Abstract
Background
Depression exhibits notable prevalence among patients affected by chronic kidney disease(CKD) and end-stage renal disease (ESRD). Emerging reports suggest a potential association between magnesium (Mg) levels and depressive symptoms, there has been a surge of interest in exploring Mg supplementation as a complementary measure in depression treatment.
Objective
In this study our aim is to investigate the correlation between depressive symptoms and serum Mg level in patients undergoing peritoneal dialysis (PD) at Handan First Hospital, China. Additionally, we assessed the diagnostic significance of this relationship and identified pertinent influencing factors.
Methods
This study comprises a cohort of 140 individuals undergoing PD for a minimum duration of 3 months at the PD center of the Handan First Hospital. The Hamilton Depression Scale (HAMD) served as the assessment tool to evaluate the psychological status of the patients. Serum Mg levels, hemoglobin (Hb), and various demographic and clinical data were collected. Logistic regression and ROC analysis were performed to identify significant predictors of depression.
Results
The prevalence of depression was higher in patients with hypomagnesemia (60%) compared to those with normal or elevated Mg levels. Notably, a correlation emerged between abnormal serum Mg levels and the presence of depressive symptoms among individuals undergoing PD. Furthermore, binary logistic regression analysis revealed that serum Mg levels, hemoglobin (Hb) concentration, and unemployment significantly influenced the likelihood of occurrence of depression in patients undergoing PD (P< 0.05).
Conclusion
In addition, serum Mg levels demonstrate significant predictive value in anticipating onset of depression, indicating that rectifying low serum Mg levels among patients undergoing PD may serve as a preventive measure against depression. Further research is recommended to explore the therapeutic potential of Mg supplementation in this population.
Keywords: biochemical marker, chronic kidney disease, depression, magnesium, peritoneal dialysis
Introduction
The global population of individuals receiving peritoneal dialysis (PD) is experiencing significant growth.1 Despite notable progress resulting in enhanced overall survival rates in recent decades, the management of multiple complications and heavy financial burden, alongside necessary lifestyle modifications such as reduced engagement in social, occupational, and recreational activities, remains a critical area requiring attention.2
Studies have consistently highlighted the heightened prevalence of depression among patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD). In comparison to the general population, where the risk of depression typically ranges from 5% to 10%, patients with CKD and ESRD exhibit a significantly elevated risk. Specifically, the prevalence of depression in this patient demographic is estimated to be 3 to 4 times greater than that of the general population and 2 to 3 times higher than in patients with other chronic diseases.3,4
Depression is a major comorbidity in patients with CKD and ESRD, particularly in those undergoing PD.5,6 Reports indicate that between 22.8% to 39.3% of patients undergoing dialysis experience depression with approximately 21.3% facing severe manifestation of depression.4,7 Depression can yield adverse clinical consequences, including compromised adherence to dialysis and medication regimes, diminished immune system functionality, and compromised nutritional status.8–11 Moreover, depression serves as a risk factor for heightened morbidity and mortality rates in these patients. Notably, the potential for suicide and self-harm among patients suffering with depression should not be underestimated.12
Prior studies have underscored the association between depressive symptoms and various factors, including inflammatory cytokines such as interleukin (IL)-6, serum levels of phosphorus, hemoglobin (Hb), and psychological stressors such as anxiety stemming from uncertainty surrounding future treatment.13–15 Additionally, magnesium (Mg) deficiency has emerged as a potential contributor to personality changes including depressive symptoms. Analysis examining serum Mg status among individuals with depression has revealed higher incidence of low serum Mg levels in these patients compared to individuals without depression.16
Mg is the fourth most abundant cation in the human body and the second most abundant cation within cells, trailing only potassium in abundance. While psychological and biological factors contribute to depression, there is growing interest in the role of electrolyte imbalances, specifically magnesium (Mg), as a potential contributor to depressive symptoms.14 Magnesium plays a crucial role in numerous physiological processes, including neuromuscular function and neurotransmitter regulation, both of which are implicated in mood disorders.16 Recent studies have suggested that low serum magnesium levels may be associated with depression, and magnesium supplementation has shown promise in improving depressive symptoms in the general population.13 However, little is known about the relationship between magnesium levels and depression in PD patients. Identifying modifiable factors such as serum magnesium levels could have significant clinical implications, potentially leading to new treatment approaches, including routine magnesium monitoring and supplementation for depression management in PD patients.
In addition, many dialysis patients take proton pump inhibitors (PPIs) for gastrointestinal symptoms, and these drugs have been shown to affect magnesium absorption, which in turn may exacerbate the occurrence of hypomagnesemia. The study found that patients who used PPIs over a long period of time may have an increased risk of depression, suggesting that the complex interaction between the drug and electrolytes may play an important role in the development of depression.7,10
Despite garnering significant attention for its potential involvement in the pathophysiology of the mood disorders, existing evidence remains inconclusive.16 Notably, Mg supplementation has exhibited promise in improving depressive symptoms, however, the precise mechanism underlying this effect remains unclear.17 The question of whether serum Mg levels serve as a biological predictor or a risk factor for depression remains unclear. Therefore, in the light of these considerations, we assessed the prevalence of depression among patients undergoing PD and delineate the relationship between depression and serum Mg levels in this patient cohort, while also exploring relevant influencing factors.
Methods
Participants and Settings
This study included a cohort of 140 patients who were undergoing peritoneal dialysis (PD) for at least 3 months at the Peritoneal Dialysis Center of the Second Department of Nephrology, Handan First Hospital, Handan, Hebei Province, China. The clinical laboratory at Handan First Hospital used the Beckman AU5800 automatic biochemical analyzer to measure various biochemical parameters, including serum magnesium (Mg) level.Data were collected from January 2021 to December 2022.
Inclusion criteria were as follows: (1) Age ranging between 20 and 80 years; (2) Minimum duration of regular PD treatment of less than 3 months; (3) Ability to comprehend and complete the Hamilton Depression Scale (HAMD) (refer Table 1) survey questionnaire and willingness to participate voluntarily; (4) Absence of any history of mental illness; (5) Availability of comprehensive clinical and biological data for collection.
Table 1.
Prevalence of Depression in PD Patients with Different Serum Magnesium
| Mg (mmol/L) | Number of Patients | Depression Group | Normal Group | Ratio % |
|---|---|---|---|---|
| <0.75 | 35 | 21 | 14 | 60.00 |
| 0.75–1.02 | 71 | 24 | 47 | 33.80 |
| >1.02 | 34 | 7 | 27 | 20.59 |
| Total | 140 | 52 | 88 | 37.14 |
Exclusion criteria were as follows: (1) Patients exhibiting severe cognitive impairment as assessed by the SIMPLE Mental State Inventory (refer Table 2); (2) Patients presenting with severe cardiovascular or cerebrovascular complications, malignant tumors, or surgical interventions within the past month; (3) Patients with history of peritonitis within the preceding month; (4) Individuals experiencing significant domestic adversity in the past month; (5) Patient with severe hearing impairment impending normal communication.
