Abstract
Calcium ions (Ca2+) play a crucial role as secondary messengers in both excitable and non-excitable cells. A complex system of proteins and molecules involved in calcium handling allows Ca2+ signals to be transduced. In cancer cells, mutations, aberrant expression, and dysregulation of these calcium handling toolkit proteins disrupt the normal Ca2+ flux between extracellular space, cytosol, endoplasmic reticulum and mitochondria, as well as the spatio-temporal patterns of Ca2+ signalling. This leads to the dysregulation of calcium-dependent effectors that control key signaling pathways involved in cancer cell proliferation, survival and invasion. Although there has been progressing in understanding the remodelling of calcium homeostasis in cancer cells and identifying key calcium transport molecules that promote malignant phenotypes, much work remains to be done to translate these fundamental findings into new tools for diagnosing and treating cancer by targeting Ca2+ homeostasis.
Keywords: Calcium homeostasis, Cancer, Therapy
INTRODUCTION
As the most versatile second messenger in vivo, calcium ions (Ca2+) are involved in a wide variety of cellular and physiological processes, including proliferation, cell death, migration, gene transcription, muscle contraction, etc. (Fiorio Pla and Gkika 2020). Notably, accumulating evidence over the past decade has shown that dysregulation of Ca2+ homeostasis is an important driving force in malignant transformation, tumour progression and angiogenesis (Marchi et al. 2020). Here, we focus on advances in the understanding of Ca2+ signalling behaviour and its role in tumour initiation, progression and treatment.
Under physiological conditions, Ca2+ concentrations in cells are maintained in a stable state with a gradient difference of Ca2+ by a series of Ca2+ channels and pumps. The cytosolic Ca2+ concentration needs to be maintained at a low level (∼100 nmol/L), approximately 104-fold lower than the extracellular milieu (Marchi et al. 2020). In addition to the exchange with the extracellular Ca2+, there are also several Ca2+ storage sites within the cell, such as the endoplasmic reticulum (ER) (∼0.5 mmol/L) (Kim et al. 2021), Golgi (∼200 μmol/L) (Lissandron et al. 2010), lysosomes (∼500 μmol/L) (Zhong et al. 2017) and mitochondria (∼100–200 nmol/L) (Giorgi et al. 2018b). Under the control of a complex set of Ca2+ pumps, channels, and exchangers distributed at either plasma membrane or organelle membranes, changes in cytosolic Ca2+ or Ca2+ transport between organelles are perceived as specific signals to activate downstream responses that affect cell fate.
Intracellular Ca2+ homeostasis is controlled by Ca2+ release from organelles or influx from extracellular spaces. ER-located Ca2+ channels, inositol 1,4,5-trisphosphate receptors (IP3Rs) or ryanodine receptors (RyRs), are responsible for ER Ca2+ release in response to elevated IP3 levels following hormone or growth factor stimulation or increased extracellular Ca2+ influx (Zheng et al. 2023). In response to ER Ca2+ depletion, the ER-resident protein stromal interaction molecule 1 (STIM1) is activated and subsequently interacts with and activates the plasma membrane-resident Ca2+ channel ORAI1, leading to extracellular Ca2+ influx, a process termed store-operated Ca2+ entry (SOCE) (Benson and Trebak 2023). On the other hand, sarcoplasmic endoplasmic reticulum-type Ca2+ ATPases (SERCAs), which are ER-resident Ca2+ pumps, are able to pump cytosolic Ca2+ back into the ER. During ER Ca2+ overload, the ER Ca2+ channel protein transmembrane and coiled-coil domains 1 (TMCO1) can be activated to release Ca2+ into the cytosol by forming a Ca2+ selective channel through monomer-to-tetramer transformation, a process termed store overload-induced Ca2+ release (SOICR) (Fig. 1A) (Sumitomo 2016).
Figure 1.
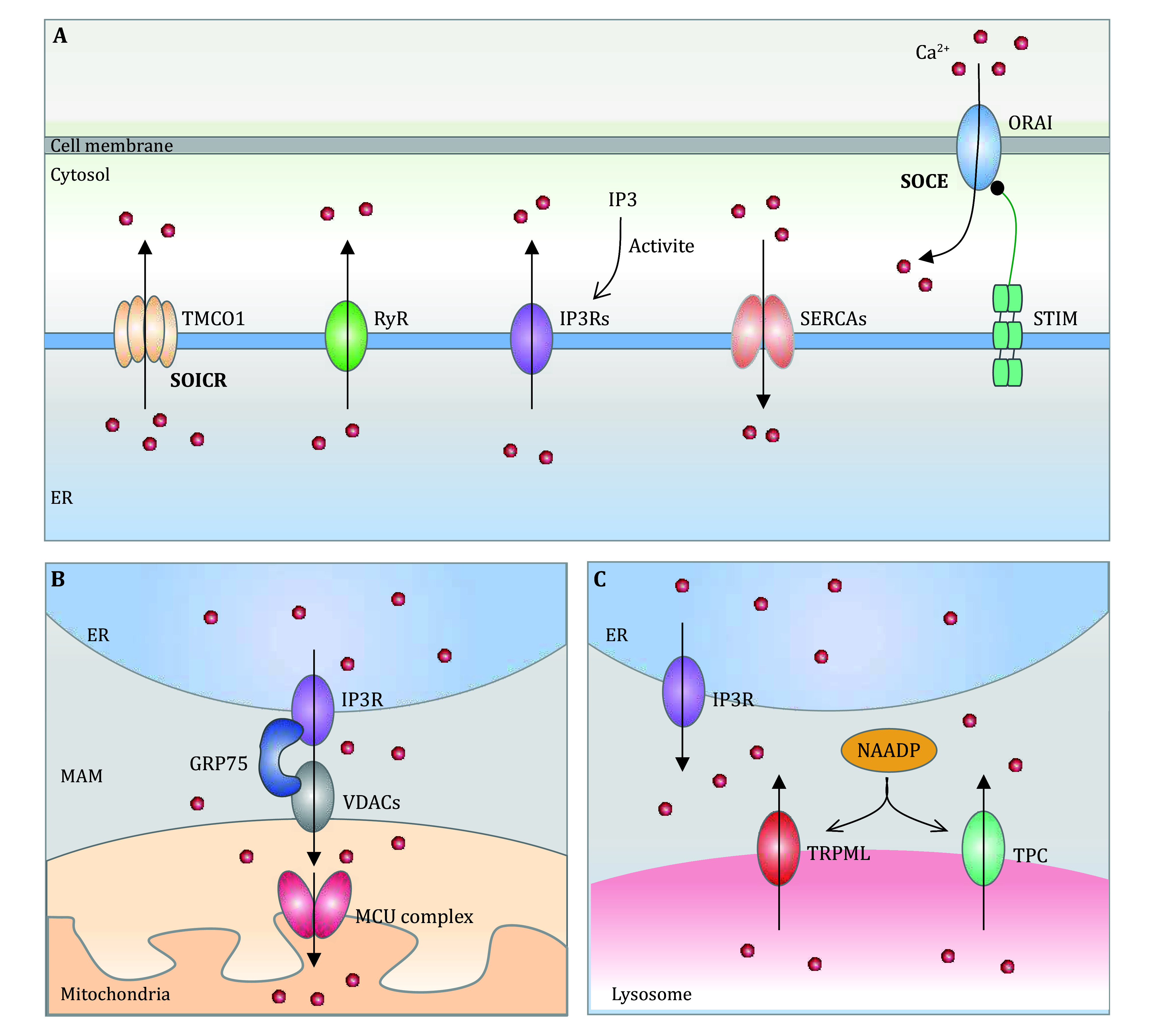
A graphical representation of calcium homeostasis attainment. A ER-Cytosol-Plasma membrane communications. ER-resident calcium pump proteins SERCAs are responsible for transporting Ca2+ from the cytosolic into the ER. IP3Rs, or RyRs mediate the release of calcium from the ER in response to the increased IP3 following hormone or growth factor stimulation, or increased cytosolic Ca2+ levels. When ER Ca2+ concentration is reduced, STIM can be activated to interact with and activate plasma membrane-localized Ca2+ channel ORAI1, leading to extracellular Ca2+ being influx and the subsequent SERCA-mediated ER Ca2+ refilling. Nevertheless, during ER Ca2+ overload, the TMCO1 can be stimulated to release Ca2+ into the cytosol by forming a selective Ca2+ channel through monomer-to-tetramer transformation, a process termed SOICR. B ER-Mitochondria communications. The ER and mitochondria are interconnected both structurally and functionally through a specialized microdomain known as the MAM. Within MAMs, several proteins such as Grp-75, VDAC1, and IP3R play a crucial role in regulating Ca2+ release from the ER and an efficient mitochondrial Ca2+ uptake. C ER–lysosome communications. Lysosomal Ca2+ depletion initiates the IP3R-mediated Ca2+ release from ER and subsequent lysosome Ca2+ refilling through the Ca2+ transporter or Ca2+/H+ exchanger (CAX). Furthermore, ER can restore Ca2+ from the lysosomes through the stimulation of two distinct mechanisms: the activation of TPC by NAADP, or the activation of TRPML1
