Abstract
β-adrenergic receptors (βARs) play significant roles in regulating Ca2+ signaling in cardiac myocytes, thus holding a key function in modulating heart performance. βARs regulate the influx of extracellular Ca2+ and the release and uptake of Ca2+ from the sarcoplasmic reticulum (SR) by activating key components such as L-type calcium channels (LTCCs), ryanodine receptors (RyRs) and phospholamban (PLN), mediated by the phosphorylation actions by protein kinase A (PKA). In cardiac myocytes, the presence of β2AR provides a protective mechanism against potential overstimulation of β1AR, which may aid in the restoration of cardiac dysfunctions. Understanding the Ca2+ regulatory signaling pathways of βARs in cardiac myocytes and the differences among various βAR subtypes are crucial in cardiology and hold great potential for developing treatments for heart diseases.
Keywords: β-adrenergic receptor (βAR), Ca2+ signaling, Cardiac myocytes, Compartmentalization
INTRODUCTION
β-adrenergic receptors (βARs) belong to the G protein-coupled receptor (GPCR) superfamily, and are essential for regulating the function of the cardiovascular system. βARs are activated by catecholamines released from sympathetic nerve terminals and adrenal medulla under stress conditions, which increase heart rate and blood pumping capability of the heart (Bers 2002). The positive chronotropic, dromotropic and inotropic effects ensure the energy supply of emergent needs.
Currently, there are three identified subtypes of βARs (β1AR, β2AR, β3AR), while the existence of a fourth subtype (β4AR) is still a subject of debate (Gauthier et al. 1996). These three isoforms exhibit different affinities for different ligands, rendering the selectivity of isoform activation (Bristow et al. 1986). The ratio of β1AR/β2AR expression in the healthy human heart is approximately 4:1, while the expression of β3AR is minimal. Both β1AR and β2AR respond to catecholamine stimulation and mediate positive inotropic effects in heart cells. β1AR plays a dominant role in increasing chronotropy and inotropy in cardiac myocytes, whereas β2AR produces only modest chronotropic effects (Xiang and Kobilka 2003; Xiao et al. 2006).
Epinephrine and norepinephrine are native catecholamine ligands of βARs (Bunemann et al. 1999; Hain et al. 1995; Nikolaev et al. 2006; Nikolaev et al. 2010). With catecholamine binding, βARs undergo conformational changes that enable its coupling to heterotrimeric G proteins, resulting in the substitution of the GDP on the Gα subunit of G-proteins by GTP and subsequent dissociation of Gβγ subunits (Wess 1997). Gα-GTP then stimulates adenylyl cyclase (AC) to catalyze the formation of cyclic AMP (cAMP). cAMP regulates a wide variety of cellular processes through activating a variety of downstream signaling molecules, including protein kinase A (PKA). PKA phosphorylates L-type Ca2+ channels (LTCCs) in the cell membrane or T-tubules, ryanodine receptor (RyR) Ca2+ release channels and phospholamban (PLN) in the sarcoplasmic reticulum (SR), and thereby up-regulates LTCC Ca2+ influx, SR Ca2+ release and cytosolic Ca2+ uptake (Fig. 1).
Figure 1.
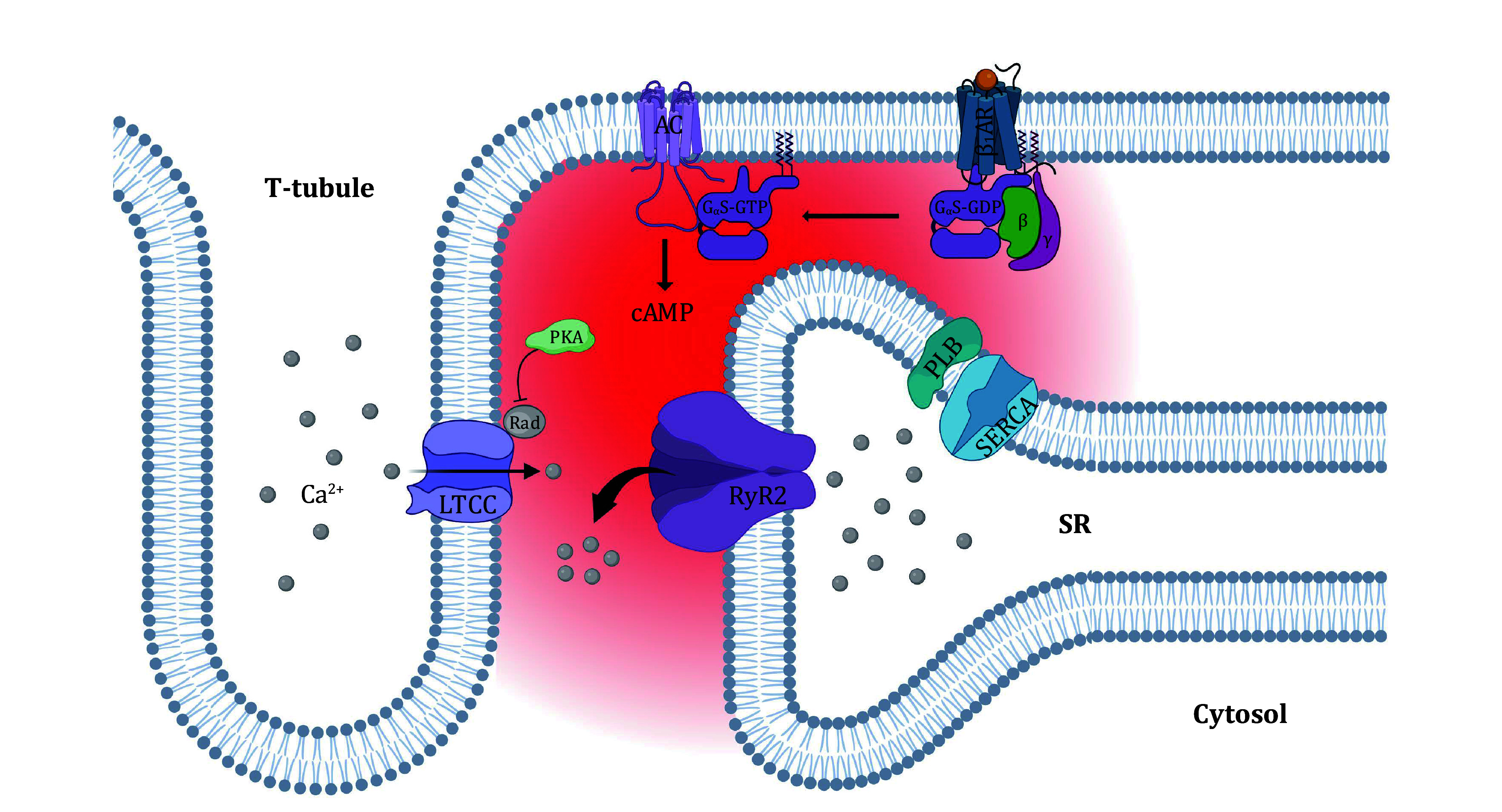
Illustration of β-adrenergic regulation of Ca2+ signaling in heart cells
REGULATION OF LTCC
LTCCs are the predominant mediator of Ca2+ influx in the cardiomyocytes playing an initiation role in the excitation-contraction coupling. In general, LTCCs are composed of α1, α2, β, δ and γ subunits. α1 is the pore-forming subunit with voltage sensors. α2/δ and β subunits modulate the expression, voltage dependence and gating kinetics of the channel (Bodi et al. 2005).
