Abstract
Healthcare services and products are rapidly changing due to the development of new technologies, offering relevant solutions to improve patient outcomes. Patient-Generated Health Data and knowledge-sharing across the European Union (EU) has a great potential of making healthcare provision more effective and efficient by putting the patient at the centre of the healthcare process. While such initiatives have been taken before, a uniting and overarching approach is still missing. The EU-funded IMPROVE project will develop an evidence-based and actual framework to effectively leverage the added value of people-centred integrated healthcare solutions, using predominantly PROMs, PPI, PREMs, and other Patient-Generated Health Data (PGHD). As a result, the project facilitates the effective and efficient implementation of Value-Based Healthcare across the EU by putting the patient central in the healthcare process.
Keywords: Patient-Generated Health Data, Patient-Reported Outcomes Measures, Patient-Reported Experience Measures, Value-Based Healthcare, Health Technology, Digital Health, Patient Quality of Life
Graphical Abstract
1. Introduction
Health and healthcare services and products are rapidly changing due to the development of new (digital) technologies, offering relevant solutions for different diseases to improve the effectiveness and efficiency of the treatment(s). Patient-Generated Health Data (PGHD) and knowledge-sharing across the European Union (EU) will make healthcare provision ‘smarter’ and accelerate the development of (cost-)effective and patient-preference based new treatments and medical devices and reducing the operational costs of integrated healthcare solutions by making the patient more central in the healthcare process [1]. Healthcare professionals, pharmacists, patients, citizens, researchers, and health regulators all over the EU generate and use large numbers of essential patient-related healthcare data that are critical to the quality and effectiveness of their work. Unfortunately, there are still complex obstacles (e.g., cross-border regulations, privacy, data protection) that make it difficult to reach the full potential of digital health and patient-related data. An important and highly relevant initiative activated by the European Commission (EC), the European Health Data Space (EHDS) [2], promises to overcome these obstacles. The EHDS is a sharing framework that establishes clear rules, common standards and practices, infrastructures, and a governance framework for using electronic health data by patients and for research, innovation, policy making, patient safety, statistics, or regulatory purposes.
In line with this, it is essential to create an accessible, functional, transferable, and (cost-) effective framework that is capable of automatically enabling and integrating the added value of PGHD integrated healthcare solutions using Patient-Reported Outcome Measures (PROMs), Patient Preference Information (PPI), and Patient-Reported Experience Measures (PREMs), and other patient-generated information. This needs to be accompanied by a management structure that can meet regulatory (e.g., AI Act [3], Data Act [4]), ethical, legal, statistical, and data requirements to facilitate decision makers, patients, researchers, and healthcare professionals with access to new methods to improve integrated healthcare solutions and achieve a Value-Based Healthcare (VBHC) [5].
1.1. Value-based healthcare
Although there is no single definition of ‘value’ within VBHC, considering value is subjective and differs between patients, clinicians, healthcare providers, policy makers, and industry stakeholders. More specifically, recently the EU Expert Panel [6] proposed a comprehensive concept built on four value-pillars to define VBHC for conveying the guiding principles underlying solidarity-based healthcare systems. VBHC is a healthcare delivery model in which providers, including hospitals and physicians, are paid based on patient health outcomes [7]. VBHC in essence links outcomes to costs and so determines value [8]. Under value-based care agreements, providers are rewarded for helping patients improve their health through improved treatment processes, reducing the effects and incidence of chronic diseases, and enabling and promoting healthier living in an evidence-based way. The “value” in VBHC is derived from measuring relevant health outcomes using predominantly, but not limited to, PROMs, PPI, and PREMs against the cost of delivering these outcomes. This information will be complemented by scientific evidence, Real-World Evidence and Real-World Data. For example, the OECD has estimated that the health expenditure per capita will increase with an average annual rate of 2.7 % across countries of the OECD, which in return will lead to 10.2 % of gross domestic product by 2030 coming from 8.8 % in 2018 [9].
One main driver of this increase is technological and digital advancement, whereby the spending of medical technology (such as medical devices, digital health tools and in vitro diagnostics) represents 5—12 % of the total healthcare costs [10]. These technologies are justifiable as they drive increase in life expectancy and health related outcomes. This leads to the second key driver of the healthcare costs, which is the demographic change that Europe is currently facing [9]. The population structure is changing globally and in 2018 for the first time more people were over 65 years old than the group of children under five years of age [11]. On top of that, the life expectancy of European men increased from an average of 74.1 years born in 2000 to an average of 77.2 [12] for the same gender group born in 2020. In the same period, the average age for women increased from 80.7 to an average of 83.2. Hence, the United Nations expects that 25 % of the population living in Europe could be 65 or above by 2050 [13].
