Abstract
Introduction:
Balance impairment contributes to gait dysfunction, falls and reduced quality of life in adults with Charcot-Marie-Tooth disease (CMT), but has been minimally examined in pediatric CMT.
Methods:
The CMT Pediatric Scale (CMTPedS) was administered to 520 children with CMT. Associations between balance function (Bruininks-Oseretsky Test of Motor Proficiency (BOT-2)), sensorimotor and gait impairments were investigated.
Results:
Daily trips/falls were reported by 42.3% of participants. Balance (BOT-2) varied by CMT subtype, was abnormal in in 42% of 4-year-olds and declined with age (p<.001). Vibration (p<.001), pinprick (p<.004), ankle dorsiflexion strength (p<.001) and foot alignment (p<.004 were associated with BOT-2 balance (adjusted R2=.28). The visual dependence of balance increased with age.
Discussion:
Balance impairment occurs from a young age in children with CMT. Balance intervention studies are needed in pediatric CMT and should consider the degree of sensorimotor impairment, foot malalignment and visual dependency.
Keywords: Balance, Charcot-Marie-Tooth, Children, Intervention, sensorimotor impairment
Introduction
Charcot-Marie-Tooth disease
Charcot-Marie-Tooth disease (CMT), is a group of genetically heterogeneous axonal or demyelinating polyneuropathies that affect approximately 1 in 1,214 to 2,500 individuals1,2 and is characterized by distally dominant muscle atrophy, weakness, sensory loss, and foot deformities. The somatosensory impairment and muscle imbalance in the foot and ankle, along with the development of Achilles tendon and plantar fascia contractures with the characteristic cavovarus foot, contribute to dysfunction of gait and to balance deficits in CMT3. The determinants of balance deficits in children with CMT has to date been minimally studied.
Sensory system maturation and balance
The integration of visual, vestibular, and somatosensory (tactile and proprioceptive) feedback contribute to the development of balance during early childhood.4,5 Initially, the inability to effectively resolve conflicting sensory information and disregard redundant sensory feedback results in inefficient integration of sensory feedback to maintain postural control. Early work established that balance could be perturbed in children under the age of 7 years 6 and more recent data indicates that in addition to the high incidence of falls in this younger population, the characteristics of sway in response to visual stimulus are altered in children as old as 12 years of age7–9. As typically developing children age, there is a reweighting process in which the primary dependence on vision is progressively supplanted by the somatosensory and vestibular systems. As a result, postural sway in childhood typically shows progressive decrement with age, but continues to be magnified with the occlusion of vision through the first decade of life4.
Impact of CMT on balance
In addition to the developmental aspects of balance in the typically developing child, the progressive sensory and motor impairments associated with CMT negatively impact standing balance. As a result, safety during activities of daily living and sports/leisure activities can also be limited10 as children with CMT are 33 times more likely to fall as compared to their typically developing peers11. Somatosensation is one determinant of balance which is diminished in patients with CMT and has been investigated both from an experimental and natural history perspective. More severely affected adults with CMT1A, the most common form of CMT, and those with CMT2 both experience increased sway in single leg stance when compared with typical controls12,13. In the adult population, the impact of tactile sensation on balance has been studiedxperimentally by inducing hypothermia, ischemia or anesthesia to better understand the role of plantar sensation on balance. The predominant consequence observed was an increase in sway.14 There is, however, a dearth of literature investigating the impact of diminished somatosensory input in the typically developing pediatric population outside of the use of a foam surface to diminish the experienced somatosensory input.15
Clinical relevance
An understanding of genotype-phenotype relationships, sex differences, and the interrelationship of age and disease related balance impairment would provide the ability to more accurately anticipate and describe the progression of balance and the factors that drive its progression. A better understanding of the development of balance and factors that influence balance in children with CMT has begun to emerge with an understanding of expectations for change over 2 years in various genetic sub types of CMT.16 This along with greater understanding of the relationships between balance and related impairments can be the basis for a thoughtful approach to impairment targeted treatment trials. The goal of this study was therefore to systematically evaluate balance and its associated factors in a large cohort of well characterized children with CMT.
Methods
Participants
Five hundred and twenty children aged 3–20 years with a diagnosis of CMT were enrolled in a prospective longitudinal cohort study17 between August 6, 2009, and July 30, 2014 as part of the Inherited Neuropathies Consortium at the following pediatric healthcare institutions: Children’s Hospital at Westmead, University of Sydney, Australia, University of Iowa Health Care, Iowa, USA, Wayne State University, Detroit, USA, Children’s Hospital of Philadelphia, Pennsylvania, USA, C. Besta Neurological Institute, Milan, Italy, Great Ormond Street Hospital and National Hospital of Neurology and Neurosurgery, London, UK, Nemours Children’s Hospital, Florida, USA, University of Rochester, New York, USA and University of Pennsylvania, Pennsylvania, USA. All sites obtained ethics approval, and written, informed consent/assent was obtained from all participants or their parent/guardian.
Measures and Procedure
The Charcot-Marie-Tooth disease Pediatric Scale (CMTPedS) was administered and a subset of items (lower extremity) from each participant’s baseline visit were selected for analysis18. Objective measures of impairment (foot alignment, ankle flexibility, plantar and dorsiflexion strength, and sensation) were assessed in addition to measures of performance (muscle power, balance, and endurance). Raw scores were compared to age and sex-matched normative reference values (NORM) to obtain a z-score3. A z-score ≥2 standard deviations below the mean was defined as impaired (impairment based measures) or described as having a deficit (performance based measures). Daily trips/falls were recorded as present or absent based on the self-report from patient/parents. Full methods are described in the CMTPedS manual19 and briefly below.
Foot Posture Index20(FPI) is a reliable method of evaluating foot alignment, allowing the foot to be scored on a continum from cavus (supinated) to planus (pronated) features. Participants were assessed in a relaxed stance with double limb support. They were asked to take several steps on the spot and then to relax and stand still, with their arms by their side and looking straight ahead. The clinician asessed 6 aspects of foot alignment, assigning each aspect a value ranging from −2(pes cavus) to +2(pes planus). The individual scores were summed and that value was recorded. Interpretation of FPI scores are as follows: −12 to −1 (Cavus), 0–5 (Neutral), 6–12 (Planus).
Standing Lunge Test21 was used to assess ankle dorsiflexion flexibilty. Participants were positioned with their foot perpindicular to a wall and asked to lunge towards the wall as far as they could, without allowing their heel to lift from the floor. The clinician measured and recorded the angle, in degrees, along the midline of the achilles tendon using the Baseline Digital Inclinometer (Fabrication Enterprises Incorporated, New York).
