Abstract
Background
Hearing impairment is a known risk factor for Alzheimer’s disease (AD), although less is known about vision impairment or dual sensory impairment (DSI) as risk factors for AD. We studied the association between diagnosed hearing impairment, visual impairment, or DSI, and the risk of AD.
Method
The Medication use and Alzheimer’s disease study (MEDALZ) is a register-based nested case-control study including 70,718 community-dwelling persons diagnosed with incident AD in 2005–2011 in Finland and their 282,845 matched controls. Sensory impairment diagnoses (limited to those that cause irreversible sensory loss designated by medical specialists) at least five years prior to AD diagnosis (or matching date) were obtained from national healthcare registers, including specialized outpatient visits. Associations were studied with cofounder-adjusted conditional logistic regression.
Results
Hearing impairment was associated with an increased risk of AD compared to people without a diagnosed sensory impairment (adjusted odds ratio (aOR) 1.15, 95% confidence interval (CI) 1.11–1.19), while no association was found in people with visual (aOR 1.02, 95% CI 0.99–1.05) or dual sensory impairment (aOR 1.05 (95% CI 0.95–1.15).
Conclusions
Hearing impairment can be a modifiable risk factor for AD, and thus its treatment in the aging population is important. Although we did not observe an association between visual impairment and AD, all sensory impairments decrease functioning and quality of life among older adults. Therefore, they should be treated, also among persons with cognitive decline or cognitive disorder.
Clinical trial number
Not Applicable.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-024-05514-z.
Keywords: Alzheimer’s disease, Dual sensory impairment, Hearing impairment, Vision impairment
Background
Alzheimer’s disease (AD), the most common form of cognitive disorders, is a neurodegenerative disease of increasing prevalence and primarily affects older adults (≥ 65 years of age) [1]. It is estimated that by 2050, about 16% of the world’s population will be over 65 years of age, up from 9% in 2019. The World Health Organization (WHO) has estimated that around 55 million people aged 65 years or older have been diagnosed with dementia worldwide, and 10 million new cases are diagnosed each year [2]. With AD’s gradual progressive nature starting decades before symptoms appear, identifying risk factors of the disease has become a priority [3].
Sensory impairments, including hearing and vision impairment, are common conditions in older adults and frequently occur in conjunction with cognitive impairment [4–6]. Vision impairment and hearing impairment are associated with various health-related adverse outcomes, including difficulties with activities of daily living and instrumental activities of daily living [7, 8], reduced social participation and social isolation [8], increased mortality risk [9], and frailty and falls [10]. The 2020 Lancet Commission report on dementia included hearing impairment on their list of the modifiable risk factors of dementia, and the 2024 report also includes vision loss [3, 11]. However, evidence of the association of the co-occurrence of dual sensory impairment (DSI), both vision and hearing impairment, has been inconclusive [12–23] with only a few studies focusing on the association between vision and/or hearing impairment and AD [15, 16, 22]. Further, several of these studies used self-reported information on sensory impairments [15, 17, 22, 24]. By limiting sensory impairments to medical diagnoses causing irreversible sensory loss, we investigated the association between having (i) either sensorineural hearing impairment or blindness leading eye diseases and the risk of AD and (ii) DSI and the risk of AD.
Methods
Study population and data sources
Community-dwelling persons who received a new clinically verified diagnosis of AD from 2005 to 2011 (N = 70,718) in Finland were included in the Medication use and Alzheimer’s disease (MEDALZ) cohort [25]. For this nationwide nested case-control study, up to four age (± 1 year), gender, and hospital district region-matched control persons without AD (N = 282,862) were identified for each case at the date of AD diagnosis (index date). Data for this study were compiled from multiple Finnish nationwide health care registers, including the Special Reimbursement Register (SRR) (1972–2015), Prescription Register (1995–2015), Care Register for Health Care (CRHC) (formerly the Hospital Discharge Register) (1972–2015), and Statistics Finland (since 1970). Data linkage of the registers was done by the Social Insurance Institution of Finland (SII) using the unique personal identification numbers assigned to each resident.
Identification of cases
People with a clinically verified AD diagnosis were identified from the SRR which contains records of all people who were granted reimbursement for antidementia drugs [25]. Predefined criteria must be fulfilled and a medical statement by a physician describing the findings must have been submitted to SII. The special reimbursement is only granted if the criteria are fulfilled. The criteria for AD reimbursement include (1) symptoms consistent with AD, (2) a decrease in social capacity over a period of at least three months, (3) a computed tomography (CT)/ magnetic resonance imaging (MRI) scan, and (4) possible alternative diagnoses excluded. The AD diagnosis was confirmed by a neurologist or geriatrician. The AD diagnosis was based on the National Institute of Neurological and Communicative Disorders and Stroke from the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) [26] and Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) [27] criteria for Alzheimer’s disease.
Identification of controls
Control persons were identified from the SII, which includes all residents of Finland. Controls were matched on the date of AD diagnosis (index date). Criteria for being a Control person included (1) community-dwelling without a clinically verified diagnosis of AD at the time of matching, and (2) they could not have had a special reimbursement for AD or antidementia medication purchases before or within 12 months after the matching date.