In addition to the modulation of cytosolic Ca2+ to maintain intracellular Ca2+ homeostasis, Ca2+ transports between organelles, mainly ER-mitochondria or ER-lysosome, are also essential. For example, the mitochondria-associated ER membrane (MAM) is the interface between the ER and mitochondria and is involved in several biological processes, mainly by modulating ER-mitochondrial Ca2+ transport (de Ridder et al. 2023). It has been shown that ER-resident IP3R and mitochondria-localized voltage-dependent anion channels (VDACs) form the Ca2+ passage to transport Ca2+ from the ER to mitochondria, resulting in enhanced mitochondrial Ca2+ uptake across the mitochondrial outer membrane (Sander et al. 2021). The subsequent Ca2+ influx into the mitochondria was enforced by a Ca2+ gradient through the mitochondrial calcium uniporter (MCU) located across the inner mitochondrial membrane (Fig. 1B) (Murphy and Steenbergen 2021). In addition, it is worth noting that ER can replenish their Ca2+ stores from the lysosomes through the activation of two different pathways: the nicotinic acid adenine dinucleotide phosphate (NAADP)-evoked activation of two-pore channels (TPC) or the activation of mammalian mucolipin TRP channel subfamily (TRPML1) (Capel et al. 2015; Marchant et al. 2022; Rosato et al. 2021). There are numerous calcium-exchange pathways that play a crucial role in the regulation of calcium homeostasis that have not been enumerated in this review (Fig. 1C).
In short, normal cells maintain calcium homeostasis and modulate calcium signalling through a complex and interconnected system that operates in different cellular compartments. This system allows Ca2+ to act as a second messenger while preventing its potentially harmful effects. Conversely, cancer cells exhibit a number of abnormalities in this machinery, providing an opportunity for the development of innovative therapeutic agents, as discussed below (Zheng et al. 2023).
Ca2+ HOMEOSTASIS DYSREGULATION AND CARCINOGENESIS IN CLINICAL SETTINGS
Cancer progression may be influenced by abnormal Ca2+ influx. Clinical evidence suggests that hypercalcaemia is often associated with the occurrence of malignant cancer. This condition has been shown to promote the growth and spread of gastric, oesophageal (Anderson et al. 2019), cervical (Lei et al. 2022), breast (Das et al. 2020) and colorectal cancers (Anderson et al. 2019) and has been considered a hallmark of end-stage disease. In colorectal cancer, the intestine has the ability to absorb significant amounts of calcium through specific ion channels, leading to hypercalcaemia in many patients. Many recent findings suggest that even a small increase in intracellular calcium signalling may contribute to the development and progression of colorectal cancer, while sustained calcium influx and overload can lead to tumour cell death (Villalobos et al. 2017). This dual function of calcium signalling adds complexity to the understanding of colorectal cancer and may offer new avenues for clinical intervention. In addition, in prostate cancer, non-small cell lung cancer and colon cancer, moderate levels of Ca2+ contribute to tumour progression, while excessive cytoplasmic Ca2+ levels also cause cell death by stimulating downstream Ca2+-dependent sensors (Cui et al. 2017; Silvestri et al. 2023).
In addition to cytosolic Ca2+ homeostasis, organellar Ca2+ homeostasis is also critical for tumour development and progression. For example, low levels of Ca2+ in mitochondria contribute to the growth of colon and non-small cell lung cancers by controlling energy production. Conversely, excessive Ca2+ accumulation in the mitochondria can also trigger cell death (Cui et al. 2017).
In conclusion, changes in Ca2+ fluxes affect a complex network of intrinsic (e.g. metabolism, redox homeostasis) and extrinsic (e.g. antigen presentation, danger signalling) processes in cancer cells, which in turn influence malignant transformation, tumour growth, and responses to therapy (Marchi et al. 2020; Monteith et al. 2017). Therefore, it is crucial to improve our understanding of the mechanisms by which perturbation of Ca2+ homeostasis leads to tumour progression.
CALCIUM HOMEOSTASIS DISRUPTION AND CANCER HALLMARKS
The role of Ca2+ signalling in carcinogenesis and clinical status of cancer has been evidenced by the fact that altered Ca2+ homeostasis has been implicated in multiple cancer pathological processes. Further mechanistic study reveals that calcium homeostasis disruption can influence tumour growth, cancer cell death, metastasis and anti-immunity by modulating Ca2+ channels or pumps (Marchi et al. 2020) (Fig. 2).
Figure 2.
