Highlights
-
•
Ultrasound speckle tracking reveals decreased diaphragm movement in amyotrophic lateral sclerosis (ALS) patients.
-
•
Diaphragm movement may offer early insights into ALS progression and management.
-
•
Our novel offline application enhances the accuracy of diaphragm function assessment.
Keywords: Amyotrophic lateral sclerosis, Diaphragm, Speckle tracking, Ultrasound
Abstract
Objective
Decreased cephalocaudal diaphragm movement may indicate respiratory dysfunction in amyotrophic lateral sclerosis (ALS). We aimed to evaluate diaphragm function in ALS using ultrasound speckle tracking, an image-analysis technology that follows similar pixel patterns.
Methods
We developed an offline application that tracks pixel patterns of recorded ultrasound video images using speckle-tracking methods. Ultrasonography of the diaphragm movement during spontaneous quiet respiration was performed on 19 ALS patients and 21 controls to measure the diaphragm moving distance (DMD) in the cephalocaudal direction during a single respiration. We compared respiratory function measures and analyzed the relationship between the clinical profiles and DMD.
Results
DMD was significantly lower in ALS patients than in the control group (0.6 ± 1.4 mm vs 2.2 ± 2.2 mm, p < 0.01) and positively correlated with phrenic nerve compound motor action potential amplitude (R = 0.63, p = 0.01). DMD was negatively correlated with the change in the ALS Functional Rating Scale-Revised scores per month after the exam (R = −0.61, p = 0.02), and those with a larger rate of decline had a significantly lower DMD (p = 0.03).
Conclusions
Diaphragm ultrasound speckle tracking enabled the detection of diaphragm dysfunction in ALS.
Significance
Diaphragm ultrasound speckle tracking may be useful for predicting prognosis.
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a rare neurodegenerative disorder characterized by progressive motor dysfunction and poor prognosis (van Es et al., 2017, Feldman et al., 2022). Patients with ALS present with restricted ventilation impairment due to respiratory muscle weakness, especially in the diaphragm, leading to respiratory acidosis and CO2 narcosis (Pinto et al., 2009, Noda et al., 2016, van Es et al., 2017, de Carvalho et al., 2019). Pulmonary function tests (PFTs) are the gold standard for monitoring respiratory function in ALS. However, accurate evaluation is difficult in patients with cognitive dysfunction or orofacial weakness because the outcome is dependent on the patient’s effort (Pinto et al., 2009, Noda et al., 2016, Iguchi et al., 2022). There is also a risk of viral infections owing to aerosol generation (Iguchi et al., 2022). Further arterial blood gas measurements are insensitive in the early stages and are highly invasive (de Carvalho et al., 2019).
Diaphragmatic ultrasonography has recently been used as a noninvasive and reproducible method for measuring respiratory function (Boussuges et al., 2020). Although this is a viable strategy for assessing respiratory function in patients with ALS (Noda et al., 2016, Pinto et al., 2016), it has yet to replace the aforementioned clinical tests (Pinto et al., 2017). Diaphragmatic ultrasound evaluates diaphragm thickness, diaphragm thickening fraction (DTF), and excursions (Pinto et al., 2016, Goutman et al., 2017, Pinto et al., 2017, Boussuges et al., 2020, Spiliopoulos et al., 2023). Diaphragm thickness is not sufficiently sensitive to detect acute changes and is thus inadequate for monitoring indicators (Pinto et al., 2017). Early-stage DTF sensitivity is also low (Spiliopoulos et al., 2023). Diaphragm excursion is a one-dimensional measurement, which poses a challenge when assessing three-dimensional diaphragm movement (Noda et al., 2016, Goutman et al., 2017). Furthermore, accurate assessment of excursion is technically challenging, not only because of beam angle issues but also due to distant signal loss and descending lung shadow.
When the ultrasound probe is placed perpendicular to the body axis in the zone of apposition (ZOA) along the anterior axillary lines, the diaphragm slides cephalocaudally. During inspiration, the diaphragm moves caudally, and the intercostal muscles pull the ribs cephalad, ultimately expanding the rib cage (Zoumot et al., 2015, Iguchi et al., 2022). The distance of the diaphragm’s cephalocaudal movement might be connected to lung capacity and consequently respiratory function and may also reflect dynamic diaphragm function compared to static thickness measurements. However, the moving distance of the diaphragm is difficult to measure using Brightness Mode (B-mode) ultrasound (Orde et al., 2016, Fritsch et al., 2022). Therefore, we used ultrasound speckle tracking, primarily used in echocardiography. This test quantifies tissue motion and deformation by tracking the speckle pattern across imaging frames, allowing left and right ventricular cardiac contractile function to be assessed (Smiseth et al., 2016). Several studies have evaluated the diaphragm using this method (Orde et al., 2016, Goutman et al., 2017, Oppersma et al., 2017, Crognier et al., 2021, Ye et al., 2021, Fritsch et al., 2022). However, these studies examined the diaphragm in a cardiac setting using existing ultrasound equipment. Therefore, the only measurement analyzed was the rate of change of the distance between two points in the diaphragm (i.e., strain), and no movement distance measurements were made. Further, to the best of our knowledge, no study has evaluated this method in ALS. We developed a video image analysis application using the Lukas–Kanade method, which is widely used as an optical flow estimation method (Lucas and Kanade, 1981), to measure the diaphragm muscle movement distance and determined the moving distance in ALS. We found that this novel measure, the diaphragmatic moving distance (DMD), was decreased in patients with ALS and may reflect a poor prognosis.
2. Methods
2.1. Study design and participants
This prospective cohort study included patients with ALS who were hospitalized at the Kobe University Hospital between October 2019 and November 2022. ALS was diagnosed by a board-certified neurologist. Progressive motor neuron failure was evidenced by clinical symptoms and electromyography, and other diseases were ruled out. Probable or definite ALS was diagnosed according to the revised El-Escorial or Awaji criteria (Brooks et al., 2000, de Carvalho et al., 2008).
We recruited age- and sex-matched healthy controls for comparison with the patients with ALS. The eligibility criteria were as follows: (1) both sexes; (2) age >20 years; and (3) no history or symptoms of neuromuscular or respiratory disease.
