Abstract
This study examines the association between posterior occlusal contact and the risk of dementia development in the Japanese population, utilizing Eichner classification to evaluate occlusal status. Data from Japanese health insurance claims were analyzed for the period from April 2016 to March 2022. Participants had undergone specific health checkups, had no prior history of dementia, and were classified according to their dental occlusal contact. Dementia diagnoses were determined using ICD-10 codes, and participants were divided into three groups—A, B, and C—based on the Eichner classification, which indicates the extent of occlusal contact. Over an average follow-up period of 35.6 months, 691 dementia were identified among 931,309 participants. Those diagnosed with dementia were more likely to belong to Eichner B and C groups, signifying reduced occlusal contact. After adjusting for covariates, the hazard ratios (95% confidence intervals) for Eichner B and C were 1.73 (1.31–2.28) and 2.10 (1.35–3.26), respectively. Sensitivity analyses confirmed these findings in adults aged 60–75. These findings suggest that reduced posterior occlusal contact correlates with an increased risk of dementia. Since the study is limited to participants under the age of 75, further research is required to determine its generalizability to older populations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-79399-8.
Keywords: Dementia, Oral health, Occlusal contacts, Eichner classification, Healthcare administrative claims
Subject terms: Epidemiology, Dental epidemiology, Dementia
Introduction
As the global population ages and life expectancy increases, the incidence of age-related conditions such as dementia increases, posing significant public health challenges1. Dementia, characterized as memory loss, language impairment, motor difficulties, impaired visual recognition, and challenges in executive functioning, disrupts daily activities2. Identifying modifiable risk factors for dementia could aid in developing prevention strategies to improve the quality of life of older adults.
The oral cavity plays a crucial role in daily life. Several meta-analyses have demonstrated an association between poor oral health and cognitive decline3–6. Cohort studies with follow-up periods ranging from 2 to 32 years have consistently shown that individuals with fewer remaining teeth are at a higher risk of developing dementia7–13. For instance, an 18-year US cohort study in a retirement community found that denture wearers with 10 or more upper and six or more lower teeth were at reduced risk10. These findings suggest that the decline in masticatory function associated with tooth loss may be important, warranting further research to explore its potential significance.
Existing studies examining the relationship between cognition and mastication have usually utilized the number of remaining teeth as an indicator of masticatory function. Notably, the risk of cognitive impairment was associated more with chewing difficulty than with the loss of multiple teeth14–17. Occlusal contact, particularly in the posterior region, is an essential aspect of oral health that plays a crucial role in effective mastication and overall dental function18,19. Some studies have shown that reduced masticatory efficiency due to poor occlusal contact can affect brain function through reduced sensory input and nutritional deficits20–22. Available evidence suggests that a higher number of total functional tooth units (FTUs) used to evaluate the posterior occlusal contact is associated with better cognitive function15,23,24. A lack of posterior occlusal support is related to cognitive decline16, whereas other studies have found no significant link with lower cognitive scores or dementia25,26. These studies, limited by small sample sizes, may have underestimated the true association due to insufficient statistical power to detect significant effects. Thus, the relationship between posterior occlusal contacts and the development of dementia remains an open research problem.
To clarify this relationship, a longitudinal study was conducted to determine whether there was an association between posterior occlusal contact and the incidence of dementia in a large Japanese population. Since the focus of this study was to evaluate occlusal support based on the existing natural teeth, we selected the Eichner classification to categorize posterior occlusal status, as it is a validated clinical tool for assessing occlusal contact based on the natural dentition. The Eichner classification has also been validated as a predictor of masticatory performance and occlusal force, with a study showing that masticatory efficiency declines as posterior occlusal contacts decrease27. We hypothesized that individuals with reduced posterior occlusal contact (Eichner B and C) exhibit a higher risk of developing dementia than those with adequate occlusal contact (Eichner A).
Result
Participant characteristics
A total of 931,309 participants (mean age: 49.4 ± 6.9 years; 526,117 male and 405,192 female) were included in the analysis. During a mean follow-up period of 35.6 months, 691 patients (mean age: 56.1 ± 8.4 years; 445 male and 246 female) were newly diagnosed with dementia. Characteristics of the study population according to the Eichner classification are presented in Table 1. The distributions of the Eichner groups A, B, and C were 901,059 (96.7%), 24,105 (2.6%), and 6,145 (0.7%), respectively. The proportion of dementia cases was higher in the Eichner B and C groups than in the Eichner A group (p < 0.001). Other variables also showed significant differences across the Eichner classifications.
Table 1.