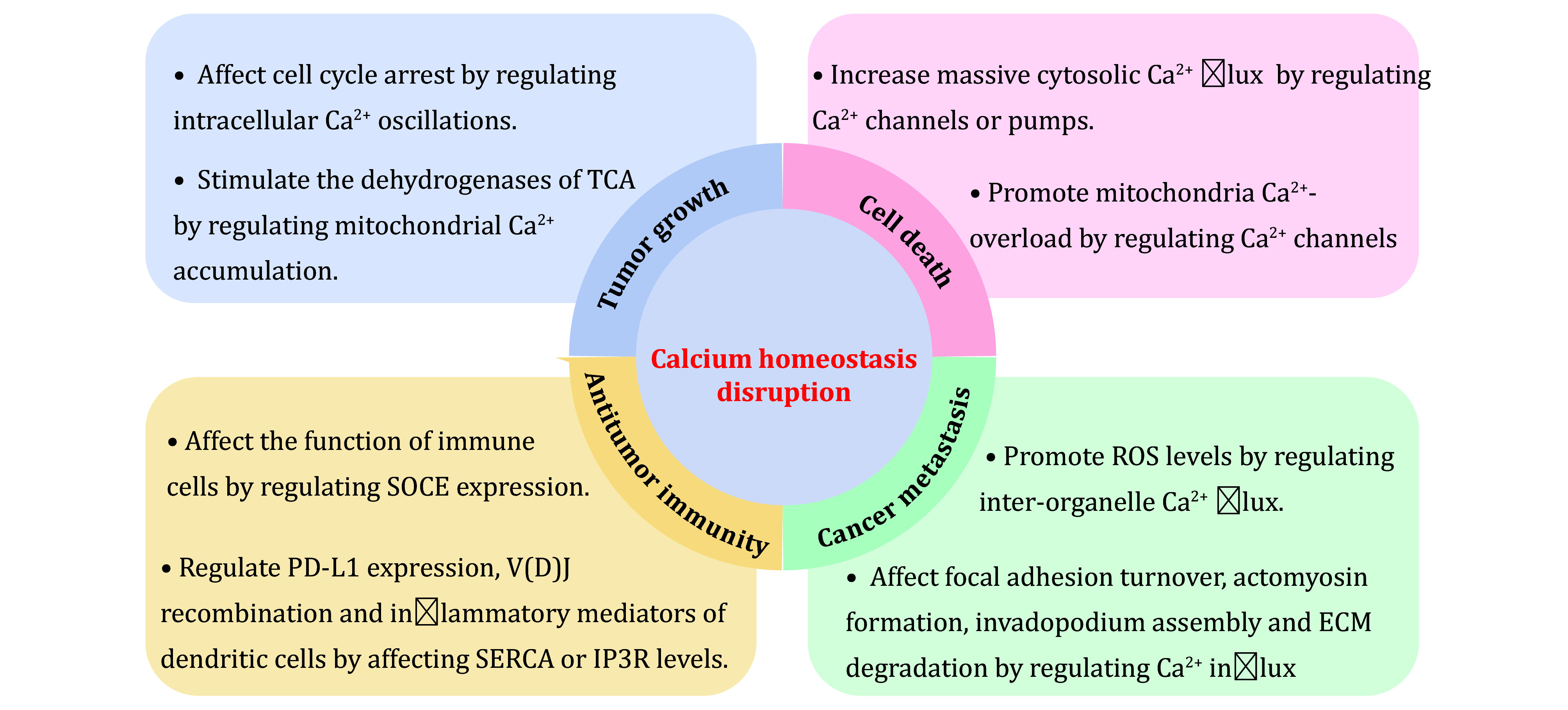
The relationship between calcium homeostasis disruption and cancer hallmarks. The examples demonstrate that disruption of calcium homeostasis is involved in mainly cancer characteristics, including tumour growth, cancer cell death, cancer metastasis, and antitumor immunity
Calcium homeostasis disruption and tumour growth
Aberrant cell cycle progression is one of the fundamental mechanisms underlying tumourigenesis (Liu et al. 2022). Changes in cytosolic Ca2+ can regulate the cell cycle by altering Ca2+ transporters, consistent with the fact that Ca2+ signalling is involved in the regulation of cancer cell proliferation. Chaochu Cui et al. reported that Orai1, as a SOCE channel protein, can regulate intracellular Ca2+ oscillations to prevent cell cycle arrest and promote cancer cell proliferation (Cui et al. 2018). A novel SOCE channel inhibitor, RP4010, has been shown to mediate intracellular Ca2+ flux to arrest the cell cycle at the G0/G1 phase, ultimately inhibiting tumour growth in oesophageal cancer (Cui et al. 2018). In addition, genetic inhibition of STIM1, the other member of the SOCE channel, can significantly inhibit cell proliferation by preventing cell cycle arrest at the S and G/M phases in cervical cancer (Chen et al. 2011). Genetic inhibition of STIM1/Orai1 or RP4010 may act as an inhibitor of SOCE-mediated Ca2+ signalling in a context-dependent manner.
In addition, genetic silencing or pharmacological inhibition (caffeine, 2-APB and xestospongin C, XeC) of IP3R3, has been shown to inhibit cancer cell proliferation by preventing the intracellular Ca2+ elevation (Guerra et al. 2019; Szatkowski et al. 2010; Ueasilamongkol et al. 2020). Similarly, it has been shown that Ca2+-dependent lysosomal exocytosis plays an important role in cancer growth and chemoresistance (Buratta et al. 2020). Inhibiting this mechanism reduces the invasiveness and chemoresistance of sarcoma cells, whereas increasing lysosomal exocytosis improves invasiveness and drug resistance (Machado et al. 2015). TRPML1 is an important regulator of lysosomal exocytosis. Indeed, cholesterol recycling via TRPML1-mediated lysosomal exocytosis promotes the growth of oncogenic HRAS-driven cancer cells. TRPML1 deletion in cancer cells inhibits cholesterol transport from lysosome to the plasma membrane, which lowers their growth rate (Jung et al. 2019). These have been developed as a potential target to inhibit tumour growth.
Low-level Ca2+ transport from the ER to the mitochondria has been shown to be essential for the maintenance of tumour growth (Cárdenas et al. 2016; Garbincius and Elrod 2022). The resulting mitochondrial Ca2+ accumulation can stimulate the key dehydrogenases of the Ca2+-regulated tricarboxylic acid cycle, suggesting that it is critical for the biosynthetic and bioenergetic needs of cancer cells (Csordás et al. 2018; Zhao et al. 2019). Consistent with the above findings, abnormal expression of MAM or MCU channels has been shown to promote cell proliferation in lung and colorectal cancers by inducing mitochondrial Ca2+ uptake (Arif et al. 2014; Liu et al. 2020; Zeng et al. 2018).
Calcium homeostasis disruption and cancer cell death
Low levels of Ca2+ are known to exert pro-survival functions by altering the cell cycle or promoting mitochondrial metabolism through Ca2+-dependent stimulation of TCA (tricarboxylic acid) cycle and OXPHOS (oxidative phosphorylation), but excessive cytosolic Ca2+ levels cause cell death (Loncke et al. 2021; Zheng et al. 2023). Indeed, increased Ca2+ influx from extracellular space through SOCE can promote Ca2+-induced apoptosis. Roberta Gualdani et al. reported that siRNA-mediated depletion of STIM1 can dramatically reduce cisplatin-induced ROS production by reducing Ca2+ entry and subsequently induce apoptosis in non-small cell lung carcinoma cells (Gualdani et al. 2019). The additional members of SOCE have been shown to elicit a similar role in prostate cancer cells. Downregulation of ORAI1 can protect cancer cells from apoptosis induced by several reagents, such as thapsigargin, oxaliplatin and tumour necrosis factor α (Flourakis et al. 2010).
Massive cytosolic Ca2+ flux, mainly from the ER, has been implicated in cancer cell death (Loncke et al. 2021). For example, Ki Cheong Park et al. showed that pharmacological inhibition of SERCA promotes Ca2+-dependent apoptosis during glucose deprivation in cancer stem-like cells (Park et al. 2018). Our data also show that TMCO1, as a SOICR channel protein, is overexpressed in colon cancer cells compared with normal controls (Zheng et al. 2022). Increased TMCO1 reduces Ca2+ levels in the ER store, which attenuates Ca2+ release from the ER to the cytosol, leading to the limited cytosolic Ca2+ dynamics upon drug treatment (TG and Stuarosporine) (Zheng et al. 2022). This mechanism may promote tumour growth and reduce staurosporine-induced apoptosis in colon cancer (Zheng et al. 2022). On the contrary, in bladder urothelial carcinoma, TMCO1 has been shown to have an opposite role, although whether its function is involved in ER Ca2+ regulation remains to be determined (Li et al. 2017). It is possible that it plays a different role in different cancer cells, depending on the context.
In addition, Ca2+ overload in the mitochondria also triggers cell death, mainly through Ca2+ transport from the ER to the mitochondria (Beaulant et al. 2022). For example, Xue Y showed that IP3R3 promotes apoptosis by enhancing Ca2+ transfer from the ER to the mitochondria, leading to the enhancement of cisplatin-induced apoptosis (Xue et al. 2021). Another MAM protein, VDAC1, has a similar function also by increasing mitochondrial Ca2+ influx (De Stefani et al. 2012). In conclusion, the promotion of massive cytosolic Ca2+ influx or mitochondrial Ca2+ overload has recently been proposed as a promising strategy to induce apoptosis in cancer cells.
