Abstract
Abstract
Objective
The objective of this study is to identify the top 10 research priorities on reducing and stopping psychiatric medication that reflect the perspectives and unmet needs of three key stakeholder groups (people with lived experience, family members/carers/supporters and healthcare professionals).
Methods
A priority-setting partnership was conducted using the James Lind Alliance’s seven-step process. This involved (1) creating an international Steering Group of key stakeholder representatives and (2) identifying potential partners; (3) gathering stakeholders’ uncertainties about reducing and stopping psychiatric medication using an online survey and summarising the survey responses; (4) checking the summary questions against existing evidence and verifying uncertainties; (5) shortlisting the questions using a second online survey; (6) determining the top 10 research questions through a prioritisation workshop; and (7) disseminating the results.
Results
A total of 3635 questions were collected in the initial survey from 884 respondents of which 32 questions were verified as uncertainties. These questions were then ranked in a second online survey by 526 respondents and the findings discussed in a final prioritisation workshop by 30 participants to produce the final top 10 list of research questions. These questions cover a range of areas including the most effective ways of safely reducing/stopping psychiatric medication and providing support to individuals undergoing the discontinuation process, as well as the best ways to educate healthcare professionals on this topic.
Conclusion
The top 10 list of research priorities was produced through extensive engagement with key stakeholders and highlights important uncertainties and gaps in the existing evidence base that need to be addressed by future research.
Keywords: PSYCHIATRY, Depression & mood disorders, Schizophrenia & psychotic disorders, Anxiety disorders
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study followed a robust and established methodology that is regarded as the gold standard in research priority-setting exercises.
This study has produced a top 10 list of research priorities on reducing and stopping psychiatric medication through extensive engagement with key stakeholders and addresses an important deficit in current practices of stakeholder engagement as involvement of people with lived experience of mental health challenges in research is not widespread, particularly in the early stages of research priority and agenda setting.
The list highlights important uncertainties and gaps in the existing evidence base on this topic that need to be addressed by future research.
The study was conducted entirely through English and the two surveys were only available in digital format which may have limited accessibility for certain groups and countries.
Introduction
The global consumption of psychiatric medication is increasing by 4% annually, with the greatest increase observed in antidepressant use.1 In England, antidepressant prescriptions almost doubled over the last decade, from 47.3 million in 2011 to 85.6 million in 2022–2023.2 Similar trends have been observed in prescribing rates of antipsychotics, gabapentinoids and mood stabilisers, while prescribing rates for benzodiazepine receptor agonists have been more variable across countries.3,5 Longer prescription duration is one of the key driving factors for increased prescribing rates of psychiatric medication.3
These rising prescription trends have called into question the appropriateness of some long-term prescribing of psychiatric medication.2 6 There is a substantial cohort of individuals looking to reduce and/or discontinue long-term use of psychiatric medication.7 8 Reasons for wanting to discontinue psychiatric medication include adverse effects, lack of perceived benefit, the desire to recapture personal autonomy and to live a life free of medication.7,9 However, psychological and physical withdrawal symptoms from some of the above-mentioned drugs are a key barrier to successful discontinuation.10 11 These symptoms can sometimes be mistaken for a return of an underlying condition or onset of a new health condition (ie, ‘relapse’).11 12
Despite the considerable cohort of individuals looking to discontinue psychiatric medication, there is a lack of high-quality evidence underpinning the process of reducing and stopping these medications.13 While gradual dose reduction, that is, tapering is the recommended approach for discontinuing psychiatric medication, there are many uncertainties about the tapering process, which poses challenges both for people who are prescribed these medications or involved in their supply or administration.13 There is a need for further research to establish an evidence base to support individuals and healthcare professionals in reducing and/or stopping psychiatric medication.
Traditionally, mental health research was solely conducted by the ‘experts’, that is, established researchers and healthcare professionals. This is now considered by many as a form of epistemic injustice, in which the perspectives of an individual diagnosed with a mental illness are wronged in their capacity as knower, by having their knowledge and expertise devalued in the knowledge production process.14,16 More recently, there has been a shift in mental health towards a more participatory and collaborative model. This revised model advocates for a framework where experiential knowledge and the voice of the service user are central to treatment, research and policy-making processes.15 16 In late 2023, a guidance document was jointly published by the WHO and United Nations which foregrounded a collaborative vision whereby individuals with mental health challenges can fully engage in their own recovery and participate in all areas of mental health, including mental health research.17 The James Lind Alliance (JLA) developed a priority-setting partnership (PSP) process which has been in use for over 15 years and aligns with this new more modern, rights-based approach to mental health research.18 The PSP process aims to identify and prioritise unanswered questions that is, ‘evidence uncertainties’, in specific conditions or areas of healthcare through working partnerships between key stakeholder groups.
This study aimed to identify the top 10 research priorities on reducing and stopping psychiatric medication that reflect the perspectives and unmet needs of three key stakeholder groups (people with lived experience of taking and/or stopping psychiatric medication, family members/carers/supporters and healthcare professionals) using a JLA PSP.
Methods
This PSP followed the JLA’s seven-step process (figure 1) and the published study protocol.18 19 The REporting guideline for PRIority SEtting of health research was used to guide the reporting of this study.20
Figure 1. Overview of the James Lind Alliance priority-setting partnership process.