Table 2.
Comparison of General Data Between Depression Group and Non-Depression Group in PD
| Characteristic | Depression | Normal | Value of t/χ2 | P value |
|---|---|---|---|---|
| Number of patients | 52 | 88 | – | – |
| Sex | 1.830 | 0.176b | ||
| Male | 24 | 51 | ||
| Female | 28 | 37 | ||
| Age | 55.71±13.95 | 54.63±13.56 | 0.453 | 0.651a |
| Body mass index (kg/m2) | 24.84±3.66 | 24.31±3.71 | 0.821 | 0.413a |
| Dialysis duration (months) | 19.23±7.83 | 20.57±6.77 | −1.065 | 0.289a |
| Hypertension | 1.645 | 0.200b | ||
| Yes | 49 | 77 | ||
| No | 3 | 11 | ||
| Diabetes | 1.391 | 0.238b | ||
| Yes | 23 | 48 | ||
| No | 29 | 40 | ||
| Employment | 10.443 | 0.001*b | ||
| Yes | 5 | 30 | ||
| No | 47 | 58 | ||
| Annual household income | 8.259 | 0.016*b | ||
| Less than thirty thousand | 14 | 8 | ||
| Thirty to ten thousand | 34 | 68 | ||
| More than ten thousand | 4 | 12 | ||
| Degree of education | 0.491 | 0.782b | ||
| Illiteracy | 4 | 10 | ||
| Primary and junior middle education | 38 | 62 | ||
| Middle school or above | 10 | 16 |
Note: aT-test using independent samples; bmeans using the chi-square test; *indicates that the difference between depression group and normal group was statistically significant (P < 0.05).
The PD solution utilized for patients in this study was manufactured by Guangzhou Baxter Medical Supplies Co., Ltd., with a Mg ion concentration 0.25 mmol/L. Prior to commencement, this study received approval from the Medical Ethics Committee of Handan First Hospital under the protocol number HDYY-LL-KY2022-H05. Moreover, all patients provided informed consent after in-depth communication.
Demographic Data
Demographic data were systematically collected including variables such as gender, age, height, weight, body mass index (BMI), education (categorized into illiterate, primary school, and junior middle school or above), employment status (classified as employed and unemployed), income level (categorized based on annual family income as below RMB 30,000, RMB 30,000 to 100,000, or exceeding RMB 100,000), duration of dialysis (measure in months), and presence of comorbidities such as diabetes and/or hypertension. These data were acquired through direct communication with patients or extracted from their medical records via the electronic data repository of the medical center.
Laboratory Data
Laboratory data pertaining to the patients undergoing PD included a comprehensive array of parameters such as Hb, serum creatinine, blood urea nitrogen, serum potassium, serum calcium, serum phosphorus, serum albumin (Alb), lipid profile, 25-hydroxyvitamin D (25-(OH)D), and intact parathyroid hormone (IPTH). Data were obtained from fasting blood draws performed in the morning. Hb levels were determined utilizing the cismeacon XN9000 full blood cell analyzer, while biochemical indices were assessed using Beckman AU5800 automatic biochemical analyzer. Serum Mg levels were measured using the dimethylaniline blue method. Measurement of 25-(OH)D was conducted using Abbott i1000 immunoassay system, whereas parathyroid hormone levels were analyzed utilizing Roche Diagnostics’ 601 electrochemical immunoanalyzer.
Dialysis adequacy and residual glomerular filtration rate (GFR) were assessed through 24-hour urine and dialysate collection to determine Kt/V (The ratio of urea clearance by dialyzer to volume during a specified dialysis duration) and creatinine clearance rate (Ccr). All utilized kits were verified to be within their validity period. On the morning of the peritoneal balance test, peripheral blood samples were extracted from the patients undergoing PD following a fasting period of at least 10 hours. The collected blood sample underwent centrifugation at 3000 rotation per minute (rpm) to isolate the relevant components.
Diagnostic Criteria
Disorder of Mg Metabolism
There are various methods for detecting serum Mg levels and results can vary depending on the specific method employed. In this study serum Mg levels were measured using dimethylaniline blue method according to the detection standard of the Mg determination kit. The normal range of serum Mg concentration was defined as 0.75 mmol/L to 1.02 mmol/L. Patients with serum Mg levels exceeding 1.02 mmol/L were diagnosed with hypermagnesemia, whereas levels falling below 0.75 mmol/L were indicative of hypomagnesemia.
Diagnosis of Depression
The HAMD, comprising 17 items, is a widely employed depression scale for diagnosing and quantifying the severity of depression in subjects. The Turkish version of the HAMD with a score range of 0 to 54 (indicating maximal impairment) was utilized to diagnose and quantify the severity of depression in those enrolled for this study. Assessors were specifically trained to ensure objectivity and consistency in scoring.
Patients were stratified into two distinct groups based on their HAMD scores: Group 1 consisted of individuals with low HAMD scores (ranging from 0 to 17), while Group 2, comprised patients with high HAMD scores (> 17). All data collection, patient interview and psychological assessment were performed in the Second Department of Nephrology, Handan First Hospital.
Statistical Analysis
All data analyses were conducted using the SPSS version 24.0 statistical software. Continuous variables with normal distribution are expressed as mean ± standard deviation (X ± S), and between-groups comparisons was performed utilizing t-test. Non-normally distributed continuous variables are expressed as median and interquartile range, with between-group comparisons conducted using Mann–Whitney U-test. Categorical data are expressed as proportions and comparisons between groups were conducted using the chi-squared (χ2) test. Binary logistic regression analysis was employed to identify factors influencing depression while receiver operating characteristic (ROC) curve analysis was utilized to determine the diagnostic efficacy. A value of P < 0.05 was considered as a statistically significant difference.