For participants with ALS, routine diaphragm ultrasonography was performed as part of clinical practice, and the stored videos were analyzed retrospectively using speckle tracking software created by GE Healthcare (Wuxi, China). Participants with ALS provided informed consent, and consent forms were completed whenever possible. Participants whose images were previously stored as part of clinical practice were informed about the study and given the option to decline participation. Healthy controls provided written informed consent. This study was approved by the Medical Ethics Committee of Kobe University Graduate School of Medicine (approval numbers: B210095 and B210308).
2.2. Clinical characteristics of patients with ALS
The Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSFRS-R) score was evaluated at study initiation and at the last visit (in patients for whom follow-up was available after the examination), and the change in ALSFRS-R score per month was calculated.
2.3. Pulmonary function tests (PFTs)
The participants underwent PFTs, including vital capacity (VC) and percent forced vital capacity (%FVC) tests, in an upright seated position. Values are expressed as percentages of the normal predicted values. For all participants, PFTs were performed after the ultrasound examination or on a different day to prevent the impact of breathing effort on quiet respiration.
2.4. Phrenic nerve conduction exam
We performed a phrenic nerve conduction exam with the participant in a supine position. The recording electrode was affixed 5 cm cephalad to the xiphoid process, and the reference electrode was affixed to the rib 16 cm from the recording electrode. The sternal and clavicular heads of the sternocleidomastoid muscles were stimulated using a bipolar stimulating electrode (MEB-2300; Nihon Kohden, Tokyo, Japan). Supramaximal stimulation with a 0.2-ms duration was administered at 0.5 Hz, and the waveforms were recorded. The peak-to-peak amplitude of the compound motor action potential (CMAP) was obtained as outcomes (Resman-Gaspersc and Podnar, 2008). Three consistent CMAP amplitudes on the right side were obtained and the mean value was analyzed.
2.5. Diaphragm ultrasound assessment
Diaphragmatic ultrasound was performed with participants in a supine position. We measured (1) diaphragm thickness at the end of expiration, (2) DTF, and (3) diaphragm excursion during quiet breathing. We then recorded video imaging for ultrasound speckle tracking. All ultrasound assessments were performed by the same examiner (S.W.) using LOGIQ e Premium (GE Healthcare) with an L4-12t-RS probe.
2.5.1. Diaphragm thickness
The diaphragm thickness was measured using a high-resolution 4–12-megahertz (MHz) linear probe set at 10 MHz in two-dimensional B mode. The probe was positioned perpendicular to the chest wall, and an ultrasound image of the diaphragm was generated at the zone of apposition located on the 8th and 11th intercostal spaces on the anterior and midaxillary lines longitudinal to the body axis. The diaphragm was depicted as a hypoechoic layer between two highly echogenic layers, the pleura and peritoneum, above the liver. We measured the diaphragm thickness at the end of expiration without including the echogenic layers (Boon et al., 2013).
2.5.2. DTF
DTF represents the change in diaphragm thickness at the end of expiration and peak inspiration. DTF was calculated as {(thickness at peak inspiration − thickness at end of expiration)/thickness at end of expiration} × 100 (%) (Wait et al., 1989, Orde et al., 2016).
2.5.3. Diaphragm excursion during quiet breathing
Diaphragmatic excursion was measured using a 4-MHz convex probe. First, we used B-mode ultrasound to obtain the best view for analyzing diaphragmatic movement using the liver as a window. The probe was placed on the anterior axillary line in the subcostal area mediodorsally and cephalad, and the posterior portion of the right diaphragm was delineated. M-mode ultrasonography was used to measure the cephalocaudal distance moved by the diaphragm during quiet breathing (Boussuges et al., 2009).
2.5.4. Video imaging for ultrasound speckle tracking
All participants underwent diaphragmatic ultrasonography in the supine position, allowing ≥30 s for quiet breathing to stabilize before examinations were performed. Diaphragmatic movements in the zone of apposition were recorded for a minimum of three quiet breaths after breathing was stabilized. An ultrasound device was set up for more accurate tracking: (1) The gain was 50–60 dB to provide some contrast; (2) the probe frequency was 8 MHz; (3) the frame rate was 50 frame per second; (4) the speckle reduction filter was turned off; and (5) the frame-averaging system, which created an average image between frames and smoothed the video, was turned off. The probe was positioned such that the diaphragm moved as far as possible in the direction of the tomographic plane.
2.6. Analysis with ultrasound images
2.6.1. Diaphragm moving distance (DMD) measurement
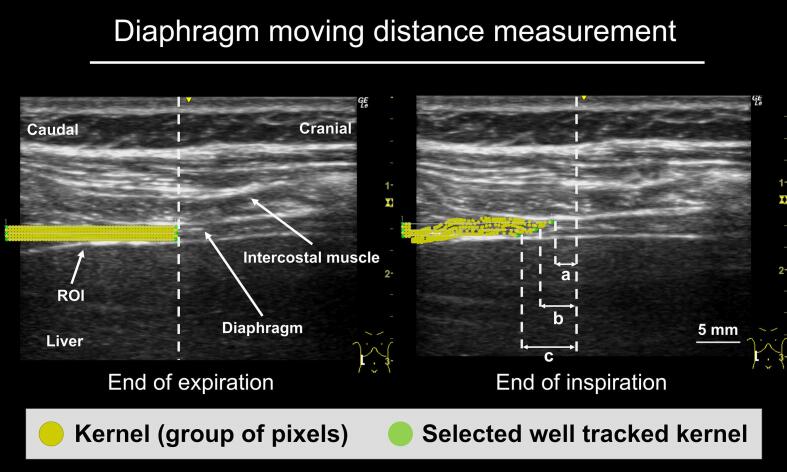
Digital clips of the ultrasound images were transferred to an external storage device in an uncompressed form in an Audio Video Interleave (AVI) container. Motion analysis was performed by dedicated prototype software developed in Microsoft Visual C++. After loading the video image, the operator set a rectangular region of interest (ROI) at a unique position on the screen, including the diaphragm layer (surface and deep layers) and the off-screen areas used as anchors. Then, a square grid with a size of 5 pixels (kernels) was displayed. The motion vector of each vertex of the grid was estimated frame-by-frame, using the Lucas–Kanade method, which is a widely used differential method for optical flow (Fig. 1) (Lucas and Kanade, 1981). We then selected three kernels on the same line and tracked central, deep, and surface diaphragmatic layers, and measured the amount of movement for one breath at each site (Fig. 1). The same analysis was performed for three different breaths, and the average was defined as the DMD.
Fig. 1.
