Abstract
Exophiala dermatitidis (E. dermatitidis), which causes skin or respiratory disease, is occasionally fatal in immunocompromised patients. Here, we report the unique antifungal potency of terbinafine (TRB), which targets squalene epoxidase, against E. dermatitidis (SQLEED) using various in vitro approaches. The versatile antifungal activities, including fungicidal activity, biofilm formation inhibition, biofilm eradication activity, and the combination effect of TRB, posaconazole (PSC), and amphotericin B (AmB) with great antifungal potency against E. dermatitidis were evaluated using crystal violet and cell viability assay. TRB formed an H-bond through Y102 in SQLEED in the binding model. E. dermatitidis hyphae elongated and attached to a cell scaffold, forming a membrane-like biofilm. TRB and PSC showed more potent antibiofilm activities than AmB, and exhibited post-antifungal effects without incubation against E. dermatitidis conidia, reducing growth at lower concentrations. In contrast, AmB exhibited strong dose- and time-dependent killing and biofilm-eradication activities. The combination of TRB and PSC was more effective than that of TRB and AmB or PSC and AmB. Although the tissue migration of TRB must be considered, these data suggest that TRB and PSC may be useful agents and a potent combination in severely immunocompromised patients with refractory and systemic E. dermatitidis infection.
Keywords: Exophiala dermatitidis, Terbinafine, Posaconazole, Amphotericin B, Biofilm
Subject terms: Translational research, Clinical microbiology, Fungi
Introduction
It is estimated that 1.7 billion people worldwide suffer from fungal infections1. Invasive fungal infections in patients undergoing organ transplantation, chemotherapy for cancer, human immunodeficiency virus (HIV) infection, or autoimmune diseases cause approximately 1.7 million deaths per year2.
Exophiala dermatitidis (E. dermatitidis) is a black fungus, a member of the Herpotrichiellaceae, that can be isolated from wet living environments such as dishwashers, humidifiers, and bathtubs3, and is commonly reported as a pathogen of black fungal infections isolated from the skin and subcutaneous tissue in dermatological treatment4. Respiratory tract infections caused by E. dermatitidis are relatively rare, with reports of underlying diseases such as bronchiectasis and cystic fibrosis5,6. Moreover, black fungal infections caused by E. dermatitidis isolated from the skin, eye, liver, central nervous system, and central venous catheters have been reported as opportunistic fatal infections in immunocompromised patients7. E. dermatitidis has a slower growth rate than the other fungi. Small black colonies of E. dermatitidis observed on Sabouraud dextrose agar (SDA) after 3 days were barely detectable. Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) is a powerful tool for the early identification of pathogenic fungal species, including late-growing fungi such as E. dermatitidis, in addition to morphological and genetic analysis of the specimen8. We previously reported E. dermatitidis pneumonia in immunocompromised patients with anorexia nervosa9. In clinical practice, E. dermatitidis is treated with azoles such as voriconazole or itraconazole for several months10,11. In this study, we analyzed characteristics of E. dermatitidis and evaluated the antifungal, antibiofilm, post-antifungal effects (PAFE), killing (fungicidal) activity, and combinations of several clinically used oral and intravenous antifungal agents such as terbinafine (TRB)12, azoles (fluconazole [FLC], miconazole [MCZ], voriconazole [VRC], itraconazole [ITC], posaconazole [PSC], isavuconazole [ISC])13, micafungin (MCF), caspofungin (CAS), and amphotericin B (AmB)14 against E. dermatitidis. The aim of this study is to elucidate the potential of TRB in vitro against E. dermatitidis.
Results
Profile of clinically isolated E. dermatitidis
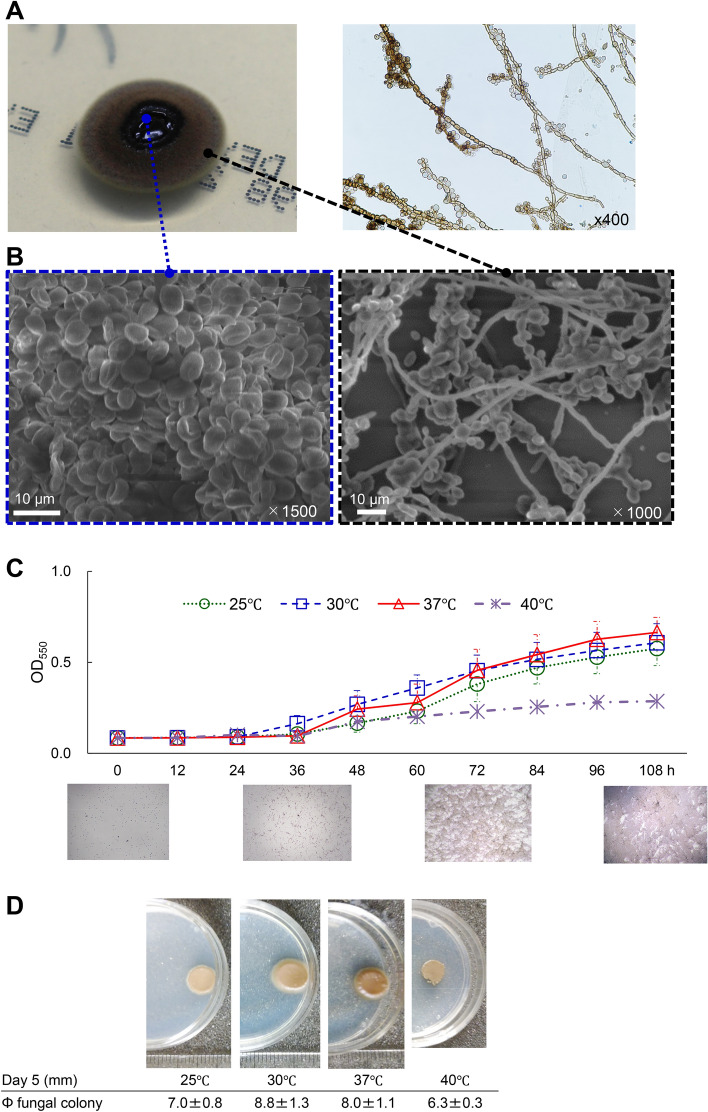
We observed giant black colonies of E. dermatitidis 1 isolated from a clinical patient9 under aerobic incubation at room temperature on SDA and performed morphological observations using an optical microscope and scanning electron microscope (SEM) (Fig. 1). The center of the giant colonies formed a black gelatinous mass and the surface and edges changed to olive brown (Fig. 1A). Microscopy revealed many yeast-like oval conidia in the center (Fig. 1B) and septate hyphae and hyphae in the outer part of the black colony (Fig. 1B). The internal transcribed spacer 1 (ITS1) and D1/D2 regions of the large subunit of the 28S ribosomal RNA gene were compared using the Basic Local Alignment Search Tool (BLAST). The identification rate of the D1/D2 region was dominated by Exophiala species, and that of E. dermatitidis was 96.9%, and the ITS region was 100% identical to E. dermatitidis (Fig. S1A). Based on genetic analysis of the ITS region, we identified the giant black fungus as E. dermatitidis genotype A (Fig. S1B), which is most commonly reported in Japan15.
Fig. 1.
Characteristics of E. dermatitidis. (A) A single colony of E. dermatitidis 1 was grown on an SDA plate for 14 days. The lactophenol cotton blue-stained specimen image was observed using the slide culture method. (B) Morphological image of the central and marginal areas of E. dermatitidis colony by SEM. The scale bar and magnification at each image are shown (C) the growth rate was determined every 12 h up to 108 ho at 25, 30, 37, and 40℃, and microscopic images at 37℃ were taken at 0, 36, 72, and 108 h in MOPS-RPMI culture medium until 108 h or (D) colonies on the SDA plate for 120 h at 25, 30, 37, and 40℃, respectively.
