Abstract
Purpose:
As per the recent World Health Organization estimates, approximately 2.2 billion people have near and distance vision impairment (VI) globally, and out of this almost 50% is avoidable.
Methods:
The Rapid Assessment of Avoidable Visual Impairment survey was a cross-sectional study conducted in September 2021, using cluster random sampling in 42 clusters with a cluster size of 140, giving a total of 6000 participants. Two teams comprising of trained optometrists and social workers conducted the ocular examination which included unaided, pinhole, and aided visual acuity assessments followed by examination of the anterior segment and lens. Distance visual acuity was measured using simplified tumbling “E” charts of different sizes for VA of 6/12, 6/18, and 6/60. The lens assessment was done in an un-dilated pupil with torch light by the optometrist.
Results:
Overall, 6520 individuals aged 6 years and above were enumerated, of whom 5440 (83.4%) were examined. The response rate for examination was better among females (93.1%) than males (73.9%), and it decreased from 93.8% in the age group 6–15 years to 77.1% in the 45+ age group. The prevalence of blindness and VI were 0.18% (95% CI: 0.06–0.29) and 4.19% (95%CI: 3.65–4.72), respectively. The major causes of VI in all age groups were uncorrected refractive error (65.4%), cataract (23.7%), cataract surgical complications (2.6%), corneal opacity (0.4%), and other posterior segment diseases (7.5%). The effective cataract surgical coverage (eCSC) was 61.8%, effective refractive error coverage (eREC) for distance vision was 59.8%, and eREC for near vision was 47.0%.
Conclusion:
The RAAVI methodology is suitable to measure effective coverage in the general population, both for baseline measurement and periodic monitoring. The 2030 targets for the surveyed district are 90% eCSC and 100% eREC. Such exercises need to be conducted in each district of the country to determine the baseline and target values of effective coverage.
Keywords: Cataract surgery, effective coverage, rapid assessment, refractive error
Vision impairment (VI), including blindness, is a serious public health problem globally with a disproportionately larger prevalence in low- to middle-income countries (LMICs).[1] World Health Organization (WHO) and World Bank Report on Universal Health Coverage (UHC) have released a list of 13 indicators, known as the Eye Care Indicator Menu (ECIM), which are important indicators for eye health and for monitoring progress toward UHC. Two of these indicators are effective cataract surgical coverage (eCSC) and effective refractive error coverage (eREC). These indicators not only capture the magnitude of coverage but also bring in the concept of “effective” coverage to ensure that people who need health services receive them with sufficient quality to produce the desired gain in vision.[2] The targets are a 30 percentage point increase in eCSC and a 40 percentage point increase in eREC by 2030.[3] Countries with baseline eCSC rates of 60% or higher and eREC rates of 70% or higher should strive for universal coverage.[4] It is vital that all countries have baseline data on eCSC and eREC to track progress and meet the above-mentioned UHC eye health targets by 2030. Unfortunately, most countries in the South-East Asia Region (SEAR) do not have this data yet.
As the denominator for these indicators is the population, therefore, the information can only be generated from population-based surveys. Regular hospital service or program output data in terms of the number of people treated will not be able to give this information. These indicators are relatively new, therefore, traditional population-based surveys do not include this in their data collection and report generation.
The most popular population-based survey methodology to obtain this information is the Rapid Assessment of Avoidable Blindness (RAAB) survey. The RAAB survey version 6 provides information on eCSC only, but the new version RAAB-7 officially launched in 2021 and was piloted in many countries in 2019 and 2020 provided detailed information on eCSC and eREC for 50 + age group. The RAAB survey methodology provides information on eCSC and eREC for 50 + age group only. It is estimated that more than 80% of cataracts occur in this age group, so eCSC data generated from the RAAB survey can be considered as representative of the total population. Similarly, the information generated for eREC—near vision impairment/presbyopia will also be representative of that population. However, distance vision impairment due to refractive error occurs in all ages and myopia is increasing in the age group of 9–15 years. Hence, RAAB survey information on the elderly age group needs to be cautiously generalized or may not be representative of all populations. In addition, a refractive error study in school children (RESC) using the WHO–RESC survey method can be used to generate eREC information in children. In countries where school enrolment is more than 90%, a well-designed school-based survey is considered as an equivalent to population-based surveys in school-going children. However, the secondary school enrolment in most of the countries of the SEAR region is less than 90%, with a huge gender gap.[5]
Hence, there is a need to develop a new methodology that is valid for all ages and provides valuable information on the coverage of cataract surgical and refractive error services. The rapid assessment of visual impairment (RAVI) survey was also a modification of the RAAB-6 for assessing coverage in the 40+ population.[6,7,8,9,10,11,12,13] However, the new methodology used in the current study would assess coverage in the general population of all ages. This methodology is modified from the RAVI studies and is known as rapid assessment of avoidable visual impairment (RAAVI). This methodology would help researchers in determining coverage of cataract surgery and refractive correction for much of their population. The results from these surveys would help in formulating recommendations to governments that will contribute not only toward achieving UHC, a target within the Sustainable Development Goals (SDGs), but also, the elimination of the main causes of avoidable blindness. Rapid assessment surveys are less expensive and less time-consuming compared to detailed and resource-intensive epidemiological studies. The current study was conducted for field testing of the novel methodology using the RAAVI method in the population aged 6+ years in a pilot district.