Diaphragm moving distance measurements. The method for measuring the diaphragm moving distance (DMD) involved setting a region of interest (ROI) at a unique position on the screen, including the diaphragm and the off-screen area at the end of expiration. The frame-by-frame motion of each grid with a size of 5 pixels (kernel) in the ROI was analyzed using the Lukas–Kanade method. Each analyzed kernel is displayed as a yellow circle. The placement of the analyzed kernel at the end of inspiration is shown. a: DMD on the surface layer. b: DMD on the central layer. c: DMD on the deep layer.
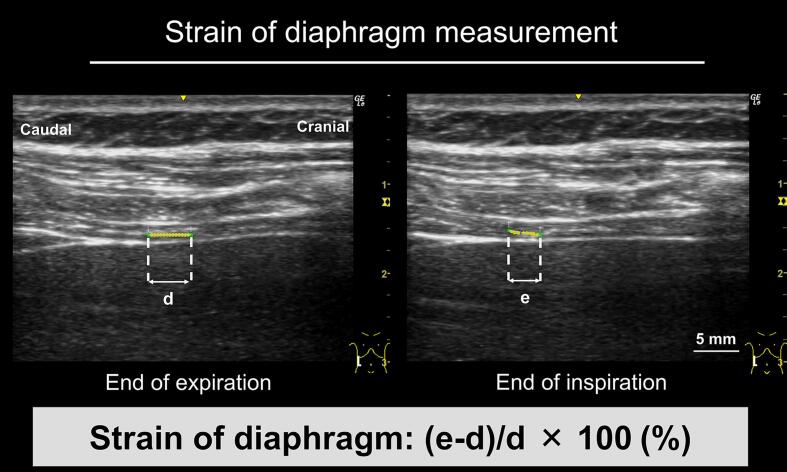
2.6.2. Strain of diaphragm measurement
Two kernels were set on the central layer of the diaphragm (interval 5 mm) around the identifiable mid-point between the two ribs, and the strain, defined as the rate of change in distance between the two kernels during expiration and inspiration, was measured using the following calculation: [(distance between two kernels at inspiration − distance between two kernels at end of expiration)/distance between two kernels at the end of expiration] × 100 (%) (Fig. 2) (Orde et al., 2016). The strain was measured three times, and the mean value was evaluated. The respiratory rate was calculated by averaging the duration of three breaths.
Fig. 2.
Diaphragm strain measurements. The method for measuring diaphragm strain involved setting two kernels 5 mm apart in the central layer of the diaphragm at the end of expiration. The distance between the two kernels at the end of inspiration was measured, and the percentage change from the end of expiration was determined. d: distance between diaphragm kernels at the end of expiration, e: distance between diaphragm kernels at the end of inspiration.
2.6.3. Reliability and reproducibility of analysis with ultrasound images
To assess reliability and reproducibility of speckle tracking software, we calculated intraclass correlation coefficients (ICC) in healthy controls. Intra-rater reliability {ICC (1,1)} and inter-rater reliability {ICC (2,1)} of DMD were calculated.
2.7. Statistical analysis
The Mann–Whitney U test was used to compare the two groups. Fisher’s exact test was used to compare sex ratios between the two groups. The Wilcoxon signed-rank test was used for comparisons within paired groups. ICC was calculated using EZR (Kanda, 2013). Correlations between each parameter and DMD or strain were calculated using Spearman correlation analysis. All analyses were performed using GraphPad Prism (version 8.0; GraphPad Software, San Diego, CA). Statistical significance was established at p < 0.05.
3. Results
3.1. Participant characteristics
Patient characteristics are described in Table 1. We enrolled 19 patients with ALS (mean age: 62.2 ± 18.1 years, 14 males) and 21 age- and sex-matched healthy controls (mean age: 54.6 ± 17.4 years, 14 males). The duration of ALS from symptom onset at evaluation was 25.2 ± 29.8 months. The mean ALSFRS-R score at evaluation was 38.1 ± 7.6 and the average monthly change in ALSFRS-R score after examination in 14 patients who were followed up was 2.09 ± 2.17 (mean follow-up, 10.9 ± 9.5 months). Riluzole was administered to twelve of the patients and edaravone to four. Three healthy controls did not undergo PFTs and %FVC evaluation could not be performed in one patient with ALS due to cognitive impairment. Both %FVC and %VC were significantly lower in patients with ALS than in healthy controls (%FVC, 86.7 ± 21.4 % vs. 103.4 ± 13.1 %, p < 0.01; %VC, 84.5 ± 21.0 % vs. 98.0 ± 12.1 %, p = 0.03, respectively). Results of phrenic nerve conduction studies showed significantly lower CMAP amplitude in patients with ALS (496.7 ± 223.4 μV vs. 804.1 ± 308.7 μV, p < 0.01). The diaphragm thickness at the end of expiration was not significantly different between healthy participants and patients with ALS (1.60 ± 0.30 mm vs. 1.58 ± 0.60 mm, p = 0.58). Similarly, DTF values were not significantly different between the groups (86.2 ± 52.0 % vs. 75.3 ± 70.9 %, p = 0.22). Neither diaphragm thickness nor DTF correlated with disease duration. Diaphragm excursion during resting respiration was 2.05 ± 0.63 cm in healthy controls, but this was evaluated in only one patient with ALS; thus, a comparison was not possible.
Table 1.
Participants’ characteristics and test results.
| Healthy controls (n = 21) (mean ± SD, range) |
ALS (n = 19) (mean ± SD, range) |
p-value | |
|---|---|---|---|
| Characteristics of participants | |||
| Age (years) | 54.6 ± 17.4 (31–77) | 62.2 ± 18.1(29–84) | 0.07 |
| Sex (male %) | 66.7 (14/21) | 73.7 (14/19) | 0.74 |
| Body mass index (kg/m2) | 22.2 ± 4.2 (16.5–31.9) | 22.4 ± 3.0 (17.7–27.5) | 0.93 |
| Disease duration at test (months) | NA | 25.2 ± 29.8 (4–125) | NA |
| Onset (upper limb: lower limb: bulbar) | NA | 8:9:2 | NA |
| ALSFRS-R (at test date) | NA | 38.1 ± 7.6 (14–45) | NA |
| Change in ALSFRS-R per month after examination | NA | 2.09 ± 2.17 (0–7.00) | NA |
| Pulmonary function test (at test date) | |||
| %FVC (%) | 103.4 ± 13.1 (77.6–122.9) | 86.7 ± 21.4 (32.4–118.7) | <0.01 ** |
| %VC (%) | 98.0 ± 12.1 (77.3–117.6) | 84.5 ± 21.0 (26.5–116.6) | 0.03 * |
| Phrenic nerve conduction test | |||
| Phrenic CMAP amplitude (μV) | 804.1 ± 308.7 (404–1367) | 496.7 ± 223.4 (214–1016) | <0.01 ** |
| Diaphragm ultrasound | |||
| Diaphragm thickness at the end of expiration (mm) | 1.60 ± 0.30 (1.1–2.2) | 1.58 ± 0.60 (0.4–2.8) | 0.58 |
| Diaphragm thickening fraction (%) | 86.2 ± 52.0 (0–171.4) | 75.3 ± 70.9 (16.7–252.9) | 0.22 |
| Diaphragm excursion at the quiet breathing (cm) | 2.05 ± 0.63 (1.30–3.08) | NA | NA |
ALS, amyotrophic lateral sclerosis; ALSFRS-R, Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised; FVC, forced vital capacity; VC, vital capacity; CMAP, compound motor action potential; SD, standard deviation.