Hand-held dynamometry (C.I.T. Technics, Rijksstraatweg, The Netherlands) 22was used to assess plantar and dorsiflexion strength. Participants were positioned in long sitting on the exam table, and the clinician provided stabilization proximal to the ankle. Three trials, using a ‘make test’, were completed and then averaged for scoring.
Pinprick sensitivity of the lower extremity was evaluated using sterile neurological examination pins (Owen Mumford, Oxfordshire, United Kingdom) Participants were asked to differentiate between a proximal (normal) reference point and distal sites when given sharp input starting distally at the great toe and working proximally to above the knee. The scoring system was adopted from the CMT Neuropathy Score23,24, hich assigns a score from 0–4 based on the distribution of sensory impairment, with a score of 0 being normal and 4 showing impairment above the knee.
Vibratory sensation of the lower extremity was evaluated using a Rydel Seiffer tuning fork, C 64 Hz / c 128 Hz, with the participant’s vision occluded. Participants were asked to report when they could no longer detect the vibratory stimuli, starting distally at the great toe and working proximally to above the knee. The scoring system was adopted from the CMT Neuropathy Score23,24, which assigns a score from 0–4 based on the distribution of sensory impairment, with a score of 0 being normal and 4 showing absent vibratory sensation at the ankle and knee.
Bruininks Oseretsky Test of Motor Proficiency 2nded. (BOT-2) Balance25 was completed following standard instructions and scoring. Participants completed timed static and dynamic balance tasks along with several items that were repeated with vision occluded. Three BOT-2 Balance Subtest items (standing with feet apart on a line, standing on 1 leg, and standing on one leg on a balance beam) were assessed both with eyes open and eyes closed. In addition, to better understand the impact of visual input on balance, further analysis of these items was completed. The time the subject could maintain the position was recorded, with a maximum cut-off of 10 seconds. The difference between time achieved with eyes open (EO) and eyes closed (EC) was calculated and recorded as a new data point (TEO-EC).
Long jump was utilized to assess lower extremity power and coordination.26 Participants were asked to stand with feet shoulder width apart and jump as far as they could. The distance from the start line to the heel of the nearest foot was recorded.27,28
6 Minute Walk Test was used to assess functional endurance capacity29,28 using a modified version of the adult guidelines from The American Thoracic Society30. Methods described in 6MWT studies in healthy children were used, including: an evaluator walking behind the participant for safety , ongoing encouragement for motivation, and verbal redirection as needed to ensurea full understanding of the test. The course was set up in accordance with the protocol established for use in pediatric neuromuscular disorders31 and the total distance walked in bare feet in 6 minutes was recorded in meters.
Statistical Analysis
Data were analyzed using Stata 14.2 (StataCorp LLC, College Station, Texas). The appropriate parametric or non-parametric tests were performed based on normality of the data distribution. A p value of <0.05 was selected to indicate significance. BOT-2 Balance performance was analyzed using: Mann-Whitney test for sex differences, one sample t-test for comparison of individual BOT-2 balance items to normative reference values for children, Kruskal-Wallis test with Bonferroni correction for differences between CMT subtypes, Pearson correlation for relationship with long jump and 6 minute walk test, and forward stepwise multiple linear regression for association with the impairment-based measures. Foot alignment was analyzed using: paired t-test to evaluate limb differences, two sample t-test for sex differences, multiple linear regression to determine association between age and foot alignment, ANOVA for comparison by CMT subtype, and Kruskal-Wallis test to compare performance on long jump, 6 minute walk test, and BOT-2 Balance based on foot alignment category (Pes Cavus, Neutral, Pes Planus). Patient/parent reported daily trips/falls were calculated as a percentage. One sample t-test was used to compare BOT (TEO-EC) times against normative reference data for age/sex matched bands from the BOT-2 validation study32, and the slopes of the regression lines were calculated. Participants unable to perform the eyes open condition were excluded from this analysis.
Results
Overview
The 520 participants (274 male) in our cohort ranged in age from 3–20 years (10.9 ± 4.4) and the following CMT types were most prevalent: CMT1A (54%), CMT1B (3%), CMT2A (6%), CMT4C (2%), and CMT1X (2%). Two hundred and twenty (42.3%) participants reported daily trips/falls, and the group mean BOT-2 z-score was (−3.25± 2.9) (Figure 1). In pre-school aged children (3–5 years old), balance deficit was prevalent. Forty two percent (15/36) of four-year-old children had a Z-score ≤−2, and mean BOT-2 scores decreased starting at five years of age (−2.1±1.5) and continued to decline through adolescence (Beta=−.15; p<.001). Significant differences (p<0.01) were observed in 139/153 (91%) of the individual BOT-2 items as compared to the normative reference values and inability to maintain balance on the feet apart (68), single leg stance (89), and single leg stance on balance beam (143) items was observed amongst participants. No sex differences were detected (p=0.30); however, balance varied by CMT subtype. Analyzed via Kruskal-Wallis test using Bonferroni correction, BOT-2 z-scores were significantly worse (p<0.0025) in CMT subtypes 1B, 2A, and 4C, as compared to CMT1A. There were no significant differences between CMT1A and 1X (p=0.400), but there were differences between CMT1X and 2A (p=0.001) (Table 1).