The aim of the study was to assess the prevalence and causes of avoidable VI in the district, to estimate the eCSC and eREC including presbyopia, and to develop a tool to assist in planning for increasing coverage in the country.
Methods
Ethical approval for the study was obtained from the Institute Ethics Committee of a tertiary care hospital and the study adhered to the tenets of the Declaration of Helsinki 2015. The study was a cross-sectional study conducted in September 2021 in one of the pilot districts in Northern India selected purposively (Gurugram, Haryana). The sample population was all those who were aged 6 years and above and were habitual residents of the district (living in the area for at least 6 months or more). Population under the age of 5 years is normally free from refractive errors and hence not included in the current study.[14] The sample size was calculated to make the study adequately powered to assess the outcome variables that is prevalence of VI in the 6+ population, eCSC among 50+ age group, eREC (distance) among all age groups, and eREC (near) among 35+ population. The first indicator yielded a sample size of 4648 assuming a 6.43% prevalence of VI in the 6+ population,[14] 80% power, 95% confidence interval, 15% relative error, 1.5 design effect for cluster sampling, and 20% non-response rate. For the study, blindness was defined as VA <3/60 in the better eye with available correction (PVA) or with best correction or pinhole (BCVA or Pin VA) and VI as VA <6/12 in the better eye with available correction (PVA) or with best correction or pinhole (BCVA or Pin VA).
The eCSC is defined as the proportion of people who have received cataract surgery and have a resultant good quality outcome (presenting visual acuity, PVA 6/12 or better) relative to the number of people in need of cataract surgery (having best corrected visual acuity, BCVA <6/12 due to cataract). Similarly, eREC is defined as the proportion of people who have received refractive error services (i.e., spectacles, contact lenses, or surgery) and have a resultant good quality outcome (PVA 6/12 or better) relative to the number of people in need of refractive error services (having PVA < 6/12 due to refractive error).[4]
Assuming 40% eCSC in 50+ population to be achieved in India, 80% power, 95% confidence interval, 8% absolute error, and design effect of 1.5 for clustering effect, the calculated sample size is 217 individuals who constitute the denominator of eCSC equation. However, not all 50+ population need cataract surgery, and it is estimated that only 24% of them need cataract surgery (those having BCVA < 6/12 due to cataracts). Also, the current pilot study is a general population survey among all age groups (excluding the under-5 population) out of which only 19.4% are in the 50+ age group. Hence, the total sample size required in all age groups will be 4661 (217/0.24 * 0.194). Assuming a 20% non-response rate, the final sample size will be 5826, rounded off to 6000.
Like the calculation for eCSC, assuming 30% eREC in 6 + population to be achieved in India, 80% power, 95% confidence interval, 6% absolute error, and design effect of 1.5 for clustering effect, the calculated sample size is 337 individuals who constitute the denominator of eREC equation. Also, the 6+ population constitutes nearly 90% of the age population and only 9% of them need refractive error services.[15] Similarly, the presbyopia population (35+) constitutes nearly 30% of the age population and only 40% of them need presbyopia services for distance vision. Hence, the total sample size after incorporating non-response will be 5200 for near vision and 3500 for distance vision. Hence, the highest sample size is 6000 among all age groups which will be adequate to measure all four indicators with sufficient power.
The sampling strategy was multi-stage cluster random sampling, comprising three stages:
In the first stage of sampling, a list of Primary Sampling Units (PSUs) was prepared within the district, comprising urban wards and villages, employing probability proportionate to size (PPS) sampling. The PSUs have a maximum population size of 1200. In case a village or ward had a population of more than 1200, it was divided into smaller PSUs of size 1200, each of which was independently entered into the sampling frame.
The second stage consisted of the selection of compact segments within the cluster. Within each PSU, the selection of households was done with the help of a compact segment sampling technique. For this, the selected PSU was divided into multiple segments of 200–300 population based on a broad area mapping, in consultation with a local health worker. Each segment had nearly 175 individuals of age 6+ and one segment was chosen randomly by draw of chits.
The third stage comprised the selection of individuals within the selected segment. In the selected segment, the survey proceeded from one corner till all contiguous houses were visited or 140–150 people were enumerated. By covering a total of 42 such segments, the target sample of 6000 in the district was achieved.
There were two survey teams, each comprising two optometrists, one enumerator, two data entry operators, and assisted by one community-based volunteer, like accredited social health activists (ASHA workers). The ASHA workers were given an honorarium of 200 INR per cluster. One ophthalmologist and epidemiologist were responsible for supervising the two survey teams and providing guidance if the optometrists faced any difficulty in diagnosis. Each team was given the target of completing one cluster per day so that two teams working in tandem could complete 42 clusters in 21 working days or 1 month. All COVID-19 appropriate precautions were followed by data collectors for example mask, face-shield, hand-sanitization after clinical examination and social distancing, etc. Before the survey, the ophthalmologist and epidemiologist provided a two-day training to the optometrists and field staff regarding standardized RAVI methodology, cluster selection and coding, enumeration methods, and clinical examination [Fig. 1].
Figure 1.