Sensory impairment diagnoses
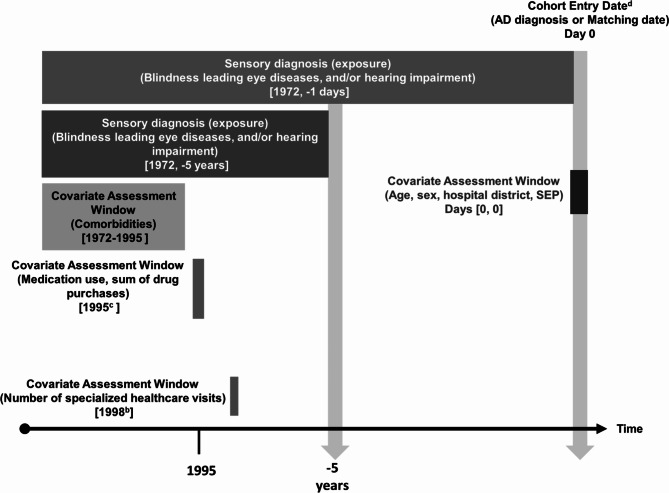
Sensory impairment diagnoses before the index date were obtained from the CRHC. Blindness leading eye diseases and sensorineural hearing impairment diagnoses were limited to those that cause irreversible sensory loss designated by medical specialists (Additional Table 1) (Fig. 1). The Special Reimbursement code 114, which is assigned to glaucoma medication, was used from the SRR (years 1972–2011) to also represent vision impairment diagnosis. Data from the CRHC included both inpatient and outpatient visits. International Statistical Classification of Diseases and Related Health Problems (ICD) 9th revision (years 1987–1995) and ICD-10 codes (1996–2011) were obtained from inpatient data. ICD-10 codes were also obtained from the outpatient visits (years 1998–2011). Both primary and secondary diagnosis codes were included. The first recorded date of vision or hearing impairment diagnosis was used. For DSI, a person needed a record of an eye disease diagnosis and sensorineural hearing impairment diagnosis documented prior to the index date. The DSI date was taken from the later sensory impairment diagnosis date that was documented prior to the index date.
Fig. 1.
Study design. a Number of hospitalizations in the year 1987 to capture data prior to exposure. b Earliest date available for outpatient specialized healthcare visits potentially prior to exposure. c Earliest date available for medication use potentially prior to exposure. d 2005 to 2011
Covariates
Socioeconomic position (SEP), defined by the occupational social class, was obtained from the census maintained by Statistics Finland. Data on comorbidities (cardiovascular disease, stroke, diabetes, asthma/ chronic obstructive pulmonary disease (COPD), cancer, head trauma, and substance abuse) were gathered from the CRHC, SRR, and the Prescription Register (PR), which is also maintained by SII. Comorbidities were only collected till the end of 1995 to capture a person’s health prior to the exposure (sensory diagnosis). Medication use and the sum of purchased drugs were obtained from the PR for the year 1995, which was the first year available in the register. The number of hospital days (inpatient care) and visits to specialized care (outpatient visits) were also obtained from the CRHC. Further details of the covariates can be found in Additional Table 1.
Statistical analysis
Descriptive statistics were conducted with means (with standard deviations (SD), medians (with interquartile ranges (IQR)), and χ2 tests for categorical variables. The association between visual and/or hearing impairment and AD was assessed with conditional logistic regression due to the matched design. To account for the prodromal period of AD, we limited sensory impairment diagnoses to those recorded at least 5 years before the index date. A sensitivity analysis, including also those sensory impairment diagnoses recorded ever before the index date, was conducted. Multivariable models were adjusted for SEP, comorbidities, medication use, and healthcare utilization. Odds ratios (OR) are presented with 95% confidence intervals (CI). The presences of sensory impairment diagnoses were compared to those without a sensory impairment for both the cases and controls. Statistical analyses were performed with STATA/MP 14.2 (StataCorp, College Station, TX, USA). All data were de-identified before submission to the research team, and therefore ethics committee approval was not required according to Finnish legislation.
Results
The mean age of people with and without AD was 80 years (SD 7.1), and the proportion of women (65%) was the same due to the matched study design. There were slight differences in the distribution of occupational social class between the AD cases and the controls without AD. Comorbidities, psychotropic medication use, the sum of medications, the sum of days hospitalized, and the sum of visits to specialized healthcare prior to exposure were more prevalent in the cases than in controls (Table 1). During the lag time (-5 years to index date) the median number of prescription drugs, the sum of days hospitalized, and the number of visits to specialized care were also higher in cases than controls.
Table 1.