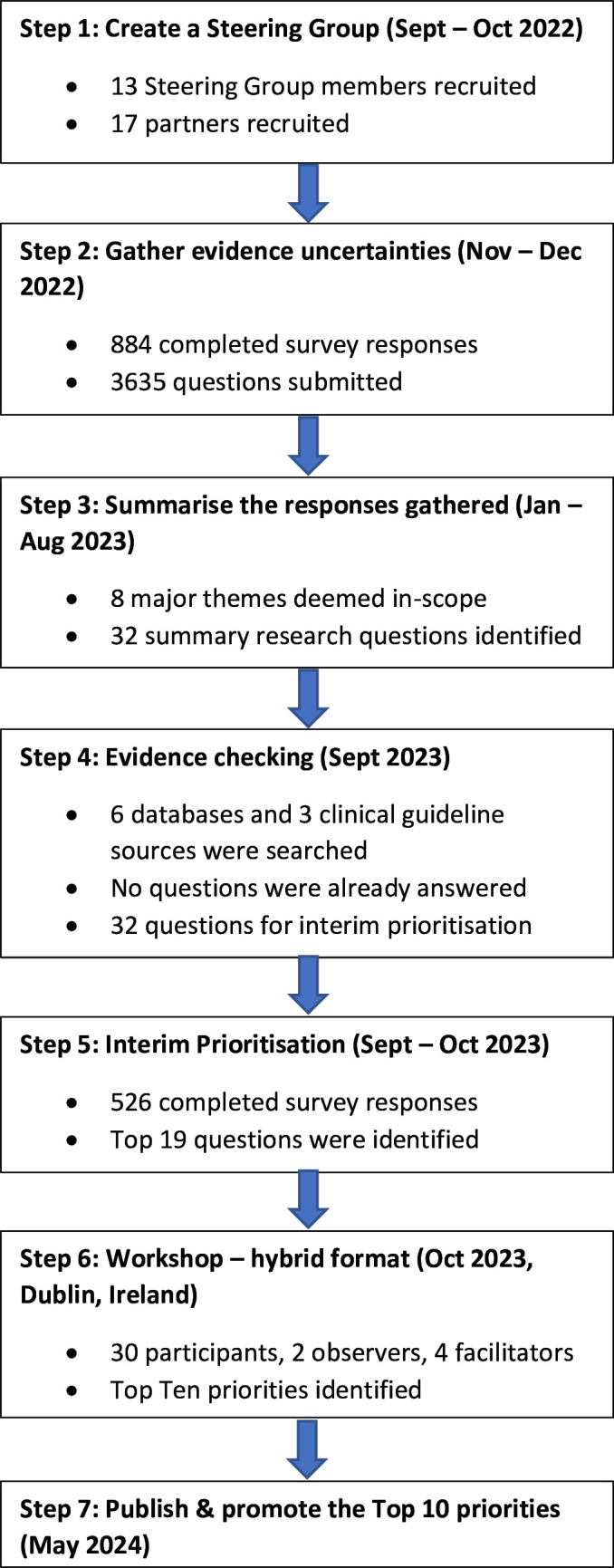
Step 1: establishing the steering group
A Steering Group was established to oversee and guide the PSP process, to discuss and agree the PSP’s strategic orientation and scope, and to disseminate and publicise the online surveys.18 The final Steering Group comprised 13 members from four countries (Ireland, Sweden, UK, USA) representing different stakeholder groups: people with lived experience of taking and/or stopping psychiatric medication (n=4), family members and/or carers/supporters (n=2) and healthcare professionals (n=7). Several Steering Group members had dual roles (eg, healthcare professional with lived experience of psychiatric medication use/discontinuation). Steering Group meetings were conducted online to facilitate international representation and scheduled at regular intervals between September 2022 and December 2023 to maintain transparency and promote engagement. These meetings were chaired by a JLA Advisor (TG), and it was agreed at the outset that a minimum of three members each representing those with lived experience and those with clinical experience would need to be present to have a quorum. Steering Group members contributed their time on a voluntary basis.
The PSP’s scope focused on reducing/stopping five classes of psychiatric medication: antidepressants, antipsychotics, benzodiazepine receptor agonists, gabapentinoids and mood stabilisers. As tapering is relevant irrespective of the clinical indication for which the medication is prescribed, the use of psychiatric medication for both mental and physical health conditions was considered within the PSP’s scope.
Step 2: gathering evidence uncertainties (round 1 survey)
An anonymous online survey was conducted between 4 November and 31 December 2022 to capture research questions/uncertainties about reducing and stopping psychiatric medication from the three key stakeholder groups (described above). The survey was created in Qualtrics, reviewed by the Steering Group and piloted by representatives from each of the three key stakeholder groups. Pilot responses were not included in the final analysis.
The main section of the survey asked respondents to share their questions/uncertainties about reducing and stopping psychiatric medication as free-text responses with no word count limit. The survey also asked respondents to provide some brief demographic information in terms of stakeholder group, age, gender and country of origin. In cases where respondents could identify with more than one stakeholder group, they were asked to select the group that best reflected their questions/uncertainties about reducing and stopping psychiatric medication. For example, if a healthcare professional also had lived experience of discontinuing a psychiatric medication, and their questions stemmed from their lived experience as opposed to their clinical/professional experience, then they were advised to select ‘people with lived experience of taking and/or stopping psychiatric medication’ as their respondent group. The survey also included an optional free-text comments section for respondents to add any comments about reducing and/or stopping psychiatric medication. The survey link which was used in all dissemination activities directed individuals to the project website (www.tapersafer.org) where they could also access an information leaflet. A narrated video was also available on the website to overcome any literacy issues and improve equity of access. Before commencing the survey, respondents were asked to provide consent by confirming that they were ≥18 years old, represented one of the key stakeholder groups described above and agreed to complete the survey voluntarily. There were no geographical restrictions on participation.
The survey was promoted using a multistrand approach which included social media, newsletters and emails. Steering Group members and organisations that agreed to formally partner with, and promote, the PSP were asked to complete the survey and to share it with their networks to maximise the response rate. There are no formal target sample sizes for PSP surveys.18 However, balanced representation of all stakeholder groups and diversity of respondents is desirable. Respondents’ demographic profile was monitored on a weekly basis, primarily in terms of stakeholder group. Various strategies were implemented to enhance engagement from specific stakeholder groups, including targeted posts on Twitter and requesting assistance from specific organisations and groups in disseminating study information within their networks.
Step 3: summarising the responses gathered
This step involved reviewing round 1 responses, removing out-of-scope questions (ie, questions not directly related to tapering psychiatric medication), and creating a list of unique, researchable questions. Survey responses were exported into Microsoft Excel and analysed using template analysis to enable the creation of a list of codes (‘template’) representing the themes identified in the data, organised in a hierarchical structure.21 Each respondent was assigned a unique response identifier (PSP001, PSP002, etc).