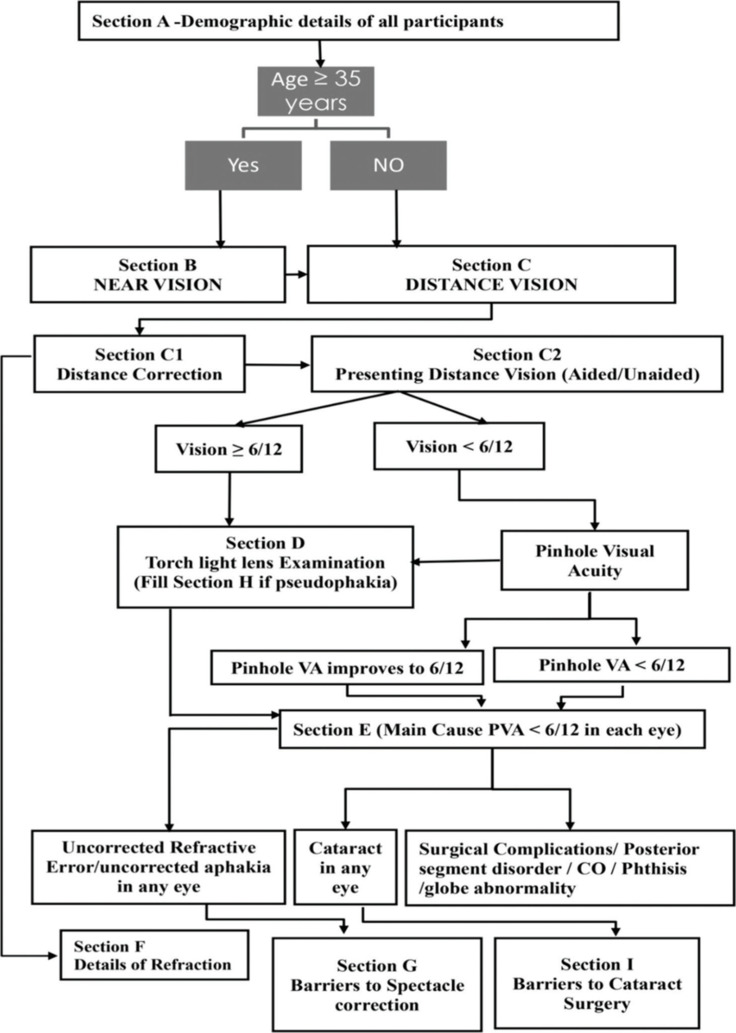
Flowchart showing the steps of the rapid assessment of avoidable visual impairment study
The steps of data collection included: enumeration, online informed consent, demographic profile, near vision testing, distance vision testing, lens examination, and barrier questions. After consent, the use of near glasses/reading glasses was enquired and details about the last refraction were asked. A simplified “E” chart consisting of five letters each of N60 and N6 optotypes was used for the near vision testing and presenting binocular near vision was recorded from 40 cm. The near vision was taken by making the participant read the N60 optotype first followed by the N6 optotype. The criteria for vision at a certain level was four correct out of five letters. After near vision, the use of distant glasses/contact lenses and history of any refractive surgery was enquired. Subsequently, distance VA was tested using the two simplified tumbling “E” charts with available correction and unaided correction. The “E” Snellen optotypes of different sizes for VA of 6/12, 6/18, and 6/60 were used at 6 m. The lens assessment was done in an un-dilated pupil with torch light. The principal disorder responsible for visual loss in each eye as well as in the individual (better eye) was recorded, as per a rank order of diseases that are most amenable to treatment or prevention [Table 1]. When there were two disorders, one of which was secondary to the other, the primary disorder was selected as the principal disorder. However, if there were co-existing primary disorders in the same or different eyes, the one that was most readily curable or preventable was noted.
Table 1.
Case definitions used in the survey for clinical examination
| Rank Order | Cause of Blindness | Case Definition | ||
|---|---|---|---|---|
| 1 | PVA 6/12 | If the patient has presenting distance vision of 6/12 or better in this eye and there is no indication to examine. | ||
| 2 | Uncorrected Refractive error/Uncorrected Aphakia | Phakic/Pseudophakia eyes with PVA <6/12, improving with pinhole or optical correction to 6/12 or better. Aphakia (absence of lens from the central pupil), improving with correction or pinhole to 6/12 or better. | ||
| 3 | Cataract | Obvious lens opacity which is likely to affect vision. Do not mark this option in cases of minor opacities, unlikely to affect vision | ||
| 4 | Cataract surgical Complications | If there is evidence that a surgical procedure has led to blindness for example PCO, secondary glaucoma, bullous keratopathy, etc., then this box should be marked. Uncorrected aphakia must be recorded as above. | ||
| 5 | Corneal opacity | Leucoma, staphyloma, or other easily visible corneal opacity present over the pupil due to any cause. | ||
| 6 | Other causes include posterior segment disease | If the VA <6/12 cannot be attributed to any of the above-mentioned causes, but a specific cause can be identified then this diagnosis will be used (DR, ARMD, Optic atrophy, glaucoma, RP, amblyopia, etc.). | ||
| 7 | Phthisis/Globe abnormalities | Small shrunken globe due to trauma or severe infection. Microphthalmos, anophthalmos, enucleated eye. |
*PVA: Presenting visual acuity, PCO: Posterior capsular opacity, DR: Diabetic retinopathy, ARMD: Age-related macular degeneration, RP: Retinitis pigmentosa
While examining children aged 6–18 years, the following precautions were observed to obtain valid results: assent was obtained from the child followed by online consent from guardian; the distance of 6 m was measured accurately; the tumbling E card was shown to the child and the procedure explained; the eye not under test was properly occluded with pinhole; the team members were trained to be non-threatening to young children; the child was given as much time as he wanted to understand the procedure; and the mother or other familiar adult was kept in attendance.
