Abstract
The mitochondrion is an essential cell organelle known as the powerhouse of the cell. Mitochondrial ribosomal proteins (MRPs) are nuclear encoded, synthesised in the cytoplasm but perform their main functions in the mitochondria, which includes translation, transcription, cell death and maintenance. However, MRPs have also been implicated in cancer, particularly advanced disease and metastasis across a broad range of cancer types, where they play a central role in cell survival and progression. For some, their altered expression has been investigated as potential prognostic markers, and/or therapeutic targets, which is the focus of this review. Several therapies targeting MRPs are currently approved by the Food and Drug Administration and the European Medicines Agency for use in other diseases, revealing the opportunity for repurposing their use in advanced and metastatic cancer. Herein, we review the evidence supporting key MRPs as molecular drivers of advanced disease in multiple cancer types. We also highlight promising avenues for future use of MRPs as precision targets in the treatment of late-stage cancers for which there are currently very limited effective treatment options.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10555-024-10216-4.
Keywords: Cancer, Metastasis, Mitochondrial ribosomal proteins, Prognostic targets, Therapeutic targets
An introduction to mitochondrial ribosomal proteins
The mitochondrion is an essential sub-cellular organelle that generates energy for cellular function. The mitochondrial proteome comprises 1,500 proteins, and over 99% of these are nuclear encoded, including the 82 mitochondrial ribosomal proteins (MRPs), some of which will be the focus of this review [1, 2]. MRPs are synthesised in the cytoplasm of the cell and transported to the mitochondria, where they assist in ribosomal assembly. The mitochondrial ribosome is essential for the translation of messenger RNA (mRNA) within the mitochondria, and, like other ribosomes, the mitochondrial ribosome comprises two subunits with distinct roles. The small 28S subunit, comprised of 30 proteins encoded by 32 small MRP (MRPS) genes, facilitates the interaction between mRNA and transfer RNA (tRNA) [3, 4]. The large 39S subunit, consisting of 52 proteins encoded by 50 large MRP (MRPL) genes, promotes the formation of peptide bonds [3, 4].
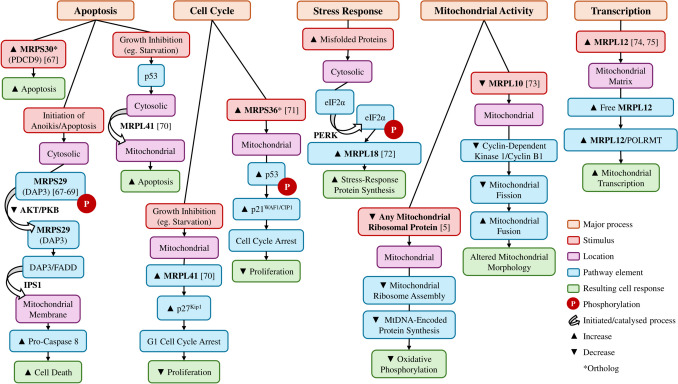
MRPs are involved in a variety of pathways crucial to cell function that extend beyond translation; from regulation of mitochondrial transcription and cell cycle to maintenance pathways, including apoptosis and cytosolic stress response (Fig. 1). As diverse pathways require MRP involvement, it is not surprising that dysfunction of these proteins has been implicated in various disorders, including cancer, neurodegenerative and metabolic disorders [5]. Without ribosomes, the mitochondria cannot produce key oxidative phosphorylation elements, and even reduced ribosome function can result in protein synthesis insufficient to maintain the basal metabolic rate [5]. Consequently, systemic disruption of MRP function can be lethal due to the critical role of mitochondrial ribosomes.
Fig. 1.
Normal cellular functions involving mitochondrial ribosomal proteins. Mitochondrial ribosomal proteins are involved in a wide range of cellular functions and responses, from apoptosis, cell cycle arrest, and stress response pathways to mitochondrial function and transcription. Although poorly characterised in humans, an increase in MRPS30 ortholog in mice fibroblasts increases apoptosis [6]. Cytosolic MRPS29 assists in activating anoikis in detached cells, through the extrinsic cell death activation pathway [6–8]. When cells are exposed to environmental changes inhibiting cell growth, MRPL41 stabilises p53 and supports its translocation into the mitochondria, where it induces apoptosis [9]. Additionally, under conditions inhibiting cell growth, MRPL41 also suppresses proliferation through G1 cell cycle arrest [9]. An increase in the MRPS36 ortholog in mice has been observed to promote the phosphorylation of p53 to induce cell cycle arrest and suppress tumour growth [10]. Cytosolic MRPL18 is increased as part of the cytosolic stress response, this then promotes increased synthesis of stress-response proteins [11]. Mitochondrial ribosomal proteins are required for mitochondrial-DNA encoded protein synthesis, which is critical for functional oxidative phosphorylation [5]. Decreased MRPL10 has been shown to decrease cyclin B1/Cdk1 activity, resulting in mitochondrial clustering and elongation [12]. Free MRPL12 within the mitochondrial matrix binds to POLRMT, an RNA polymerase, to increase promoter-dependent and -independent mitochondrial transcription [13, 14]
Mitochondrial ribosomal proteins in cancer metastasis
MRPs have been implicated in tumorigenesis across a broad range of cancer types, with altered expression resulting in an increase in tumour cell heterogeneity, survival and progression to metastasis [15]. The process of metastasis, where cancer cells spread from the primary tumour site to distal organs, requires cells to adapt to vastly different and often changing environments. Specifically, the mitochondria of invasive cancer cells must undergo metabolic shifts to enable them to grow, survive and colonise at distal sites [16]. Traditionally, cancer cells are thought to switch from an epithelial state to acquire mesenchymal features, a key characteristic of cancer cell development (EMT; epithelial to mesenchymal transition). However, how cancer cells acquire these metabolic and mesenchymal features is poorly understood. In this review, we will discuss the current literature surrounding MRPSs and MRPLs in the context of metastatic cancer.
