Abstract
Purinergic signaling is a crucial determinant in the regulation of pulmonary vascular physiology and presents a promising avenue for addressing lung diseases. This intricate signaling system encompasses two primary receptor classes: P1 and P2 receptors. P1 receptors selectively bind adenosine, while P2 receptors exhibit an affinity for ATP, ADP, UTP, and UDP. Functionally, P1 receptors are associated with vasodilation, while P2 receptors mediate vasoconstriction, particularly in basally relaxed vessels, through modulation of intracellular Ca2+ levels. The P2X subtype receptors facilitate extracellular Ca2+ influx, while the P2Y subtype receptors are linked to endoplasmic reticulum Ca2+ release. Notably, the primary receptor responsible for ATP-induced vasoconstriction is P2X1, with α,β-meATP and UDP being identified as potent vasoconstrictor agonists. Interestingly, ATP has been shown to induce endothelium-dependent vasodilation in pre-constricted vessels, associated with nitric oxide (NO) release. In the context of P1 receptors, adenosine stimulation of pulmonary vessels has been unequivocally demonstrated to induce vasodilation, with a clear dependency on the A2B receptor, as evidenced in studies involving guinea pigs and rats. Importantly, evidence strongly suggests that this vasodilation occurs independently of endothelium-mediated mechanisms. Furthermore, studies have revealed variations in the expression of purinergic receptors across different vessel sizes, with reports indicating notably higher expression of P2Y1, P2Y2, and P2Y4 receptors in small pulmonary arteries. While the existing evidence in this area is still emerging, it underscores the urgent need for a comprehensive examination of the specific characteristics of purinergic signaling in the regulation of pulmonary vascular tone, particularly focusing on the disparities observed across different intrapulmonary vessel sizes. Consequently, this review aims to meticulously explore the current evidence regarding the role of purinergic signaling in pulmonary vascular tone regulation, with a specific emphasis on the variations observed in intrapulmonary vessel sizes. This endeavor is critical, as purinergic signaling holds substantial promise in the modulation of vascular tone and in the proactive prevention and treatment of pulmonary vascular diseases.
Keywords: Pulmonary circulation, Pulmonary vascular tone, Purinergic agonists, Purinergic receptors, Purinergic signaling
Introduction
To ensure adequate blood perfusion of the alveoli, dynamic and regulated pulmonary vascular tone is necessary and a ubiquitous signaling pathway can be identified in humans and other species: the purinergic signaling pathway. Recent investigations using endogenous and exogenous purinergic receptor agonists and antagonists have expanded the knowledge related to this field. In addition, there is growing evidence concerning the role of purinergic signaling in vascular pathophysiology of lung diseases, such as pulmonary arterial hypertension and chronic obstructive pulmonary disease. This review will address the characteristics of purinergic signaling and its role in pulmonary vascular dynamics, highlighting the differences between different sizes and types of pulmonary vessels [1–6].
Purinergic signaling pathways
Receptors and ligands
Purinergic receptors (PRs) can be divided into two groups; P1 receptors, which bind adenosine and P2 receptors, which bind nucleotides, such as ATP (adenosine 5′-triphosphate) and ADP (adenosine 5′-diphosphate) [7]. P1 receptors, also known as adenosine (A) receptors, are G protein-coupled receptors [8]. A1 and A3 receptors are coupled to Gi protein, whereas A2A and A2B receptors are coupled to Gs and Gq proteins, respectively [8]. On the other hand, P2 receptors are divided into P2X and P2Y receptors [8]. P2X receptors are non-selective, monovalent, and divalent cation channels with a preference for Na+, K+, and Ca2+ [9]. They are activated by ATP and consist of 7 subtypes: P2X1, P2X2, P2X3, P2X4, P2X5, P2X6, and P2X7 receptors [9]. They form trimers and have similarities in their aminoacidic structure ranging from 41 to 55.4% in humans [9]. P2Y receptors are G protein-coupled receptors and there are eight subtypes: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14 receptors [10]. P2Y receptors have affinity not just for purine nucleotides but also for pyrimidine (uracil) nucleotides, such as UTP (uridine triphosphate) and UDP (uridine diphosphate) [11, 12].
Ligand metabolism
Regarding purinergic ligands and their effects on the pulmonary circulation, it is important to consider that they are subject to extracellular metabolism because this will determine which receptor and pathway will be activated. Studies have shown that this is conducted by nucleotidase enzymes (ectonucleotidases) in the extracellular medium, changing the available circulating ligands to PRs at the cell membrane. Ectonucleotidases are a family of oligomerizable glycosylated proteins with two transmembrane domains, and they can be grouped into two: ecto-nucleoside triphosphate diphosphohydrolase (e-NTPDase) and ecto-5′-nucleotidase [13]. Conversion of ATP to ADP and ADP to adenosine 5′-monophosphate (AMP) by e-NTPDase has been demonstrated, while adenosine production from AMP by ecto-5′-nucleotidase has been described [14, 15]. For uracil nucleotides (UTP, UDP, UMP) the metabolic pathway is similar although with different effectiveness [13, 16]. Four of the eight existing e-NTPDs have been located on the cell surface with an extracellular catalytic site: e-NTPDase1, e-NTPDase2, e-NTPDase3, and e-NTPDase8 [13, 16]. All of them require Ca2+ and Mg2+ in the millimolar concentration range to function [13, 16] and to hydrolyze nucleoside triphosphate, including ATP and UTP. In vessels, e-NTPD1 has been described as the main e-NTPD [13, 16], and e-NTPD2 has also been associated with the vascular system, where both e-NTPDases participate in hemostatic regulation [16]. In the lung, ecto-5′-nucleotidase has been observed in capillary endothelium and erythrocytes. Additionally, increased expression of e-NTPDase and ecto-5–nucleotidase mRNA has been observed in human bronchial epithelial cell lines following exposure to cyclic mechanical stress, and increased e-NTPDase1/ecto-5′-nucleotidase mRNA expression and protein levels in the lungs of mechanical ventilated mice have also been observed, where e-NTPDase1/ecto-5′-nucleotidases were located in the epithelium and pulmonary endothelium [17]. However, there is still little evidence about the distribution of e-NTPDases in the lungs [18].
The interpretation of functional test results should consider the action of e-NTPDases when endogenous purinergic agonists are used, but not with non-hydrolyzable agonists (e.g., α,β-meATP and 2-meSATP). For example, released ATP can induce vasoconstriction by P2X receptors and vasodilation by adenosine, which has been observed as a product of hydrolysis by e-NTPDases [15].
Expression and function of purinergic receptors in regulation the tone of pulmonary vessels
General considerations
The interpretation of studies on pulmonary vascular tone regulation is influenced by experimental heterogeneity. Among the variables that can determine differences in the results are as follows:
Vascular segment: To analyze the response of intrapulmonary vessels, these can be divided into vessels of small internal caliber (less than 500 μm approximately) and large internal caliber (greater than 1 mm approximately). Also, vessels can be divided into pulmonary arteries (PA) and pulmonary veins (PV). Thus, four different groups can be analyzed: small pulmonary arteries (SPA), large pulmonary arteries (LPA), small pulmonary veins (SPV), and large pulmonary veins (LPV). This classification has been based on histological and molecular differences between different types of vessels [19–21]. Despite this, most studies have used SPA [21–25] or LPA [20, 21, 24, 26, 27] and a few specific investigations have studied veins of small caliber [28], large caliber [27], or capillaries [29].
Experimental model: Differences in experimental results among species have been reported [7]; among them, models derived from rats, rabbits, cats, and guinea pigs are the most frequently used, consequently, leading to heterogeneous experimental designs. Moreover, for the same species, different responses have been reported depending on the experimental model, i.e., perfused lungs, vascular rings, and isolated cells [30]. For example, previous reports have shown different outcomes in studies with arterial rings and patch clamp isolated cells [25], possibly due to the activity of e-NTPDases, which is higher in arterial rings. In the perfused lung model, variables such as the flow rate used [31], the presence of erythrocytes [32], and the physical barrier that represents the wall of vessels for agonists coming from the lumen to stimulate smooth muscle cells (SMC) may also affect the outcome [29].