1.2. Increasing health expenditures
The combination between this change in population structure and the fact that long-term care needs and health expenses increase with age, increases the overall healthcare expenditures [14], [15]. Additionally, with this change in population structure, the demand for healthcare increases as healthcare providers need to treat more people – and less caregivers will be available. Therefore, there is an urgent need to develop more efficient and (cost-)effective healthcare systems, otherwise the costs will not only increase, but patients will face longer waiting times for their treatment as well [16], [17]. Although technological innovations are partly accountable for the rising healthcare costs, at the same time, smart technological solutions are considered the main solution to reduce healthcare costs by improving both efficiency and outcomes [15]). To exemplify this, a study showed that treating 5570 patients with telehealth-enabled care reduced medically unnecessary emergency department visits by 6.7 %, leading to a $928,000 annual cost saving [18]. Another burden for healthcare expenses is chronic diseases [19], since these diseases affect approximately 36 % of the European population and contribute 70 % to 80 % of the costs of the healthcare system [20]. Hence, improved prevention, personalized recommendations, and monitoring can have a great impact on overall health spending. Thus, in order to meet these challenges, only focusing on efficiency is insufficient. Hence, VBHC can be a promising approach, as it ensures the sustainability of the system by maximizing patients’ value of care within the resources that are available [21], [22]. Value-based systems aim to improve understanding of the patient’s experience, critically evaluate where and how care is provided, and reduce the number of unwarranted clinical variations.
2. Project description
To address the forementioned issues, a recent Innovative Health Initiative entitled “Framework to IMPROVE the Integration of Patient-Generated Health Data to Facilitate Value-Based Healthcare" has been launched. It will develop an evidence-based and real-time framework to effectively leverage integrated added value of people-centred integrated healthcare solutions, using predominantly, but not limited to, PROMs, PPI, and PREMs. This information will be complemented by scientific evidence, Real-World Evidence and Real-World Data. Together, this generates a more comprehensive understanding of how patient-generated evidence can best be used to improve outcomes, support decision making, and accelerate innovation by providing tailored solutions to industry. Developing approaches for such comprehensive data collection frameworks is timely in view of the challenge and ambition formulated in the EHDS for both primary and secondary data use. IMPROVE’s framework will be integrated into an online platform facilitating the development and implementation of integrated healthcare solutions using patient inputs, including patient and real-world data. The IMPROVE platform will enable scientific advances with and from patient input and patient-generated evidence to facilitate the faster market entry of patient-centric and cost-effective advanced integrated care solutions. In turn, this improves the return on research and innovation investments and health system sustainability.
IMPROVE will use patient input gathered via m-health and e-health technologies to gain improved insights into the real-life behaviour of, and challenges faced by, patients of all ages with complex, chronic diseases and comorbidities. The IMPROVE project will support the usage of such data enabling clinical innovation, better health outcomes, and advancing and consolidating evidence-based decision making for further acceleration of innovation and health system sustainability. The vision is to improve the integration of in-clinic and out-of-clinic PGHD and experiences to harness VBHC through improving the use of PROMs, PPI, PREMs, and other PGHD to enhance healthcare enabling accelerated innovation of cost-effective and personalized patient journeys. This will be based on accurate insight into health conditions and treatment options in relation to foreseeable outcomes, patient experiences and preferences, which are integrated for informed decision making by the patient, family members, and healthcare professionals.
Already today, a wealth of patient and citizen information is available, but fragmented, and therefore not coming to its full utility and value. The IMPROVE platform that the consortium will build will enable the smart use of patient input and patient generated evidence to 1) advance the role of patient preference and patient experience in the context of treatment selection, 2) improve medical device design based on patient preferences and experiences, and 3) facilitate faster market entry of patient-centric and cost-effective advanced integrated care solutions.