Figure 1:
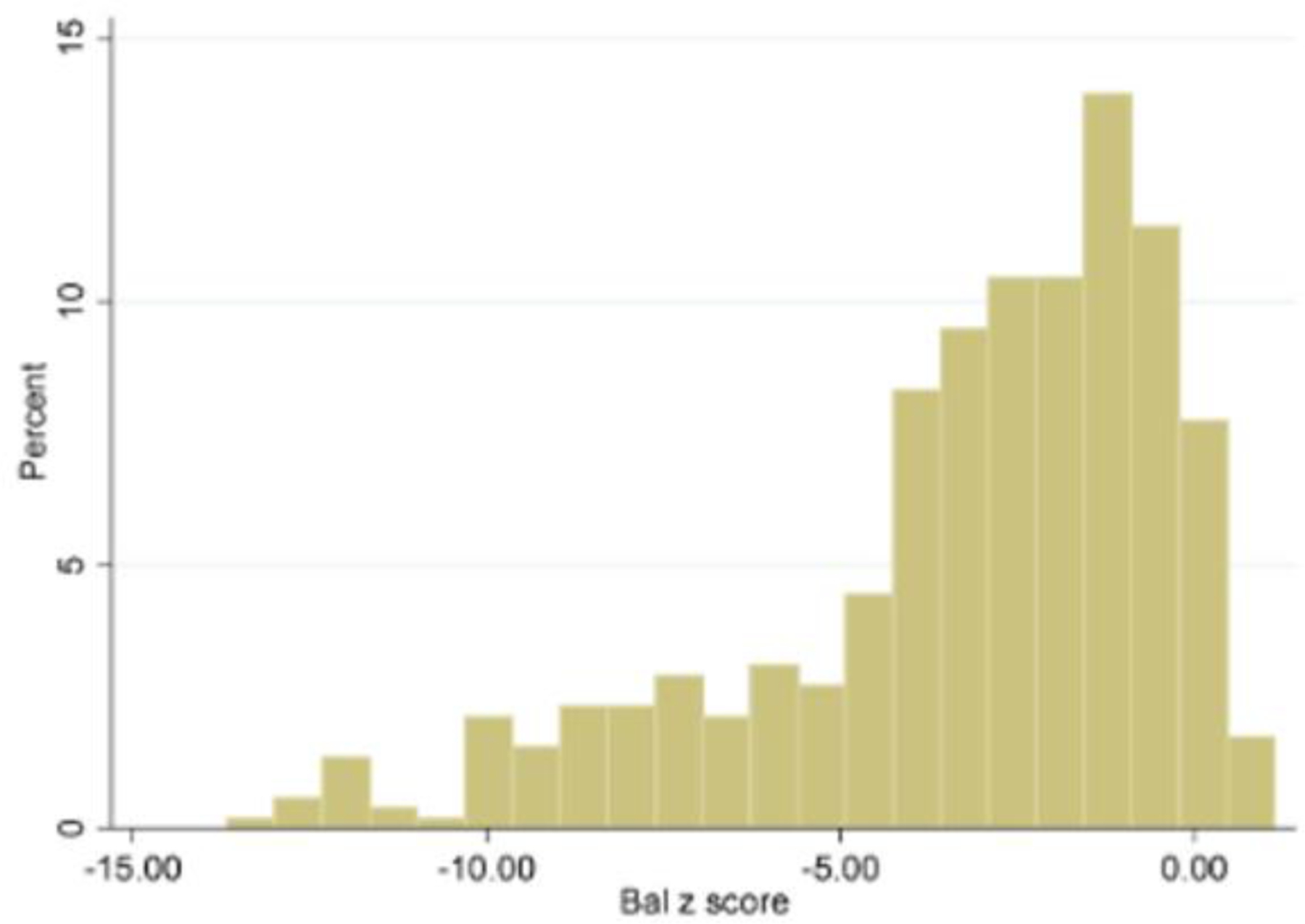
The histogram displays the Bruininks Oseretsky Test of Motor Proficiency 2nded. (BOT-2) balance Z-score distribution of our Charcot-Marie-Tooth (CMT) cohort which is left skewed with the center around −3.25 and a wide spread that ranges from −13.67 to 1.14.
Table 1.
Balance, Foot Alignment, and Function in Pediatric CMT
| Functional Test | BOT-2 Balance Associations | Performance Measures Z- Score & S.D.by Sub-type | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (r) | p-value | Z-Score | 1A | 1B | 2A | 4C | 1X | |||
| 6 Minute Walk Test | 0.51 | <0.001 | −3.2 ± 2.91 | −2.4±2.4 | −4.3±3.7 | −5.1±3.7 | −4.9±3.1 | −1.7±1.6 | ||
| Long Jump | 0.58 | <0.001 | −3.1 ± 1.79 | −2.6±1.4 | −3.5±2.0 | −3.8±2.0 | −4.6±2.0 | −2.0±1.2 | ||
| BOT - 2 | −2.2±2.2 | −4.1±2.9 | −5.1±3.3 | −5.7±3.4 | −2.0±1.9 | |||||
| Foot Alignment and Performance Measures | ||||||||||
| Functional Test | Foot Alignment | Z- Score & S.D. | Group Differences of significance | |||||||
| Bot −2 Balance Subtest | Neutral n= 218 | −3.03 ± 2.99 | Neutral – Non-Neutral p=0.003* Neutral – Planus p= 0.002* |
|||||||
| Pes Cavus n= 233 | −3.28 ± 2.97 | |||||||||
| Pes Planus n= 65 | −3.86 ± 2.78 | |||||||||
| 6 Minute Walk Test | Neutral n= 216 | −2.96 ± 2.93 | Neutral – Non-Neutral p=0.002* Neutral – Cavus p= 0.005* |
|||||||
| Pes Cavus n= 229 | −3.47 ± 2.86 | |||||||||
| Pes Planus n=61 | −3.00 ± 2.99 | |||||||||
| Long Jump | Neutral n= 217 | −2.88 ± 1.75 | Neutral – Non-Neutral p=0.003* | |||||||
| Pes Cavus n= 227 | −3.27 ± 1.79 | |||||||||
| Pes Planus n= 64 | −3.04 ± 1.89 | |||||||||
Bonferroni adjusted p value= 0.008
Association of impairment, activity, and performance related components
Vibratory sensation, pinprick sensitivity, ankle dorsiflexion strength, and foot alignment were significantly associated with BOT-2 balance performance. Vibratory sensation, plantar flexion strength, dorsiflexion strength, and BOT −2 balance were significantly associated with 6 minute walk test performance. BOT −2 balance, plantar flexion strength, and vibratory sensation were significantly associated with long jump (Figure 2). There was no association between performance on the lunge test and BOT-2 balance, long jump, and six minute walk test.