Results
Basic Clinical Data
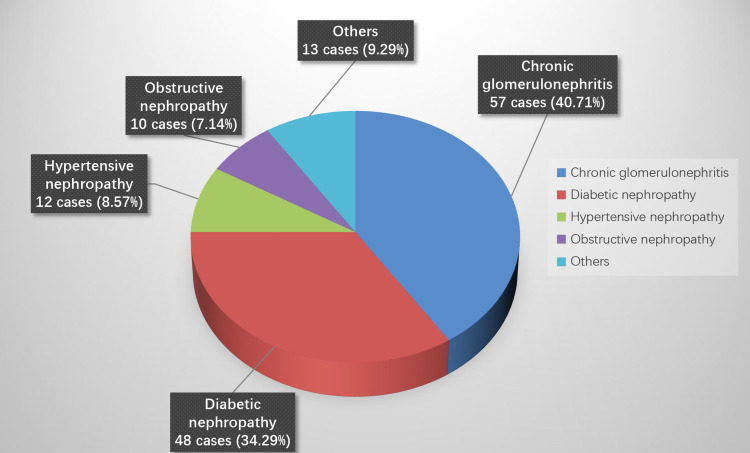
A total of 140 patients undergoing PD were enrolled in this study. The cohort comprised 75 males (53.57%) and 65 females (46.43%), with ages ranging from 21 to 80 years (average age 55.03 ± 13.67 years). The mean duration of dialysis was (20.07 ± 7.18) months. The primary etiologies of ESRD included chronic glomerulonephritis in 57 cases (40.71%), diabetic nephropathy in 48 cases (34.29%), hypertensive nephropathy in 12 cases (8.57%), obstructive nephropathy in 10 cases (7.14%), primary small vessel vasculitis in 4 cases (2.86%), systemic lupus erythematosus nephropathy in 4 cases (2.86%), polycystic kidney disease in 3 cases (2.14%), and amyloid nephritis in 2 cases (1.43%) (see Figure 1). Notably, in this study we found that chronic glomerulonephritis emerged as the predominant case of ESRD, consistent with findings from related studies.
Figure 1.
Constituent ratio of primary disease in patients undergoing PD.
Serum Mg Levels
In this study, serum Mg levels of all patients undergoing PD were detected using the ditoluidine blue method. There were 34 patients (24.29%) with hypermagnesemia, 71 patients (50.71%) with normal serum Mg level, and 35 patients (25%) with hypomagnesemia.
Prevalence of Depression in Patients with Different Serum Mg Levels
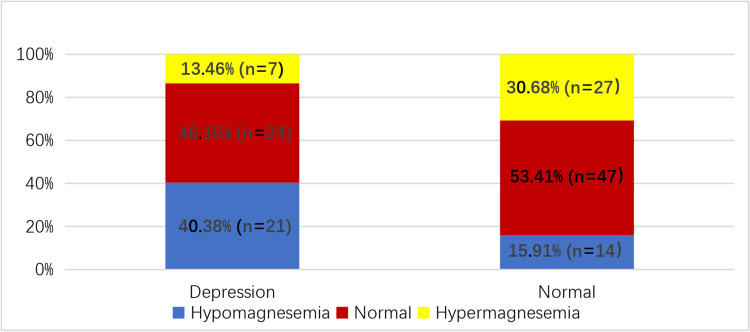
Among those enrolled, the prevalence of depression within the hypomagnesemia group was 60% (n = 21), while in the normal serum Mg group it was 33.80% (n = 24), and in the hypermagnesemia group, it was 20.59% (n = 7). The total number of patients with depression was 52. The prevalence of depression across all groups was 37.1%, with significant differences observed among the three groups (P<0.05) (refer to Table 3 and Figure 2). Pairwise comparison revealed a statistically significant difference between the hypomagnesemia group and both the normal and hypermagnesemia groups (P<0.05). However, no significant difference was noted between the normal serum Mg group and the hypermagnesemia groups (P<0.05).
Table 3.
Comparison of Laboratory Indexes Between Depression Group and Non-Depression Group in PD Patients
| Clinical Index | Depression Group | Normal Group | t/z | P value |
|---|---|---|---|---|
| Hb(g/L) | 83.46±22.80 | 96.36±21.11 | −3.392 | 0.001*a |
| Serum magnesium (mmol/L) | 0.79 (0.27) | 0.91 (0.30) | −4.506 | 0.000*c |
| Serum calcium (mmol/L) | 2.11 (0.55) | 2.10 (0.55) | −0.677 | 0.498c |
| Serum phosphorus (mmol/L) | 2.01 (0.31) | 2.12 (0.34) | −1.510 | 0.131c |
| 25-(OH)D (ng/mL) | 18.80 (18.35) | 27.00 (15.28) | −3.235 | 0.001*c |
| Serum creatinine (umol/L) | 746.00 (429.50) | 604.50 (358.75) | −1.596 | 0.111c |
| Urea nitrogen (mmol/L) | 17.60 (11.53) | 17.10 (9.48) | −0.326 | 0.745c |
| Parathyroid stimulation (pg/mL) | 201.85 (282.27) | 174.35 (221.95) | −0.567 | 0.571c |
| Serum albumin (g/L) | 32.56±7.27 | 35.44±6.68 | −2.385 | 0.018*a |
| Cholesterol (mmol/L) | 4.46±1.51 | 4.33±0.93 | 0.553 | 0.582a |
| Triglyceride (mmol/L) | 1.57 (0.88) | 1.53 (1.05) | −0.304 | 0.761c |
| High density lipoprotein (mmol/L) | 0.84 (0.49) | 1.06 (0.58) | −2.711 | 0.007*c |
| Low density lipoprotein (mmol/L) | 2.49±0.74 | 2.33±0.68 | 1.265 | 0.208a |
| The Kt/V of residual kidney | 0.08 (0.05) | 0.07 (0.05) | −0.316 | 0.752c |
| The Kt/V of peritoneum | 0.17 (0.08) | 0.18 (0.07) | −0.015 | 0.988c |
Note: at test using independent samples; cdenotes the use of rank sum test; *indicates that the difference between depression group and normal groups was statistically significant (P < 0.05).
Figure 2.
Proportion of different serum Mg levels in patients undergoing PD in depression group and non-depression group.
Comparison of General Data Between Depressed and Non-Depressed Patients
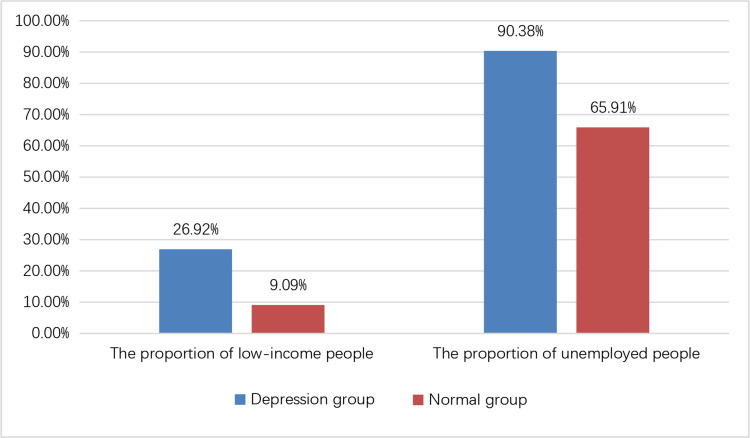
In the depression group, the number of employed people was significantly lower compared to the normal group, whereas the proportion of individuals with low-income was significantly higher in the depression group compared to the normal group (P<0.05). However, no significant differences were observed in terms of gender, age, BMI, duration of dialysis, education, hypertension, or diabetes between the two groups (P>0.05) (refer Table 4, Figure 3).