The IMPROVE consortium consists of 26 partners from ten European countries. It consists of experts from academia, economy and politics. The coordinating partner of the IMPROVE project is Universidad Politecnica de Madrid in Spain. IMPROVE is a project supported by the Innovative Health Initiative Joint Undertaking (IHI JU) under grant agreement No. 101132847. The JU receives support from the European Union’s Horizon Europe research and innovation programme and COCIR, EFPIA, and MedTech Europe, Vaccines Europe. The contributing partners of IMPROVE are Universidad Politecnica de Madrid (Spain), PredictBy (Spain), Danish Medicine Agency (Belgium), Roche (Switzerland), Institute for Economic Research (Slovenia), Copenhagen Institute for Futures Studies (Denmark), Fundació Institut d′Investigació Biomèdica de Bellvitge (Spain), Philips Medical System Nederland BV (The Netherlands), Heinrich-Heine-Universitaet Duesseldorf (Germany), Tilburg University (The Netherlands), Dedalus (Italy), Fondazione Italiana Sclerosi Multipla Fism Onlus (Italy), AReSS Puglia (Italy), MultiMed (Italy), iserundschmidt GmbH (Germany), Better (Slovenia), The Netherlands Cancer Institute (The Netherlands), University of Applied Sciences St. Pölten (Austria), Eye Hospital, University Medical Centre Ljubljana (Slovenia), Utrecht University (The Netherlands), UDG Alliance (Switzerland), Medtronic Iberica SA (Spain), Fundacio Hospital Universitari Vall D′Hebron – Institut de Recerca (VHIR), Splosna Bolnisnica Celje (Slovenia), Ortopedska Bolniscnica Valdotra (Slovenia), and Ethniko, Kentro Erevnas Kai Technologikis Anaptyxis (Greece).
3. Impact
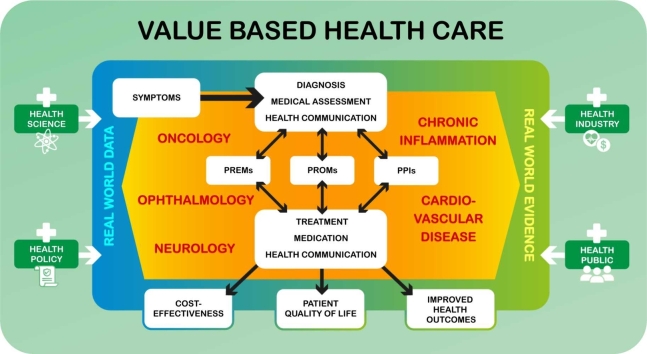
The IMPROVE project will provide improved clinical adoption of Value-Based Health Care, and enhanced return on research and innovation investments will be demonstrated in different care settings across the EU, for 10 use cases in at least 5 different disease areas (e.g., ophthalmology, oncology, cardiovascular disease, chronic inflammation, and neurology). The use cases in this project will be conducted using a large variety of implementation strategies, building on a design thinking approach, to optimally test the innovative framework of data gathering and translation into controlled change and action. In addition, a significant contribution from implementation science is planned to reach out to all stakeholders that are relevant for this initiative and maximize the impact to IMPROVE healthcare provision.
IMPROVE will become an implementable and ready-to-use framework that is able to better integrate the fragmented patient reported information. It will be constantly updating, improving and expanding with the most recent scientific evidence and existing frameworks available, tailored to individual’s needs, using PGHD and health system information (e.g., clinical information digital health date, digital biomarkers, EHR). IMPROVE will be applicable in a cost-effective manner to multiple treatment conditions in real settings with the minimum burden for all the actors involved. Additionally, it will develop and perform extensive data and evidence search that will be integrated into one framework, modelled and validated by empirical evidence, supported by Artificial Intelligence (AI), Natural Language Processing (NLP), and Machine Learning (ML) to be continuously updated and optimized by new insights and tested by relevant stakeholders in real settings.
