Abstract
Objectives
Previous studies have reported that mode of delivery, particularly cesarean delivery (CD), is associated with neurodevelopmental outcomes in children. This study evaluates behavioral and neuropsychological test scores in children based on mode of delivery.
Methods
Children enrolled in the Raine Study from Western Australia, born between 1989 and 1992 by instrumental vaginal delivery (IVD), elective CD, and non-elective CD, were compared to those with spontaneous vaginal delivery (SVD). The primary outcome was the Child Behavior Checklist (CBCL) administered at age 10. Secondary outcomes included evaluations of language, motor function, cognition, and autistic traits. Multivariable linear regression was used to evaluate score differences by mode of delivery adjusted for sociodemographic and clinical characteristics, and Poisson regression was used to evaluate for increased risk of clinical deficit.
Results
Of 2,855 children, 1770 (62.0 %) were delivered via SVD, 480 (16.8 %) via IVD, 346 (12.1 %) via elective CD, and 259 (9.1 %) via non-elective CD. Non-elective CD was associated with higher (worse) CBCL Internalizing (+2.09; 95 % CI 0.49, 3.96; p=0.01) scores, and elective CD was associated with lower (worse) McCarron Assessment of Neuromuscular Development (MAND) (−3.48; 95 % CI −5.61, −1.35; p=0.001) scores. Differences were not seen in other outcomes, and increased risk of clinical deficit was not observed with either the CBCL Internalizing or MAND scores.
Conclusions
Differences in behavior and motor function were observed in children delivered by CD, but given that score differences were not associated with increased incidence of clinical deficit, clinical significance may be limited.
Keywords: cesarean delivery, mode of delivery, behavioral deficit, neurodevelopment
Introduction
The decision to proceed with vaginal vs. operative delivery considers obstetrical and fetal factors, with increasing consideration for maternal preferences. Currently, in developed countries, 5–20 % of births involve instrumental deliveries, and 20–50 % are cesarean deliveries (CD) 1], [2], [3], [4], [5. While instrumental delivery rates have decreased in recent years, CD rates have increased, with global rates doubling to over 20 % from 2000 to 2015 [6], with reported rates of 56 % in Brazil and up to 62 % in parts of China [6]. Regarding reasons for CD, a study of French women found that 42 % of CDs were elective in nature [7]. Based on the high rates of CDs in some countries, concerns have been raised regarding iatrogenic neonatal morbidity associated with operative delivery [8, 9]. While immediate perinatal complications associated with each mode of delivery can be readily observed, long-term implications are more difficult to determine. In this context, long-term neurodevelopmental outcomes have been evaluated with mixed results.
In some studies, no differences in IQ, motor skills, and physical development [10], maternal reports of developmental milestones [11], or increased rates of adverse neurodevelopmental diagnoses at age 4 years [12], were reported based on mode of delivery. However, in others, particularly studies comparing CD to vaginal delivery, lower developmental scores [13], lower performance on standardized testing [14], higher levels of inattention and social problems [15], diagnoses for autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) [16], and lower motor function scores [17], have been reported.
Given the inconsistency in the published studies, the relationship between mode of delivery, particularly CD and neurodevelopmental outcomes remains unclear. Reasons for this include methodological limitations of prior studies such as small sample size, the lack of differentiation between elective and non-elective CD and indication for CD, limited follow-up, and limited adjustment for confounders including sociodemographic and perinatal factors. To address these concerns, we evaluated a large established birth cohort which differentiates between multiple modes of delivery, with the ability to adjust for a wide variety of sociodemographic and clinical covariates, including perinatal factors, and evaluated behavioral problems and other neurodevelopmental domains at ages 10 and 20 years.
Methods
The Raine study
The Raine Study is a multigenerational cohort from Perth, Western Australia, which recruited 2,900 women (Gen1) in their 16th–20th week of pregnancy between the years of 1989 and 1991, resulting in data from 2,868 live births [18]. Mothers were assessed at 18 and 34 weeks of pregnancy as well as at the time of delivery. During the prenatal evaluations, detailed maternal and paternal questionnaires were completed and mothers received clinical assessments. At the time of delivery, clinical data was also collected from mothers. The children (Gen 2) were evaluated at the time of birth and during multiple follow-up visits.
Mode of delivery and covariates
The Raine Study Gen1 and Gen2 antenatal and perinatal data were evaluated, including the mode of delivery, classified as unassisted vaginal delivery, instrumental vaginal delivery, elective CD, and non-elective CD. Unassisted vaginal delivery included spontaneous vertex, spontaneous direct occipital posterior, face, and instrumental vaginal breech. Instrumental vaginal delivery included various modes of operative/instrumental deliveries including breech extraction and vacuum and forceps instrumental deliveries. 80 covariates were selected a priori to control for confounding, including sociodemographic characteristics, maternal pre-existing medical characteristics, maternal psychosocial characteristics, antenatal characteristics, perinatal characteristics, preterm labor or birth, duration of first stage of labor, and fetal characteristics (sex, birthweight, congenital abnormalities). Characteristics occurring after delivery were not considered for covariate adjustment in order to avoid overadjustment bias.