*, p < 0.05; **, p < 0.01; NA, not available.
3.2. Ultrasound speckle tracking results in healthy controls
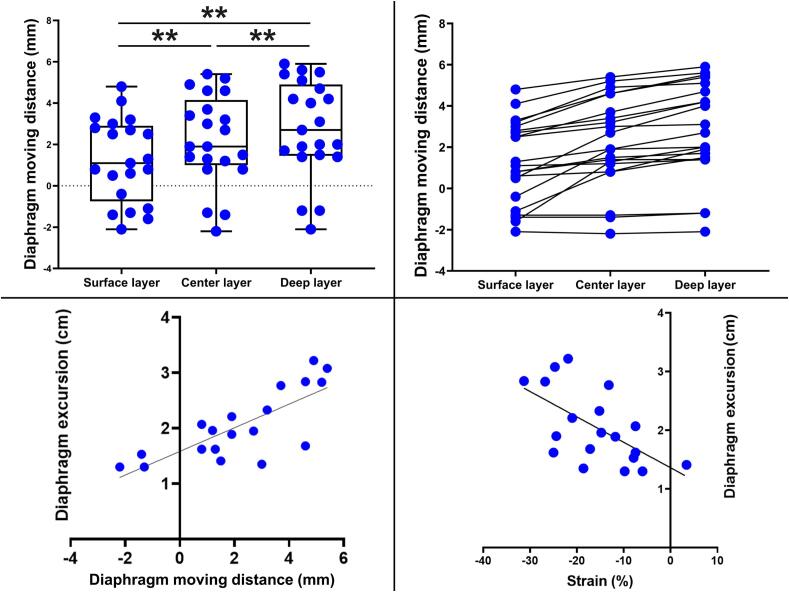
Evaluation of the DMD and strain using speckle tracking was performed for all participants. The tracking results varied slightly for each speckle of the different layers within the diaphragm, which was set as the kernel. The distance moved tended to be greatest in the deep layer and least in the surface layer (Table 2, Fig. 3). Therefore, for accuracy, we performed the following analysis using a kernel placed in the central layer of the diaphragm. Reproducibility of analyze with ultrasound images using speckle tracking software was good with ICC (1,1) of 0.985 and (2,1) of 0.972. The average DMD during quiet breathing was 2.2 ± 2.2 mm and the average strain was −15.9 ± 8.4 % (Table 2, Fig. 3, Fig. 4). DMD was not correlated with age. Both DMD and strain were significantly correlated with diaphragmatic excursion (DMD, R = 0.76, p < 0.01; strain, R = −0.61, p < 0.01, Table 3, Fig. 3), and DMD and strain were significantly correlated (R = −0.67, p < 0.01, Table 3). DMD did not correlate with age, %FVC, phrenic CMAP amplitude, diaphragmatic thickness, or DTF (Table 3). In three cases, the diaphragm shifted cephalad during inspiration (paradoxical abdominal DMD) (Iguchi et al., 2022).
Table 2.
Ultrasound speckle tracking results of right hemidiaphragm analysis.
| Healthy controls (mean ± SD, range) |
ALS (mean ± SD, range) |
p-value | |
|---|---|---|---|
| DMD on the surface layer (mm) | 1.2 ± 2.0 (−2.1–4.8) | 0.4 ± 1.4 (−3.1–2.7) | 0.23 |
| DMD on the central layer (mm) | 2.2 ± 2.2 (−2.2–5.4) | 0.6 ± 1.4 (−3.1–2.8) | <0.01 ** |
| DMD on the deep layer (mm) | 2.8 ± 2.4 (−2.1–5.9) | 0.8 ± 1.6 (−3.3–2.8) | <0.01 ** |
| Strain of the diaphragm (%) | −15.9 ± 8.4 (−31.3–3.4) | −11.0 ± 6.2 (−21.2–-1.3) | 0.04 * |
| Respiratory rate (/min) | 14.1 ± 3.8 (8.5–20.3) | 17.7 ± 3.6 (12.5–22.7) | <0.01 ** |
ALS, amyotrophic lateral sclerosis; DMD, diaphragm moving distance; SD, standard deviation.
*, p < 0.05; **, p < 0.01.
Fig. 3.
Ultrasound speckle tracking results in healthy controls. Moving distance of each site of different layers within the diaphragm in healthy controls. The distance moved tended to be greatest in the deep layer and least in the surface layer. Diaphragm moving distance (DMD) at each site indicated by connecting lines for each case. Both DMD and strain were significantly correlated with diaphragmatic excursion in healthy controls. *, p < 0.05; **, p < 0.01.