Preliminary coding was undertaken on a subset of 50 responses by the leading researchers (MB, CC, AH) to develop a provisional coding template. Once lower-order codes (level 2) were identified and defined, groups of similar codes were clustered and given a higher-order code title (level 1). Level 1 codes represented major themes. The provisional coding template was piloted on an additional 200 responses and modified on an iterative basis with input from the Steering Group. This group had oversight of the entire coding process and was sent the full dataset of coded responses to review to enhance rigour, credibility and transparency. Once the final coding template was agreed, it was applied to the full dataset by the lead researcher (MB). General queries that were not directly related to research or answerable through research (eg, how to take legal action against a prescriber) were coded under an out-of-scope code and excluded from the final analysis.
Most survey responses contained multiple questions/uncertainties and were not always written in the format of a research question. Once level 1 and level 2 coding were complete, responses were split into individual questions/uncertainties and assigned a unique number which correlated to their response identifier. For example, if PSP001 submitted three questions, questions were split and named 1.1, 1.2 and 1.3 accordingly. Individual questions/uncertainties were grouped by major themes. In line with previous research, level 3 codes were created to facilitate the grouping of questions.21
Once levels 1–3 coding was completed, the coded data were converted into clear, indicative questions that were addressable by research using the PICO format (Patient, population, or problem; Intervention; Comparison or control; Outcome or objective) while retaining the sentiment of the original submission.22 Similar questions were merged to minimise duplication and reduce the volume of data. Once formulated, the questions were reviewed by the Steering Group and refined to improve clarity.
Step 4: evidence checking
To verify that each indicative question had not previously been answered, relevant electronic databases (Medline, Embase, CINAHL, PsycINFO, Web of Science, Cochrane Database of Systematic Reviews) and organisational websites (National Institute for Health and Care Excellence (NICE), Scottish Intercollegiate Guidelines Network guidelines, Royal College of Psychiatrists) were searched for high-quality evidence (ie, systematic reviews, evidence-based guidelines) published within the last 3 years18 using relevant keywords and search terms with input from an information retrieval specialist (GS).
All titles and abstracts were independently screened for inclusion by two researchers (MB and CC) in Covidence against inclusion criteria: systematic review or evidence-based guideline published within the last 3 years. Data were extracted by the lead researcher relating to the main aim/purpose of the reference source, as well as key results and author conclusions. This information was used to determine whether any of the summary questions had already been answered for any of the individual classes of psychiatric medication. Unless a reference source that addressed a summary question covered all classes of psychiatric medication, the question was considered only partially answered by existing evidence and retained as an existing uncertainty. To maintain transparency within the PSP process, a summary of the evidence-checking process findings linked to the list of unanswered summary questions was shared with the Steering Group for review before inclusion in the round 2 survey (step 5).
Step 5: interim prioritisation (round 2 survey)
A second anonymous online survey was conducted between 14 September and 16 October 2023 to identify which of the unanswered summary questions were considered most important by the key stakeholder groups. Similar to round 1, the survey was created in Qualtrics, reviewed by the Steering Group and piloted by representatives from each of the three key stakeholder groups. Pilot responses were not included in the final analysis.
The main component of the survey comprised two parts. Part 1 asked respondents to review the full list of summary questions and shortlist those that they felt were most important. Part 2 asked respondents to review their shortlisted questions and select up to 10 questions from that list. The same processes for dissemination, monitoring engagement and obtaining consent were used as per round 1. Individuals could respond to the survey irrespective of whether they completed the previous survey. Similar to the round 1 survey, in cases where respondents could identify with more than one stakeholder group, they were asked to select the group that best reflected their questions/uncertainties about reducing and stopping psychiatric medication. The response data were analysed in Microsoft Excel using descriptive summary statistics. Using the demographic information submitted by respondents, responses were grouped for each stakeholder group, and the questions were ranked based on the frequency with which they had been selected. This enabled the ranked priorities across the different stakeholder groups to be compared and contrasted. Once the analysis was complete, the Steering Group reviewed the findings and the 19 most highly ranked questions across the three key stakeholder groups were taken forward to the final workshop (step 6).
Step 6: workshop (final prioritisation)
A final prioritisation workshop was held in Trinity College Dublin in October 2023 to prioritise through consensus the most highly ranked questions about reducing and stopping psychiatric medication from the round 2 survey. This 1-day workshop was held as a hybrid event to enable participants to join remotely from any location and enhance international representation. Workshop participants (n=30) consisted of representatives from each of the three key stakeholder groups from six countries (Belgium, Canada, Ireland, Sweden, UK and US). They were identified through two processes. First, the round 2 survey respondents could express their interest in participating in the workshop through a dedicated email address. Second, all Steering Group members were invited to attend and asked to nominate other individuals within their networks. The response obtained exceeded expectations. Workshop participants were purposively selected to achieve a representation of voices, genders and nationalities. Workshop participants contributed their time on a voluntary basis; however, travel and accommodation were reimbursed for those attending the workshop in person.
The workshop was facilitated by two JLA advisors and two JLA-trained facilitators who chaired the small group sessions. Prior to the workshop, participants were provided with the list of 19 shortlisted questions and asked to select their top three and bottom three questions. An adapted Nominal Group Technique was used to make decisions and ensure that all participants’ opinions were considered.18 The workshop involved three small group sessions during which participants completed several ranking exercises. In the first session, participants were split into four groups, balanced according to participant background, and asked to share their top three and bottom three priorities. Similarities and differences between the individual rankings were documented. During the second session, each group was tasked with ranking all 19 shortlisted questions in order of priority. Questions were assigned to gold, silver and bronze categories and then ordered within each category to assign a score to each question. A combined rank was calculated by arithmetic averaging of the individual group rankings and shared with the entire group of participants. For the final small group session, participants were split into three groups and groupings were revised to ensure that participants were exposed to different stakeholders and perspectives. During this session, each group started with the combined ranking, and was given the opportunity to revise this as they wished. The rankings from each group were again collated using Microsoft Excel. The workshop concluded with a whole group plenary discussion to review and reflect on the final top 10 priorities.
Patient and public involvement
People with lived experience of taking and/or stopping psychiatric medication and family members and/or carers/supporters were involved as equal members of the Steering Group members and had a key role in all stages of this research (design, conduct, reporting and dissemination plans). People with lived experience of taking and/or stopping psychiatric medication and family members and/or carers/supporters were also involved in deciding on the top 10 priorities at the final workshop.