Calcium homeostasis disruption and cancer metastasis
Approximately 90% of cancer-related deaths are due to metastasis (Bakir et al. 2020). Metastasis consists of a number of processes, including the migration of cancer cells from their original growth sites to colonize distant organs (Gerstberger et al. 2023; Pastushenko and Blanpain 2019). SOCE, as a major influx route of intracellular Ca2+, can affect focal adhesion turnover during cancer cell migration, which was an early event in cancer metastasis. Indeed, Sang Kwon Lee et al. reported that decreased SOCE disrupted focal adhesion turnover and actomyosin formation by impairing Ca2+ influx in breast cancer cells (Lee et al. 2022). In addition, Jianwei Sun et al. showed that STIM1- and Orai1-mediated Ca2+ oscillations can increase invadopodium assembly and extracellular matrix (ECM) degradation, leading to the promotion of melanoma invasion, the later event in cancer metastasis (Sun et al. 2014). These findings provide a mechanism and shed new light on SOCE-mediated Ca2+ signalling in cancer cell metastasis.
In addition, cancer cell metastasis can be regulated by SERCA-mediated intracellular Ca2+ flux. As a result, phosphorylated cofilin failed to regulate F-actin polymerisation and lamellipodium formation, thereby impairing lamellipodium-based migration (Shi et al. 2018).
MAM-associated proteins are also involved in cancer cell metastasis by regulating inter-organelle Ca2+ flux. As a master gatekeeper gene that regulates Ca2+ transport from the ER to the mitochondria, VDAC1 contributes to cell migration by promoting ROS levels produced by the electron transport chain (Arif et al. 2014). In addition, Xiuchao Wang et al. have shown that another mitochondrial protein, MCU, promotes pancreatic ductal adenocarcinoma cell migration and invasion by activating the Keap1-Nrf2 antioxidant program (Wang et al. 2022).
Calcium homeostasis disruption and antitumor immunity
SOCE-induced intracellular Ca2+ influx is essential for antitumour immunity, as abnormal SOCE proteins lead to the dysfunction of various immune cells, including T cells, B cells, natural killer (NK) cells, dendritic cells, mast cells, macrophages and neutrophils (Xie et al. 2016). Carl Weidinger et al. have shown that SOCE in CD8+ T cells is required to prevent the development of melanoma and colon cancer cells to control tumour growth (Weidinger et al. 2013). SOCE can regulate (cytotoxic T-lymphocyte) CTL degranulation, Fas ligand expression and TNF-α and IFN-γ production, which affect CTL cytotoxic function both in vitro and in vivo (Weidinger et al. 2013). In addition, antigen cross-presentation by dendritic cells (DCs) activates cytotoxic T-cell stimulation to enhance immunity against cancer (Nunes-Hasler et al. 2017). Deletion of SOCE impairs cross-presentation and DC migration by reducing Ca2+ signaling (Nunes-Hasler et al. 2017). Alternatively, lymphocytes mediate cytotoxicity by polarized release of cytotoxic granule contents towards their target cells (Maul-Pavicic et al. 2011). NK cells from SOCE-deficient patients exhibited defective SOCE and severely impaired exocytosis of cytotoxic granules, leading to impaired target cell lysis by reducing SOCE-mediated Ca2+ influx (Maul-Pavicic et al. 2011). These findings highlight an important role of SOCE in anti-tumour immunity and support the use of SOCE activity regulators in cancer therapy.
In addition, Ca2+ modulators are essential for anti-tumour immunity. Jun-Kyu Byun et al. showed that impaired SERCA activity can reduce cellular (Glutathione) GSH levels and subsequently upregulate PD-L1 expression under glutamine-limited conditions (Byun et al. 2020). This has been shown to suppress T cell-mediated anti-tumour activity (Byun et al. 2020). SERCA2 has also been reported to regulate V(D)J recombination. For example, Chun-Chin Chen et al. showed that mice with SERCA2-deficient B cells have decreased ER Ca2+ levels and increased cytosolic Ca2+ levels, resulting in a profound block in V(D)J recombination (Chen et al. 2021). Alternatively, IP3R-mediated Ca2+ mobilization can regulate NFAT activation, which suppresses cell survival and promotes the production of inflammatory mediators of dendritic cells (DC) (Marongiu et al. 2021). This suggests that Ca2+ mobilization may be a promising target for anti-inflammatory therapy or immunotherapies.
THERAPEUTIC VALUES OF TARGETING Ca2+ HOMEOSTASIS
Chemotherapy, the basic cancer treatment, is beneficial for patients with early-stage tumours but is inevitably associated with the emergence of drug resistance during treatment (Bukowski et al. 2020). Therefore, elucidating the mechanism of action of chemotherapeutic drugs may provide a better basis for improving the sensitivity of chemotherapeutic drugs to improve the prognosis of patients. Although chemotherapy drugs have multiple mechanisms of action, many drugs have the function of regulating Ca2+ homeostasis, which is achieved by regulating Ca2+ modulators. For example, paclitaxel, cisplatin and doxorubicin, which are common chemotherapy drugs, increase the cytosolic Ca2+ levels or promote Ca2+ overload in mitochondria, thereby enhancing Ca2+-induced apoptosis (Varghese et al. 2019).
In addition to the function of chemotherapeutic drugs in regulating Ca2+ homeostasis, the combination of Ca2+-related regulators and chemotherapeutic drugs to kill tumours has also come to our attention. Given the important role of Ca2+ modulators in cancer development, a number of Ca2+ activators or inhibitors have been developed. For example, Celastrol, the curcumin analogue F36, JQ-FT and stemphol, which are SERCA inhibitors, are able to target SERCA and inhibit its activity, thereby inducing apoptosis (Fan et al. 2014; Ji et al. 2018; Roti et al. 2018; Xu et al. 2020). In addition, inhibitors that target calcium channels have also been shown to kill tumours by inhibiting cell metastasis or increasing apoptosis in vitro or in vivo, including the imidazole derivative SKF-96365 (Yang et al. 2009), a SOCE inhibitor, Synta66 (Waldherr et al. 2020), an ORAI1 inhibitor, the IP3R blocker 2-aminoethoxydiphenyl borate (2APB) (Waldherr et al. 2020). Moreover, the combination of some FDA-approved inhibitors with chemotherapy drugs has also shown promising effects in cancer treatments. For example, amlodipine, an FDA-approved Ca2+ channel blocker, can significantly enhance the therapeutic response of tumor cells to gemcitabine (Principe et al. 2022). Alternatively, Heejin Lee reported that Ca2+ channel inhibitors (manidipine, lacidipine, benidipine and lometzine) screened from the FDA-approved compound library show a clear inhibitory effect on the growth of ovarian (cancer stem cells) CSCs (Lee et al. 2020). Other chemotherapeutic agents have been shown to regulate Ca2+ signalling and exert anti-cancer effects, although the underlying molecular mechanisms require further investigation.
Immunotherapy has become one of the most promising tumour treatment strategies in advanced cancer (Sharma et al. 2017). As mentioned above, activation of Ca2+ channels is necessary for the immune response, while dysregulation of Ca2+ homeostasis can lead to immune cell evasion in cancer cells (Shi et al. 2013). Therefore, based on the research on Ca2+ and tumour characteristics, it is reasonable to design a clinical strategy that combines the regulation of Ca2+ homeostasis with immunotherapy to achieve better therapeutic effects. It is worth noting that an ongoing project has used nanotechnology to deliver cytotoxic doses of Ca2+ into the tumour, followed by a combination with immunotherapy, which has so far shown promising anti-tumour effects (Zhao et al. 2021; Zheng et al. 2021) (Fig. 3).
Figure 3.
