Abstract
Mitochondrial dysfunction with oxidative stress contributes to metabolic dysfunction-associated steatotic liver disease (MASLD) progression. We aimed to evaluate the fibrosis predictive efficacy of a novel non-invasive diagnostic panel using metabolic stress biomarkers. From a population-based general cohort, 144 subjects with MASLD were recruited in the development group and underwent magnetic resonance imaging-based liver examinations, anthropometric and laboratory tests. As an external validation group, 41 patients enrolled in a biopsy-evaluated MASLD cohort participated in this study. Liver fat content and stiffness were measured by magnetic resonance (MR) imaging-proton density fat fraction and MR elastography (MRE), respectively. Serologic stress biomarkers were quantitated by ELISA. Multivariate regression showed that waist-to-height ratio, growth differentiation factor-15 (GDF15), γ-glutamyltransferase, decorin, and alkaline-phosphatase were independent predictors of hepatic fibrosis (rank-ordered by Wald). The area under receiver-operator characteristics curve [AUROC (95% CI)) of the metabolic stress index for fibrosis (MSI-F) was 0.912 (0.85‒0.98) and 0.977 (0.92‒1.00) in development and validation groups, respectively. MSI-F also had better diagnostic accuracy (82.6‒92.4%) than other fibrosis indices in the both study cohorts. MSI-F consistently differentiated fibrosis severities across cohorts of MRE-evaluated general population and biopsy-proven patients with MASLD, while other indices showed no or less discrimination. MSI-F, as a novel non-invasive index based on a stress-stimulated protective hormone GDF15 and decorin, effectively predicted hepatic fibrosis. Furthermore, MSI-F may serve as pre-screening tool to increase the population that could be excluded from further evaluation, reducing unnecessary invasive investigations more effectively than other indices.
Keywords: Hepatic fibrosis, Mitochondrial stress, Serum biomarker, Growth differentiation factor-15, Decorin
Subject terms: Physiology, Endocrinology, Medical research
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) is the most prevalent chronic liver disease, progressing from simple steatosis to NASH, fibrosis, cirrhosis, and hepatocellular carcinoma. Simple hepatic steatosis has a benign nature, whereas NASH is more likely to progress to cirrhosis and cancer1. Liver cirrhosis is characterized by progressive accumulation of extracellular matrix proteins, leading to distortion of the liver architecture and loss of liver function. Because of the high prevalence and serious progression, reliable diagnostic and prognostic strategies are required to prevent the progression of MASLD and improve patient outcomes2. In the last 20 years, noninvasive serum biomarkers to identify liver fibrosis in patients with MASLD have been developed and validated against liver biopsy, the gold standard for determining the presence of tissue fibrosis3.
The pathogenesis of MASLD has been explained with the multiple parallel hits hypothesis, which suggests that several factors act in concert to cause the accumulation of fat in the liver and subsequent liver damage4. Mitochondrial dysfunction and oxidative stress are considered two of the second hits that can cause liver injury and progression from simple steatosis to nonalcoholic steatohepatitis (NASH)5. Our group has previously suggested that oxidative stress inflicts prolonged mitochondrial and endoplasmic reticulum (ER) stress via Ca2+ dysregulation, leading to further excessive ROS generation from the mitochondria and ER6,7. This ‘vicious cycle’ between oxidative stress and organellar dysfunction results in hepatic inflammation and further pathologic progression8,9.
In response to mitochondrial and ER stresses, cells are known to exhibit an adaptive and protective response mediated by the integrated stress response (ISR)10,11. Growth differentiation factor-15 (GDF15) and fibroblast growth factor-21 (FGF21) are representative humoral factors induced by activating transcription factor 4 (ATF4) or other components of the ISR10,12. Although GDF15 and FGF21 have differential roles in systemic adaptation to mitochondrial and ER stress, both have a common metabolic benefit in overcoming integrated stresses13. Furthermore, the therapeutic application of GDF15 has been shown to ameliorate hepatic inflammation and metabolic deterioration in an animal model of MASLD14.
Serum levels of GDF15 have been suggested as a promising biomarker for mitochondrial diseases and age-related disorders15,16. Recently, Koo et al. demonstrated that serum GDF15 in MASLD is positively correlated with the severity of hepatic inflammation and fibrosis17. Elevated serum GDF15 indicates unmet demand for protective stress responses in the pathologic progression of MASLD. However, there are controversies regarding the functional consequences of serum GDF15 elevation on hepatic fibrosis based on opposite findings in fibrogenic protein regulation in vitro14,17. Here, without any prejudices or selection bias, we aimed to elucidate the correlation between serum levels of 10 biomarkers known as metabolic humoral factors and clinical hepatic fibrosis phenotype estimated with magnetic resonance elastography (MRE) or tissue biopsy in a general cohort and liver cirrhosis patients. Interestingly, we demonstrated that serum levels of GDF15 and decorin are potent and independent predicting factors of liver cirrhosis in MASLD patients. In addition, a novel non-invasive index based on serum predictors including GDF15 and decorin not only effectively predicts fibrotic progression but also excludes biopsy candidates with a lower risk of liver cirrhosis.