Small mitochondrial ribosomal proteins
In total, five MRPSs have been implicated in metastasis of more than one cancer type, which equates to one seventh of all MRPSs (Table 1); whilst additional MRPSs have been associated with metastasis of only a single cancer type (one third of all MRPSs; see Supplementary Material). Of the MRPSs associated with more than one cancer type, MRPS23 has been the most well-studied, however studies have found a conflicting role for the protein within and between cancer types [17–23].
Table 1.
Overview of the small mitochondrial ribosomal proteins associated with metastasis in more than one cancer type
| MRPS | Associated Cancer(s) | Associated Protein(s) or Gene(s) | Associated Pathway(s) | Association of Metastatic Traits with Patterns of Expression |
|---|---|---|---|---|
| MRPS12 | Breast [24], Ovarian [25] | p53 [25] |
Cell cycle [25], PI3K/Akt/mTOR [25], Immune infiltration [25] |
|
| MRPS16 |
Ovarian [26], Lung [27] (LUAD (Proximal-proliferative subtype) & LUSC) |
HE4 [26] | ▲ Poor survival [26, 27] | |
| MRPS18B |
Endometrial [28], |
PIP3/AKT [30], Oestrogen signalling [30], |
▲ EMT [28, 29], ▲ Invasion [28, 29], ▲ Metastatic phenotype [29], ▲ Proliferation in endometrial cancer [28], ▼ Proliferation in prostate cancer [29] |
|
| MRPS23 |
Cervical [21], Breast [17–20, 22, 31] (Luminal B subtype [19]), Colorectal [32], Hepatocellular [23] |
Cell cycle [21] |
▼ Progression free survival [21], ▲ Proliferation [18–23], ▲ EMT [18, 22], ▲ Metastatic phenotype [19, 21, 23], ▼ Invasion in breast cancer [20], ▼ Metastatic phenotype in breast cancer [20], ▼ High grade in breast cancer [20], ▲ Methylation at k108me2 (SETD6) [20], ▼ Methylation at r21me1 (PRMT7) [20], ▲ Poor prognosis [32], |
|
| MRPS31 | Ovarian [26], Breast (Luminal A, Triple Negative) [30] |
Fatty acid oxidation [30], BDNF [30], Folate biosynthesis [30], EGFR1 [30], PIP3/AKT [30], Hedgehog signalling [30], Wnt signalling [30], Oestrogen signalling [30] |
▲ Poor survival [26], ▲ Poor progression free survival [26] ▲ Metastatic cell lines [30] |
▲ Increased expression of MRPS is associated with the trait; ▼ Decreased expression of MRPS is associated with the trait. Abbreviations: LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; EMT, epithelial to mesenchymal transition
An overview of the small mitochondrial ribosomal proteins associated with metastasis in only one cancer type can be found in Supplementary Table 1
MRPS23
Lyng et al. (2006) first identified an association between MRPS23 expression and metastasis in cervical cancer [21]. Specifically, MRPS23 was upregulated in cervical tumours with increased recurrence and lymph node metastases, and was associated with rapid growth and invasive capacity [21]. Moreover, a rat breast cancer model found similar results; long-term MRPS23 depletion reduced tumour growth and metastasis [18] and the authors suggested that MRPS23 may be involved in reversing the apoptotic pathway to assist metastatic progression [18]. Investigations in patient breast cancer samples have observed heightened MRPS23 expression in high-grade, aggressive tumour samples [19], as well as an increase in tumour compared to normal tissue [22]. Although both studies did not reveal an association with patient survival, altered expression levels support a potential role of MRPS23 in an aggressive breast cancer phenotype [19, 22]. Intriguingly, Oviya and colleagues (2021) found a breast cancer-specific MRPS23 isoform (or altered post-translational modification) that was expressed almost exclusively in breast cancer samples [22]. This altered post-translational modification was later defined to be methylation of two sites within MRPS23 (K108me2, R21me1) and was observed to alter the expression of the resulting protein, with the rate of oxidative phosphorylation dependent on MRPS23 expression [20]. In contrast to the aforementioned studies, they also found that low MRPS23 was associated with high-grade disease and some metastatic traits [20]. Additionally, hypermethylation of MRPS23 was associated with poor survival in breast cancer patients in a separate study [31], thus further supporting the suspected role of epigenetic regulation of MRPS23 in breast cancer progression [31].
MRPS23 also has prognostic value in colorectal cancer, where it was identified as one of 12 prognostic RNA binding proteins [32]. Although expression was increased in colorectal cancer, and was considered prognostic, the authors considered this expression profile to be low-risk (HR = 0.589) and did not elucidate the specific function of MRPS23 in colorectal cancer progression [32]. In hepatocellular carcinoma, increased MRPS23 expression is associated with larger, later stage tumours, and poor survival [23]. Despite this, knockdown has not been shown to effect the metastatic capability of hepatocellular carcinoma cells (unlike its effects in breast cancer), suggesting the change in expression may be a response to increased metastatic capacity, not causative [18, 23]. These results could also suggest that modifications to the expression of MRPS23 may only occur in, or only provide a survival advantage in certain cancer types, such as breast cancer.