Characteristics of the study population including cases with Alzheimer’s disease (AD) and controls without AD
| Persons with AD N = 70,718 | Persons without AD N = 282,858 | p value | ||
|---|---|---|---|---|
| Sociodemographic factors | ||||
| Age at beginning of follow-up, mean (SD) | 80.05 (7.1) | 80.02 (7.1) | matched | |
| Sex | matched | |||
| Women | 46,116 (65) | 184,448 (65) | ||
| Men | 24,602 (35) | 98,397 (35) | ||
| Highest occupational Social class | < 0.001 | |||
| Managerial/ professional | 14,692 (20.8) | 60,524 (21.4) | ||
| Office worker | 5,973 (8.5) | 23,567 (8.3) | ||
| Farming/forestry | 13,439 (19.0) | 55,490(19.6) | ||
| Sales/industry/ cleaning | 30,148 (42.6) | 110,872 (39.2) | ||
| unknown/ did not respond | 6,446 (9.1) | 32,392 (11.5) | ||
| Comorbidities | ||||
| Any cardiovascular disease | 24,461 (34.6) | 92,027 (32.5) | < 0.001 | |
| Stroke | 1,545 (2.2) | 5,756 (2.0) | 0.012 | |
| Diabetes | 3,596 (5.1) | 10,393 (3.7) | < 0.001 | |
| Asthma/ COPD | 4,034 (5.7) | 15,520 (5.5) | 0.024 | |
| Hip fracture | 377 (0.5) | 1,498 (0.5) | 0.909 | |
| Cancer | 6,130 (8.7) | 23,650 (8.4) | 0.009 | |
| Rheumatoid arthritis | 2,205 (3.1) | 8,477 (3.0) | 0.093 | |
| Head trauma | 2,452 (3.5) | 8,406 (2.9) | < 0.001 | |
| Psychosis | 1,365 (1.9) | 5,252 (1.9) | 0.198 | |
| Substance abuse | 787 (1.1) | 2,728 (0.9) | < 0.001 | |
| Medication Use | ||||
| Benzodiazipine and related drugs use | 11,720 (16.6) | 42,080 (14.9) | < 0.001 | |
| Antidepressant use | 3,465 (4.9) | 11,204 (3.9) | < 0.001 | |
| Antipsychotic use | 1,504 (2.1) | 5,452 (1.9) | 0.001 | |
| Sum of purchased drugs at the beginning of the exposure | < 0.001 | |||
| 0 | 16,524 (23.4) | 77,023 (27.2) | ||
| 1–2 | 18,263 (25.8) | 72,549 (25.7) | ||
| 3–5 | 19,704 (27.9) | 76,061 (26.9) | ||
| 6+ | 16,227 (22.9) | 57,212 (20.2) | ||
| Other | ||||
| Number of hospital days at the beginning of the exposure | < 0.001 | |||
| 0 | 62,268 (88.0) | 251,816 (89.0) | ||
| 1–3 | 3,033 (4.3) | 11,397 (4.0) | ||
| 4–8 | 2,773 (3.9) | 9,823 (3.5) | ||
| 9+ | 2,644 (3.7) | 9,809 (3.5) | ||
| Number of visits to specialized healthcare at the beginning of the exposure | < 0.001 | |||
| 0 | 45,193 (63.9) | 19,0127 (67.2) | ||
| 1 | 8,611 (12.2) | 32,341 (11.4) | ||
| 2–3 | 9,119 (12.9) | 33,615 (11.9) | ||
| 4+ | 7,795 (11.0) | 26,762 (9.5) | ||
| Mediators | ||||
| Number of purchased drugs during the lag time, median (IQR) | 11 (7–17) | 11 (6–16) | ||
| categories: | < 0.001 | |||
| 0 | 1,303 (1.8) | 13,894 (4.9) | ||
| 1–8 | 22,491 (31.8) | 94,037 (33.3) | ||
| 9–15 | 24,927 (35.3) | 94,776 (33.5) | ||
| 16+ | 21,997 (31.1) | 80,138 (28.3) | ||
| Number of hospital days during the lag time, median (IQR) | 8 (1–30) | 3 (0–15) | ||
| categories: | < 0.001 | |||
| 0 | 16,487 (23.3) | 94,452 (33.4) | ||
| 1–7 | 17,909 (25.3) | 83,200 (29.4) | ||
| 8–30 | 18,665 (26.4) | 64,904 (22.9) | ||
| 31+ | 17,657 (24.9) | 40,289 (14.2) | ||
| Number of admissions to specialized healthcare during the lag time, median (IQR) | 5 (2–11) | 4 (1–10) | ||
| categories: | < 0.001 | |||
| 0 | 8,078 (11.4) | 57,643 (20.4) | ||
| 1–4 | 23,535 (33.3) | 90,586 (32.0) | ||
| 5–9 | 17,900 (25.3) | 61,731 (21.8) | ||
| 10+ | 21,205 (29.9) | 72,885 (25.8) | ||
Note. Data are given as n (%) unless otherwise indicated
Abbreviations: AD, Alzheimer’s Disease; COPD, Chronic Obstructive Pulmonary Disease; IQR, Interquartile Range; SD, Standard Deviation
In the analyses limited to sensory impairments diagnosed at least 5 years before the index date, a diagnosed sensorineural hearing impairment was associated with an increased risk of AD compared to people without a recorded sensorineural hearing impairment diagnosis (confounder-adjusted odds ratio (aOR) 1.15, 95% CI 1.11–1.19), and the association remained after accounting for increased healthcare contact in the lag time (Table 2). A diagnosis of a blindness leading eye disease was weakly associated with an increased risk of AD compared to those without a recorded eye disease diagnosis (comorbidity-adjusted OR 1.03, 95% CI 1.00-1.06), although the results were attenuated in the conditional logistic regression model that was adjusted for the increased healthcare contact in the lag window (aOR 1.02, 95% CI 0.98–1.04).
Table 2.