Figure 2:
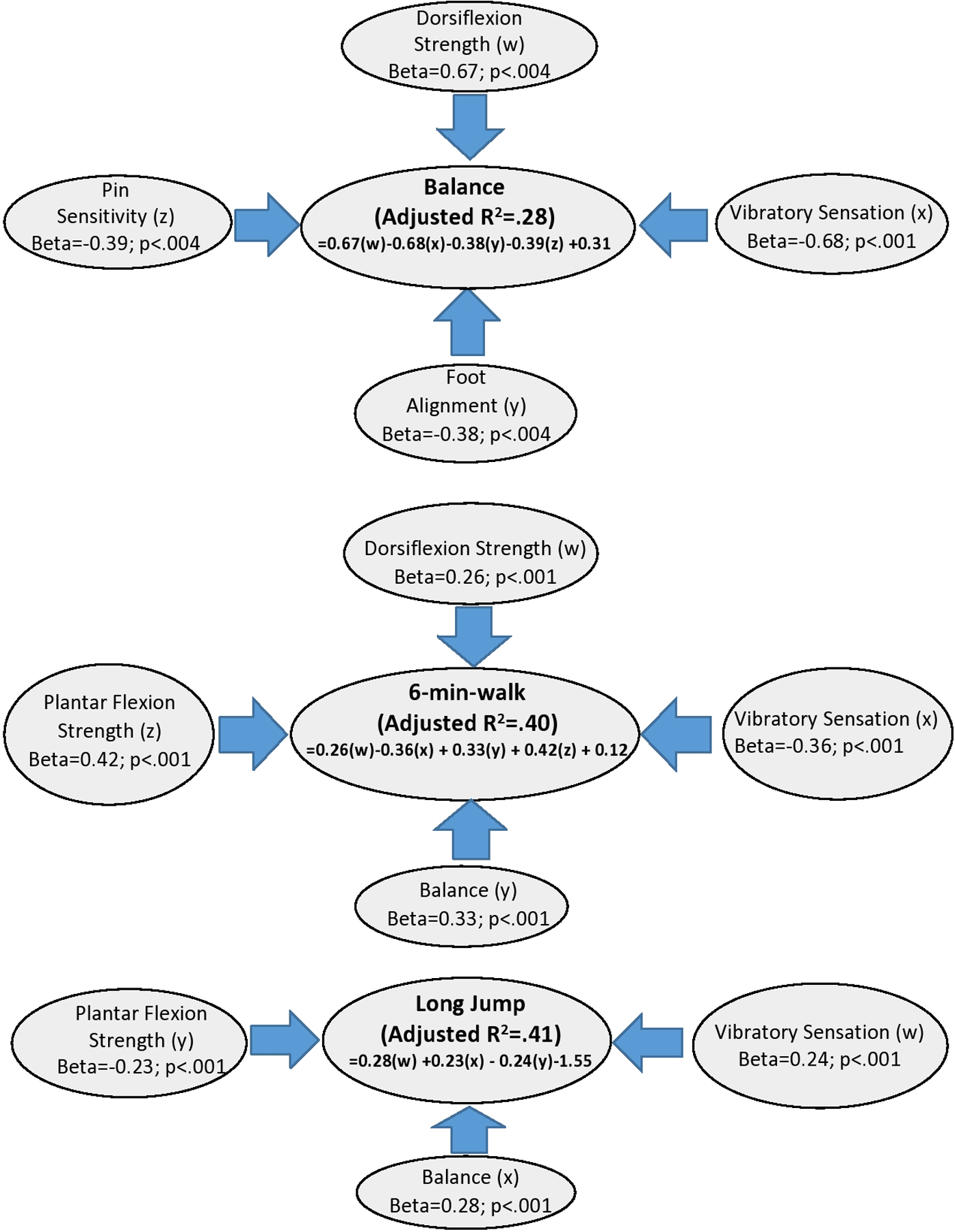
Step-wise regression results of Bruininks Oseretsky Test of Motor Proficiency 2nded. (BOT-2) balance, 6 minute walk test, and long jump. Identifies impairments for each functional performance outcome.
Impact of foot alignment
Children and adolescents with a neutral foot alignment performed best on all functional measures. Neutral alignment was significantly different than planus/cavus alignment based on long jump, 6-min walk, and BOT-2 balance performance. Comparison of the three alignment groups (neutral, planus, and cavus) showed significant differences between the neutral and planus groups with respect to balance, and neutral and cavus groups with respect to the 6 minute walk test, but not for long jump (p=0.08) (Table 1). Those with cavus feet performed the worst on the long jump and 6 minute walk test. There was no significant difference in FPI scores between CMT subtypes or between limbs (p=0.06). Although normal, females had a more cavus foot (0.6 ± 4.1) as compared to males (FPI: male: (1.9 ± 4.3), p<0.001). Foot alignment was also significantly associated with age, where it became more cavoid over time (Figure 3).
Figure 3:
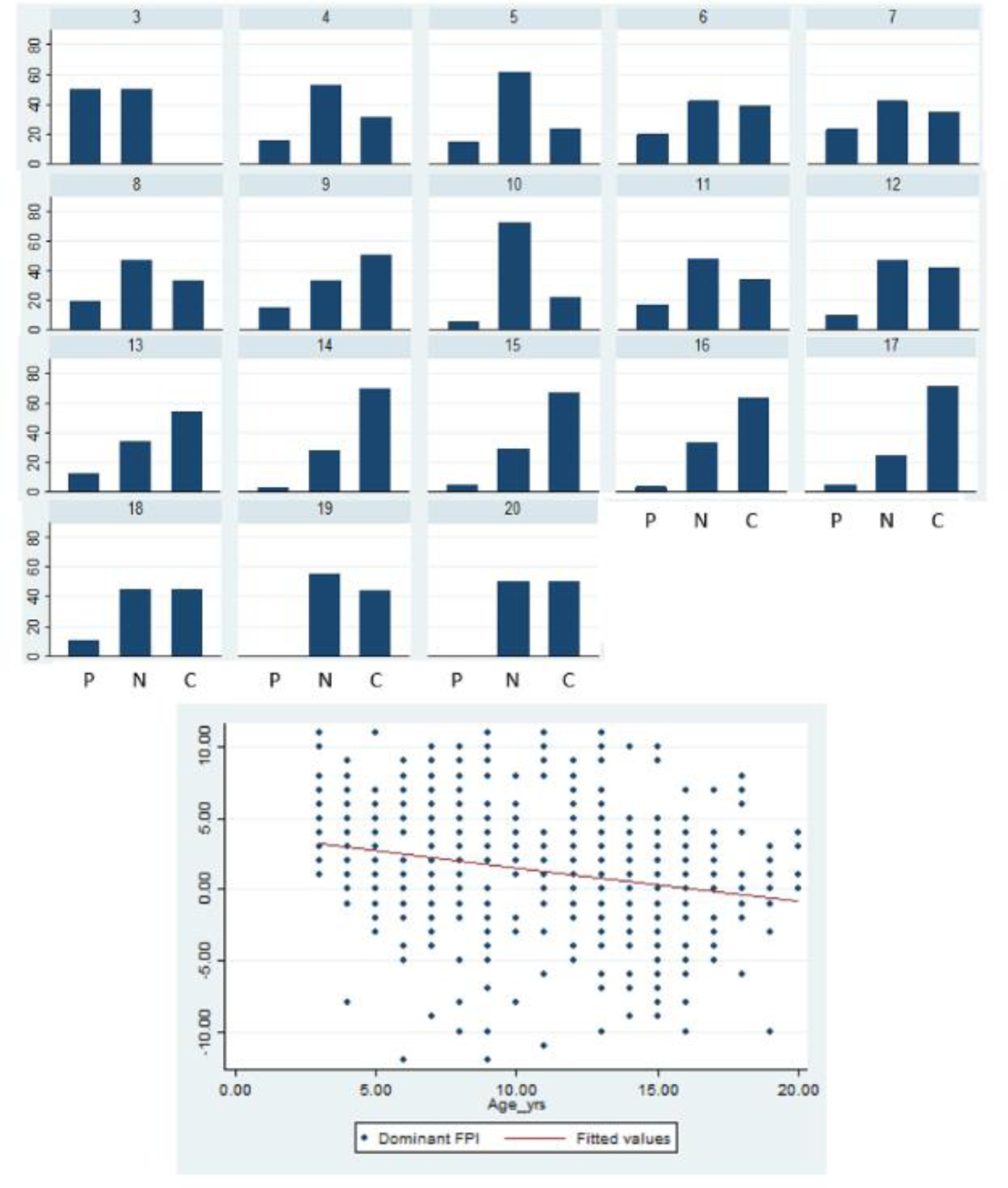
The histogram illustrates the prevalence of foot alignment groups (Pes Cavus, Neutral, Pes Planus) by age. The scatterplot demonstrates the wide variability of Foot Posture Index (FPI) scores across our cohort and association between age and foot alignment, which is more cavoid in older children.