Characteristics of the subjects according to the Eichner classification.
| Eichner A | Eichner B | Eichner C | P-value† | ||||
|---|---|---|---|---|---|---|---|
| N = 901,059 | N = 24,105 | N = 6145 | |||||
| Insurance status (N, %) | |||||||
| Employee | 713,113 | 79.1% | 19,840 | 82.3% | 5254 | 85.5% | < 0.001 |
| Dependent | 187,946 | 20.9% | 4265 | 17.7% | 891 | 14.5% | |
| Age group (N, %) | |||||||
| 40–59 years | 825,940 | 91.7% | 13,582 | 56.3% | 2379 | 38.7% | < 0.001 |
| 60–75 years | 75,119 | 8.3% | 10,523 | 43.7% | 3766 | 61.3% | |
| Sex (N, %) | |||||||
| Male | 505,961 | 56.2% | 15,932 | 66.1% | 4224 | 68.7% | < 0.001 |
| Female | 395,098 | 43.8% | 8173 | 33.9% | 1921 | 31.3% | |
| BMI (N, %) | |||||||
| 18.5–24.9 | 604,546 | 67.1% | 14,879 | 61.8% | 3758 | 61.2% | < 0.001 |
| < 18.5 | 65,990 | 7.3% | 1287 | 5.3% | 441 | 7.2% | |
| ≥ 25.0 | 230,523 | 25.6% | 7939 | 32.9% | 1946 | 31.6% | |
| CCI (N, %) | |||||||
| 0 | 632,233 | 70.1% | 14,531 | 60.3% | 3430 | 55.8% | < 0.001 |
| 1 | 188,815 | 21.0% | 5585 | 23.2% | 1466 | 23.9% | |
| > 2 | 80,011 | 8.9% | 3989 | 16.5% | 1249 | 20.3% | |
| Depression (N, %) | |||||||
| No | 832,098 | 92.3% | 22,553 | 93.6% | 5830 | 94.9% | < 0.001 |
| Yes | 68,961 | 7.7% | 1552 | 6.4% | 315 | 5.1% | |
| Alcohol (N, %) | |||||||
| No | 221,677 | 24.6% | 7,981 | 33.1% | 2128 | 34.6% | < 0.001 |
| Yes | 679,382 | 75.4% | 16,124 | 66.9% | 4017 | 65.4% | |
| Smoking (N, %) | |||||||
| No | 737,606 | 81.9% | 16,589 | 68.8% | 3555 | 57.9% | < 0.001 |
| Yes | 163,453 | 18.1% | 7516 | 31.2% | 2590 | 42.1% | |
| Physical activity (N, %) | |||||||
| No | 557,916 | 61.9% | 14,387 | 59.7% | 3702 | 60.2% | < 0.001 |
| Yes | 343,143 | 38.1% | 9718 | 40.3% | 2443 | 39.8% | |
| Dementia (N, %) | |||||||
| No | 900,451 | 99.9% | 24,044 | 99.7% | 6123 | 99.6% | < 0.001 |
| Yes | 608 | 0.1% | 61 | 0.3% | 22 | 0.4% | |
BMI body mass index, CCI Charlson comorbidity index.
†Chi-squared test.
Multivariate Cox proportional hazards analyses
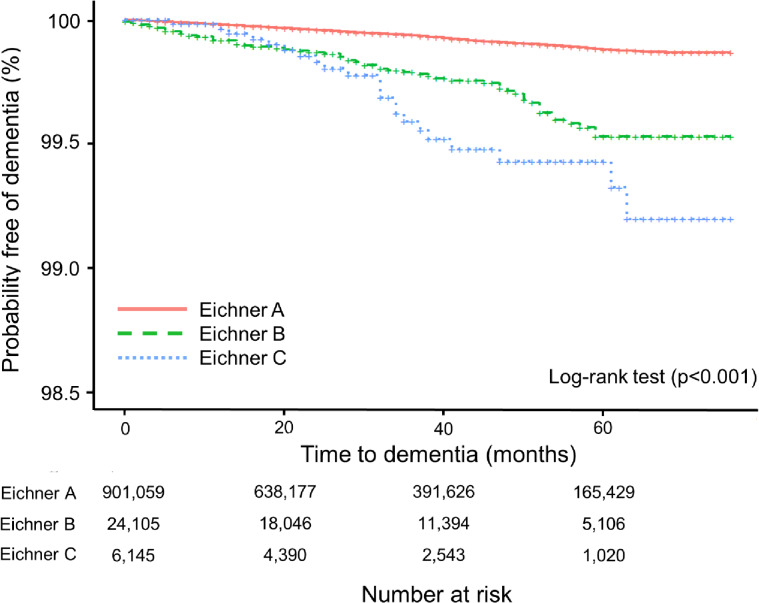
Figure 1 shows the Kaplan–Meier curves. The log-rank test indicated a significantly higher cumulative risk of dementia during follow-up among patients classified as Eichner B or C (p < 0.001). The log-log plots confirmed that the proportional hazards assumption was appropriate. Table 2 presents the results of the Cox proportional hazard analyses for dementia. The HRs with 95% CIs for participants in the Eichner B and Eichner C groups were 1.73 (1.31–2.28) and 2.10 (1.35–3.26), respectively. The cumulative incidence of dementia was higher among the Eichner B and Eichner C groups compared to those in the Eichner A group, with absolute differences of 0.2% and 0.3%, respectively.