Desensitization: Receptor desensitization is a biological phenomenon that occurs when a receptor’s responsiveness to repeated or continuous stimulation decreases, and the rate of desensitization of purinergic receptors has been reported to vary [25, 33, 34]. For example, the P2Y2 and P2Y4 receptors desensitized faster (minutes) than the P2Y6 receptors (hours) [35–37]. Some P2X receptors can be classified as fast desensitizers (less than 1 s), such as P2X1 and P2X3 receptors, and slow desensitizers (several seconds), such as P2X2, P2X4, P2X5, and P2X7 receptors [9, 38–40]. These differences in P2Y and P2X receptors can determine the outcome in similar experimental conditions.
Basal vessel tone: This can be modulated by pathways other than purinergic [41, 42] and determines the response of a vessel to the same agonist.
Agonist administration route: Although in perfused lung models, agonists are mainly administered intravascularly, administration by the airway has also been reported as a delivery route, resulting in effects on capillary vessels [29]. In addition, the models based on isolated vessels allow the perfusion with drugs directly reaching the SMC, avoiding physiological barriers, such as endothelial cells (ECs).
Purinergic receptors in pulmonary vessels
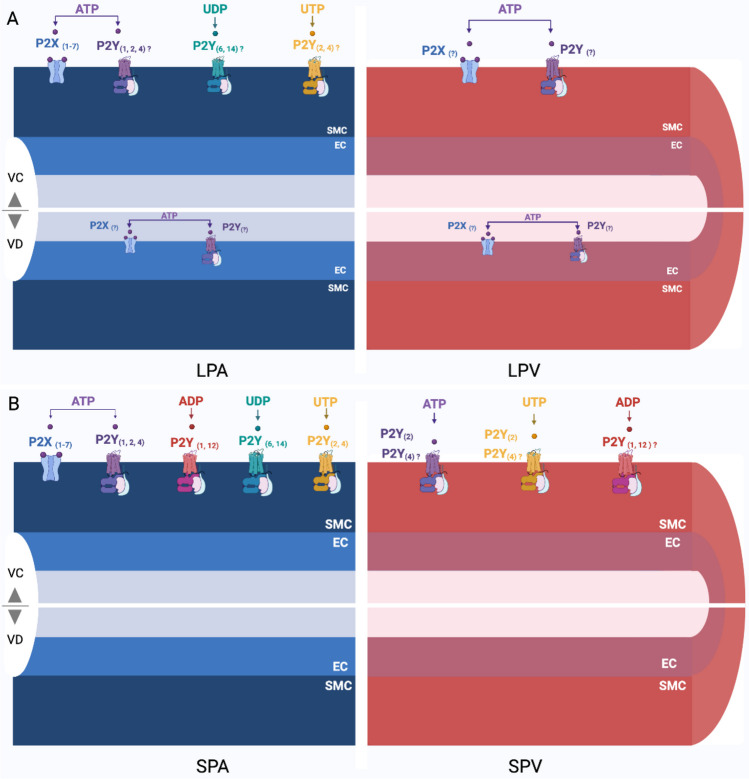
PRs are distributed in the pulmonary vessels differently depending on their caliber. In SPA, higher expression of P2Y1, P2Y2, and P2Y4 receptors has been reported [22]. These receptors have been functional [23], although inhibition of the P2Y1 receptor did not affect the contraction induced by ATP, so this receptor does not seem to be relevant for SPA contraction [22, 23]. In rat SPV, P2Y2 and P2Y4 receptors have been reported, although ATP-induced contraction mainly depended on the P2Y2 receptor [28]. One study showed rat LPA contractions following stimulation with UTP and UDP, both in endothelium-intact and endothelium-denuded vessels, suggesting expression of P2Y receptors in LPA, specifically in SMC [20] (Table 1 and Fig. 1). More studies are required to elucidate the expression of P2Y-specific receptors in each vascular segment.
Table 1.
Expression of P2 receptors according to vascular segment in rat lungs (21, 23, 24, 28, 29, 31, 34)
| LPA | SPA | Capillary | SPV | LPV | ||
|---|---|---|---|---|---|---|
| mRNA | Smooth muscle cell | |||||
| P2X1 (24) | P2X1 (24, 34) | P2Y1 (23, 34) | - | |||
| P2X2 (24) | P2X2 (24, 34) | P2Y2 (34) | - | |||
| P2X3 (24) | P2X3 (24, 34) | P2Y4 (34) | - | |||
| P2X4 (24) | P2X4 (24, 34) | P2Y6 (23, 31) | - | |||
| P2X5 (24) | P2X5 (24, 34) | P2Y12 (23) | - | |||
| P2X6 (24) | P2X6 (24) | P2Y13 (34) | - | |||
| P2X7 (24) | P2X7 (24, 34) | P2Y14 (34) | - | |||
| Immunofluorescence | Smooth muscle cell | |||||
| P2X1 (24) | P2X1 (24) | - | P2Y2 (28) | |||
| P2X2 (24) | P2X2 (24) | - | ||||
| P2X4 (24) | P2X4 (24) | - | ||||
| P2X5 (24) | P2X5 (24) | - | ||||
| Smooth muscle cell + endothelial cell | ||||||
| P2X1 (21) | P2X1 (21) | P2Y2 (29) | ||||
| P2X3 (21) | P2X4 (21) | |||||
| P2X4 (21) | P2X5 (21) | |||||
| P2X5 (21) | P2X7 (21) | |||||
| P2X7 (21) | ||||||
Fig. 1.
Schematic representation of the presence of purinergic receptors and respective physiological agonists response in pulmonary vessels. A Vasoconstriction or vasodilation has been described in response to ATP in LPA and LPV, while vasoconstriction has been reported to UTP and UDP in LPA. B Vasoconstriction has been demonstrated in response to ATP, UTP, UDP, and ADP. Vasodilator response needs further studies in SPA and SPV. In both figures (A and B), the specific receptors that mediate the response to agonists are mainly unknown. LPA large pulmonary arteries, LPV large pulmonary veins, SPA small pulmonary arteries, SPV small pulmonary veins, SMC smooth muscle cells, EC endothelium, VC vasoconstriction, VD vasodilation. Figure created using BioRender software (21, 23, 24, 28, 29, 31, 34)
Regarding the P2X receptors, all receptor subtypes have been reported to be expressed in rat SPA and LPA [22, 24] (Table 1 and Fig. 1), being P2X1 the most expressed subtype in both structures [21, 22, 24]. In addition, the use of P2X1 receptor antagonists has been reported to exert an inhibition of agonist-induced contractions [24]. In the endothelium of mouse lung micro-vessels, P2X4 was the most expressed receptor [43], which resembles humans, where P2X4 receptor mRNA has been described as the most expressed receptor in the endothelium of PA [44–47]. In rat endothelium-denuded PA, the expression of P2X4 receptors has been observed next to P2X1 receptors [24]. No reports have been found of the presence of P2X receptors in PV.
Expression of P1 receptors has also been described in pulmonary vessels, although the evidence only includes human pulmonary vessels. The expression of A1, A2A, A2B, and A3 receptors mRNA has been observed in smooth muscle cells isolated from the human pulmonary artery, accordingly with the protein levels of A2A and A2B in healthy individuals. However, this remains unknown for different species [48].
Role of P2 receptor signaling in the regulation of pulmonary vascular tone
ATP is one of the most studied purinergic ligands in the regulation of pulmonary vascular tone. As reported in rats PA and PV, the direct stimulation of SMC with ATP leads to an increase in vascular tone and an increased intracellular Ca2+ concentration [28, 22]; similar effects have been observed after the stimulation of EC from PA with ATP in different species [34, 49, 50]. These effects have also been demonstrated for other P2 receptor agonists, such as UTP and ADP [34, 49, 50].