Neurodevelopmental outcomes
A battery of neuropsychological assessments was used to evaluate Gen2 participants. The primary outcome was the Child Behavioral Checklist (CBCL). The CBCL is a parental questionnaire assessing the child’s internalizing problems (e.g., depression and somatic complaints) and externalizing problems (e.g., aggressive behavior and rule breaking), and generates Total scores, and Internalizing and Externalizing subscale subscores [19]. Six additional assessments were evaluated as secondary outcomes. Language was evaluated with two tests. The Clinical Evaluation of Language Fundamentals (CELF) is a language test that assesses higher-order semantic, grammatical, and verbal memory abilities and reports three scores, a Receptive language score measuring listening comprehension, and Expressive language score tracking speaking ability, and a Total score representing total language ability [20]. The Peabody Picture Vocabulary Test (PPVT) is a receptive vocabulary test that also assesses language [21]. Cognition was evaluated using the Raven’s Colored Progressive Matrices (CPM), which measures global cognitive performance, nonverbal intelligence, and visuospatial functions, and the Symbol Digit Modality Test (SDMT), which evaluates visual tracking, attention, and motor skills, and generates oral and written scores [22, 23]. Fine and gross motor function were evaluated with the McCarron Assessment of Neuromuscular Development (MAND) [24]. All the above outcomes including the CBCL were evaluated at the Raine Study Gen2-10 year follow-up. Finally, the Autism Spectrum Quotient (AQ) at the Raine Study Gen2-20 year follow-up was also evaluated, which is a fifty-item self-reported questionnaire soliciting the degree of autistic traits exhibited and has been validated in Australian populations [25].
Statistical analysis
The mean scores for all neurodevelopmental outcomes were initially evaluated in each exposure group. Linear regression was then used to perform unadjusted analyses comparing mean score differences between exposure groups. Given the presence of four separate exposure groups, an F-statistic was first calculated for each outcome, with a p-value threshold of 0.05 used to determine whether a statistically significant difference between any groups was present. If an F-statistic p-value <0.05 was observed, individual comparisons between each exposure group and the reference, assigned as the unassisted vaginal delivery group, were then evaluated. Following unadjusted analyses, missing covariate values were imputed using multiple imputation, generating five imputed datasets. Multivariable linear regression using stepwise covariate selection was then used to evaluate each of the five datasets. Covariates with a p-value <0.05 were selected in addition to child sex, race, family income, prematurity status, and parity of the mother. Adjusted analyses from the imputed datasets were pooled using Rubin’s rules [26]. For any score showing a statistically significant score difference in the adjusted analyses, we additionally determined whether children were more likely to be classified as having a clinical deficit. For CBCL we evaluated whether children in any exposure group were more likely to have a score >60, which is one standard deviation above the population mean score. For AQ, 29 was the pre-established threshold score for deficit. For the remaining scores, the threshold was defined as one standard deviation below the mean in this cohort, which represents children with approximately the worst 16th percentile of scores. In this analysis, a Poisson regression was performed adjusting for covariates using stepwise covariate selection similar to the primary analyses.
Two sub-analyses based on patient characteristics were also performed. The first evaluated only singletons, and the second evaluated only full term births in order to determine whether the results persisted when only looking at these populations.
Three additional sub-analyses of specific modes of delivery were also performed. In the first, in order to also account for duration of labor, only laboring women were included, with women with elective CD excluded. In this sub-analysis, the type of labor onset, duration of first stage of labor, duration of second stage of labor, oxytocin for induction of labor, oxytocin for augmentation of labor, and use of tocolytics were included as potential covariates for stepwise selection. In the second sub-analysis, only women with CD were evaluated with elective CD designated as the reference group. In this sub-analysis, use of general and epidural anesthesia during pregnancy, and epidural duration were included as potential covariates for stepwise selection. In the third sub-analysis, only women with non-elective CD were evaluated to compare the indication for CD being presumed fetal hypoxia or fetal distress compared to other reasons.
Results
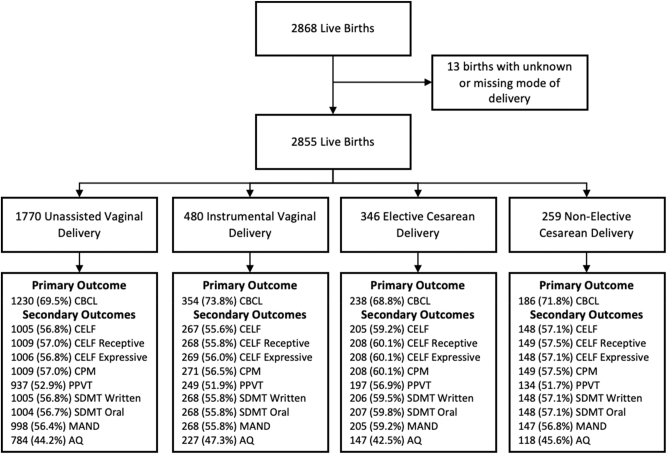
Of the 2,868 children in the Raine Study, 13 were excluded due to lack of data on mode of delivery (Figure 1). Of the remaining 2,855 children, 1,770 (62.0 %) were delivered via normal spontaneous vaginal delivery, 480 (16.8 %) via instrumental vaginal delivery, 346 (12.1 %) via elective CD, and 259 (9.1 %) via non-elective CD. The most common indications for instrumental vaginal delivery were delay in the second stage of labor (41.9 %, n=201) and fetal distress (29.6 %, n=142) (Supplementary Table 1). The most common indications for elective CD were: previous CD/presumed cephalopelvic disproportion or maternal request (25.1 %, n=87) and two previous CD (20.2 %, n=70), and the most common indications for intrapartum or non-elective CD were failure to progress due to reasons other than definitive cephalopelvic disproportion (27.4 %, n=71) and presumed fetal hypoxia (25.9 %, n=67).