Inter-observer agreement among the optometrists for clinical diagnosis and for distant and near visual acuity testing was performed in hospital and field settings. An algorithm was used for allocating causes of blindness and visual impairment and the case definitions were the same as those used in the RAAB methodology, except that the number of categories was reduced from thirteen in RAAB to seven in the current study. The category of uncorrected aphakia was merged with uncorrected refractive error, non-trachomatous, and trachomatous corneal opacity were merged, globe abnormalities and phthisis were merged, and the categories of glaucoma, diabetic retinopathy, and age-related macular degeneration were merged with posterior segment disorders [Table 1]. Good inter-observer agreement was found for all survey procedures (kappa >0.8) among optometrists. The data entry was done in a specially designed open data kit (ODK)-based online form with all checks in place for validation and data consistency. Data cleaning was done to remove all inconsistent findings and outliers and analysis was done using the Stata 15.1 software package (Stata Corp., College Station, Texas, USA). Age and gender disaggregated prevalence of VI along with 95% CI were calculated.
Results
Overall, 6520 individuals aged 6+ years were enumerated, of whom 5440 (83.4%) were examined. The response rate for examination was better among females (93.1%) compared to males (73.9%), and it decreased from 93.8% in the age group 6–15 years to 77.1% in the 45+ age group [Table 2]. The mean age of the examined study population was 29.7 (±17.1) years with a range from 6 to 99 years. Among the people examined, nearly three-fourths of the respondents (74.1%) were aged 39 years and below, and nearly 11.5% of respondents were aged 40–49 years. Nearly 1555 (28.6%) of the participants were either illiterate or had received no formal education [Table 2].
Table 2.
Socio-demographic profile of the study population along with response rates (n=6520)
| Variable | Male (%) | Female (%) | Total (%) | Enumerated | Response Rate | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age Group (Years) | ||||||||||
| 6–15 years | 661 (27.1) | 636 (21.2) | 1297 (23.8) | 1382 (21.2) | 93.9% | |||||
| 16–25 years | 600 (24.6) | 642 (21.4) | 1242 (22.8) | 1411 (21.6) | 88.0% | |||||
| 26–35 years | 514 (21.1) | 696 (23.2) | 1210 (22.2) | 1501 (23.0) | 80.6% | |||||
| 36–45 years | 288 (11.8) | 429 (14.3) | 717 (13.2) | 962 (14.8) | 74.5% | |||||
| 45+years | 374 (15.3) | 600 (20.0) | 974 (17.9) | 1264 (19.4) | 77.1% | |||||
| Educational Status | ||||||||||
| Illiterate | 156 (6.4) | 551 (18.3) | 707 (13.0) | 859 (13.2) | 82.3% | |||||
| Up to 4th pass | 400 (16.4) | 448 (14.9) | 848 (15.6) | 916 (14.0) | 92.6% | |||||
| 5th to 9th pass | 669 (27.5) | 872 (29.0) | 1541 (28.3) | 1841 (28.2) | 83.7% | |||||
| 10th and above | 1212 (49.7) | 1132 (37.7) | 2344 (43.1) | 2904 (44.5) | 80.7% | |||||
| Total | 2437 (100) | 3003 (100) | 5440 (100) | 6520 | 83.4% |
Based on the WHO definition of blindness (PVA <3/60 in the better eye), ten individuals were blind, all in the 45+ age group. The prevalence of blindness was 0.18% (95% CI: 0.06–0.29) and that of VI was 4.19% (95% CI: 3.65–4.72) in the 6+ population [Table 3]. The prevalence of blindness in the 45+ age group was 1.03%. The prevalence of VI increased with age from 1.5% in the 6–15 years age group to 16.7% in the 45+ years; however, it decreased as the literacy level of the study participants increased that is from 16.4% in illiterate participants to 2.2% in those educated up to 10th class and above [Table 4]. The prevalence of VI in rural participants was 5.8%, which was higher than that of urban participants (3.6%).
Table 3.
Prevalence of blindness, severe (SVI), moderate (MVI) and mild visual impairment-all causes
| Male | Females | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | |||||||
| Blindness—VA<3/60 in the better eye with available correction (presenting VA) | ||||||||||||
| Blindness | 7 | 0.28 (0.07–0.49) | 3 | 0.09 (0.01–0.21) | 10 | 0.18 (0.06–0.29) | ||||||
| Blind eyes | 42 | 0.86 (0.60–1.12) | 48 | 0.79 (0.57–1.02) | 90 | 0.82 (0.65–0.99) | ||||||
| Severe visual impairment (SVI)—VA<6/60—3/60 in the better eye with available correction | ||||||||||||
| Severe VI | 0 | 0 | 6 | 0.19 (0.04–0.36) | 6 | 0.11 (0.02–0.19) | ||||||
| SVI eyes | 8 | 0.16 (0.05–0.27) | 37 | 0.61 (0.41–0.81) | 45 | 0.41 (0.29–0.53) | ||||||
| Moderate visual impairment (MVI) VA<6/18–6/60 in the better eye with available correction | ||||||||||||
| Moderate VI | 22 | 0.90 (0.52–1.27) | 47 | 1.56 (1.12–2.00) | 69 | 1.26 (0.97–1.56) | ||||||
| MVI eyes | 68 | 1.39 (1.062–1.72) | 132 | 2.19 (1.82–2.56) | 200 | 1.83 (1.58–2.09) | ||||||
| Mild visual impairment (EVI)—VA<6/12–6/18 in the better eye with available correction | ||||||||||||
| Mild VI | 55 | 2.25 (1.66–2.84) | 88 | 2.93 (2.32–3.53) | 143 | 2.62 (2.20–3.05) | ||||||
| Mild VI eyes | 122 | 2.50 (2.06–2.94) | 198 | 3.29 (2.84–3.74) | 320 | 2.94 (2.62–3.25) | ||||||
| Visual impairment (VI)—VA<6/12 in the better eye with available correction | ||||||||||||
| VI | 84 | 3.44 (2.72–4.17) | 144 | 4.79 (4.03–5.55) | 228 | 4.19 (3.65–4.72) | ||||||
| VI eyes | 240 | 4.92 (4.31–5.53) | 415 | 6.90 (6.26–7.55) | 655 | 6.02 (5.57–6.46) | ||||||
Table 4.