Next, we analyzed the growth of E. dermatitidis 1 at 25, 30, 37, and 40 ℃. The growth gradually increased from 36 to 108 h and was more efficient at 30 and 37 ℃ than at 25 and 40 ℃ in MOPS-RPMI (Fig. 1C) and sabouraud medium (Fig. S1C). In addition, the diameter of the black colonies of E. dermatitidis 1 on SDA incubated for a week was 8.8, 8.0, 7.0, and 6.3 mm at 30, 37, 25, and 40 ℃, respectively, in line with the growth results at these temperatures (Fig. 1D). These results suggest that the growth of E. dermatitidis was affected by incubation temperature; in particular, incubation at 40℃ prevented the growth. The benefit of thermotherapy in cutaneous or subcutaneous infections has been previously reported16.
Structural modeling of E. dermatitidis squalene epoxidase
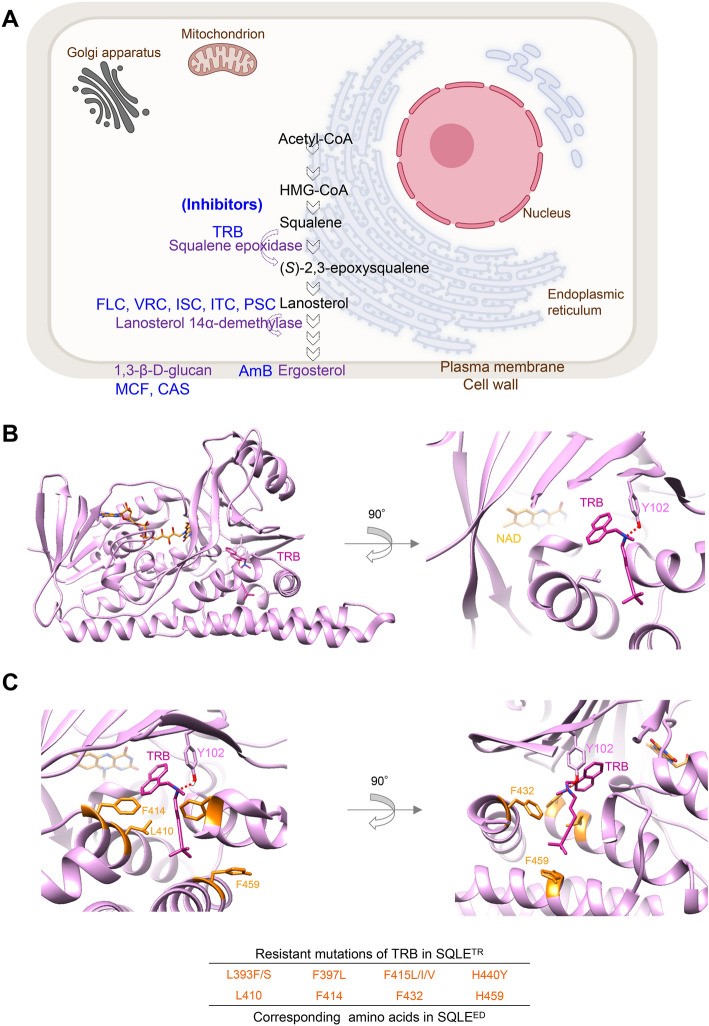
Target proteins of various antifungal drugs in clinical use are shown in Fig. 2A. TRB target squalene epoxidase (SQLE), also known as squalene monooxygenase, which catalyzes the conversion of squalene to (S)-2,3-epoxy squalene, is a key enzyme in ergosterol biosynthesis in fungal membranes17,18. Azoles can also exert anti-fungal activity by inhibiting a different target enzyme, lanosterol 14α-demethylase in the same pathway as shown in Fig. 2A.19.
Fig. 2.
Targets of antifungal drugs and SQLE structures. (A) Schematic illustration of antifungal target proteins in fungal cells. (B) The best binding model of TRB to the SQLE structure of E. dermatitidis with nicotinamide adenine dinucleotide (NAD). TRB interacts with Y102 through an H-bond in SQLEED. (C) The location of putative TRB-resistant amino acid residues (L410, F414, F432, and H459) in the SQLEED structure corresponding to clinically TRB-resistant mutations (L393F/S, F397L, F415L/I/V, and H440Y) in the SQLETR.
To investigate the structural antifungal efficacy of TRB against E. dermatitidis, we identified the SQLEED derived from three E. dermatitidis (Table S1) with the same amino acid (AA) sequences. The AA sequences of SQLEs from humans, E. dermatitidis, and Trichophyton rubrum (T. rubrum) were compared using Clustal omega (multiple sequence alignment tool), as shown in Table S1. Moreover, the structural modeling of SQLEED was produced by SWISS-MODEL based on the crystallography of human SQLE (SQLEHum) (PDB:6C6N) as previously reported18 and the full-length SQLEHum model shown in Fig. 2B and S2A. Next, we performed a docking simulation of TRB to SQLEED (Fig. 2B) based on the crystal structure of Cmpd-4 bound to SQLEHum (PDB:6C6N) using SeeSAR (see Methods). The top 20 structures of the TRB bound to SQLEED are shown in Fig. S2C. The TRB-binding structures of SQLEHum and SQLEED formed hydrogen bonds with the side chains of Tyr 122 and 102, respectively (Fig. S2). Important AA residues (L393, F397, F415, and H440) of SQLE (SQLETR) that bind to TRB have been reported in the resistance profiles of clinical isolates of T. rubrum (Fig. S2D)20. TRB is surrounded by L410, F414, F432, and H459 of SQLEED, which correspond to L393, F397, F415, and H440 of SQLETR in the binding as shown in Fig. 2C. These results indicate that TRB can effectively interact with SQLEED, resulting in a potent antifungal activity against E. dermatitidis (Table 1 and Table S2).
Table 1.
The susceptibility of E. dermatitidis 1 to the drugs.
| OD530 | OD440 (WST-1) | |||||
|---|---|---|---|---|---|---|
| Drugs | MIC50 | MIC90 | MIC50 | MIC90 | MIC | MEC |
| TRB | 0.13 | 0.25 | 0.13 | 0.25 | 0.25 | – |
| FLC | 4 | 8 | 8 | 16 | 16 | – |
| MCZ | 0.5 | 0.5 | 0.25 | 0.5 | 0.5 | – |
| VRC | 0.063 | 0.13 | 0.13 | 0.13 | 0.13 | – |
| ISC | 0.13 | 1 | 0.25 | 1 | 1 | – |
| ITC | 0.25 | 0.25 | 0.13 | 0.25 | 0.25 | – |
| PSC | 0.063 | 0.13 | 0.031 | 0.13 | 0.13 | – |
| AmB | 0.063 | 0.13 | 0.063 | 0.13 | 0.13 | – |
| MCF | 8 | 32 | 16 | 32 | – | 8 |
| CAS | 8 | 32 | 16 | 32 | – | 8 |
Minimum inhibitory concentration, MIC (mg/L) is defined as the lowest concentration of drugs that can be visually observed to inhibit the growth of clinical isolate E. dermatitidis 1. MIC50 and MIC90 values are determined as the lowest drug concentrations capable of inhibiting the E. dermatitidis 1 growth by 50% or 90%, respectively, as measured at OD530 nm. MIC50 and MIC90 of WST-1 are determined as the lowest drug concentrations capable of inhibiting 50% or 90%, respectively, of the E. dermatitidis 1 viability, as measured at OD440 nm after staining with WST-1. Minimum effective concentration (MEC) is defined as the lowest concentration of drugs that have any effect on E. dermatitidis 1.