Results
Step 2: gathering evidence uncertainties (round 1 survey)
There were 884 responses to the survey containing at least one question/uncertainty. All three stakeholder groups were represented in the responses: people with lived experience of taking and/or stopping psychiatric medication (69%), healthcare professionals (21%), family members/carers/supporters (10%). Most respondents resided in the US (42%), UK (21%) and Ireland (10%) with the remainder spread across Europe (13%), Canada (6%), Oceania (5%), Africa (<1%), Asia (2%) and Latin America (<1%). Full details of respondents’ demographics are reported in table 1.
Table 1. Demographic characterises of respondents to the round 1 and round 2 surveys.
| Round 1 survey | Round 2 survey | |
| N (%) | N (%) | |
| Total respondents | 884 | 526 |
| Gender | ||
| Male | 232 (26%) | 143 (27%) |
| Female | 629 (71%) | 371 (71%) |
| Non-binary | 21 (2%) | 11 (2%) |
| Did not specify | 2 (<1%) | 1 (<1%) |
| Stakeholder group | ||
| Person with lived experience | 609 (69%) | 343 (65%) |
| Family member, friend, carer, supporter | 86 (10%) | 32 (6%) |
| Healthcare professional | 186 (21%) | 151 (29%) |
| Other | 3 (<1%) | 0 |
| Healthcare professional group | ||
| General practitioner/primary care physician | 11 (6%) | 7 (5%) |
| Nurse | 47 (25%) | 66 (44%) |
| Pharmacist | 22 (12%) | 23 (15%) |
| Psychiatrist | 64 (34%) | 17 (11%) |
| Psychologist/psychotherapist/ counsellor | 24 (13%) | 17 (11%) |
| Social worker | 5 (3%) | 4 (3%) |
| Specialist physician | 4 (2%) | 1 (<1%) |
| Occupational therapist | 0 | 7 (5%) |
| Other | 9 (5%) | 9 (6%) |
| Age range (years) | ||
| 18–24 | 22 (2%) | 7 (1%) |
| 25–34 | 88 (10%) | 67 (13%) |
| 35–44 | 164 (19%) | 120 (23%) |
| 45–54 | 195 (22%) | 127 (24%) |
| 55–64 | 236 (27%) | 129 25%) |
| 65–74 | 137 (15%) | 62 (12%) |
| 75–84 | 39 (4%) | 14 (3%) |
| >85 | 2 (<1%) | 0 |
| Did not specify | 1 (<1%) | 0 |
| Country of origin | ||
| USA | 371 (42%) | 156 (30%) |
| UK | 188 (21%) | 147 (28%) |
| Ireland | 88 (10%) | 79 (15%) |
| Canada | 49 (6%) | 35 (7%) |
| Oceania | 41 (5%) | 36 (7%) |
| Europe | 118 (13%) | 44 (8%) |
| Latin America | 7 (<1%) | 7 (1%) |
| Africa | 5 (<1%) | 2 (<1%) |
| Asia | 15 (2%) | 9 (2%) |
| Did not specify | 2 (<1%) | 11 (2%) |
Step 3: summarising the responses gathered
Round 1 respondents submitted 3635 individual questions/uncertainties. These were reviewed in consultation with the Steering Group and out-of-scope questions (n=1458) were removed, leaving 2177 questions (figure 1).
The in-scope questions were then coded into the framework generating eight themes (level 1 codes): (1) tapering aids/supports; (2) tapering process; (3) post-taper; (4) withdrawal symptoms and adverse effects; (5) accountability/responsibility; (6) acknowledgement/recognition of problems/issues; (7) communication/decision-making; and (8) healthcare professional knowledge/training. Examples of questions linked to each of these themes are provided in online supplemental table 1.
Once coding was complete, the coded data were converted into researchable summary questions. Submitted questions were reviewed for similarity, combined and rephrased to create summary questions. Initially, there were 105 summary questions. These questions underwent three rounds of review and refinement involving the Steering Group (table 2). This included exclusion, merging and/or rewording of questions. After the review, 32 ‘indicative questions’ (online supplemental table 2) were taken forward to the evidence check (step 4).
Table 2. Overview of process of iterative review and refinement of summary questions following round 1 survey.
| Theme | Number of summary questions per round of review | |||
| Prereview | Review 1 | Review 2 | Review 3 | |
| 1. Tapering aids and supports | 18 | 8 | 7 | 6 |
| 2. Tapering process | 23 | 17 | 8 | 8 |
| 3. Post-taper | 12 | 7 | 4 | 4 |
| 4. Withdrawal symptoms and adverse effects | 11 | 9 | 7 | 5 |
| 5. Accountability | 14 | 8 | 3 | 2 |
| 6. Acknowledgement of issues | 17 | 4 | 3 | 3 |
| 7. Communication and decision-making | 7 | 6 | 3 | 3 |
| 8. Healthcare professional | 3 | 1 | 1 | 1 |
| Total number of questions | 105 | 60 | 36 | 32 |
Step 4: evidence checking
Following the evidence review (online supplemental table 3), all 32 questions were considered as ‘verified uncertainties’ and were entered into the round 2 survey (step 5) for prioritisation.
Step 5: interim prioritisation (round 2 survey)
In total, 526 respondents completed the survey. The respondent profile was broadly similar to round 1 in terms of stakeholder group and geographical location. Full details of respondents' demographics are reported in table 1.
The ranked order of each question is outlined across each stakeholder group in table 3. In consultation with the Steering Group, the 19 highest ranked questions across the stakeholder groups were taken forward to the final prioritisation workshop (step 6) (online supplemental table 4).
Table 3. Ranked order of questions from round 2 survey.
| Ranked order | People with lived experience | Family members/carers/supporters | Healthcare professionals |
| 1 | Q9 | Q3 | Q3 |
| 2 | Q32 | Q32 | Q16 |
| 3 | Q3 | Q9, Q23 | Q32 |
| 4 | Q16 | Q4*, Q25, Q28 | Q6, Q25 |
| 5 | Q28 | Q2, Q26 | Q9 |
| 6 | Q26 | Q22 | Q7 |
| 7 | Q19 | Q6, Q14, Q18, Q24 | Q31 |
| 8 | Q15 | Q12, Q15, Q16, Q31 | Q4* |
Due to a high level of overlap/similarity between Q3 and Q4, the Steering Group agreed to exclude Q4 and retain Q3.