Association of visual impairment with socio-demographic variables in the study population
| Age Groups | Male (P%) | Total | Female (P%) | Total | VI | Total | P% (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6–15 years | 11 (1.7) | 661 | 9 (1.4) | 636 | 20 | 1297 | 1.5 (0.9–2.4) | |||||||
| 16–25 years | 9 (1.5) | 600 | 12 (1.9) | 642 | 21 | 1242 | 1.7 (1.1–2.6) | |||||||
| 26–35 years | 2 (0.4) | 514 | 5 (0.7) | 696 | 7 | 1210 | 0.6 (0.2–1.2) | |||||||
| 36–45 years | 4 (1.4) | 288 | 13 (3.0) | 429 | 17 | 717 | 2.4 (1.4–3.8) | |||||||
| 45+years | 58 (15.5) | 374 | 105 (17.5) | 600 | 163 | 974 | 16.7 (14.4–19.2) | |||||||
| Education | ||||||||||||||
| Illiterate | 28 (17.9) | 156 | 88 (16.0) | 551 | 116 | 707 | 16.4 (13.8–19.4) | |||||||
| Up to 4th class | 7 (1.8) | 400 | 7 (1.6) | 448 | 14 | 848 | 1.7 (0.9–2.8) | |||||||
| 5th–9th class | 20 (3.0) | 669 | 27 (3.1) | 872 | 47 | 1541 | 3.1 (2.3–4.0) | |||||||
| 10th pass and above | 29 (2.4) | 1212 | 22 (1.9) | 1132 | 51 | 2344 | 2.2 (1.6–2.9) | |||||||
| Total | 84 (3.4) | 2437 | 144 (4.8) | 3003 | 228 | 5440 | 4.2 (3.7–4.8) |
*P%: Prevalence in percentage, VI: Visually impaired
Among the 10 blind individuals, the causes of blindness were cataracts (6, 60.0%), cataract surgical complications (CSC, 2, 20.0%), posterior segment pathology (1, 10.0%), and corneal opacity (CO, 1, 10.0%). Similarly, out of a total of 228 participants with VI, the causes identified were uncorrected refractive error (URE, 149, 65.4%), cataract (54, 23.7%), posterior segment diseases (17, 7.5%), CSC (6, 2.6%), and CO (1, 0.4%). On segregating the causes of VI by age groups, all the blind and 163 (71.5%) of the VI participants belonged to the 45+ age group. The remaining 65 VI participants belonged to the age groups of 16–25 years (21, 9.2%), 6–15 years (20, 8.8%), 36–45 years (17, 7.5%), and 26–35 years (7, 3.1%). The important causes of VI in the younger age groups (<45 years) were posterior segment pathology (13, 76.5%), URE (50, 33.6%), and cataracts (2, 1.9%) [Fig. 2].
Figure 2.
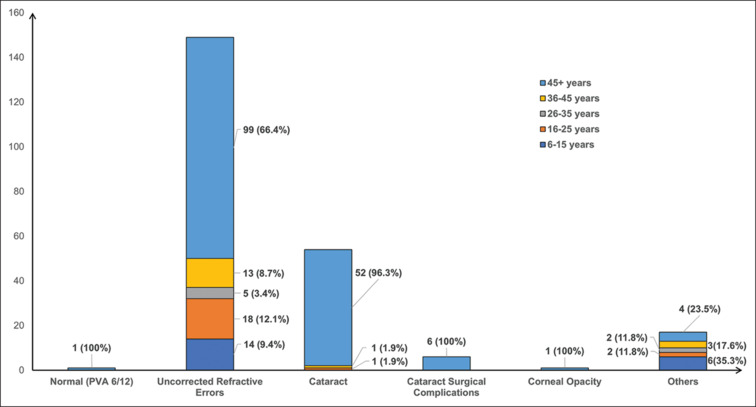
Causes of visual impairment in the study population aged 6 years and above
Cataract surgical coverage (CSC) in the 50+ population among cataract blind persons (PVA < 3/60 in the better eye) was 96.24% (males 93.18% and females 97.75%). The CSC among persons with VA < 6/18 in better eye due to cataract was 87.5% (males 87.76% and females 87.38%). The eCSC among the 50+ population in Gurugram was 61.8% and was higher in females than males (65.25% vs 55.0%) [Table 5].