Results
Baseline characteristics of the study participants
The median (IQR) age and BMI in the development and validation cohorts were 65.5 years (61.0‒71.0) and 26.8 kg/m2 (24.9‒28.4), and 47.0 years (32.5‒59.0) and 27.7 kg/m2 (25.9‒31.3), respectively. Of the development cohort, 15.3% had hepatic fibrosis of stage 1 or greater (MRE ≥ 2.9 kPa). In the validation cohort, 61.0% and 24.4% of subjects were diagnosed with biopsy-proven moderate-to-severe steatosis and significant-to-advanced fibrosis, respectively. The clinical and laboratory characteristics of the subjects are described in Supplementary Tables S1, S2.
Development of metabolic stress index for liver fibrosis
Independent predictive variables derived from logistic regression analyses with significant liver fibrosis as a dependent variable are described in Table 1. In the determination of liver stiffness, intriguingly, GDF15 as a mitochondrial stress biomarker had the highest significance coefficient (Wald χ2; 12.2, p < 0.001) after the γ-glutamyltransferase (γ-GT) level (15.4, p < 0.001) in univariate logistic analyses. Multivariate logistic analysis showed that WHtR and natural logarithms of serologic markers, including GDF15, γ-GT, decorin, and alkaline-phosphatase (ALP), were selected as significant independent predictors (rank-ordered by Wald), and were used to develop a metabolic stress index for fibrosis (MSI-F): ex/(1 + ex) ∙ 100. [x = 21.921∙WHtR + 2.392∙ln(GDF15, pg/mL) + 1.513∙ln(γ-GT, IU/L) + 2.576∙ln(decorin, ng/mL) + 3.226∙ln(ALP, IU/L)–55.69].
Table 1.
Univariate and multivariate (stepwise forward) logistic regression analyses for the prediction of hepatic fibrosis.
| Variable | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (95% CI) | S.E | Wald | p-value | Coefficient (95% CI) | S.E | Wald | p-value | |
| Significant factors | ||||||||
| Body mass index (kg/m2) | 0.197 (0.046‒0.347) | 0.077 | 6.523 | 0.011 | ||||
| Waist (cm) | 0.094 (0.035‒0.154) | 0.03 | 9.698 | 0.002 | ||||
| WHtR | 10.997 (1.848‒20.145) | 4.668 | 5.55 | 0.018 | 21.921 (8.771‒35.072) | 6.709 | 10.675 | 0.001 |
| WHR | 9.32 (0.1‒18.54) | 4.704 | 3.925 | 0.048 | ||||
| Ln [fasting insulin (mU/L)] | 0.66 (0.023‒1.298) | 0.325 | 4.118 | 0.042 | ||||
| Ln [AST (IU/L)] | 2.236 (0.943‒3.528) | 0.659 | 11.495 | < 0.001 | ||||
| Ln [ALT (IU/L)] | 1.06 (0.176‒1.944) | 0.451 | 5.524 | 0.019 | ||||
| Ln [γ-GT (IU/L)] | 1.445 (0.724‒2.166) | 0.368 | 15.414 | < 0.001 | 1.513 (0.542‒2.483) | 0.495 | 9.335 | 0.002 |
| Ln [ALP (IU/L)] | 1.645 (0.065‒3.225) | 0.806 | 4.166 | 0.041 | 3.226 (0.77‒5.682) | 1.253 | 6.629 | 0.01 |
| Ln [calcium (mg/dL)] | 14.78 (0.317‒29.243) | 7.379 | 4.012 | 0.045 | ||||
| Ln [platelet count (109/L)] | -3.136 (-5.13‒-1.143) | 1.017 | 9.51 | 0.002 | ||||
| Ln [GDF15 (pg/mL)] | 1.574 (0.691‒2.456) | 0.45 | 12.209 | < 0.001 | 2.392 (0.894‒3.891) | 0.765 | 9.79 | 0.002 |
| Ln [FGF21 (pg/mL)] | 0.708 (0.077‒1.339) | 0.322 | 4.834 | 0.028 | ||||
| Ln [IL6 (pg/mL)] | 0.959 (0.257‒1.661) | 0.358 | 7.177 | 0.007 | ||||
| Ln [decorin (ng/mL)] | 2.184 (0.652‒3.715) | 0.781 | 7.807 | 0.005 | 2.576 (0.747‒4.405) | 0.933 | 7.622 | 0.006 |
| Constant | − 55.689 (− 80.504‒ − 30.873) | 12.661 | 19.347 | < 0.001 | ||||