Association between recorded sensory impairment diagnoses until five years before index date and risk of AD
| Persons with AD, no. (%) | Persons without AD, no. (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI)a | Adjusted OR (95% CI)b | Adjusted OR (95% CI)c | |
|---|---|---|---|---|---|---|
| Eye disease diagnosis | ||||||
| No | 63,543 (89.8) | 255,465 (90.3) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Yes | 7,175 (10.2) | 27,380 (9.7) | 1.05 (1.02–1.08) | 1.03 (1.00-1.06) | 1.01 (0.98–1.04) | 1.02 (0.98–1.04) |
| Sensorineural hearing impairment diagnosis | ||||||
| No | 66,131 (93.5) | 266,826 (94.3) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Yes | 4,587 (6.5) | 16,019 (5.7) | 1.16 (1.12–1.20) | 1.15 (1.11–1.19) | 1.13 (1.09–1.17) | 1.14 (1.10–1.19) |
| Eye disease diagnosis, sensorineural hearing impairment or both | ||||||
| None | 59,552 (84.2) | 241,624 (85.4) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Eye disease diagnosis only | 6,579 (9.3) | 25,202 (8.9) | 1.06 (1.03–1.09) | 1.04 (1.01–1.07) | 1.02 (0.99–1.05) | 1.02 (0.99–1.06) |
| Sensorineural hearing impairment diagnosis only | 3,991 (5.6) | 13,841 (4.9) | 1.18 (1.13–1.22) | 1.17 (1.12–1.21) | 1.15 (1.11–1.19) | 1.16 (1.12–1.20) |
| Both | 596 (0.8) | 2,178 (0.8) | 1.12 (1.02–1.23) | 1.08 (0.99–1.19) | 1.05 (0.95–1.15) | 1.05 (0.95–1.16) |
Note.
a adjusted for Occupational socioeconomic class, cardiovascular disease, stroke, diabetes, asthma/COPD, cancer, Head trauma, substance abuse, medication use
b adjusted for occupational socioeconomic class, comorbidities, medication use, sum of purchased medications, number of days hospitalized, and sum of outpatient specialized healthcare visits
c adjusted for occupational socioeconomic class, comorbidities, medication use, sum of purchased medications in lag period (0 to -5 years), number of days hospitalized in lag period, and sum of outpatient specialized healthcare visits in lag period
Abbreviations: AD, Alzheimer’s disease; CI, Confidence Interval; no., Number; OR, Odds Ratio; Ref, Reference
Similar results for blindness-leading eye disease diagnosis and sensorineural hearing impairment diagnosis were observed with the categorization of singular or dual sensory impairment (Table 2). In the unadjusted analyses, persons with either hearing or eye disease diagnosis, or both had a higher risk of AD compared to those with no recorded sensory impairment diagnosis, but the association of dual sensory impairment attenuated after adjusting for comorbidities (aOR 1.08, 95% CI 0.99–1.19). The association of blindness leading eye disease diagnosis or dual sensory impairment was not observed after additional adjustments for healthcare utilization during the lag time. Similar results were observed when sensory impairment diagnoses until the index date were considered (Table 3).
Table 3.
Association between recorded sensory impairment diagnoses until index date and risk of AD
| Persons with AD, no. (%) | Persons without AD, no. (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI)a | Adjusted OR (95% CI)b | Adjusted OR (95% CI)c | |
|---|---|---|---|---|---|---|
| Eye disease diagnosis | ||||||
| No | 42,942 (84.7) | 239,903 (84.8) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Yes | 10,830 (15.3) | 42,942 (15.2) | 1.01 (0.98–1.03) | 0.98 (0.96–1.01) | 0.97 (0.95-1.00) | 0.98 (0.95-1.00) |
| Sensorineural hearing impairment diagnosis | ||||||
| No | 60,870 (86.1) | 247,329 (87.4) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Yes | 9848 (13.9) | 35,516 (12.6) | 1.13 (1.10–1.16) | 1.12 (1.10–1.15) | 1.11 (1.08–1.14) | 1.12 (1.09–1.15) |
| Eye disease diagnosis, sensorineural hearing impairment diagnosis or dual sensory impairment | ||||||
| None | 51,851 (73.3) | 211,407 (74.7) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Eye disease diagnosis only | 9019 (12.8) | 35,922 (12.7) | 1.03 (1.0-1.05) | 1.00 (0.98–1.03) | 0.99 (0.96–1.01) | 0.99 (0.98-1.00) |
| Sensorineural hearing impairment diagnosis only | 8037 (11.4) | 28,496 (10.1) | 1.16 (1.13–1.19) | 1.15 (1.12–1.18) | 1.13 (1.10–1.17) | 1.15 (1.12–1.18) |
| Both | 1811 (2.6) | 7020 (2.5) | 1.06 (1.0-1.12) | 1.03 (0.98–1.09) | 1.01 (0.95–1.06) | 1.01 (0.96–1.07) |
Note.
a adjusted for Occupational socioeconomic class, cardiovascular disease, stroke, diabetes, asthma/COPD, cancer, Head trauma, substance abuse, medication use
b adjusted for occupational socioeconomic class, comorbidities, medication use, sum of purchased medications, number of days hospitalized, and sum of outpatient specialized healthcare visits
c adjusted for occupational socioeconomic class, comorbidities, medication use, sum of purchased medications in lag period (0 to -5 years), number of days hospitalized in lag period, and sum of outpatient specialized healthcare visits in lag period
Abbreviations: AD, Alzheimer’s disease; CI, Confidence Interval; no., Number; OR, Odds Ratio; Ref, Reference
Table 3. Association between recorded sensory impairment diagnoses until index date and risk of AD (placement).