Several PKA phosphorylation sites in the α1 subunit have been identified (Fu et al. 2014; Hulme et al. 2006; Yang et al. 2016). βAR agonists, such as isoproterenol, increase the phosphorylation level of S1928 in the distal C-terminal domain (Hulme et al. 2006), which can be blocked by βAR antagonists. Interestingly, LTCCs with S1928 mutated to alanine still retain 70%–80% response to βAR stimulation, indicating that S1928 is not the major phosphorylation site for βAR stimulation (Benitah et al. 2010; Ganesan et al. 2006; Hulme et al. 2003, 2006). S1700 and T1704 located at the interface between the proximal and distal C-terminal domain are also phosphorylated in βAR regulation of LTCCs (Fu et al. 2013, 2014). Again, mutations of both S1700 and T1704 cannot eliminate βAR effects (Fu et al. 2013).
The regulatory β2 subunit plays a crucial role in LTCCs regulation in response to βAR stimulation (Haase et al. 1993). S478 and S479 were identified as the phosphorylation sites of PKA in β2 subunit (Gerhardstein et al. 1999). Mutating S478 and S479 to alanine in the β2 subunit inhibits PKA-mediated Ca2+ current increase in transfected cells (Bunemann et al. 1999). This result suggests that phosphorylation of S478 or S479 contributes to PKA-mediated regulation of LTCCs.
Monomeric G proteins, such as Rem and Rad, function as endogenous LTCC inhibitors (Beguin et al. 2001; Finlin et al. 2003). Recent analysis from a proximity proteomics screen provided solid evidence that Rad is enriched in the LTCC microenvironment but is depleted during β-adrenergic stimulation. Phosphorylation by PKA decreases Rad affinity for β subunits and increases LTCC open probability (Liu et al. 2020). Four serines in Rad have been identified as PKA phosphorylation sites, and mutation of these four serines or disrupting the interaction between LTCC β subunit and Rad reduced heart rate and basal contractility, and greatly diminished β-adrenergic contractile response (Papa et al. 2022, 2024).
A kinase anchoring protein 15 (AKAP15) is a lipid-anchored protein with a single amphipathic helix that binds PKA. AKAP15 colocalizes and associates with LTCC in T-tubules (Gray et al. 1998). PKA tethered to a leucine zipper motif in the C-terminal domain of the LTCC α1 subunit via AKAP15 (Hulme et al. 2002), which is essential for β-adrenergic regulation of LTCC (Hulme et al. 2003).
LTCCs are also phosphorylated by Ca2+/calmodulin-dependent kinase II (CaMKII). CaMKII is activated by βAR stimulation via guanine nucleotide exchange protein directly activated by cAMP (Epac) (Curran et al. 2007; Grimm and Brown 2010). Mutations at sites S1512 and S1570 of α1 subunit (Hudmon et al. 2005) and T498 of β2 subunit (Koval et al. 2010) reduce Ca2+ influx.
Besides, cardiac phosphatase activities also play important roles in the regulation of Ca2+ homeostasis. Phosphatase type 1 (PP1) and 2A (PP2A) are the major isotypes of cardiac phosphatases, comprising over 90% of the protein phosphatases in cardiomyocytes (Lüss et al. 2000). PP1 is reported to contribute to the dephosphorylation of LTCC, RyR, and PLB. Whereas, PP2A is mainly involved in the dephosphorylation of myofibrillar proteins, including troponin I and myosin-binding protein C (Metzger and Westfall 2004).
In recent years, a few proteins have been reported to modify the β-adrenergic regulation of LTCCs. Sphingosine-1-phosphate (S1P), a circulating bioactive sphingolipid, has been implicated in the regulation of several cellular processes including cardiac Ca2+ handling (Means and Brown 2009). S1P does not affect the basal LTCC current, but partially reverses the regulation of βAR activation on LTCCs through a signaling pathway involving the interaction between P21-activated kinase 1 (Pak1) and protein phosphatase 2A (PP2A) (Egom et al. 2016). Ahnak functions as a suppressor of LTCCs by sequestering the β2 subunit through a strong binding to the LTCC β2 subunit (Hohaus et al. 2002). Rem GTPase interacts with LTCC β2 subunit and inhibits LTCC currents. The inhibitor effects can be rescued by LTCC activators such as BayK8644, but not by the βAR stimulation (Xu et al. 2010). Besides the functional coupling regulators, there were some structural coupling factors, such as Bridging Integrator 1 (BIN1) and caveolin-3. BIN1 is essential for the localization of LTCCs to T-tubules in cardiomyocytes and affects LTCC regulation by βAR stimulation (Kumari et al. 2018). In heart cells, a subpopulation of LTCCs localizes in caveolae. Caveolae are specialized membrane microdomains and are supported by the structural protein caveolin-3. It is well known that β2AR is enriched in caveolae. There is evidence showing that regulation of LTCCs by β2AR, but not β1AR, is eliminated when caveolae were disrupted (Balijepalli et al. 2006). This indicates that LTCCs are coupled to β1AR signaling outside of caveolae.
REGULATION OF RyRs
RyRs are major Ca2+ release channels in the SR of striated myocytes or endoplasmic reticulum (ER) of other cells. RyRs bind to ryanodine in their open state. Early studies using radiolabeled ryanodine have shown that phosphorylation of RyR2 by PKA increased channel activity (Takasago et al. 1991). However, the identification of the phosphorylation site critical for βAR response has been highly controversial. It has been proposed that the phosphorylation of S2808 by PKA sensitizes the response of RyRs to cytosolic Ca2+ change (Wehrens et al. 2004a). However, the mouse model harboring the S2808A mutation has normal inotropic and chronotropic responses to βAR stimulation (MacDonnell et al. 2008). S2808A cardiomyocytes exhibit blunted enhancement of systolic Ca2+ transients at 3 Hz but not at lower frequencies (Benkusky et al. 2007). There is also evidence that the phosphorylation of S2030 by PKA enhances RyR2 responsiveness to luminal Ca2+ (Xiao et al. 2005, 2007). However, data from different labs questioned Ser2030 as a physiological PKA phosphorylation site (Huke and Bers 2008; Wehrens et al. 2006).
Besides PKA-mediated phosphorylation, βAR-activated CaMKII specifically phosphorylates S2815 in RyRs (Kushnir et al. 2010; Wehrens et al. 2004b). Phosphorylation at S2815 increases the open probability of RyR2 by sensitizing the channel (Wehrens et al. 2004b). While cardiac-specific CaMKII overexpression enhances SR Ca2+ fractional release (Maier et al. 2003), cardiac-specific inhibition of CaMKII reduces isoproterenol-induced responses in SR Ca2+ release and heart rate (Wu et al. 2009).