| Non-significant factors | ||||||||
| Sex (man = 0, woman = 1) | 0.694 (0.279‒1.726) | 0.464 | 0.616 | 0.432 | ||||
| Age (years) | 1.036 (0.971‒1.105) | 0.033 | 1.144 | 0.285 | ||||
| Ln [SBP (mmHg)] | 0.466 (0.009‒23.40) | 1.998 | 0.146 | 0.702 | ||||
| Ln [DBP (mmHg)] | 1.105 (0.014‒88.08) | 2.234 | 0.002 | 0.964 | ||||
| Ln [Triglyceride (mg/dL)] | 1.138 (0.484‒2.678) | 0.437 | 0.088 | 0.767 | ||||
| Total cholesterol (mg/dL) | 0.987 (0.975‒1.002) | 0.007 | 3.832 | 0.051 | ||||
| Ln [HDL-C (mg/dL)] | 0.272 (0.048‒1.545) | 0.886 | 2.159 | 0.142 | ||||
| Ln [Fasting glucose (mg/dL)] | 0.433 (0.042‒4.470) | 1.191 | 0.494 | 0.482 | ||||
| Ln [HOMA-IR] | 1.519 (0.899‒2.566) | 0.267 | 2.446 | 0.118 | ||||
| Ln [Total bilirubin (mg/dL)] | 1.497 (0.486‒4.605) | 0.573 | 0.494 | 0.482 | ||||
| Ln [Creatinine (mg/dL)] | 5.137 (0.849‒31.09) | 0.919 | 3.174 | 0.075 | ||||
| Ln [Phosphorus (mg/mL)] | 0.457 (0.011‒18.86) | 1.898 | 0.17 | 0.68 | ||||
| Ln [C-peptide (ng/mL)] | 2.057 (0.878‒4.816) | 0.434 | 2.759 | 0.097 | ||||
| Ln [FGF19 (pg/mL)] | 1.771 (0.941‒3.334) | 0.323 | 3.137 | 0.077 | ||||
| Ln [Leptin (ng/mL)] | 1.016 (0.561‒1.837) | 0.302 | 0.003 | 0.959 | ||||
| Ln [Adiponectin (μg/mL)] | 1.195 (0.628‒2.277) | 0.329 | 0.295 | 0.587 | ||||
| Ln [RBP4 (μg/mL)] | 0.421 (0.079‒2.232) | 0.852 | 1.034 | 0.309 | ||||
| Ln [TGF-β1 (ng/mL)] | 0.456 (0.112‒1.862) | 0.717 | 1.196 | 0.274 | ||||
| Ln [Myostatin (ng/mL)] | 0.829 (0.272‒2.528) | 0.569 | 0.108 | 0.742 | ||||
ALP alkaline phosphatase, ALT alanine aminotransferase, AST aspartate aminotransferase, DBP diastolic blood pressure, FGF fibroblast growth factor, γ-GT gamma-glutamyl transferase, GDF growth differentiation factor, HDL-C high-density lipoprotein cholesterol, HOMA-IR homeostasis model assessment of insulin resistance, IL6 interleukin 6, Ln natural logarithm, RBP4 retinol-binding protein 4, SBP systolic blood pressure, TGF-β1 transforming growth factor beta 1, WHR waist-to-hip ratio, WHtR waist-to-height ratio.
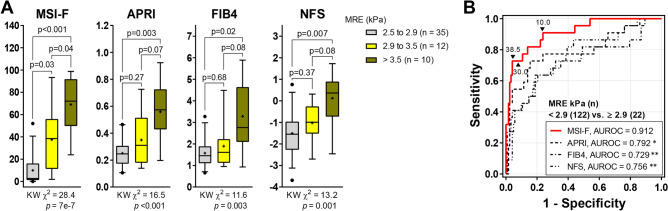
The Hosmer–Lemeshow statistic (χ2 = 10.55, p = 0.229) and the Nagelkerke index (R2 = 0.535) indicate a good fitness of the MSI-F model. The AUROC [95% CI] of the MSI-F (0.912 [0.85‒0.98]) shows a marked excellence in diagnostic performance compared with previously suggested indices for liver fibrosis, including the aspartate aminotransferase to platelet ratio index (APRI) (0.792 [0.67‒0.91]), the fibrosis-4 index (FIB4) (0.729 [0.59‒0.86]), and the non-alcoholic fatty liver disease fibrosis score (NFS) (0.756 [0.64‒0.87]) (Fig. 1). Even when the MSI-F was applied to the entire cohort, including 199 subjects without MASLD, its superiority in the discrimination of fibrosis was maintained (0.944 [0.90‒0.99]) (Supplementary Fig. S1A). At the optimal cut-off value of 38.53, the MSI-F could detect fibrosis with a specificity of 86% (95% CI 79‒92) and a positive likelihood ratio of 5.9 (95% CI 3.6‒9.5), and could rule out hepatic fibrosis with a sensitivity of 82% (95% CI 60‒95) and a negative likelihood ratio of 0.21 (95% CI 0.09‒0.51). The diagnostic accuracy of the MSI-F (92.4%) was noticeably superior to other fibrosis indices, as shown in Table 2.
Fig. 1.