MRPS12
Another MRPS associated with metastasis, which is a promising prognostic and therapeutic target is MRPS12. Increased expression has been consistently linked to metastatic traits as well as therapeutic resistance [24, 25]. Sotgia and colleagues (2017) investigated the association between nuclear-encoded mitochondrial-associated genes and high-risk estrogen receptor-positive breast cancer and found that increased expression of MRPS12 was associated with tumour recurrence and tamoxifen-resistance [24], both of which are precursors to metastatic disease. Likewise, in ovarian cancer, the expression of MRPS12 is also associated with recurrence and advanced disease stage [25]. Gene set enrichment analysis of 426 ovarian cancer tumours from The Cancer Genome Atlas (TCGA) revealed MRPS12 overexpression was correlated with the activation of biological pathways such as cell cycle activation, PI3K/Akt/mTOR, and p53 [25]. Association with these pathways suggests that MRPS12 may be involved in limiting apoptosis in cancer cells, which may explain the increased tumour recurrence in breast and ovarian cancers.
In terms of therapeutics, MRPS12 has been associated with tamoxifen resistance in breast cancer [24], where combination therapies including tigecycline inhibited proliferation and enabled selective toxicity in cancer cells [33, 34]. In ovarian cancer, tigecycline improves response to chemotherapy in chemo-resistant and metastatic cells, and has minimal effect on normal ovarian cells, which can be attributed to a greater reliance on oxidative phosphorylation in these cells [35]. Interestingly, in the same study, tigecycline was found to suppress a range of signalling pathways, including mTOR signalling, and the cell cycle [35], which were upregulated in response to increased MRPS12 expression in another study [25]. Further investigation into the effects of tigecycline and other tetracycline analogues on MRPS12 function may provide insight into this potential relationship.
MRPS16
Although the literature supporting a role for MRPS16 is limited, there is evidence that this MRP may also prove to be a useful biomarker. In high-risk ovarian and lung cancers, the use of MRPS16 as a biomarker of cancer progression has been demonstrated [26, 27]. Xu et al. (2021) examined the expression of six MRPs in a variety of ovarian cancer datasets, including TCGA, GTEx (Genotype-Expression Tissue Portal), and Oncomine, and found a strong positive correlation between increased MRPS16 and poor survival in ovarian cancer [26]. Notably, MRPS16 was upregulated in response to HE4 (human epididymis protein 4), an established ovarian cancer biomarker associated with metastasis [26]. MRPS16 was among 40 mitochondrial-associated genes previously found to have genomic copy number variations in lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) [27]. Interestingly, MRPS16 expression was consistently upregulated across both LUAD (ncases = 533; ncontrols = 59) and LUSC (ncases = 502; ncontrols = 49) TCGA samples, with significant differences between LUAD subtypes [27]. It is well documented that LUAD subtypes are differentiated by expression signatures and clinical outcomes [17]. This study by Hertweck et al. (2023) identified significant upregulation of MRPS16 in the proximal-proliferative subtype compared to both the proximal-inflammatory and terminal respiratory unit groups [27]. Given the features of these subtypes, this suggests that MRPS16 may contribute to decreased infiltration and increased proliferation in LUAD.
MRPS18B
One of the most interesting groups of MRPSs is the MRPS18 protein family which is comprised of three isoforms (MRPS18A, MRPS18B, MRPS18C) that bind to different sites of the mitochondrial ribosome [36]. MRPS18A is upregulated in non-small cell lung cancer (NSCLC) subgroups, while upregulation of MRPS18C is associated with poor prognosis in breast cancer [27, 31] (see Supplementary Material). Comparatively, MRPS18B has been associated with more than one cancer type. An increase in MRPS18B expression is also associated with EMT and metastatic capacity in endometrial and prostate cancer [28, 29]. Two studies by the same group used patient endometrium and prostate samples, as well as cell line and animal models, to study the relationship between MRPS18B and metastatic capacity [28, 29]. In endometrial cancer, they found that expression of MRPS18B was positively correlated with free E2F1 in 84 patient samples, with expression of both proteins significantly increased compared to normal and hyperplasia samples [28] [E2F1 is a potential driver of metastasis and is highly expressed in late-stage endometrial tumours [37]]. Additionally, morphological changes in endometrial cancer cells expressing high levels of MRPS18B suggested progression of EMT, which was not observed in cells with lower MRPS18B expression [28]. In mouse endometrial cancer xenografts, MRPS18B overexpressing cells produced larger and more vascularised tumours, suggesting high expression increases proliferation and tumour aggressiveness in vivo [28]. Whereas, using a prostate cancer zebrafish xenograft model, experimental fish developed smaller tumours that migrated faster compared to those microinjected with low MRPS18B expressing cells [29]. The same study also found that increased expression of MRPS18B improved the migratory ability of prostate cancer cells through the induction of EMT, via chemokine signalling (CXCL12-CXCR4), and increased EMT transcription factor (TWIST2) expression [29]. Increased MRPS18B was correlated with disease stage, which suggests that EMT-induced migration increases metastasis [29]. In breast cancer, pathway enrichment analysis found that MRPS18B had roles in cancer-associated pathways including PIP3/AKT, oestrogen signalling, cell cycle, and circadian rhythm [30], however functional studies are needed to understand how MRPS18B impacts these pathways. Given the clear evidence that MRPS18B plays a crucial role in EMT progression and the development of metastatic capacity in multiple cancers, there is a clear need for further mechanistic studies manipulating MRPS18B expression to test its utility as a therapeutic target. The potential use as a biomarker of advanced disease requires larger cohort studies to understand the extent to which MRPS18B contributes to cancer progression, more broadly.