Antifungal activity of clinically used drugs against E. dermatitidis
Various orally and intravenously administered antifungal drugs are available for clinical use. In this study, we evaluated the antifungal effects of ten drugs (FLC, MCZ, VRC, ITC, PSC, ISC, TRB, AmB, CAS, and MCF) (Fig. 3A and Fig. S3) against clinically isolated E. dermatitidis 1 (Table 1) based on the CLSI M38-Ed3. Additionally, the susceptibilities of the other two E. dermatitidis strains (E.D.2, clinically isolated, and E.D.3, ATCC28869) to TRB, PSC, and AmB were evaluated (Table S2). The minimum inhibitory concentration (MIC), 50% inhibition of growth (MIC50), and 90% inhibition of growth (MIC90) were determined using OD530, or OD440 after WST-1 reagent staining to evaluate the viability of E. dermatitidis (WST-1 assay)21. As previously reported22,23, the MICs of FLC, MCF, and CAS against E. dermatitidis exceeded 16 mg/L. PSC, VRC, AmB, TRB, and ITC showed potent inhibitory activity from 0.031 to 0.25 mg/L against E. dermatitidis at MIC by visual observation or at MIC50 and MIC90 by measurement of OD530 or OD440 (WST-1) (Table 1). MCZ at 0.25 to 0.5 mg/L inhibited the growth similarly to ISC. The MIC of all drugs was found to be very close to the MIC90 value obtained from WST-1 assay. E. dermatitidis exhibited different growth rates at different temperatures (Fig. 1C and Fig. S1C). To examine the effect of the incubation temperature on antifungal activity, we determined MIC50 and MIC90 of drugs by measuring E. dermatitidis growth at OD530. (Table S3). The findings indicated that incubation temperature did not significantly affect the antifungal activity of these drugs.
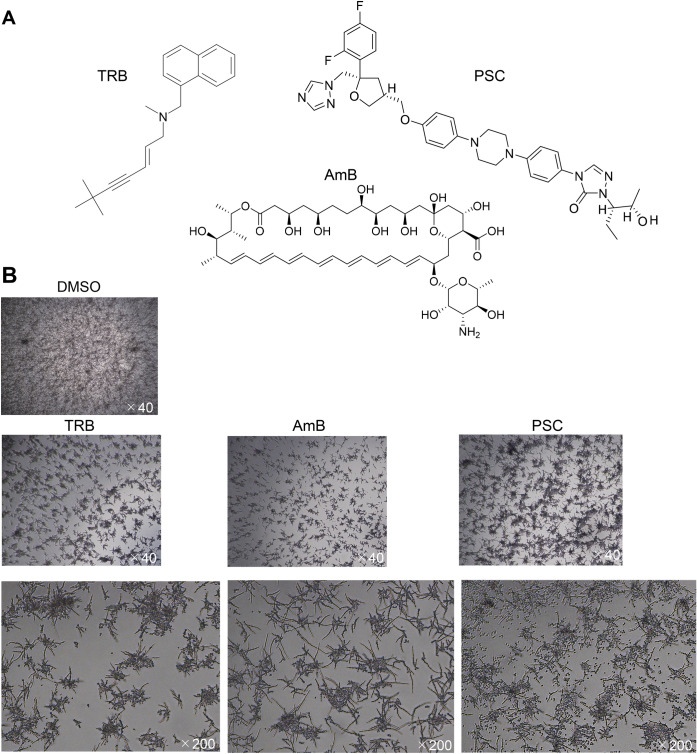
Fig. 3.
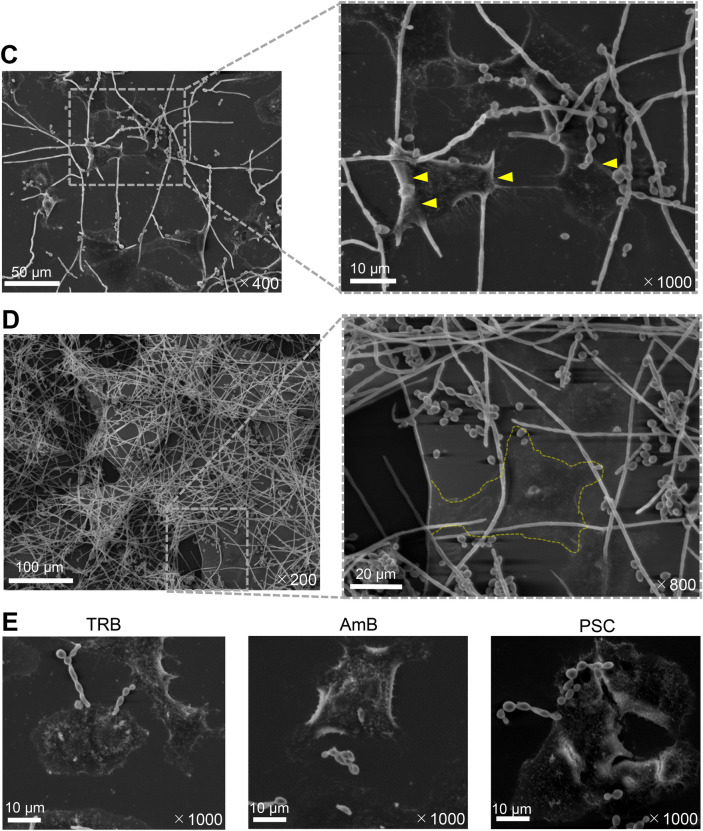
Morphology of E. dermatitidis and drug efficacy of TRB, PSC, and AmB. (A) Chemical structures of TRB, PSC, and AmB. (B) Morphology of E. dermatitidis 1 in the presence of TRB, AmB, and PSC at 0.125 mg/L after incubation at 35℃ for 48 h in MOPS-RPMI buffer. (C) The E. dermatitidis hyphae were elongated and attached to the 549 cells indicated by yellow arrows after incubation at 35℃ for 24 h by SEM. (D) Gray-membrane biofilm containing A549 cells circled at the dotted yellow line formed on enriched E. dermatitidis 1 after incubation at 35℃ for 48 h. (E) E. dermatitidis 1 incubated on the A549 cells at 35℃ for 24 h in the presence of TRB, AmB, and PSC at 0.25 mg/L. The scale bar and magnification at each image are shown in white.
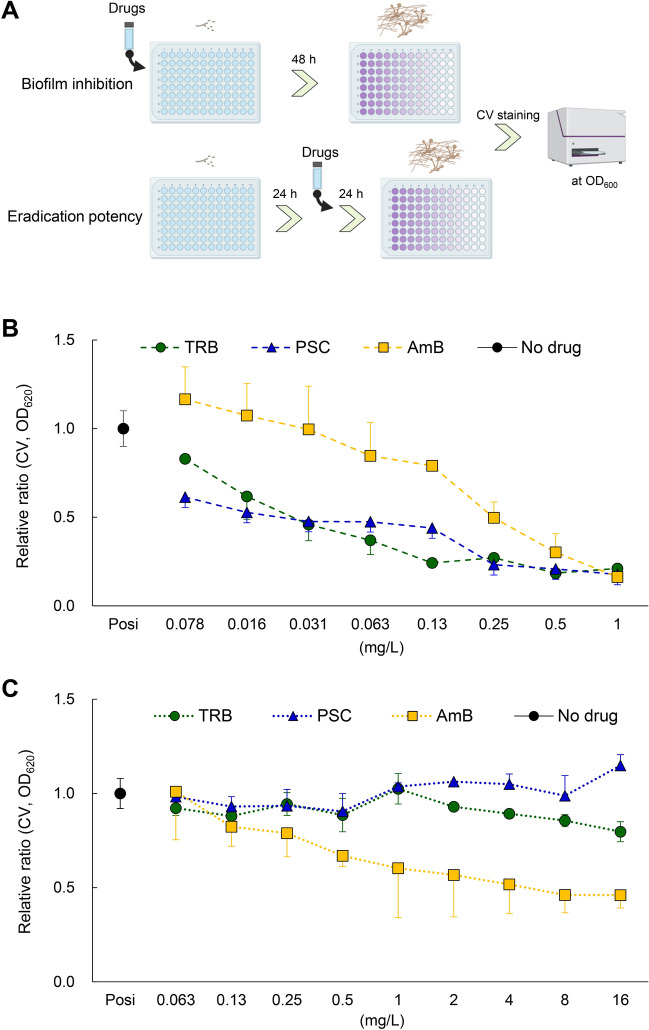
Inhibition and eradication of E. dermatitidis-induced biofilm
Bacterial and fungal biofilms form in infected organs, particularly on medical devices such as intravascular catheters, artificial heart valves, and artificial joints, causing intractable chronic infections, which is resistant to antibiotics and antifungals24. It has been reported that E. dermatitidis can produce biofilm formation25.