Predictive ability of MSI-F for liver stiffness compared with other fibrosis indices. (A) Non-invasive prediction scores according to liver stiffness grades (Kruskal–Wallis [KW] test with post hoc Dunnett’s T3 test). (B) ROC curves of non-invasive scores for predicting hepatic fibrosis. *p < 0.05, **p < 0.01 vs. MSI-F (DeLong’s tests). MSI-F metabolic stress index of liver fibrosis, APRI AST to platelet ratio index, FIB-4 Fibrosis-4 index, NFS nonalcoholic fatty liver disease fibrosis score, MRE magnetic resonance elastography, AUROC area under the ROC (receiver operating characteristic) curve.
Table 2.
Diagnostic performance of non-invasive prediction scores for hepatic fibrosis.
| SN | SP | LR + | LR‒ | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|---|---|
| Development cohort | |||||||
| MSI-F | 72.7 (49.8–89.3) | 95.9 (90.7–98.7) | 17.8 (7.3–43.5) | 0.28 (0.14–0.56) | 76.2 (56.6–88.7) | 95.1 (90.8–97.5) | 92.4 (86.7–96.1) |
| APRI | 72.7 (49.8‒89.3) | 84.4 (76.8‒90.4) | 4.8 (2.9‒7.6) | 0.32 (0.16‒0.64) | 45.1 (34.1‒57.8) | 94.5 (89.6‒97.2) | 82.6 (75.5‒88.4) |
| FIB-4 | 63.6 (40.7‒82.8) | 79.5 (71.3‒86.3) | 3.1 (1.9‒5.0) | 0.46 (0.26‒0.80) | 35.9 (25.9‒47.3) | 92.4 (87.4‒95.5) | 77.1 (69.4‒83.7) |
| NFS | 63.6 (40.7‒82.8) | 81.2 (73.1‒87.7) | 3.4 (2.1‒5.5) | 0.45 (0.26‒0.78) | 37.8 (27.3‒49.7) | 92.5 (87.6‒95.6) | 78.5 (70.9‒84.9) |
| Validation cohort | |||||||
| MSI-F | 100 (69.2–100) | 69.2 (38.6–90.9) | 3.3 (1.4–7.4) | – | 71.4 (52.5–85.0) | 100 | 82.6 (61.2–95.1) |
| APRI | 70.0 (34.8–93.3) | 38.5 (13.9–68.4) | 1.1 (0.6–2.1) | 0.78 (0.24–2.51) | 46.7 (32.6–61.2) | 62.5 (34.1–84.3) | 52.2 (30.6–73.2) |
| IB-4 | 30.0 (6.67–65.3) | 100 (75.3–100) | – | 0.7 (0.47–1.05) | 100 | 65.0 (55.3–73.6) | 69.6 (47.1–86.8) |
| NFS | 50.0 (18.7–81.3) | 100 (75.3–100) | – | 0.5 (0.27–0.93) | 100 | 72.2 (58.3–82.9) | 78.3 (56.3–92.5) |
Data are presented as percentages (95% CI).
MSI-F metabolic stress index for liver fibrosis, APRI AST to platelet ratio index, FIB-4 Fibrosis-4 index, NFS nonalcoholic fatty liver disease fibrosis score, SN sensitivity, SP specificity, LR + positive likelihood ratio, LR‒ negative likelihood ratio, PPV positive predictive value, NPV negative predictive value.
To evaluate the effectiveness of the indices in reflecting the progression of liver stiffness stages, a subgroup analysis was performed, including only participants with clinical signs of inflammation or more advanced stages, while excluding those with normal stiffness (MRE < 2.5 kPa). As a result, the Kruskal–Wallis chi-square value (KW χ2 = 28.4) for the MSI-F was higher than those for other indices. The MSI-F values for moderate-to-severe stiffness (> 3.5 kPa) were significantly higher than those for mild stiffness (2.9 to 3.4 kPa) (median [95% CI]; 38.5 [18.3‒56.6] vs. 72.1 [50.7‒87.3] for moderate-to-severe stiffness; p = 0.038). By comparison, other fibrosis indices could not differentiate the severity of fibrosis (Fig. 1A).
To further support the association of the MSI-F with quantitative measures, a multivariate forward stepwise linear regression analysis was performed to build an estimating model for liver stiffness, using the same variables employed in the hepatic fibrosis scoring system. The estimated values from the linear regression model showed a notable correlation with the measured stiffness obtained through MRE (Supplementary Table S3), with an intraclass correlation coefficient of 0.75 (95% CI 0.65–0.82) (Supplementary Fig. S2A). Additionally, 95.8% of the data for MASLD patients fell within the acceptable limits of the mean difference, as illustrated in the Bland–Altman plot (Supplementary Fig. S2B).