In intact cells, βAR modulation of RyR function is difficult to measure, because βAR also increases LTCC Ca2+ current and SR Ca2+ loading. With a high-affinity Ca2+ indicator combined with a slow Ca2+ buffer agent EGTA to elicit Ca2+ spikes, it is demonstrated that isoproterenol synchronizes the Ca2+ release from RyR clusters (Song et al. 2001). When the SR Ca2+ load and Ca2+ current were controlled, isoproterenol stimulation of β1AR accelerates SR Ca2+ release kinetics without altering the amplitude of Ca2+ transients (Ginsburg and Bers 2004), agreeing well with the PKA-mediated synchronization of RyR Ca2+ release (Lakatta 2004; Wang and Wehrens 2010). However, experiments using UV photolysis to activate RyRs showed that isoproterenol enhances both the speed and the magnitude of Ca2+ transients in cells with controlled SR Ca2+ load (Ogrodnik and Niggli 2010). Using the loose-sealed patch clamp to trigger individual RyR Ca2+ release units, manifested as a Ca2+ spark, we observed that selective βAR stimulation enhances the amplitude of triggered sparks in an LTCC unitary current-independent manner. The Ca2+ release flux that underlies a Ca2+ spark is enhanced when the SR Ca2+ content is controlled to a comparable level. These results demonstrate unequivocally that the activation of RyRs is expedited and synchronized under βAR stimulation (Zhou et al. 2009).
REGULATION OF PLN
The rapid removal of Ca2+ from the cytoplasm is primarily facilitated by the sarco(endo)plasmic reticulum Ca2+ ATPase SERCA2a, which pumps Ca2+ back into the SR cavity and thus controls the amount of Ca2+ in the SR (Zhihao et al. 2020). PLB is the endogenous regulatory protein of SERCA2a activity and is the only regulatory protein of SERCA2a that is directly involved in the development of heart disease, including heart failure (Shanmugam et al. 2011; Weber et al. 2021).
There are two phosphorylation sites in PLN, Ser16 and Thr17, which are phosphorylated by PKA and CaMKII respectively (Kuschel et al. 1999; Simmerman et al. 1986; Xiao et al. 1994). Experiments with phosphorylation of PLN at either site increase SR Ca2+ load, and thus enhance SR Ca2+ release and accelerate cardiomyocyte relaxation (Li et al. 2002). Different from that of Ser16, the phosphorylation of Thr17 by β1AR is enhanced with increased frequency of electrical stimulation possibly because frequency-dependent accumulation of intracellular Ca2+ facilities CaMKII activation (Hagemann et al. 2000).
DIFFERENCE BETWEEN β1AR AND β2AR SIGNALING
The amino acid sequences of human β1AR and β2AR share only 71% identity in the transmembrane domains and 54% identity overall (Dixon et al. 1986). In the heart, β1AR-activated cAMP signaling increases the phosphorylation of sarcolemmal LTCCs and a multitude of intracellular regulatory proteins, including RyR, PLB and myofilaments (Xiao 2001). However, β2AR-mediated cAMP signaling specifically modulates LTCCs without affecting PLB and myofilaments in most mammalian species (Fig. 2) (Xiao and Lakatta 1993). Although in the human heart, β2AR stimulation increases PKA-dependent phosphorylation of intracellular regulatory proteins, its effects are much smaller than that induced by β1AR stimulation (Altschuld et al. 1995). Furthermore, β2ARs are expressed preferentially in the T-Tubule membrane, while β1ARs are distributed in both T-tubules and surface membrane (Nikolaev et al. 2010).
Figure 2.
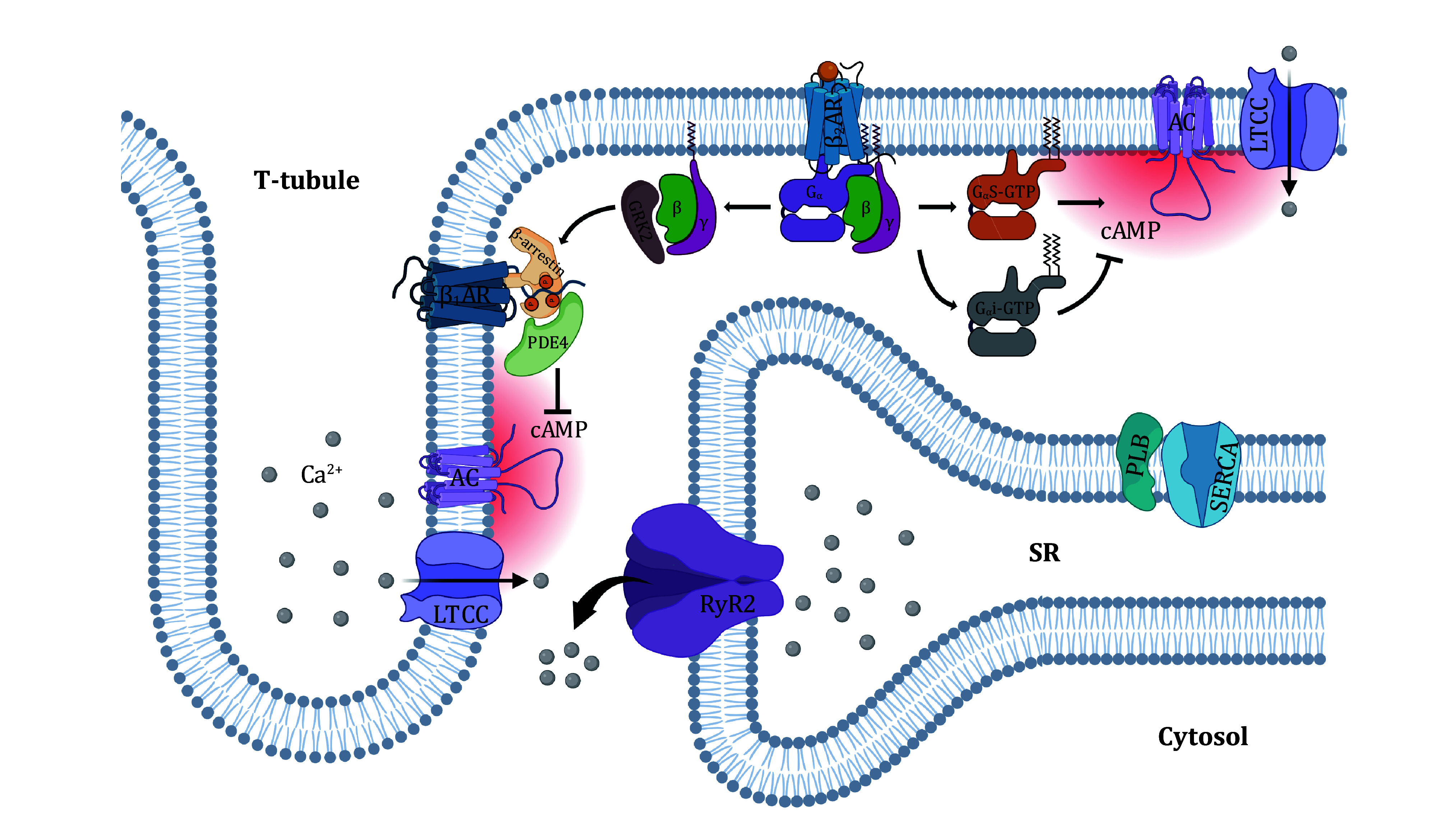
Illustration of compartmentalized β2AR-cAMP signaling in heart cells
βARs are G protein-coupled receptors. β1AR and β2AR both couple to Gs protein, while β2AR also couples to Gi protein (Fig. 2). Selective β2AR stimulation by zinterol does not enhance cardiomyocyte contraction in both wild-type (WT) mice and transgenic mice overexpressing human β2AR (TG4) (Zhou et al. 1999). After incubating cells with pertussis toxin (PTX), which abrogates Gi/Go function via ADP ribosylation, zinterol markedly increases contraction amplitude in both WT and TG4 cardiomyocytes, which can be completely abolished by the specific β2AR antagonist (Xiao et al. 1999). In cell-attached patch clamp experiment, β2AR agonist in bath solution outside the patch pipette cannot cause a discernible change in LTCC activity in the patch membrane, while local β2AR agonist in the pipette markedly increases the open probability of the patched channel. This sophisticated experiment indicates that β2AR signaling is confined in a highly localized microdomain. After PTX treatment, the channels in the patch membrane became responsive to agonist in the bath solution, suggesting that Gi plays an essential role in the compartmentalized β2AR signaling (Chen-Izu et al. 2000).