Fig. 1.
Kaplan–Meier survival curve of the dementia-free rates in participants by Eichner classification.
Table 2.
Univariable and multivariate Cox proportional hazards model for dementia.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Eichner classification (reference: Eichner_A) | ||||||
| Eichner_B | 3.42 | 2.63–4.45 | < 0.001 | 1.73 | 1.31–2.28 | < 0.001 |
| Eichner_C | 5.06 | 3.31–7.74 | < 0.001 | 2.10 | 1.35–3.26 | < 0.001 |
| Insurance status (reference: Employee) | ||||||
| Dependent | 1.25 | 1.06–1.48 | 0.009 | 2.04 | 1.55–2.69 | < 0.001 |
| Age group (reference: 40–59 years) | ||||||
| 60–75 years | 6.08 | 5.19–7.12 | < 0.001 | 5.31 | 4.46–6.33 | < 0.001 |
| Sex (reference: Male) | ||||||
| Female | 0.75 | 0.64–0.87 | < 0.001 | 0.47 | 0.36–0.61 | < 0.001 |
| BMI (reference: 18.5–24.9) | ||||||
| < 18.5 | 1.43 | 1.11–1.83 | 0.005 | 1.63 | 1.25–2.11 | < 0.001 |
| ≥ 25.0 | 0.99 | 0.83–1.18 | 0.92 | 0.93 | 0.78–1.12 | 0.46 |
| CCI (reference: 0) | ||||||
| 1 | 1.36 | 1.15–1.61 | < 0.001 | 1.30 | 1.09–1.56 | 0.004 |
| ≥ 2 | 2.75 | 2.29–3.30 | < 0.001 | 1.76 | 1.44–2.15 | < 0.001 |
| Depression (reference: No) | ||||||
| Yes | 4.99 | 4.25–5.85 | < 0.001 | 5.07 | 4.29–5.98 | < 0.001 |
| Smoking (reference: No) | ||||||
| Yes | 0.91 | 0.75–1.11 | 0.37 | 0.91 | 0.74–1.11 | 0.35 |
| Alcohol (reference: No) | ||||||
| Yes | 0.95 | 0.80–1.13 | 0.55 | 1.05 | 0.88–1.26 | 0.56 |
| Physical activity (reference: Yes) | ||||||
| No | 1.02 | 0.87–1.19 | 0.81 | 1.09 | 0.94–1.28 | 0.26 |
The multivariate Cox proportional hazards model included dementia as the outcome and Eichner classification as the explanatory variable. HR hazard ratio, CI confidence interval, BMI body mass index, CCI Charlson comorbidity index.
Sensitive analyses
First, to exclude individuals with latent dementia at baseline, we included only those with follow-up periods longer than 1 year. The main findings in this cohort were consistent (Table S1). Second, our main findings remained consistent even after performing multiple imputations for missing data (Table S2). Third, the main results persisted when the definition of dementia was combined with the use of medication (Table S3). Fourth, multivariate models using continuous age values yielded results similar to those relative to the use of age groups to examine the relationship between the risk factors and incident dementia (Table S4). Fifth, the effect of occlusal contact loss was more pronounced in adults aged 60–75 years. Eichner B (HR = 1.59, 95% CI: 1.14–2.23) and Eichner C (HR = 2.19, 95% CI: 1.37–3.52) were significantly associated with an increased risk of dementia (Table S5).
Discussion
The main findings of this study indicate that individuals in the Eichner B and C groups, characterized by reduced or absent posterior occlusal contact, had a higher risk of developing dementia than those in the Eichner A group. Therefore, promoting access to dental care and prosthetic rehabilitation, particularly for maintaining and restoring posterior tooth occlusion, could be an important clinical intervention to prevent the onset of dementia.
Our results are consistent with those of previous research demonstrating a significant association between chewing difficulties and cognitive impairment in older adults28–30. Additionally, this study corroborates evidence from cross-sectional studies that show objective measures of masticatory ability, such as the number of FTUs, are correlated with cognitive test scores and cognitive impairment15,23,24. Nevertheless, our results contrast with those from a 22-year longitudinal study, which found no increased risk of dementia based on posterior occlusal contact, as assessed by the Eichner classification25. A key factor that may explain this discrepancy is the methodological differences between the studies, including its small sample size of 544 participants, which likely reduced statistical power. Moreover, the higher average age of participants (63 years at baseline) and the extended follow-up period may have introduced selective survival bias, as evidenced by the high mortality rates across the Eichner groups: 53.1%, 68.6%, and 90.4% for Eichners A, B, and C, respectively25. Consequently, the participants who survived to later stages of the study may have represented a healthier subset of the population, potentially underestimating the association between posterior occlusal contact and dementia risk. In contrast, our study leveraged a larger sample size and a more contemporary cohort, enhancing both its statistical power and relevance. These factors suggest that our findings provide a robust and accurate representation of the association between chewing function and cognitive impairment in older adults.