Table 4.
Logistic Regression Analysis of Related Factors of Depression in Peritoneal Dialysis Patients
| Characteristic | B | SE | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Hb | −0.415 | 0.012 | 11.115 | 0.001* | 0.959 | 0.936–0.938 |
| Serum magnesium | −6.266 | 1.728 | 13.155 | 0.000* | 0.002 | 0.000–0.056 |
| 25-(OH)D | −0.037 | 0.027 | 1.963 | 0.161 | 0.963 | 0.914–1.015 |
| Serum albumin | −0.060 | 0.034 | 3.064 | 0.080 | 0.942 | 0.880–1.007 |
| High density lipoprotein | 0.578 | 0.879 | 0.432 | 0.511 | 1.782 | 0.318–9.985 |
| Annual household Income | −0.956 | 0.680 | 1.976 | 0.160 | 0.384 | 0.101–1.458 |
| employment | −2.797 | 0.751 | 13.875 | 0.000* | 0.061 | 0.014–0.266 |
Note: *indicates P < 0.05, indicating statistical significance.
Figure 3.
Comparison of general data between depression group and non-depression group in patients undergoing PD.
Note: The difference was statistically significant (P < 0.05).
Comparison of Blood Biochemical Data Between Depression Group and Normal Group
In this study, individuals in the depression group exhibited significantly lower levels of Hb, serum Mg ion, 25-(OH)D, serum Alb, and high-density lipoprotein (HDL) compared to those in the normal group (P<0.05). However, there were no significant differences observed between the two groups in terms of serum calcium, serum phosphorus, serum creatinine, blood urea nitrogen, parathyroid hormone, cholesterol, triglyceride, low-density lipoprotein (LDL), residual kidney Kt/V, and peritoneal Kt/V (P>0.05) (refer Table 5).
Table 5.
Diagnostic Value of Serum Magnesium and Hemoglobin in Depression with PD Patients
| Index | AUC | P | 95% CI | Sensitivity (%) | Specificity (%) | Youden Index | Cut-off |
|---|---|---|---|---|---|---|---|
| Serum magnesium (mmol/L) | 0.728 | 0.000 | 0.641–0.816 | 0.808 | 0.591 | 0.399 | 0.855 |
| Hb (g/L) | 0.653 | 0.002 | 0.558–0.748 | 0.692 | 0.591 | 0.283 | 89.500 |
Note: *indicates P < 0.05, indicating statistical significance.
Logistic Regression of Risk Factors for Depression in Patients Undergoing PD
Indicators with a significance level of P < 0.1 in Table 4 and Table 5 were incorporated into the logistic regression analysis. Binary logistic regression was then employed to evaluate the impact of employment status, annual household income, Hb level, serum Mg ion level, 25-(OH)D level, serum Alb level, and HDL level on depression occurrence in patients undergoing PD. The results revealed that a decrease of serum Mg ion level, Hb level, and unemployment status were significantly associated with the occurrence of depression in patients undergoing PD (P<0.05) (refer Table 4).
Diagnostic Value of Serum Mg and Hb for Depression
The area under the ROC curve (AUC) serves as a metric of test accuracy. A value exceeding 0.9 indicates high diagnostic value, while AUCs between 0.7 and 0.9 indicate moderate diagnostic value. AUC ranging from 0.5 to 0.7 indicate low diagnostic value, and AUCs below 0.5 indicate no diagnostic value.
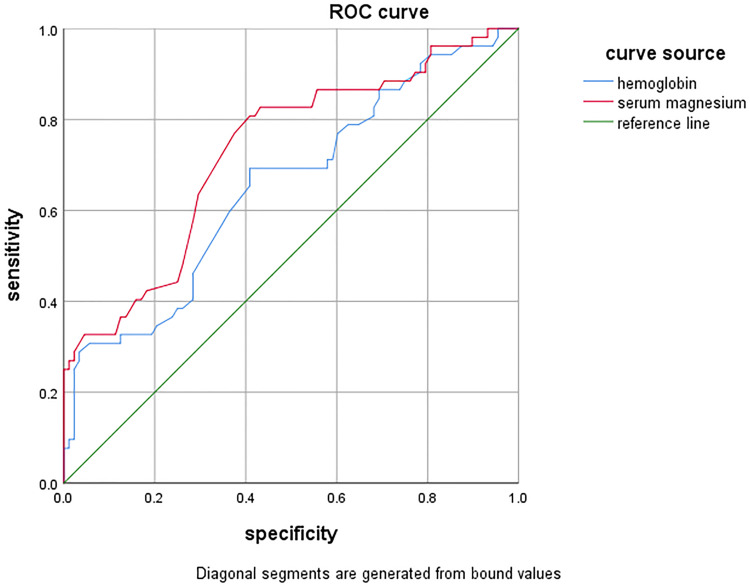
In this study, the AUC for serum Mg and Hb in predicting depression among patients undergoing PD was found to be AUCMg = 0.728 and AUCHb = 0.653 respectively. Both AUCMg and AUCHb values were greater than 0.5, indicating diagnostic value (all P values <0.05). Specifically, AUCMg > AUCHb, indicating that serum Mg may possess higher predictive utility for depression in patients undergoing PD compared to Hb levels. The ROC curves for serum Mg and Hb were further analyzed to determine the Youden’s index, cut-off values, and corresponding specificity and sensitivity (refer Figure 4 and Table 5).
Figure 4.
ROC curve of serum Mg and Hb.
Discussion
In a 2020 report by the World Health Organization, depression is projected to emerge as the second leading cause of disability globally among non-communicable diseases.18 Depression ranks as the most prevalent psychiatric disorder following PD and is associated with adverse outcomes. Studies have found that abnormalities in Mg homeostasis have been correlated with various personality changes, including depression. Despite the high incidence of abnormal serum Mg levels among patients undergoing PD, there is a dearth of research investigating the potential relationship between serum Mg abnormalities and alterations in mental well-being induced by dialysis.