Impact of vision occlusion on balance
Individual BOT-2 balance item raw times increased (improved) with age for both CMT and NORM groups; however, when factoring in the impact of visual input, opposite trends were observed for 4/6 regressions. With increasing age, healthy peers showed a decrease in difference between time achieved with eyes open (EO) and eyes closed (EC) (TEo-Ec), as opposed to those with CMT who show an increase in TEo-Ec (Figure 4). Age/sex matched mean TEo-Ec comparisons were as follows: standing with feet apart was significantly different (p<0.05) for 24/26 of the respective age/sex groups; all groups 11 years and older were significantly different on single leg stance (12/24 groups overall); 3/24 groups differed significantly on single leg stance while on a beam.
Figure 4:
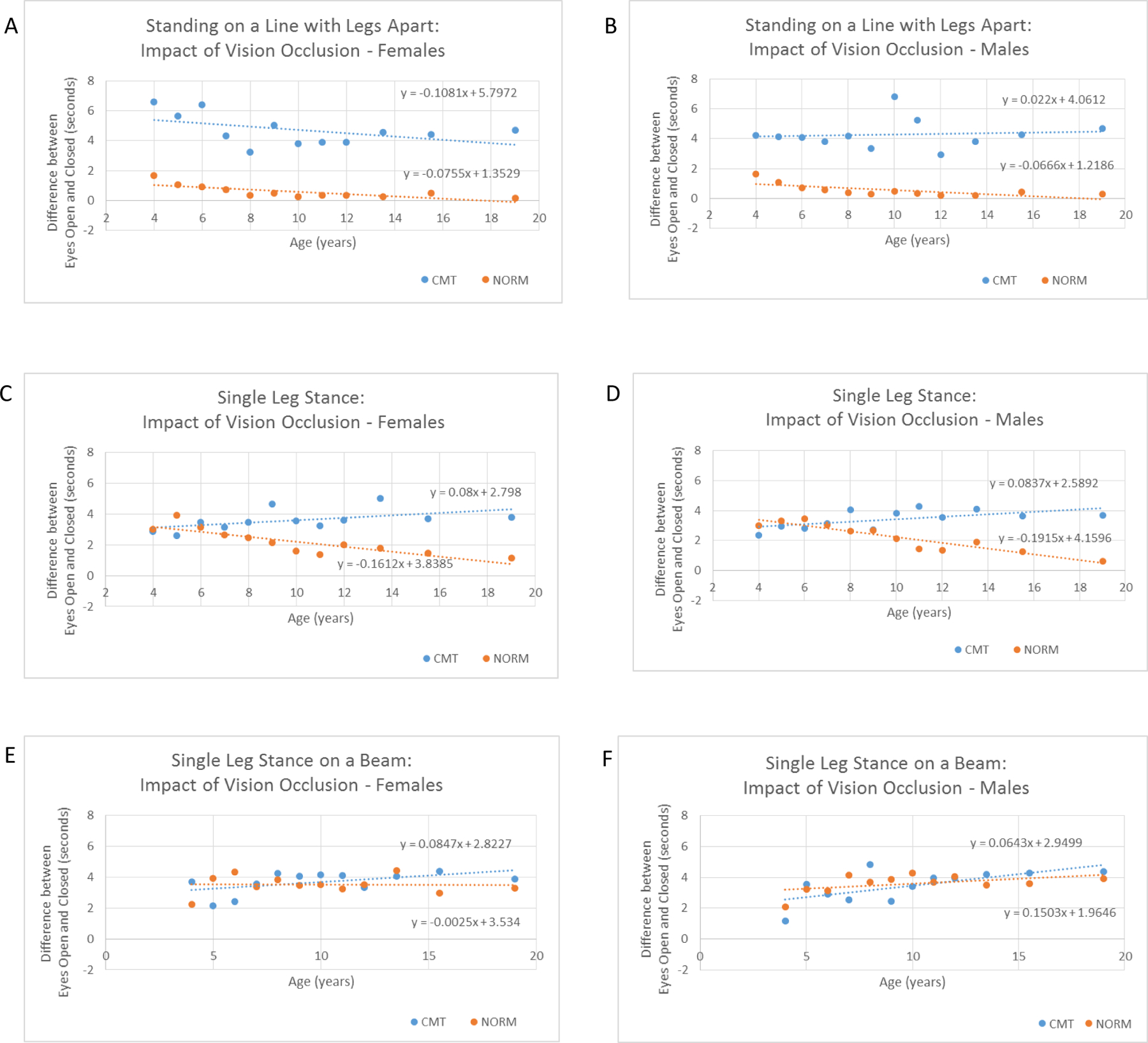
The regression equations utilize mean age/group (TEo-Ec) times to illustrate the change in balance performance over time between the Charcot-Marie-Tooth (CMT) and age and sex-matched normative reference (NORM) groups.
Discussion
Evolution of balance in CMT
Children with CMT, like their typically developing peers, show an increase in their raw times for the BOT-2 balance items as they age and begin to integrate their visual, vestibular, and somatosensory systems. However, from the age of 4, the youngest we can compare to NORM, they show balance deficits that increase with age, despite the continued integration of their visual and vestibular systems. This early balance deficit we identify is consistent with previous findings of increased falls for 4 year old children with CMT, as compared to their typically developing peers (2.2 vs. 0.004 falls per day)11. This is likely due to the progressive sensory impairment that can manifest in CMT.
