuracy of the device.
Individuals with a baseline systolic blood pressure of 160 mmHg or higher, or a diastolic blood pressure of 100 mmHg or higher, were excluded to mitigate risks associated with cardiovascular complications. For similar reasons, participants identified as having a high total cardiovascular risk or any clinical condition that could pose a risk during HIIT were also excluded.
Additional exclusion criteria included the inability to wear the CardioWatch 287–2 (e.g., due to allergies, wounds, or amputations), and the inability or unwillingness to sign the informed consent form.
Investigational device
The investigational device (CardioWatch 287–2 [14]) employs photoplethysmography (PPG) for non-invasive measurement of blood volume changes in the microvascular bed on the dorsal side of the wrist. This method utilizes light, with a PPG sensor comprising green, red and infrared light-emitting diodes (LEDs), along with two photo-diodes directed toward the patient’s skin. The photo-diodes capture the light emitted by the LEDs and reflected by the skin operating at a sampling rate of 128 Hz. Spectrophotometry, facilitated by red and infrared LEDs, is employed for SpO2 monitoring, while PR is assessed based on the intensity, amplitude, and frequency characteristics of the detected waveform using green LEDs. Additionally, an accelerometer is integrated to mitigate movement artifacts during monitoring. Wirelessly, data are transmitted via Bluetooth to the Corsano App Application on a mobile device before being forwarded to the Health Cloud and Research Portal for comprehensive data analysis.
Study description
To evaluate the accuracy of CardioWatch 287–2 measurements for PR, IB-I, and SpO2, data from these parameters were compared with measurements obtained from the Covidien Nellcor pulse oximeter (PM10N) and Vivalink’s wearable ECG patch monitor [15, 16]. This comparative analysis involved all included healthy participants engaging in HIIT. During each measurement, the responsible physician was present to provide supervision.
A single, 20 min, measurement session was conducted for each participant. The following measurement set-up was applied in all participants: the CardioWatch 287–2 on the right wrist, the ECG patch on the left side of the chest according to placement instructions, and the PM10N on the right index finger. Subsequently, participants commenced the HIIT on a LifeSpan R3i stationary bike [17]. The HIIT protocol involved cycling with increments in bike resistance to elevate the participant’s heart rate. The following study protocol was applied: 2-min warm-up (own pace), increase pace/bike resistance to achieve five heartbeat ranges (120–130 beats per minute (bpm); 130–140 bpm; 140–160 bpm; > 160 bpm) and hold each range for 30 s; 2-min cool-down (own pace).
The CardioWatch 287–2 operated at a sampling frequency of 128 Hz throughout the entire measurement period. Specifically, the CardioWatch 287–2, obtained pulse rate once every 8 s, IB-I every second, and SpO2 once every 14 s. In contrast, reference devices measured parameters every second.
Baseline characteristics (age, gender, weight, height, skin color) were gathered before the measurement session.
Endpoints
The study aimed to assess the average root mean square (Arms) of PR monitoring to ensure an Arms ≤ 4 bpm. Additionally, the Arms criteria target values of ≤ 50 ms, and ≤ 3 percentage points for IB-I and SpO2, respectively.
Accuracy requirements are in line with ISO 80601-2-61:2017 [18].
Data pre-processing
The continuous data from the CardioWatch 287–2 was time aligned with the pulse oximeter and ECG patch. First the timestamps of the pulse oximeter were aligned with the timestamps of the Corsano mobile app, to which both the CardioWatch 287–2 and the ECG patch were connected. No time drift occurred in the devices.
The PPG data extracted by the CardioWatch was pre-processed using a bandpass filter between 0.5 and 5 Hz. After this, each pulse was assessed on signal quality according to the criteria proposed by Liu et al. (2020) [19]. Pulses not meeting these criterion for the SpO2 and IB-I analyses were subsequently excluded. However, for the PR analyses no pulses were discarded as this vital parameter is more robust.