Step 6: final prioritisation workshop
Thirty individuals representing each of the key stakeholder groups took part in the final prioritisation workshop: people with lived experience of taking and/or stopping psychiatric medication (n=10), family members/carers/supporters (n=4) and healthcare professionals (n=16). Most participants attended in person (n=21).
The top 10 priorities were agreed with workshop participants and are listed in table 4. The remaining priorities are listed in online supplemental table 5. The two highest ranked questions focused on the most effective ways of safely reducing/stopping psychiatric medication and providing support to individuals undergoing the discontinuation process. Another question asked about the best ways to educate healthcare professionals about reducing and stopping psychiatric medication. Other questions focused on understanding barriers and enablers to reducing and stopping psychiatric medication, and the views and experiences of those who had reduced/stopped or were currently doing so. Questions also asked about the perspectives of key stakeholders on shared decision-making, as well as the professional, ethical and legal responsibilities of healthcare professionals and the pharmaceutical industry in relation to reducing and stopping psychiatric medication. Other questions focused on the consequences (positive and negative) of reducing and stopping psychiatric medication on an individual’s physical and mental health, as well as understanding the withdrawal symptoms.
Table 4. Top 10 priorities for reducing and stopping psychiatric medication.
| Final ranking (after workshop) | Question |
| 1 | What is the most effective way to safely reduce and stop psychiatric medication in terms of tapering approach, rate of taper and duration of taper? What individual service user characteristics (eg, age, gender, pregnancy, other medical conditions/diseases) and drug characteristics (eg, medication type, duration of treatment, use of other medication) determine these? |
| 2 | What are the most effective ways to provide support to individuals who are reducing and stopping psychiatric medication? These may include, but are not limited to, family/peer support, educational support, financial support, psychological support, and healthcare support. |
| 3 | What are the best ways to educate current and future healthcare professionals about reducing and stopping psychiatric medication in terms of the tapering process, associated risks/difficulties, withdrawal symptoms and supporting shared decision-making? What is the impact of education on clinical practice? |
| 4 | What are the views and experiences of individuals who have reduced/stopped psychiatric medication or are currently reducing/stopping psychiatric medication on the tapering process and accessing tapering support? |
| 5 | What are the views and experiences of service users, family members/carers and healthcare professionals around shared decision-making in relation to starting and stopping psychiatric medication? This includes informed consent. How can the process of implementing shared decision-making be improved when starting and stopping psychiatric medication? What factors influence this process? |
| 6 | What are the positive and negative long-term consequences of reducing and stopping psychiatric medication on an individual’s physical and mental health status? For individuals who experience negative consequences, what are the best ways to manage these difficulties? Negative consequences may include withdrawal symptoms, relapse and protracted withdrawal syndromes. |
| 7 | What are the perspectives of key stakeholders on the professional, ethical and legal responsibilities of healthcare professionals and/or the pharmaceutical industry in relation to reducing and stopping psychiatric medication? Stakeholders include service users, family members/carers and healthcare professionals. What are the best ways to enact these responsibilities? |
| 8 | Which factors influence the prevalence, duration and severity of withdrawal effects that appear during or after reducing and stopping psychiatric medication? What is the best way to control these factors and reduce an individual’s risk of developing withdrawal effects or relapsing? |
| 9 | How best can the withdrawal symptoms that appear during or after reducing and stopping psychiatric medication be identified and differentiated from other causes (eg, relapse/return of underlying condition, distress)? |
| 10 | What are the barriers and enablers to reducing and stopping psychiatric medication? These may include, but are not limited to, the service user, the healthcare professional, family and society. |
Discussion
This study has produced a top 10 list of research priorities on reducing and stopping psychiatric medication through extensive engagement with key stakeholders representing people with lived experience of taking and/or stopping psychiatric medication, family members/carers/supporters and healthcare professionals. The list highlights important uncertainties and gaps in the existing evidence base on this topic that need to be addressed by future research. To our knowledge, this is the first JLA PSP to focus on psychiatric medication. The process by which these priorities have been developed also addresses an important deficit in current practices of stakeholder engagement as involvement of people with lived experience of mental health challenges in research is not widespread, particularly in the early stages of research priority and agenda setting.23 For example, a survey commissioned by the International Alliance of Mental Health Research Funders found that most involvement of people with lived experience in health research has traditionally taken place at the level of feedback and information giving (ie, ‘consultation’ stages).24 Consequently, people with lived experience of mental health challenges report difficulties in sharing their views and influencing the research agenda.23 A hallmark of the JLA approach is the representation of all stakeholder groups throughout the priority-setting process. Equitable involvement of different stakeholders has been shown to facilitate the process of setting research priorities by developing a more holistic understanding of the ‘unknown unknowns’.25
The highest ranked question focused on the most effective ways of safely reducing/stopping psychiatric medication. This is a long-standing issue that has not been answered by previous research. To date, there is no standard approach on how best to taper which has created many uncertainties regarding the tapering process.26 Studies have shown that the rate of taper is typically based on prescribers’ clinical experience as opposed to high-quality empirical evidence.13 Key organisations in the UK, such as the Royal College of Psychiatrists and the NICE, recognise the importance of gradual dosage reduction at a rate that is tailored to the individual.27 There is a pressing need for further comprehensive research to establish an evidence base to support individuals looking to reduce and/or stop psychiatric medication.