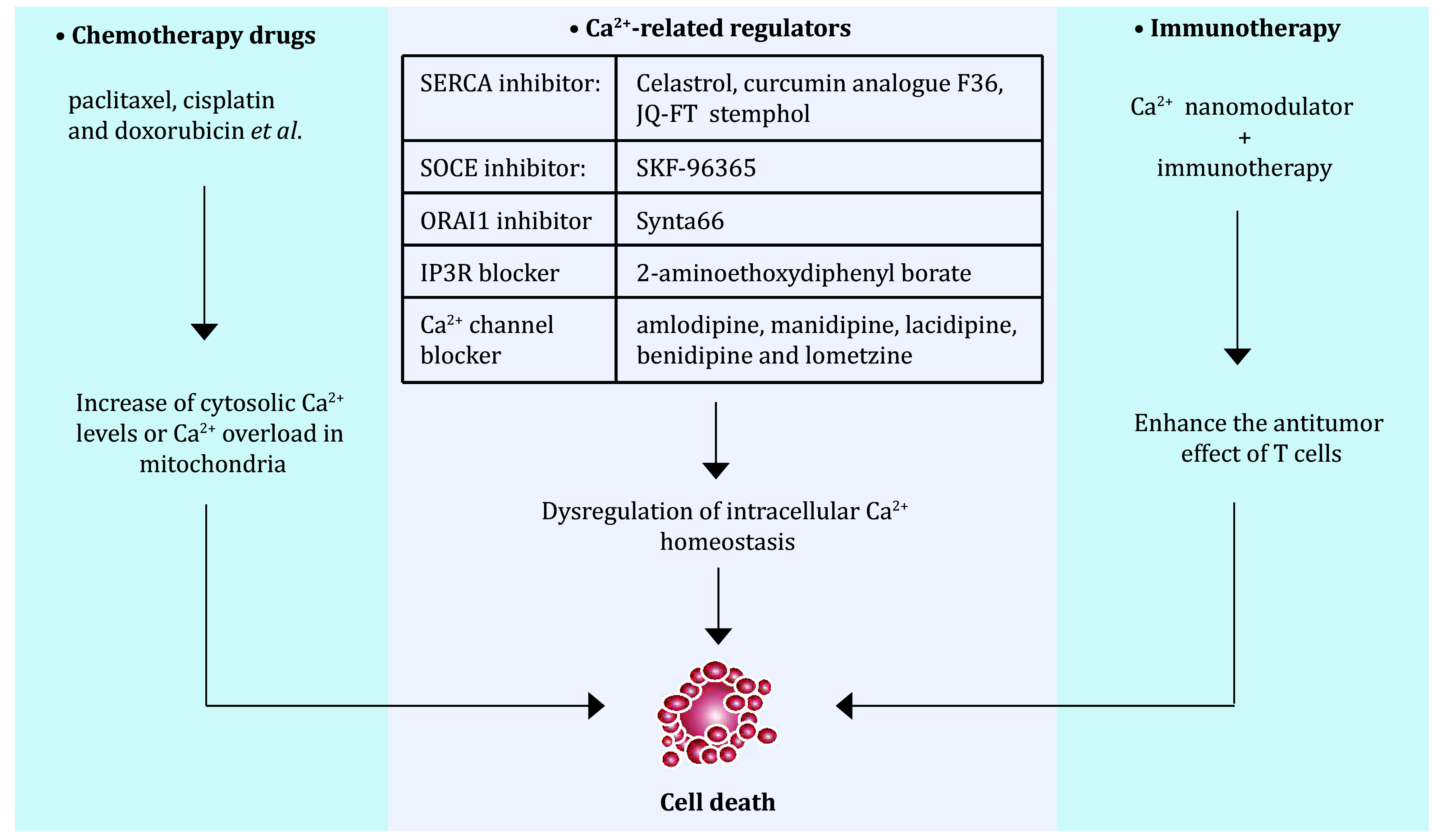
Targeting Ca2+ homeostasis as a potential therapeutic value. Relationship between targeted calcium homeostasis and tumor therapy, which contains Ca2+-related chemotherapy, combined-therapy and immunotherapy
REMARKS
Ca2+ homeostasis is a necessary factor in maintaining normal growth and development of normal cells (Garbincius and Elrod 2022). Intracellular Ca2+ homeostasis requires a variety of Ca2+ channels and transporters to be maintained (Giorgi et al. 2018a). It is obvious that the imbalance of Ca2+ homeostasis caused by the abnormality of these proteins, is one of the major culprits that cause cells from a normal physiological state to a pathological state, resulting in the occurrence of various diseases, including cancer (Zheng et al. 2023). Regulating Ca2+ homeostasis was a double-edged sword for cancer cells. Modulating Ca2+ levels can promote tumour growth by interfering with cell cycle arrest. Conversely, massive cytosolic Ca2+ or mitochondrial Ca2+ overload promotes cell death (Marchi et al. 2020). It is therefore crucial to increase our knowledge of the mechanisms by which Ca2+ modulator functions lead to such opposite cellular outcomes.
Increased understanding of the role of Ca2+ homeostasis has greatly advanced the field of cancer, and Ca2+-based cancer therapeutics have attracted increasing attention. Although common chemotherapeutic drugs targeting Ca2+ have not yet been developed, more and more studies have shown that a variety of chemotherapeutic drugs can regulate Ca2+ homeostasis and thereby affect the fate of cancer cells (Varghese et al. 2019). In addition, several inhibitors of Ca2+ modulators screened from the FDA's library of compounds can be combined with chemotherapeutic drugs to kill cancer cells. These suggest that disrupting Ca2+ homeostasis to promote cancer cell death has become a remarkable effect. What's more, Ca2+-based therapy combined with immunotherapy has become a hot topic, while the underlying molecular mechanism needs to be further explored.
Conflict of interest
Min Su, Shanliang Zheng, Hao Liu, Tie-Shan Tang and Ying Hu declare that they have no conflict of interest.
Compliance with Ethical Standards
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Shanliang Zheng, Email: zhengshanliang@hit.edu.cn.
Ying Hu, Email: huying@hit.edu.cn.