The chemical structures of TRB, PSC, and AmB are shown in Fig. 3A. When E. dermatitidis 1 was treated with TRB, PSC, and AmB at 0.125 mg/L, different morphologies of E. dermatitidis conidia were observed. The E. dermatitidis conidia and hyphae were dense in TRB and AmB, while the E. dermatitidis conidia were diffuse in PSC (Fig. 3B). In addition, E. dermatitidis 1 was incubated with or without A549 cells in a glass-bottomed slide chamber plate. The E. dermatitidis without A549 cells floated in the buffer, whereas the E. dermatitidis with A549 cells firmly attached to the bottom of the slide (Fig. S3). Next, we investigated the morphology of the E. dermatitidis with or without A549 cells by optical microscopy (Fig. S3A) and SEM. (Fig. 3). The coadunate filamentous biofilm-like morphology of the E. dermatitidis was sparsely observed without A549 cells as observed by SEM (Fig. S3B). On the other hand, the E. dermatitidis hyphae extended cohesively below and above the cells, and the oval conidia were diffusely attached to the cells for 24 h of incubation (Fig. 3C). Moreover, membrane biofilm-like morphology including the cells appeared where the E. dermatitidis was highly enriched for 48 h of incubation (Fig. 3D and Fig. S3B). Treatment with TRB, PSC, and AmB at 0.25 mg/L inhibited hypha growth and showed pseudohyphae of the E. dermatitidis (Fig. 3E). There were no clear differences in the E. dermatitidis morphology between these drug treatments in this condition.
Next, we examined the inhibitory and eradication abilities of TRB, PSC, and AmB against E. dermatitidis-induced biofilms using a CV assay (Fig. 4 and Fig. S4). Interestingly, TRB and PSC showed more potent inhibitory activity against the biofilm formation by E. dermatitidis 1 (Fig. 4B), and the antibiofilm activity of these drugs was similar to their antifungal activity (Fig. S4). In contrast, AmB sufficiently and TRB slightly at high concentration eradicated the E. dermatitidis-induced biofilm compared to PSC (Fig. 4C). These results indicate that TRB and PSC can inhibit biofilm formation at lower concentrations than AmB. However, a higher concentration of AmB can moderately eradicate the biofilm-formed E. dermatitidis 1, suggesting that TRB and PSC may be useful in the early treatment of acute E. dermatitidis infection and that AmB may be effective in the late or prolonged treatment of chronic biofilm-formed E. dermatitidis. 1.
Fig. 4.
Inhibition and eradication of biofilm activity of TRB, PSC, and AmB. (A) Schematic illustration shows assay procedures to confirm inhibition and eradication of TRB, PSC, and AmB against E. dermatitidis 1 biofilm. (B) Biofilm inhibition and (C) eradication potency of TRB (green circle), PSC (blue triangle), and AmB (yellow square) are shown as a relative ratio from 0.078 to 1 mg/L and from 0.063 to 16 mg/L, respectively. All assays were performed independently in triplicate. Means (± S.D.) of all data are presented.
Fungicidal activity and combination effects of antifungal drugs against E. dermatitidis
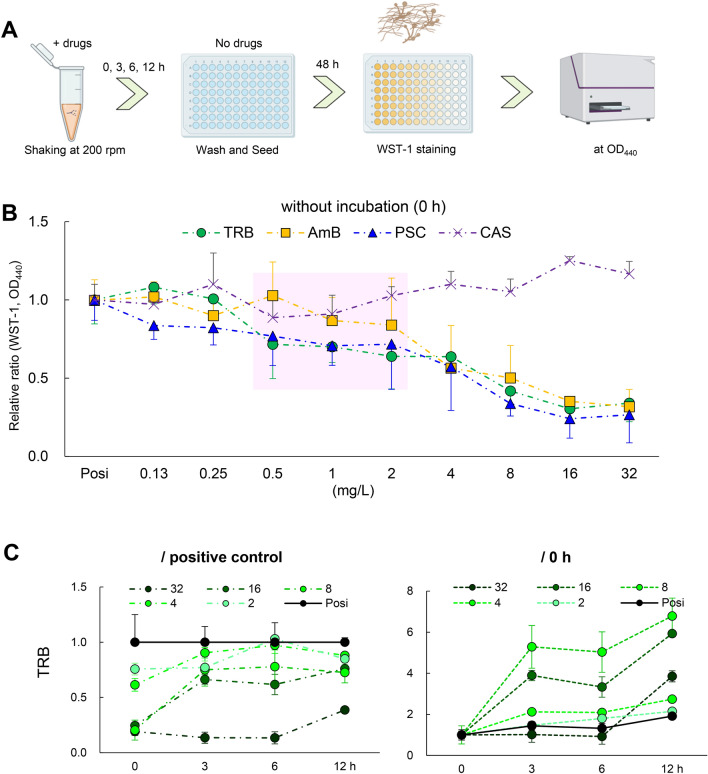
The ability to kill invasive fungi is important in immunocompromised conditions26. We examined various antifungal effects of TRB, PSC, and AmB on E. dermatitidis using WST-1 reagent to examine living E. dermatitidis.
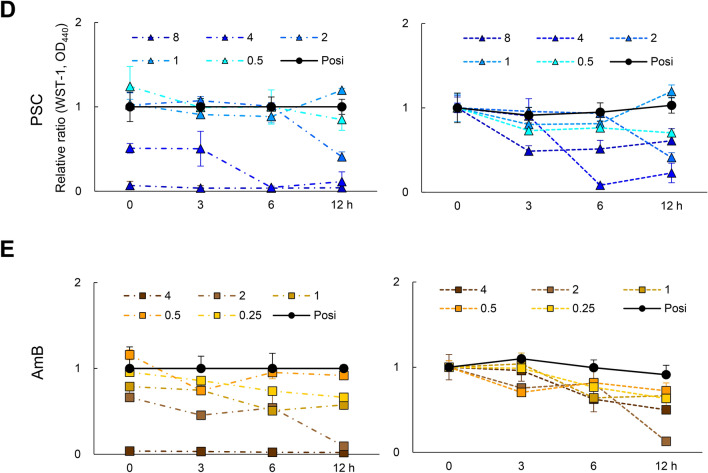
First, we evaluated the residual potency of the drugs, PAFE27, on E. dermatitidis 1. Notably, the growth of E. dermatitidis conidia could be inhibited by TRB, PSC, and AmB without incubation, which washed the drugs immediately after administration (see Materials and Methods). (Fig. 5A, B). TRB and PSC at low concentrations (from 0.5 to 2.0 mg/L) decreased the growth of the E. dermatitidis compared to AmB, indicating that TRB and PSC can rapidly interact with and maintain the target proteins. TRB has been reported to have fungicidal activity against dermatophytes including T. rubrum28. In contrast, TRB did not show a time- and dose-dependent ability to kill the E. dermatitidis conidia, even at the highest concentration of 32 mg/L (Fig. 5C) in line with Exophiala spinifera27. The viability of E. dermatitidis 1 after treatment with TRB decreased compared to positive controls (no drug) up to 12 h, but incubation for 3, 6, and 12 h at all tested TRB concentrations increased compared to no incubation (0-h), indicating a reactive response of the E. dermatitidis to TRB. These data suggest that TRB has a fungistatic effect on E. dermatitidis conidia (Fig. 5C), whereas PSC and AmB killed E. dermatitidis conidia in a dose- and time-dependent manner (Fig. 5D, E).
Fig. 5.
PAFE and killing activity of TRB, PSC, and AmB. (A) Schematic illustration of assay procedures to confirm the PAFE and killing potency of TRB, PSC, and AmB against E. dermatitidis 1. (B) PAFE of TRB (green circle), PSC (blue triangle), AmB (yellow square), and CAS (purple × mark) treated with no incubation time (0 h) is shown as a relative ratio from 0.125 to 32 mg/L. Assays were performed independently in triplicate. Means (± S.D.) of all data are presented. The killing potency of (C) TRB, (D) PSC, and (E) AmB were evaluated and shown as relative ratios at each appropriate concentration and incubation time as assessed by cell viability using WST-1 staining. Assays were performed independently in triplicate. Means (± S.D.) of representative data are shown.