MRPS31
In ovarian and breast cancer, MRPS31 is known to interact with metastasis-associated proteins [26, 30]. Specifically, MRPS31 expression is associated with the established ovarian cancer biomarker, HE4 [26] and increased expression is associated with poor survival and poor progression free survival in ovarian cancer [26]. Which together suggests that it plays a role in ovarian tumour recurrence. In breast cancer, MRPS31 is overexpressed in metastatic cell lines (MDA-MB231, MDA-MB468), and is known to interact with ACADSB [30] [ACADSB is involved in fatty acid oxidation and has been associated with EMT and progression in a range of cancers, including breast cancer [38–41]]. In triple negative breast cancer, MRPS31 also interacts with CES1 and NPAS2, both of which are involved in pathways that have been implicated in the progression and metastasis of a variety of cancers [30]. Additionally, there are multiple reports that MRPS31 is associated with a range of other pathways associated with breast cancer progression, including PIP3/AKT, hedgehog signalling, and wnt signalling [30, 42–44].
MRPS Summary
In summary, over half of all MRPSs have been associated with metastatic capacity. Of the five that have been associated with multiple cancer types, MRPS23 presents as an interesting candidate for further study. Given the broad foundation of work that has been performed across breast, cervical and hepatocellular carcinoma, as well as an association with numerous metastatic traits, this gene and protein clearly plays a role in metastatic capacity. Furthermore, there is compelling emerging evidence that increased MRPS12, MRPS16 and MRPS18B expression and association with metastatic capacity are observed across five different cancer types, collectively. Therefore, it is apparent that MRPSs contribute to disease aggressiveness across the cancer spectrum.
Large mitochondrial ribosomal proteins
Of the 52 proteins encoded by the large MRP genes, almost one quarter have been implicated in metastasis of more than one cancer type (Table 2) and the same proportion of MRPLs have been associated with metastasis of a single cancer type (see Supplementary Material). One of the most promising prognostic targets of cancer metastasis is MRPL4, with studies consistently demonstrating that increased expression is associated with a higher risk of metastasis in both breast and prostate cancer [24, 45, 46].
Table 2.
Overview of the large mitochondrial ribosomal proteins associated with metastasis in more than one cancer type
| MRPL | Associated Cancer(s) | Associated Protein(s) or Gene(s) | Associated Pathway(s) | Association of Metastatic Traits with Patterns of Expression |
|---|---|---|---|---|
| MRPL1 |
Breast [31], Lung [47] (LCLC), Colorectal [48] |
SLC25A10 [48], Metastasis inhibition network (MRPL19, MRPL20, MRPL37, MRPL38, MRPL39, MRPL50, ICT1) [48] |
▲ Poor Prognosis [31], ▲/▼ Risk of metastasis in lung cancer [47], ▼ Risk of metastasis in colorectal cancer [48] |
|
| MRPL4 | Breast [24, 46] (ER + [46]), Prostate [45] | Part of a prognostic nine gene signature (MRPL3, MRPL13, MRPL15, MRPL17, MRPL18, MRPL24, MRPL46, MRPL48) [46] |
RNA/mRNA binding [45], Ribosome signalling [45] |
▲ Recurrence [24, 46], ▲ Risk of metastasis [45], ▲ Poor survival [45], ▲ Distant metastasis [46] |
| MRPL9 |
Lung [49], Hepatocellular [50] |
E-cadherin [49], Part of a prognostic two gene signature (SMG5) [50] |
c-MYC signalling [49], Cell cycle [50], Mismatch repair signalling [50], Spliceosome signalling [50], Immune infiltration [50] |
▼ Poor sphere formation [49], ▲ Risk of metastasis [49], ▲ Poor survival [49, 50], ▲ Recurrence [49, 50],▼ Poor proliferation [49, 50], ▼ Poor migration [49, 50], ▲ Drug resistance [50], |
| MRPL10 |
Ovarian [26], Lung [27] (LUAD; Proximal-proliferative subtype & LUSC; Classical subtype) |
HE4 [26] |
▲ Progression free survival [26], ▲ Poor survival [27] |
|
| MRPL12 |
Lung [51] (LUAD), Breast [52] |
Immune infiltration [51] |
▲ Poor prognosis [51], ▼ Poor proliferation [51], |
|
| MRPL13 |
(Triple-negative breast cancer [54, 55]), Lung |
Part of multiple prognostic gene signatures [46], Bcl-2 [56] |
▲ Recurrence [24, 46, 54, 52, 55, 57], ▲ Poor Prognosis [31], ▲ Distant metastasis [46], ▼ Poor cell viability [52], ▼ Poor migration [52, 55, 57], ▲ Advanced stage [53, 55], ▲ Poor survival [53–57], ▲ Risk of metastasis [53, 55, 57], ▲ Proliferation [55–57], ▲ EMT [55, 57], |
|
| MRPL15 | Breast [24, 46] (ER + [46]), Ovarian [26] |