Linked to this question about the most effective ways of safely reducing/stopping psychiatric medication is the question about what the most effective ways are to support individuals undergoing the discontinuation process. The prioritisation of this question highlights a fundamental shift in the recognition of the potential challenges of discontinuing psychiatric medication and associated withdrawal symptoms, which were previously underacknowledged. For many years, guidelines described antidepressant withdrawal symptoms as mild and self-limiting, typically lasting only 1–2 weeks.28 Only in recent years have professional bodies and prescribing guidelines acknowledged that a proportion of individuals taking psychiatric medication, such as antidepressants, may experience significant withdrawal symptoms following discontinuation.27 28 Although gradual dosage reduction is a core feature of many interventions, there is less robust evidence about additional measures such as psychological support.29 30 In addition to this, the availability of dedicated withdrawal services is lacking.31 In the absence of empirical evidence, individuals are increasingly turning to online sources, such as discussion fora and peer support groups, for guidance and support while tapering psychiatric medication.32
Another key question asked about the best ways to educate healthcare professionals about reducing and stopping psychiatric medication. Previous research has highlighted important perceived deficits in healthcare professionals’ knowledge of psychiatric medication and tapering approaches.33 This aligns with the views and reported experiences of people taking psychiatric medication.34 For example, a survey of members of an online discussion forum, who have stopped or tried to stop antidepressant use, found that 71% of respondents (n=906/1276) felt their doctors’ advice regarding stopping an antidepressant was unhelpful.35 Reasons included their doctor recommended an abrupt taper and/or were unfamiliar with the concept of withdrawal. Similar findings were reported by another survey involving antidepressant users in which 64% (n=205/319) of respondents reported receiving a lack of information about the potential for withdrawal symptoms from their doctor and 40% were advised to withdraw from their medication rapidly.36 Gaps in healthcare professionals’ knowledge and training may create barriers to the safe discontinuation of psychiatric medication. According to this survey, the most frequently cited recommendation for future withdrawal services was to improve healthcare professionals' knowledge.36
Other questions focused on the consequences of reducing and stopping psychiatric medication on an individual’s physical and mental health, as well as understanding the withdrawal symptoms, and key stakeholders’ perspectives on shared decision-making. Various adverse effects are associated with the use and discontinuation of different psychiatric medications, about which individuals have reported not being informed before starting.37 According to the WHO, shared decision-making and informed consent are crucial to the provision of person-centred and recovery-oriented care.17 Service users have reported challenges in having their autonomy and choice respected and being involved in decisions around their medication.38 This does not align with a rights-based, recovery-oriented approach to mental healthcare that seeks to promote open discussion about medication.17 Another related area of importance was the professional, ethical and legal responsibilities of healthcare professionals and the pharmaceutical industry in relation to reducing and stopping psychiatric medication. This has not been examined by previous research.
Although the PSP’s key output was the top 10 list of research priorities, it is important to note that several other shortlisted questions were discussed at length during the final prioritisation workshop. These questions may also provide a useful starting point for future research on reducing and stopping psychiatric medication. For example, the question about improving the availability of psychiatric medication in formulations and dosage ranges that facilitate the tapering process was deemed a key uncertainty among many survey respondents and workshop participants. There is currently a lack of evidence informing clinicians or individuals as to what extent existing marketed formulations of psychiatric medication, which primarily consist of oral solid dosage forms (ie, tablets, capsules, oral liquids/drops) can be further manipulated (eg, split/crushed/diluted) to achieve smaller doses with due consideration to the physicochemical properties of individual medication and their formulations. Consequently, many individuals attempting dosage reduction use do-it-yourself methods that are shared online or through peer support forms that involve crushing tablets and making liquid preparations.11 32 The accuracy, efficacy and safety of these approaches have not been evaluated. Tapering strips, consisting of psychiatric medication packaged into pouches of individual daily doses, have been developed in the Netherlands to enable gradual dosage reduction.39 However, as in the Netherlands, tapering strips are not widely available or accessible on public health schemes via existing reimbursement mechanisms. This is therefore an important area for future research, but ultimately, the stakeholder collaboration determined that other questions about the tapering process were of higher priority.
While the prioritisation of research uncertainties was the principle objective of the PSP, it also sought to enhance and strengthen the manner in which research questions are identified. By engaging key stakeholders in a meaningful and structured way in jointly identifying research priorities, the PSP process enabled the coproduction of research priorities.25 40 Coproduction has been widely advocated as a means of valuing and respecting knowledge from different sources and stakeholders, and thus promoting inclusive research practices and strengthening research impact.40 In this sense, the PSP process aligns with more modern, rights-based approach to mental health research.41
A key strength of this study was the robustness of the JLA PSP methodology which has been used internationally across a range of healthcare domains and is regarded as the gold standard in research priority-setting exercises.42 The research prioritisation process was further strengthened through extensive engagement with the three key stakeholder groups across the Steering Group members, survey respondents and workshop attendees. The involvement of a diverse and international mix of stakeholders with varying demographics at every step of the process gives the resultant priorities legitimacy. Moreover, the high level of interest and engagement with the study, as evidenced by the volume of responses submitted to both the round 1 and 2 surveys, adds to the credibility of the findings as the study captured uncertainties/questions from hundreds of individuals worldwide.
A limitation of this study was that it was conducted entirely through English and the two surveys were only available in digital format due to resource and logistical constraints. This may have limited accessibility for certain groups and countries where English is not widely spoken. Furthermore, in consultation with the Steering Group, it was decided not to capture information on the ethnicity of survey respondents as this information would not have contributed directly to the selection of the top 10 list of research priorities. It must also be noted that the round 1 survey submissions primarily consisted of free-text responses. Despite efforts to minimise interpretative bias while coding, the subjective nature of coding qualitative data always creates a potential for bias. However, the Steering Group had oversight of the entire coding process and were sent the coded responses to review to enhance rigour, credibility and transparency of the coding process.
Conclusion
This PSP has produced a top 10 list of research priorities on reducing and stopping psychiatric medication through extensive engagement with key stakeholders representing people with lived experience of taking and/or stopping psychiatric medication, family members, carers/supporters and healthcare professionals. The list highlights important uncertainties and gaps in the existing evidence base on this topic that should be addressed by future research. This top 10 list of research priorities is relevant to research funding agencies and could help to guide future research and deliver responsive and strategic allocation of research resources, with a view to improving the future health and well-being of individuals who are taking psychiatric medication.
supplementary material
Acknowledgements
The authors are grateful to all participants (survey respondents and workshop participants), and partner organisations for supporting this study. The authors would also like to acknowledge the support provided by Maryrose Tarpey from the JLA, and Clodagh O’Donovan, Trinity College Dublin in facilitating the final workshop; and Greg Sheaf, Trinity College Dublin, for creating a comprehensive search strategy as part of the evidence checking process.