Ca2+ dynamics downstream of P2 receptors
Movement of Ca2+ has been related to the type of activated PR. Thus, P2X receptors (ionotropic) have been associated with the entry of Ca2+ from the extracellular medium through ion channels [51]. On the other hand, P2Y receptors have been related to the release of Ca2+ from the endoplasmic reticulum to the cytosol [52]. The second mechanism induces an increase in Ca2+ concentration which can be independent of extracellular Ca2+ and that has been recorded as Ca2+ oscillations [53].
Continuous stimulation of arteries with ATP for 30–50 s has shown oscillations of intracellular Ca2+ concentration with decreasing amplitude, which was slightly decreased but not abolished by the removal of external Ca2+ and strongly affected by inhibition of sarcoplasmic reticulum Ca2+ pump [53]. Therefore, oscillations of intracellular Ca2+ depended on the entry of Ca2+ from the extracellular medium via P2X receptors, followed by release of Ca2+ from intracellular reservoirs after activation of P2Y receptors and IP3 formation [53]. The phenomenon has also been induced by UTP, but in this case, all the effects have been attributed to the Ca2+ released from the sarcoplasmic reticulum [53].
The role of ATP concentration in Ca2+ response has been described in SMC of rat PA. Low ATP concentrations (10–300 nM) only induced a transient response with an increase in intracellular Ca2+ concentration, which dropped to baseline values in 20–40 s, while high ATP concentrations (1 μM–1 mM) induced a biphasic response, with a transient response followed by a sustained elevation phase, which was maintained for several minutes [22]. This biphasic response was also induced by ADP, although ATP was more potent [22]. The transient response to low ATP concentrations depended on both P2X receptor-mediated Ca2+ mobilization from the extracellular medium and P2Y receptor-mediated Ca2+ mobilization from the endoplasmic reticulum [22, 30], whereas the transient response to high ATP concentrations was mainly mediated by P2Y receptors [28, 22, 50, 53]. The sustained response was mediated by P2X and P2Y receptors [22], although one study showed that stimulation with ATP in a Ca2+-free medium was not able to affect the sustained phase [30], suggesting that it depended on P2Y receptors. The biphasic response to the single stimulus was also observed using UTP, where the intracellular Ca2+ concentration was increased in a concentration-dependent manner [50].
In the pulmonary vessels, the Ca2+ dynamics described above have been reported in bovine EC of PA [34, 48], rabbit SMC, rat SPA and LPA [50], and SMC of rat SPV [28]. A higher amplitude of transient phase in rabbit SPA compared to LPA was found [50], and a greater response to ATP than UTP and UDP was observed in rat SPA [54]. In the SMC of rat SPV, a sustained stimulation with ATP induced a transient increase in intracellular Ca2+ concentration, followed by immediate oscillations, which ceased rapidly after ATP withdrawal. This response was not dependent on P2X receptors, but instead P2Y receptors, since IP3 receptor inhibition led to a single low-amplitude Ca2+ signal that quickly returned to baseline values, and phospholipase C-β inhibition blocked the contraction [28]. In the SMC of rat PA, biphasic Ca2+ currents were induced by ATP and UTP [54, 55]. Similarly, in SMC of human PA, ATP also induced a biphasic response [56], and the sustained phase in EC was mediated by Ca2+ influx via P2X4 receptors [47]. The latter was observed especially following an increase in shear stress and the concomitant ATP release [44].
Studies have shown that P2Y receptors can interact with ion channels [57]. Thus, the activation of both, P2X and P2Y receptors, led to membrane depolarization with the activation of voltage-dependent ion channels, triggering or enhancing muscle contraction, or the production of vasodilation mediators in the endothelium. In this regard, the participation of the Cav1.2 channel in the Ca2+ entry after activation of P2Y receptors in SMC of rats SPA has been previously observed [33]. Additionally, a study has shown that the SMC contraction involves Cl− channels activated by Ca2+ (CaCC), where the opening of these channels led to membrane depolarization by Cl− output. This induced the opening of voltage-dependent Ca2+ channels and subsequent contraction. The initial stimulus was the increase in intracellular Ca2+ concentration induced by an agonist. The opening of CaCC has also been observed after an increase in the concentration of intracellular Ca2+ triggered by the release of Ca2+ from intracellular reservoirs, which created a spontaneous transient inward currents (STICs, a Ca2+-activated chloride current), leading to a state of increased contractility [58]. Thus, the induction of oscillating input currents of Cl− after stimulation with ATP, UTP, and UDP has been reported in rat SMC of SPA and LPA, which were dependent on the release of Ca2+ from the sarcoplasmic reticulum [54].
Vascular dynamics in response to the stimulation of P2 receptor
In experiments of vascular functional dynamics, the rat has been the most studied species, followed by rabbit, cat, human, and guinea pig. The effect of purinergic agonists on P2 receptors usually has been vasoconstriction if the vessel is basally relaxed, or vasodilation if it is previously constricted [27]. Predominantly, vasoconstriction depends on the agonist action on SMC, and the relaxation depends on the action on EC [12].
In rats, the most potent vasoconstrictor described in SPA with intact endothelium (E +), SPA with endothelium-denuded (E-), LPA E-, and LPV E- has been α,β-meATP, a non-selective P2 agonist [38, 59] (Table 2 and Fig. 1). In LPA E + , UDP has shown the highest vasoconstrictor potency [20]. In SPV E + , ATP-γ-S has shown the highest vasoconstrictor potency [28], although α,β-meATP effect on these vessels has not been described. ATP has shown greater potency in SPA than LPA [20], similar to UDP which had a slightly higher potency in SPA than LPA, although in LPA E + , studies have observed differences in UDP potency [20, 26]. UTP has shown a high potency in LPA E- and SPA, except for LPA E + , where a low potency is observed [20, 26]. The agonist with the lowest vasoconstrictor potency reported has been ADP in SPA/LPA E + and SPA/LPA E- compared to α,β-meATP, UDP, UTP, ATP, and 2-meSATP [20, 28].
Table 2.
Purinergic agonist potency for vasoconstriction or vasodilation according to vascular segment in rat lungs (20, 26, 27, 28)
| Potency | LPA | SPA | SPV | LPV | |||||
|---|---|---|---|---|---|---|---|---|---|
| E + a | E- | E + | E- | E + | E- | E + | E- | ||
| Vasoconstriction | Higher potency | α,β-meATP (20) | α,β-meATP (20) | ||||||
|
α,β-meATP [0.5 µM]b (20, 27) UTP (20) |
UTP (20) ATP (20) 2-meSATP (20) |
UDP (20) UTP (20) ATP (20) 2-meSATP (20) |
α,β-meATP [0.1 µM] (27) | ||||||
| UDP (20) |
β,γ-meATP [2.8 µM] (27) UDP (20) |
UDP (20) | ATPγ-S [2.5 µM] (28) | ||||||
| 2-meSATP [17.8 µM] (20, 27) |
UTP [16,4 µM] (28) ATP [28.5 µM] (28) |
2-meSATP [58.9 µM] (27) β,γ-meATP [95.4 µM] (27) |
|||||||
| ATP [169.8 µM] (20, 27) | ADP [117 µM] (28) | ATP [245.5 µM] (27) | |||||||
|
α,β-meATP (20) UDP (26) UTP (20, 26) |
ADP (20) | ADP (20) | |||||||
| Lower potency/no effect |
ATP (20, 26) 2-meSATP (20) [ADP] (20) |
[ADP] (20) | |||||||
| Vasodilation | Higher potency | 2-meSATP [0.04 µM] (27) | |||||||
|
ATP [0.4 µM] (27) β,γ-meATP [0.9 µM] (27) |
|||||||||
| 2-meSATP [1.6 µM] (27) | |||||||||
| α,β-meATP [0.1 µM] (27) |
ATP [16.6 µM] (27) β,γ-meATP [55.0 µM] (27) |
||||||||
| Lower potency/no effect |
UTP (26) UDP (26) |
α,β-meATP (27) | |||||||
aAbbreviations: E + presence of endothelium, E- absence of endothelium
bEC50 or EC40 is shown in brackets when data was available
Conversely, in pre-constricted rat lung vessels, ATP has been able to induce endothelium-dependent vasodilation [26], while UTP and UDP seem to have no effects in these vessels [26] (Table 2 and Fig. 1). This is more prominent in arteries than in veins [42, 60], and it was observed following pre-constriction using different stimuli, such as hypoxia [42], thromboxane A2 receptor agonists [41, 42, 60], and sympathomimetics such as methoxamine [26], phenylephrine [27], and serotonin [27]. In rabbit models, endothelium-dependent vasodilation in pre-constricted LPA using the alpha-1 adrenergic agonist, phenylephrine [50], or with U-46619 [32] has been observed with UTP stimulus [32, 50]. Additionally, vasodilation has been recorded in endothelium-denuded and pre-constricted PA of rabbits in response to ATP and 2-meSATP, suggesting a SMC intrinsic vasodilation mechanism [50]. In rats, a greater ATP-induced vasodilation has been demonstrated in endothelium-intact arteries compared to endothelium-denuded, while vasodilation induced by 2-meSATP has been similar in both situations [50]. Strikingly, in pre-constricted LPV E + , α,β-meATP induced greater vasoconstriction rather than vasodilation [61].