Discussion
Our study showed that persons with a diagnosed sensorineural hearing impairment had an increased risk of AD. We did not find an association between the most common blindness leading eye diseases, such as age-related macular degeneration, glaucoma, or diabetic retinopathy, and the presence of hearing impairment. Similar results were found when sensory diagnoses were not limited to the 5-year lag period before the AD diagnosis. This lag period was used to control for the prodromal phase of AD. These findings have important public health implications, given hearing impairment is a potentially modifiable risk factor for AD.
To the best of our knowledge, this is the first study that investigated the association of diagnosed sensory impairments that are limited to irreversible sensory loss and the risk of AD. Previous research on dual sensory impairment and its association with AD and dementia has been inconsistent. Definitions of hearing or vision impairment have varied greatly across studies (e.g., self-reported [4, 15–17, 19, 22–24, 28], measured [12, 14, 19, 29], diagnoses from medical records [13, 18]) and most studies used all-cause dementia or cognitive impairment as the outcome. In a large case-control study by Michalowsky et al., [13] ICD-10 codes were used to identify sensory impairment and dementia. When compared to people without a sensory impairment diagnosis, persons with a hearing impairment only and DSI, but not those with only a vision impairment were associated with dementia. Of the studies limited to AD as the outcome, Hwang et al., [15, 22] found DSI associated with AD; however, they reported on single impairment versus dual impairment and not on hearing or vision. Byeon et al., [16] found no association between DSI and AD, and they also only reported on single impairment versus dual impairment.
According to the Lancet Commission, hearing impairment was the strongest potentially modifiable risk factor for dementia among nine health and lifestyle factors [3]. A systematic review and meta-analysis by Loughrey et al., [30] reported an association of hearing impairment measured by pure tone audiometry and dementia in cohorts studies (3 studies, OR 1.28 (95% CI 1.02–1.59); however, the results were attenuated when the outcome was restricted to AD (2 studies, OR 1.69 (95% CI 0.7-4). Of the studies in the meta-analysis Lin et al., [31] did not report a lag time between hearing impairment and AD diagnosis; however, Gallacher et al. [32] had at least six years from the hearing examination till they screened for dementia. A case-control study by Hung et al., [33] defined hearing impairment using similar ICD-9 codes to our study and found that hearing impairment was associated with an increased risk of AD (OR 1.39 (95% CI 1.05–1.84)); however, they did not utilize a lag period prior to the AD diagnosis, potentially introducing a time-related bias.
Very few studies have reported eye diseases as a risk factor of AD and other types of dementia. Michalowsky et al., [13] did not find an association between vision impairment, based on ICD-10 codes, and dementia. However, this study was based on general practitioner’s medical records where vision impairment diagnoses were found to be underreported compared to the prevalence of vision impairments among older adults leading to exposure misclassification bias. Associations between individual eye diseases and AD have been reported with mixed findings [5, 34]. Previous research has focused on individual diseases of the eye, including glaucoma [35–38], age-related macular degeneration [5, 34, 38, 39], diabetic retinopathy [5, 34, 38], and cataracts [5, 34, 38], as a risk factor of AD. A recent systematic review and meta-analysis by Feng et al., [34] found glaucoma (10 studies, RR 1.18, 95% CI 1.02–1.38, I2 = 91%), diabetic retinopathy (4 studies, RR 1.21, 95% CI 1.04–1.41, I2 = 72%), and age-related macular degeneration (7 studies, RR 1.27, 95% CI 1.06–1.52, I2 = 70%) to be associated with AD. Age-related macular degeneration, glaucoma, and diabetic retinopathy are the most common blindness-leading eye diseases in Western countries [40–42]. These diseases lead to decreased central and/or peripheric visual acuity during disease progression. In 2024, the Lancet Commission report added untreated vision loss as a potential modifiable risk factor for dementia [3]. The report was based on the meta-analysis of cohort studies on vision impairment as a risk factor form dementia by Shang et al. [36]. As that meta-analysis was restricted to studies assessing visual impairment and 11 studies on conditions causing visual impairment as dementia risk factor were excluded, our findings are not directly comparable with the meta-analysis. Although we did not find an association between diagnosed blindness leading eye diseases, which included diabetic retinopathy, glaucoma, and age-related macular degeneration, and AD, our results were similar to the meta-analysis by Kuźma et al. [5] on the association between various eye conditions and risk of dementia. In that meta-analysis, cataracts and diabetic retinopathy were associated with higher risk of dementia, while no association between glaucoma or age-related macular degeneration and dementia was observed.
Several potential mechanisms have been suggested for the relationship between sensory impairment and AD, including a common cause theory [43], sensory deprivation theory [44], and occupation of cognitive resources theory [45]. The common cause theory suggests that common pathology and risk factors shared between AD and sensory impairment may result in their strong association. As hearing loss and AD may have common factors it has been proposed that a third variable (e.g., aging, mitochondrial dysfunction, microvascular factors, and inflammation) results in impairment on both hearing loss and cognitive impairment.