In addition to the impact of Gi protein, it is proposed that β2ARs reside in caveolae, which compartmentalize β2AR signaling. Indeed, caveolin-3 is of vital importance for the localization of β2AR and compartmentation of β2AR-cAMP signaling in healthy cardiomyocytes (Wright et al. 2014). Also, phosphodiesterase 4D (PDE4D) is recruited by β-arrestin2 to the vicinity of β2AR. Its hydrolysis of cAMP restricts the spatial diffusion of β2AR-activated cAMP signal (Fischmeister et al. 2006; Richter et al. 2008; Shi et al. 2017). Endogenous catecholamine ligands of βARs, epinephrine and norepinephrine, induced distinct β2AR signaling through G protein-coupled receptor kinase 2 (GRK2) phosphorylation and selective binding of Gs or Gi (Heubach et al. 2004; Wang et al. 2008), which further revealed the complexity of β2AR downstream signaling.
β2AR-MEDIATED OFFSIDE COMPARTMENTALIZATION OF β1AR SIGNALING
Accumulative evidence suggests that β1AR and β2AR pathways may have crosstalk. The activation of β2AR has been found to blunt the signaling of β1AR in failing heart cells (He et al. 2005). In transgenic mice overexpressing β2ARs, the contractility of cardiomyocytes is enhanced through spontaneous β2AR-cAMP signaling. However, these cells lose their ability to respond to β1AR stimulation (Zhang et al. 2000).
Recently, we have analyzed the interaction between β2AR and β1AR signaling. While isoproterenol normally up-regulates Ca2+ transients during cardiomyocyte excitation, salbutamol, a selective β2AR agonist, hinders the ability of isoproterenol to regulate Ca2+ transients (Yang et al. 2019). This effect can be eliminated either by rolipram, a PDE4 inhibitor, or by peptides that antagonize β-arrestin1. In the rat model harboring mutations of the phosphorylation sites in the C-terminus of β1AR, a putative binding domain for β-arrestin1 and GRK2, β2AR agonist no longer interferes with β1AR signaling. This study suggests that β2AR stimulation activates GRK2 to phosphorylate the C-terminus of β1AR, facilitates the recruitment of PDE4 to the phosphorylated β1AR, and compartmentalizes β1AR-cAMP signals within a sub-membrane nanodomain, preventing the PKA-dependent regulation of RyR and PLB. Because the compartmentalization of the β1AR pathway is rendered by the β2AR pathway in an offside manner, this signaling process is described as “offside compartmentalization” (Fig. 2) (Yang et al. 2019).
It is important to mention that the activation of offside compartmentalization can occur in vivo through the use of epinephrine, which hinders the regulation of heart contraction by norepinephrine (Yang et al. 2020). Epinephrine exhibits a limited preference for β2AR over β1AR as an adrenal hormone (Baker 2010), while norepinephrine predominantly stimulates β- and α1ARs as a sympathetic neurotransmitter (Minneman et al. 1981) and exhibits selectivity for β1AR over β2AR due to different entrance pathways to the extracellular binding pockets (Xu et al. 2021). Epinephrine and a less quantity of norepinephrine are tonically released from the adrenal glands (Paur et al. 2012). As prolonged activation of β1AR leads to cytotoxicity (Wu et al. 2017; Zhu et al. 2003), the offside compartmentalization initiated by β2AR signaling can serve as a negative feed-forward mechanism preventing the tonic β1AR activation by circulating catecholamines. Under the offside compartmentalization, βAR signaling is still able to synchronize SR Ca2+ release by up-regulating LTCC Ca2+ influx (Yang et al. 2020) and enhance the transient response of β1AR to norepinephrine during sympathetic excitation. Hence, in contrast to the robust and predictable E-C coupling regulation through overall β1AR signaling, the compartmentalized βAR regulation of E-C coupling, while being moderate, exhibits an "autoadaptive" nature in response to various physiological and pathological circumstances.
PATHOLOGICAL IMPLICATIONS
While βARs play essential roles in the physiological operation of Ca2+ signaling, their malfunction is implicated in a variety of pathological processes. Prolonged β1AR stimulation induces apoptosis in a CaMKII-dependent manner, and β2AR blockade exaggerates β1AR-induced apoptosis (Communal et al. 1999) possibly due to the absence of offside compartmentalization. In contrast, stimulation of β2AR protects cardiac myocytes against a wide range of apoptotic insults, including enhanced β1AR signaling, hypoxic treatment or induction of reactive oxygen species (ROS) (Zhu et al. 2001). Inhibition of β2AR-activated Gi–Gβγ–PI3K–PKB signaling eliminates these protective effects, and transforms β2AR signaling from anti-apoptotic to pro-apoptotic (Zhu et al. 2001; Chesley et al. 2000). Emerging evidence suggests that mitogen-activated protein kinase (MAPK) and extracellular signal-regulated protein kinases (ERK1 and ERK2) are also involved in β2AR-mediated anti-apoptotic signaling (Shizukuda and Buttrick 2002).
During the early-stage development of heart failure, the sympathetic nervous system adjusts its activity to increase cardiac output to compensate for the alterations of cardiac and peripheral hemodynamics (Toschi-Dias et al. 2017). However, the continuous hemodynamic stress promotes the chronic release of catecholamines. The elevated level of catecholamine (Bristow et al. 1982; Ungerer et al. 1993) leads to sustained and toxic β1AR-CaMKII signaling, which exacerbates the decline in cardiac function as observed in mid- and late-stages of heart failure (Brede et al. 2002; Johnson and Antoons 2018; Zhu et al. 2003).
CONCLUDING REMARKS
Heart disease is the leading cause of death globally. β-adrenergic signaling plays a pivotal role in the modulation of cardiac function in physiological and pathological conditions. Understanding the molecular mechanism of β-adrenergic signaling is fundamental for heart disease therapy. A recent discovery of Rad, a novel endogenous regulator of LTCC, brought new insights into the βAR-cAMP-PKA signaling pathway, and well explained the controversial evidence on the functional phosphorylation sites on LTCC subunits. It is well known that β1AR mediates global cAMP signaling while β2AR generates localized cAMP signaling. Recent findings suggest that β2AR may also blunt β1AR signaling through GRK2 mediated “offside compartmentalization” mechanism, which can also serve as a negative feed-forward mechanism preventing the cell toxicity of tonic β1AR activation by circulating catecholamines. This underscores the critical protective role of β2AR against the detrimental effects of β1AR overstimulation. Further discoveries of βAR signaling mechanisms will contribute to novel and effective diagnostic and therapeutic heart disease targets.