In models of human endothelium-denuded SPA, the order of vasoconstrictor potency was described as follows: α,β-meATP = β,γ-meATP > ATP > 2-meSATP. On the other hand, in endothelium-intact SPA and pre-constricted with prostaglandin F2a, the vasodilator potency follows a slightly different order: 2-meSATP > ATP > > β,γ-meATP = α,β-meATP [62]. However, the removal of the endothelium did not modify the vasodilator response in SPA to ATP and ATP analogs; therefore, a SMC intrinsic vasodilation mechanism has been suggested in humans [62]. This has also been reported in LPA, where relaxation of LPA induced by ATP partially decreased with the removal of endothelium [62, 63]. On the other hand, vasodilation in response to ADP was described in arterial rings pre-constricted with adrenaline; however, this was abolished in endothelium-denuded PA and was independent of prostaglandin production [64].
A major mechanism that mediates endothelium-dependent vasodilation is the release of nitric oxide (NO) [65]. In this sense, studies have described a reduction in vasodilation induced by ATP and 2-meSATP in previously constricted rat pulmonary artery and a reduction in vasodilation induced by ATP and UTP in previously constricted rabbit pulmonary artery, after treatment with L-NAME, a nitric oxide synthesis inhibitor [41, 42, 50]. On the other hand, the release of prostacyclin (PGI2) from EC after perfusion with ATP and UTP was reported for the bovine pulmonary artery [49], although the functional impact of this finding has not been evaluated. In addition, endothelium-dependent hyperpolarization has been proposed as an alternative mechanism of vasodilation [66]. Within this context, a study reported that endothelium-dependent hyperpolarization was activated by P2Y2 receptors and mediated by K+ channels, myoendothelial gap junctions, and connexins 37 and 40 [66]. Another mechanism of vasodilation, the intrinsic mechanism of vascular SMC relaxation, has been suggested [67, 68]. This has been supported by experiments where the removal of endothelium did not change the vasodilator response to ATP and ATP analogs [67, 68].
Regarding the specific P2 receptor involved in the regulation of vascular tone, P2X1 receptor has been described as the main mediator of vasoconstriction induced by ATP in rat SPA [69] and LPA [24]. P2Y12 receptor may also have a role in vascular tone control. This has been suggested by experiments that observed a reduced contraction in response to ATP when rat intrapulmonary artery is treated with cangrelor, a P2Y12 antagonist [22, 23]. Combined exposure to P2Y12 and P2X1 receptor antagonists abolished vasoconstriction induced by ATP [23]. P2Y1 receptor was present and functional in studies using rat SPA; however, they did not seem to be involved in vascular tone regulation [22, 23]. In veins, a study reported that vasoconstriction of rat SPV was mediated by P2Y2 receptors, based on the inhibitory effect observed by selective antagonists, the affinity of the agonists, and receptor expression in the tissue [28].
Role of P1 receptor signaling in the regulation of pulmonary vascular tone
Conversion of ATP, which is released by stressed, apoptotic, and/or necrotic cells, to adenosine is performed by ecto-5′-mucleotidase, and activation of adenosine receptors allows biological effects of adenosine. The balance between ATP and ADP determines the final effect. In this sense, studies have observed that the regulation of adenosine signaling depends on ATP release, enzymatic conversion, expression of adenosine receptors, and termination of extracellular adenosine signaling by equilibrative nucleoside transporters (ENTs) [70–72].
In guinea pig vessels pre-constricted with noradrenaline, adenosine presents a biphasic action consisting of a fast constrictor response[73, 74] and a slow relaxation phase [74]. The vasoconstrictor response was inhibited by a selective A1 receptor antagonist and an A1 receptor agonist-induced vasoconstriction, suggesting that the smooth muscle contraction phase is A1 receptor-dependent [74]. On the other hand, the vasodilator response was inhibited by A1 and A2 receptor antagonists, in the presence of complete A1 receptor blockade by a selective antagonist and remained present after the addition of the selective A2A receptor antagonist, CP66713, after endothelium removal and in the presence of NO synthase inhibitor, L-NOARG [74]. This suggests that vasodilation is A2B receptor-dependent and A2A receptor-independent, with no participation of an endothelium-mediated mechanism or production of NO [74].
In rats, a dose-dependent vasodilator response to adenosine has been described in perfused lungs and in PA pre-constricted with norepinephrine. In endothelium-intact arterial rings, 10−6 M adenosine induced an increase in tension; however, vasodilation was observed if the concentration was increased to 10−4 M [75]. In endothelium-denuded arterial rings, the addition of adenosine induced vasodilation [75]. Similarly, adenosine induced vasodilation in perfused lungs where previous vasoconstriction was induced by hypoxia [76]. This process was mediated by A2B receptors and independent of NO generation [76].
A vasodilator response to adenosine has also been reported in rabbit PA pre-constricted with the thromboxane mimetic U-46619 [77, 78], phenylephrine [78], serotonin [79], and acetylcholine [79]. Interestingly, exposure to adenosine receptor antagonists increased the contraction force induced by phenylephrine [78]. The adenosine vasodilator response was partially dependent on the endothelium in a study using rabbit PA, since vasodilation induced by adenosine was attenuated in endothelium-denuded arteries [78]. The effect of adenosine was partially mediated by NO, since arteries pre-treated with a nitric oxide synthase inhibitor shifted rightward the adenosine concentration–response curve [78]. A greater vasodilator response induced by adenosine has been reported in endothelium-denuded vessels, possibly because this can decrease the release of endothelium-derived constrictor factors [79]. Additionally, the action of adenosine could be mediated by cyclic guanidine monophosphate (cGMP), since its vasodilator effect was inhibited by methylene blue, a guanylyl cyclase inhibitor [79].
Vasodilation after stimulation of ventilated dogs with adenosine administered by infusion has been observed [80]. However, vasoconstriction induced by adenosine administered by infusion was reported in sheep, an effect that was associated with an increase in plasma levels of thromboxane A2 [81].
Vasoconstriction induced by infusion of adenosine under low resting tone conditions in intact-chest cats has been reported [82–84], and this may depend on A1 receptors [84], cyclooxygenase activity [82–84], and thromboxane receptors [82, 83]. On the other hand, a vasodilator effect of adenosine has also been reported in vessels pre-constricted with U-46619 [82, 83], which appears to involve A2 receptors [84], but not NO, guanylyl cyclase nor K+ATP channels [84]. ATP also induced vasoconstriction in the same experiment, which may partly be due to its degradation to adenosine, since A1 antagonists inhibited the ATP effect [83], and this inhibition was less when the degradation was blocked [83].