Due to hearing impairment, there is degradation and loss of input to the brain which progressively results in changes in function and structure of auditory and cognitive systems. Functional changes such as speech-in-noise processing, are associated with decreased social interaction and social withdrawal/isolation which are known risk factors of AD [46]. Hearing impairment has been associated with a decrease in cortical volume and decreased density of grey matter in the superior temporal lobe, frontal lobe, and hippocampus. These areas are crucial for semantic memory and contribute to the progression of AD [47].
The occupation of cognitive resources theory suggests that the connection between hearing impairment and AD stems from heightened cognitive processing required to offset the decline in sensory input. When auditory input is reduced, more neural resources, such as working memory, language processing, and attention, are required for auditory processing, leading to fewer resources available for higher cognitive functions including memory retention and retrieval [48].
Strengths and limitations
Strengths of our study include a nationwide cohort of persons with a clinically verified AD diagnosis in Finland [49]. Studies assessing the internal validity of CRHC and comparing register information with patient records or other information from primary sources have confirmed that the coverage and accuracy of these registers are well-suited to epidemiological research [49, 50]. This study used sensory impairment diagnoses, avoiding self-report bias. A 5-year lag period was investigated to avoid the prodromal phase of AD since AD pathology is known to begin years before the first signs and symptoms are present. During this lag time, people with AD had more contact with the healthcare system compared to people without AD; however, the results did not change when we adjusted for this.
Unfortunately, we do not have information on the stage or severity of sensory impairment which may be interpreted as a study limitation. However, blindness leading eye disease diagnoses were done by ophthalmologists. With regard to hearing impairment, most subjects presented with at least a moderate degree of hearing impairment (PTA 0.5–4 kHz [HL]) since it represents the national indication for hearing aid provision and when also the diagnosis is coded by an Ears, Nose, and Throat specialist. Hearing impairment diagnoses may be under-recorded due to the lack of adult hearing screening. Many patients with significant hearing impairment may go undetected. We limited our study to diagnoses that have significant sensory impairment that cannot be corrected or are degenerative in nature. The majority of the sensory diagnoses were obtained from the outpatient specialized care visits that were first recorded in the CRHC in 1998, so sensory diagnoses prior to 1998 were minimally captured in the inpatient records.
Conclusion
Hearing impairment was associated with an increased risk of AD. Considering hearing impairment can be a modifiable risk factor for AD, screening and treatment in the aging population needs to be prioritized. Although we did not find an association between blindness leading eye diseases all sensory impairments decrease functioning and quality of life among older adults. Clinical practice guidelines may need to be revised to recommend screenings for sensory impairments and implement effective, evidence-based care and interventions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
An oral presentation of this research was given at the 20th International Congress of the European Geriatric Medicine Society.
Abbreviations
- AD
Alzheimer’s disease
- aOR
Adjusted Odds Ratio
- CI
Confidence Interval
- COPD
Chronic Obstructive Pulmonary Disease.
- CRHC
Care Register for Health Care
- CT
Computed Tomography
- DSI
Dual Sensory Impairment
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- ICD
International Classification of Diseases
- IQR
Interquartile Range
- MEDALZ
Medication use and Alzheimer’s disease study
- MRI
Magnetic Resonance imaging
- NINCDS-ADRDA
National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association
- OR
Odds Ratio.
- PR
Prescription Register
- RR
Risk Ratio
- SD
Standard Deviation
- SEP
Socioeconomic position
- SRR
Special Reimbursement Register
- WHO
World Health Organization
Author contributions
Study concept and design- BR, KH, AD, KK, SH, and AMT Acquisition, analysis, and interpretation of data- BR, KH, AD, KK, SH, and AMTPreparation of manuscript- BR, AD, KK, SH, and AMTCritical revision for relevant intellectual content: BR, AD, KK, SH, and AMT.
Funding
No funding was received for conducting this research.
Data availability
The data that support the findings of this study are available from the Social Insurance Institution (SII) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors (contact AMT) upon reasonable request and with permission of SII.
Declarations
Ethics approval
Ethics committee approval or informed consent were not required according to the Finnish legislation (Personal Data Act) because only de-identified, routinely collected register data was used and the study participants were not contacted. The MEDALZ study protocol was approved by the register maintainers (Statistics Finland, SII, and National Institute of Health and Welfare) and the University of Eastern Finland.
Consent for publication
Not applicable.
Competing interests
AMT reports a research grant from Amgen, paid through the institution she is employed by, outside of the submitted work. BR, KH, AD, KK and SH have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alzheimer’s A. 2023 Alzheimer’s disease facts and Fig. 2023;19:alz.13016.
- 2.World Health Organization (WHO). Dementia Fact Sheet. 2021.