Validation of metabolic stress index for liver fibrosis
Of the validation subjects, median (IQR) values of MSI-F were 3.2 (0.3‒50.8) in no fibrosis, 31.0 (1.8‒80.6) in mild fibrosis, 80.6 (64.0‒95.8) in significant fibrosis, and 97.9 (92.8‒97.9) in advanced fibrosis (Fig. 2). The AUROCs of MSI-F for detecting significant fibrosis (F0 vs. ≥ F2) was 0.977 (95% CI 0.924‒1.00), which indicates superior diagnostic performance compared with other hepatic fibrosis indices (Fig. 2). Even when the MSI-F was applied to the overall validation group, including 18 MASLD patients with mild fibrosis (F1), its superiority in the discrimination of significant fibrosis (F0‒1 vs. ≥ F2) was maintained (0.926 [0.84‒0.99]) (Supplementary Fig. S1B). By applying the optimal cut-off value calculated from the development cohort, the MSI-F consistently had a higher diagnostic accuracy of (82.6% [95% CI 61.2‒95.1]) than other fibrosis indices which all showed an accuracy of less than 80% (Table 2). Of predictive fibrosis indices in the present study, only MSI-F values for both stages of significant and advanced fibrosis were significantly higher than those for no and mild fibrosis stages, whereas other fibrosis indices could not distinguish the severity of fibrosis.
Fig. 2.
Validation of MSI-F in patients with biopsy-proven MASLD. (A) Non-invasive prediction scores according to histological fibrosis stages (Kruskal–Wallis [KW] test with post hoc Dunnett’s T3 test). Bars and circles represent the mean with standard error of the mean and individual values, respectively. (B) ROC curves of non-invasive scores for predicting significant fibrosis. *p < 0.05, **p < 0.01 vs. MSI-F (DeLong’s tests).
Clinical applicability of metabolic stress index for liver steatosis and fibrosis
To improve the clinical applicability of the predictive indices, we performed further analyses assuming that a liver biopsy would not be necessary in those who had a true positive or true negative. Cut-off values of MSI-F with a sensitivity and specificity of ≥ 90% were 10 and 30; thus, 75.7% and 78.3% of cases in the development and validation groups would have avoided a liver biopsy or further investigations, respectively (Fig. 3 and Supplementary Table S4). Therefore, MSI-F is more effective to reduce unnecessary invasive evaluations than other currently available indices.
Fig. 3.
The potential clinical utility model of MSI-F. (A) Clinical utility of MSI-F and other indices predicting hepatic fibrosis with sensitivity and specificity of 90% in the development cohort. (B) Clinical utility of the indices in the validation cohort by applying the thresholds derived from the development cohort. Blue, true positive and true negative; red, indeterminate; grey, false positive and false negative. MSI-F metabolic stress index of liver fibrosis, APRI AST to platelet ratio index, FIB-4 Fibrosis-4 index, NFS nonalcoholic fatty liver disease fibrosis score, AUROC area under the ROC (receiver operating characteristic) curve.
Discussion
In this study, we developed a novel metabolic stress index (MSI-F) for non-invasive evaluation of fibrosis using serum biomarkers and MASLD-related parameters. Compared to pre-existing indices, MSI-F showed superior predictiveness and usefulness. Notably, GDF15 as a mitochondrial stress indicator had high associations with liver stiffness and significantly contributed to the high predictive power of this index. Our results emphasize the importance of mitochondrial stress in the progression of MASLD into serious fibrosis. Elevated serum GDF15 implies heavy oxidative and organellar stresses and unmet demand for protective humoral factors of the ISR. Our novel approach to developing a diagnostic/prognostic biomarker index including mitochondrial stress hormones could be applied to other metabolic, cardiovascular, and neurodegenerative diseases18.
We used MRE as a quantitative analysis of liver stiffness, which is considered one of the most accurate non-invasive diagnostic modalities for fibrosis19,20. MRE is an imaging technique that evaluates mechanical stiffness based on wave propagation. Its diagnostic reliability has been demonstrated through clinical comparison with other serological or imaging biomarkers. Moreover, MRE has recently been shown to be useful in predicting complications of decompensation such as ascites, variceal bleeding, and encephalopathy in MASLD patients21. However, despite its precision and use as a reference standard, MRE’s high cost, time-consuming processes, and unavailability in many global regions preclude its widespread applicability in the general population, especially for screening large cohorts.
In our result, GDF15 correlated with waist-to-hip ratio, liver function enzymes (AST, ALT and γ-GT), the homeostatic model assessment of insulin resistance, and pro-inflammatory cytokine (Interleukin-6), which are identified as the main risk factors of MASLD. Intriguingly, GDF15 was the most robust serologic predictor for fibrosis progression among MASLD patients. The AUROCs of GDF15 in determining fibrosis were 0.753 in the development cohort and 0.816 in the validation cohort, comparable to previous fibrosis indices (Supplementary Fig. S3). Recent studies have reported significant sex-specific differences, with higher GDF15 concentrations being linked to reduced muscle mass in men but not in women22, and showing distinct patterns across metabolic conditions such as obesity and diabetes23. Thus, we conducted subgroup analyses to assess whether the elevated circulating GDF15 levels in liver fibrosis were dependent on sex. The results indicated that MASLD patients with liver fibrosis in both sexes showed significantly elevated GDF15 levels compared to those without fibrosis, although GDF15 levels were higher in men compared to women in the non-fibrotic subgroup (Supplementary Fig. S4A). These findings suggest that there are no sex-specific differences in GDF15 levels, at least in the context of predicting the presence of liver fibrosis. Serum GDF15 also reflects the severity of fibrosis (Supplementary Fig. S5). These findings are consistent with a previous report that circulating GDF15 levels could independently discriminate the presence and the absence of advanced hepatic fibrosis among patients with MASLD17.