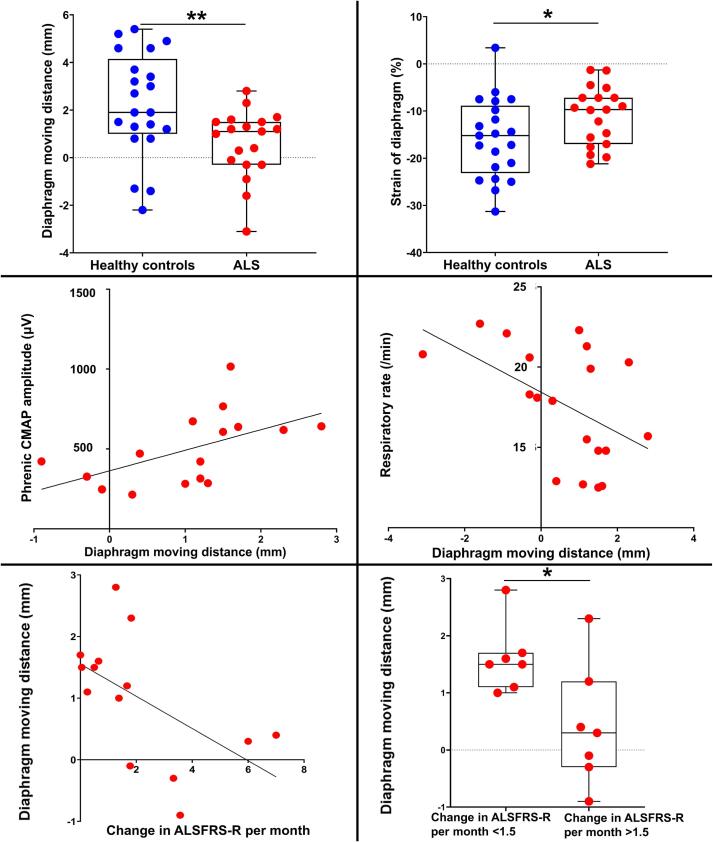
Fig. 4.
Ultrasound speckle tracking results in ALS. Both the central DMD and the strain of the diaphragm were significantly lower than in healthy controls. DMD was positively associated with phrenic CMAP amplitude and negatively correlated with respiratory rate. DMD and the change in ALSFRS-R scores per month were significantly negatively correlated after the examination. The group with an ALSFRS-R score change > 1.5 per month had significantly lower DMD. DMD, diaphragm moving distance; ALS, amyotrophic lateral sclerosis; CMAP, compound motor action potential; FVC, forced vital capacity. *, p < 0.05; **, p < 0.01.
Table 3.
Correlation of each parameter with DMD and strain.
| Healthy controls |
ALS |
|||
|---|---|---|---|---|
| R | p-value | R | p-value | |
| DMD and age | −0.35 | 0.12 | −0.09 | 0.71 |
| DMD and respiratory rate | −0.33 | 0.15 | −0.55 | 0.02 * |
| DMD and %FVC | −0.23 | 0.35 | 0.35 | 0.15 |
| DMD and phrenic CMAP amplitude | 0.32 | 0.19 | 0.63 | 0.01 * |
| DMD and diaphragm thickness | 0.40 | 0.08 | −0.30 | 0.22 |
| DMD and DTF | 0.21 | 0.38 | 0.35 | 0.32 |
| DMD and diaphragm excursion | 0.76 | <0.01 ** | NA | NA |
| DMD and duration of illness | NA | NA | −0.07 | 0.78 |
| DMD and ALSFRS-R score | NA | NA | −0.02 | 0.93 |
| DMD and change in ALSFRS-R score per month | NA | NA | −0.61 | 0.02 * |
| DMD and strain | −0.67 | <0.01 ** | −0.64 | <0.01 ** |
| Strain and age | 0.10 | 0.66 | −0.01 | 0.97 |
| Strain and respiratory rate | 0.46 | 0.04 | 0.10 | 0.69 |
| Strain and %FVC | 0.20 | 0.43 | 0.04 | 0.87 |
| Strain and phrenic CMAP amplitude | 0.01 | 0.98 | −0.31 | 0.21 |
| Strain and diaphragm thickness | −0.13 | 0.59 | 0.43 | 0.07 |
| Strain and DTF | −0.31 | 0.18 | −0.52 | 0.12 |
| Strain and diaphragm excursion | −0.61 | <0.01 ** | NA | NA |
| Strain and duration of illness | NA | NA | −0.05 | 0.83 |
| Strain and ALSFRS-R score | NA | NA | 0.17 | 0.49 |
| Strain and change in ALSFRS-R score per month | NA | NA | 0.20 | 0.48 |
ALS, amyotrophic lateral sclerosis; ALSFRS-R, Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised; DMD, diaphragm moving distance; CMAP, compound motor action potential; DTF, diaphragm thickening fraction; FVC, forced vital capacity.
*, p < 0.05; **, p < 0.01; NA, not available.
3.3. Ultrasound speckle tracking results in ALS
In patients with ALS, as in healthy controls, the moving distance of each layer within the diaphragm was greatest in the deep layer and least in the surface layer (Table 2), and the DMD and strain were significantly correlated (R = −0.64, p < 0.01, Table 3). Likewise, DMD was not associated with age (p = 0.71) or illness duration (p = 0.78, Table 3). The central DMD during quiet breathing was 0.6 ± 1.4 mm and the strain of the diaphragm was −11.0 ± 6.2 %, both significantly lower than in healthy controls (p < 0.01 and p = 0.04, respectively; Table 2, Fig. 4). In patients with ALS, DMD was positively associated with phrenic CMAP amplitude (R = 0.63, p = 0.01, Table 3, Fig. 4), and negatively correlated with respiratory rate (R = −0.55, p = 0.02, Table 3, Fig. 4). Conversely, strain was not significantly correlated with phrenic CMAP amplitude and respiratory rate (Table 3). Paradoxical abdominal movements were observed in six patients with ALS. The respiratory rate was 17.7 ± 3.6 breaths/min, significantly higher than in healthy controls (p < 0.01, Table 2). In some patients, DMD decreased even when the %FVC was within the normal range. No significant correlation was observed between DMD and diaphragm thickness or DTF (Table 3).
3.4. DMD and clinical course in patients with ALS
Although no correlation was observed between ALSFRS-R scores at the time of examination and DMD (p = 0.93, Table 3), DMD and the change in ALSFRS-R scores per month were significantly negatively correlated after the examination (R = −0.61, p = 0.02; Table 3, Fig. 4). ALS was divided into two groups based on the median value of change in ALSFRS-R score per month (1.5), and the group with an ALSFRS-R score change >1.5 per month had significantly lower DMD (Fig. 4). However, the duration of illness (<1.5 group: 42.1 ± 34.2 months vs. >1.5 group: 17.4 ± 5.8 months, p = 0.27) and ALSFRS-R scores at the time of the examination (<1.5 group: 41.4 ± 3.6 vs. > 1.5 group: 39.3 ± 5.7, p = 0.64) were not different.