To further explore the role of visual input in the setting of balance development in CMT, we analyzed the impact of occluding vision. Younger, typically developing, children under the age of 7 years primarily depend on vision to maintain standing6–9 and qualitative balance differences can be observed up to 12 years of age when vision is occluded 5–7. As a result of this visual dependence, the balance time difference with eyes open vs. eyes closed was more similar between typically developing children and those with CMT up to around 6 years of age for the standing with feet apart item, and 11 years for single leg stance; where children with CMT diverge from the typical population and significant time differences exist.
The normative data available show that older children have more similar standing balance times with eyes open and eyes closed as they age. In our population of children with CMT the opposite was true and the older children were progressively more limited in their standing balance times with their eyes closed as compared to with eyes open in a number of tests. Of interest, is the possibility that the difference noted between the typically developing group and the children with CMT may suffer from a ceiling effect in the typically developing group, where at many ages, the balance cut off maximum time of 10 seconds is easily reached. This factor would have the effect of diminishing any association that was found. It appears that children with CMT are able to utilize visual and vestibular input to compensate somewhat for a lack of appropriate somatosensory feedback.
Impact of decreased somatosensory feedback
The (TEo-Ec) measure, which assesses somatosensory impairment, was associated with the 6 min walk test linking it with ambulation ability, an important function. This impairment can result in difficulty navigating uneven surfaces (grass, ball fields, city sidewalk, etc.) impact the ability to maintain balance in reduced vision environments (night time, dimly lit areas, bathing etc.), create social challenges (difficulty maintaining conversation while walking) and limit the ability to multi-task, as focused visual attention is required to accommodate for the somatosensory impairment.
Phenotypic Variation
CMT is divisible into three large sub-groups; CMT1 (dominantly inherited demyelinating neuropathies), CMT2 (dominantly inherited axonal neuropathies), and CMT4 (recessively inherited neuropathies). Mutations in more than 90 genes cause CMT1, CMT2 and CMT4 and different subtypes are given a letter based on the causal gene33. We recognized there may be significant differences between CMT subtypes with respect to balance and that some types of CMT present with a more severe course. Here we establish significant differences in balance between CMT1A and 1B, 2A, and 4C. Although CMT1X was not different from CMT1A, possibly due to the later onset of symptom progression, it did demonstrate a significant difference when compared to CMT 2A. These findings allow clinicians to provide anticipatory guidance based on CMT subtype and may be helpful in selecting appropriate patient populations for clinical trials.
Clinical Implications
The early onset of balance deficits and resultant impact on ambulation demonstrated here support the need for early therapeutic intervention for children with CMT. The underlying impairments in strength (dorsiflexion) and sensation (vibratory and pinprick) in addition to foot alignment explained 28% of the variance in balance and are consistent with findings from previous work identifying balance and foot dorsiflexion strength as having fair negative correlations with total falls11. This forms a basis for understanding the factors that contribute to balance in CMT, which is significantly associated with ambulation, as measured by the 6 minute walk test, and creates a framework to begin to investigate causative relationships and to create a context in which one might frame an investigation of an impairment based treatment approach with a focus on those impairments that might be impacted with exercise34 or appropriate orthotic management35. Additionally, interventions targeted at habilitating the visual and vestibular systems may help those with CMT to compensate better for their balance deficit, and would be important to study.
Along with the developmental and sensorimotor aspects of balance, the impact of structural changes to the lower extremity need to be considered as well. Most typically developing children have a neutral or slightly pronated foot alignment as a base of support, with no sex differences noted36. However, in children with CMT, we observed (and expected) that with increasing age, the neutral alignment of the foot is disturbed and posture shifts toward a more supinated foot. In addition those with more of a cavus foot deformity performed worse on the standing long jump and 6 minute walk test measures. This association of foot posture with balance - when sensation, strength and range of motion were controlled - brings up questions related to how the tripod representation of foot function relates to balance.
In typically developing adults, foot alignment is related to unilateral sway velocity.37 The foot postures investigated here; pronation, neutral, and supination, each create different plantar pressure distributions38. In addition, the length tension relationships of the agonist and antagonist prime movers are altered in each postural deviation. As a result there is a potential impact on muscle force generating capacity and resulting plantar pressure39. Other studies have looked at balance in disorders such as Down syndrome that include pronation and have found that orthotic management of foot posture improves balance measured with the BOT-2 balance sub test and function on the GMFM39. Others have evaluated balance and gait in idiopathic flat foot and found a relationship between foot posture and sway.37 An impact of foot orthoses on medial lateral sway40 and a variety of frontal plane gait parameters41 has been identified. In the CMT population the cavus foot can be associated with pain and increased fore foot pressure and pressure along the lateral margin of the mid foot42,43 and may limit activity.
Limitations and ongoing research
The current investigation expands the literature on the contribution of the biomechanics of the foot to standing balance. The associations between sensory/motor impairment, foot deformity and balance identified here in children with CMT provide the basis for investigating rational therapeutic interventions to address strength, flexibility, and foot posture to improve balance and ambulation in children with CMT. This cross-sectional data is limited in its predictive abilities, and longitudinal studies are needed to better understand how changes in sensorimotor impairment and foot structure relate to changes in balance and performance measures. We have continued to collect longitudinal data in this cohort, and plan to analyze the data to further explore the relationships discussed in this paper. Our exploration of visual, vestibular, and somatosensory integration and its impact on CMT also was limited, and we have since started collecting pilot data on a subgroup of patients with CMT1A using computerized balance assessment (Natus Medical Incorporated, Pleasanton, California) to better understand how children with CMT integrate these systems.
Summary
In summary, children with CMT frequently exhibit quantifiable deficits in balance function early in the course of their disease that varies in degree according to CMT subtype and sensorimotor impairment. Balance dysfunction in childhood CMT is increasingly apparent at older ages, with more pronounced differences in balance with eyes open versus closed. Examination of the relationships between functional and impairment level (strength, range of motion, sensation, etc.) measures has allowed the creation of a framework to conceptualize the development of multimodal rehabilitation strategies that target balance and ambulation in children with CMT.