Serum Mg is the second most abundant divalent cation within cellular content. In a healthy human body, the intake and excretion of Mg ions are meticulously balanced, relying on joint regulation involving dietary intake, gastrointestinal absorption, and renal metabolism.19 The kidneys play a pivotal role in regulating Mg balance through glomerular filtration and tubular reabsorption. The concentration of extracellular Mg, crucial for overall Mg balance, is chiefly influenced by dietary intake and renal excretion.20
In patients with CKD, disruptions in Mg metabolism arise due to impaired renal excretion. In conditions of impaired kidney function, particularly in patients with end-stage renal disease, imbalances in Mg ion levels occur frequently, often resulting in varying degree of persistent hypermagnesemia clinically.21 International studies have corroborated the common occurrence of hypermagnesemia.22,23 However, factors such as thiazide and loop diuretics, as well as lower Mg concentration in dialysate, contribute to Mg loss from the body. Additionally, dietary restrictions imposed on patients undergoing PD may limit their intake of Mg-rich foods. Consequently, hypomagnesemia is not uncommon among patients with ESRD undergoing PD. Studies by Tsai et al24 and Ye et al25 have reported hypermagnesemia in 33% of patients undergoing PD and hypomagnesemia in 40.5% of patients undergoing PD.
At the Hemodialysis Center of Peking University People’s Hospital, a strikingly high proportion of patients undergoing hemodialysis exhibit concomitant hypermagnesemia, reaching 81%. However, it is noteworthy that all these patients have Mg levels below 2 mmol/L, with none experiencing hypomagnesemia.26 Conversely, in the present study, the incidence of hypomagnesemia among patients undergoing PD was 25%, while the incidence of hypermagnesemia was 24.3%. In contrast, Cai et al27 reported a lower hypomagnesemia prevalence of 14.2% among patients undergoing PD.
These divergent findings across studies concerning serum Mg levels in patients undergoing PD may be attributed to differences in PD fluid compositions. Specifically, individuals in this study exclusively utilized PD fluid containing 0.25 mmol/L Mg ions from Guangzhou BaiTe Company, whereas other studies employed different types of PD solutions with varying Mg concentrations. These variations influenced by national and regional differences, can affect Mg clearance rates in PD effluent and consequently lead to discrepancies in blood Mg levels.
Additionally, disparities in test results for serum Mg levels may be influenced by variations in race and differences in the methods and institutions employed for measurement. Additionally, common medications prescribed for patients with CKD, such as vitamin D and diuretics, alongside the widespread use of acid suppressants for gastrointestinal symptoms, further complicate Mg homeostasis.
In our study, all participants were patients undergoing PD with a dialysis vintage exceeding 3 months. Diuretic usage is prevalent in this patient group to preserve residual urine volume and safeguard residual kidney function. Furthermore, a subset of these individuals commonly employ acid suppressants to alleviate gastrointestinal symptoms, which may increase the incidence of hypomagnesemia. However, the clinical significance of Mg homeostasis disorder and the actual risks associated with hypermagnesemia and hypomagnesemia remain subjects of controversy.
Rodríguez et al17 and Jacka et al28 identified a correlation between low blood Mg levels and heightened depressive symptoms across various racial groups. Additionally, it has been reported that very low Mg intake is significantly associated with depression.29 Depression is the most prevalent psychological concern encountered in clinical practice among patients undergoing PD. Research suggests that the prevalence of depression among dialysis patients ranges from approximately 13.1% to 76.3%, significantly surpassing rates observed in the general population.30
Depression represents a prevalent mental health condition characterized by persistent feelings of low mood, diminished energy levels, and a loss of interest or pleasure in usual activities. A study in southern China revealed a depression prevalence of 34% among patients undergoing PD,31 while a cross-sectional study conducted in Qatar reported a depression prevalence of 45.8%.32 In this study, the overall incidence of depression among patients was 37.1% (refer Figure 2 and Table 1), a finding consistent with previous research. However, a study by Traisathit et al reported a lower depression incidence of only 11% among patients undergoing PD.33
The disparities observed in these findings may stem from the utilization of various measurement scales to assess depression. These scales include Patient Health Questionnaire(PHQ)-9 depression screening scale, Beck Depression Inventory, Self-Rating Depression Scale(SDS), Hospital Anxiety and Depression Scale(HADS) depression scale, and the HAMD depression scale. Among these, the HAMD depression scale is one of the earliest semi-structured interview tools employed for clinical assessment of adult depression. Its applicability extends even to patients with lower cultural literacy.
In our study, a notable proportion of patients exhibited limited educational qualifications, with a substantial proportion having completed only primary or secondary school education or were illiterate. Utilizing the HAMD depression scale for assessment conferred advantages due to the participants’ lower literacy levels. Furthermore, this scale was tailored to evaluate emotional changes within three months of exposure to a stressor such as peritoneal dialysis. The selection of different assessment scales may contribute to the observed variability in depression incidence rates among patients undergoing PD across variable studies.
The study also revealed a widespread lack of awareness and suboptimal treatment of depression among patients. Participants expressed a perception that clinical doctors did not adequately acknowledge their mental health concerns. These findings emphasize the necessity for nephrologists to prioritize psychological health support and counseling services for patients undergoing PD.
Further examination of the association between serum Mg levels and depression reveals consistent findings across variable studies. Some investigations have reported significantly lower Mg levels in both cerebrospinal fluid and serum among patients diagnosed with depression compared to the control groups.34 Moreover, animal experiments have validated the correlation between serum Mg levels and depression. Mirzaian et al35 demonstrated that mice fed with a Mg-deficient diet exhibited depression-related behaviors in their daily activities. Similarly, Cardoso et al36 found that Mg supplementation improved depressive-like behavior in mice, suggesting an inverse relationship between serum Mg ions and depressive behavior in these animals. The findings of this study also align with previous research indicating a gradual increase in the incidence of depression among patients as serum Mg ion levels decrease (see Figure 2 and Table 1). However, it is essential to recognize that depression in humans engaged in complex social activities, may not stem from a singular factor.
Studies investigating factors contributing to the development of anxiety and depression in hemodialysis patients have identified several risk factors. These include low income,37 unemployment and low educational levels,38 decreased levels of hemoglobin and serum albumin,39 and vitamin D deficiency.40 Addressing these risk factors is considered an effective strategy for preventing and treating depression in dialysis patients.
A comparison was made between the general characteristics (Table 2) and biochemical data (Table 3) of the depression group and the non-depression group. Significant differences were observed in the unemployment and low-income group (as depicted in Figure 3). Furthermore, Hb, serum Mg ions, 25-(OH)D, serum albumin, and high-density lipoprotein were all notably lower in the depression group compared to the non-depression group.