In human PA pre-constricted with serotonin, vasodilation induced by adenosine has been observed, with no significant differences between endothelium-denuded and endothelium-intact vessels, nor between SPA (200–400 μm internal diameter) and LPA (7–10 mm internal diameter) [85], suggesting that the vasodilation was not mediated by the release of relaxant factors from endothelial cells. In male subjects, intravenous administration of adenosine reduced pulmonary vascular resistance and mean pulmonary arterial pressure, without affecting systemic circulation [86]. No changes in arterial O2 partial pressure were found in this study, so it is suggested that adenosine-induced vasodilation does not significantly affect the ventilation-perfusion ratio [86]. In conscious subjects, a decreased pulmonary vascular resistance has been reported after a low dose of adenosine, with no hemodynamic effects on systemic circulation, while the administration of a high dose of adenosine reduced both pulmonary and systemic vascular resistance. It is worth mentioning that this study reported an increase in the end pressure of the left ventricular diastole, pulmonary capillary pressure, and mean pulmonary arterial pressure [87].
Innervation and purinergic regulation
Simultaneous release of ATP and catecholamines at sympathetic nerve terminals of pulmonary vessels has been reported in rabbits [88, 89]. A study observed a reduction in contractions of rabbit pulmonary artery induced by field stimulation when the artery was treated with ATP and ADP [90]. In this sense, in fixed blood vessels of rats, clusters of P2X1 and P2X2 receptors in SMC were colocalized with nerve varicosities [91]. On the other hand, in rings of guinea pig pulmonary artery, a vasoconstrictor effect of adenosine and P1 receptor agonists has been reported, which potentiated the vasoconstriction in response to nerve stimulation or noradrenaline, and this effect was mediated by postsynaptic receptors [74]. Thus, in the neuromuscular synapses, there are regulatory mechanisms where purinergic signaling seems to participate, and this determines the physiological response to the stimulus of neurotransmitters.
Perspectives of purinergic signaling in pulmonary vascular disease
In the pulmonary circulation, purinergic signaling could play an important role in regulating vascular tone and preventing the development of pulmonary vascular diseases (PVD). For example, adenosine released from endothelial cells can activate A2A receptors on smooth muscle cells, leading to vasodilation and decrease in pulmonary vascular resistance [92]. ATP released from platelets and other cells can activate P2Y1 receptors on smooth muscle cells, leading to vasoconstriction [23]. However, purinergic signaling is often dysregulated in patients with PVD. For example, an increase in ATP release or a decrease in ecto-nucleotidase activity may lead to increased levels of extracellular ATP [93–95]. This can trigger P2 receptors on smooth muscle cells, inducing vasoconstriction and the development of pulmonary hypertension. In addition, purinergic signaling may also play a role in the inflammation which is associated with PVD. For example, ATP released from immune cells can activate P2X7 receptors on endothelial cells, leading to the release of pro-inflammatory cytokines. These cytokines could then further promote vascular remodeling and progression of PVD [96]. Adenosine and adenosine receptors, expressed in the epithelium and endothelium of different organs, have also been associated with inflammatory diseases and hypoxia, which have been shown to enhance the production and signaling effects of adenosine [97, 98]. While the potential therapeutic benefits of targeting purinergic signaling in PVD are promising, further research is needed to fully understand this pathway and to develop safe and effective therapies. Some key research areas are as follows: specific mechanisms of purinergic signaling deregulation in PVD, the role of purinergic signaling in the development and progression of PVD, and the efficacy and safety of drugs that target purinergic signaling in PVD.
Conclusions
Research on the purinergic pathway is moving forward. Recent investigations describing endogenous and exogenous PR agonists and antagonists have been published [91, 92, 96, 99–102], expanding the knowledge in this field. In addition, there is growing evidence concerning the role of purinergic signaling in the vascular pathophysiology of lung diseases, such as pulmonary arterial hypertension [93, 94, 103–105] and chronic obstructive pulmonary disease [106, 107]. Remarkably, purinergic agonists can induce different responses depending on the previous condition of the tissue; thus, the use of compounds that target purinergic receptors as regulators of pulmonary vascular tone represents a challenge.
The current research efforts predominantly concentrate on arteries, with limited attention given to venous segments and distal circulation within the pulmonary vasculature. This imbalance underscores the critical need for studies that specifically address venous segments and small vessels to gain a comprehensive understanding of pulmonary vascular function and regulation. Additionally, there is a scarcity of evidence regarding vasodilation following different mechanisms of pre-contraction. Therefore, further research is essential to elucidate the purinergic mechanisms involved in regulating pulmonary vascular tone and to determine the contribution of purinergic signaling to pulmonary vascular diseases, such as pulmonary hypertension.
Acknowledgements
We thank Santiago Ramirez (UTHealth | The University of Texas Health Science Center at Houston) for English correction of the manuscript.
Marco Alveal Bahamóndez
is a medical student at the University of Chile. He joined Dr. M Henriquez’s Laboratory at the University of Chile for his assistantship, where he studied the role of purinergic receptors in the regulation of pulmonary vascular tone. Currently, he is doing his internship at Hospital del Salvador.

Author contribution
MA made substantial contributions to the conception and design of the work and data interpretation; drafted, revised, and approved the submitted version; and has agreed to be accountable.
AM made substantial contributions to the review design and data interpretation, revised and approved the submitted version of the manuscript, and has agreed to be accountable.
AG has drafted and revised the manuscript, approved the submitted version, and has agreed to be accountable.
MH made substantial contributions to the conception and design of the work and data interpretation; drafted, revised, and approved the submitted version; and has agreed to be accountable.
Data availability
No datasets were generated or analyzed during the current study.
Declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weibel ER (2017) Lung morphometry: the link between structure and function. Cell Tissue Res 367(3):413–426. 10.1007/s00441-016-2541-4 [DOI] [PubMed] [Google Scholar]
- 2.Hsia CCW, Hyde DM, Weibel ER (2016). Lung structure and the intrinsic challenges of gas exchange. Comprehensive physiology. Hoboken, NJ, USA: John Wiley & Sons, Inc 827–95. 10.1002/cphy.c150028 [DOI] [PMC free article] [PubMed]
- 3.Clark A, Tawhai M (2019). Pulmonary vascular dynamics. In: Comprehensive physiology Wiley 1081–100. 10.1002/cphy.c180033 [DOI] [PubMed]