As evidenced in Table S5, a significant association between posterior occlusal contact loss and dementia onset was found in individuals aged not only 60–75 years but also 40–59 years, indicating a potential risk even for those over 40 years of age. Several mechanisms have been proposed to explain why loss of posterior occlusal support is a risk factor for dementia. First, reduced occlusal contact is associated with impaired nutritional intake, particularly that of fruits, vegetables, and essential nutrients31,32. This nutritional deficiency may contribute to cognitive decline. A 6-year longitudinal study conducted in Japan found that vegetable and fruit intake partially mediates the relationship between declining oral health and the onset of dementia12. The study observed that adjusting for nutritional status in analytical models significantly attenuated the association between cognitive function and posterior teeth occlusion, underscoring the role of nutritional status in elucidating this relationship15. Second, the occlusal condition affects neural activity in the brain through blood perfusion33, which may positively influence memory and enhance cognitive abilities34. Chewing has been demonstrated to increase blood oxygenation level-dependent (BOLD) signals bilaterally in the sensorimotor cortex, supplementary motor area, insula, thalamus, and cerebellum35,36. Notably, in animal models, chewing immediately before cognitive tasks enhances BOLD signals in the prefrontal cortex and hippocampus, potentially improving task performance37. In aged mice, the impairment of learning ability induced by the absence of molars was reversed by restoring lost molars with artificial crowns38. Third, reduced posterior occlusal support leads to difficulty in speaking and reduced self-confidence, which may contribute to the loss of social interaction and decreased cognitive engagement, thereby exacerbating the risk of cognitive decline39. Limited social interaction is recognized as a risk factor for dementia, and recent research indicates that social factors such as social networks and homeboundness partly mediate the link between poor oral health and dementia onset12,40. The results of age-stratified analyses are presented in Table S5. Among individuals aged 60–75, the loss of occlusal contact, particularly in Eichner B (HR = 1.59) and Eichner C (HR = 2.19), significantly increased the risk of dementia, indicating a heightened vulnerability to cognitive decline. Older adults often experience a decline in social interactions after retirement for various reasons, including physical limitations and changes in social roles. Therefore, the loss of posterior occlusal contact’s impact on the development of dementia in individuals aged 60–75 may be exacerbated by concurrent reductions in social interactions. These mechanisms suggest a multifaceted effect of occlusal health on brain function, emphasizing the importance of preserving occlusal contact. Proactive management of occlusal health could play a crucial role in dementia prevention by addressing key factors such as reduced nutritional intake and diminished brain stimulation, both of which are frequently linked to aging.
This study has several limitations. First, because this database primarily includes Japan’s working-age population and their family members, it excludes individuals aged over 75 years. This limitation restricts our ability to evaluate the relationship between risk factors and dementia in latter-stage older adults; consequently, the findings may be skewed towards early-stage dementia cases, affecting the broader applicability of our results. Second, administrative claims only capture diagnoses linked to medical treatment, potentially leading to the underreporting of dementia cases among individuals who do not seek medical care. Moreover, the study’s focus on newly diagnosed patients with dementia likely overlooked variations in symptom severity, possibly skewing the findings towards milder cases. We also did not distinguish between different types of dementia, which is a common limitation in studies that use claims data41. For example, vascular dementia might be underreported due to the lack of specific therapeutic interventions and could also be misclassified as unspecified dementia. Furthermore, we did not adjust for baseline cognitive assessments. Although we included only participants without a dementia diagnosis, the potential presence of undiagnosed or subclinical dementia in the population should be recognized as a limitation. Third, while adjustments were made for conventional confounders available in the database, residual confounding may persist, given the association of dementia with factors such as family history, education, and socioeconomic status42. Socioeconomic status, in particular, has been shown to affect healthcare access, including dental care, which may lead to disparities in both dental health and dementia risk43. Therefore, the association between occlusal contacts and dementia might be overestimated due to the lack of adjustment for socioeconomic status and family history. Fourth, we did not account for the duration of the loss of posterior occlusal support. If this condition affects cognitive function, it is important to know for how long the participants have been without such support. Moreover, while we utilized dental claims data to estimate posterior occlusal contact, it is important to acknowledge that relying on claims data may introduce inaccuracies. Dental claims data typically assume that if both upper and lower teeth are recorded in corresponding positions, occlusal contact is automatically inferred. In contrast, clinical practice relies on a dentist’s direct examination to determine whether the upper and lower teeth make contact during biting. Consequently, using claims data may overestimate the number of posterior occlusal contacts, especially in Eichner A and B. Additionally, this study did not account for the presence or severity of periodontitis despite its association with inflammatory processes and immune responses related to the pathogenesis of cognitive impairment44. Future research should aim to address these limitations through more diverse populations and detailed assessments.