Conflict of interest
Bo Yang, Shi-Qiang Wang and Hua-Qian Yang declare that they have no conflict of interest.
Compliance with Ethical Standards
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Altschuld RA, Starling RC, Hamlin RL, Billman GE, Hensley J, Castillo L, Fertel RH, Hohl CM, Robitaille PM, Jones LR, Xiao R-P, Lakatta EG Response of failing canine and human heart cells to beta 2-adrenergic stimulation. Circulation. 1995;92(6):1612–1618. doi: 10.1161/01.CIR.92.6.1612. [DOI] [PubMed] [Google Scholar]
- Baker JG The selectivity of beta-adrenoceptor agonists at human beta1-, beta2- and beta3-adrenoceptors. Br J Pharmacol. 2010;160(5):1048–1061. doi: 10.1111/j.1476-5381.2010.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ Localization of cardiac L-type Ca2+ channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation. Proc Natl Acad Sci USA. 2006;103(19):7500–7505. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beguin P, Nagashima K, Gonoi T, Shibasaki T, Takahashi K, Kashima Y, Ozaki N, Geering K, Iwanaga T, Seino S Regulation of Ca2+ channel expression at the cell surface by the small G-protein kir/Gem. Nature. 2001;411(6838):701–706. doi: 10.1038/35079621. [DOI] [PubMed] [Google Scholar]
- Benitah JP, Alvarez JL, Gomez AM L-type Ca2+ current in ventricular cardiomyocytes. J Mol Cell Cardiol. 2010;48(1):26–36. doi: 10.1016/j.yjmcc.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Benkusky NA, Weber CS, Scherman JA, Farrell EF, Hacker TA, John MC, Powers PA, Valdivia HH Intact beta-adrenergic response and unmodified progression toward heart failure in mice with genetic ablation of a major protein kinase A phosphorylation site in the cardiac ryanodine receptor. Circ Res. 2007;101(8):819–829. doi: 10.1161/CIRCRESAHA.107.153007. [DOI] [PubMed] [Google Scholar]
- Bers DM Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Bodi I, Mikala G, Koch SE, Akhter SA, Schwartz A The L-type calcium channel in the heart: the beat goes on. J Clin Invest. 2005;115(12):3306–3317. doi: 10.1172/JCI27167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brede M, Wiesmann F, Jahns R, Hadamek K, Arnolt C, Neubauer S, Lohse MJ, Hein L Feedback inhibition of catecholamine release by two different α2-adrenoceptor subtypes prevents progression of heart failure. Circulation. 2002;106(19):2491–2496. doi: 10.1161/01.CIR.0000036600.39600.66. [DOI] [PubMed] [Google Scholar]
- Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB Decreased catecholamine sensitivity and β-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307(4):205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986;59(3):297–309. doi: 10.1161/01.RES.59.3.297. [DOI] [PubMed] [Google Scholar]
- Bunemann M, Gerhardstein BL, Gao T, Hosey MM Functional regulation of L-type calcium channels via protein kinase A-mediated phosphorylation of the beta(2) subunit. J Biol Chem. 1999;274(48):33851–33854. doi: 10.1074/jbc.274.48.33851. [DOI] [PubMed] [Google Scholar]
- Chen-Izu Y, Xiao RP, Izu LT, Cheng H, Kuschel M, Spurgeon H, Lakatta EG G(i)-dependent localization of beta(2)-adrenergic receptor signaling to L-type Ca2+ channels. Biophys J. 2000;79(5):2547–2556. doi: 10.1016/S0006-3495(00)76495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, Crow MT The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3'-kinase. Circ Res. 2000;87(12):1172–1179. doi: 10.1161/01.RES.87.12.1172. [DOI] [PubMed] [Google Scholar]
- Communal C, Singh K, Sawyer DB, Colucci WS (1999) Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis : role of a pertussis toxin-sensitive G protein. Circulation 100(22): 2210−2212
- Curran J, Hinton MJ, Rios E, Bers DM, Shannon TR Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res. 2007;100(3):391–398. doi: 10.1161/01.RES.0000258172.74570.e6. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Kobilka BK, Strader DJ, Benovic JL, Dohlman HG, Frielle T, Bolanowski MA, Bennett CD, Rands E, Diehl RE, Mumford RA, Slater EE, Sigal IS, Caron MG, Lefkowitz RJ, Strader CD Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986;321(6065):75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Egom EE, Bae JS, Capel R, Richards M, Ke Y, Pharithi RB, Maher V, Kruzliak P, Lei M Effect of sphingosine-1-phosphate on L-type calcium current and Ca2+ transient in rat ventricular myocytes. Mol Cell Biochem. 2016;419(1-2):83–92. doi: 10.1007/s11010-016-2752-8. [DOI] [PubMed] [Google Scholar]
- Finlin BS, Crump SM, Satin J, Andres DA Regulation of voltage-gated calcium channel activity by the Rem and Rad GTPases. Proc Natl Acad Sci USA. 2003;100(24):14469–14474. doi: 10.1073/pnas.2437756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischmeister R, Castro LR, Abi-Gerges A, Rochais F, Jurevicius J, Leroy J, Vandecasteele G Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circ Res. 2006;99(8):816–828. doi: 10.1161/01.RES.0000246118.98832.04. [DOI] [PubMed] [Google Scholar]
- Fu Y, Westenbroek RE, Scheuer T, Catterall WA Phosphorylation sites required for regulation of cardiac calcium channels in the fight-or-flight response. Proc Natl Acad Sci USA. 2013;110(48):19621–19626. doi: 10.1073/pnas.1319421110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Westenbroek RE, Scheuer T, Catterall WA (2014) Basal and beta-adrenergic regulation of the cardiac calcium channel CaV1.2 requires phosphorylation of serine 1700. Proc Natl Acad Sci USA 111(46): 16598−16603
- Ganesan AN, Maack C, Johns DC, Sidor A, O'Rourke B Beta-adrenergic stimulation of L-type Ca2+ channels in cardiac myocytes requires the distal carboxyl terminus of alpha1C but not serine 1928. Circ Res. 2006;98(2):e11–18. doi: 10.1161/01.RES.0000202692.23001.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier C, Tavernier G, Charpentier F, Langin D, Le Marec H Functional beta3-adrenoceptor in the human heart. J Clin Invest. 1996;98(2):556–562. doi: 10.1172/JCI118823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardstein BL, Puri TS, Chien AJ, Hosey MM Identification of the sites phosphorylated by cyclic AMP-dependent protein kinase on the beta 2 subunit of L-type voltage-dependent calcium channels. Biochemistry. 1999;38(32):10361–10370. doi: 10.1021/bi990896o. [DOI] [PubMed] [Google Scholar]