Figure 1:
Flowchart of children included for analysis in this study, listing completion rates of primary and secondary outcomes by exposure status. CBCL, child behaviour checklist; CELF, clinical evaluation of language fundamentals; PPVT, peabody picture vocabulary test; MAND, McCarron’s assessment of neuromuscular development; CPM, coloured progressive matrices; SDMT, symbol digit modality test; AQ, autism spectrum quotient.
Characteristics of mothers and children by mode of delivery
Selected patient characteristics by mode of delivery are displayed in Table 1 with a complete list of all characteristics in Supplementary Table 2. Mother’s age at the time of birth for each mode of delivery followed similar distributions with peak ages being in the 25–<30 and 30–<35 age ranges. There were several variables that differed significantly between mode of delivery groups. For example, BMI >25 was found to be more common in elective and non-elective CD children compared to unassisted or instrumental vaginal deliveries. Also, mothers reporting emotional, physical, or other trauma during pregnancy were more common in the elective CD group.
Table 1:
Selected characteristics of mothers and children by mode of delivery.
| Mode of delivery | |||||
|---|---|---|---|---|---|
| Unassisted vaginal delivery | Instrumental vaginal delivery | Elective cesarean delivery |
Non-elective cesarean delivery |
||
| n (%) | n (%) | n (%) | n (%) | ||
| Maternal sociodemographic characteristics | |||||
|
| |||||
| Gravidity | 1 | 511 (28.9) | 234 (48.8) | 7 (20.2) | 112 (43.2) |
| 2 | 506 (28.6) | 136 (28.3) | 95 (27.5) | 71 (27.4) | |
| 3 | 335 (18.9) | 6 (12.5) | 75 (21.7) | 34 (13.1) | |
| 4 | 215 (12.2) | 34 (7.1) | 62 (17.9) | 25 (9.7) | |
| ≥5 | 202 (11.4) | 16 (3.3) | 44 (12.7) | 17 (6.6) | |
| Missing | 1 (0.1) | – | – | – | |
| Parity | 0 | 745 (42.1) | 349 (72.7) | 107 (30.9) | 165 (63.7) |
| 1 | 558 (31.5) | 87 (18.1) | 124 (35.8) | 56 (21.6) | |
| 2 | 3 (16.9) | 34 (7.1) | 7 (20.2) | 21 (8.1) | |
| ≥3 | 166 (9.4) | 1 (2.1) | 45 (13.0) | 17 (6.6) | |
| Missing | 1 (0.1) | – | – | – | |
| BMI pre-pregnancy | BMI <18.5 | 206 (11.6) | 65 (13.5) | 32 (9.2) | 17 (6.6) |
| BMI 18.5 – <25 | 1,235 (69.8) | 343 (71.5) | 215 (62.1) | 171 (66.0) | |
| BMI 25 – <30 | 197 (11.1) | 45 (9.4) | 43 (12.4) | 38 (14.7) | |
| BMI ≥30 | 11 (6.2) | 13 (2.7) | 34 (9.8) | 27 (10.4) | |
| Missing | 22 (1.2) | 14 (2.9) | 22 (6.4) | 6 (2.3) | |
|
| |||||
| Paternal sociodemographic characteristics | |||||
|
| |||||
| Father highest level of education | None | 507 (28.6) | 114 (23.8) | 102 (29.5) | 62 (23.9) |
| Trade certificate or apprenticeship | 419 (23.7) | 119 (24.8) | 68 (19.6) | 72 (27.8) | |
| Professional registration | 55 (3.1) | 14 (2.9) | 13 (3.8) | 9 (3.5) | |
| College diploma or degree | 169 (9.6) | 47 (9.8) | 32 (9.2) | 26 (10.0) | |
| University degree | 213 (12.0) | 101 (21.0) | 56 (16.2) | 32 (12.4) | |
| Other | 67 (3.8) | 8 (1.7) | 15 (4.3) | 7 (2.7) | |
| Missing | 34 (19.2) | 77 (16.0) | 6 (17.3) | 51 (19.7) | |
|
| |||||
| Maternal health characteristics | |||||
|
| |||||
| Treated for hypertension | No | 1,715 (96.9) | 459 (95.6) | 318 (91.9) | 25 (96.5) |
| Yes | 54 (3.0) | 21 (4.4) | 28 (8.1) | 9 (3.5) | |
| Missing | 1 (0.1) | – | – | – | |
|
| |||||
| Maternal emotional and traumatic characteristics | |||||
|
| |||||
| Emotional upsets: problems with your children | No | 1,654 (93.5) | 465 (96.9) | 309 (89.3) | 246 (95.0) |
| Yes | 115 (6.5) | 15 (3.1) | 37 (10.7) | 13 (5.0) | |
| Missing | 1 (0.1) | – | – | – | |
| Trauma: suffered any other physical trauma since becoming pregnant | No | 1,538 (86.9) | 427 (89.0) | 294 (85.0) | 214 (82.6) |
| Yes | 9 (5.1) | 17 (3.5) | 27 (7.8) | 12 (4.6) | |
| Missing | 142 (8.0) | 36 (7.5) | 25 (7.2) | 33 (12.7) | |
|
| |||||
| Maternal antenatal characteristics | |||||
|
| |||||