- 4.Vaillancourt M, Chia P, Sarji S, Nguyen J, Hoftman N, Ruffenach G et al (2017) Autonomic nervous system involvement in pulmonary arterial hypertension. Respir Res 18:201. 10.1186/s12931-017-0679-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sylvester JT, Shimoda LA, Aaronson PI, Ward JPT (2012) Hypoxic pulmonary vasoconstriction. Physiol Rev 92(1):367–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldenberg NM, Kuebler WM (2015). Endothelial cell regulation of pulmonary vascular tone, inflammation, and coagulation. Comprehensive physiology. Hoboken, NJ, USA: John Wiley & Sons, Inc 531–59. 10.1002/cphy.c140024 [DOI] [PubMed]
- 7.Burnstock G (2017) Purinergic signaling in the cardiovascular system. Circ Res 120(1):207–228. 10.1161/CIRCRESAHA.116.309726 [DOI] [PubMed] [Google Scholar]
- 8.Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE (2011) International union of basic and clinical pharmacology. LXXXI. Nomenclature and classification of adenosine receptors - An update. Pharmacol Rev 63(1):1–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.North RA (2002) Molecular physiology of P2X receptors. Physiol Rev Am Physiol Soc 82:1013–1067 [DOI] [PubMed] [Google Scholar]
- 10.von Kügelgen I (2019) Pharmacology of P2Y receptors. Brain Res Bull 151:12–24. 10.1016/j.brainresbull.2019.03.010 [DOI] [PubMed] [Google Scholar]
- 11.O’Connor SE, Dainty IA, Leff P (1991) Further subclassification of ATP receptors based on agonist studies. Trends Pharmacol Sci 12(C):137–41 [DOI] [PubMed] [Google Scholar]
- 12.Burnstock G, Ralevic V (2013) Purinergic signaling and blood vessels in health and disease. Pharmacol Rev 66(1):102–192 [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann H (2021) Ectonucleoside triphosphate diphosphohydrolases and ecto-5′-nucleotidase in purinergic signaling: how the field developed and where we are now. Purinergic Signal 17(1):117–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goueli SA, Hsiao K (2019) Monitoring and characterizing soluble and membrane-bound ectonucleotidases CD73 and CD39. PLoS ONE 14(10):1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allard B, Longhi MS, Robson SC, Stagg J (2017) The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol Rev 276:121–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robson SC, Sévigny J, Zimmermann H (2006) The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal 2(2):409–430. 10.1007/s11302-006-9003-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckle T, Füllbier L, Wehrmann M, Khoury J, Mittelbronn M, Ibla J et al (2007) Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J Immunol 178(12):8127–8137 [DOI] [PubMed] [Google Scholar]
- 18.Burch LH, Picher M (2006) E-NTPDases in human airways: regulation and relevance for chronic lung diseases. Purinergic Signalling 2:399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens T, Phan S, Frid MG, Alvarez D, Herzog E, Stenmark KR (2008) Lung vascular cell heterogeneity: endothelium, smooth muscle, and fibroblasts. Proc Am Thorac Soc 5(7):783–791. 10.1513/pats.200803-027HR [DOI] [PMC free article] [PubMed]
- 20.Chootip K, Ness KF, Wang Y, Gurney AM, Kennedy C (2002) Regional variation in P2 receptor expression in the rat pulmonary arterial circulation. Br J Pharmacol 137(5):637–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis CJ, Evans RJ (2001) P2X receptor immunoreactivity in different arteries from the femoral, pulmonary, cerebral, coronary and renal circulations. J Vasc Res 38(4):332–340 [DOI] [PubMed] [Google Scholar]
- 22.Huang C, Hu J, Subedi KP, Lin AHY, Paudel O, Ran P et al (2015) Extracellular adenosine diphosphate ribose mobilizes intracellular Ca 2+ via purinergic-dependent Ca 2+ pathways in rat pulmonary artery smooth muscle cells. Cell Physiol Biochem 37(5):2043–2059 [DOI] [PubMed] [Google Scholar]
- 23.Mitchell C, Syed NH, Tengah A, Gurney AM, Kennedy C (2012) Identification of contractile P2Y1, P2Y6, and P2Y12 receptors in rat intrapulmonary artery using selective ligands. J Pharmacol Exp Ther 343(3):755–762 [DOI] [PubMed] [Google Scholar]
- 24.Syed N-H, Tengah A, Paul A, Kennedy C (2010) Characterisation of P2X receptors expressed in rat pulmonary arteries. Eur J Pharmacol 649(1–3):342–348 [DOI] [PubMed] [Google Scholar]
- 25.Hartley SA, Kato K, Salter KJ, Kozlowski RZ (1998) Functional evidence for a novel suramin-insensitive pyrimidine receptor in rat small pulmonary arteries. Circ Res 83(9):940–946 [DOI] [PubMed] [Google Scholar]
- 26.Rubino A, Ziabary L, Burnstock G (2019) Regulation of vascular tone by UTP and UDP in isolated rat intrapulmonary arteries. Eur J Pharmacol 370(2):139–143 [DOI] [PubMed] [Google Scholar]
- 27.Liu SF, McCormack DG, Evans TW, Barnes PJ (1989) Characterization and distribution of P2-purinoceptor subtypes in rat pulmonary vessels. J Pharmacol Exp Ther 251(3):1204–1210 [PubMed] [Google Scholar]
- 28.Henriquez M, Fonseca M, Perez-Zoghbi JF (2018) Purinergic receptor stimulation induces calcium oscillations and smooth muscle contraction in small pulmonary veins. J Physiol 596(13):2491–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiefmann R, Islam MN, Lindert J, Parthasarathi K, Bhattacharya J (2009) Paracrine purinergic signaling determines lung endothelial nitric oxide production. Am J Physiol Cell Mol Physiol 296(6):L901–L910. 10.1152/ajplung.90549.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baek EB, Yoo HY, Park SJ, Kim HS, Kim SD, Earm YE et al (2008) Luminal ATP-induced contraction of rabbit pulmonary arteries and role of purinoceptors in the regulation of pulmonary arterial pressure. Pflügers Arch - Eur J Physiol 457(2):281–291. 10.1007/s00424-008-0536-z [DOI] [PubMed] [Google Scholar]
- 31.Hasséssian H, Bodin P, Burnstock G (1993) Blockade by glibenclamide of the flow-evoked endothelial release of ATP that contributes to vasodilatation in the pulmonary vascular bed of the rat. Br J Pharmacol 109(2):466–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sprague RS, Olearczyk JJ, Spence DM, Stephenson AH, Sprung RW, Lonigro AJ (2003) Extracellular ATP signaling in the rabbit lung: erythrocytes as determinants of vascular resistance. Am J Physiol Circ Physiol 285(2):H693-700 [DOI] [PubMed] [Google Scholar]
- 33.Mitchell C, Syed NIH, Gurney AM, Kennedy C (2012) A Ca 2+-dependent chloride current and Ca 2+ influx via Ca v1.2 ion channels play major roles in P2Y receptor-mediated pulmonary vasoconstriction. Br J Pharmacol 166(4):1503–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moerenhout M, Himpens B, Vereecke J (2001) Intercellular communication upon mechanical stimulation of CPAE-endothelial cells is mediated by nucleotides. Cell Calcium 29(2):125–136 [DOI] [PubMed] [Google Scholar]
- 35.Robaye B, Boeynaems JM, Communi D (1997) Slow desensitization of the human P2Y6 receptor. Eur J Pharmacol 329(2–3):231–236 [PubMed] [Google Scholar]
- 36.Brinson AE, Harden TK (2001) Differential regulation of the uridine nucleotide-activated P2Y4 and P2Y6 receptors. Ser-333 and Ser-334 in the carboxyl terminus are involved in agonist-dependent phosphorylation desensitization and internalization of the P2Y4 receptor. J Biol Chem 276(15):11939–48 [DOI] [PubMed] [Google Scholar]
- 37.Flores RV, Hernández-Pérez MG, Aquino E, Garrad RC, Weisman GA, Gonzalez FA (2005) Agonist-induced phosphorylation and desensitization of the P2Y2 nucleotide receptor. Mol Cell Biochem 280(1–2):35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jarvis MF, Khakh BS (2009) ATP-gated P2X cation-channels. Neuropharmacology 56:208–215 [DOI] [PubMed] [Google Scholar]
- 39.Koshimizu TA, Koshimizu M, Stojilkovic SS (1999) Contributions of the C-terminal domain to the control of P2X receptor desensitization. J Biol Chem 274(53):37651–37657 [DOI] [PubMed] [Google Scholar]