- 3.Livingston G, Huntley J, Liu KY, Costafreda SG, Selbæk G, Alladi S, et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet. 2024;404:572–628. [DOI] [PubMed] [Google Scholar]
- 4.Kuo P-L, Huang AR, Ehrlich JR, Kasper J, Lin FR, McKee MM, et al. Prevalence of Concurrent Functional Vision and Hearing Impairment and Association with Dementia in Community-Dwelling Medicare beneficiaries. JAMA Netw Open. 2021;4:e211558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuźma E, Littlejohns TJ, Khawaja AP, Llewellyn DJ, Ukoumunne OC, Thiem U. Visual impairment, Eye diseases, and dementia risk: a systematic review and Meta-analysis. J Alzheimer’s Disease. 2021;83:1073–87. [DOI] [PubMed] [Google Scholar]
- 6.Wang H-F, Zhang W, Rolls ET, Li Y, Wang L, Ma Y-H et al. Hearing impairment is associated with cognitive decline, brain atrophy and tau pathology. eBioMedicine. 2022;86. [DOI] [PMC free article] [PubMed]
- 7.Chen DS, Betz J, Yaffe K, Ayonayon HN, Kritchevsky S, Martin KR, et al. Association of hearing impairment with declines in physical functioning and the risk of disability in older adults. J Gerontol Biol Sci Med Sci. 2015;70:654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polku H, Mikkola TM, Rantakokko M, Portegijs E, Törmäkangas T, Rantanen T, et al. Self-reported hearing difficulties and changes in life-space mobility among community-dwelling older adults: a two-year follow-Up study. BMC Geriatr. 2015;15:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schubert CR, Fischer ME, Pinto AA, Klein BEK, Klein R, Tweed TS, et al. Sensory impairments and risk of mortality in older adults. J Gerontol Biol Sci Med Sci. 2017;72:710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamil RJ, Betz J, Powers BB, Pratt S, Kritchevsky S, Ayonayon HN, et al. Association of hearing impairment with Incident Frailty and Falls in older adults. J Aging Health. 2016;28:644–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo Y, He P, Guo C, Chen G, Li N, Zheng X. Association between Sensory Impairment and Dementia in older adults: evidence from China. J Am Geriatr Soc. 2018;66:480–6. [DOI] [PubMed] [Google Scholar]
- 13.Michalowsky B, Hoffmann W, Kostev K. Association between Hearing and Vision Impairment and Risk of Dementia: results of a case-control study based on secondary data. Front Aging Neurosci. 2019;11. [DOI] [PMC free article] [PubMed]
- 14.Hu W, Wang Y, Wang W, Zhang X, Shang X, Liao H, et al. Association of Visual, hearing, and dual sensory impairment with Incident Dementia. Front Aging Neurosci. 2022;14:872967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang PH, Longstreth WT Jr, Thielke SM, Francis CE, Carone M, Kuller LH, et al. Longitudinal changes in hearing and visual impairments and risk of dementia in older adults in the United States. JAMA Netw Open. 2022;5:e2210734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byeon G, Oh GH, Jhoo JH, Jang J-W, Bae JB, Han JW, et al. Dual sensory impairment and cognitive impairment in the Korean Longitudinal Elderly Cohort. Neurology. 2021;96:e2284–95. [DOI] [PubMed] [Google Scholar]
- 17.Maharani A, Dawes P, Nazroo J, Tampubolon G, Pendleton N, Sense-Cog WP1 Group. Associations between Self-reported sensory impairment and risk of Cognitive decline and impairment in the Health and Retirement Study Cohort. Journals Gerontology: Ser B. 2020;75:1230–42. [DOI] [PubMed] [Google Scholar]
- 18.Dintica CS, Calderón-Larrañaga A, Vetrano DL, Xu W. Association between Sensory Impairment and dementia: the roles of Social Network and Leisure Activity. J Alzheimers Dis. 2023;94:585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenowitz WD, Kaup AR, Lin FR, Yaffe K. Multiple sensory impairment is Associated with increased risk of Dementia among Black and White older adults. J Gerontol Biol Sci Med Sci. 2019;74:890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pabst A, Bär J, Röhr S, Löbner M, Kleineidam L, Heser K, et al. Do self-reported hearing and visual impairments predict longitudinal dementia in older adults? J Am Geriatr Soc. 2021;69:1519–28. [DOI] [PubMed] [Google Scholar]
- 21.Maruta M, Tabira T, Sagari A, Miyata H, Yoshimitsu K, Han G, et al. Impact of sensory impairments on dementia incidence and symptoms among Japanese older adults. Psychogeriatrics. 2020;20:262–70. [DOI] [PubMed] [Google Scholar]
- 22.Hwang PH, Longstreth WT, Brenowitz WD, Thielke SM, Lopez OL, Francis CE, et al. Dual sensory impairment in older adults and risk of dementia from the GEM study. Alzheimers Dement (Amst). 2020;12:e12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran EM, Stefanick ML, Henderson VW, Rapp SR, Chen J-C, Armstrong NM, et al. Association of Visual Impairment with Risk of Incident Dementia in a women’s Health Initiative Population. JAMA Ophthalmol. 2020;138:624–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuller-Thomson E, Nowaczynski A, MacNeil A. The Association between Hearing Impairment, Vision Impairment, dual sensory impairment, and Serious Cognitive Impairment: findings from a Population-based study of 5.4 million older adults. J Alzheimer’s Disease Rep. 2022;6:211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tolppanen AM, Taipale H, Koponen M, Lavikainen P, Tanskanen A, Tiihonen J, et al. Cohort profile: the Finnish medication and Alzheimer’s disease (MEDALZ) study. BMJ Open. 2016;6:012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease. Neurology. 1984;34:939–44. [DOI] [PubMed] [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV). American Psychiatric Publishing; 1994.