| Attend antenatal classes | Did not attend | 796 (45.0) | 113 (23.5) | 183 (52.9) | 69 (26.6) |
| Yes already attended | 654 (37.0) | 284 (59.2) | 1 (28.9) | 14 (54.0) | |
| Yes will attend | 76 (4.3) | 31 (6.5) | 1 (2.9) | 14 (5.4) | |
| Haven’t decided | 94 (5.3) | 14 (2.9) | 15 (4.3) | 5 (1.9) | |
| Missing | 15 (8.5) | 38 (7.9) | 38 (11.0) | 31 (12.0) | |
| Medical professional treatment or advice to help become pregnant | No | 1714 (96.8) | 452 (94.2) | 318 (91.9) | 245 (94.6) |
| Yes | 55 (3.1) | 28 (5.8) | 28 (8.1) | 14 (5.4) | |
| Missing | 1 (0.1) | – | – | – | |
| Antenatal admissions | No | 1,368 (77.3) | 345 (71.9) | 175 (50.6) | 16 (61.8) |
| Yes | 402 (22.7) | 135 (28.1) | 171 (49.4) | 99 (38.2) | |
| Antenatal admissions >20 weeks | No | 1,397 (78.9) | 352 (73.3) | 179 (51.7) | 167 (64.5) |
| Yes | 373 (21.1) | 128 (26.7) | 167 (48.3) | 92 (35.5) | |
| Severity of pre-eclampsia and hypertension | None | 1,406 (79.4) | 324 (67.5) | 234 (67.6) | 177 (68.3) |
| BP <140/90 consistently | 166 (9.4) | 6 (12.5) | 34 (9.8) | 38 (14.7) | |
| BP <140/90 mostly but not always | 32 (1.8) | 22 (4.6) | 29 (8.4) | 11 (4.2) | |
| BP >170/110 sometimes or frequently | 103 (5.8) | 49 (10.2) | 34 (9.8) | 21 (8.1) | |
| BP only elevated sporadically | 63 (3.6) | 25 (5.2) | 15 (4.3) | 12 (4.6) | |
| Proteinuric pre-eclampsia | No | 1747 (98.7) | 463 (96.5) | 312 (90.2) | 251 (96.9) |
| Yes | 23 (1.3) | 17 (3.5) | 34 (9.8) | 8 (3.1) | |
|
| |||||
| Maternal perinatal characteristics | |||||
|
| |||||
| Prostaglandins | No | 171 (96.6) | 443 (92.3) | 346 (100.0) | 22 (84.9) |
| Yes | 6 (3.4) | 37 (7.7) | 39 (15.1) | ||
| Pre-term birth (<37 wks) | No | 1,662 (93.9) | 456 (95.0) | 262 (75.7) | 218 (84.2) |
| Yes | 108 (6.1) | 24 (5.0) | 84 (24.3) | 41 (15.8) | |
| Pre-term labour requiring admission | No | 1703 (96.2) | 471 (98.1) | 315 (91.0) | 232 (89.6) |
| Yes | 67 (3.8) | 9 (1.9) | 31 (9.0) | 27 (10.4) | |
| Corticosteroids | No | 1739 (98.2) | 474 (98.8) | 303 (87.6) | 247 (95.4) |
| Yes | 31 (1.8) | 6 (1.2) | 43 (12.4) | 12 (4.6) | |
| Multiple pregnancy | Singleton | 1725 (97.5) | 455 (94.8) | 302 (87.3) | 247 (95.4) |
| Twins/triplets | 45 (2.5) | 25 (5.2) | 44 (12.7) | 12 (4.6) | |
|
| |||||
| Maternal post-exposure mediators | |||||
|
| |||||
| Antibiotics for fever | No | 1725 (97.5) | 44 (91.7) | 346 (100.0) | 225 (86.9) |
| Yes | 45 (2.5) | 4 (8.3) | 34 (13.1) | ||
|
| |||||
| Child neonatal characteristics | |||||
|
| |||||
| Neonate birth weight | < 2,500 g | 105 (5.9) | 22 (4.6) | 85 (24.6) | 4 (15.4) |
| ≥ 2,500 g – <4,000 g | 1,505 (85.0) | 419 (87.3) | 24 (69.4) | 184 (71.0) | |
| ≥4,000 g | 16 (9.0) | 39 (8.1) | 21 (6.1) | 35 (13.5) | |
|
| |||||
| Neonatal post-exposure mediators: fetal heart rate abnormalities | |||||
|
| |||||
| Fetal heart rate – severe abnormalities | No | 1,518 (85.8) | 278 (57.9) | 344 (99.4) | 147 (56.8) |
| Yes | 252 (14.2) | 202 (42.1) | 2 (0.6) | 112 (43.2) | |
| Fetal heart rate – mild or moderate abnormalities | No | 152 (85.9) | 333 (69.4) | 345 (99.7) | 172 (66.4) |
| Yes | 25 (14.1) | 147 (30.6) | 1 (0.3) | 87 (33.6) | |
Neuropsychological test scores by mode of delivery
CBCL scores were available in 1,230 (69.5 %) of children born via unassisted vaginal delivery, 354 (73.8 %) via instrumental vaginal delivery, 238 (68.8 %) via elective CD, and 186 (71.8 %) via non-elective CD. Supplementary Table 3 compares characteristics of mothers and children who did and did not complete the CBCL at 10 years of age. Children without CBCL scores were similar with regard to mode of delivery, but were more likely to come from mothers who were younger with unplanned pregnancies, and families with lower income and parental education status. See Figure 1 for availability of secondary outcomes in each group. Mean scores by mode of delivery for all outcomes is reported in Supplementary Table 4.