- 40.Burnstock G, Kennedy C (2011) P2X receptors in health and disease. Adv Pharmacol 61:333–72. 10.1016/B978-0-12-385526-8.00011-4 [DOI] [PubMed] [Google Scholar]
- 41.Hasséssian H, Burnstock G (1995) Interacting roles of nitric oxide and ATP in the pulmonary circulation of the rat. Br J Pharmacol 114(4):846–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hakim TS, Ferrario L, Freedman JC, Carlin RE, Camporesi EM (1997) Segmental pulmonary vascular responses to ATP in rat lungs: role of nitric oxide. J Appl Physiol 82(3):852–858 [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto K, Sokabe T, Matsumoto T, Yoshimura K, Shibata M, Ohura N et al (2006) Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med 12(1):133–137 [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto K, Sokabe T, Ohura N, Nakatsuka H, Kamiya A, Ando J (2003) Endogenously released ATP mediates shear stress-induced Ca2+ influx into pulmonary artery endothelial cells. Am J Physiol - Hear Circ Physiol 285(2 54-2):793–803 [DOI] [PubMed] [Google Scholar]
- 45.Schwiebert LM, Rice WC, Kudlow BA, Taylor AL, Schwiebert EM (2002) Extracellular ATP signaling and P2X nucleotide receptors in monolayers of primary human vascular endothelial cells. Am J Physiol - Cell Physiol 282(2 51-2):289–301 [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto K, Korenaga R, Kamiya A, Ando J (2000) Fluid shear stress activates Ca 2+ influx into human endothelial cells via P2X4 purinoceptors. Circ Res 87(5):385–391. 10.1161/01.RES.87.5.385 [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto K, Korenaga R, Kamiya A, Qi Z, Sokabe M, Ando J (2000) P2X4 receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am J Physiol - Hear Circ Physiol 279(1 48-1):285–92 [DOI] [PubMed] [Google Scholar]
- 48.Mertens TCJ, Hanmandlu A, Tu L, Phan C, Collum SD, Chen NY et al (2018) Switching-off ADORA2B in vascular smooth muscle cells halts the development of pulmonary hypertension. Front Physiol 9:555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lustig KD, Erb L, Landis DM, Hicks-Taylor CS, Zhang X, Sportiello MG et al (1992) Mechanisms by which extracellular ATP and UTP stimulate the release of prostacyclin from bovine pulmonary artery endothelial cells. Biochim Biophys Acta - Mol Cell Res 1134(1):61–72 [DOI] [PubMed] [Google Scholar]
- 50.Qasabian RA, Schyvens C, Owe-Young R, Killen JP, Macdonald PS, Conigrave AD et al (1997) Characterization of the P2 receptors in rabbit pulmonary artery. Br J Pharmacol 120(4):553–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.North RA (2016) P2X receptors. Philos Trans R Soc B Biol Sci 371(1700):20150427. 10.1098/rstb.2015.0427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Von Kügelgen I, Hoffmann K (2016) Pharmacology and structure of P2Y receptors. Neuropharmacology 104:50–61 [DOI] [PubMed] [Google Scholar]
- 53.Guibert C, Pacaud P, Loirand G, Marthan R, Savineau JP (1996) Effect of extracellular ATP on cytosolic Ca2+ concentration in rat pulmonary artery myocytes. Am J Physiol 271(3 Pt 1):L450–L458 [DOI] [PubMed] [Google Scholar]
- 54.Chootip K, Gurney AM, Kennedy C (2005) Multiple P2Y receptors couple to calcium-dependent, chloride channels in smooth muscle cells of the rat pulmonary artery. Respir Res 6(1):124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hartley SA, Kozlowski RZ (1997) Electrophysiological consequences of purinergic receptor stimulation in isolated rat pulmonary arterial myocytes. Circ Res 80(2):170–178. 10.1161/01.res.80.2.170 [DOI] [PubMed] [Google Scholar]
- 56.Zheng YM, Wang QS, Rathore R, Zhang WH, Mazurkiewicz JE, Sorrentino V et al (2005) Type-3 ryanodine receptors mediate hypoxia-, but not neurotransmitter- induced calcium release and contraction in pulmonary artery smooth muscle cells. J Gen Physiol 125(4):427–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C et al (2006) International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58:281–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berg J, Yang H, Jan LY (2012) Ca2+-activated Cl-channels at a glance. J Cell Sci 125(6):1367–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Erlinge D, Burnstock G (2008) P2 receptors in cardiovascular regulation and disease. Purinergic Signalling 4(1):1–20. 10.1007/s11302-007-9078-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eichinger MR, Walker BR (1994) Segmental heterogeneity of NO-mediated pulmonary vasodilation in rats. Am J Physiol 267(2 Pt 2):H494–H499 [DOI] [PubMed] [Google Scholar]
- 61.Liu SF, McCormack DG, Evans TW, Barnes PJ (1989) Characterization and distribution of P2-purinoceptor subtypes in rat pulmonary vessels. J Pharmacol Exp Ther 251(3):1204–1210 [PubMed] [Google Scholar]
- 62.Liu SF, McCormack DG, Evans TW, Barnes PJ (1989) Evidence for two P2-purinoceptor subtypes in human small pulmonary arteries. Br J Pharmacol 98(3):1014–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greenberg B, Rhoden K, Barnes PJ (1987) Endothelium-dependent relaxation of human pulmonary arteries. Am J Physiol Circ Physiol 252(2):H434–H438 [DOI] [PubMed] [Google Scholar]
- 64.DinhXuan AT, Higenbottan TW, Clelland C, Pepke-Zaba J, Wells FC, Wallwork J (1990) Acetylcholine and adenosine diphosphate cause endothelium-dependent relaxation of isolated human pulmonary arteries. Eur Respir J 3(6):633–8 [PubMed] [Google Scholar]
- 65.Liu SF, Barnes PJ (1994) Role of endothelium in the control of pulmonary vascular tone. Endothelium 2(1):11–33. 10.3109/10623329409024631 [Google Scholar]
- 66.Dominguez Rieg JA, Burt JM, Ruth P, Rieg T (2015) P2Y2 receptor activation decreases blood pressure via intermediate conductance potassium channels and connexin 37. Acta Physiol 213(3):628–641. 10.1111/apha.12446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiménez M, Clavé P, Accarino A, Gallego D (2014) Purinergic neuromuscular transmission in the gastrointestinal tract; functional basis for future clinical and pharmacological studies. Br J Pharmacol 171:4360–4375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paquola A, Mañé N, Giron MC, Jimenez M (2019) Diadenosine tetraphosphate activates P2Y1 receptors that cause smooth muscle relaxation in the mouse colon. Eur J Pharmacol 855:160–166 [DOI] [PubMed] [Google Scholar]
- 69.Dales MO, Mitchell C, Gurney AM, Drummond RM, Kennedy C (2022) Characterisation of P2Y receptor subtypes mediating vasodilation and vasoconstriction of rat pulmonary artery using selective antagonists. Purinergic Signal 18(4):515–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bowser JL, Phan LH, Eltzschig HK (2018) The hypoxia-adenosine link during intestinal inflammation. J Immunol 200(3):897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eltzschig HK (2013) Extracellular adenosine signaling in molecular medicine. J Mol Med (Berl) 91(2):141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aherne CM, Collins CB, Rapp CR, Olli KE, Perrenoud L, Jedlicka P et al (2018) Coordination of ENT2-dependent adenosine transport and signaling dampens mucosal inflammation. JCI Insight 3(20):e121521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wiklund NP, Cederqvist B, Matsuda H, Gustafsson LE (1987) Adenosine can excite pulmonary artery. Acta Physiol Scand 131(3):477–478 [DOI] [PubMed] [Google Scholar]
- 74.Szentmiklósi AJ, Ujfalusi A, Cseppentő Á, Nosztray K, Kovacs P, Szabó JZ (1995) Adenosine receptors mediate both contractile and relaxant effects of adenosine in main pulmonary artery of guinea pigs. Naunyn Schmiedebergs Arch Pharmacol 351(4):417–425. 10.1007/BF00169083 [DOI] [PubMed] [Google Scholar]