4. Discussion
We developed novel methods for analyzing dynamic diaphragm dysfunction using ultrasonography and found decreased diaphragm movement and compensatory respiratory changes with increased respiratory rates in patients with ALS during quiet breathing. Diaphragmatic ultrasonography has been used to assess respiratory function in patients with ALS; however, several issues remain unresolved. In patients with ALS, diaphragm thickness correlates with phrenic CMAP amplitude both on inspiration and expiration (Noda et al., 2016, Pinto et al., 2016), and DTF < 120 % suggests a severe impairment of the diaphragm (Spiliopoulos et al., 2023). However, diaphragm thickness is inferior to phrenic CMAP amplitude as a monitoring index because significant changes cannot be detected in a short time (Pinto et al., 2017), and DTF values remain normal until the advanced stage (Spiliopoulos et al., 2023).
The primary function of the diaphragm is to dilate the rib cage by caudally pulling the dome (Fritsch et al., 2022, Iguchi et al., 2022). As such, diaphragm excursion rather than thickness would be a more direct assessment of dynamic diaphragm function; however, the ability of M-mode ultrasound to depict three-dimensional diaphragmatic movement is limited (Noda et al., 2016, Goutman et al., 2017). Therefore, we used ultrasound speckle tracking, which quantifies tissue movement and deformation by tracking speckle patterns across imaging frames. We developed an original application that can analyze two-dimensional B-mode ultrasound images to measure the amount of movement of any kernel of the thin diaphragm muscle layer, unlike previous studies performed by diverting programs for cardiac use, which differ from the diaphragm in terms of pixel pattern or frame rate. Previous studies using speckle tracking in the diaphragm were performed on healthy participants (Orde et al., 2016, Goutman et al., 2017, Oppersma et al., 2017, Crognier et al., 2021, Ye et al., 2021), except for one study that reported diaphragm dysfunction and the process of recovery after cardiac surgery (Fritsch et al., 2022). We first used this method to measure the diaphragm moving distance in the ZOA of patients with ALS. We only observed a portion of the diaphragm through a small intercostal window from the anterior axillary line; however, the small sliding portion which is the muscle attachment area on the chest wall, may reflect the movement of the entire diaphragm during quiet respiration (Kondo et al., 2000).
In healthy controls, DMD significantly correlated with diaphragmatic excursion, suggesting a link with the actual movement of the diaphragm. In patients with ALS, DMD was decreased compared to healthy controls and was associated with phrenic nerve CMAP amplitude, indicating that DMD may reflect diaphragmatic dysfunction in ALS. Moreover, DMD was negatively correlated with the respiratory rate and change in the ALSFRS-R score per month. DMD in those with a change of >1.5 in ALSFRS-R score per month was significantly lower than in those with a change of <1.5. Strain also correlated significantly with diaphragm excursion in healthy controls and was significantly lower in patients with ALS but was not correlated with phrenic CMAP amplitude or respiratory rate in ALS.
Although %FVC < 50 % has traditionally been associated with a poor prognosis and considered an indicator for non-invasive ventilation induction, it is thought that patients with ALS may have moderate to severe diaphragmatic dysfunction before %FVC reaches this point (Lechtzin et al., 2002, Pihtili et al., 2021). Biopsy specimens of the diaphragm showed muscle atrophy, and compensatory remodeling by re-innervation occurred in ALS even before it was detected by routine PFTs (Guimarães-Costa et al., 2019). Although respiratory symptoms are not apparent in the early stages of ALS (Lechtzin et al., 2002, Pihtili et al., 2021), indirect signs of diaphragmatic dysfunction, such as tachypnea, decreased thoracic dilation, and utilization of accessory respiratory muscles are still observed (Braun et al., 2018). One explanation is that patients with ALS have decreased tidal volume due to diaphragmatic dysfunction and decreased thoracic compliance (Lechtzin et al., 2006), which is compensated for by an increased respiratory rate (Siirala et al., 2012). In ALS, respiratory dysfunction progresses sub-clinically, even in the absence of symptoms, and compensatory mechanisms may delay the manifestation of symptoms. In our study, diaphragm movement was lower in patients with ALS than in healthy controls and was negatively correlated with the respiratory rate even if %FVC was preserved, possibly indicating respiratory compensation in ALS (Fig. 4).
Conversely, the onset of respiratory symptoms signifies limitations of compensation by re-innervation, at which point ALS is considered to be in an advanced stage with a poor prognosis. In the present study, the negative correlation between the change in the ALSFRS-R score per month after the examination and DMD as well as the lower DMD scores in the group with a greater change in ALSFRS-R per month, suggest a worse subsequent prognosis in those patients. The rate of ALSFRS-R decline is not constant, even in individual cases (Swinnen and Robberecht, 2014), and the rate of disease progression depends on the metabolic status of the patient (Cattaneo et al., 2022). Patients with low DMD are considered to be in a hypermetabolic state due to tachypnea, as they compensate for diaphragmatic dysfunction with an increased respiratory rate (Ichihara et al., 2012). Anatomical studies have also shown that the anterolateral and posterior portions of the diaphragm are developmentally distinct, with the cell bodies of the innervating nerve being more rostral (C4) in the anterior portion than caudal (C6) in the posterior portion (Fogarty and Sieck, 2019). Aran–Duchenne type ALS, which is a common form of ALS with initially wasting hands, has a poor prognosis when the area of involvement spreads cephalad from the hands to the shoulders. Therefore, the presence of abnormalities in the diaphragm observed from the anterior axillary line may be associated with a more extensive lesion.
Although strain was the endpoint in previous reports (Orde et al., 2016, Goutman et al., 2017, Oppersma et al., 2017, Crognier et al., 2021, Ye et al., 2021, Fritsch et al., 2022), it was not a useful endpoint in that it did not correlate with phrenic CMAP amplitude or respiratory rate in the present study compared to DMD. Strain seemed appropriate as an evaluation parameter since the heart muscle is not fixed at either end or moves in a centripetal direction. In contrast, one end of the diaphragmatic muscle is fixed to the ZOA; therefore, strain may not be the ideal measurement. It is necessary to create a program specifically for the diaphragm, rather than the heart. In addition, the speckle-tracking algorithm also refers to the movement of surrounding pixels (Lucas and Kanade, 1981), which may cause errors in the measurement results when there are surrounding tissues that move differently, such as the intercostal muscles or the liver, as in the present study. To use ultrasound examination as a marker of deterioration, these complexities need to be overcome.