Unadjusted analyses reported that non-elective CD was associated with higher, indicating worse CBCL total (+2.22; 95 % confidence interval [CI] 0.46, 3.98; p=0.01) and CBCL internalizing (+2.46; 95 % CI 0.83, 4.08; p=0.003) scores relative to unassisted vaginal deliveries (Table 2). In the unadjusted analyses of the secondary outcomes, relative to unassisted vaginal deliveries, instrumental vaginal delivery was found to be associated with higher, indicating better CELF receptive (+2.28; 95 % CI 0.10, 4.47; p=0.04) and expressive (+2.88; 95 % CI 0.79, 4.97; p=0.01) as well as PPVT (+2.40; 95 % CI 0.70, 4.10; p=0.01) language scores. Elective CD however was associated with lower, indicating worse scores on the MAND (−3.74; 95 % CI −5.84, −1.64; p=0.0005).
Table 2:
Unadjusted score differences by mode of delivery.
|
Outcomes |
F-statistic p-Value |
Unassisted vaginal delivery (n=1,770) [reference] |
Instrumental vaginal delivery (n=480) |
Elective caesarean delivery (n=346) |
Non-elective caesarean delivery (n=259) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | n | Estimate (95 % CI) | p-Value | n | Estimate (95 % CI) | p-Value | n | Estimate (95 % CI) | p-Value | ||
| Primary outcome | |||||||||||
|
| |||||||||||
| CBCL total | 0.04 | 1,230 | 354 | −0.67 (−2.02, 0.68) | 0.33 | 238 | 0.73 (−0.86, 2.31) | 0.37 | 186 | 2.22 (0.46, 3.98) | 0.01 |
| CBCL externalizing | 0.36 | 1,230 | 354 | – | – | 238 | – | – | 186 | – | – |
| CBCL internalizing | 0.004 | 1,230 | 354 | −0.81 (−2.06, 0.43) | 0.2 | 238 | 0.85 (−0.61, 2.32) | 0.25 | 186 | 2.46 (0.83, 4.08) | 0.003 |
|
| |||||||||||
| Secondary outcomes | |||||||||||
|
| |||||||||||
| CELF total | 0.06 | 1,005 | 267 | – | – | 205 | – | – | 148 | – | – |
| CELF receptive | 0.04 | 1,009 | 268 | 2.28 (0.10, 4.47) | 0.04 | 208 | −0.75 (−3.17, 1.67) | 0.55 | 149 | 2.62 (−0.16, 5.41) | 0.06 |
| CELF expressive | 0.04 | 1,006 | 269 | 2.88 (0.79, 4.97) | 0.01 | 208 | −0.61 (−2.93, 1.72) | 0.61 | 148 | 1.42 (−1.26, 4.11) | 0.3 |
| CPM | 0.06 | 1,009 | 271 | – | – | 208 | – | – | 149 | – | – |
| PPVT | 0.02 | 937 | 249 | 2.40 (0.70, 4.10) | 0.01 | 197 | −0.20 (−2.07, 1.67) | 0.83 | 134 | 1.61 (−0.59, 3.82) | 0.15 |
| SDMT written | 0.58 | 1,005 | 268 | – | – | 206 | – | – | 148 | – | – |
| SDMT oral | 0.52 | 1,004 | 268 | – | – | 207 | – | – | 148 | – | – |
| MAND | 0.003 | 998 | 268 | −0.11 (−2.00, 1.77) | 0.91 | 205 | −3.74 (−5.84, −1.64) | 0.0005 | 147 | −2.10 (−4.52, 0.31) | 0.09 |
| Autism quotient | 0.84 | 784 | 227 | – | – | 147 | – | – | 118 | – | – |
CI, confidence interval; CBCL, child behavior checklist; CELF, clinical evaluation of language fundamentals; CPM, coloured progressive matrices; PPVT, peabody picture vocabulary test; SDMT, symbol digit modality test; MAND, McCarron’s assessment of neuromuscular development; AQ, autism spectrum quotient. AQ is completed at age 19–20. All other assessments are completed at age 10. Higher scores on CBCL and AQ indicate worse scores; lower scores on CELF, PPVT, MAND, CPM, and SDMT indicate poorer performance.