Growth differentiation factor 15 (GDF15) belongs to the TGFβ/bone morphogenetic protein superfamily member and is widely expressed in various tissues, with the highest levels in the liver and placenta24. Mitochondrial or ER stresses, as well as tissue injuries, upregulate GDF15 expression and release into circulation. Thus, serum GDF15 has been known as a useful biomarker for mitochondrial disorders resulting from respiratory chain deficiency, which is extended to other chronic diseases having mitochondrial dysfunction as an important pathogenic mechanism16,18. As a component of the ISR, secreted GDF15 is suggested to have a protective role against pathologic stressful conditions; however, the exact mechanism is still not clearly understood15. Regarding hepatic fibrosis, recombinant GDF15 application to LX2 cells treatment resulted in upregulation of fibrogenic proteins with activating TGFβ signalling. This is not consistent with another report showing that GDF15 directly suppressed expression of fibrosis-related genes in hepatic stellate cells in vitro and in the liver of mice in vivo14. The therapeutic potential of exogenous GDF15 should be investigated further in detail.
Decorin, a small leucine-rich proteoglycan, is one of the major endogenous inhibitors of bioactive TGFβ25. Interestingly, decorin increases with hepatic inflammation and fibrosis, and plays a protective role against fibrogenesis26–28. Therefore, the amount of compensatory secretion of decorin may reflect the severity of pathologic fibrosis, in the same way mitochondrial stress markers do. To our knowledge, this study provides the first clinical evidence that decorin is an independent serologic predictor of liver fibrosis. The serum decorin level could differentiate between mild and moderate-to-severe fibrosis (Supplementary Fig. S5) and contributed to the improvement of the MSI-F’s predictive ability. Moreover, subgroup analyses of serum decorin levels, as conducted for GDF15, revealed no significant sex differences in MASLD patients, both with and without liver fibrosis (Supplementary Fig. S4B). However, the fibrotic group in both sexes showed significantly higher circulating decorin concentrations compared to the non-fibrotic group, suggesting that decorin does not show a sex-specific difference in relation to fibrosis status.
One striking advantage of MSI-F is its effectiveness in reducing unnecessary further investigations, including liver biopsy and MR studies. Compared to currently available indices for hepatic fibrosis, MSI-F had markedly fewer patients with ‘indeterminate’ scores, as shown in Fig. 3 and Supplementary Table S4. We suggest that MSI-F is an efficient tool for predicting fibrosis in clinical settings where MR equipment or invasive diagnostic methods are inaccessible.
A limitation of our study was that the evaluation of fibrosis for the development cohort was according to MR image-based evaluation, not invasive liver biopsy, which is ethically unfeasible to obtain biopsy samples from healthy general cohort subjects. Instead, we validated the predictability of MSI-F with liver biopsy-evaluated patients, which was superior to other indices. Another limitation of this study is the relatively small sample size, particularly in the validation cohort, which may constrain the generalizability of the findings, especially when considering the broad spectrum of fibrosis severities, including advanced stages and cirrhosis. The absence of long-term follow-up data further restricts the ability to fully evaluate the MSI-F’s predictive efficiency over time. Despite these limitations, the findings warrant further validation in longitudinal and larger-scale clinical studies to confirm the robustness of MSI-F in predicting fibrosis progression in MASLD patients.
Conclusion
The present study has demonstrated that serum levels of humoral factors protecting from fibrotic stresses based on the pathophysiologic mechanisms of steatohepatitis and fibrosis could have high predictive power for liver cirrhosis in MASLD patients. The inclusion of mitochondrial stress hormone GDF15 and antifibrotic humoral factor decorin in the scoring system for fibrotic progression of MASLD patients markedly enhanced predictive performance, resulting in a more accurate and precise algorithm than other existing indices. Notably, our approach to predicting fibrosis using the MSI-F was narrowed to the MASLD population at risk of progressing to cirrhosis. The high negative predictive value of our MSI-F may allow one to avoid unnecessary biopsy in patients with MASLD.
Methods
Study participants
The present study initially enrolled 348 volunteers. After excluding five participants due to missing data, 343 individuals from a population-based general cohort, KoGES-ARIRANG (the Korean Genome and Epidemiology Study on Atherosclerosis Risk of Rural Areas in the Korean General Population)29, were evaluated for MASLD. The development cohort was then comprised solely of 144 subjects (66 men [45.8%]; aged 51‒80 years) diagnosed with MASLD. The validation cohort comprised 41 patients (16 men [39.0%]; aged 22‒67 years) from a biopsy-evaluated MASLD cohort. Study population recruitment and selection procedures are detailed in Fig. 4 and Supplementary Methods. The study protocol was reviewed and approved by the Institutional Review Board of Wonju Severance Christian Hospital (approval No. CR317131 and CR318003). All study participants were informed about the rationale and possible risks of the study and provided written informed consent before participation.