Acknowledgement:
This study was supported by grant U54NS065712 from the National Institutes of Neurological Diseases and Stroke and office of Rare Diseases (Drs Herrmann, Pareyson, Muntoni, Burns, Laurá, M.E. Shy, Finkel, and Yum); grant G0601943 from the Medical Research Council (Dr Reilly); the National Institute for Health Research University College London Hospitals Biomedical Research Centre (Drs Reilly and Laurá); the Medical Research Council Neuromuscular Centre, the National Institute for Health Research Biomedical Research Centre at Great Ormond Street Hospital for Children National Health Service Foundation Trust, and University College London (Dr Muntoni); the Charcot-Marie-Tooth Association (Dr Muntoni); the Muscular Dystrophy Association (Drs Muntoni and Burns); the National Health and Medical Research Council of Australia, Centre of Research Excellence 1031893 (Dr Burns); Charcot-Marie-Tooth Association of Australia (Dr Burns); the Natural Sciences and Engineering Research Council of Canada (Ms Cornett); and the University of Sydney International Scholarship (Ms Cornett
Abbreviations:
- BOT-2
Bruininks Oseretsky Test of Motor Proficiency 2nded. Balance Sub-test
- CMT
Charcot-Marie-Tooth
- CMTPedS
Charcot-Marie-Tooth disease Pediatric Scale
- CMTNS
CMT Neuropathy Score
- EC
Eyes closed
- EO
Eyes open
- TEO_EC
Time difference between eyes open and eyes closed conditions
- NORM
Age and sex-matched normative reference values
Footnotes
CMTPedS Study group: Kayla MD Cornett, PhD, Manoj P Menezes, PhD, Robert Ouvrier, MD, Gyula Acsadi, MD, PhD, Rosemary R Shy, MD, Daniela Calabrese, Maria Foscan, Roberta Sala, Isabella Moroni,MD, Emanuela Pagliano, MD, Davide Pareyson, MD,Timothy Estilow, OTR/L, Sabrina W Yum, MD, TruptiBhandari, PT, Francesco Muntoni, MD, FRCPCH, Matilde Laura, PhD, Mary M Reilly, MD, FRCP, Richard SFinkel, MD, Kate J Eichinger, DPT, David N Herrmann, MBBCh, Paula Bray, PhD, Kristy Rose, PhD, Mark Halaki, PhD, Julie Pallant, PhD, Monkol Lek, PhD, Michael E Shy, MD and Joshua Burns, PhD
Ethical Statement: We confirm that we have read the Journal’s position on issues involved in ethical publishing and affirm that this report is consistent with those guidelines
Disclosure: FM is partly supported by the NIHR GOSH BRC. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
References
- 1.Braathen GJ, Sand JC, Lobato A, Hoyer H, Russell MB. Genetic epidemiology of Charcot-Marie-Tooth in the general population. Eur J Neurol 2011;18(1):39–48. [DOI] [PubMed] [Google Scholar]
- 2.Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth’s disease. Clin Genet 1974;6(2):98–118. [DOI] [PubMed] [Google Scholar]
- 3.Tozza S, Aceto MG, Pisciotta C, Bruzzese D, Iodice R, Santoro L, Manganelli F. Postural instability in Charcot-Marie-Tooth 1A disease. Gait Posture 2016;49:353–357. [DOI] [PubMed] [Google Scholar]
- 4.Verbecque E, Vereeck L, Hallemans A. Postural sway in children: A literature review. Gait Posture 2016;49:402–410. [DOI] [PubMed] [Google Scholar]
- 5.Gouleme N, Ezane MD, Wiener-Vacher S, Bucci MP. Spatial and temporal postural analysis: a developmental study in healthy children. Int J Dev Neurosci 2014;38:169–177. [DOI] [PubMed] [Google Scholar]
- 6.Forssberg H, Nashner LM. Ontogenetic development of postural control in man: adaptation to altered support and visual conditions during stance. J Neurosci 1982;2(5):545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rinaldi NM, Polastri PF, Barela JA. Age-related changes in postural control sensory reweighting. Neurosci Lett 2009;467(3):225–229. [DOI] [PubMed] [Google Scholar]
- 8.Shumway-Cook A, Woollacott MH. The growth of stability: postural control from a development perspective. J Mot Behav 1985;17(2):131–147. [DOI] [PubMed] [Google Scholar]
- 9.Woollacott M, Debu B, Mowatt M. Neuromuscular control of posture in the infant and child: is vision dominant? J Mot Behav 1987;19(2):167–186. [DOI] [PubMed] [Google Scholar]
- 10.Eichinger K, Odrzywolski K, Sowden J, Herrmann DN. Patient Reported Falls and Balance Confidence in Individuals with Charcot-Marie-Tooth Disease. J Neuromuscul Dis 2016;3(2):289–292. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy RA, Carroll K, Hepworth G, Paterson KL, Ryan MM, McGinley JL. Falls in paediatric Charcot-Marie-Tooth disease: a 6-month prospective cohort study. Arch Dis Child 2018. [DOI] [PubMed] [Google Scholar]
- 12.Nardone A, Grasso M, Schieppati M. Balance control in peripheral neuropathy: are patients equally unstable under static and dynamic conditions? Gait Posture 2006;23(3):364–373. [DOI] [PubMed] [Google Scholar]
- 13.Nardone A, Tarantola J, Miscio G, Pisano F, Schenone A, Schieppati M. Loss of large-diameter spindle afferent fibres is not detrimental to the control of body sway during upright stance: evidence from neuropathy. Exp Brain Res 2000;135(2):155–162. [DOI] [PubMed] [Google Scholar]
- 14.Kars HJ, Hijmans JM, Geertzen JH, Zijlstra W. The effect of reduced somatosensation on standing balance: a systematic review. J Diabetes Sci Technol 2009;3(4):931–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu YS, Kuan CC, Young YH. Assessing the development of balance function in children using stabilometry. Int J Pediatr Otorhinolaryngol 2009;73(5):737–740. [DOI] [PubMed] [Google Scholar]
- 16.Cornett KMD, Menezes MP, Shy RR, Moroni I, Pagliano E, Pareyson D, Estilow T, Yum SW, Bhandari T, Muntoni F, Laura M, Reilly MM, Finkel RS, Eichinger KJ, Herrmann DN, Bray P, Halaki M, Shy ME, Burns J, Group CMS. Natural history of Charcot-Marie-Tooth disease during childhood. Ann Neurol 2017;82(3):353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornett KM, Menezes MP, Bray P, Halaki M, Shy RR, Yum SW, Estilow T, Moroni I, Foscan M, Pagliano E, Pareyson D, Laura M, Bhandari T, Muntoni F, Reilly MM, Finkel RS, Sowden J, Eichinger KJ, Herrmann DN, Shy ME, Burns J, Inherited Neuropathies C. Phenotypic Variability of Childhood Charcot-Marie-Tooth Disease. JAMA Neurol 2016;73(6):645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burns J, Ouvrier R, Estilow T, Shy R, Laura M, Pallant JF, Lek M, Muntoni F, Reilly MM, Pareyson D, Acsadi G, Shy ME, Finkel RS. Validation of the Charcot-Marie-Tooth disease pediatric scale as an outcome measure of disability. Ann Neurol 2012;71(5):642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CMTPedS Calculator Volume 2018: Murdoch Children’s Research Institute; 2015. [Google Scholar]