Part of multiple prognostic gene signatures [46] |
Cell cycle [26] |
▲ Drug resistance [24], ▲ Recurrence [24, 46], ▲ Poor progression free survival [26], |
| MRPL19 |
Colorectal [48], Lung [58] (LUAD) |
Metastasis inhibition network (MRPL20, MRPL37, MRPL38, MRPL39, MRPL50, ICT1) [48] |
Proliferative signalling pathways [58], Cell cycle [58], Immune infiltration [58] |
▼ Risk of metastasis in colorectal cancer [48], ▲ Risk of metastasis in LUAD [58], ▲ Invasion [58], ▲ Migration [58], ▲ Proliferation [58], ▲ Poor Survival [58], ▲ High grade [58] |
| MRPL20 |
Colorectal [48], Prostate [59] |
Metastasis inhibition network (MRPL19, MRPL37, MRPL38, MRPL39, MRPL50, ICT1) [48] |
Hormone independence [59] |
▼ Risk of metastasis [48], ▲ Androgen independence [59] |
| MRPL35 |
Colorectal [62], |
▲ Poor prognosis [60], ▲ Increased tumour size [60], ▼ Poor invasion [60], ▼ Low glutamine metabolism [60], ▼ Poor viability [60], ▲ Poor survival [60, 62, 63], ▼ Apoptosis [60, 62, 63], ▼ Poor proliferation [60–63], ▲ Metastasis [60, 63], ▼ Slowed tumour progression [61], ▼ Poor colony formation [62], ▼ DNA damage [62], ▼ Increased ROS [62], ▼ Poor tumour formation [63] |
||
| MRPL36 |
Ovarian [26], (ER-/Basal [46]) |
HE4 [26], Part of a prognostic six gene signature (MRPL13, MRPL22, MRPL41, MRPL42, MRPL54) [46], Part of a prognostic four gene signature (FEZ1, BMF, AFG1L) [65] |
▲ Poor progression free survival [26], ▲ Risk of metastasis [26, 65], ▲ Distant metastasis [46], ▲ Mutation burden [65], ▼ Drug resistance [65], ▲ Advanced stage [65] |
|
| MRPL37 |
Lung [27] (LUAD; Terminal respiratory unit), Colorectal [48] |
MRPL1[48], SLC25A10 [48], Metastasis inhibition network (MRPL19, MRPL20, MRPL38, MRPL39, MRPL50, ICT1) [48] |
▼ Favourable prognosis [27], ▼ Risk of metastasis [48] |
|
| MRPL38 |
Lung [47] (LCLC), Colorectal [48], Ovarian [66] |
MRPL1 [48], SLC25A10 [48], Metastasis inhibition network (MRPL19, MRPL20, MRPL37, MRPL39, MRPL50, ICT1) [48] |
▼ Risk of metastasis in colorectal and lung cancer [47, 48], ▲ Risk of metastasis in ovarian cancer [66], ▲ Migration [66] |
|
| MRPL39 |
Ovarian [26], Lung [47] (LCLC), Colorectal [48] |
Metastasis inhibition network (MRPL19, MRPL20, MRPL37, MRPL38, MRPL50, ICT1) [48] |
||
| MRPL42 |
(ER-/Basal [46]), Lung [67] (LUAD) |
Part of a prognostic six gene signature (MRPL13, MRPL22, MRPL36, MRPL41, MRPL54) [46], |
Cell cycle [67] |
▲ Recurrence [24], ▲ Distant metastasis [46], |
| MRPL44 | Breast [24], Thyroid [68] | Oxidative Phosphorylation [68] | ▲ Recurrence [24], ▲ Risk of metastasis [68] | |
| MRPL49 | Lung [27] (LUAD & LUSC), Breast [69] | ▲ Advanced stage [27], ▼ Invasion [69] | ||
| MRPL54 |
Breast [46] (ER-/Basal) |
Part of a prognostic six gene signature (MRPL13, MRPL22, MRPL36, MRPL41, MRPL42) [46], Part of a prognostic six gene signature (CNOT6, UPF3B, ZC3H13, IFIT5, PPARGC1A) [70], Part of a prognostic four gene risk model (EZH2, PPARGC1A, EIF2AK4) [71] |
▲ Distant metastasis [46] |
▲Increased expression of MRP is associated with the trait; ▼ Decreased expression of MRP is associated with the trait. Abbreviations: LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; ER, estrogen receptor; NSCLC, non-small cell lung cancer; LCLC, large cell lung cancer; EMT, epithelial to mesenchymal transition
An overview of the large mitochondrial ribosomal proteins associated with metastasis in only one cancer type can be found in Supplementary Table 1
MRPL4
In prostate cancer, comparison of expression between benign prostatic hyperplasia and low- and high-risk primary prostate cancer samples, revealed 56 proteins, including MRPL4, which were highly expressed in the high-risk samples compared to the other two groups [45]. MRPL4 gene expression in high-risk samples was also more than double that of low-risk and benign prostatic hyperplasia samples [45]. Interestingly, expression of MRPL4 was found to have a greater association with high-risk samples than AMACR, an established biomarker of prostate cancer [72], with MRPL4 overexpression also associated with increased mortality and poorer survival [45, 72]. Similarly, Sotgia et al. (2017) observed, through an informatics-based approach, that MRPL4 expression was associated with tumour recurrence and hormone resistance in breast cancer, further suggesting a role in aggressive cancer phenotypes [24]. Microarray data from 3,951 breast cancer tumours additionally revealed that increased expression of MRPL4 was associated with distant metastasis in estrogen-receptor positive patients [46].