References
- Anderson KJ, Cormier RT, Scott PM Role of ion channels in gastrointestinal cancer. World J Gastroenterol. 2019;25(38):5732–5772. doi: 10.3748/wjg.v25.i38.5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif T, Vasilkovsky L, Refaely Y, Konson A, Shoshan-Barmatz V Silencing VDAC1 expression by siRNA inhibits cancer cell proliferation and tumor growth in vivo. Mol Ther Nucleic Acids. 2014;3(4):e159. doi: 10.1038/mtna.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakir B, Chiarella AM, Pitarresi JR, Rustgi AK EMT, MET, plasticity, and tumor metastasis. Trends Cell Biol. 2020;30(10):764–776. doi: 10.1016/j.tcb.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulant A, Dia M, Pillot B, Chauvin M-A, Ji-Cao J, Durand C, Bendridi N, Chanon S, Vieille-Marchiset A, Da Silva CC, Patouraux S, Anty R, Iannelli A, Tran A, Gual P, Vidal H, Gomez L, Paillard M, Rieusset J Endoplasmic reticulum-mitochondria miscommunication is an early and causal trigger of hepatic insulin resistance and steatosis. J Hepatol. 2022;77(3):710–722. doi: 10.1016/j.jhep.2022.03.017. [DOI] [PubMed] [Google Scholar]
- Benson JC, Trebak M Too much of a good thing: the case of SOCE in cellular apoptosis. Cell Calcium. 2023;111:102716. doi: 10.1016/j.ceca.2023.102716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski K, Kciuk M, Kontek R Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci. 2020;21(9):3233. doi: 10.3390/ijms21093233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratta S, Tancini B, Sagini K, Delo F, Chiaradia E, Urbanelli L, Emiliani C Lysosomal exocytosis, exosome release and secretory autophagy: the autophagic- and endo-lysosomal systems go extracellular. Int J Mol Sci. 2020;21(7):2576. doi: 10.3390/ijms21072576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun J-K, Park M, Lee S, Yun JW, Lee J, Kim JS, Cho SJ, Jeon H-J, Lee I-K, Choi Y-K, Park K-G Inhibition of glutamine utilization synergizes with immune checkpoint inhibitor to promote antitumor immunity. Mol Cell. 2020;80(4):592–606. doi: 10.1016/j.molcel.2020.10.015. [DOI] [PubMed] [Google Scholar]
- Capel RA, Bolton EL, Lin WK, Aston D, Wang Y, Liu W, Wang X, Burton RA, Bloor-Young D, Shade KT, Ruas M, Parrington J, Churchill GC, Lei M, Galione A, Terrar DA Two-pore channels (TPC2s) and nicotinic acid adenine dinucleotide phosphate (NAADP) at lysosomal-sarcoplasmic reticular junctions contribute to acute and chronic β-Adrenoceptor signaling in the heart. J Biol Chem. 2015;290(50):30087–30098. doi: 10.1074/jbc.M115.684076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas C, Müller M, McNeal A, Lovy A, Jaňa F, Bustos G, Urra F, Smith N, Molgó J, Diehl JA, Ridky TW, Foskett JK Selective vulnerability of cancer cells by inhibition of Ca(2+) transfer from endoplasmic reticulum to mitochondria. Cell Reports. 2016;14(10):2313–2324. doi: 10.1016/j.celrep.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-C, Chen B-R, Wang Y, Curman P, Beilinson HA, Brecht RM, Liu CC, Farrell RJ, de Juan-Sanz J, Charbonnier L-M, Kajimura S, Ryan TA, Schatz DG, Chatila TA, Wikstrom JD, Tyler JK, Sleckman BP Sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) activity is required for V(D)J recombination. J Exp Med. 2021;218(8):e20201708. doi: 10.1084/jem.20201708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-F, Chiu W-T, Chen Y-T, Lin P-Y, Huang H-J, Chou C-Y, Chang H-C, Tang M-J, Shen M-R Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc Natl Acad Sci USA. 2011;108(37):15225–15230. doi: 10.1073/pnas.1103315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordás G, Weaver D, Hajnóczky G Endoplasmic reticulum-mitochondrial contactology: structure and signaling functions. Trends Cell Biol. 2018;28(7):523–540. doi: 10.1016/j.tcb.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Chang Y, Zhang X, Choi S, Tran H, Penmetsa KV, Viswanadha S, Fu L, Pan Z Targeting Orai1-mediated store-operated calcium entry by RP4010 for anti-tumor activity in esophagus squamous cell carcinoma. Cancer Lett. 2018;432:169–179. doi: 10.1016/j.canlet.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Merritt R, Fu L, Pan Z Targeting calcium signaling in cancer therapy. Acta Pharm Sin B. 2017;7(1):3–17. doi: 10.1016/j.apsb.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Clézardin P, Kamel S, Brazier M, Mentaverri R The CaSR in pathogenesis of breast cancer: a new target for early stage bone metastases. Front Oncol. 2020;10:69. doi: 10.3389/fonc.2020.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ridder I, Kerkhofs M, Lemos FO, Loncke J, Bultynck G, Parys JB The ER-mitochondria interface, where Ca(2+) and cell death meet. Cell Calcium. 2023;112:102743. doi: 10.1016/j.ceca.2023.102743. [DOI] [PubMed] [Google Scholar]
- De Stefani D, Bononi A, Romagnoli A, Messina A, De Pinto V, Pinton P, Rizzuto R VDAC1 selectively transfers apoptotic Ca2+ signals to mitochondria. Cell Death Differ. 2012;19(2):267–273. doi: 10.1038/cdd.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Li A, Li W, Cai P, Yang B, Zhang M, Gu Y, Shu Y, Sun Y, Shen Y, Wu X, Hu G, Wu X, Xu Q Novel role of Sarco/endoplasmic reticulum calcium ATPase 2 in development of colorectal cancer and its regulation by F36, a curcumin analog. Biomed Pharmacother. 2014;68(8):1141–1148. doi: 10.1016/j.biopha.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Fiorio Pla A, Gkika D Ca2+ channel toolkit in neuroendocrine tumors. Neuroendocrinology. 2020;110(1-2):147–154. doi: 10.1159/000501397. [DOI] [PubMed] [Google Scholar]
- Flourakis M, Lehen'kyi V, Beck B, Raphaël M, Vandenberghe M, Abeele FV, Roudbaraki M, Lepage G, Mauroy B, Romanin C, Shuba Y, Skryma R, Prevarskaya N Orai1 contributes to the establishment of an apoptosis-resistant phenotype in prostate cancer cells. Cell Death Dis. 2010;1(9):e75. doi: 10.1038/cddis.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbincius JF, Elrod JW Mitochondrial calcium exchange in physiology and disease. Physiol Rev. 2022;102(2):893–992. doi: 10.1152/physrev.00041.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstberger S, Jiang Q, Ganesh K Metastasis. Cell. 2023;186(8):1564–1579. doi: 10.1016/j.cell.2023.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C, Danese A, Missiroli S, Patergnani S, Pinton P Calcium dynamics as a machine for decoding signals. Trends Cell Biol. 2018a;28(4):258–273. doi: 10.1016/j.tcb.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Giorgi C, Marchi S, Pinton P The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol. 2018b;19(11):713–730. doi: 10.1038/s41580-018-0052-8. [DOI] [PubMed] [Google Scholar]