The intended outcome of this large European project is enabling healthcare stakeholders to better understand patient behaviours and, consequently, to allow the development of more effective strategies to enable the implementation of effective and smart integrated healthcare delivery. Outputs, outcomes, and solutions will be generated in a data-driven and living lab fashion, while the entire modelling framework and ecosystem of solutions will be based on a scalable approach, to facilitate the integration of new modules in the future. IMPROVE will have a knowledge extraction module that will identify, process, analyse and categorize the current and future secondary sources from the scientific and grey literature using NLP. This will be the starting point of the Data Lab, where the data will be stored for the rest of the project. In addition to these secondary sources, IMPROVE will collect a significant amount of Real-World Data (RWD), considering data from patients (e.g., IoT solutions, Apps) as well as health system information (e.g., EHR registries; (digital) biomarkers). The data collection tools will minimize as much as possible the burden of reporting by patients. Engaging patients as key stakeholders via the MULTI-ACT model will enable us to measure the impact of research on outcomes that matter most to patients (science of patient input), making health research and care more sustainable. PROMs included in the MULTI-ACT Master Scorecard (MSC) [23] are increasingly instrumental in making stakeholders co-accountable for patient engagement in research and care. A co-creation scenario process enables decision makers to discuss key developments within a contextual environment on the bases of shared understanding. It also provides a more solid understanding of critical drivers (uncertainties) determining the trajectory and speed of the developments. Scenarios provide a solid fundament for a future strategy and for evaluating the resilience of existing strategy. Moreover, due to the modular and open access strategy, a full ecosystem of trusted and evidence-based applications will be delivered. Always complying to the highest ethical and data protection standards.
Through improving the use of PROMs, PPI, PREMs, and other PGHD we will accelerate innovation of cost-effective and personalized patient journeys, based on accurate patient perspective’s insight into health conditions and treatment options in relation to foreseeable outcomes. In using patient experiences and preferences, which are integrated for informed decision making by the patient, caregivers, family members, and health care professionals, IMPROVE will be able to reinforce the implementation of the VBHC. The project will be executed in four different phases.
| Phase 1. Conceptualizing |
|---|
|
| Phase 2. Modelling |
|
| Phase 3. Testing and validating |
|
| Phase 4. Implementing |
|
3.1. From conceptualisation to modelling phase 1 conceptualising
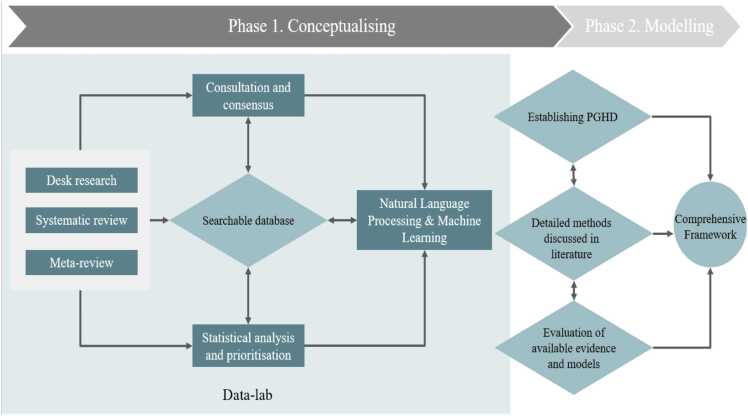
The first activity of the IMPROVE journey will be an extensive combination of desk research, systematic review, and meta-reviews. This allows us to identify and collect the state-of-the-art evidence and existing frameworks and models that are published, categorize it in an open-access, searchable database, using traditional (statistical analysis) and advanced analytics (AI, NLP, ML) in the Data Lab. As a result, an annotated “corpus” will be built and the relevant knowledge will be extracted. By setting up a Data Lab using AI, NLP, and ML we are able to screen and include more information than the traditional way of screening; we can feed the framework with all state-of-the-art findings and therefore better integrate the outcome, resulting in an eclectic overview of the findings in this field. For IMPROVE, this means that we can achieve a higher quality and accuracy than traditional approaches and immediately prioritize findings as well as incorporate new evidence. This process will allow us to identify and assess the available models, methods and their potential biases and how we can IMPROVE them, for example FDA guidelines for Patient Focused Drug Development and Medical Device Development, several IMI projects such as PREFER, SISAQOL, and PARADIGM. Next, we will conduct consultation with relevant stakeholders (e.g., patients, support network of formal and informal caregivers, associations, researchers, healthcare professionals, healthcare system regulators, and industry) in a co-creation and living lab approach, in order to create consensus about these findings, including the relevance of the current models and definitions, and factors driving effective and efficient use of PGHD, to elicit stakeholder needs and capabilities in situations, contexts of use, etc. This will facilitate the identification of the knowledge gaps, strengths, and weaknesses, including the need to incorporate additional data (see Fig. 1).
Fig. 1.
From conceptualisation to modelling.