Statistical analysis
To compare the values obtained by the CardioWatch 287–2 and the reference devices, the average root mean square (Arms) was calculated for heart rate, IB-I and SpO2. The Arms captures the bias (defined as the difference between the test results and an accepted reference value) and the precision (the closeness of agreement between independent test results obtained under stipulated conditions). Arms values of the reference devices (Covidien Nellcor pulse oximeter PM10N, Vivalink ECG patch) are reported with a zero-bias. To compute the Arms, the following formula was used:
| 1 |
where Ri is the reference value of sample i and CW2i the value computed by the CardioWatch 287–2 Software of sample i. All primary endpoint calculations were repeated per gender category (male/female) and per skin color category (Fitzpatrick I-IV and V-VI).
Furthermore, the bias and 95% limits of agreement (LoA) of CardioWatch 287–2 PR, IB-I, and SpO2 compared to Covidien Nellcor pulse oximeter and Vivalink ECG patch reference measurements were calculated. The bias and LoA are calculated according to the paper by Bland et al. [20] which considers multiple observations per individual and which considers the within and between-subject variance. For the bias and LoA parameters of the pooled analysis, the 95% confidence interval (CI) was determined. Furthermore, guidelines for Bland–Altman applied to multiple measurements per participant [21] and for calculation of the CI [22] were followed. Differences between reference and test device determinations showed a normal distribution for all included vital parameters.
Acknowledgements
We wish to acknowledge the contribution of the medical ethics assessment committee and the participants who were involved in this study.
Abbreviations
- Arms
Average root mean square
- bpm
Beats per minute
- CI
Confidence interval
- CVD
Cardiovascular disease
- HIIT
High-intensity interval training
- LEDs
Light-emitting diodes
- LoA
Limits of agreement
- PPG
Photoplethysmography
- PR
Pulse rate
- IB-I
Interbeat-intervals
- SpO2
Oxygen saturation
Author contributions
TV: conceptualization; investigation; methodology; project administration; writing—original draft; MM: investigation; writing—original draft; KE: conceptualization; investigation; methodology; project administration; software; visualization; data curation; writing—review & editing; CvE: investigation; writing—review and editing; ER: conceptualization; investigation; supervision; writing—review and editing.
Funding
This work was funded by Corsano Health B.V., The Hague, Netherlands.
Availability of data and materials
All data generated and analyzed during this study will be made available by the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the regional Dutch Medical Ethical Committee on the 22nd of January 2024. The study is registered under the MREC number P23.093.
Competing interests
T. Vijgeboom, C. van Eijck and M. Muller are Technical Physicians and part-time employees of Corsano Health. K. Ebrahimkheil is a Data Scientist at Corsano Health. E. Ronner is a Cardiologist at the Reinier de Graaf Hospital and received consultancy fees from Corsano Health.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. “Cardiovascular diseases (cvds),” https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed 3 July 2024
- 2.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. AHA/ACC/HFSA guideline for the management of heart failure: a report of the American college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation. 2022. 10.1161/cir.0000000000001063. [Google Scholar]
- 3.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction. Circulation. 2010;121(4):586–613. 10.1161/circulationaha.109.192703. [DOI] [PubMed] [Google Scholar]
- 4.Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients. Circulation. 2007;115(24):3086–94. 10.1161/circulationaha.106.675041.106.675041. [DOI] [PubMed] [Google Scholar]
- 5.Almarshad MA, Islam MS, Al-Ahmadi S, et al. Diagnostic features and potential applications of PPG Signal in Healthcare: a systematic review. Healthcare. 2022;10(3):547. 10.3390/healthcare10030547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghamari M, Castaneda D, Esparza A, et al. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int J Biosens Bioelect. 2018. 10.15406/ijbsbe.2018.04.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park J, Seok HS, Kim S-S, et al. Photoplethysmogram analysis and applications: an integrative review. Front Physiol. 2022. 10.3389/fphys.2021.808451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Measure. 2007. 10.1088/0967-3334/28/3/r01. [DOI] [PubMed] [Google Scholar]
- 9.Hertzman AB. Photoelectric plethysmography of the fingers and toes in man. Exp Biol Med. 1937;37(3):529–34. 10.3181/00379727-37-9630. [Google Scholar]
- 10.Hertzman AB. The blood supply of various skin areas as estimated by the Photoelectric plethysmograph. Am J Physiol-Legacy Content. 1938;124(2):328–40. 10.1152/ajplegacy.1938.124.2.328. [Google Scholar]
- 11.Beer. Versuch die absorptions-verhältnisse des cordierites für Rothes Licht zu bestimmen. Annalen der Physik und Chemie. 1851. 160(9):37–44. 10.1002/andp.18511600904
- 12.Subbe CP, Kruger M, Rutherford P, et al. Validation of a modified early warning score in medical admissions. QJM An Int J Med. 2001;94(10):521–6. 10.1093/qjmed/94.10.521. [DOI] [PubMed] [Google Scholar]
- 13.Nagarajah S, Krzyzanowska MK, Murphy T. Early warning scores and their application in the inpatient oncology settings. JCO Oncol Pract. 2022;18(6):465–73. 10.1200/op.21.00532. [DOI] [PubMed] [Google Scholar]
- 14.CardioWatch 287–2, Corsano Health. Corsano Health; 2024. https://corsano.com/products/bracelet-2/. Accessed 31 May 2024.