Conclusion and implications
This study is the first to demonstrate a significant association between loss of posterior occlusal contact, as assessed using the Eichner classification, and the development of dementia. These findings emphasize the importance of maintaining occlusal function, which may reduce the risk of dementia. From a public health perspective, focusing on the location of tooth loss at health checkups may help in the early identification of individuals at an increased risk of developing dementia. Future research should investigate whether dental treatments, such as prosthetics, can reduce the risk of dementia or delay its onset. Furthermore, it is necessary to determine whether these findings are applicable to other populations with different healthcare systems and dietary habits.
Methods
Study database
This was a retrospective observational study based on data from the JMDC Claims Database (JMDC Inc., Tokyo, Japan), which provides longitudinal healthcare data, including demographic characteristics, monthly medical and dental health insurance claims, and annual health checkups. The database contains encrypted personal identifiers, age, sex, treatment details, International Classification of Diseases, 10th Revision (ICD-10) codes, and standardized disease classification codes. It primarily includes the records of individuals insured by Japanese companies and their dependents (excluding those aged > 75 years). Health checkup data and medical and dental claims were integrated using patient identification numbers for analysis. To ensure anonymity, all personal names and identification numbers in the JMDC claims database were replaced with unique numerical codes.
Study participants
The study was conducted from April 2016 to March 2022. Figure 2 shows a flowchart of the participant selection method. Initially, individuals with available health checkup data were identified (6,741,176 participants). Participants were excluded if they were under 40 years of age (N = 2,399,923), had missing values in their health checkup data (N = 1,128,414), had no dental codes for 28 teeth within 1 year prior to obtaining health checkup data (N = 2,280,978), or had been diagnosed with dementia within the previous 12 months before the index diagnosis (N = 552). This ensured that only patients initially diagnosed during the follow-up period were included.
Fig. 2.
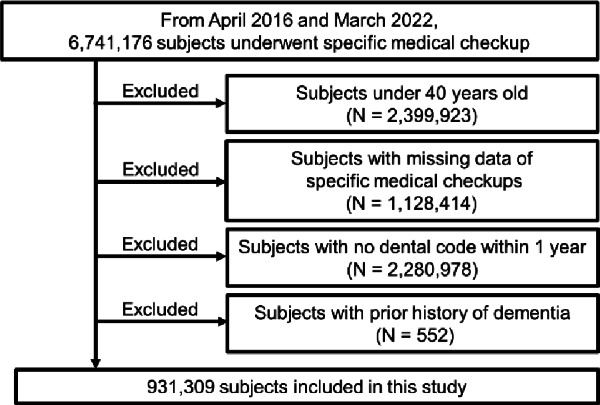
Flow diagram of sample selection. This flowchart outlines the process of selecting participants from the JMDC claims database. The initial pool included 6,741,176 individuals with available health checkup data. Exclusion criteria were applied in the following order: participants under 40 years of age (N = 2,399,923), those with missing health checkup data (N = 1,128,414), those lacking dental codes for 28 teeth within 1 year before the health checkup data (N = 2,280,978), and individuals diagnosed with dementia within 12 months preceding the index diagnosis (N = 552).
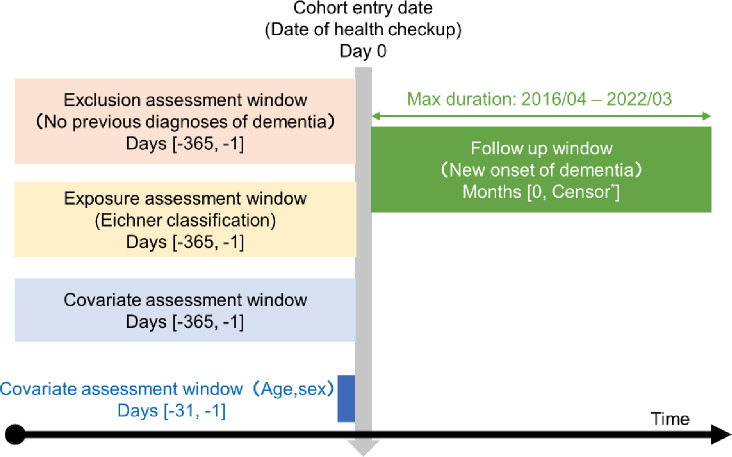
In the identification of dementia diagnosis, we classified candidates for whom the ICD-10 codes F00–F03 and G30–G31, except G319, were detailed in a previous report45. These codes encompassed a range of dementia types, such as Alzheimer’s disease, vascular dementia, dementia from specified diseases, unspecified dementia, and other degenerative nervous system disorders. Finally, 931,309 participants were included in subsequent analyses. The cohort study design is shown in Fig. 3.