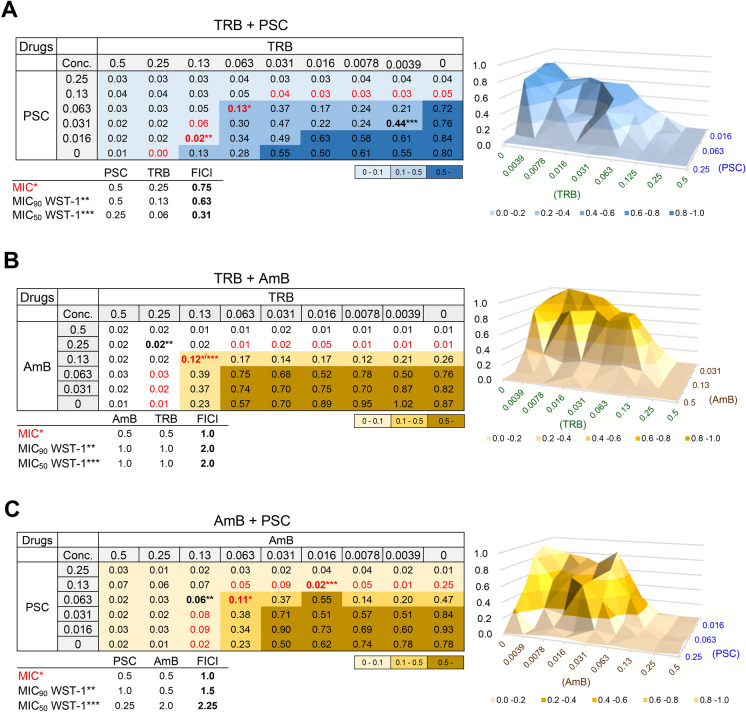
In vitro and in vivo combination therapies are effective in treating refractory and chronic infections29. TRB and other antifungal combinations have been reported against E. dermatitidis and other fungi.22,29,30 We investigated the combinations of antifungal drugs such as TRB and azoles (PSC, VRC, and ITC), TRB and AmB, and TRB and CAS against E. dermatitidis 1 (Fig. 6 and Fig. S5). A previous study22 showed a synergistic effect against E. dermatitidis when CAS was combined with azoles (VRC and ITC) in vitro. The fractional inhibitory concentration index (FICI) was calculated from the MIC, MIC50, and MIC90 values of WST-1 assay. The values for CAS and PSC were < 0.5, indicating a synergistic effect (Fig. S5). The value for the combination of TRB and AmB was between 1.0 and 2.0, and that for PSC and AmB between 1.0 and 2.2, resulting in no interaction effects (Fig. 6). However, when a combination of TRB with other azoles was examined, the FICI of TRB and PSC showed better efficacy between 0.31 and 0.75, TRB and ISC between 0.63 and 1.0, TRB and ITC at 0.5, and TRB and VRC between 0.63 and 1.0, indicating synergistic or no interaction effects (Fig. 6 and Fig. S5). These results suggest that the combination of TRB with azoles that inhibit different target proteins in the same pathway29, (Fig. 2A) particularly ITC and PSC, was more favorable than AmB against E. dermatitidis.
Fig. 6.
Combination effect of TRB, PSC, and AmB on E. dermatitidis. A combination of (A) TRB and PSC, (B) TRB and AmB, and (C) AmB and PSC tables and 3D surface plots consist of relative ratios at each appropriate concentration as assessed by E. dermatitidis 1 viability using WST-1 staining. FIC indices (FICI) were evaluated as synergy; FICI < 0.5, no interaction (additive); 0.5 < FICI < 4, or antagonism; FICI > 4, which calculated from MIC (*in red) data or MIC50 (***) and MIC90 (**) of WST-1 staining results. All assays were performed independently in triplicate and representative data are shown.
Taken together, TRB may be a therapeutic agent with potent antifungal, anti-biofilm, PAFE, and fungistatic inhibition against E. dermatitidis, further when combined with azoles including PSC (Table 2).
Table 2.
Summary of versatile anti-E. dermatitidis activity of TRB, PSC, and AmB.
| Drugs | Growth Inhibition | Biofilm Inhibition | Combination | ||||
|---|---|---|---|---|---|---|---|
| MIC | MIC50 | MIC90 | Killing | Inhibition | Eradication | Synergy or Additive | |
| TRB | 0.25 | 0.13 | 0.25 | − | + + + | ± | PSC, VRC, ITC |
| PSC | 0.13 | 0.063 | 0.13 | + + | + + + | − | TRB |
| AmB | 0.13 | 0.063 | 0.13 | + + + | + | + + | − |
MIC50 and MIC90 values were determined by measuring at OD530 nm. The effect of the drugs on E. dermatitidis 1 is shown as follows: + + + ; very strong, + + ; strong, + ; mild, ± ; weak, and −; none.
Discussion
Systemic and invasive E. dermatitidis infections such as pneumonia and sepsis require a relatively long treatment period10,11, and have been reported to result in death8. Therefore, potent antifungal activity including PAFE and fungicidal activity as well as tissue migration of antifungals to the site of infection are essential factors for antifungal treatment when treating immunocompromised patients. TRB has been available as a useful therapeutic agent for trichophytosis in clinical practice20,31.
In this study, TRB showed potent antifungal activity against E. dermatitidis in various in vitro assays, similar to that of PSC. In addition, TRB and PSC showed a stronger anti-biofilm effect of E. dermatitidis than AmB. Treatment of E. dermatitidis conidia with TRB of 0 h incubation decreased the growth of E. dermatitidis as a PAFE (Fig. 4A); however, TRB did not show a time- and dose-dependent killing ability against E. dermatitidis conidia even at the highest concentration, 32 mg/L (Fig. 5C). TRB can suppress the growth of E. dermatitidis from the conidia, but cannot kill them, suggesting that TRB has fungistatic effects against E. dermatitidis conidia as previously report of same genus, E. spinifera27.
On the other hand, the biofilm eradication ability of TRB shown in Fig. 4C is slightly higher than that of PSC. Moreover, when we performed the growth inhibition assay to determine MIC, MIC50, and MIC90 at OD530 and OD440 (WST-1 assay). TRB was found to inhibit the growth of E. dermatitidis at low concentrations similar to PSC with fungicidal activity against E. dermatitidis (Fig. S4).
TRB targets SQLE, a key enzyme, in ergosterol biosynthesis in fungal membranes17,18. The important AA residues (L393, F397, F415, and H440) of SQLE that interact with TRB have been reported in the resistance profiles of clinical isolates of T. rubrum32. We identified the AA residues of SQLEED from the genome and confirmed that the AA residues at L410, F414, F432, and H459 of SQLEED corresponded to those at L393, F397, F415, and H440 of SQLETR, respectively, indicating that there were no TRB resistance mutations against E. dermatitidis (Table S1 and Table S2). In the binding model, TRB formed an H-bond with Y102 and was surrounded by TRB resistance-related residues at L410, F414, F432, and H459, showing a better interaction of TRB with SQLEED (Fig. 2 and Fig. S2). These data suggested that TRB can strongly bind to SQLEED, eliciting a potent antifungal effect.
TRB shows sufficient serum concentrations33,34 and drug transfer to the skin and nails35, resulting in efficacy against dermatomycosis in clinical studies, but insufficient transfer to lung tissue36,37 in animal models. Nevertheless, Oral administration of TRB has been reported to be effective in a few patients with chronic Aspergillus lung infections38,39. Clinical trials with larger numbers of patients are needed to evaluate the significant efficacy of TRB in the treatment of fungal pneumonia.
In this study, PSC, with a potent antifungal activity profile, has a different mechanism of action from that of TRB against E. dermatitidis. According to a report on tissue concentrations in biopsy specimens obtained at autopsy from seven patients receiving PSC prophylaxis, lung concentrations were higher than those in the plasma40. The formulation of PSC is mixed with hydroxy-β-cyclodextrin, which improves its antifungal activity and pharmacokinetics by enhancing its solubility and oral bioavailability41 as well as ITC42. These results suggest that PSC with improved tissue migration may also be a suitable therapeutic option for invasive E. dermatitidis infections.