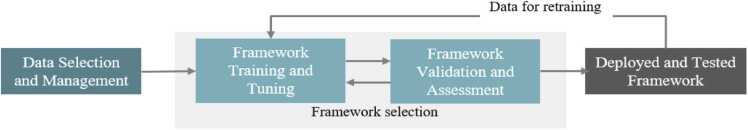
IMPROVE’s conceptual model framework approach will emerge from the consensus achieved during this process and represents the starting point of Phase 2: Modelling. Following the consensual definition and approach, traditional statistical analyses and advanced analytics (AI, ML, NLP) will be deployed to prioritize relevant factors that have a reciprocal relation with the database, allowing the insights gained in one task to automatically feed the other tasks. Automatic learning procedures can make use of statistical inference algorithms to produce robust models from unfamiliar or irrelevant input at a split-second, while manually conducting this work would be impossible. As a result of these exercises, a comprehensive understanding of existing frameworks will be developed, independently from the therapeutic area in a real-world context and to identify, categorize, quantify, and assess the most significant theories, models and frameworks explaining the usage of patient reported outcomes in healthcare delivery. As a result of Phase 1, an IMPROVE framework conceptual approach will be delivered, including the main components of the AI/ML model design, development, and validation process as described in the next paragraph (see Fig. 2).
Fig. 2.
The main components of the AI/ML model design, development and validation processes.
Data selection and management concerns the curation of measurement and data collection issues (e.g., estimation of missing values), aiming at ensuring the quality of the training set, and standard data pre-processing tasks (e.g., aggregation, sampling, feature creation, dimensionality reduction, feature selection, discretization, and variable transformation). Model selection is the core building block of an AI/NLP/ML strategy; given a class of AI/ML models, (e.g., a kernel-based method or an expert system), its parameters are leant by proper optimization algorithms applied on the training set, treating, in parallel, the tuning of model hyperparameters via rigid approaches, and, evaluating subsequently the model’s performance on the test set using suitable performance metrics (e.g., sensitivity, specificity, positive predictive value, area under the ROC curve).
3.2. From modelling to testing and validation
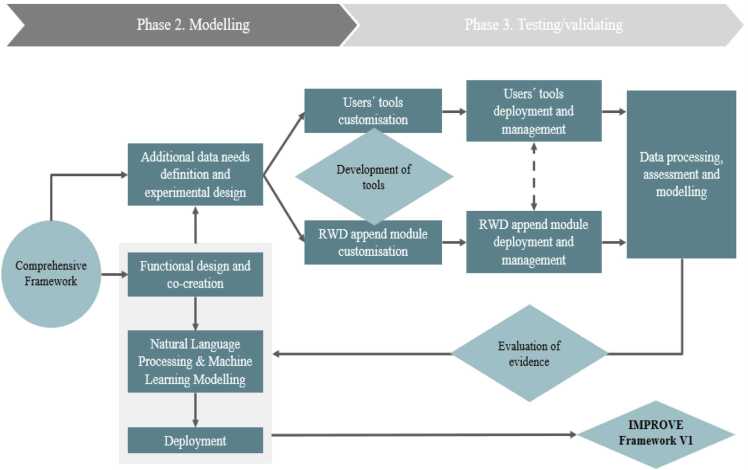
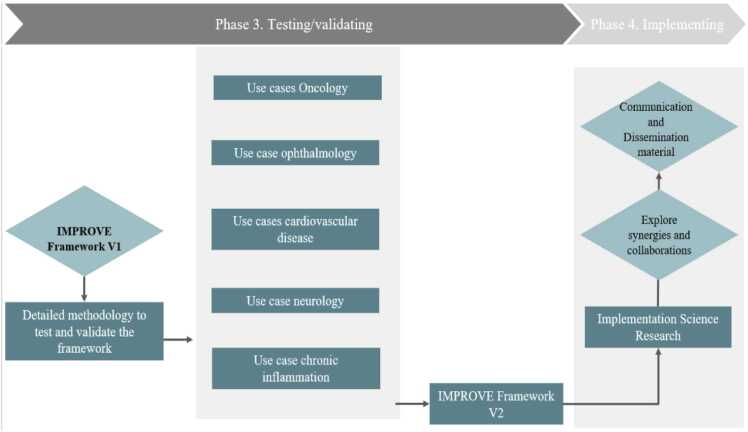
Phase 2 Modelling also includes the deployment of the conceptual framework (see Fig. 3). This will be coherently to phase 1, defined in co-creation modality, using the Living Lab together with the additional data needs definition and design for new data collection and existing RWD to optimize IMPROVE framework v.1. The definition of the validation studies to be conduct in phase 3 will be supported by the development of specifications for the data collection technologies and federated RWD collectors that aims to identify correlations of attributes in various RWD and the interfaces for data collection, including patients’ reported data (e.g., PROMs, IPP, PREMS) and the consolidation of multivariable RWD (e.g., EHR, digital biomarkers, digital tools). The output will be stored and visualized enabling the interaction with the obtained evidence. These modules will be built and refined through a final IMPROVE data driven performance evaluation and the delivery of IMPROVE framework v.2. All these activities will be supported by the deployment of the model (WP2 – T2.3), including the initial evaluation of the gaps in the existing framework.