- Ginsburg KS, Bers DM (2004) Modulation of excitation-contraction coupling by isoproterenol in cardiomyocytes with controlled SR Ca2+ load and Ca2+ current trigger. J Physiol 556(Pt 2): 463−480
- Gray PC, Johnson BD, Westenbroek RE, Hays LG, Yates JR, 3rd, Scheuer T, Catterall WA, Murphy BJ Primary structure and function of an A kinase anchoring protein associated with calcium channels. Neuron. 1998;20(5):1017–1026. doi: 10.1016/S0896-6273(00)80482-1. [DOI] [PubMed] [Google Scholar]
- Grimm M, Brown JH Beta-adrenergic receptor signaling in the heart: role of CaMKII. J Mol Cell Cardiol. 2010;48(2):322–330. doi: 10.1016/j.yjmcc.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase H, Karczewski P, Beckert R, Krause EG Phosphorylation of the L-type calcium channel beta subunit is involved in beta-adrenergic signal transduction in canine myocardium. FEBS Lett. 1993;335(2):217–222. doi: 10.1016/0014-5793(93)80733-B. [DOI] [PubMed] [Google Scholar]
- Hagemann D, Kuschel M, Kuramochi T, Zhu W, Cheng H, Xiao R-P Frequency-encoding Thr17 phospholamban phosphorylation is independent of Ser16 phosphorylation in cardiac myocytes. J Biol Chem. 2000;275(29):22532–22536. doi: 10.1074/jbc.C000253200. [DOI] [PubMed] [Google Scholar]
- Hain J, Onoue H, Mayrleitner M, Fleischer S, Schindler H Phosphorylation modulates the function of the calcium release channel of sarcoplasmic reticulum from cardiac muscle. J Biol Chem. 1995;270(5):2074–2081. doi: 10.1074/jbc.270.5.2074. [DOI] [PubMed] [Google Scholar]
- He JQ, Balijepalli RC, Haworth RA, Kamp TJ Crosstalk of beta-adrenergic receptor subtypes through Gi blunts beta-adrenergic stimulation of L-type Ca2+ channels in canine heart failure. Circ Res. 2005;97(6):566–573. doi: 10.1161/01.RES.0000181160.31851.05. [DOI] [PubMed] [Google Scholar]
- Heubach JF, Ravens U, Kaumann AJ Epinephrine activates both Gs and Gi pathways, but norepinephrine activates only the Gs pathway through human beta2-adrenoceptors overexpressed in mouse heart. Mol Pharmacol. 2004;65(5):1313–1322. doi: 10.1124/mol.65.5.1313. [DOI] [PubMed] [Google Scholar]
- Hohaus A, Person V, Behlke J, Schaper J, Morano I, Haase H The carboxyl-terminal region of ahnak provides a link between cardiac L-type Ca2+ channels and the actin-based cytoskeleton. FASEB J. 2002;16(10):1205–1216. doi: 10.1096/fj.01-0855com. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol. 2005;171(3):537–547. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huke S, Bers DM Ryanodine receptor phosphorylation at Serine 2030, 2808 and 2814 in rat cardiomyocytes. Biochem Biophys Res Commun. 2008;376(1):80–85. doi: 10.1016/j.bbrc.2008.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme JT, Ahn M, Hauschka SD, Scheuer T, Catterall WA A novel leucine zipper targets AKAP15 and cyclic AMP-dependent protein kinase to the C terminus of the skeletal muscle Ca2+ channel and modulates its function. J Biol Chem. 2002;277(6):4079–4087. doi: 10.1074/jbc.M109814200. [DOI] [PubMed] [Google Scholar]
- Hulme JT, Lin TW, Westenbroek RE, Scheuer T, Catterall WA (2003) Beta-adrenergic regulation requires direct anchoring of PKA to cardiac CaV1.2 channels via a leucine zipper interaction with A kinase-anchoring protein 15. Proc Natl Acad Sci USA 100(22): 13093−13098
- Hulme JT, Westenbroek RE, Scheuer T, Catterall WA (2006) Phosphorylation of serine 1928 in the distal C-terminal domain of cardiac CaV1.2 channels during beta1-adrenergic regulation. Proc Natl Acad Sci USA 103(44): 16574−16579
- Johnson DM, Antoons G Arrhythmogenic mechanisms in heart failure: linking β-adrenergic stimulation, stretch, and calcium. Front Physiol. 2018;9:1453. doi: 10.3389/fphys.2018.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval OM, Guan X, Wu Y, Joiner ML, Gao Z, Chen B, Grumbach IM, Luczak ED, Colbran RJ, Song LS, Hund TJ, Mohler PJ, Anderson ME (2010) CaV1.2 beta-subunit coordinates CaMKII-triggered cardiomyocyte death and afterdepolarizations. Proc Natl Acad Sci USA 107(11): 4996−5000
- Kumari N, Gaur H, Bhargava A Cardiac voltage gated calcium channels and their regulation by β-adrenergic signaling. Life Sci. 2018;194:139–149. doi: 10.1016/j.lfs.2017.12.033. [DOI] [PubMed] [Google Scholar]
- Kuschel M, Zhou YY, Spurgeon HA, Bartel S, Karczewski P, Zhang SJ, Krause EG, Lakatta EG, Xiao RP beta2-adrenergic cAMP signaling is uncoupled from phosphorylation of cytoplasmic proteins in canine heart. Circulation. 1999;99(18):2458–2465. doi: 10.1161/01.CIR.99.18.2458. [DOI] [PubMed] [Google Scholar]
- Kushnir A, Shan J, Betzenhauser MJ, Reiken S, Marks AR Role of CaMKIIdelta phosphorylation of the cardiac ryanodine receptor in the force frequency relationship and heart failure. Proc Natl Acad Sci USA. 2010;107(22):10274–10279. doi: 10.1073/pnas.1005843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG Beyond Bowditch: the convergence of cardiac chronotropy and inotropy. Cell Calcium. 2004;35(6):629–642. doi: 10.1016/j.ceca.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Li Y, Kranias EG, Mignery GA, Bers DM Protein kinase A phosphorylation of the ryanodine receptor does not affect calcium sparks in mouse ventricular myocytes. Circ Res. 2002;90(3):309–316. doi: 10.1161/hh0302.105660. [DOI] [PubMed] [Google Scholar]
- Liu G, Papa A, Katchman AN, Zakharov SI, Roybal D, Hennessey JA, Kushner J, Yang L, Chen BX, Kushnir A, Dangas K, Gygi SP, Pitt GS, Colecraft HM, Ben-Johny M, Kalocsay M, Marx SO (2020) Mechanism of adrenergic Ca(V)1.2 stimulation revealed by proximity proteomics. Nature 577(7792): 695−700
- Lüss H, Klein-Wiele O, BokníK P, Herzig S, Knapp J, Linck B, Müller FU, Scheld HH, Schmid C, Schmitz W, Neumann J Regional expression of protein phosphatase type 1 and 2A catalytic subunit isoforms in the human heart. J Mol Cell Cardiol. 2000;32(12):2349–2359. doi: 10.1006/jmcc.2000.1265. [DOI] [PubMed] [Google Scholar]
- MacDonnell SM, Garcia-Rivas G, Scherman JA, Kubo H, Chen X, Valdivia H, Houser SR Adrenergic regulation of cardiac contractility does not involve phosphorylation of the cardiac ryanodine receptor at serine 2808. Circ Res. 2008;102(8):e65–72. doi: 10.1161/CIRCRESAHA.108.174722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92(8):904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- Means CK, Brown JH Sphingosine-1-phosphate receptor signalling in the heart. Cardiovasc Res. 2009;82(2):193–200. doi: 10.1093/cvr/cvp086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger JM, Westfall MV Covalent and noncovalent modification of thin filament action. Circ Res. 2004;94(2):146–158. doi: 10.1161/01.RES.0000110083.17024.60. [DOI] [PubMed] [Google Scholar]