Footnotes
Funding: Miriam Boland was supported by a 1252 Scholarship from the School of Pharmacy and Pharmaceutical Sciences, Trinity College Dublin. The workshop was funded by a grant received by the Health Research Board Ireland (CES-2023-008). The funding sources had no role in study design, data collection, data analysis, data interpretation, writing of the report or decision to submit for publication.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-088266).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and was approved by Faculty of Health Sciences Research Ethics Committee, Trinity College Dublin (Ref: 220509). Participants gave informed consent to participate in the study before taking part.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Contributor Information
Miriam Boland, Email: bolandm7@tcd.ie.
Agnes Higgins, Email: ahiggins@tcd.ie.
Claire Beecher, Email: beecher.claire@gmail.com.
Pat Bracken, Email: patjbracken@gmail.com.
Wendy Burn, Email: wendy.burn@rcpsych.ac.uk.
Anne Cody, Email: anne@hrb.ie.
Adele Framer, Email: survivingads@gmail.com.
Toto Gronlund, Email: toto.jla@microwells.uk.
Mark Horowitz, Email: markhoochowitz@gmail.com.
Christy Huff, Email: christy@benzoinfo.com.
Sandra Jayacodi, Email: sandragcg@gmail.com.
Dolores Keating, Email: dolores.keating@sjog.ie.
David Kessler, Email: david.kessler@bristol.ac.uk.
Åsa Konradsson-Geuken, Email: asa.konradsson-geuken@farmbio.uu.se.
Nicole Lamberson, Email: nicole@benzoinfo.com.
Luke Montagu, Email: lukemontagu@me.com.
Ruth Smith, Email: ruth.counter-smith@hotmail.com.
Cathal Cadogan, Email: cathal.cadogan@tcd.ie.
Data availability statement
Data are available upon reasonable request.
References
- 1.Brauer R, Alfageh B, Blais JE, et al. Psychotropic medicine consumption in 65 countries and regions, 2008-19: a longitudinal study. Lancet Psychiatry. 2021;8:1071–82. doi: 10.1016/S2215-0366(21)00292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies J, Read J, Kruger D, et al. Politicians, experts, and patient representatives call for the UK government to reverse the rate of antidepressant prescribing. BMJ. 2023;383:2730. doi: 10.1136/bmj.p2730. [DOI] [PubMed] [Google Scholar]
- 3.Ilyas S, Moncrieff J. Trends in prescriptions and costs of drugs for mental disorders in England, 1998-2010. Br J Psychiatry. 2012;200:393–8. doi: 10.1192/bjp.bp.111.104257. [DOI] [PubMed] [Google Scholar]
- 4.Roberts R, Neasham A, Lambrinudi C, et al. A quantitative analysis of antipsychotic prescribing trends for the treatment of schizophrenia in England and Wales. JRSM Open. 2018;9:2054270418758570. doi: 10.1177/2054270418758570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cadogan CA, Ryan C, Cahir C, et al. Benzodiazepine and Z-drug prescribing in Ireland: analysis of national prescribing trends from 2005 to 2015. Br J Clin Pharmacol. 2018;84:1354–63. doi: 10.1111/bcp.13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gøtzsche PC, Young AH, Crace J. Does long term use of psychiatric drugs cause more harm than good? BMJ. 2015;350:h2435. doi: 10.1136/bmj.h2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crellin NE, Priebe S, Morant N, et al. An analysis of views about supported reduction or discontinuation of antipsychotic treatment among people with schizophrenia and other psychotic disorders. BMC Psychiatry. 2022;22:185. doi: 10.1186/s12888-022-03822-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watts MD, Murphy E, Keogh BD, et al. Deciding to discontinue prescribed psychotropic medication: A qualitative study of service users’ experiences. Int J Mental Health Nurs. 2021;30:1395–406. doi: 10.1111/inm.12894. [DOI] [PubMed] [Google Scholar]
- 9.Keogh B, Murphy E, Doyle L, et al. Mental health service users experiences of medication discontinuation: a systematic review of qualitative studies. J Ment Health. 2022;31:227–38. doi: 10.1080/09638237.2021.1922644. [DOI] [PubMed] [Google Scholar]
- 10.Horowitz MA, Jauhar S, Natesan S, et al. A Method for Tapering Antipsychotic Treatment That May Minimize the Risk of Relapse. Schizophr Bull. 2021;47:1116–29. doi: 10.1093/schbul/sbab017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Framer A. What I have learnt from helping thousands of people taper off antidepressants and other psychotropic medications. Ther Adv Psychopharmacol. 2021;11:2045125321991274. doi: 10.1177/2045125321991274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hengartner MP, Plöderl M. Prophylactic effects or withdrawal reactions? An analysis of time-to-event data from antidepressant relapse prevention trials submitted to the FDA. Ther Adv Psychopharmacol. 2021;11:20451253211032051. doi: 10.1177/20451253211032051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sørensen A, Juhl Jørgensen K, Munkholm K. Clinical practice guideline recommendations on tapering and discontinuing antidepressants for depression: a systematic review. Ther Adv Psychopharmacol. 2022;12:20451253211067656. doi: 10.1177/20451253211067656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fricker M. Oxford, UK: Oxford University Press; 2007. Epistemic injustice: power and the ethics of knowing. [Google Scholar]
- 15.Miller R, Pavlo A. In: Recovering the us mental healthcare system: The past, present, and future of psychosocial interventions for psychosis. Davidson CA, Stacy M, editors. Cambridge, UK: Cambridge University Press; 2022. From deinstitutionalization to deprescribing and beyond. [Google Scholar]
- 16.Rose D. Service user produced knowledge. J Ment Health. 2008;17:447–51. doi: 10.1080/09638230802453682. [DOI] [Google Scholar]
- 17.Geneva: World Health Organization and the United Nations (represented by the Office of the United Nations High Commissioner for Human Rights); 2023. [13-Feb-2024]. Mental health, human rights, and legislation: guidance and practice, Licence: CCBY-NC-SA 3.0 IGO.https://www.who.int/publications/i/item/9789240080737 Available. accessed. [Google Scholar]
- 18.James Lind Alliance . The James Lind Alliance Guidebook. Version 10. Southampton, United Kingdom; 2021. [13-Feb-2024]. https://www.jla.nihr.ac.uk/jla-guidebook/ Available. accessed. [Google Scholar]