We did not evaluate DMD at deep inspiration for two reasons. First, tracking was not possible in deep inspiration because the ROI would be off-screen. Second, deep inspiration depends on patient effort. Therefore, it may be difficult to perform, especially in patients with cognitive dysfunction. In addition, although thoracic compliance and central ventilatory drive may be involved in quiet breathing, it is largely dependent on diaphragm function itself, such as muscle contractility and length. The advantage of this method is that the function of the diaphragm itself can be evaluated, and quiet breathing was chosen because it is less affected by individual differences in inspiratory effort.
This study had several limitations. First, it was a single-center, single-examiner study with a small sample size, making it difficult to validate each subtype of ALS. Second, some patients were treated with riluzole and edaravone, which may have affected the prognosis. Third, paradoxical abdominal movements were observed in some middle-aged women in the healthy controls. Diaphragm excursion is lower in women who have given birth or have stress urinary incontinence and is compensated for by intercostal and other muscles. Thus, diaphragm function may be reduced in a disuse manner (Hwang et al., 2021). In this group, diaphragmatic evaluation may not have been initially useful; however, further studies with a larger number of cases are required.
5. Conclusion
Evaluation of DMD using ultrasound speckle tracking may detect diaphragmatic dysfunction in patients with ALS. In addition, DMD measurements may predict prognosis.
Author contributions
Kenji Sekiguchi: Conceptualization, Methodology, Writing- review and editing, Funding acquisition. Shunsuke Watanabe: Writing – Original draft, Data curation, Formal analysis. Hirotomo Suehiro: Data curation. Masaaki Yoshikawa: Data curation. Yoshikatsu Noda: Data curation. Naohisa Kamiyama: Software. Riki Matsumoto: Writing – review & editing. All authors have read and agreed to the published version of manuscript.
Funding
This work was supported by JSPS KAKENHI (grant number 20K11207).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Kenji Sekiguchi is currently receiving a grant (number 20K11207, 24K14297) from JSPS KAKENHI.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cnp.2024.10.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Diaphragm ultrasound movie analyzed using speckle tracking software. Video of the diaphragm moving distance measurement described in Fig. 1 caption in the text.
Data availability
Raw data were generated at Kobe University. Derived data supporting the findings of this study are available from the corresponding author (K.S.) upon request.
References
- Boon A.J., Harper C.J., Ghahfarokhi L.S., Strommen J.A., Watson J.C., Sorenson E.J. Two-dimensional ultrasound imaging of the diaphragm: quantitative values in normal subjects. Muscle Nerve. 2013;47:884–889. doi: 10.1002/mus.23702. [DOI] [PubMed] [Google Scholar]
- Boussuges A., Gole Y., Blanc P. Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest. 2009;135:391–400. doi: 10.1378/chest.08-1541. [DOI] [PubMed] [Google Scholar]
- Boussuges A., Rives S., Finance J., Brégeon F. Assessment of diaphragmatic function by ultrasonography: current approach and perspectives. World J. Clin. Cases. 2020;8:2408–2424. doi: 10.12998/wjcc.v8.i12.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A.T., Caballero-Eraso C., Lechtzin N. Amyotrophic lateral sclerosis and the respiratory system. Clin. Chest Med. 2018;39:391–400. doi: 10.1016/j.ccm.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Brooks B.R., Miller R.G., Swash M., Munsat T.L. World federation of neurology research group on motor neuron diseases, El escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Motor Neuron. Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Cattaneo M., Jesus P., Lizio A., Fayemendy P., Guanziroli N., Corradi E., et al. The hypometabolic state: a good predictor of a better prognosis in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatr. 2022;93:41–47. doi: 10.1136/jnnp-2021-326184. [DOI] [PubMed] [Google Scholar]
- Crognier L., Poette M., Conil J.M., Lairez O., Minville V. Diaphragmatic speckle tracking imaging for 2D-strain assessment in mechanical ventilation weaning test. Med. Hypotheses. 2021;152 doi: 10.1016/j.mehy.2021.110593. [DOI] [PubMed] [Google Scholar]
- de Carvalho M., Dengler R., Eisen A., England J.D., Kaji R., Kimura J., et al. Electrodiagnostic criteria for diagnosis of ALS. Clin. Neurophysiol. 2008;119:497–503. doi: 10.1016/j.clinph.2007.09.143. [DOI] [PubMed] [Google Scholar]
- de Carvalho M., Swash M., Pinto S. Diaphragmatic neurophysiology and respiratory markers in ALS. Front. Neurol. 2019;10:143. doi: 10.3389/fneur.2019.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman E.L., Goutman S.A., Petri S., Mazzini L., Savelieff M.G., Shaw P.J., et al. Amyotrophic lateral sclerosis. Lancet. 2022;400:1363–1380. doi: 10.1016/S0140-6736(22)01272-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty M.J., Sieck G.C. Evolution and functional differentiation of the diaphragm muscle of mammals. Compr. Physiol. 2019;9:715–766. doi: 10.1002/cphy.c180012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch S.J., Hatam N., Goetzenich A., Marx G., Autschbach R., Heunks L., et al. Speckle tracking ultrasonography as a new tool to assess diaphragmatic function: a feasibility study. Ultrasonography. 2022;41:403–415. doi: 10.14366/usg.21044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutman S.A., Hamilton J.D., Swihart B., Foerster B., Feldman E.L., Rubin J.M. Speckle tracking as a method to measure hemidiaphragm excursion. Muscle Nerve. 2017;55:125–127. doi: 10.1002/mus.25380. [DOI] [PubMed] [Google Scholar]
- Guimarães-Costa R., Similowski T., Rivals I., Morélot-Panzini C., Nierat M.C., Bui M.T., et al. Human diaphragm atrophy in amyotrophic lateral sclerosis is not predicted by routine respiratory measures. Eur. Respir. J. 2019;53:1801749. doi: 10.1183/13993003.01749-2018. [DOI] [PubMed] [Google Scholar]