Subsequently, data exhibiting statistically significant differences were subjected to binary logistic regression analysis, revealing that Hb, serum Mg levels, and employment status were associated with depressive disorder in patients undergoing PD (see Table 4), while other factors demonstrated no significant correlation. This contrast with findings from previous research may be attributed to factors such as limited sample size and regional disparities in this study. Additionally, variations in nationality, race, and lifestyle could also influence research outcomes. It is important to note that this study is cross-sectional in nature. Given that majority of patients undergoing PD typically exhibit vitamin D deficiency and may experience hypoalbuminemia, many undergo clinical treatment involving alpha-calcidol and protein supplementation. These could be confounding factors potentially contributing to differences in research findings.
For patients undergoing PD, the factors contributing to their depression are multifaceted. The necessity for dialysis treatment due to kidney function loss significantly restricts patients’ social activities. The lifestyle adjustments necessitated by PD, performed multiple times daily, intensify the mental strain on patients, leading to diminished confidence. Moreover, various complications associated with PD, such as peritonitis, exit site infections, catheter malfunction, dialysate leakage, and cardiovascular complications, further exacerbate psychological burden on already vulnerable patients.
Additionally, PD often requires assistance and care from family members. Certain patients may harbor feelings of guilt regarding burdening their caregivers and worry about disrupting the lives of their family members. This explains the heightened prevalence of depression among patients undergoing dialysis compared to individuals with other chronic diseases. Therefore, actively guiding patients with work capacity to reintegrate into the workforce or encouraging their participation in social activities is paramount. Such interventions aid in fostering the acknowledgment of their social roles.
Finally, to evaluate the diagnostic value of the screened serum Mg and Hb levels for depression in patients undergoing PD, we generated an ROC curve using serum Mg levels as an indicator (AUC = 0.728, P < 0.001). The findings indicate that serum Mg levels possess a certain predictive value for such patients (see Figure 4 and Table 5).
A study investigating the role of Mg supplements in patients with depression confirmed the efficacy of Mg supplementation in treating the condition.41 Furthermore, a cross-sectional analysis involving 3,604 adults attending primary care clinics in the United States demonstrated an association between lower serum Mg levels and depressive symptoms.42 A study by Barragan et al34 also identified a correlation between the degree of Mg deficiency in serum and the severity of depressive symptoms. However, studies conducted by George et al43 and Camardese et al44 suggested no clear correlation between serum Mg levels and depressive symptoms. The discrepancies between studies may arise from the fact that Mg in the blood constitutes only 1% of the total Mg in the body. It can swiftly exchange with Mg in bones, muscles, and soft tissues through compensatory mechanisms leading to redistribution. Hence, serum Mg levels may not accurately reflect the true Mg storage in the body. Additionally, dietary habits and ethnic variations across different countries may also influence research outcomes. Nevertheless, the significance of the observed differences should not be disregarded.
Previous research conducted both domestically and internationally has consistently suggested that low blood Mg levels may contribute to the onset of depression. However, to date there has been no similar study focusing on patients undergoing PD. This study marks the first attempt in China to explore the correlation between serum Mg levels and depression in patients undergoing PD. The findings reveal that abnormal serum Mg levels and depression are prevalent in patients undergoing PD. Notably, the decrease in serum Mg levels exhibits a significant correlation with the incidence of depression and demonstrates a certain predictive value.
The findings of this study demonstrate a significant association between low serum magnesium levels and depression in patients undergoing peritoneal dialysis (PD), consistent with the expected results and in alignment with the study’s objectives. These results not only contribute to our understanding of the relationship between electrolyte imbalances and mood disorders but also highlight magnesium as a potential biomarker for depression in this population. The study confirms previous research indicating a link between magnesium deficiency and depressive symptoms, particularly in chronic illness populations such as patients with CKD and ESRD.16,45
To strengthen the study, it is important to consider the specific mechanisms through which low magnesium levels might contribute to depression, particularly in PD patients.Magnesium plays a critical role in modulating neurotransmitter systems that are involved in mood regulation. One key pathway is through its interaction with the N-methyl-D-aspartate (NMDA) receptor, a receptor that plays a pivotal role in glutamatergic neurotransmission. Dysregulation of the NMDA receptor has been implicated in the pathophysiology of depression.16,46 Magnesium acts as a natural antagonist to NMDA receptors, preventing excessive calcium influx into neurons, which is associated with neurotoxicity and mood disturbances.47 In cases of magnesium deficiency, this inhibitory effect is diminished, leading to overactivation of NMDA receptors, which may result in neuronal damage and the development of depressive symptoms.45 Magnesium is also essential for the synthesis of serotonin and norepinephrine, two neurotransmitters crucial for mood regulation.16 A deficiency in magnesium can reduce the production of these neurotransmitters, leading to imbalances that may contribute to depressive states.47 Several studies have suggested that magnesium supplementation can increase serotonin levels and improve mood, further supporting the role of magnesium in emotional well-being.17,29
Chronic inflammation and oxidative stress have been well-documented in both CKD and depression. Magnesium deficiency has been shown to exacerbate inflammatory responses by increasing the production of pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α).48 Inflammation is known to contribute to depressive symptoms by influencing neuroendocrine function and neurotransmitter metabolism.49 Additionally, oxidative stress resulting from low magnesium levels can lead to mitochondrial dysfunction and neurodegeneration, further exacerbating mood disorders.50
Given the association between low magnesium levels and depression in PD patients, regular monitoring of magnesium status in this population is crucial. This study highlights the potential therapeutic value of magnesium supplementation, which has shown promise in alleviating depressive symptoms in both general and CKD populations.17,29 Future studies should investigate the efficacy of magnesium supplementation in reducing depression specifically in PD patients, and whether correction of magnesium deficiency can improve both mood and overall quality of life.
Additionally, exploring the interactions between magnesium and other electrolytes, such as potassium and sodium, could provide a more comprehensive understanding of the role of electrolyte imbalances in mental health.51 Medication use, particularly proton pump inhibitors (PPIs), which are known to reduce magnesium absorption, should also be considered in future research.52
These findings underscore the need for healthcare professionals to broaden their focus beyond assessing the adequacy of peritoneal dialysis and the management of associated complications. They should also vigilantly monitor symptoms related to renal failure, including sleep disturbances, fatigue, pain, low mood, nausea, and poor appetite. Given the high prevalence of depression among patients undergoing PD and its significant impact on patient survival and prognosis, clinicians should incorporate psychological counseling into the care and regimen for patients. The establishment of a multidisciplinary team, including nephrologists, mental health specialists and dietitians, will help to comprehensively assess patients’ physical and psychological conditions and develop personalized treatment plans. Moreover, early assessments for individuals displaying depressive symptoms are imperative.
Monitoring and stabilizing blood Mg levels can offer new insights for the prevention and treatment of depression in patients with PD. The approach provides clinicians with novel targets for directing treatment interventions for this condition.
Acknowledgments
We are particularly grateful to all the people who have given us help on our article.