Recently, amikacin liposomal inhalation suspension (ALIS)43 was developed for the treatment of refractory nontuberculous mycobacterial infectious pulmonary disease (NTM-PD) and demonstrated better efficacy in the CONVERT trial44. Direct administration at the site of infection, such as inhalation, can increase drug concentrations at the tissue level, resulting in greater antifungal efficacy than that of systemic administration, and leading to a reduction in the side effects of the drug. Inhalable and spray-dried microparticles of TRB45,46 have been studied for the treatment of pulmonary fungal infections to enhance the beneficial effects of antifungal drugs as well as AmB47. TRB, which does not require particularly prolonged exposure (Fig. 4), would be a suitable inhaled drug for the treatment of E. dermatitidis pneumonia, which has been reported in approximately 6% of patients with bronchiectasis and cystic fibrosis5. In addition, TRB may be an additional option for therapeutic agents combined with azoles to cure invasive E. dermatitidis infections in immunocompromised patients20. We believe that re-evaluating potent old antifungals such as TRB with established efficacy and safety profiles can not only provide efficient and cost-effective treatment options for patients, leading to sustainable development goals but also help people in developing countries.
In conclusion, TRB could potentially be an even more useful and attractive antifungal drug if new routes of administration, such as inhalation or novel drug delivery systems, are developed to enhance TRB tissue migration.
Methods
Fungus and cells
E. dermatitidis 1 and 2 isolated from the patients with pneumonia were identified by ESI–MS and ITS gene analysis15, and E. dermatitidis 3 (NBRC6421, ATCC28869) was purchased from Biological Resource Center, NITE (NBRC, Japan) (Table S2). The fungus was incubated in Sabouraud buffer (5 g of meat peptone, 5 g of casein peptone, and 20 g of glucose in 1L dH2O) and Sabouraud dextrose agar (SDA) plate (5 g of meat peptone, 5 g of casein peptone, 40 g of glucose, and 1.5% agar in 1L H2O) supplemented with chloramphenicol (Cam) and kanamycin (K), or 0.25 µm filtered RPMI (Nissui, Japan) without NaHCO3 at pH 6.8, 0.165 M 3-morpholinopropane-1-sulfonic acid, MOPS (MOPS-RPMI) supplemented with Cam and K. A549 cells isolated from a male patient with lung cancer were purchased from the Japanese Collection of Research Bioresources (JCRB) Cell Bank, Japan, and cultured in DMEM medium (FUJIFILM Wako Pure Chemical Corporation, Japan) supplemented with 10% fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, USA), penicillin (P), and K.
DNA and RNA extraction, and identification of SQLE sequences
A collection of E. dermatitidis incubated on SDA with a small medicine spoon was completely frozen in liquid nitrogen. After grinding with a masher tube, total RNA and DNA were extracted using TRIzole (Invitrogen, Thermo Fisher Scientific). RNA was converted into complementary DNA (cDNA) using ReverTra Ace® (TOYOBO, Japan). These primers (ITS4R: TCC TCC GCT TAT TGA TAT GC NS7F: GAG GCA ATA ACA GGT CTG TGA TGC) provided the best combination for identification with the strain of E. dermatitidis using the ITS sequence. PCR amplifications were performed in an Eppendorf thermocycler (Eppendorf® Mastercycler) in a final volume of 40 μL with 10–50 ng of the DNA as a template using KOD one polymerase (TOYOBO). The SQLE sequence of E. dermatitidis 1, 2, and 3 was identified from the cDNA using these primers (ED.SE.SF: ATG CCT CTC ATA CTC GAT TCG TCG TC, ED.SE.ER: TCA AAT CCT CAG TTC GGC AAA TAT ATA CG, ED.SE.SQF: TCT GAT TCT GGG TGT GGA GTC C, ED.SE.SQR: TCA GGT ACG TCG ACC AGG ACA CG).
SQLE 3D structure and docking simulation
The 3D structure model of SQLEED was produced as a template of the crystal structure of SQLEHum (PDB accession number, 6C6N) using the SWISS model (https://swissmodel.expasy.org/). A nicotinamide adenine dinucleotide (NAD) was docked to the SQLEED in the same manner as the crystal structure of SQLEHum using SeeSAR v13.1 software (BioSolveIT GmbH, Sankt Augustin, Germany) (https://www.biosolveit.de/products/seesar/)48. Subsequently, the binding pocket of TRB was identified through PDB:6C6N and clinically isolated TRB-resistant amino acid mutations of T. rubrum. The docking simulation of TRB to the SQLEED was conducted. Molecular graphics and analyses were performed using UCSF Chimera (https://www.rbvi.ucsf.edu/chimera).
Giemsa and WST-1 staining procedures
Giemsa staining was used to observe E. dermatitis on A549 cells. A549 cells with or without E. dermatitis was washed with phosphate-buffered saline (PBS) and dried at room temperature (RT) for 30 min. They were then fixed with methanol for 5 min, stained with Giemsa dilution buffer (Merck KGaA, Darmstadt, Germany) for 20 min, washed with phosphate buffer (pH 7.2), and dried at RT for 30 min.
WST-1 staining was employed to assess the viability of E. dermatitidis. WST-1 staining solution was composed of 0.2 mM 1-methoxy-5-methylphenazinium methylsulfate (Dojindo, Japan), and 5 mM 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt (Dojindo, Japan) in 20 mM HEPES buffer (pH 7.2)21. Before the assay, the WST-1 staining solution was stored at -80℃. A volume of 10 μL of WST-1 staining solution was added to the samples in a 96-well plate and incubated at 35℃ for 2 h in a shaking incubator. The plate absorbance was quantified at OD440 using an absorbance spectrometer (FLUOstar Omega, BMG Labtech, Germany).
Minimum inhibitory concentration
According to CLSI M38 3rd edition, drugs were adjusted by 1/2 dilution to the concentration tested, and E. dermatitidis 1, 2, and 3 were inoculated at 1.0 × 105 CFU/ml and incubated at 35℃ for 48 or 72 h in a flat-bottomed 96-well polystyrene plate with MOPS-RPMI. MIC (mg/L) represents the lowest concentration of antifungal agents that inhibited the visible growth of E. dermatitidis. The lowest drug concentration that inhibited 50% (MIC50) or 90% (MIC90) of E. dermatitidis growth was determined by measuring fungal growth at OD530 nm or fungal viability following WST-1 staining at OD440 nm using an absorbance spectrometer (FLUOstar Omega) in comparison with the positive control (E. dermatitidis without drugs in MOPS-RPMI) and the negative control (MOPS-RPMI only).
E. dermatitis morphology with or without A549 cells
The adhesion details of E. dermatitis on A549 cells were observed by scanning electron microscope (SEM), JSM-IT300 InTouchScope™9,49. A549 cells at 1.5 × 105/ml were inoculated into an 18 × 18 mm coverslip on a chamber slide II (IWAKI, Japan) filled with DMEM containing 10% FBS, P, and K. After overnight incubation in 5% CO2 at 37℃, the final concentration of E. dermatitis 1 was added at 1.0 × 106 /mL to these plates, which were changed to DMEM containing 1% FBS, Cam, and K, and incubated in 5% CO2 at 35℃. The tested drugs at 0.25 mg/L were added to the plate after 6 h. These samples were further incubated in 5% CO2 at 35℃ for 24 or 48 h. The samples on the coverslip in the chamber slide II were fixed with 2% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for 24 or 48 h and dehydrated through 50, 75, 90, 95, and 100% ethanol sequentially. The 100% ethanol was replaced with t-butyl alcohol to cover the samples, which were then stored at − 20℃. The frozen sample was lyophilized under a vacuum and subsequently coated with platinum for observation under SEM.