- Hwang U.J., Lee M.S., Jung S.H., Ahn S.H., Kwon O.Y. Effect of pelvic floor electrical stimulation on diaphragm excursion and rib cage movement during tidal and forceful breathing and coughing in women with stress urinary incontinence: a randomized controlled trial. Medicine (baltimore) 2021;100 doi: 10.1097/MD.0000000000024158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara N., Namba K., Ishikawa-Takata K., Sekine K., Takase M., Kamada Y., et al. Energy requirement assessed by doubly-labeled water method in patients with advanced amyotrophic lateral sclerosis managed by tracheotomy positive pressure ventilation. Amyotroph. Lateral Scler. 2012;13:544–549. doi: 10.3109/17482968.2012.699968. [DOI] [PubMed] [Google Scholar]
- Iguchi N., Mano T., Iwasa N., Ozaki M., Yamada N., Kikutsuji N., et al. Thoracic excursion is a biomarker for evaluating respiratory function in amyotrophic lateral sclerosis. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.853469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. Epub 2012 Dec 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T., Kobayashi I., Taguchi Y., Ohta Y., Yanagimachi N. A dynamic analysis of chest wall motions with MRI in healthy young subjects. Respirology. 2000;5:19–25. doi: 10.1046/j.1440-1843.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- Lechtzin N., Wiener C.M., Shade D.M., Clawson L., Diette G.B. Spirometry in the supine position improves the detection of diaphragmatic weakness in patients with amyotrophic lateral sclerosis. Chest. 2002;121:436–442. doi: 10.1378/chest.121.2.436. [DOI] [PubMed] [Google Scholar]
- Lechtzin N., Shade D., Clawson L., Wiener C.M. Supramaximal inflation improves lung compliance in subjects with amyotrophic lateral sclerosis. Chest. 2006;129:1322–1329. doi: 10.1378/chest.129.5.1322. [DOI] [PubMed] [Google Scholar]
- Lucas B.D., Kanade T. 1981. An Iterative Image Registration Technique with an Application to Stereo Vision. [Google Scholar]
- Noda Y., Sekiguchi K., Kohara N., Kanda F., Toda T. Ultrasonographic diaphragm thickness correlates with compound muscle action potential amplitude and forced vital capacity. Muscle Nerve. 2016;53:522–527. doi: 10.1002/mus.24902. [DOI] [PubMed] [Google Scholar]
- Oppersma E., Hatam N., Doorduin J., van der Hoeven J.G., Marx G., Goetzenich A., et al. Functional assessment of the diaphragm by speckle tracking ultrasound during inspiratory loading. J. Appl. Physiol. 2017;123:1063–1070. doi: 10.1152/japplphysiol.00095.2017. [DOI] [PubMed] [Google Scholar]
- Orde S.R., Boon A.J., Firth D.G., Villarraga H.R., Sekiguchi H. Diaphragm assessment by two dimensional speckle tracking imaging in normal subjects. BMC Anesthesiol. 2016;16:43. doi: 10.1186/s12871-016-0201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihtili A., Bingol Z., Durmus H., Parman Y., Kiyan E. Diaphragmatic dysfunction at the first visit to a chest diseases outpatient clinic in 500 patients with amyotrophic lateral sclerosis. Muscle Nerve. 2021;63:683–689. doi: 10.1002/mus.27200. [DOI] [PubMed] [Google Scholar]
- Pinto S., Geraldes R., Vaz N., Pinto A., de Carvalho M. Changes of the phrenic nerve motor response in amyotrophic lateral sclerosis: longitudinal study. Clin. Neurophysiol. 2009;120:20825. doi: 10.1016/j.clinph.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Pinto S., Alves P., Pimentel B., Swash M., de Carvalho M. Ultrasound for assessment of diaphragm in ALS. Clin. Neurophysiol. 2016;127:892–897. doi: 10.1016/j.clinph.2015.03.024. [DOI] [PubMed] [Google Scholar]
- Pinto S., Alves P., Swash M., de Carvalho M. Phrenic nerve stimulation is more sensitive than ultrasound measurement of diaphragm thickness in assessing early ALS progression. Neurophysiol. Clin. 2017;47:69–73. doi: 10.1016/j.neucli.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Resman-Gaspersc A., Podnar S. Phrenic nerve conduction studies: technical aspects and normative data. Muscle Nerve. 2008;37:36–41. doi: 10.1002/mus.20887. [DOI] [PubMed] [Google Scholar]
- Siirala W., Saaresranta T., Vuori A., Salanterä S., Olkkola K.T., Aantaa R. Using respiratory rate and thoracic movement to assess respiratory insufficiency in amyotrophic lateral sclerosis: a preliminary study. BMC Palliat. Care. 2012;11:26. doi: 10.1186/1472-684X-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiseth O.A., Torp H., Opdahl A., Haugaa K.H., Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? Eur. Heart J. 2016;37(15):1196–1207. doi: 10.1093/eurheartj/ehv529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiliopoulos K.C., Lykouras D., Veltsista D., Skaramagkas V., Karkoulias K., Tzouvelekis A., Chroni E. The utility of diaphragm ultrasound thickening indices for assessing respiratory decompensation in amyotrophic lateral sclerosis. Muscle Nerve. 2023;68(6):850–856. doi: 10.1002/mus.27980. Epub 2023 Oct 10. [DOI] [PubMed] [Google Scholar]
- Swinnen B., Robberecht W. The phenotypic variability of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2014;10:661–670. doi: 10.1038/nrneurol.2014.184. [DOI] [PubMed] [Google Scholar]
- van Es M.A., Hardiman O., Chio A., Al-Chalabi A., Pasterkamp R.J., Veldink J.H., et al. Amyotrophic lateral sclerosis. Lancet. 2017;390:2084–2098. doi: 10.1016/S0140-6736(17)31287-4. [DOI] [PubMed] [Google Scholar]
- Wait J.L., Nahormek P.A., Yost W.T., Rochester D.P. Diaphragmatic thickness-lung volume relationship in vivo. J. Appl. Physiol. 1989;67:1560–1568. doi: 10.1152/jappl.1989.67.4.1560. [DOI] [PubMed] [Google Scholar]
- Ye X., Liu Z., Ma Y., Song Y., Hu L., Luo J., et al. A novel normalized cross-correlation speckle-tracking ultrasound algorithm for the evaluation of diaphragm deformation. Front Med (lausanne) 2021;8 doi: 10.3389/fmed.2021.612933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoumot Z., LoMauro A., Aliverti A., Nelson C., Ward S., Jordan S., et al. Lung volume reduction in emphysema improves chest wall asynchrony. Chest. 2015;148:185–195. doi: 10.1378/chest.14-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Diaphragm ultrasound movie analyzed using speckle tracking software. Video of the diaphragm moving distance measurement described in Fig. 1 caption in the text.
Data Availability Statement
Raw data were generated at Kobe University. Derived data supporting the findings of this study are available from the corresponding author (K.S.) upon request.