Fig. 3.
From modelling to testing and validation.
Therefore, Phase 3 Testing / validating comprises the collection and analysis of additional real-world data from PGHD (e.g., PROMs, IPP, PREMS) or health system information (e.g., openEHR, digital biomarkers) to test and finetune IMPROVE model framework 1.0, see Fig. 4). IMPROVE will use PGHD via m-health and e-health technologies to gain improved insights into the real-life behaviour of, and challenges faced by, patients of all ages with complex, chronic diseases and comorbidities. Several Use Cases (N = 10) are planned to be conducted in across different Member States of the EU. As part of the concept, we are integrating the eConsented approach to capture RWD from patients and generate PGHD to move towards personalized medicine developed in the PharmaLedger project. The blockchain based ecosystem will be integrated to the IMPROVE platform to enable creating evidence in the blockchain for PGHD using RWD from medical history and devices, thus allowing to improve outcomes and decision making through data that have been consented to be use for both primary and secondary usage. The use case includes a module for e-consent and dynamic consent, as well as collection of data from medical and lifestyle devices. The development arrived to TRL 6–7 supporting creation of evidence using PGHD. This has been validated in a demonstrator and could provide a very good base for supporting PGHD in this project supported by a multistakeholder blockchain based-trusted ecosystem. Currently, a wealth of information is available, although fragmented, and therefore not coming to its full utility. IMPROVE will improve the usage of these data to improve health outcomes, make evidence-based decision making more available, thereby providing solutions for the acceleration of innovation. IMPROVE will focus on applicability of PGHD in industrial innovation in the following clinical areas and use cases:
-
•
Ophthalmology – 1) macular degeneration
-
•
Oncology – 1&2) incorporation of emerging MRI biomarkers for treatment counselling; 3) breast cancer; 4) head and neck cancer
-
•
Cardiovascular diseases – 1&2) Heart failure; 2) coronary artery diseases; 3) atrial fibrillation; 4) aortic stenosis
-
•
Chronic Inflammation – 1) Rhinosinusitis/allergic rhinitis
-
•
Neurology – 1) Multiple Sclerosis
Fig. 4.
From testing and validation to implementation.
Importantly, validation of the functionality of the IMPROVE model framework will start with the co-creation of the exact methodological design with all the actors involved and the use of complementary methods to collect additional insights on functionality of the methodology and how to complement existing frameworks.
4. Discussion
Currently, innovative and digitalized healthcare services and products are rapidly being developed, to address all the needs and challenges that high quality healthcare provision provides. Central to this is the perception of patients of the newly developed tools and solutions (e.g., PGHD) that is essential to better understand their impact and make implementation of effective and efficient solutions faster. Until now, knowledge-sharing across the EU has been limited and fragmented, therefore decreasing its effectiveness and efficiency. To facilitate and implement VBHC, it is necessary to provide all stakeholders with a comprehensive framework and tools to effectively make use of PGHD. The IMPROVE project will fill this gap.