- 20.Redmond AC, Crosbie J, Ouvrier RA. Development and validation of a novel rating system for scoring standing foot posture: the Foot Posture Index. Clin Biomech (Bristol, Avon) 2006;21(1):89–98. [DOI] [PubMed] [Google Scholar]
- 21.O’Shea S, Grafton K. The intra and inter-rater reliability of a modified weight-bearing lunge measure of ankle dorsiflexion. Man Ther 2013;18(3):264–268. [DOI] [PubMed] [Google Scholar]
- 22.Burns J, Redmond A, Ouvrier R, Crosbie J. Quantification of muscle strength and imbalance in neurogenic pes cavus, compared to health controls, using hand-held dynamometry. Foot Ankle Int 2005;26(7):540–544. [DOI] [PubMed] [Google Scholar]
- 23.Shy ME, Blake J, Krajewski K, Fuerst DR, Laura M, Hahn AF, Li J, Lewis RA, Reilly M. Reliability and validity of the CMT neuropathy score as a measure of disability. Neurology 2005;64(7):1209–1214. [DOI] [PubMed] [Google Scholar]
- 24.Murphy SM, Herrmann DN, McDermott MP, Scherer SS, Shy ME, Reilly MM, Pareyson D. Reliability of the CMT neuropathy score (second version) in Charcot-Marie-Tooth disease. J Peripher Nerv Syst 2011;16(3):191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deitz JC, Kartin D, Kopp K. Review of the Bruininks-Oseretsky Test of Motor Proficiency, Second Edition (BOT-2). Phys Occup Ther Pediatr 2007;27(4):87–102. [PubMed] [Google Scholar]
- 26.Castro-Pinero J, Gonzalez-Montesinos JL, Mora J, Keating XD, Girela-Rejon MJ, Sjostrom M, Ruiz JR. Percentile values for muscular strength field tests in children aged 6 to 17 years: influence of weight status. J Strength Cond Res 2009;23(8):2295–2310. [DOI] [PubMed] [Google Scholar]
- 27.Rose KJ, Burns J, North KN. Relationship between foot strength and motor function in preschool-age children. Neuromuscul Disord 2009;19(2):104–107. [DOI] [PubMed] [Google Scholar]
- 28.Geiger R, Strasak A, Treml B, Gasser K, Kleinsasser A, Fischer V, Geiger H, Loeckinger A, Stein JI. Six-minute walk test in children and adolescents. J Pediatr 2007;150(4):395–399, 399 e391–392. [DOI] [PubMed] [Google Scholar]
- 29.Lammers AE, Hislop AA, Flynn Y, Haworth SG. The 6-minute walk test: normal values for children of 4–11 years of age. Arch Dis Child 2008;93(6):464–468. [DOI] [PubMed] [Google Scholar]
- 30.Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166(1):111–117. [DOI] [PubMed] [Google Scholar]
- 31.McDonald CM, Henricson EK, Han JJ, Abresch RT, Nicorici A, Elfring GL, Atkinson L, Reha A, Hirawat S, Miller LL. The 6-minute walk test as a new outcome measure in Duchenne muscular dystrophy. Muscle Nerve 2010;41(4):500–510. [DOI] [PubMed] [Google Scholar]
- 32.Bruininks RHB, B.D. Bruininks-Oseretsky Test of Motor Proficiency. 2nd ed. . Minneapolis, MN NCS Pearson; 2005. [Google Scholar]
- 33.Fridman V, Bundy B, Reilly MM, Pareyson D, Bacon C, Burns J, Day J, Feely S, Finkel RS, Grider T, Kirk CA, Herrmann DN, Laura M, Li J, Lloyd T, Sumner CJ, Muntoni F, Piscosquito G, Ramchandren S, Shy R, Siskind CE, Yum SW, Moroni I, Pagliano E, Zuchner S, Scherer SS, Shy ME, Inherited Neuropathies C. CMT subtypes and disease burden in patients enrolled in the Inherited Neuropathies Consortium natural history study: a cross-sectional analysis. J Neurol Neurosurg Psychiatry 2015;86(8):873–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sman AD, Hackett D, Fiatarone Singh M, Fornusek C, Menezes MP, Burns J. Systematic review of exercise for Charcot-Marie-Tooth disease. J Peripher Nerv Syst 2015;20(4):347–362. [DOI] [PubMed] [Google Scholar]
- 35.Najafi B, Wrobel JS, Burns J. Mechanism of orthotic therapy for the painful cavus foot deformity. J Foot Ankle Res 2014;7(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jimenez-Cebrian AM, Morente-Bernal MF, Roman-Bravo PD, Saucedo-Badia JF, Alonso-Rios JA, Montiel-Luque A. Influence of Age, Sex, and Anthropometric Determinants on the Foot Posture Index in a Pediatric Population. J Am Podiatr Med Assoc 2017;107(2):124–129. [DOI] [PubMed] [Google Scholar]
- 37.Angin S, Ilcin N, Yesilyaprak SS, Simsek IE. Prediction of postural sway velocity by foot posture index, foot size and plantar pressure values in unilateral stance. Eklem Hastalik Cerrahisi 2013;24(3):144–148. [DOI] [PubMed] [Google Scholar]
- 38.Mohd Said A, Justine M, Manaf H. Plantar Pressure Distribution among Older Persons with Different Types of Foot and Its Correlation with Functional Reach Distance. Scientifica (Cairo) 2016;2016:8564020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanchez-Rodriguez R, Martinez-Nova A, Escamilla-Martinez E, Pedrera-Zamorano JD. Can the Foot Posture Index or their individual criteria predict dynamic plantar pressures? Gait Posture 2012;36(3):591–595. [DOI] [PubMed] [Google Scholar]
- 40.Rome K, Brown CL. Randomized clinical trial into the impact of rigid foot orthoses on balance parameters in excessively pronated feet. Clin Rehabil 2004;18(6):624–630. [DOI] [PubMed] [Google Scholar]
- 41.Novick A, Kelley DL. Position and Movement Changes of the Foot with Orthotic Intervention during the Loading Response of Gait. J Orthop Sports Phys Ther 1990;11(7):301–312. [DOI] [PubMed] [Google Scholar]
- 42.Crosbie J, Burns J, Ouvrier RA. Pressure characteristics in painful pes cavus feet resulting from Charcot-Marie-Tooth disease. Gait Posture 2008;28(4):545–551. [DOI] [PubMed] [Google Scholar]
- 43.Burns J, Crosbie J, Ouvrier R, Hunt A. Effective orthotic therapy for the painful cavus foot: a randomized controlled trial. J Am Podiatr Med Assoc 2006;96(3):205–211. [DOI] [PubMed] [Google Scholar]