Table 5.
Effective cataract surgical and effective refractive error coverage in the study population
| Variables for eCSC Calculation | Male | Female | Total | |||
|---|---|---|---|---|---|---|
| Numerator | ||||||
| A=Unilateral operated cataract achieving PVA ≥6/12 in the operated eye and have BCVA <6/12 with cataract as the main cause of vision impairment or blindness in the other eye; | 7 | 14 | 21 | |||
| B=Bilateral operated cataract achieving PVA ≥6/12 in at least one eye | 26 | 63 | 89 | |||
| Denominator | ||||||
| C=Unilateral operated cataract and BCVA <6/12 with cataract as the main cause of vision impairment or blindness in the other eye | 12 | 19 | 31 | |||
| D=Bilateral operated cataract, regardless of visual acuity | 31 | 73 | 104 | |||
| E=BCVA<6/12 with cataracts as the main cause of vision impairment or blindness in both eyes | 17 | 26 | 43 | |||
| eCSC=(a+b/c+d+e) *100 (%) | 55.0 | 65.25 | 61.80 | |||
| Variables for eREC for Distance Calculation | ||||||
| Numerator | ||||||
| A=Individuals with UCVA <6/12 in the better eye who present with spectacles or contact lenses for distance and whose PVA is ≥6/12 in the better eye (Met Need) | 83 | 123 | 206 | |||
| B=Individuals with a history of refractive surgery whose UCVA is ≥6/12 in the better eye (Met Need); | 1 | 3 | 4 | |||
| Denominator | ||||||
| C=Individuals with UCVA <6/12 in the better eye who present with spectacles or contact lenses for distance or a history of refractive surgery and a PVA of <6/12 in the better eye, but who improve to ≥6/12 on pinhole VA or refraction (Undermet Need); | 4 | 9 | 13 | |||
| D=Individuals with UCVA <6/12 in the better eye who do not have distance correction and who improve to ≥6/12 on pinhole VA or refraction (Unmet Need) | 50 | 78 | 128 | |||
| eREC for distance=(a+b/a+b+c+d) *100 (%) | 60.87 | 59.15 | 59.83 | |||
| Variables for eREC for Near Calculation | ||||||
| A=Individuals with UCVA <N6 at 40 cm who present with spectacles for near and whose PVA is ≥N6 in the better eye (Met Need); | 195 | 301 | 496 | |||
| B=Individuals with distance BCVA of ≥6/12 in at least one eye who present with spectacles for near and whose PVA was <N6 in the better eye (Undermet Need); | 5 | 17 | 22 | |||
| C=Individuals with distance BCVA of ≥6/12 in at least one eye who do not have correction for near and whose UCVA was <N6 in the better eye (Unmet Need) | 192 | 344 | 536 | |||
| eREC for near=(a/a+b+c) *100 (%) | 49.74 | 45.47 | 47.06 |
The eREC for distance vision was assessed among the 6 + population and the eREC for near vision was assessed in the 35+ population. The eREC for distance was 59.83% and was higher in males than females (60.87% vs 59.15%). The eREC for near was 47.06% and was higher in males than females (49.74% vs 45.47%) [Table 5].
Discussion
The pilot implementation of the RAAVI study identified its numerous strengths as an easily administered online tool for determining baseline coverage, as well as for monitoring improvements in coverage periodically. The RAAB methodology focuses predominantly on the elderly population and is not suited for younger age groups. A blindness survey on all age groups requires a minimum sample size of 20,000, which also with a high margin of error. This issue can be circumvented if the primary objective is changed from blindness prevalence to coverage of cataract surgical services or VI. Then, the study becomes adequately powered to detect coverage as well as VI in the total population, while it becomes less powered for blindness burden estimation.
Some of the modifications made in the RAAB-6 methodology to develop the RAAVI are as follows: change of age group from 50+ to 6+ for distance visual acuity assessment and 35+ for near vision assessment, measurement of presenting as well as uncorrected visual acuity (UCVA), measurement of near vision first using the N60 optotype followed by N6 optotype, addition of history of any refractive surgery like Laser-Assisted In-Situ Keratomileusis (LASIK) or Small Incision Lenticule Excision (SMILE), history of use of contact lenses in addition to glasses and merging of causes of PVA <6/12 from thirteen to seven categories only.
The current pilot study field tested the survey protocol for assessing eCSC and eREC as indicators for UHC utilizing the novel RAAVI methodology. According to the National Blindness Survey (NBS),[1] the prevalence of VI in the 50+ age population in India was 13.76% and cataracts (71.2%) was the most important cause of VI followed by refractive error (13.4%). Click or tap here to enter text. In the current study, the age-sex-adjusted prevalence of MVI was 1.26% and VI was 4.19% among the 6+ population. The NBS was conducted among the 50+ age population in one district of Haryana (Yamuna Nagar) and the prevalence of MVI and VI reported were 7.28% and 10.6%, respectively.[1] Although the prevalence figures in the current study are lower as compared to NBS, it might be due to the lower age group (6+) and other differences among the survey participants.
Numerous studies from around the globe have reported that females are at higher risk of VI as compared to their male counterparts.[16,17,18] A significant difference was observed in the current study also which can be explained by the socio-cultural disadvantage of the female gender leading to a poor continuum of care. Refractive error (RE) and cataracts remain the leading causes of VI in the current study. These findings are corroborated by numerous studies from Asia and Africa, as well as across India.[19,20,21,22] Cataracts and REs combined contributed to nearly 90% of VI both of which are amenable to treatment as compared to the other causes.