CRediT authorship contribution statement
Chiara Macagnano: Writing – review & editing, Writing – original draft, Conceptualization. Franco Chiarugi: Writing – review & editing, Writing – original draft, Conceptualization. Joe-Max Wakim: Writing – review & editing, Writing – original draft, Conceptualization. Martin Ernst: Writing – review & editing, Writing – original draft, Conceptualization. Aron Szpisjak: Writing – review & editing, Writing – original draft, Conceptualization. Giuseppe Fico: Writing – review & editing, Writing – original draft, Supervision, Funding acquisition. Bogi Eliasen: Writing – review & editing, Writing – original draft, Conceptualization. Kathrin Scheckenbach: Writing – review & editing, Writing – original draft, Conceptualization. Seldag Gunes Peschke: Writing – review & editing, Writing – original draft, Conceptualization. Martin Wagenmann: Writing – review & editing, Writing – original draft, Data curation, Conceptualization. Lutz Peschke: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. Giampaolo Brichetto: Writing – review & editing, Writing – original draft, Conceptualization. Eva Podovšovnik: Writing – review & editing, Writing – original draft, Conceptualization. Vesna Levašič: Writing – review & editing, Writing – original draft, Conceptualization. Paola Zaratin: Writing – review & editing, Writing – original draft, Conceptualization. Johannes Peeters: Writing – review & editing, Writing – original draft, Conceptualization. Marina Ramiro-Pareta: Writing – review & editing, Writing – original draft, Conceptualization. Ludovico Pedullà: Writing – review & editing, Writing – original draft, Validation, Conceptualization. Gerard Carot-Sans: Writing – review & editing, Writing – original draft, Conceptualization. Yvonne Prinzellner: Writing – review & editing, Writing – original draft, Conceptualization. Caridad Pontes: Writing – review & editing, Writing – original draft, Conceptualization. Eva Turk: Writing – review & editing, Writing – original draft, Conceptualization. Luciano Benetti: Writing – review & editing, Writing – original draft, Conceptualization. Jordi Piera-Jiménez: Writing – review & editing, Writing – original draft, Conceptualization. Laura Pinna: Writing – review & editing, Writing – original draft, Conceptualization. Liss Hernandez Gonzalez: Writing – review & editing, Writing – original draft, Conceptualization. Carmela Genovese: Writing – review & editing, Writing – original draft, Conceptualization. Rens van de Schoot: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. Laia Juan: Writing – review & editing, Writing – original draft, Conceptualization. Manuel Ottaviano: Writing – review & editing, Writing – original draft, Funding acquisition, Conceptualization. Davide Guerri: Writing – review & editing, Writing – original draft, Conceptualization. Diego Carvajal: Writing – review & editing, Writing – original draft, Supervision, Project administration, Conceptualization. Emiel Krahmer: Writing – review & editing, Writing – original draft, Conceptualization. Frans Folkvord: Writing – review & editing, Writing – original draft, Supervision, Formal analysis, Conceptualization. Clàudia Navarro: Writing – review & editing, Writing – original draft, Conceptualization. Jim Ingebretsen Carlson: Writing – review & editing, Writing – original draft, Methodology, Funding acquisition, Conceptualization. Nadine Bol: Writing – review & editing, Writing – original draft, Conceptualization. Linwei He: Writing – review & editing, Writing – original draft, Conceptualization.
Declaration of Competing Interest
I am enclosing a manuscript for the “Computational and Structural Biotechnology Journal” entitled, “Using Patient-Generated Health Data more efficient and effectively to facilitate the implementation of Value-Based Healthcare in the EU – Innovation Report” and want to confirm that none of the authors have a conflict of interest.
Ackowledgements
The IMPROVE project is an Innovative Health Initiative project that has been granted by the European Commission under Grant agreement ID:101132847.
References
- 1.Tian S., Yang W., Le Grange J.M., Wang P., Huang W., Ye Z. Smart healthcare: making medical care more intelligent. Glob Health J. 2019;3(3):62–65. [Google Scholar]
- 2.: European Commission (2024). European Health Data Space. Obtained on 12 June 2024, retrieved from: https://health.ec.europa.eu/ehealth-digital-health-and-care/european-health-data-space_en.
- 3.: European Commission (2021). Regulation of the European Parliament and of the council laying down harmonized rules on artificial intelligence (Artificial Intelligence Act) and amending certain union legislative acts. Obtained on 12 June 2024, retrieved from https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52021PC0206.
- 4.: European Commission (2022). Data Act: Commission proposes measures for a fair and innovative data economy. Obtained on 12 June 2024, retrieved from https://ec.europa.eu/commission/presscorner/detail/en/ip_22_1113.