Neuropsychological test scores after adjustment
Following imputation of missing covariate values, stepwise regression was performed to select covariates for each outcome for each imputed dataset. A total of 23 variables were selected in the various models and are listed in Supplementary Table 5. After covariate adjustment, non-elective CD was associated with higher, indicating worse CBCL Internalizing (+2.09; 95 % CI 0.49, 3.96; p=0.01) scores (Table 3). For secondary outcomes, elective CD was associated with lower, indicating worse scores for MAND (−3.48; 95 % CI −5.61, −1.35; p=0.001). No differences were found in any other outcomes. In the subanalysis evaluating singleton births (Supplementary Table 6), as well the subanalysis evaluating full term births (Supplementary Table 7), differences in the CBCL Internalizing and MAND scores were similar the those reported in the primary analysis. In the subanalysis only including laboring women, non-elective CD was associated with higher scores for CBCL Internalizing (2.06; 95 % CI 0.47, 3.65; p=0.01) (Supplementary Table 8). In the subanalysis including only women who underwent a CD, no significant differences were found between elective and non-elective CD (Supplementary Table 9). In the subanalysis including only women who underwent a non-elective CD, worse scores were observed where the indication for CD was presumed fetal hypoxia and fetal distress compared to any other indications. These scores included higher CBCL Externalizing (4.26; 95 % CI 0.57, 7.94; p=0.01, lower CELF Total (−8.02; 95 % CI −14.0, −1.99; p=0.01) CPM (−1.51; 95 % CI −2.85, −0.17; p=0.03), SDMT Written (−3.72; 95 % CI −6.49, −0.95; p=0.01) and SDMT Oral (−4.80; 95 % CI −8.69, −0.90; p=0.02) scores (Supplementary Table 10).
Table 3:
Adjusted score differences by mode of delivery.
|
Outcomes |
F-statistic p-Value |
Unassisted vaginal delivery (n=1,770) [reference] |
Instrumental vaginal delivery (n=480) | Elective caesarean delivery (n=346) |
Non-elective caesarean delivery (n=259) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | n | Estimate (95 % CI) | p-Value | n | Estimate (95 % CI) | p-Value | n | Estimate (95 % CI) | p-Value | ||
| Primary outcome | |||||||||||
|
| |||||||||||
| CBCL total | 0.18 | 1,230 | 354 | – | – | 238 | – | – | 186 | – | – |
| CBCL externalizing | 0.78 | 1,230 | 354 | – | – | 238 | – | – | 186 | – | – |
| CBCL internalizing | 0.003 | 1,230 | 354 | −1.00 (−2.25, 0.25) | 0.12 | 238 | 1.32 (−0.15, 2.78) | 0.08 | 186 | 2.09 (0.49, 3.69) | 0.01 |
|
| |||||||||||
| Secondary outcomes | |||||||||||
|
| |||||||||||
| CELF total | 0.5 | 1,005 | 267 | – | – | 205 | – | – | 148 | – | – |
| CELF receptive | 0.46 | 1,009 | 268 | – | – | 208 | – | – | 149 | – | – |
| CELF expressive | 0.54 | 1,006 | 269 | – | – | 208 | – | – | 148 | – | – |
| CPM | 0.58 | 1,009 | 271 | – | – | 208 | – | – | 149 | – | – |
| PPVT | 0.64 | 937 | 249 | – | – | 197 | – | – | 134 | – | – |
| SDMT written | 0.96 | 1,005 | 268 | – | – | 206 | – | – | 148 | – | – |
| SDMT oral | 0.84 | 1,004 | 268 | – | – | 207 | – | – | 148 | – | – |
| MAND | 0.005 | 998 | 268 | −0.38 (−2.30, 1.54) | 0.7 | 205 | −3.48 (−5.61, −1.35) | 0.001 | 147 | −2.41 (−4.82, 0.01) | 0.05 |
| Autism quotient | 0.48 | 784 | 227 | – | – | 147 | – | – | 118 | – | – |
CI, confidence interval; CBCL, child behavior checklist; CELF, clinical evaluation of language fundamentals; CPM, coloured progressive matrices; PPVT, peabody picture vocabulary test; SDMT, symbol digit modality test; MAND, McCarron’s assessment of neuromuscular development; AQ, autism spectrum quotient. AQ is completed at age 19–20. All other assessments are completed at age 10. Higher scores on CBCL and AQ indicate worse scores; lower scores on CELF, PPVT, MAND, CPM, and SDMT indicate poorer performance. Covariates were selected using stepwise regression with specific covariates selected for each outcome listed in Supplementary Table 3.
In scores showing a statistically significant difference based on mode of delivery in our primary analysis following adjustment for covariates, we evaluated whether these differences were associated with an increased risk of clinical deficit. For CBCL internalizing in the non-elective CD group, the adjusted risk ratio (aRR) of clinical deficit was 1.32 (95 % CI 0.91, 1.90; p=0.14). For MAND in the elective CD group, the aRR was 1.27 (95 % CI 0.89, 1.82; p=0.18).
Discussion
Our results report that relative to spontaneous vaginal deliveries, non-elective CD was associated with higher, indicating worse internalizing behavioral scores. For secondary outcomes, children with elective CD had lower MAND, indicating worse motor function scores. The differences in CBCL internalizing scores persisted in our first sub-analysis which only included laboring women and adjusted for additional perinatal covariates. No other score differences were observed, and a statistically significant increased risk of clinical deficit for either the CBCL internalizing or the MAND score in children with CD were also not observed. Worse scores in several neurodevelopmental domains however were observed in children of mothers with non-elective CD for presumed fetal hypoxia or fetal distress.
Prior studies have shown mixed results, but in the studies that have reported differences based on mode of delivery, children with CD have exhibited higher levels of inattention and social problems as measured by the CBCL at ages 7–15 years [15], and a meta-analysis of 61 studies increased odds for ASD and ADHD [16]. Regarding motor function, delays in motor function have been reported in infant assessments, but resolved by early childhood [9, 17].