The CSC in the 50+ population among cataract blind persons was 96.2%, among persons with severe VI due to cataract was 95.5%, and among VI due to cataract was 87.5%. In the NBS, a CSC (persons) of 85.6% at a VA cut-off of 6/18 was reported in Yamuna Nagar, Haryana, with males having higher CSC compared to females, like the findings of the current study. The relatively higher CSC of Haryana as compared to the national average (74.0%) indicates better access to surgical services.
The eCSC reported in the current study was 61.8% and it was higher in females as compared to males. A previous study in two coastal districts of Odisha in Eastern India had reported eCSC of 35.0% only, being higher in females than males.[9] A preliminary analysis (unpublished) of 47 population-based surveys from 11 countries revealed a significant range in eCSC between countries, from 2.8% to 88.5%. Data from repeated population-based surveys within four LMICs revealed an average annual percentage point increase in eCSC of 1.1% (range = 0.8–1.4%). In addition, gender inequities in eCSC have been reported: men have better coverage as compared to females.[23] However, the gender differential was reversed in India, indicating greater longevity of women and their better health-seeking behavior.
To know the refractive error coverage, UCVA needs to be measured (without spectacles or contact lenses), followed by BCVA (with pinhole or spectacles). The current study employed this novel methodology, and the eREC for distance was 59.8%, while for near vision it was 47.0%. In 2021, distance eREC among 50+ population was 79.1% (95% CI: 72.4–85.0) in the high-income countries compared to 40.0% (31.7–48.2) in SEAR.[24] The study in Eastern India among a 40+ population reported 40.0% eREC for distance and 35.7% eREC for near vision.[9] As far as the targets are concerned, Gurugram district can aspire for 90% eCSC and 100% eREC for distance by 2030. This seems feasible considering the resources available at the disposal of the district, and its good health infrastructure.
The Government of India launched the National Program for Control of Blindness and Visual Impairment (NPCB and VI) in 1976 and it currently has the provision of free services for cataract surgery and other sub-specialties; however, with the increase in the number of RE, there is a need to introduce a provision of subsidized/free spectacles also into the program to alleviate some of the additional costs added.[25] Demand generation of services and addressing various barriers to accessing those services is necessary to scale up the provision of cataract surgical and refractive error services to the population.
Conclusion
The RAAVI study has a few limitations. First, it is not adequately powered to determine the prevalence of blindness. The sample size was deduced based on the estimated coverage and could only provide rough estimates for blindness. Second, the findings of the study cannot be extrapolated to other districts of India, as Gurugram has a much better socio-economic milieu as compared to the rest of the country. Some of the major recommendations from the current study include, graded scaling up of cataract and RE services over the next few years to achieve UHC targets by 2030 and reducing the cost of services by incorporating services in insurance packages for example health benefits packages (HBP2.0) of Ayushman Bharat scheme. Also, RAAVI studies need to be conducted in all the districts of India so that accurate estimation of coverage and district-specific interventions for improving coverage can be planned.
Financial support and sponsorship:
WHO-SEARO funded the project in the form of grants, equipment, drugs, etc.
Conflicts of interest:
There are no conflicts of interest.
Acknowledgment
WHO India office, Dr. Patanjali Dev Nayar Regional advisor WHO SEARO, Dr Virendar Yadav Civil Surgeon Gurugram Haryana, Dr. Isha Narang District Program Manager, Blindness, and Visual Impairment Gurugram.
References
- 1.Vashist P, Senjam SS, Gupta V, Gupta N, Shamanna BR, Wadhwani M, et al. Blindness and visual impairment and their causes in India: Results of a nationally representative survey. PLoS One. 2022;17:1–14. doi: 10.1371/journal.pone.0271736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng M, Fullman N, Dieleman JL, Flaxman AD, Murray CJL, Lim SS. Effective coverage: A metric for monitoring universal health coverage. PLoS Med. 2014;11:e1001730. doi: 10.1371/journal.pmed.1001730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramke J, Gilbert CE, Lee AC, Ackland P, Limburg H, Foster A. Effective cataract surgical coverage: An indicator for measuring quality-of-care in the context of Universal Health Coverage. PLoS One. 2017;12:e0172342. doi: 10.1371/journal.pone.0172342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keel S, Müller A, Block S, Bourne R, Burton MJ, Chatterji S, et al. Keeping an eye on eye care: Monitoring progress towards effective coverage. Lancet Glob Health. 2021;9:e1460–4. doi: 10.1016/S2214-109X(21)00212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou H, Mo D, Zhou C, Medina A, Shi Y, Zhang L, et al. The gender gap among school children in poor rural areas of western China: Evidence from a multi-province data set. Int J Equity Health. 