- 28.Liljas AEM, Walters K, de Oliveira C, Wannamethee SG, Ramsay SE, Carvalho LA. Self-reported sensory impairments and changes in cognitive performance: a longitudinal 6-Year Follow-Up study of English Community-Dwelling adults aged ⩾50 years. J Aging Health. 2020;32:243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong T, Mitchell P, Burlutsky G, Liew G, Wang JJ. Visual impairment, hearing loss and cognitive function in an older Population: longitudinal findings from the Blue mountains Eye Study. PLoS ONE. 2016;11:e0147646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of Age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and Meta-analysis. JAMA Otolaryngology–Head Neck Surg. 2018;144:115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin FR, Metter EJ, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and Incident Dementia. Arch Neurol. 2011;68:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallacher J, Ilubaera V, Ben-Shlomo Y, Bayer A, Fish M, Babisch W, et al. Auditory threshold, phonologic demand, and incident dementia. Neurology. 2012;79:1583–90. [DOI] [PubMed] [Google Scholar]
- 33.Hung S-C, Liao K-F, Muo C-H, Lai S-W, Chang C-W, Hung H-C. Hearing loss is Associated with risk of Alzheimer’s Disease: a case-control study in older people. J Epidemiol. 2015;25:517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng J, Huang C, Liang L, Li C, Wang X, Ma J, et al. The Association between Eye Disease and Incidence of Dementia: systematic review and Meta-analysis. J Am Med Dir Assoc. 2023;24:1363–e13736. [DOI] [PubMed] [Google Scholar]
- 35.Xu X-H, Zou J-Y, Geng W, Wang A-Y. Association between glaucoma and the risk of Alzheimer’s disease: a systematic review of observational studies. Acta Ophthalmol. 2019;97:665–71. [DOI] [PubMed] [Google Scholar]
- 36.Shang X, Zhu Z, Wang W, Ha J, He M. The Association between Vision Impairment and Incidence of Dementia and cognitive impairment: a systematic review and Meta-analysis. Ophthalmology. 2021;128:1135–49. [DOI] [PubMed] [Google Scholar]
- 37.Lee CS, Larson EB, Gibbons LE, Lee AY, McCurry SM, Bowen JD, et al. Associations between recent and established ophthalmic conditions and risk of Alzheimer’s disease. Alzheimers Dement. 2019;15:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang PH, Longstreth WT, Thielke SM, Francis CE, Carone M, Kuller LH, et al. Ophthalmic conditions associated with dementia risk: the Cardiovascular Health Study. Alzheimers Dement. 2021;17:1442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rong SS, Lee BY, Kuk AK, Yu XT, Li SS, Li J, et al. Comorbidity of dementia and age-related macular degeneration calls for clinical awareness: a meta-analysis. Br J Ophthalmol. 2019;103:1777–83. [DOI] [PubMed] [Google Scholar]
- 40.Purola P, Kaarniranta K, Ojamo M, Gissler M, Uusitalo H. Visual impairment due to age-related macular degeneration during 40 years in Finland and the impact of novel therapies. Acta Ophthalmol. 2023;101:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaajanen A, Purola P, Ojamo M, Gissler M, Uusitalo H. Changes in incidence and severity of visual impairment due to glaucoma during 40 years – a register-based study in Finland. Acta Ophthalmol. 2022;100:534–40. [DOI] [PubMed] [Google Scholar]
- 42.Heloterä H, Arffman M, Sund R, Keskimäki I, Kaarniranta K. The incidence and prevalence of diabetic macular edema and proliferative diabetic retinopathy, their progression to visual impairment and patterns in their intravitreal treatment in the Finnish population. Acta Ophthalmologica. n/a n/a. [DOI] [PubMed]
- 43.Valentijn SAM, van Boxtel MPJ, van Hooren SAH, Bosma H, Beckers HJM, Ponds RWHM, et al. Change in sensory functioning predicts change in cognitive functioning: results from a 6-year follow-up in the maastricht aging study. J Am Geriatr Soc. 2005;53:374–80. [DOI] [PubMed] [Google Scholar]
- 44.Panza F, Lozupone M, Sardone R, Battista P, Piccininni M, Dibello V, et al. Sensorial frailty: age-related hearing loss and the risk of cognitive impairment and dementia in later life. Therapeutic Adv Chronic Disease. 2019;10:2040622318811000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griffiths TD, Lad M, Kumar S, Holmes E, McMurray B, Maguire EA, et al. How Can Hear Loss Cause Dementia? Neuron. 2020;108:401–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sommerlad A, Kivimäki M, Larson EB, Röhr S, Shirai K, Singh-Manoux A, et al. Social participation and risk of developing dementia. Nat Aging. 2023;3:532–45. [DOI] [PubMed] [Google Scholar]
- 47.Thompson PM, Hayashi KM, de Zubicaray G, Janke AL, Rose SE, Semple J, et al. Dynamics of Gray Matter Loss in Alzheimer’s Disease. J Neurosci. 2003;23:994–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fulton SE, Lister JJ, Bush ALH, Edwards JD, Andel R. Mechanisms of the hearing–cognition relationship. Semin Hear. 2015;36:140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sund R. Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health. 2012;40:505–15. [DOI] [PubMed] [Google Scholar]
- 50.Solomon A, Ngandu T, Soininen H, Hallikainen MM, Kivipelto M, Laatikainen T. Validity of dementia and Alzheimer’s disease diagnoses in Finnish national registers. Alzheimer’s Dement. 2014;10:303–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the Social Insurance Institution (SII) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors (contact AMT) upon reasonable request and with permission of SII.