Fig. 4.
Flow chart of participant recruitment and analyzed subgroups in this study. KoGES-ARIRANG Korean Genome and Epidemiology Study on the Atherosclerosis Risk of Rural Areas in the Korean General Population, MASLD metabolic dysfunction-associated steatotic liver disease, MRI-PDFF magnetic resonance imaging-proton density fat fraction, MRE magnetic resonance elastography.
Evaluation of hepatic fibrosis
Quantitative assessments of MRE was performed in the development cohort. Hepatic fibrosis was staged according to the following criteria: normal, stiffness < 2.5 kPa; normal to inflammation, 2.5 kPa ≤ stiffness < 2.9 kPa; stage 1 to 2 fibrosis, 2.9 kPa ≤ stiffness < 3.5 kPa; stage 2 to 3, 3.5 kPa ≤ stiffness < 4.0 kPa, stage 3 to 4, 4.0 kPa ≤ stiffness < 5.0 kPa30. Histological examinations of liver biopsy specimens were carried out in the validation cohort and their features were classified according to criteria outlined by Kleiner et al.31. Fibrosis staging was defined as no fibrosis (F0), zone 3 perisinusoidal or periportal fibrosis (F1), perisinusoidal plus portal/periportal fibrosis (F2), bridging fibrosis (F3), and cirrhosis (F4). Further detailed diagnostic processes are provided in Supplementary Methods.
Clinical and laboratory assessments
Anthropometric measurements such as weight, height, and waist and hip circumference were taken, and then the body mass index (BMI), waist-to-height ratio (WHtR), and waist-to-hip ratio (WHR) were calculated. Routine biochemical tests, including parameters of liver test, were performed using automated clinical chemistry analysers. Serum concentrations of 10 metabolic biomarkers, including adiponectin, leptin, retinol-binding protein 4 (RBP4), interleukin 6 (IL6), GDF15, FGF21, FGF19, transforming growth factor-β (TGFβ), myostatin, and decorin were quantified by using commercially available ELISA kits according to the manufacturer’s instructions. The details of clinical and laboratory assessment and the criteria for metabolic diseases are provided in the Supplementary methods. We also calculated several predictive scores derived from clinical and laboratory indices to compare their diagnostic performance in significant hepatic fibrosis. The details of clinico-laboratory assessments, the criteria of metabolic diseases, and the scoring formulae are provided in the Supplementary methods.
Statistical analysis
Continuous data are presented as medians with interquartile ranges (IQR) and the categorical data are presented as frequencies with proportions. All variables collected in the development cohort were included in a multivariate forward stepwise logistic regression analysis to identify variables independently associated with presence or absence of hepatic fibrosis. Non-parametric data were used as independent variables after natural logarithmic transformation. The contribution strength of each variable to the multivariate model was evaluated by the Wald chi-square value (Wald χ2), which was calculated by squaring the ratio of the regression coefficient divided by its standard error. The diagnostic powers of prediction models were evaluated by area under the receiver operator characteristic curve (AUROC) analyses with assessments of likelihood ratios, predictive values, and diagnostic accuracy. Several cut-off values were calculated for the diagnosis of steatosis and fibrosis: the one that corresponded to the highest Youden index, which maximizes sensitivity and specificity, and the others that corresponded to ≥ 90% sensitivity (low threshold for ruling-out) and ≥ 90% specificity (upper threshold for ruling-in). All statistical tests were 2-tailed and p values < 0.05 were considered significant. Data were analysed using IBM SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, NY, USA). For further details regarding the statistical methods used, please refer to the Supplementary methods.
Supplementary Information
Author contributions
JSC, MYK and K-SP conceptualized and designed the study; JSC, J-HA, and MYK have conducted the experimental and clinical investigations; JSC, J-HA, MYK and K-SP have collected and interpreted data, JSC, and K-SP have drafted the manuscript; JSC, MYK, and K-SP have revised and finalized the manuscript. All authors read, edited and approved the final manuscript.
Funding
This work was supported by the Medical Research Center Program (NRF-2017R1A5A2015369 and RS-2024-00409403) and in part by the Basic Science Research Program (NRF-2018R1C1B6005036) from the Ministry of Science and ICT, Republic of Korea.
Data availability
The data sets used and/or analysed during the current study are not available in public because of privacy and confidentially of the study participants and patients, but are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics statement
All aspects of this study, including research methods were conducted in strict accordance with relevant guidelines and regulations. This study was conducted in compliance with the ethical principles outlined in the Declaration of Helsinki. All data were de-identified, and all study protocols and procedures were reviewed and approved by the Institutional Review Board of Wonju Severance Christian Hospital (approval No. CR317131).
Informed consent
Written informed consent was obtained from all participants, who have consented to the publication of any data included in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Moon Young Kim, Email: drkimmy@yonsei.ac.kr.
Kyu-Sang Park, Email: qsang@yonsei.ac.kr.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-77719-6.