- 19.Boland M, Higgins A, Beecher C, et al. Priorities for future research on reducing and stopping psychiatric medicines using a James Lind Alliance priority setting partnership: The PROTECT study protocol. HRB Open Res. 2022;5:72. doi: 10.12688/hrbopenres.13649.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tong A, Synnot A, Crowe S, et al. Reporting guideline for priority setting of health research (REPRISE) BMC Med Res Methodol. 2019;19:243. doi: 10.1186/s12874-019-0889-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooks J, McCluskey S, Turley E, et al. The Utility of Template Analysis in Qualitative Psychology Research. Qual Res Psychol. 2015;12:202–22. doi: 10.1080/14780887.2014.955224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratan SK, Anand T, Ratan J. Formulation of Research Question - Stepwise Approach. J Indian Assoc Pediatr Surg. 2019;24:15–20. doi: 10.4103/jiaps.JIAPS_76_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghisoni M, Wilson CA, Morgan K, et al. Priority setting in research: user led mental health research. Res Involv Engagem. 2017;3:4. doi: 10.1186/s40900-016-0054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White R, van den Eijnden M, Donskoy A-L, et al. Lived experience involvement in research funding: taking a more systematic approach. Nat Mental Health. 2023;1:157–9. doi: 10.1038/s44220-023-00029-9. [DOI] [Google Scholar]
- 25.Pollock A, St George B, Fenton M, et al. Development of a new model to engage patients and clinicians in setting research priorities. J Health Serv Res Policy. 2014;19:12–8. doi: 10.1177/1355819613500665. [DOI] [PubMed] [Google Scholar]
- 26.Groot PC, van Os J. How user knowledge of psychotropic drug withdrawal resulted in the development of person-specific tapering medication. Ther Adv Psychopharmacol. 2020;10:2045125320932452. doi: 10.1177/2045125320932452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Institute for Health and Care Excellence NICE guideline: medicines associated with dependence or withdrawal symptoms: safe prescribing and withdrawal management for adults. 2022. [08-Mar-2024]. https://www.nice.org.uk/guidance/ng215 Available. Accessed. [PubMed]
- 28.Davies J, Read J. A systematic review into the incidence, severity and duration of antidepressant withdrawal effects: Are guidelines evidence-based? Addict Behav. 2019;97:111–21. doi: 10.1016/j.addbeh.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 29.Van Leeuwen E, van Driel ML, Horowitz MA, et al. Approaches for discontinuation versus continuation of long-term antidepressant use for depressive and anxiety disorders in adults. Cochrane Database Syst Rev. 2021;4:CD013495. doi: 10.1002/14651858.CD013495.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darker CD, Sweeney BP, Barry JM, et al. Psychosocial interventions for benzodiazepine harmful use, abuse or dependence. Cochrane Database Syst Rev. 2015;2015:CD009652. doi: 10.1002/14651858.CD009652.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guy A, Brown M, Lewis S, et al. The “patient voice”: patients who experience antidepressant withdrawal symptoms are often dismissed, or misdiagnosed with relapse, or a new medical condition. Ther Adv Psychopharmacol. 2020;10:2045125320967183. doi: 10.1177/2045125320967183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White E. Tapering antidepressants: why do tens of thousands turn to Facebook groups for support? Br J Gen Pract. 2021;71:315. doi: 10.3399/bjgp21X716309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Begum F, Mutsatsa S, Gul N, et al. Antipsychotic medication side effects knowledge amongst registered mental health nurses in England: A national survey. J Psychiatr Ment Health Nurs. 2020;27:521–32. doi: 10.1111/jpm.12600. [DOI] [PubMed] [Google Scholar]
- 34.Read J, Renton J, Harrop C, et al. A survey of UK general practitioners about depression, antidepressants and withdrawal: implementing the 2019 Public Health England report. Ther Adv Psychopharmacol. 2020;10:2045125320950124. doi: 10.1177/2045125320950124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Read J, Moncrieff J, Horowitz MA. Designing withdrawal support services for antidepressant users: Patients’ views on existing services and what they really need. J Psychiatr Res. 2023;161:298–306. doi: 10.1016/j.jpsychires.2023.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Read J, Lewis S, Horowitz M, et al. The need for antidepressant withdrawal support services: Recommendations from 708 patients. Psychiatry Res. 2023;326:115303. doi: 10.1016/j.psychres.2023.115303. [DOI] [PubMed] [Google Scholar]
- 37.Read J, Gee A, Diggle J, et al. Staying on, and coming off, antidepressants: The experiences of 752 UK adults. Addict Behav. 2019;88:82–5. doi: 10.1016/j.addbeh.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 38.Cooper RE, Hanratty É, Morant N, et al. Mental health professionals’ views and experiences of antipsychotic reduction and discontinuation. PLoS ONE. 2019;14:e0218711. doi: 10.1371/journal.pone.0218711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groot PC, van Os J. Successful use of tapering strips for hyperbolic reduction of antidepressant dose: a cohort study. Ther Adv Psychopharmacol. 2021;11:20451253211039327. doi: 10.1177/20451253211039327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliver K, Kothari A, Mays N. The dark side of coproduction: do the costs outweigh the benefits for health research? Health Res Policy Syst. 2019;17:33. doi: 10.1186/s12961-019-0432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allemang B, Sitter K, Dimitropoulos G. Pragmatism as a paradigm for patient-oriented research. Health Expect. 2022;25:38–47. doi: 10.1111/hex.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hart AL, Lomer M, Verjee A, et al. What Are the Top 10 Research Questions in the Treatment of Inflammatory Bowel Disease? A Priority Setting Partnership with the James Lind Alliance. J Crohns Colitis. 2017;11:204–11. doi: 10.1093/ecco-jcc/jjw144. [DOI] [PMC free article] [PubMed] [Google Scholar]