While there is some consistency with previously published studies, our study has a number of limitations. First despite our ability to account for a wide range of potential confounders, unmeasured confounding may still be present. Indication bias is of particular concern, or the idea that characteristics may predispose mothers to have a certain mode of delivery and also influence their children to have a specific neurodevelopmental outcome. Second, while we were able to assess long-term outcomes at 10 years after birth, there was some level of attrition, which may result in selection bias. Third, the CBCL assessment was completed by the children’s parents, and factors that influence mode of delivery could also influence parental survey responses. Fourth, the mothers with elective CD were composed of mothers with a variety of indications from clinical reasons such as pre-eclampsia, to reasons based on maternal request. Finally, these deliveries occurred decades ago and clinical management of labor and delivery may have changed during that time. Future studies of more recent cohorts may provide information about outcomes following contemporary clinical management.
In summary, after accounting for a range of covariates, children delivered via non-elective CD had higher, indicating worse internalizing behavioral scores compared to those with spontaneous vaginal delivery, and those with elective CD had lower, indicating worse motor function scores at 10 years of age. While there is consistency between these results and published studies, these differences may still be due to confounding factors. In addition, the score differences were not associated with more children being classified in the deficit range. This is reassuring as this could indicate that any differences may be too small to be recognized clinically, suggesting that the differences may have limited clinical significance.
Supplementary Material
Supplementary Material
Acknowledgments
We would like to acknowledge the Raine Study participants and their families for their ongoing participation in the study and the Raine Study team for study co-ordination and data collection. We also thank the NHMRC and the Raine Medical Research Foundation for their long term contribution to funding the study over the last 30 years. The core management of the Raine Study is funded by The University of Western Australia, Curtin University, Telethon Kids Institute, Women and Infants Research Foundation, Edith Cowan University, Murdoch University and The University of Notre Dame Australia. The Raine Study Gen1-and Gen2-antenatal and perinatal data collections are funded by the Raine Medical Research Foundation. The Raine Study Gen2-10-year follow-up and the autism quotient data of the Gen2-20-year follow-up are funded by the NHMRC and the Raine Medical Research Foundation.
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/jpm-2024-0188).
Footnotes
Research ethics: This study was approved by the Institutional Review Board at Columbia University Irving Medical Center, with the requirement of written informed consent waived. Data collection and storage were approved by Ethics Committees at King Edward Memorial Hospital, Princess Margaret Hospital, and the University of Western Australia.
Informed consent: Not applicable.
Author contributions: Study design and conceptualization: AM, RT, RL, BVUS, AW, GL, CP, CI. Statistical analysis: ZY, OI, CM, GL, CI. Interpretation of data: all authors. Drafting of manuscript: AM, CI. Critical revision of manuscript: all authors. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Use of Large Language Models, AI and Machine Learning Tools: None declared.
Conflict of interest: The authors state no conflicts of interest.
Research funding: The Raine Study is funded by project and program grants from the National Health and Medical Research Council of Australia (NHMRC, Canberra, Australia). Core management funding is provided by the Raine Medical Research Foundation, the Telethon Kids Institute, the University of Western Australia (UWA), the Women and Infants Research Foundation, Curtin University, Murdoch University, Edith Cowan University, and the University of Notre Dame Australia. Dr. Caleb Ing is supported by the Agency for Healthcare Research and Quality (AHRQ) under award number R01HS026493. The content is solely the responsibility of the authors and does not necessarily represent the official views of the AHRQ. Professor Andrew Whitehouse is supported by an Investigator Grant from the National Health and Medical Research Council (APP1173896). Professor Britta S. von Ungern-Sternberg is supported by the Stan Perron Charitable Foundation and through a National Health and Medical Research Council Investigator Grant (2009322).
Data availability: The data that support the findings of this study are available from The Raine Study. Restrictions apply to the availability of these data, which were used under license for this study. Data are available with the permission of The Raine Study from https://rainestudy.org.au/information-for-researchers/available-data.