References
- 1.Starley, B. Q., Calcagno, C. J. & Harrison, S. A. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology51(5), 1820–1832 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Wong, V. W., Adams, L. A., de Ledinghen, V., Wong, G. L. & Sookoian, S. Noninvasive biomarkers in NAFLD and NASH - current progress and future promise. Nat. Rev. Gastroenterol. Hepatol.15(8), 461–478 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Reinson, T., Buchanan, R. M. & Byrne, C. D. Noninvasive serum biomarkers for liver fibrosis in NAFLD: current and future. Clin. Mol. Hepatol.29(Suppl), S157–S170 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tilg, H. & Moschen, A. R. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology52(5), 1836–1846 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Karkucinska-Wieckowska, A. et al. Mitochondria, oxidative stress and nonalcoholic fatty liver disease: A complex relationship. Eur. J. Clin. Investig.52(3), e13622 (2022). [DOI] [PubMed] [Google Scholar]
- 6.Ly, L. D. et al. Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp. Mol. Med.49(2), e291 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu, S. et al. Palmitate induces ER calcium depletion and apoptosis in mouse podocytes subsequent to mitochondrial oxidative stress. Cell Death Dis.6(11), e1976 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machado, M. V. & Diehl, A. M. Pathogenesis of nonalcoholic steatohepatitis. Gastroenterology150(8), 1769–1777 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sunny, N. E., Bril, F. & Cusi, K. Mitochondrial adaptation in nonalcoholic fatty liver disease: Novel mechanisms and treatment strategies. Trends Endocrinol. Metab.28(4), 250–260 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Kim, K. H. et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat. Med.19(1), 83–92 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Pakos-Zebrucka, K. et al. The integrated stress response. EMBO Rep.17(10), 1374–1395 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung, H. K. et al. Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. J. Cell Biol.216(1), 149–165 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang, S. G. et al. Differential roles of GDF15 and FGF21 in systemic metabolic adaptation to the mitochondrial integrated stress response. iScience24(3), 102181 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, K. H. et al. Growth differentiation factor 15 ameliorates nonalcoholic steatohepatitis and related metabolic disorders in mice. Sci. Rep.8(1), 6789 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujita, Y., Taniguchi, Y., Shinkai, S., Tanaka, M. & Ito, M. Secreted growth differentiation factor 15 as a potential biomarker for mitochondrial dysfunctions in aging and age-related disorders. Geriatr. Gerontol. Int.16(Suppl 1), 17–29 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Yatsuga, S. et al. Growth differentiation factor 15 as a useful biomarker for mitochondrial disorders. Ann. Neurol.78(5), 814–823 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koo, B. K. et al. Growth differentiation factor 15 predicts advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease. Liver Int.38(4), 695–705 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Wang, D. et al. GDF15: emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat. Rev. Endocrinol.17(10), 592–607 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Park, C. C. et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology152(3), 598-607 e592 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao, G. et al. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology66(5), 1486–1501 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Han, M. A. T. et al. MR elastography-based liver fibrosis correlates with liver events in nonalcoholic fatty liver patients: A multicenter study. Liver Int.40(9), 2242–2251 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Herpich, C. et al. Associations between serum GDF15 concentrations, muscle mass, and strength show sex-specific differences in older hospital patients. Rejuvenation Res.24(1), 14–19 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Asrih, M. et al. Sex-specific modulation of circulating growth differentiation factor-15 in patients with type 2 diabetes and/or obesity. Endocr. Connect.10.1530/EC-22-0054 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang, E. et al. Multidimensional biomarker analysis including mitochondrial stress indicators for nonalcoholic fatty liver disease. Gut Liver16(2), 171–189 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baghy, K., Iozzo, R. V. & Kovalszky, I. Decorin-TGFbeta axis in hepatic fibrosis and cirrhosis. J. Histochem. Cytochem.60(4), 262–268 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dudas, J. et al. Expression of decorin, transforming growth factor-beta 1, tissue inhibitor metalloproteinase 1 and 2, and type IV collagenases in chronic hepatitis. Am. J. Clin. Pathol.115(5), 725–735 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Baghy, K. et al. Ablation of the decorin gene enhances experimental hepatic fibrosis and impairs hepatic healing in mice. Lab. Investig.91(3), 439–451 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jang, Y. O. et al. Effect of function-enhanced mesenchymal stem cells infected with decorin-expressing adenovirus on hepatic fibrosis. Stem Cells Transl. Med.5(9), 1247–1256 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim, J. Y. et al. Prospective study of serum adiponectin and incident metabolic syndrome: The ARIRANG study. Diabetes Care36(6), 1547–1553 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guglielmo, F. F., Venkatesh, S. K. & Mitchell, D. G. Liver MR elastography technique and image interpretation: Pearls and pitfalls. Radiographics39(7), 1983–2002 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology41(6), 1313–1321 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets used and/or analysed during the current study are not available in public because of privacy and confidentially of the study participants and patients, but are available from the corresponding author upon reasonable request.