- 75.Roepke JE, Patterson CE, Packer CS, Rhoades RA (1991) Response of perfused lung and isolated pulmonary artery to adenosine. Exp Lung Res 17(1):25–37 [DOI] [PubMed] [Google Scholar]
- 76.Haynes Jr J, Obiako B, Thompson WJ, Downey J (1995) Adenosine-induced vasodilation: receptor characterization in pulmonary circulation. Am J Physiol 268(5 Pt 2):H1862–H1868. 10.1152/ajpheart.1995.268.5.H1862 [DOI] [PubMed]
- 77.Pearl RG (1994) Adenosine produces pulmonary vasodilation in the perfused rabbit lung via an adenosine A2 receptor. Anesth Analg 79(1):46–51 [DOI] [PubMed] [Google Scholar]
- 78.Steinhorn RH, Morin FC, Van Wylen DG, Gugino SF, Giese EC, Russell JA (1994) Endothelium-dependent relaxations to adenosine in juvenile rabbit pulmonary arteries and veins. Am J Physiol Circ Physiol 266(5):H2001–H2006 [DOI] [PubMed] [Google Scholar]
- 79.El-Kashef H, Elmazar MM, Al-Shabanah OA, Al-Bekairi AM (1999) Effect of adenosine on pulmonary circulation of rabbits. Gen Pharmacol 32(3):307–313 [DOI] [PubMed] [Google Scholar]
- 80.Mentzer RM, Rubio R, Berne R (1975) Release of adenosine by hypoxic canine lung tissue and its possible role in pulmonary circulation. Am J Physiol Content 229(6):1625–1631. 10.1152/ajplegacy.1975.229.6.1625 [DOI] [PubMed] [Google Scholar]
- 81.Biaggioni I, King LS, Enayat N, Robertson D, Newman JH (1989) Adenosine produces pulmonary vasoconstriction in sheep. Evidence for thromboxane A2/prostaglandin endoperoxide-receptor activation. Circ Res 65(6):1516–25. 10.1161/01.RES.65.6.1516 [DOI] [PubMed] [Google Scholar]
- 82.Lippton HL, Hao Q, Hauth T, Hyman A (1992) Mechanisms of signal transduction for adenosine and ATP in pulmonary vascular bed. Am J Physiol 262(3 Pt 2):H926–H929 [DOI] [PubMed] [Google Scholar]
- 83.Neely CF (1993) Purinergic responses in the feline pulmonary vascular bed. Drug Dev Res 28(3):328–335. 10.1002/ddr.430280326 [Google Scholar]
- 84.Cheng DY, Dewitt BJ, Suzuki F, Neely CF, Kadowitz PJ (1996). Adenosine A1 and A2 receptors mediate tone-dependent responses in feline pulmonary vascular bed. Am J Physiol - Hear Circ Physiol 270(1 39–1). 10.1152/ajpheart.1996.270.1.H200 [DOI] [PubMed]
- 85.McCormack DG, Clarke B, Barnes PJ (1989) Characterization of adenosine receptors in human pulmonary arteries. Am J Physiol Circ Physiol 256(1):H41–H46 [DOI] [PubMed] [Google Scholar]
- 86.Fullerton DA, Kirson LE, Jones SD, McIntyre RC (1996) Adenosine is a selective pulmonary vasodilator in cardiac surgical patients. Chest 109(1):41–46 [DOI] [PubMed] [Google Scholar]
- 87.Reid PG, Fraser AG, Watt AH, Henderson AH, Routledge PA (1990) Acute haemodynamic effects of intravenous infusion of adenosine in conscious man. Eur Heart J 11(11):1018–1028 [DOI] [PubMed] [Google Scholar]
- 88.Katsuragi T, Su C (1980) Purine release from vascular adrenergic nerves by high potassium and a calcium ionophore, A-23187. J Pharmacol Exp Ther 215(3):685–690 [PubMed] [Google Scholar]
- 89.Mohri K, Takeuchi K, Shinozuka K, Bjur RA, Westfalli DP (1993) Simultaneous determination of nerve-induced adenine nucleotides and nucleosides released from rabbit pulmonary artery. Anal Biochem 210(2):262–267 [DOI] [PubMed] [Google Scholar]
- 90.Husted SE, Nedergaard OA (1985) Dual inhibitory action of ATP on adrenergic neuroeffector transmission in rabbit pulmonary artery. Acta Pharmacol Toxicol (Copenh) 57(3):204–213 [DOI] [PubMed] [Google Scholar]
- 91.Hansen MA, Dutton JL, Balcar VJ, Barden JA, Bennett MR (1999) P2X (purinergic) receptor distributions in rat blood vessels. J Auton Nerv Syst 75(2–3):147–155 [DOI] [PubMed] [Google Scholar]
- 92.Alencar AKN, Pereira SL, Montagnoli TL, Maia RC, Kümmerle AE, Landgraf SS et al (2013) Beneficial effects of a novel agonist of the adenosine A2A receptor on monocrotaline-induced pulmonary hypertension in rats. Br J Pharmacol 169(5):953–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Helenius MH, Vattulainen S, Orcholski M, Aho J, Komulainen A, Taimen P et al (2015) Suppression of endothelial CD39/ENTPD1 is associated with pulmonary vascular remodeling in pulmonary arterial hypertension. Am J Physiol Cell Mol Physiol 308(10):L1046–L1057. 10.1152/ajplung.00340.2014 [DOI] [PubMed] [Google Scholar]
- 94.Visovatti SH, Hyman MC, Bouis D, Neubig R, McLaughlin VV, Pinsky DJ (2012) Increased CD39 nucleotidase activity on microparticles from patients with idiopathic pulmonary arterial hypertension. PLoS ONE 7(7):e40829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang W, Zhang Y, Wang W, Dai Y, Ning C, Luo R et al (2013) Elevated ecto-5′-nucleotidase-mediated increased renal adenosine signaling via A2B adenosine receptor contributes to chronic hypertension. Circ Res 112(11):1466–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yin J, You S, Liu H, Chen L, Zhang C, Hu H et al (2017) Role of P2X7R in the development and progression of pulmonary hypertension. Respir Res 18(1):127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aherne CM, Saeedi B, Collins CB, Masterson JC, McNamee EN, Perrenoud L et al (2015) Epithelial-specific A2B adenosine receptor signaling protects the colonic epithelial barrier during acute colitis. Mucosal Immunol 8(6):1324–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yuan X, Lee JW, Bowser JL, Neudecker V, Sridhar S, Eltzschig HK (2018) Targeting hypoxia signaling for perioperative organ injury. Anesth Analg 126(1):308–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rafehi M, Müller CE (2018) Tools and drugs for uracil nucleotide-activated P2Y receptors. Pharmacol Ther 190:24–80 [DOI] [PubMed] [Google Scholar]
- 100.Xu P, Feng X, Luan H, Wang J, Ge R, Li Z et al (2018) Current knowledge on the nucleotide agonists for the P2Y2 receptor. Bioorg Med Chem 26:366–375 [DOI] [PubMed] [Google Scholar]
- 101.Jacobson KA, Müller CE (2016) Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology 104:31–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abdelrahman A, Yerande SG, Namasivayam V, Klapschinski TA, Alnouri MW, El-Tayeb A et al (2020) Substituted 4-phenylthiazoles: development of potent and selective A1, A3 and dual A1/A3 adenosine receptor antagonists. Eur J Med Chem 186:111879. 10.1016/j.ejmech.2019.111879 [DOI] [PubMed] [Google Scholar]
- 103.Hennigs JK, Lüneburg N, Stage A, Schmitz M, Körbelin J, Harbaum L et al (2019) The P2-receptor-mediated Ca2+ signalosome of the human pulmonary endothelium - implications for pulmonary arterial hypertension. Purinergic Signal 15(3):299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Visovatti SH, Hyman MC, Goonewardena SN, Anyanwu AC, Kanthi Y, Robichaud P et al (2016) Purinergic dysregulation in pulmonary hypertension. Am J Physiol - Hear Circ Physiol 311(1):H286–H298. 10.1152/ajpheart.00572.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li LZ, Yue LH, Zhang ZM, Zhao J, Ren LM, Wang HJ et al (2020) Comparison of mRNA expression of P2X receptor subtypes in different arterial tissues of rats. Biochem Genet 58(5):677–690 [DOI] [PubMed] [Google Scholar]
- 106.Aliagas E, Muñoz-Esquerre M, Cuevas E, Careta O, Huertas D, López-Sánchez M et al (2018) Is the purinergic pathway involved in the pathology of COPD? Decreased lung CD39 expression at initial stages of COPD. Respir Res 19(1):1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Careta O, Cuevas E, Muñoz-Esquerre M, López-Sánchez M, Pascual-González Y, Dorca J et al (2019) Imbalance in the expression of genes associated with purinergic signalling in the lung and systemic arteries of COPD patients. Sci Rep 9(1):1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.