References
- 1.Aiken CE, Aiken AR, Brockelsby JC, Scott JG. Factors influencing the likelihood of instrumental delivery success. Obstet Gynecol. 2014;123:796–803. doi: 10.1097/aog.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osterman MJK, Hamilton BE, Martin JA, Driscoll AK, Valenzuela CP. Births: final data for 2021. Natl Vital Stat Rep. 2023;72:1–53. [PubMed] [Google Scholar]
- 3.Yerrabelli RS, Peterman N, Kaptur B, Yeo E, Carpenter K. Geospatial distribution of relative cesarean section rates within the USA. BMC Res Notes. 2022;15:247. doi: 10.1186/s13104-022-06141-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowley CM, Lang NA, O’Leary BD, Geary MP. Trends in instrument preference for operative vaginal delivery in a tertiary referral center: 2008-2021. Int J Gynaecol Obstet. 2023;162:752–8. doi: 10.1002/ijgo.14736. [DOI] [PubMed] [Google Scholar]
- 5.Betran AP, Ye J, Moller AB, Souza JP, Zhang J. Trends and projections of caesarean section rates: global and regional estimates. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2021-005671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boerma T, Ronsmans C, Melesse DY, Barros AJD, Barros FC, Juan L, et al. Global epidemiology of use of and disparities in caesarean sections. Lancet. 2018;392:1341–8. doi: 10.1016/s0140-6736(18)31928-7. [DOI] [PubMed] [Google Scholar]
- 7.Zbiri S, Rozenberg P, Goffinet F, Milcent C. Cesarean delivery rate and staffing levels of the maternity unit. PLoS One. 2018;13:e0207379. doi: 10.1371/journal.pone.0207379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takács L, Putnam SP, Monk C, Dahlen HG, Thornton C, Bartoš F, et al. Associations between mode of birth and neuropsychological development in children aged 4 Years: results from a birth cohort study. Child Psychiatr Hum Dev. 2021;52:1094–105. doi: 10.1007/s10578-020-01084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al Khalaf SY, O’Neill SM, O’Keeffe LM, Henriksen TB, Kenny LC, Cryan JF, et al. The impact of obstetric mode of delivery on childhood behavior. Soc Psychiatr Psychiatr Epidemiol. 2015;50:1557–67. doi: 10.1007/s00127-015-1055-9. [DOI] [PubMed] [Google Scholar]
- 10.McBride WG, Black BP, Brown CJ, Dolby RM, Murray AD, Thomas DB. Method of delivery and developmental outcome at five years of age. Med J Aust. 1979;1:301–4. doi: 10.5694/j.1326-5377.1979.tb112116.x. [DOI] [PubMed] [Google Scholar]
- 11.Bahl R, Patel RR, Swingler R, Ellis M, Murphy DJ. Neurodevelopmental outcome at 5 years after operative delivery in the second stage of labor: a cohort study. Am J Obstet Gynecol. 2007;197:147.e1–6. doi: 10.1016/j.ajog.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 12.Macharey G, Väisänen-Tommiska M, Gissler M, Ulander VM, Rahkonen L, Nuutila M, et al. Neurodevelopmental outcome at the age of 4 years according to the planned mode of delivery in term breech presentation: a nationwide, population-based record linkage study. J Perinat Med. 2018;46:323–31. doi: 10.1515/jpm-2017-0127. [DOI] [PubMed] [Google Scholar]
- 13.Zaigham M, Hellström-Westas L, Domellöf M, Andersson O. Prelabour caesarean section and neurodevelopmental outcome at 4 and 12 months of age: an observational study. BMC Pregnancy Childbirth. 2020;20:564. doi: 10.1186/s12884-020-03253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polidano C, Zhu A, Bornstein JC. The relation between cesarean birth and child cognitive development. Sci Rep. 2017;7:11483. doi: 10.1038/s41598-017-10831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi XY, Wang J, Zhang WN, Zhao M, Ju J, Li XY, et al. Cesarean section due to social factors affects children’s psychology and behavior: a retrospective cohort study. Front Pediatr. 2020;8:586957. doi: 10.3389/fped.2020.586957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang T, Sidorchuk A, Sevilla-Cermeño L, Vilaplana-Pérez A, Chang Z, Larsson H, et al. Association of cesarean delivery with risk of neurodevelopmental and psychiatric disorders in the offspring: a systematic Review and meta-analysis. JAMA Netw Open. 2019;2:e1910236. doi: 10.1001/jamanetworkopen.2019.10236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun YF, Huang K, Hu YB, Gao H, Niu Y, Tao XY, et al. [Association between elective cesarean section and infants’ developmental behaviors: a cohort study] Zhonghua Yu Fang Yi Xue Za Zhi. 2017;51:1069–73. doi: 10.3760/cma.j.issn.0253-9624.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Straker L, Mountain J, Jacques A, White S, Smith A, Landau L, et al. Cohort profile: the western Australian pregnancy cohort (raine) study-generation 2. Int J Epidemiol. 2017;46:1384–5j. doi: 10.1093/ije/dyw308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Achenbach TM, Edelbrock CS. Manual for the child behavior checklist/4-19 and 1991 profile. Burlington, VT: University of Vermont Department of Psychiatry; 1991. p. 1991. [Google Scholar]
- 20.Semel E, Wiig E, Secord W. Clinical evaluation of language fundamentals. 3rd ed. San Antonio, TX: Psychological Corporation Harcourt Brace Co; 1995. [Google Scholar]
- 21.Dunn LM, Dunn LM. Peabody picture vocabulary test III. Circle Pines, MN: American Guidance Services Inc; 1997. [Google Scholar]
- 22.Raven JC, Raven JE, Court JH, Oxford Psychologists P. Manual for Raven’s progressive matrices and vocabulary scales. Oxford: Oxford Psychologists Press Oxford; 1972. [Google Scholar]
- 23.Smith A. Symbol digit modalities test. Los Angeles: Western psychological services; 1973. [Google Scholar]
- 24.McCarron LT. Mand: McCarron assessment of neuromuscular development, fine and gross motor abilities. Rev ed. Dallas, Tex.: McCarron-Dial Systems, Inc.; 1997. [Google Scholar]
- 25.Broadbent J, Galic I, Stokes MA. Validation of autism spectrum quotient adult version in an Australian sample. Autism Res Treat. 2013;2013:984205. doi: 10.1155/2013/984205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall A, Altman DG, Holder RL, Royston P. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol. 2009;9:57. doi: 10.1186/1471-2288-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material