MRPL13
In addition to MRPL4, MRPL13 has been widely studied across breast and lung cancer and holds promise as a putative metastatic prognostic target. MRPL13 was first identified as a potential prognostic biomarker in breast cancer [24]. Using an informatics-based approach, one study observed that MRPL13 expression was associated with breast cancer tumour recurrence and hormone resistance across several Gene Expression Omnibus (GEO) datasets (n = 3,455) [24]. Analyses of breast cancer TCGA data showed high MRPL13 expression correlated with poor prognosis, as well as advanced tumour stage, increased immune infiltration and metastasis [53]. Analysis of microarray data from 3,951 breast cancer tumours also found that recurrence and distant metastasis were associated with increased MRPL13 [46]. These findings indicate that increased MRPL13 expression likely contributes to aggressive cancer phenotypes with potential clinical applications in predicting response to therapy [31, 46, 53, 54]. Furthermore, in vitro studies have found that downregulation of MRPL13 inhibited migration and decreased cell viability in bone and lung metastasised breast cancer cell lines [52]. Another study found that MRPL13 knockdown inhibits invasion in both non-invasive (MCF-7, T47D) and invasive (MDA-MB-231) breast cancer cells [53, 55], partly through diminished EMT processes [55], which suggests that MRPL13 expression may contribute to the acquisition of metastatic traits in breast cancer. Upregulation of MRPL13 is also associated with lung cancer [54, 56, 57]. Specifically, a TCGA Pan-Cancer Atlas analysis revealed that MRPL13 is significantly upregulated in LUAD patient samples [54]. Likewise, an additional study found increased MRPL13 mRNA and protein expression in NSCLC tumours compared to normal tissue, with the highest expression observed in a metastatic NSCLC cell line (H1299) [56]. This study also found an association between MRPL13 expression and MYC, PI3K/AKT/mTOR, metabolism, cell cycle pathways, and increased proliferation, suggesting links to cancer progression and potentially EMT promotion [56]. This was additionally supported by analysis of the TCGA LUAD dataset that found MRPL13 expression was associated with immune infiltration, metastasis, and EMT, with gene knockdown in vitro decreasing cell survival and metastasis, and increasing apoptosis [57]. Furthermore, Zhong et al. (2023) found that by employing MRPL13 as a diagnostic biomarker, the accuracy of diagnostic predictions across multiple cancer types, including LUAD, were improved [57], which is a promising area of future research.
Recently, Li et al. (2023) suggested the use of synthetic nucleic acid drugs to disrupt the production of the MRPL13 protein (as well as MRPL9; see relevant section below) [49]. The use of synthetic nucleic acids as therapeutics is relatively new but provides a compelling opportunity to directly target genes that promote metastatic capacity in cancers. The therapy selectively targets tissues or cells with oligonucleotides that only bind to target RNA, which modulates function or promotes degradation, thus allowing the synthesis of specific proteins to be inhibited [73]. Several antisense oligonucleotide therapies have been approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA), although none are currently approved for cancer treatment [74]. Therefore, repurposing them to target MRPs, such as MRPL13 and MRPL9 in aggressive lung cancer is a promising area of future research. We suggest that studies should firstly aim to assess the underlying mechanisms by which these MRPLs promote metastasis using in vitro models. Such therapies can then be tested in these models to determine whether they specifically target MRPL9 and MRPL13, and whether they adequately suppress the metastatic phenotype, before they can enter clinical trials.
MRPL36
MRPL36 is another example of a promising risk predictor, particularly in relation to breast and ovarian cancer metastasis [26, 46, 65]. Recently, MRPL36 was included in a four gene breast cancer risk prediction model and, of the four genes, was determined to be the most predictive of risk [65]. This risk score was capable of predicting not only survival of breast cancer patients, but also tumour stage, including metastasis [65]. In a second study, increased expression of MRPL36 was associated with distant metastasis in estrogen-receptor negative breast cancer [46]. Similarly, Xu et al. (2021) revealed an apparent association between high MRPL36 expression and upregulation of HE4 in ovarian cancer [26]. Collectively, increased expression of MRPL36 has been linked to a wide variety of metastatic traits (Table 2), thus further evidence of its importance in cancer metastasis across a broader range of cancer types would be of value.
MRPL35
As for the most promising MRPL therapeutic targets, studies have targeted MRPL35 and MRPL42 with 18β-glycyrrhetinic acid and a YYI inhibitor, respectively. Increased MRPL35 expression is associated with poor survival outcomes across NSCLC, colorectal, and gastric cancers [60–64]. In NSCLC, high expression is correlated with advanced stage and metastasis, with knockdown inhibiting proliferation, invasion, and glutamine metabolism [60]. The authors found that MRPL35 knockdown caused downregulation of SLC7A5 [60], which is a lung cancer-specific prognostic biomarker [75]. Additionally, knockdown promoted apoptosis and impeded proliferation, likely through the modulation of several cell regulatory proteins (CDK1, BIRC5, CHEK1, STMN1 and MCM2) and the activation of the p53 signalling pathway [61]. In colorectal cancer, downregulation of MPRL35 increased reactive oxygen species production leading to DNA damage, cell cycle arrest, decreased mitochondrial membrane potential, and ultimately, apoptosis [62]. As a result, knockdown of MRPL35 inhibited proliferation and colony formation, in vitro and in vivo, using a xenograft mouse model [62]. In gastric cancer, increased expression of MRPL35 is associated with metastasis and advanced stage, with in vivo knockdown inhibiting tumour formation and promoting apoptosis [63]. Later investigation by the same authors found that treatment of gastric cancer cell lines (BGC-823, MGC80-3) with 18β-glycyrrhetinic acid, a promising anti-inflammatory and antioxidant agent, inhibited MRPL35 expression and induced cancer cell apoptosis and cell cycle arrest [64]. Together, these findings suggest MRPL35 may have an application as a therapeutic target across a range of cancers, however further investigations beyond gastric cancer are required.