- Minneman KP, Pittman RN, Molinoff PB beta-adrenergic receptor subtypes: properties, distribution, and regulation. Annu Rev Neurosci. 1981;4(1):419–461. doi: 10.1146/annurev.ne.04.030181.002223. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Bunemann M, Schmitteckert E, Lohse MJ, Engelhardt S Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching beta1-adrenergic but locally confined beta2-adrenergic receptor-mediated signaling. Circ Res. 2006;99(10):1084–1091. doi: 10.1161/01.RES.0000250046.69918.d5. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, Lohse MJ, Korchev YE, Harding SE, Gorelik J Beta2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science. 2010;327(5973):1653–1657. doi: 10.1126/science.1185988. [DOI] [PubMed] [Google Scholar]
- Ogrodnik J, Niggli E (2010) Increased Ca2+ leak and spatiotemporal coherence of Ca2+ release in cardiomyocytes during beta-adrenergic stimulation. J Physiol 588(Pt 1): 225−242
- Papa A, Del Rivero Morfin PJ, Chen BX, Yang L, Katchman AN, Zakharov SI, Liu G, Bohnen MS, Zheng V, Katz M, Subramaniam S, Hirsch JA, Weiss S, Dascal N, Karlin A, Pitt GS, Colecraft HM, Ben Johny M, Marx SO A membrane-associated phosphoswitch in Rad controls adrenergic regulation of cardiac calcium channels. J Clin Invest. 2024;134(5):e176943. doi: 10.1172/JCI176943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa A, Zakharov SI, Katchman AN, Kushner JS, Chen BX, Yang L, Liu G, Jimenez AS, Eisert RJ, Bradshaw GA, Dun W, Ali SR, Rodriques A, Zhou K, Topkara V, Yang M, Morrow JP, Tsai EJ, Karlin A, Wan E, Kalocsay M, Pitt GS, Colecraft HM, Ben-Johny M, Marx SO (2022) Rad regulation of Ca(V)1.2 channels controls cardiac fight-or-flight response. Nat Cardiovasc Res 1(11): 1022−1038
- Paur H, Wright PT, Sikkel MB, Tranter MH, Mansfield C, O'Gara P, Stuckey DJ, Nikolaev VO, Diakonov I, Pannell L, Gong H, Sun H, Peters NS, Petrou M, Zheng Z, Gorelik J, Lyon AR, Harding SE High levels of circulating epinephrine trigger apical cardiodepression in a beta2-adrenergic receptor/Gi-dependent manner: a new model of Takotsubo cardiomyopathy. Circulation. 2012;126(6):697–706. doi: 10.1161/CIRCULATIONAHA.112.111591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter W, Day P, Agrawal R, Bruss MD, Granier S, Wang YL, Rasmussen SG, Horner K, Wang P, Lei T, Patterson AJ, Kobilka B, Conti M Signaling from beta1- and beta2-adrenergic receptors is defined by differential interactions with PDE4. EMBO J. 2008;27(2):384–393. doi: 10.1038/sj.emboj.7601968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam M, Gao S, Hong C, Fefelova N, Nowycky MC, Xie LH, Periasamy M, Babu GJ Ablation of phospholamban and sarcolipin results in cardiac hypertrophy and decreased cardiac contractility. Cardiovasc Res. 2011;89(2):353–361. doi: 10.1093/cvr/cvq294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Li M, Mika D, Fu Q, Kim S, Phan J, Shen A, Vandecasteele G, Xiang YK Heterologous desensitization of cardiac beta-adrenergic signal via hormone-induced betaAR/arrestin/PDE4 complexes. Cardiovasc Res. 2017;113(6):656–670. doi: 10.1093/cvr/cvx036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shizukuda Y, Buttrick PM Subtype specific roles of beta-adrenergic receptors in apoptosis of adult rat ventricular myocytes. J Mol Cell Cardiol. 2002;34(7):823–831. doi: 10.1006/jmcc.2002.2020. [DOI] [PubMed] [Google Scholar]
- Simmerman HK, Collins JH, Theibert JL, Wegener AD, Jones LR Sequence analysis of phospholamban. Identification of phosphorylation sites and two major structural domains. J Biol Chem. 1986;261(28):13333–13341. [PubMed] [Google Scholar]
- Song LS, Wang SQ, Xiao RP, Spurgeon H, Lakatta EG, Cheng H β-Adrenergic stimulation synchronizes intracellular Ca2+ release during excitation-contraction coupling in cardiac myocytes. Circ Res. 2001;88(8):794–801. doi: 10.1161/hh0801.090461. [DOI] [PubMed] [Google Scholar]
- Takasago T, Imagawa T, Furukawa K, Ogurusu T, Shigekawa M Regulation of the cardiac ryanodine receptor by protein kinase-dependent phosphorylation. J Biochem. 1991;109(1):163–170. doi: 10.1093/oxfordjournals.jbchem.a123339. [DOI] [PubMed] [Google Scholar]
- Toschi-Dias E, Rondon MUPB, Cogliati C, Paolocci N, Tobaldini E, Montano N Contribution of autonomic reflexes to the hyperadrenergic state in heart failure. Front Neurosci. 2017;11:162. doi: 10.3389/fnins.2017.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerer M, Böhm M, Elce JS, Erdmann E, Lohse MJ Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation. 1993;87(2):454–463. doi: 10.1161/01.CIR.87.2.454. [DOI] [PubMed] [Google Scholar]
- Wang W, Wehrens XH (2010) Stress synchronizes calcium release and promotes SR calcium leak. J Physiol 588(Pt 3): 391−392
- Wang Y, De Arcangelis V, Gao X, Ramani B, Jung YS, Xiang Y Norepinephrine- and epinephrine-induced distinct beta2-adrenoceptor signaling is dictated by GRK2 phosphorylation in cardiomyocytes. J Biol Chem. 2008;283(4):1799–1807. doi: 10.1074/jbc.M705747200. [DOI] [PubMed] [Google Scholar]
- Weber DK, Reddy UV, Wang S, Larsen EK, Gopinath T, Gustavsson MB, Cornea RL, Thomas DD, De Simone A, Veglia G Structural basis for allosteric control of the SERCA-Phospholamban membrane complex by Ca2+ and phosphorylation. Elife. 2021;10:e66226. doi: 10.7554/eLife.66226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci USA. 2006;103(3):511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, Coromilas J, Landry DW, Marks AR Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004a;304(5668):292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken SR, Marks AR Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004b;94(6):e61–70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- Wess J G-protein-coupled receptors: molecular mechanisms involved in receptor activation and selectivity of G-protein recognition. FASEB J. 1997;11(5):346–354. doi: 10.1096/fasebj.11.5.9141501. [DOI] [PubMed] [Google Scholar]