Funding Statement
Hebei Province Talent Training Program in 2019 [Grant number A201905016]; Science and Technology Research and Development Program of Handan City [Grant number 1723208066-4].
Abbreviations
PD, peritoneal dialysis; CKD, chronic kidney disease; ESRD, end-stage renal disease; IL, interleukin; Hb, hemoglobin; Mg, Magnesium; HAMD, Hamilton Depression Scale; BMI, body mass index; Alb, albumin; IPTH, intact parathyroid hormone; GFR, glomerular filtration rate; Ccr, creatinine clearance rate; ROC, receiver operating characteristic curve; AUC, area under the ROC curve; PHQ, patient health questionnaire; SDS, self-rating depression scale; HADS, hospital anxiety and depression scale.
Data Sharing Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the Declaration of Helsinki (as was revised in 2013). The study was approved by Ethics Committee of the Handan First Hospital. The written, informed consent was obtained from the participant for the publication.
Disclosure
The authors declare no conflict of interest.
References
- 1.Jain AK, Blake P, Cordy P, et al. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol. 2012;23:533–544. doi: 10.1681/ASN.2011060607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perl J, Wald R, Bargman JM, et al. Changes in patient and technique survival over time among incident peritoneal dialysis patients in Canada. Clin J Am Soc Nephrol. 2012;7:1145–1154. doi: 10.2215/CJN.01480212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katon WJ. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci. 2011;13(1):7–23. doi: 10.31887/DCNS.2011.13.1/wkaton [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer S, Vecchio M, Craig JC, et al. Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int. 2013;84(1):179–191. doi: 10.1038/ki.2013.77 [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the national comorbidity survey replication (NCS-R). JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095 [DOI] [PubMed] [Google Scholar]
- 6.Jiang W, Alexander J, Christopher E, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med. 2001;161(15):1849–1856. doi: 10.1001/archinte.161.15.1849 [DOI] [PubMed] [Google Scholar]
- 7.Shirazian S, Grant CD, Aina O, et al. Depression in chronic kidney disease and end-stage renal disease: similarities and differences in diagnosis, epidemiology, and management. Kidney Int Rep. 2017;2:94–110. doi: 10.1016/j.ekir.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimmel PL. Depression in patients with chronic renal disease: what we know and what we need to know. J Psychosom Res. 2002;53:951–956. doi: 10.1016/S0022-3999(02)00310-0 [DOI] [PubMed] [Google Scholar]
- 9.Kimmel PL, Phillips TM, Simmens SJ, et al. Immunologic function and survival in hemodialysis patients. Kidney Int. 1998;54:236–244. doi: 10.1046/j.1523-1755.1998.00981.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen SD, Kimmel PL. Nutritional status, psychological issues and survival in hemodialysis patients. Contrib Nephrol. 2007;155:1–17. [DOI] [PubMed] [Google Scholar]
- 11.Szeto C-C, Chan GC-K, Ng J-C, et al. Depression and physical frailty have additive effect on the nutritional status and clinical outcome of Chinese peritoneal dialysis. Kidney Blood Press Res. 2018;43:914–923. doi: 10.1159/000490470 [DOI] [PubMed] [Google Scholar]
- 12.Pompili M, Venturini P, Montebovi F, et al. Suicide risk in dialysis: review of current literature. Int J Psychiatry Med. 2013;46:85–108. doi: 10.2190/PM.46.1.f [DOI] [PubMed] [Google Scholar]
- 13.Taraz M, Taraz S, Dashti-Khavidaki S. Association between depression and inflammatory/anti-inflammatory cytokines in chronic kidney disease and end-stage renal disease patients: a review of literature. Hemodial Int. 2015;19:11–22. doi: 10.1111/hdi.12200 [DOI] [PubMed] [Google Scholar]
- 14.Cirilloa L, Cutruzzulàa R, Somma C, et al. Depressive symptoms in dialysis: prevalence and relationship with uremia-related biochemical parameters. Blood Purif. 2018;46(4):286–291. doi: 10.1159/000491014 [DOI] [PubMed] [Google Scholar]
- 15.Iida H, Fujimoto S, Wakita T, et al. Despite psychological flexibility and depression in advanced CKD and dialysis. Kidney Med. 2020;2(6):684–691. doi: 10.1016/j.xkme.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serefko A, Szopa A, Poleszak E. Magnesium and depression. Magnes Res. 2016;29(3):112–119. doi: 10.1684/mrh.2016.0407 [DOI] [PubMed] [Google Scholar]
- 17.Barragán-Rodríguez L, Rodríguez-Morán M, Guerrero-Romero F. Efficacy and safety of oral magnesium supplementation in the treatment of depression in the elderly with type 2 diabetes: a randomized, equivalent trial. Magnes Res. 2008;21(4):218–223. [PubMed] [Google Scholar]
- 18.de la Santé OM. World Health Statistics 2020: Monitoring Health for the Sdgs, Sustainable Development Goals. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 19.de Baaij JH, Hoenderop JG, Bindels RJ. Regulation of magnesium balance: lessons learned from human genetic disease. Clin Kidney J. 2012;5(Suppl 1):i15–i24. doi: 10.1093/ndtplus/sfr164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen S, Mao G. Method of magnesium detection and its clinical significance. J Clin Lab Med. 1984;(3):38–39. [Google Scholar]
- 21.Cunningham J, Rodríguez M, Messa P. Magnesium in chronic kidney disease stages 3 and 4 and in dialysis patients. Clin Kidney J. 2012;5(Suppl 1):i39–i51. doi: 10.1093/ndtplus/sfr166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saha H, Harmoinen A, Pietilä K, et al. Measurement of serum ionized versus total levels of magnesium and calcium in hemodialysis patients. Clin Nephrol. 1996;46(5):326–331. [PubMed] [Google Scholar]
- 23.Navarro JF, Mora C, Macia M, et al. Serum magnesium concentration is an independent predictor of parathyroid hormone levels in peritoneal dialysis patients. Perit Dial Int. 1999;19(5):455–461. doi: 10.1177/089686089901900509 [DOI] [PubMed] [Google Scholar]
- 24.Tsai S, Zhao H, Wu B, et al. Serum magnesium abnormality and influencing factors of serum magnesium level in peritoneal dialysis patients: a single-center study in Northern China. Blood Purif. 2018;45(1–3):110–117. doi: 10.1159/000485315 [DOI] [PubMed] [Google Scholar]