Fig. 3.
Cohort study design of this study. *Earliest onset of dementia, loss of health insurance coverage, or end of follow-up.
Outcome variable
The primary outcome was new-onset dementia. Dementia diagnoses were established based on the following criteria: (i) individuals were identified as those with recorded diagnoses of F00–F03 and G30–G31, excluding G319, in their medical claims data during the follow-up period (April 2016 to March 2022), and (ii) those flagged as suspected for these diagnoses were excluded. Follow-up concluded on the date of diagnosis, death, or the end of the study period.
Explanatory variables
The posterior occlusal status was used as an explanatory variable. The JMDC Claims Database, which lacks specific data on the number of teeth present, includes records of dental visits for procedures such as periodontal examinations and mechanical tooth cleaning. These records feature dental codes indicating the presence and condition of each tooth. The number of present teeth was calculated using these codes, a method validated by comparing claims data with community oral health screening data by dentists in Japan46. Occlusal contact was evaluated according to the Eichner classification47. The Eichner classification is based on natural tooth contact in the premolar and molar regions of the bilateral maxilla and mandible. The Eichner classification categorizes occlusal contact conditions into three groups: A (contact in all four posterior zones), B (contact in one–three posterior zones or in the anterior region), and C (absence of tooth contact).
Other variables
There are 12 risk factors for dementia48. We adjusted for demographic factors, health behaviors, and comorbidities that are likely associated with dementia and accessible through claims data.
The demographic information included insurance status (employee or dependent), age, and sex (male or female). Age was derived by subtracting the date of birth from the date of the medical checkup. Because the Anderson-Darling test determined that age did not have a normal distribution (p < 0.001), the participants were stratified into two age groups: 40–59 years and 60–75 years.
Health behaviors included body mass index (BMI), smoking, alcohol consumption, and physical activity. BMI categories were defined as follows: <18.5 kg/m² for underweight, 18.5–24.9 kg/m² for normal weight, and ≥ 25.0 kg/m² for overweight. Current smokers were defined as individuals who had smoked more than five packs (100 cigarettes) in their lifetime and smoked daily or occasionally within the last 28 days. Alcohol use was categorized based on the frequency of alcohol consumption, and current drinkers were defined as those who consumed alcohol daily or occasionally. Physical activity was assessed using a standardized questionnaire in which ideal physical activity was defined as engaging in at least 30 min of exercise at least twice per week or walking for at least 1 h per day.
The comorbidity burden was evaluated using the Charlson Comorbidity Index (CCI). Participants were stratified into three CCI groups: 0, 1, and 2. Depression diagnoses were identified using the ICD-10 codes F32 (depressive episode) and F33 (major depressive disorder, recurrent), as described previously49.
Statistical analyses
The study employed descriptive analysis which was used to present data as means ± standard error for continuous variables and as counts and percentages for categorical variables. Baseline characteristics were compared among the three Eichner classifications (A, B, and C) using the chi-square test. Kaplan-Meier curves were used to compare the dementia incidence rates across the Eichner classifications, and the log-rank test was used to evaluate differences between the groups. Log-log plots were used to verify the assumptions of the Cox proportional hazards model. Multivariate Cox proportional hazards analyses were conducted to determine the relationship between Eichner classification and dementia. We calculated the hazard ratios (HRs) and corresponding 95% confidence intervals (95% CIs) in an unadjusted model after adjusting for covariates such as insurance status, age group, sex, BMI, CCI, depression, smoking, alcohol use, and physical activity.
Five sensitivity analyses were performed: (1) analysis with a 1-year induction period to exclude individuals with latent dementia (N = 751,224); (2) multiple imputations of missing health checkup data using 10 imputed datasets (N = 1,275,701); (3) new dementia onset defined by ICD-10 codes and dementia medication prescriptions; (4) age replaced as a continuous variable in the model; and (5) division into age groups of 40–59 and 60–75 years due to general retirement age in Japan.
All analyses were performed using R Studio, version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria), with p-values < 0.05 indicating statistical significance.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge the valuable contributions of other researchers, staff, and participants in the study using the JMDC database. We would like to thank Editage (www.editage.jp) for editing.
Author contributions
The paper was coauthored by Takashi Miyano (T.M), Yudai Tamada (Y.T), Taro Kusama (T.K), Ken Osaka (K.O), and Kenji Takeuchi (K.T). All authors meet the criteria for authorship polycy for Nature as follows:-Study concept and design: T.M, Y.T, T.K, K.O, and K.T -Acquisition of data: K.T-Analysis and interpretation of data: T.M, Y.T, T.K, K.O, and K.T-Drafting of the manuscript: T.M-Critical revision of the manuscript for important intellectual content: Y.T, T.K, K.O, and K.T.