- Wright PT, Nikolaev VO, O'Hara T, Diakonov I, Bhargava A, Tokar S, Schobesberger S, Shevchuk AI, Sikkel MB, Wilkinson R, Trayanova NA, Lyon AR, Harding SE, Gorelik J Caveolin-3 regulates compartmentation of cardiomyocyte beta2-adrenergic receptor-mediated cAMP signaling. J Mol Cell Cardiol. 2014;67:38–48. doi: 10.1016/j.yjmcc.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Gao Z, Chen B, Koval OM, Singh MV, Guan X, Hund TJ, Kutschke W, Sarma S, Grumbach IM, Wehrens XH, Mohler PJ, Song LS, Anderson ME Calmodulin kinase II is required for fight or flight sinoatrial node physiology. Proc Natl Acad Sci USA. 2009;106(14):5972–5977. doi: 10.1073/pnas.0806422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Li J, Xu L, Lin L, Chen Y-H Mechanistic and therapeutic perspectives for cardiac arrhythmias: beyond ion channels. Sci China Life Sci. 2017;60(4):348–355. doi: 10.1007/s11427-016-9005-6. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Kobilka BK Myocyte adrenoceptor signaling pathways. Science. 2003;300(5625):1530–1532. doi: 10.1126/science.1079206. [DOI] [PubMed] [Google Scholar]
- Xiao B, Jiang MT, Zhao M, Yang D, Sutherland C, Lai FA, Walsh MP, Warltier DC, Cheng H, Chen SR Characterization of a novel PKA phosphorylation site, serine-2030, reveals no PKA hyperphosphorylation of the cardiac ryanodine receptor in canine heart failure. Circ Res. 2005;96(8):847–855. doi: 10.1161/01.RES.0000163276.26083.e8. [DOI] [PubMed] [Google Scholar]
- Xiao B, Tian X, Xie W, Jones PP, Cai S, Wang X, Jiang D, Kong H, Zhang L, Chen K, Walsh MP, Cheng H, Chen SR Functional consequence of protein kinase A-dependent phosphorylation of the cardiac ryanodine receptor: sensitization of store overload-induced Ca2+ release. J Biol Chem. 2007;282(41):30256–30264. doi: 10.1074/jbc.M703510200. [DOI] [PubMed] [Google Scholar]
- Xiao RP Beta-adrenergic signaling in the heart: dual coupling of the beta2-adrenergic receptor to G(s) and G(i) proteins. Sci STKE. 2001;2001(104):re15. doi: 10.1126/stke.2001.104.re15. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Avdonin P, Zhou YY, Cheng H, Akhter SA, Eschenhagen T, Lefkowitz RJ, Koch WJ, Lakatta EG Coupling of beta2-adrenoceptor to Gi proteins and its physiological relevance in murine cardiac myocytes. Circ Res. 1999;84(1):43–52. doi: 10.1161/01.RES.84.1.43. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Hohl C, Altschuld R, Jones L, Livingston B, Ziman B, Tantini B, Lakatta EG Beta 2-adrenergic receptor-stimulated increase in cAMP in rat heart cells is not coupled to changes in Ca2+ dynamics, contractility, or phospholamban phosphorylation. J Biol Chem. 1994;269(29):19151–19156. doi: 10.1016/S0021-9258(17)32287-1. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Lakatta EG Beta 1-adrenoceptor stimulation and beta 2-adrenoceptor stimulation differ in their effects on contraction, cytosolic Ca2+, and Ca2+ current in single rat ventricular cells. Circ Res. 1993;73(2):286–300. doi: 10.1161/01.RES.73.2.286. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Zhu W, Zheng M, Cao C, Zhang Y, Lakatta EG, Han Q Subtype-specific alpha1- and beta-adrenoceptor signaling in the heart. Trends Pharmacol Sci. 2006;27(6):330–337. doi: 10.1016/j.tips.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Xu X, Kaindl J, Clark MJ, Hubner H, Hirata K, Sunahara RK, Gmeiner P, Kobilka BK, Liu X Binding pathway determines norepinephrine selectivity for the human beta(1)AR over beta(2)AR. Cell Res. 2021;31(5):569–579. doi: 10.1038/s41422-020-00424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Marx SO, Colecraft HM (2010) Molecular mechanisms, and selective pharmacological rescue, of Rem-inhibited CaV1.2 channels in heart. Circ Res 107(5): 620−630
- Yang HQ, Wang LP, Gong YY, Fan XX, Zhu SY, Wang XT, Wang YP, Li LL, Xing X, Liu XX, Ji GS, Hou T, Zhang Y, Xiao RP, Wang SQ beta2-adrenergic stimulation compartmentalizes beta1 signaling into nanoscale local domains by targeting the C-terminus of beta1-adrenoceptors. Circ Res. 2019;124(9):1350–1359. doi: 10.1161/CIRCRESAHA.118.314322. [DOI] [PubMed] [Google Scholar]
- Yang HQ, Zhou P, Wang LP, Zhao YT, Ren YJ, Guo YB, Xu M, Wang SQ Compartmentalized beta1-adrenergic signalling synchronizes excitation-contraction coupling without modulating individual Ca2+ sparks in healthy and hypertrophied cardiomyocytes. Cardiovasc Res. 2020;116(13):2069–2080. doi: 10.1093/cvr/cvaa013. [DOI] [PubMed] [Google Scholar]
- Yang L, Dai DF, Yuan C, Westenbroek RE, Yu H, West N, de la Iglesia HO, Catterall WA (2016) Loss of beta-adrenergic-stimulated phosphorylation of CaV1.2 channels on Ser1700 leads to heart failure. Proc Natl Acad Sci USA 113(49): E7976−E7985
- Zhang SJ, Cheng H, Zhou YY, Wang DJ, Zhu W, Ziman B, Spurgoen H, Lefkowitz RJ, Lakatta EG, Koch WJ, Xiao RP Inhibition of spontaneous beta 2-adrenergic activation rescues beta 1-adrenergic contractile response in cardiomyocytes overexpressing beta 2-adrenoceptor. J Biol Chem. 2000;275(28):21773–21779. doi: 10.1074/jbc.M909484199. [DOI] [PubMed] [Google Scholar]
- Zhihao L, Jingyu N, Lan L, Michael S, Rui G, Xiyun B, Xiaozhi L, Guanwei F SERCA2a: a key protein in the Ca2+ cycle of the heart failure. Heart Fail Rev. 2020;25(3):523–535. doi: 10.1007/s10741-019-09873-3. [DOI] [PubMed] [Google Scholar]
- Zhou P, Zhao YT, Guo YB, Xu SM, Bai SH, Lakatta EG, Cheng H, Hao XM, Wang SQ Beta-adrenergic signaling accelerates and synchronizes cardiac ryanodine receptor response to a single L-type Ca2+ channel. Proc Natl Acad Sci USA. 2009;106(42):18028–18033. doi: 10.1073/pnas.0906560106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YY, Song LS, Lakatta EG, Xiao RP, Cheng H (1999) Constitutive beta2-adrenergic signalling enhances sarcoplasmic reticulum Ca2+ cycling to augment contraction in mouse heart. J Physiol 521 Pt 2(Pt 2): 351−361
- Zhu WZ, Wang SQ, Chakir K, Yang D, Zhang T, Brown JH, Devic E, Kobilka BK, Cheng H, Xiao RP Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J Clin Invest. 2003;111(5):617–625. doi: 10.1172/JCI200316326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci USA. 2001;98(4):1607–1612. doi: 10.1073/pnas.98.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]