- 15.Nellcor™ Portable SpO₂ Patient Monitoring System, PM10N. Medtronic; 2024. https://www.medtronic.com/covidien/en-us/products/pulse-oximetry/nellcor-portable-spo2-patient-monitoring-system.html. Accessed 31 May 2024.
- 16.Wearable ECG monitor: Continuous cardiac & heart patch. Vivalink; 2024. https://www.vivalink.com/wearable-ecg-monitor Bike. Accessed 31 May 2024
- 17.Lifespan Fitness Recumbent Bike r3i. LifeSpan Europe; 2024. https://www.lifespaneurope.com/products/lifespan-fitness-recumbent-bike-r3i. Accessed 31 may 2024
- 18.ISO 80601–2–61:2017. Medical electrical equipment — Part 2–61: Particular requirements for basic safety and essential performance of pulse oximeter equipment. https://www.iso.org/standard/67963.html. Accessed 11 Nov 2023
- 19.Liu S-H, Liu H-C, Chen W, et al. Evaluating quality of photoplethysmographic signal on wearable forehead pulse oximeter with supervised classification approaches. IEEE Access. 2020. 10.1109/access.2020.3029842. [Google Scholar]
- 20.Douglas G. Altman and John Martin Bland. measurement in medicine: the analysis of method compari- son studies. Statistician. 1983;32:307–17. [Google Scholar]
- 21.Hamilton C, Lewis S. The importance of using the correct bounds on the bland–Altman limits of agreement when multiple measurements are recorded per patient. J Clin Monit Comput. 2010;24(3):173–5. 10.1007/s10877-010-9230-8. [DOI] [PubMed] [Google Scholar]
- 22.Zou GY. Confidence interval estimation for the Bland-Altman limits of agreement with multiple observations per individual. Stat Methods Med Res. 2013;22(6):630–42. 10.1177/0962280211402548. [DOI] [PubMed] [Google Scholar]
- 23.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124(6):869–71. 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 24.Fine J, Branan KL, Rodriguez AJ, et al. Sources of inaccuracy in photoplethysmography for continuous cardiovascular monitoring. Biosensors. 2021;11(4):126. 10.3390/bios11040126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Specifications corsano vitals [Internet]. Corsano Health; 2022. https://corsano.com/wp-content/uploads/2023/04/Corsano-CardioWatch-Vital-Parameters.pdf
- 26.Avolio A, Shirbani F, Tan I, et al. Cuffless blood pressure monitoring and the advent of a new era in medicine and society. Handbook Cuffless Blood Pressure Monit. 2019;1:7. 10.1007/978-3-030-24701-0_1. [Google Scholar]
- 27.Blok S, Piek MA, Tulevski II, Somsen GA, Winter MM. The accuracy of heartbeat detection using photoplethysmography technology in cardiac patients. J Electrocardiol. 2021;67:148–57. 10.1016/j.jelectrocard.2021.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and analyzed during this study will be made available by the corresponding author on reasonable request.