MRPL42
In LUAD, MRPL42 is known to support metastatic properties, and is crucial for tumour growth and development [67]. Jiang and colleagues (2021) demonstrated that knockdown of MRPL42 decreased migration, invasion, and proliferation in LUAD cells of varying metastatic capacities [67]. Additionally, expression of MRPL42 is correlated with the presence of lymph node metastases and tumour size in patient samples [67]. This study found that the transcription factor, YY1, was likely responsible for increased MRPL42 expression, with knockdown of YY1 associated with decreased MRPL42 expression [67]. Consistent with these findings, high levels of MRPL42 are associated with tumour recurrence in breast cancer patients and are predictive of distant metastasis in estrogen-receptor negative basal breast cancer [24, 46], as shown in Table 2. We suggest that if MRPL42 expression could be therapeutically targeted through the inhibition of YY1 [67], then the size of MRPL42-overexpressing LUAD tumours could be reduced and the presence of lymph node metastases significantly diminished [47]. The same may be possible in breast cancer, where MRPL42 also appears to play a significant role in aggressive disease.
MRPL1
Although the literature supporting a role for MRPL1 is limited, there is evidence that this MRP may also prove to be a useful biomarker. MRPL1 has been implicated in the progression of breast, colorectal, and large cell lung cancer (LCLC) [31, 47, 48]. In breast cancer, through the analysis of 1,056 TCGA tumours, increased expression of MRPL1 was associated with poor prognosis and over 50% increased risk of mortality [31]. Conversely, a broad investigation into metastasis-associated genes in colorectal cancer proposed that MRPL1 was linked to decreased metastatic risk via SLC25A10 [48] [SLC25A10 is a mitochondrial translocation protein that is often overexpressed in tumour cells, and is associated with rapid proliferation [76]]. This suggests that MRPL1 may play a role in excessive proliferation in primary cancers, in turn suppressing the migratory capacity of metastatic cells. Analysis of mitochondrial protein profiles from LCLC cell lines with varying metastatic potential, revealed 64 differentially expressed proteins, including MRPL1 [47]. Though, contrary to the findings in breast and colorectal cancer, the role of MRPL1 in LCLC remains inconclusive.
MRPL9
In lung cancer and hepatocellular carcinoma, MRPL9 overexpression is associated with poor survival and recurrence, and knockdown in an in vitro cell model inhibited pro-metastatic capabilities such as migration, spheroid formation, and proliferation [49, 50]. MRPL9 was found to modulate c-MYC transcription, which alters ZEB1 expression to regulate the expression of e-cadherin in lung cancer [49]. High MRPL9 expression is known to be associated with decreased e-cadherin expression, which is essential for cancer-specific EMT [77]. In hepatocellular carcinoma, MRPL9 was used in a two gene prognostic model that could predict prognosis, immune infiltration, and chemoresistance [50]. This model was effective in identifying patients suitable for immunotherapy and those who were likely to have better survival outcomes post chemotherapy [50]. MRPL9 was found to be associated with chemoresistance pathways in hepatocellular carcinoma, including cell cycle, mismatch repair, and spliceosome signalling [50]. Together, these studies suggest MRPL9 may play a key role in aggressive cancer phenotypes and could be a viable therapeutic target.
MRPL10
MRPL10 has been linked to lower tumour recurrence in ovarian cancer, however it has also been associated with aggressive molecular subtypes in LUAD and LUSC [26, 27]. In ovarian cancer, through gene expression analysis of tumours, significant upregulation of MRPL10 in response to HE4 overexpression has been observed, although MRPL10 overexpression was also correlated with progression free survival [26]. This suggests that increased expression of MRPL10 may be a protective response to upregulation of HE4 in ovarian cancer. Whereas, high expression of MRPL10 was observed to correlate with aggressive molecular subtypes of both LUAD and LUSC, through an analysis of TCGA data by Hertweck and colleagues (2023) [27]. In LUAD, MRPL10 expression was higher in the proximal-proliferative subtype, which is often associated with poorer outcomes [27, 78, 79]. Similarly, in LUSC, higher MRPL10 expression was observed in the classical subtype [27], which is often associated with drug resistance [79, 80]. Thus, these studies suggest that the role of MRPL10 may vary between cancer types.
MRPL12
Knockdown of MRPL12 is associated with decreased metastatic capacity, such as proliferation, migration and cell viability, in aggressive lung and breast cancer cell lines [51, 52]. This response to loss of MRPL12, in both LUAD and breast cancer, suggest that it plays a key role in promoting metastatic behaviour and tumour growth. Further supporting this, the same study found that high expression of MRPL12 was associated with worse prognosis, immune infiltration and poor survival in LUAD tumours [51, 52]. Due to MRPL12’s association with prognostic factors and metastatic capacity in breast cancer, Liu et al. incorporated MRPL12 into a three-protein prognosis prediction signature, alongside MRPL13 and POP1 [52]. Combined with age and stage data, this signature was able to determine short-term survival for breast cancer patients and highlighted these genes as possible therapeutic targets [52].