- Gualdani R, de Clippele M, Ratbi I, Gailly P, Tajeddine N Store-operated calcium entry contributes to cisplatin-induced cell death in non-small cell lung carcinoma. Cancers. 2019;11(3):430. doi: 10.3390/cancers11030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra MT, Florentino RM, Franca A, Lima Filho AC, Dos Santos ML, Fonseca RC, Lemos FO, Fonseca MC, Kruglov E, Mennone A, Njei B, Gibson J, Guan F, Cheng Y-C, Ananthanarayanan M, Gu J, Jiang J, Zhao H, Lima CX, Vidigal PT, Oliveira AG, Nathanson MH, Leite MF Expression of the type 3 InsP3 receptor is a final common event in the development of hepatocellular carcinoma. Gut. 2019;68(9):1676–1687. doi: 10.1136/gutjnl-2018-317811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S, Lee J-Y, Schrör J, Mazumder A, Jang DM, Chateauvieux S, Schnekenburger M, Hong CR, Christov C, Kang HJ, Lee Y, Han BW, Kim K-W, Shin H-Y, Dicato M, Cerella C, König GM, Orlikova B, Diederich M The dialkyl resorcinol stemphol disrupts calcium homeostasis to trigger programmed immunogenic necrosis in cancer. Cancer Lett. 2018;416:109–123. doi: 10.1016/j.canlet.2017.12.011. [DOI] [PubMed] [Google Scholar]
- Jung J, Cho KJ, Naji AK, Clemons KN, Wong CO, Villanueva M, Gregory S, Karagas NE, Tan L, Liang H, Rousseau MA, Tomasevich KM, Sikora AG, Levental I, van der Hoeven D, Zhou Y, Hancock JF, Venkatachalam K HRAS-driven cancer cells are vulnerable to TRPML1 inhibition. EMBO Rep. 2019;20(4):e46685. doi: 10.15252/embr.201846685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Lee DM, Seo MJ, Lee HJ, Choi KS Intracellular Ca2+ imbalance critically contributes to paraptosis. Front Cell Dev Biol. 2021;8:607844. doi: 10.3389/fcell.2020.607844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Kim JW, Kim DK, Choi DK, Lee S, Yu JH, Kwon O-B, Lee J, Lee D-S, Kim JH, Min S-H Calcium channels as novel therapeutic targets for ovarian cancer stem cells. Int J Mol Sci. 2020;21(7):2327. doi: 10.3390/ijms21072327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Kweon YC, Lee AR, Lee YY, Park CY Metastasis enhancer PGRMC1 boosts store-operated Ca2+ entry by uncoiling Ca2+ sensor STIM1 for focal adhesion turnover and actomyosin formation. Cell Rep. 2022;38(3):110281. doi: 10.1016/j.celrep.2021.110281. [DOI] [PubMed] [Google Scholar]
- Lei J, Deng F, Ding H, Fu M, Xu T, Ji B, Feng L, Li M, Qiu J, Gao Q Recent developments on the roles of calcium signals and potential therapy targets in cervical cancer. Cells. 2022;11(19):3003. doi: 10.3390/cells11193003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-F, Wu W-R, Chan T-C, Wang Y-H, Chen L-R, Wu W-J, Yeh B-W, Liang S-S, Shiue Y-L Transmembrane and coiled-coil domain 1 impairs the AKT signaling pathway in urinary bladder urothelial carcinoma: a characterization of a tumor suppressor. Clin Cancer Res. 2017;23(24):7650–7663. doi: 10.1158/1078-0432.CCR-17-0002. [DOI] [PubMed] [Google Scholar]
- Lissandron V, Podini P, Pizzo P, Pozzan T Unique characteristics of Ca2+ homeostasis of the trans-Golgi compartment. Proc Natl Acad Sci USA. 2010;107(20):9198–9203. doi: 10.1073/pnas.1004702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Peng Y, Wei W Cell cycle on the crossroad of tumorigenesis and cancer therapy. Trends Cell Biol. 2022;32(1):30–44. doi: 10.1016/j.tcb.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Jin M, Wang Y, Zhu J, Tan R, Zhao J, Ji X, Jin C, Jia Y, Ren T, Xing J MCU-induced mitochondrial calcium uptake promotes mitochondrial biogenesis and colorectal cancer growth. Signal Transduct Target Ther. 2020;5(1):59. doi: 10.1038/s41392-020-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loncke J, Kaasik A, Bezprozvanny I, Parys JB, Kerkhofs M, Bultynck G Balancing ER-mitochondrial Ca2+ fluxes in health and disease. Trends Cell Biol. 2021;31(7):598–612. doi: 10.1016/j.tcb.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado E, White-Gilbertson S, van de Vlekkert D, Janke L, Moshiach S, Campos Y, Finkelstein D, Gomero E, Mosca R, Qiu X, Morton CL, Annunziata I, d'Azzo A Regulated lysosomal exocytosis mediates cancer progression. Sci Adv. 2015;1(11):e1500603. doi: 10.1126/sciadv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant JS, Gunaratne GS, Cai X, Slama JT, Patel S NAADP-binding proteins find their identity. Trends Biochem Sci. 2022;47(3):235–249. doi: 10.1016/j.tibs.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi S, Giorgi C, Galluzzi L, Pinton P Ca2+ fluxes and cancer. Mol Cell. 2020;78(6):1055–1069. doi: 10.1016/j.molcel.2020.04.017. [DOI] [PubMed] [Google Scholar]
- Marongiu L, Mingozzi F, Cigni C, Marzi R, Di Gioia M, Garrè M, Parazzoli D, Sironi L, Collini M, Sakaguchi R, Morii T, Crosti M, Moro M, Schurmans S, Catelani T, Rotem R, Colombo M, Shears S, Prosperi D, Zanoni I, Granucci F Inositol 1, 4, 5-trisphosphate 3-kinase B promotes Ca2+ mobilization and the inflammatory activity of dendritic cells. Sci Signal. 2021;14(676):eaaz2120. doi: 10.1126/scisignal.aaz2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul-Pavicic A, Chiang SCC, Rensing-Ehl A, Jessen B, Fauriat C, Wood SM, Sjöqvist S, Hufnagel M, Schulze I, Bass T, Schamel WW, Fuchs S, Pircher H, McCarl C-A, Mikoshiba K, Schwarz K, Feske S, Bryceson YT, Ehl S ORAI1-mediated calcium influx is required for human cytotoxic lymphocyte degranulation and target cell lysis. Proc Natl Acad Sci USA. 2011;108(8):3324–3329. doi: 10.1073/pnas.1013285108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteith GR, Prevarskaya N, Roberts-Thomson SJ The calcium-cancer signalling nexus. Nat Rev Cancer. 2017;17(6):367–380. doi: 10.1038/nrc.2017.18. [DOI] [PubMed] [Google Scholar]
- Murphy E, Steenbergen C Regulation of mitochondrial Ca(2+) uptake. Annu Rev Physiol. 2021;83:107–126. doi: 10.1146/annurev-physiol-031920-092419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes-Hasler P, Maschalidi S, Lippens C, Castelbou C, Bouvet S, Guido D, Bermont F, Bassoy EY, Page N, Merkler D, Hugues S, Martinvalet D, Manoury B, Demaurex N STIM1 promotes migration, phagosomal maturation and antigen cross-presentation in dendritic cells. Nat Commun. 2017;8(1):1852. doi: 10.1038/s41467-017-01600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KC, Kim SW, Jeon JY, Jo AR, Choi HJ, Kim J, Lee HG, Kim Y, Mills GB, Noh SH, Lee MG, Park ES, Cheong J-H Survival of cancer stem-like cells under metabolic stress via CaMK2α-mediated upregulation of sarco/endoplasmic reticulum calcium atpase expression. Clin Cancer Res. 2018;24(7):1677–1690. doi: 10.1158/1078-0432.CCR-17-2219. [DOI] [PubMed] [Google Scholar]
- Pastushenko I, Blanpain C EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29(3):212–226. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Principe DR, Aissa AF, Kumar S, Pham TND, Underwood PW, Nair R, Ke R, Rana B, Trevino JG, Munshi HG, Benevolenskaya EV, Rana A Calcium channel blockers potentiate gemcitabine chemotherapy in pancreatic cancer. Proc Natl Acad Sci USA. 2022;119(18):e2200143119. doi: 10.1073/pnas.2200143119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato AS, Tang R, Grimm C Two-pore and TRPML cation channels: regulators of phagocytosis, autophagy and lysosomal exocytosis. Pharmacol Ther. 2021;220:107713. doi: 10.1016/j.pharmthera.2020.107713. [DOI] [PubMed] [Google Scholar]
- Roti G, Qi J, Kitara S, Sanchez-Martin M, Saur Conway A, Varca AC, Su A, Wu L, Kung AL, Ferrando AA, Bradner JE, Stegmaier K Leukemia-specific delivery of mutant NOTCH1 targeted therapy. J Exp Med. 2018;215(1):197–216. doi: 10.1084/jem.20151778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander P, Gudermann T, Schredelseker J (2021) A calcium guard in the outer membrane: is VDAC a regulated gatekeeper of mitochondrial calcium uptake? Int J Mol Sci 22(2): 946. https://doi.org/10.3390/ijms22020946
- Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Cai Y, Li Y, Li Y, Hu N, Ma S, Hu S, Zhu P, Wang W, Zhou H Yap promotes hepatocellular carcinoma metastasis and mobilization via governing cofilin/F-actin/lamellipodium axis by regulation of JNK/Bnip3/SERCA/CaMKII pathways. Redox Biol. 2018;14:59–71. doi: 10.1016/j.redox.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Bi Y, Yang W, Guo X, Jiang Y, Wan C, Li L, Bai Y, Guo J, Wang Y, Chen X, Wu B, Sun H, Liu W, Wang J, Xu C Ca2+ regulates T-cell receptor activation by modulating the charge property of lipids. Nature. 2013;493(7430):111–115. doi: 10.1038/nature11699. [DOI] [PubMed] [Google Scholar]
- Silvestri R, Nicolì V, Gangadharannambiar P, Crea F, Bootman MD Calcium signalling pathways in prostate cancer initiation and progression. Nat Rev Urol. 2023;20(9):524–543. doi: 10.1038/s41585-023-00738-x. [DOI] [PubMed] [Google Scholar]
- Sumitomo N Current topics in catecholaminergic polymorphic ventricular tachycardia. J Arrhythm. 2016;32(5):344–351. doi: 10.1016/j.joa.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Lu F, He H, Shen J, Messina J, Mathew R, Wang D, Sarnaik AA, Chang W-C, Kim M, Cheng H, Yang S STIM1- and Orai1-mediated Ca(2+) oscillation orchestrates invadopodium formation and melanoma invasion. J Cell Biol. 2014;207(4):535–548. doi: 10.1083/jcb.201407082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatkowski C, Parys JB, Ouadid-Ahidouch H, Matifat F Inositol 1, 4, 5-trisphosphate-induced Ca2+ signalling is involved in estradiol-induced breast cancer epithelial cell growth. Mol Cancer. 2010;9:156. doi: 10.1186/1476-4598-9-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueasilamongkol P, Khamphaya T, Guerra MT, Rodrigues MA, Gomes DA, Kong Y, Wei W, Jain D, Trampert DC, Ananthanarayanan M, Banales JM, Roberts LR, Farshidfar F, Nathanson MH, Weerachayaphorn J Type 3 inositol 1, 4, 5-trisphosphate receptor is increased and enhances malignant properties in cholangiocarcinoma. Hepatology. 2020;71(2):583–599. doi: 10.1002/hep.30839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese E, Samuel SM, Sadiq Z, Kubatka P, Liskova A, Benacka J, Pazinka P, Kruzliak P, Büsselberg D Anti-cancer agents in proliferation and cell death: the calcium connection. Int J Mol Sci. 2019;20(12):3017. doi: 10.3390/ijms20123017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos C, Sobradillo D, Hernández-Morales M, Núñez L Calcium remodeling in colorectal cancer. Biochim Biophys Acta Mol Cell Res. 2017;1864(6):843–849. doi: 10.1016/j.bbamcr.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Waldherr L, Tiffner A, Mishra D, Sallinger M, Schober R, Frischauf I, Schmidt T, Handl V, Sagmeister P, Köckinger M, Derler I, Üçal M, Bonhenry D, Patz S, Schindl R Blockage of store-operated Ca2+ influx by Synta66 is mediated by direct inhibition of the Ca2+ selective orai1 pore. Cancers. 2020;12(10):2876. doi: 10.3390/cancers12102876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li Y, Li Z, Lin S, Wang H, Sun J, Lan C, Wu L, Sun D, Huang C, Singh PK, Hempel N, Trebak M, DeNicola GM, Hao J, Yang S Mitochondrial calcium uniporter drives metastasis and confers a targetable cystine dependency in pancreatic cancer. Cancer Res. 2022;82(12):2254–2268. doi: 10.1158/0008-5472.CAN-21-3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidinger C, Shaw PJ, Feske S STIM1 and STIM2-mediated Ca(2+) influx regulates antitumour immunity by CD8(+) T cells. EMBO Mol Med. 2013;5(9):1311–1321. doi: 10.1002/emmm.201302989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Pan H, Yao J, Zhou Y, Han W SOCE and cancer: recent progress and new perspectives. Int J Cancer. 2016;138(9):2067–2077. doi: 10.1002/ijc.29840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S-W, Law BYK, Qu SLQ, Hamdoun S, Chen J, Zhang W, Guo J-R, Wu A-G, Mok SWF, Zhang DW, Xia C, Sugimoto Y, Efferth T, Liu L, Wong VKW SERCA and P-glycoprotein inhibition and ATP depletion are necessary for celastrol-induced autophagic cell death and collateral sensitivity in multidrug-resistant tumor cells. Pharmacol Res. 2020;153:104660. doi: 10.1016/j.phrs.2020.104660. [DOI] [PubMed] [Google Scholar]
- Xue Y, Morris JL, Yang K, Fu Z, Zhu X, Johnson F, Meehan B, Witkowski L, Yasmeen A, Golenar T, Coatham M, Morin G, Monast A, Pilon V, Fiset PO, Jung S, Gonzalez AV, Camilleri-Broet S, Fu L, Postovit L-M, Spicer J, Gotlieb WH, Guiot M-C, Rak J, Park M, Lockwood W, Foulkes WD, Prudent J, Huang S SMARCA4/2 loss inhibits chemotherapy-induced apoptosis by restricting IP3R3-mediated Ca2+ flux to mitochondria. Nat Commun. 2021;12(1):5404. doi: 10.1038/s41467-021-25260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Zhang JJ, Huang X-Y Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009;15(2):124–134. doi: 10.1016/j.ccr.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Zeng F, Chen X, Cui W, Wen W, Lu F, Sun X, Ma D, Yuan Y, Li Z, Hou N, Zhao H, Bi X, Zhao J, Zhou J, Zhang Y, Xiao R-P, Cai J, Zhang X RIPK1 binds MCU to mediate induction of mitochondrial Ca2+ uptake and promotes colorectal oncogenesis. Cancer Res. 2018;78(11):2876–2885. doi: 10.1158/0008-5472.CAN-17-3082. [DOI] [PubMed] [Google Scholar]
- Zhao H, Li T, Wang K, Zhao F, Chen J, Xu G, Zhao J, Li T, Chen L, Li L, Xia Q, Zhou T, Li H-Y, Li A-L, Finkel T, Zhang X-M, Pan X AMPK-mediated activation of MCU stimulates mitochondrial Ca2+ entry to promote mitotic progression. Nat Cell Biol. 2019;21(4):476–486. doi: 10.1038/s41556-019-0296-3. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Gong Z, Li Z, Wang J, Zhang J, Zhao Z, Zhang P, Zheng S, Miron RJ, Yuan Q, Zhang Y Target reprogramming lysosomes of CD8+ T Cells by a mineralized metal-organic framework for cancer immunotherapy. Adv Mater. 2021;33(17):e2100616. doi: 10.1002/adma.202100616. [DOI] [PubMed] [Google Scholar]
- Zheng P, Ding B, Shi R, Jiang Z, Xu W, Li G, Ding J, Chen X A multichannel Ca2+ nanomodulator for multilevel mitochondrial destruction-mediated cancer therapy. Adv Mater. 2021;33(15):e2007426. doi: 10.1002/adma.202007426. [DOI] [PubMed] [Google Scholar]
- Zheng S, Wang X, Zhao D, Liu H, Hu Y Calcium homeostasis and cancer: insights from endoplasmic reticulum-centered organelle communications. Trends Cell Biol. 2023;33(4):312–323. doi: 10.1016/j.tcb.2022.07.004. [DOI] [PubMed] [Google Scholar]
- Zheng S, Zhao D, Hou G, Zhao S, Zhang W, Wang X, Li L, Lin L, Tang T-S, Hu Y iASPP suppresses Gp78-mediated TMCO1 degradation to maintain Ca2+ homeostasis and control tumor growth and drug resistance. Proc Natl Acad Sci USA. 2022;119(6):e2111380119. doi: 10.1073/pnas.2111380119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong XZ, Yang Y, Sun X, Dong X-P Methods for monitoring Ca2+ and ion channels in the lysosome. Cell Calcium. 2017;64:20–28. doi: 10.1016/j.ceca.2016.12.001. [DOI] [PubMed] [Google Scholar]