Funding
This study was supported by Grants-in-Aid for Scientific Research (22H03299, 23K24557) from the Japan Society for the Promotion of Science (JSPS) KAKENHI and Health Labor Sciences Research Grants (23FA1022) from the Ministry of Health, Labor, and Welfare. The funders had no role in the study design, data analysis, data interpretation, manuscript preparation, or decision to submit the manuscript for publication. The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policies or positions of the respective funding organizations.
Data availability
Data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
We used anonymized information obtained from the JMDC Claims Database, which is commercially available in accordance with the Japan Act on the Protection of Personal Information. Informed consent was not required for its provision or use. The anonymization procedure was based on the Next-Generation Medical Infrastructure Act. According to the ethical guidelines for clinical research in Japan, studies using anonymized processed information do not require review by an ethics review committee. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhao, M., Chen, Z., Xu, T., Fan, P. & Tian, F. Global prevalence of polypharmacy and potentially inappropriate medication in older patients with dementia: a systematic review and meta-analysis. Front. Pharmacol.14, 1221069 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grand, J. H. G., Caspar, S. & MacDonald, S. W. S. Clinical features and multidisciplinary approaches to dementia care. J. Multidiscipl. Healthc.4, 125–147 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daly, B. et al. Evidence summary: the relationship between oral health and dementia. Br. Dent. J.223, 846 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Shen, T., Lv, J., Wang, L., Wang, W. & Zhang, D. Association between tooth loss and dementia among older people: a meta-analysis. Int. J. Geriatr. Psychiatry31, 953–955 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Cerutti-Kopplin, D. et al. Tooth loss increases the risk of diminished cognitive function: a systematic review and meta-analysis. JDR Clin. Trans. Res.1, 10–19 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Li, L. et al. Tooth loss and the risk of cognitive decline and dementia: a meta-analysis of cohort studies. Front. Neurol.14, 1103052 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim, J. M. et al. Dental health, nutritional status and recent-onset dementia in a Korean community population. Int. J. Geriatr. Psychiatry22, 850–855 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Kaye, E. K. et al. Tooth loss and periodontal disease predict poor cognitive function in older men. J. Am. Geriatr. Soc.58, 713–718 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto, T. et al. Association between self-reported dental health status and onset of dementia: a 4-year prospective cohort study of older Japanese adults from the Aichi Gerontological Evaluation Study (AGES) project. Psychosom. Med.74, 241–248 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Paganini-Hill, A., White, S. C. & Atchison, K. A. Dentition, dental health habits, and dementia: the Leisure World cohort study. J. Am. Geriatr. Soc.60, 1556–1563 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi, K. et al. Tooth loss and risk of Dementia in the community: the Hisayama Study. J. Am. Geriatr. Soc.65, e95–e100 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Kiuchi, S. et al. Oral status and dementia onset: mediation of nutritional and social factors. J. Dent. Res.101, 420–427 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Yang, H. L. et al. Tooth loss, denture use, and cognitive impairment in Chinese older adults: A Community Cohort Study. J. Gerontol. Ser. Biol. Sci. Med. Sci.77, 180–187 (2022). [DOI] [PubMed] [Google Scholar]
- 14.Lexomboon, D., Trulsson, M., Wãrdh, I. & Parker, M. G. Chewing ability and tooth loss: association with cognitive impairment in an elderly population study. J. Am. Geriatr. Soc.60, 1951–1956 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi, K. et al. Posterior teeth occlusion Associated with cognitive function in nursing home older residents: a cross-sectional observational study. PLoS One10, e0141737 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatta, K. et al. Influence of lack of posterior occlusal support on cognitive decline among 80-year-old Japanese people in a 3-year prospective study. Geriatr. Gerontol. Int.18, 1439–1446 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Miyano, T. et al. Association between reduced posterior occlusal contact and Alzheimer’s Disease Onset in older Japanese adults: results from the LIFE Study. J. Alzheimers Dis.97, 871–881 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delwel, S. et al. Chewing efficiency, global cognitive functioning, and dentition: a cross-sectional observational study in older people with mild cognitive impairment or mild to moderate dementia. Front. Aging Neurosci.12, (2020). [DOI] [PMC free article] [PubMed]
- 19.Kosaka, T. et al. Factors influencing the changes in Masticatory Performance: the Suita Study. JDR Clin. Trans. Res.3, 405–412 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Momose, T. et al. Effect of mastication on regional cerebral blood flow in humans examined by positron-emission tomography with 15O-labelled water and magnetic resonance imaging. Arch. Oral Biol.42, 57–61 (1997). [DOI] [PubMed] [Google Scholar]
- 21.Miura, H., Yamasaki, K., Kariyasu, M., Miura, K. & Sumi, Y. Relationship between cognitive function and mastication in elderly females. J. Oral Rehabil.30, 808–811 (2003). [DOI] [PubMed] [Google Scholar]