- 5.Rivera S.C., Liu X., Hughes S.E., Dunster H., Manna E., Denniston A.K., et al. Embedding patient-reported outcomes at the heart of artificial intelligence health-care technologies. Lancet Digit Health. 2023;5(3):e168–e173. doi: 10.1016/S2589-7500(22)00252-7. [DOI] [PubMed] [Google Scholar]
- 6.: European Commission (2012). Expert Panel on effective ways of investing in health. Obtained on 12 June 2024, retrieved from. https://ec.europa.eu/health/expert_panel/home_en.
- 7.Mjåset C., Ikram U., Nagra N.S., Feeley T.W. Value-based health care in four different health care systems. NEJM Catal Innov Care Deliv. 2020;1(6) [Google Scholar]
- 8.Porter M.E., Teisberg E.O. Harvard Business School Press; Boston, MA: 2006. Redefining Health Care: Creating Value-Based Competition on Results. [Google Scholar]
- 9.OECD (2019). Health spending set to outpace GDP growth to 2030 Obtained on 12 June 2024, retrieved from https://www.oecd.org/health/health-spending-set-to-outpace-gdp-growth-to-2030.htm.
- 10.MedTech(2023). The European Medical Technology in Figures. Obtained on 12 June 2024,retrieved from https://www.medtecheurope.org/datahub/expenditure/.
- 11.Ritchie, H., & Roser, M.2024. Age structure. Our World in Data.
- 12.Roffia P., Bucciol A., Hashlamoun S. Determinants of life expectancy at birth: a longitudinal study on OECD countries. Int J Health Econ Manag. 2023;23(2):189–212. doi: 10.1007/s10754-022-09338-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.: United Nations 2024. Ageing. Obtained on 9 September 2024, retrieved from 〈https://www.un.org/en/global-issues/ageing〉.
- 14.Cylus J. Figueras J. Normand C. Sagan A., Richardson E. North J., & White C. (Eds.). (2019). Will population ageing spell the end of the welfare state?: A review of evidence and policy options. European Observatory on Health Systems and Policies. [PubMed]
- 15.: World Economic Forum 2023. Global health and healthcare strategic outlook: Shaping the future of health and healthcare. Obtained on 12 June 2024, retrieved from https://www3.weforum.org/docs/WEF_Global_Health_and_Healthcare_Strategic_Outlook_2023.pdf.
- 16.Folkvord F., Würth A.R.U., van Houten K., Liefveld A.R., Carlson J.I., Bol N., et al. A systematic review on experimental studies about patient adherence to treatment. Pharmacol Res Perspect. 2024;12(1) doi: 10.1002/prp2.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anastasiadou D., Folkvord F., Lupiañez‐Villanueva F. A systematic review of mHealth interventions for the support of eating disorders. Eur Eat Disord Rev. 2018;26(5):394–416. doi: 10.1002/erv.2609. [DOI] [PubMed] [Google Scholar]
- 18.Langabeer J.R., Champagne-Langabeer T., Alqusairi D., Kim J., Jackson A., Persse D., et al. Cost–benefit analysis of telehealth in pre-hospital care. J Telemed Telecare. 2017;23(8):747–751. doi: 10.1177/1357633X16680541. [DOI] [PubMed] [Google Scholar]
- 19.Raveendran A.V., Jayadevan R., Sashidharan S. Long COVID: an overview. Diabetes Metab Syndr Clin Res Rev. 2021;15(3):869–875. doi: 10.1016/j.dsx.2022.102660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viegi G., Maio S., Fasola S., Baldacci S. Global burden of chronic respiratory diseases. J Aerosol Med Pulm Drug Deliv. 2020;33(4):171–177. doi: 10.1089/jamp.2019.1576. [DOI] [PubMed] [Google Scholar]
- 21.Koff E., Lyons N. Implementing value‐based health care at scale: the NSW experience. Med J Aust. 2020;212(3):104–106. doi: 10.5694/mja2.50470. [DOI] [PubMed] [Google Scholar]
- 22.Withers K., Palmer R., Lewis S., Carolan-Rees G. First steps in PROMs and PREMs collection in Wales as part of the prudent and value-based healthcare agenda. Qual Life Res. 2021;30:3157–3170. doi: 10.1007/s11136-020-02711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaratin P., Bertorello D., Guglielmino R., et al. The MULTI-ACT model: the path forward for participatory and anticipatory governance in health research and care. Health Res Policy Syst. 2022;20(22) doi: 10.1186/s12961-022-00825. [DOI] [PMC free article] [PubMed] [Google Scholar]