- 25.Ye H, Zhang X, Guo Q, et al. Prevalence and factors associated with hypomagnesemia in Southern Chinese continuous ambulatory peritoneal dialysis patients. Perit Dial Int. 2013;33(4):450–454. doi: 10.3747/pdi.2012.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin Y, Lei J, Wang Y, et al. Analysis of blood magnesium level and related nutritional status in hemodialysis patients. China Blood Purification. 2015;14(11):658–661. [Google Scholar]
- 27.Cai K, Luo Q, Dai Z, et al. Hypomagnesemia is associated with increased mortality among peritoneal dialysis patients. PLoS One. 2016;11(3):e0152488. doi: 10.1371/journal.pone.0152488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacka FN, Maes M, Pasco JA, et al. Nutrient intakes and the common mental disorders in women. J Affect Disord. 2012;141(1):79–85. doi: 10.1016/j.jad.2012.02.018 [DOI] [PubMed] [Google Scholar]
- 29.Tarleton EK, Littenberg B. Magnesium intake and depression in adults. J Am Board Fam Med. 2015;28(2):249–256. doi: 10.3122/jabfm.2015.02.140176 [DOI] [PubMed] [Google Scholar]
- 30.Tian N, Chen N, Li PK. Depression in dialysis. Curr Opin Nephrol Hypertens. 2021;30(6):600–612. doi: 10.1097/MNH.0000000000000741 [DOI] [PubMed] [Google Scholar]
- 31.Lin J, Guo Q, Ye X, et al. The effect of social support and coping style on depression in patients with continuous ambulatory peritoneal dialysis in southern China. Int Urol Nephrol. 2013;45(2):527–535. doi: 10.1007/s11255-012-0309-7 [DOI] [PubMed] [Google Scholar]
- 32.Al-Ali F, Elshirbeny M, Hamad A, et al. Prevalence of depression and sleep disorders in patients on dialysis: a cross-sectional study in Qatar. Int J Nephrol. 2021;2021(5533416):1–7. doi: 10.1155/2021/5533416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Traisathit P, Moolkham K, Maneeton N, et al. Associated factors for depressive disorder in patients with end-stage renal disease treated with continuous ambulatory peritoneal dialysis. Ther Clin Risk Manag. 2019;15:541–548. doi: 10.2147/TCRM.S186394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barragán-Rodríguez L, Rodríguez-Morán M, Guerrero-Romero F. Depressive symptoms and hypomagnesemia in older diabetic subjects. Arch Med Res. 2007;38(7):752–756. doi: 10.1016/j.arcmed.2007.03.008 [DOI] [PubMed] [Google Scholar]
- 35.Haj-Mirzaian A, Amiri S, Kordjazy N, et al. Blockade of NMDA receptors reverses the depressant, but not anxiogenic effect of adolescence social isolation in mice. Eur J Pharmacol. 2015;750:160–166. doi: 10.1016/j.ejphar.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 36.Cardoso CC, Lobato KR, Binfaré RW, et al. Evidence for the involvement of the monoaminergic system in the antidepressant-like effect of magnesium. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(2):235–242. doi: 10.1016/j.pnpbp.2008.11.007 [DOI] [PubMed] [Google Scholar]
- 37.Gadia P, Awasthi A, Jain S, et al. Depression and anxiety in patients of chronic kidney disease undergoing haemodialysis: a study from western Rajasthan. J Family Med Prim Care. 2020;9(8):4282–4286. doi: 10.4103/jfmpc.jfmpc_840_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feroze U, Martin D, Kalantar-Zadeh K, et al. Anxiety and depression in maintenance dialysis patients: preliminary data of a cross-sectional study and brief literature review. J Ren Nutr. 2012;22(1):207–210. doi: 10.1053/j.jrn.2011.10.009 [DOI] [PubMed] [Google Scholar]
- 39.Mahajan S, Tiwari SC, Kalra V, et al. Analysis of depression and its effect on outcome among adult Indian peritoneal dialysis patients. Perit Dial Int. 2007;27(1):94–96. doi: 10.1177/089686080702700121 [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Zhang P, Ni X, et al. Vitamin D status in chronic dialysis patients with depression: a prospective study. BMC Psychiatry. 2014;14:125–131. doi: 10.1186/1471-244X-14-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryszewska-Pokraśniewicz B, Mach A, M SM, et al. Effects of magnesium supplementation on unipolar depression: a placebo-controlled study and review of the importance of dosing and magnesium status in the therapeutic response. Nutrients. 2018;10(8):1014–1027. doi: 10.3390/nu10081014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarleton EK, Kennedy AG, Rose GL, et al. The association between serum magnesium levels and depression in an adult primary care population. Nutrients. 2019;11(7):1475–1483. doi: 10.3390/nu11071475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.George MS, Rosenstein D, Rubinow DR, et al. CSF magnesium in affective disorder: lack of correlation with clinical course of treatment. Psychiatry Res. 1994;51(2):139–146. doi: 10.1016/0165-1781(94)90033-7 [DOI] [PubMed] [Google Scholar]
- 44.Camardese G, De Risio L, Pizi G, et al. Plasma magnesium levels and treatment outcome in depressed patients. Nutr Neurosci. 2012;15(2):78–84. doi: 10.1179/1476830512Y.0000000002 [DOI] [PubMed] [Google Scholar]
- 45.Eby GA, Eby KL. Rapid recovery from major depression using magnesium treatment. Med Hypotheses. 2006;67(2):362–370. doi: 10.1016/j.mehy.2006.01.047 [DOI] [PubMed] [Google Scholar]
- 46.Barbagallo M, Dominguez LJ. Magnesium and aging. Curr Pharm Des. 2010;16(7):832–839. doi: 10.2174/138161210790883679 [DOI] [PubMed] [Google Scholar]
- 47.Murck H. Magnesium and affective disorders. Nutr Neurosci. 2002;5(6):375–389. doi: 10.1080/1028415021000039194 [DOI] [PubMed] [Google Scholar]
- 48.Wienecke T, Fuss J, Gertz K, et al. Immunomodulatory effects of magnesium on monocytes: a potential role for magnesium in resolving inflammation. Nutrients. 2021;13(2):350. doi: 10.3390/nu13020350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berk M, Kapczinski F, Andreazza AC, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress, and neurotrophic factors. Psychopathology. 2011;44(2):85–96. [DOI] [PubMed] [Google Scholar]
- 51.Sakaguchi Y, Iwatani H, Hamano T, et al. Magnesium modifies the cardiovascular mortality risk associated with hyperphosphatemia in patients undergoing hemodialysis: a cohort study. PLoS One. 2014;9(12):e116273. doi: 10.1371/journal.pone.0116273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zipursky J, Macdonald EM, Hollands S, et al. Proton pump inhibitors and hospitalization with hypomagnesemia: a population-based case-control study. PLoS Med. 2014;11(9):e1001736. doi: 10.1371/journal.pmed.1001736 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.