- 22.González, S., Huerta, J. M., Fernández, S., Patterson, Á. M. & Lasheras, C. The relationship between dietary lipids and cognitive performance in an elderly population. Int. J. Food Sci. Nutr.61, 217–225 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Cardoso, M. G. et al. Relationship between functional masticatory units and cognitive impairment in elderly persons. J. Oral Rehabil. 46, 417–423 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Han, J. H. et al. Loss of functional dentition is associated with cognitive Impairment. J. Alzheimers Dis.73, 1313–1320 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Dintica, C. S. et al. The relation of poor mastication with cognition and dementia risk: a population-based longitudinal study. Aging12, 8536–8548 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jockusch, J., Hopfenmüller, W. & Nitschke, I. Chewing function and related parameters as a function of the degree of dementia: is there a link between the brain and the mouth? J. Oral Rehabil. 48, 1160–1172 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakatsuka, Y. et al. Location of main occluding areas and masticatory ability in patients with reduced occlusal support. Aust. Dent. J.55, 45–50 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Kim, E. K. et al. Relationship between chewing ability and cognitive impairment in the rural elderly. Arch. Gerontol. Geriatr.70, 209–213 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Park, T. et al. More teeth and posterior balanced occlusion are a key determinant for cognitive function in the Elderly. Int. J. Environ. Res. Public. Health18, 1–10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim, M. S., Oh, B., Yoo, J. W. & Han, D. H. The association between mastication and mild cognitive impairment in Korean adults. Medicine99, e20653 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samnieng, P. et al. Oral health status and chewing ability is related to mini-nutritional assessment results in an older adult population in Thailand. J. Nutr. Gerontol. Geriatr.30, 291–304 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Kossioni, A. E. The Association of Poor Oral Health Parameters with malnutrition in older adults: a review considering the potential implications for cognitive impairment. Nutrients10, 1709 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura, T., Zou, K., Shibuya, Y. & Michikawa, M. Oral dysfunctions and cognitive impairment/dementia. J. Neurosci. Res.99, 518–528 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Hirano, Y. et al. Effects of chewing in working memory processing. Neurosci. Lett.436, 189–192 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Onozuka, M. et al. Mapping brain region activity during chewing: a functional magnetic resonance imaging study. J. Dent. Res.81, 743–746 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Quintero, A., Ichesco, E., Myers, C., Schutt, R. & Gerstner, G. E. Brain activity and human unilateral chewing: an FMRI study. J. Dent. Res.92, 136–142 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kubo, K., Chen, H. & Onozuk, M. The relationship between mastication and cognition. In Senescence and Senescence-Related Disorders (InTech, 2013).
- 38.Watanabe, K. et al. The molarless condition in aged SAMP8 mice attenuates hippocampal Fos induction linked to water maze performance. Behav. Brain. Res.128, 19–25 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Yun, S., Ogawa, N., Izutsu, M. & Yuki, M. The association between social isolation and oral health of community-dwelling older adults-A systematic review. Jpn. J. Nurs. Sci.20, e12524 (2023). [DOI] [PubMed] [Google Scholar]
- 40.Evans, I. E. M., Martyr, A., Collins, R., Brayne, C. & Clare, L. Social isolation and cognitive function in later life: a systematic review and meta-analysis. J. Alzheimers Dis.70, S119–S144 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hessler, J. B. et al. Predicting dementia in primary care patients with a cardiovascular health metric: a prospective population-based study. BMC Neurol.16, 116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saito, M. et al. Risk factors for tooth loss in adult Japanese dental patients: 8020 Promotion Foundation Study. J. Investig. Clin. Dent.10, e12392 (2019). [DOI] [PubMed] [Google Scholar]
- 43.McMaughan, D. J., Oloruntoba, O. & Smith, M. L. Socioeconomic Status and Access to Healthcare: interrelated drivers for healthy aging. Front. Public. Health8 (2020). [DOI] [PMC free article] [PubMed]
- 44.Lutshumba, J., Nikolajczyk, B. S. & Bachstetter, A. D. Dysregulation of systemic immunity in aging and dementia. Front. Cell. Neurosci.15 (2021). [DOI] [PMC free article] [PubMed]
- 45.Sakata, N. & Okumura, Y. Job loss after diagnosis of early-onset dementia: a matched cohort study. J. Alzheimers Dis.60, 1231–1235 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamada, Y. et al. Validity of claims-based definition of number of remaining teeth in Japan: results from the longevity improvement and fair evidence study. PLoS One19 (2024). [DOI] [PMC free article] [PubMed]
- 47.Eichner, K. Über Eine Gruppeneinteilung des Lückengebisses für die Prothetik. Deutsch Zahnärtl Z.10, 1831–1834 (1995). [Google Scholar]
- 48.Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet396, 413–446 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akechi, T., Mishiro, I., Fujimoto, S. & Murase, K. Risk of major depressive disorder in Japanese cancer patients: a matched cohort study using employer-based health insurance claims data. Psychooncology29, 1686–1694 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.