Biofilm inhibition
Biofilm inhibition was determined using the crystal violet (CV) staining assay24. The conidium of E. dermatitidis 1 was seeded at 1.0 × 105 CFU/ml in a flat-bottomed, 96-well plate with the tested drugs and incubated at 35℃ for 48 h. The antifungal activity of the tested drugs was also determined by measuring OD530 before CV staining. The samples were washed twice with 200 μL of PBS, and stained with 100 μL of a 0.1% CV solution for 20 min at RT. The samples were washed with 200 μL of PBS and dried at 35℃ overnight. A solution of 100 μL of 30% acetic acid was incubated for 30 min at RT to extract CV staining from the biofilm. A volume of 80 μL of the solution was transferred to a fresh 96-well plate and the samples were measured at OD620 nm. Biofilm inhibition by the drugs was expressed as a relative ratio compared to the positive control (E. dermatitidis without drugs in MOPS-RPMI) and the negative control (MOPS-RPMI only).
Biofilm eradication
A modified biofilm eradication assay was performed according to the previous report50. Briefly, the conidium of E. dermatitidis 1 was seeded at 5.0 × 105 cells/ml in a 96-well plate without the tested drugs in 100 μL of MOPS-RPMI and incubated at 35℃ for 24 h. Then, the tested drugs were added to the plate at each concentration and further incubated at 35℃ for 24 h. The eradication ability of the tested drugs was determined by the CV staining assay described above.
The post-antifungal effects (PAFE) and time-kill assay
The PAFE and time-kill assay of E. dermatitidis (1.0 × 106 CFU/ml) was performed in a microtube tube with 100 μL of MOPS-RPMI medium. The PAFE of TRB, PSC, and AmB were tested at concentrations ranging from 0.13 to 32 mg/L. In the time-kill assay, a series of concentrations of TRB (ranging from 2 to 32 mg/L), PSC (from 0.5 to 8 mg/L), and AmB (from 0.25 to 4 mg/L) were prepared. The samples were shaken at 200 rpm, incubated at 35 °C for 0, 3, 6, and 12 h, respectively, and then were washed twice with 1000 μL of PBS at centrifugation of 2500 rpm for 2 min. The E. dermatitidis samples were duplicated (final concentration, 2.5 × 105 CFU/ml) and inoculated into a 96-well plate with 200 μL of fresh MOPS-RPMI medium without drugs at 35 °C for 48 h. The viable E. dermatitidis was evaluated by the WST-1 staining assay (measuring at OD440). The PAFE was determined using the samples incubated for 0 h. In the time-kill assay, the relative ratios were determined by dividing the data set by the positive control (E. dermatitidis with dimethyl sulfoxide [DMSO] in MOPS-RPMI) or the 0-h samples E. dermatitidis washed after the drug treatment without incubation).
Drug combination
The drug combination assay was based on the previous report26. Briefly, combinations of TRB and azoles, AmB and azoles, TRB and AmB, or TRB and CAS at the tested concentration were prepared in a flat-bottomed 96-well plate with MOPS-RPMI medium. The conidium of E. dermatitidis was seeded at 1.0 × 105 CFU/mL and incubated at 35℃ for 48 or 72 h. MIC (mg/L) was determined by visual observation of E. dermatitidis growth. The MIC50 and MIC90 (mg/L) values were determined by quantifying E. dermatitidis viability at OD440 nm following WST-1 staining. The inhibition ratios of the drug combination were calculated by dividing the data set by the positive control (E. dermatitidis without agents in MOPS-RPMI). The FIC index (FICI) of the tested combinations was determined according to previous reports26,51. Synergy effect was defined as FICI < 2.0, no interaction (additive effect); 2.0 < FICI < 4.0, and antagonistic effect; FICI > 4.0 from each MIC, MIC50 (WST-1), and MIC90 (WST-1) data.
Illustration
Illustrations accompanying the experimental procedure and explanations were created using BioRender (https://www.biorender.com/): Scientific Image and Illustration Software (Simplified Science Publishing, LLC [https://www.simplifiedsciencepublishing.com/]).
Drugs
TRB, MCZ, VRC, ITC, and PSC were purchased from the Tokyo Chemical Industry Japan, FLC and AmB from Wako Japan, ISC and CAS from Selleck Chemicals USA, and MCFG from Cayman Chemical USA. These tested drugs (5–20 mM) in DMSO or appropriate solutions were stored at − 80℃. Before the assays, these drugs were prepared at appropriate concentrations in MOPS-RPMI.
Statement of experimental samples
Clinical isolate samples of organisms are not human tissue. The fungi isolated in this study were analyzed for the fungi themselves, not for events related to human health. All methods were carried out under relevant guidelines and regulations of Kumamoto University.
Supplementary Information
Acknowledgements
We thank the bacteriological examination staff of the Department of Laboratory Medicine at Kumamoto University Hospital.
Author contributions
T.N. and T.Y. designed the research and, T.N., T.Y., and M.O. performed all the experiments. T.Y., D.M., and H.N. discussed the data and supported the preparation of the research. Y.J. and Y.T. supervised the personnel and the study. T.N. and H.N. obtained the necessary funding. T.N. and T.Y. wrote the manuscript, and T.Y., D.M., Y.J., and Y.T. advised or edited the manuscript. All authors read, commented on, and approved the final manuscript.
Funding
This work was supported by the Japan Society for the Promotion of Science, KAKENHI grant number JP21K16324 (T.N.), and a grant from Kobayashi Foundation, Kobayashi pharmacy-related grant (H.N.).
Data availability
Human SQLE sequences (Gene ID: 6713) and T. rubrum SQLE sequences (Gene ID: 10376061) were used in this study. The SQLE sequence of E. dermatitidis 1 identified in this study was deposited in the DNA Data Bank of Japan (DDBJ) and the NCBI GenBank database under the accession number LC829653 (https://www.ncbi.nlm.nih.gov/nuccore/LC829653). DDBJ is linked to the International Nucleotide Sequence Database, INSD. The data generated during and/or analyzed in the current study are available from the corresponding authors upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tomofumi Nakamura and Tatsuya Yoshinouchi.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-78815-3.