2016;15:1–11. doi: 10.1186/s12939-016-0442-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta N, Vashist P, Malhotra S, Senjam SS, Misra V, Bhardwaj A. Rapid assessment of visual impairment in urban population of Delhi, India. PLoS One. 2015;10:e0124206. doi: 10.1371/journal.pone.0124206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senjam SS, Vashist P, Gupta N, Malhotra S, Misra V, Bhardwaj A, et al. Prevalence of visual impairment due to uncorrected refractive error: Results from Delhi-Rapid Assessment of Visual Impairment Study. Indian J Ophthalmol. 2016;64:387–90. doi: 10.4103/0301-4738.185614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marmamula S, Madala SR, Rao GN. Rapid assessment of visual impairment (RAVI) in marine fishing communities in South India--study protocol and main findings. BMC Ophthalmol. 2011;11:26. doi: 10.1186/1471-2415-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhardwaj A, Vashist P, Singh Senjam S, Gupta V, Gupta N, Manna S, et al. Rapid assessment of avoidable visual impairment in two coastal districts of Eastern India for determining effective coverage: A cross-sectional study. J Ophthalmic Vis Res. 2023;18:182–91. doi: 10.18502/jovr.v18i2.13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marmamula S, Narsaiah S, Shekhar K, Khanna RC, Rao GN. Visual impairment in the South Indian State of Andhra Pradesh: Andhra Pradesh - Rapid assessment of visual impairment (AP-RAVI) project. PLoS One. 2013;8:e70120. doi: 10.1371/journal.pone.0070120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marmamula S, Khanna RC, Shekhar K, Rao GN. Outcomes of cataract surgery in urban and rural population in the South Indian State of Andhra Pradesh: Rapid assessment of visual impairment (RAVI) project. PLoS One. 2016;11:e0167708. doi: 10.1371/journal.pone.0167708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marmamula S, Khanna RC, Shekhar K, Rao GN. A population-based cross-sectional study of barriers to uptake of eye care services in South India: The Rapid Assessment of Visual Impairment (RAVI) project. BMJ Open. 2014;4:1–5. doi: 10.1136/bmjopen-2014-005125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marmamula S, Khanna RC, Kunkunu E, Rao GN. Population-based assessment of prevalence and causes of visual impairment in the state of Telangana, India: A cross-sectional study using the Rapid Assessment of Visual Impairment (RAVI) methodology. BMJ Open. 2016;6:1–7. doi: 10.1136/bmjopen-2016-012617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashemi H, Yekta A, Jafarzadehpur E, Doostdar A, Ostadimoghaddam H, Khabazkhoob M. The prevalence of visual impairment and blindness in underserved rural areas: A crucial issue for future. Eye. 2017;31:1221–8. doi: 10.1038/eye.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheeladevi S, Seelam B, Nukella P, Borah R, Ali R, Keay L. Prevalence of refractive errors, uncorrected refractive error, and presbyopia in adults in India: A systematic review. Indian J Ophthalmol. 2019;67:583–92. doi: 10.4103/ijo.IJO_1235_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye Q, Chen Y, Yan W, Wang W, Zhong J, Tang C, et al. Female gender remains a significant barrier to access cataract surgery in South Asia: A systematic review and meta-analysis. J Ophthalmol. 2020;2020:1–14. doi: 10.1155/2020/2091462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pant HB, Bandyopadhyay S, John N, Chandran A, Gudlavalleti MVS. Differential cataract blindness by sex in India: Evidence from two large national surveys. Indian J Ophthalmol. 2017;65:160–4. doi: 10.4103/ijo.IJO_28_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aninye IO, Digre K, Hartnett ME, Baldonado K, Shriver EM, Periman LM, et al. The roles of sex and gender in women’s eye health disparities in the United States. Biol Sex Differ. 2021;12:1–8. doi: 10.1186/s13293-021-00401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neena J, Rachel J, Praveen V, Murthy GVS. Rapid assessment of avoidable blindness in India. PLoS One. 2008;3:e2867. doi: 10.1371/journal.pone.0002867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu M. Rapid assessment of avoidable blindness in Kunming, China. Community Eye Health. 2007;20:10. [PMC free article] [PubMed] [Google Scholar]
- 21.Isipradit S, Sirimaharaj M, Charukamnoetkanok P, Thonginnetra O, Wongsawad W, Sathornsumetee B, et al. The first rapid assessment of avoidable blindness (RAAB) in Thailand. PLoS One. 2014;9:e114245. doi: 10.1371/journal.pone.0114245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müller A, Zerom M, Limburg H, Ghebrat Y, Meresie G, Fessahazion K, et al. Results of a rapid assessment of avoidable blindness (RAAB) in Eritrea. Ophthalmic Epidemiol. 2011;18:103–8. doi: 10.3109/09286586.2010.545932. [DOI] [PubMed] [Google Scholar]
- 23.McCormick I, Butcher R, Evans JR, Mactaggart IZ, Limburg H, Jolley E, et al. Effective cataract surgical coverage in adults aged 50 years and older: Estimates from population-based surveys in 55 countries. Lancet Glob Health. 2022;10:e1744–53. doi: 10.1016/S2214-109X(22)00419-3. [DOI] [PubMed] [Google Scholar]
- 24.Bourne RRA, Cicinelli MV, Sedighi T, Tapply IH, McCormick I, Jonas JB, et al. Effective refractive error coverage in adults aged 50 years and older: Estimates from population-based surveys in 61 countries. Lancet Glob Health. 2022;10:e1754–63. doi: 10.1016/S2214-109X(22)00433-8. [DOI] [PubMed] [Google Scholar]
- 25.MoHFW National Programme for Control of Blindness. National Health Portal of India. Available from: https://www.nhp.gov.in/national-programme-for-control-of-blindness_pg . [Last accessed on 2024 Mar 23] [Google Scholar]