References
- 1.Bongomin, F., Gago, S., Oladele, R. O. & Denning, D. W. Global and multi-national prevalence of fungal diseases-estimate precision. J. Fungi3, 57 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown, G. D. et al. Hidden killers: human fungal infections. Sci. Transl. Med.4, 165rv13 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Döğen, A. et al. Dishwashers are a major source of human opportunistic yeast-like fungi in indoor environments in Mersin, Turkey. Med. Mycol.51, 493–498 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Isa-Isa, R., García, C., Isa, M. & Arenas, R. Subcutaneous phaeohyphomycosis (mycotic cyst). Clin. Dermatol.30, 425–431 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Lebecque, P. et al. Exophiala (Wangiella) dermatitidis and cystic fibrosis prevalence and risk factors. Med. Mycol.48, S4-9 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Vasquez, A. et al. Management of an outbreak of Exophiala dermatitidis bloodstream infections at an outpatient oncology clinic. Clin. Infect. Dis.66, 959–962 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Kirchhoff, L., Olsowski, M., Rath, P. M. & Steinmann, J. Exophiala dermatitidis: Key issues of an opportunistic fungal pathogen. In Virulence 984–998 (Taylor and Francis Inc, 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kondori, N. et al. Analyses of black fungi by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS): species-level identification of clinical isolates of Exophiala dermatitidis. FEMS Microbiol. Lett.362, 1–6 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Yoshinouchi, T. et al. Diagnosis and clinical management of Exophiala dermatitidis pneumonia in a patient with anorexia nervosa: A case report. Med. Mycol. Case Rep.42, 100617 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mpakosi, A. et al. A fatal neonatal case of fungemia due to Exophiala dermatitidis-case report and literature review. BMC Pediatr.22, 482 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva, W. C. et al. Species diversity, antifungal susceptibility and phenotypic and genotypic characterisation of Exophiala spp. infecting patients in different medical centres in Brazil. Mycoses60, 328–337 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Petranyi, G., Ryder, N. S. & Stütz, A. Allylamine derivatives: new class of synthetic antifungal agents inhibiting fungal squalene epoxidase. Science224, 1239–1241 (1984). [DOI] [PubMed] [Google Scholar]
- 13.Shafiei, M., Peyton, L., Hashemzadeh, M. & Foroumadi, A. History of the development of antifungal azoles: A review on structures, SAR, and mechanism of action. Bioorg. Chem.104, 104240 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Brüggemann, R. J., Jensen, G. M. & Lass-Flörl, C. Liposomal amphotericin B-the past. J. Antimicrob. Chemother.77, ii3–ii10 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alimu, Y., Ban, S. & Yaguchi, T. Molecular phylogenetic study of strains morphologically identified as Exophiala dermatitidis from clinical and environmental specimens in Japan. Med. Mycol. J.63, 1–9 (2022). [DOI] [PubMed] [Google Scholar]
- 16.Naka, W., Harada, T. & Nishikawa, T. Growth temperature of pathogenic dematiaceous fungi and skin surface temperature. Jpn. J. Med. Mycol.27, 245–250 (1986). [Google Scholar]
- 17.Ryder, N. S. Specific inhibition of fungal sterol biosynthesis by SF 86–327, a new allylamine antimycotic agent. Antimicrob. Agents Chemother.27, 252–256 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padyana, A. K. et al. Structure and inhibition mechanism of the catalytic domain of human squalene epoxidase. Nat. Commun.10, 97 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghannoum, M. A. & Rice, L. B. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev.12, 501–517 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramzi, S. H. T. et al. Efficacy of terbinafine and itraconazole combination therapy versus terbinafine or itraconazole monotherapy in the management of fungal diseases: A systematic review and meta-analysis. Cureus15, e48819 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishiyama, M., Miyazono, Y., Sasamoto, K., Ohkura, Y. & Ueno, K. A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta4, 1299–1305 (1994). [DOI] [PubMed] [Google Scholar]
- 22.Sun, Y., Liu, W., Wan, Z., Wang, X. & Li, R. Antifungal activity of antifungal drugs, as well as drug combinations against Exophiala dermatitidis. Mycopathologia171, 111–117 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Badali, H., de Hoog, G. S., Sudhadham, M. & Meis, J. F. Microdilution in vitro antifungal susceptibility of Exophiala dermatitidis, a systemic opportunist. Med. Mycol.49, 819–824 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Sharma, S. et al. Microbial biofilm: A review on formation, infection, antibiotic resistance, control measures, and innovative treatment. Microorganisms11, 1614 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirchhoff, L. et al. Biofilm formation of the black yeast-like fungus Exophiala dermatitidis and its susceptibility to antiinfective agents. Sci. Rep.7, 42886 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaller, M. A., Sheehan, D. J. & Rex, J. H. Determination of fungicidal activities against yeasts and molds: lessons learned from bactericidal testing and the need for standardization. Clin. Microbiol. Rev.17, 268–280 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vitale, R. G., De Hoog, G. S. & Verweij, P. E. In vitro activity of amphotericin B, itraconazole, terbinafine and 5-fluocytosine against Exophiala spinifera and evaluation of post-antifungal effects. Med. Mycol.41, 301–307 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Hazen, K. C. Fungicidal versus fungistatic activity of terbinafine and itraconazole: an in vitro comparison. J. Am. Acad. Dermatol.38, S37-41 (1998). [DOI] [PubMed] [Google Scholar]
- 29.Cuenca-Estrella, M. Combinations of antifungal agents in therapy- what value are they?. J. Antimicrob. Chemother.54, 854–869 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Meletiadis, J., Mouton, J. W., Meis, J. F. & Verweij, P. E. In vitro drug interaction modeling of combinations of azoles with terbinafine against clinical Scedosporium prolificans isolates. Antimicrob. Agents Chemother.47, 106–117 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sigurgeirsson, B. et al. Long-term effectiveness of treatment with terbinafine vs itraconazole in onychomycosis: a 5-year blinded prospective follow-up study. Arch. Dermatol.138, 353–357 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Yamada, T. et al. Terbinafine resistance of trichophyton clinical isolates caused by specific point mutations in the squalene epoxidase gene. Antimicrob. Agents Chemother.61, e00115-e117 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rojo-Solís, C. et al. Pharmacokinetics of single dose oral terbinafine in common Shelducks (Tadorna tadorna). J. Vet. Pharmacol. Ther.44, 510–515 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Leyden, J. Pharmacokinetics and pharmacology of terbinafine and itraconazole. J. Am. Acad. Dermatol.38, S42–S47 (1998). [DOI] [PubMed] [Google Scholar]
- 35.Faergemann, J., Zehender, H. & Millerioux, L. Levels of terbinafine in plasma, stratum corneum, dermis-epidermis (without stratum corneum), sebum, hair and nails during and after 250 mg terbinafine orally once daily for 7 and 14 days. Clin. Exp. Dermatol.19, 121–126 (1994). [DOI] [PubMed] [Google Scholar]
- 36.Bechert, U., Christensen, J. M., Poppenga, R., Fahmy, S. A. & Redig, P. Pharmacokinetics of terbinafine after single oral dose administration in red-tailed hawks (Buteo jamaicensis). J. Avian Med. Surg.24, 122–130 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Schmitt, H. J. et al. Inactivity of terbinafine in a rat model of pulmonary aspergillosis. Eur. J. Clin. Microbiol. Infect. Dis.9, 832–835 (1990). [DOI] [PubMed] [Google Scholar]
- 38.Schiraldi, G. F. et al. Terbinafine versus itraconazole: a long-term, randomized, double-blind, clinical trial in chronic pulmonary aspergillosis. A pilote study. J. Health Soc. Sci.1, 47–56 (2016). [Google Scholar]
- 39.Schiraldi, G. F. et al. Refractory pulmonary aspergillosis: compassionate trial with terbinafine. Br. J. Dermatol.134, 25–29 (1996). [DOI] [PubMed] [Google Scholar]
- 40.Blennow, O. et al. Posaconazole concentrations in human tissues after allogeneic stem cell transplantation. Antimicrob. Agents Chemother.58, 4941–4943 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greer, N. D. Posaconazole (noxafil): a new triazole antifungal agent. Bayl. Univ. Med. Cent. Proc.20, 188–196 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mouton, J. W. et al. Pharmacokinetics of itraconazole and hydroxyitraconazole in healthy subjects after single and multiple doses of a novel formulation. Antimicrob. Agents Chemother.50, 4096–4102 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang, J. et al. Amikacin liposome inhalation suspension (ALIS) penetrates non-tuberculous mycobacterial biofilms and enhances amikacin uptake into macrophages. Front. Microbiol.9, 915 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griffith, D. E. et al. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium avium Complex (CONVERT). A prospective, open-label, randomized study. Am. J. Respir. Crit. Care Med.198, 1559–1569 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Almansour, K. et al. Inhalable, spray-dried terbinafine microparticles for management of pulmonary fungal infections: optimization of the excipient composition and selection of an inhalation device. Pharmaceutics14, 87 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brunet, K., Martellosio, J. P., Tewes, F., Marchand, S. & Rammaert, B. Inhaled antifungal agents for treatment and prophylaxis of bronchopulmonary invasive mold infections. Pharmaceutics14, 641 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Pablo, E. et al. Targeting lung macrophages for fungal and parasitic pulmonary infections with innovative amphotericin B dry powder inhalers. Int. J. Pharm.635, 122788 (2023). [DOI] [PubMed] [Google Scholar]
- 48.Nakamura, T. et al. Conversion of raltegravir carrying a 1,3,4-oxadiazole ring to a hydrolysis product upon pH changes decreases its antiviral activity. PNAS Nexus3, pgad446 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ichikawa, T. et al. Adherence and cytotoxicity of Candida spp. to HaCaT and A549 cells. Med. Mycol. J.60, 5–10 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Pierce, C. G. et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc.3, 1494–1500 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Odds, F. C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother.52, 1 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Human SQLE sequences (Gene ID: 6713) and T. rubrum SQLE sequences (Gene ID: 10376061) were used in this study. The SQLE sequence of E. dermatitidis 1 identified in this study was deposited in the DNA Data Bank of Japan (DDBJ) and the NCBI GenBank database under the accession number LC829653 (https://www.ncbi.nlm.nih.gov/nuccore/LC829653). DDBJ is linked to the International Nucleotide Sequence Database, INSD. The data generated during and/or analyzed in the current study are available from the corresponding authors upon reasonable request.