MRPL15
MRPL15 has shown prognostic potential in both breast and ovarian cancers, with expression correlating with recurrence and risk of metastatic disease [24, 26, 46]. Among 12 MRPs examined in breast cancer, MRPL15 had the best prognostic value [24]. Additionally, MRPL15 was incorporated into a four gene signature, where patients with a high signature expression were over five times more likely to experience tumour recurrence and over three times more likely to develop metastasis, compared to those with a low signature expression [24]. Similarly, Xu and colleagues (2021) demonstrated the potential use of MRPL15 as an ovarian cancer prognostic biomarker and therapeutic target [26]. MRPL15 was consistently highly expressed in ovarian tumours compared to normal tissue; significantly associated with late stage disease, and was associated with poor survival when compared to patients with low MRPL15 expression [26]. Additionally, MRPL15 is known to correlate with HE4, and their interaction is thought to promote oncogenesis via increased metastatic capacity and drug resistance [26].
MRPL19
In LUAD, MRPL19 has been suggested as a potential biomarker as it is associated with markers of cancer progression and metastasis [58]. Upregulation of MRPL19 in LUAD samples is associated with poor prognosis, including increased differentiation, tumour stage, and metastasis [58]. Notably, knockdown of MRPL19 in LUAD cells inhibited cell growth, migration, and invasion, further supporting links to metastatic capacity [58]. This study additionally identified correlations between MRPL19 expression and proliferative signalling pathways, as well as cell cycle, adhesion molecules, and immune infiltration pathways [58]. Considered together, these findings provide a very compelling connection between MRPL19 and metastasis in LUAD cells. Conversely, a broad investigation into metastasis-associated genes in colorectal cancer proposed that MRPL19 was associated with decreased metastasis risk through SLC25A10 and MRPL1 [48]. This suggests that the role of MRPL19 may be multifaceted, with different functions across a range of cancers.
MRPL20
Differential expression of MRPL20 has been observed in prostate and colorectal cancers [48, 59]. In prostate cancer, comparison of gene expression profiles between newly-diagnosed, androgen-dependent and androgen-independent primary tumours from patients with metastatic disease [59], revealed higher MRPL20 expression in androgen-independent samples, suggesting a possible role in aggressive prostate cancer [59]. Conversely, MRPL20, was found to limit metastasis in colorectal cancer [48].
MRPL37
In LUAD, MRPL37 appears to contribute to more aggressive tumour subtypes, while it has been indicated as part of a metastasis inhibition network in colorectal cancer [27, 48]. Upregulation of MPRL37 has been observed in both LUAD and LUSC cases compared to unaffected controls [27] and downregulation of MRPL37 was significantly associated with the terminal respiratory unit subtype in LUAD [27], which has a favourable prognosis. This suggests that upregulation of MRPL37 may contribute to aggressive lung tumour subtypes [17]. Contrarily, MRPL37 was associated with metastasis inhibition in colorectal cancer, with interactions with SLC25A10 through MRPL1 also identified [48].
MRPL38
MRPL38 is associated with fewer metastatic lesions in both lung and colorectal cancers [47, 48]. Analysis of mitochondrial protein profiles from LCLC cell lines, revealed that cells with high metastatic potential had lower MRPL38 expression compared to cells with low metastatic potential [47]. Likewise, in colorectal cancer, analysis of genes associated with metastasis found that MRPL38 was associated with lower levels of metastasis through SLC25A10 [48]. Conversely, MRPL38 is associated with heightened metastatic capacity in ovarian cancer cells, where a more invasive cell line exhibited a three-fold increase in expression compared to a paired non-invasive cell line [66]. These findings suggest a more nuanced role for MRPL38 in metastatic progression, which is dependent on tumour characteristics.
MRPL39
Studies in ovarian, lung, and colorectal cancers, provide some insight into the potential mechanisms of MRPL39 in tumour progression and metastasis [26, 47, 48]. In ovarian cancer, MRPL39 was among the top six MRPs whose expression correlated with tumorigenicity [26], including advanced stage and poor survival [26]. A study in LCLC cells by Liu et al. (2019), revealed 64 differentially expressed proteins, including MRPL39, when comparing cells with low and high metastatic potential [47]. Higher levels of MRPL39 were observed in cells with lower metastatic potential, suggesting a tumour suppressive role [47]. Additionally, this study proposed that MRPL39 functions in a similar manner to TRAP1, which is a regulator of mitochondrial respiration. High expression of TRAP1 in LUAD is known to increase proliferation but inhibit metastasis [47, 81]. Notably, in a colorectal cancer study, MRPL39 was associated with reduced metastasis [47, 48, 81]. As previously mentioned, this study uncovered metastasis inhibiting protein–protein interactions, connecting MRPL39 to SLC25A10, through MRPL1 [48]. These findings indicate that MRPL39 favours metastatic progression, particularly in ovarian cancer, but appears disadvantageous to metastasis in others, such as colorectal and lung cancers.
MRPL44
In thyroid cancer, patients with unaltered MRPL44 expression between tumour and benign tissue were observed to have an increased risk of lymph-node metastasis compared to those with decreased expression in tumour tissue [68]. Additionally, low MRPL44 expression correlated with a glycolytic metabolic phenotype and a lower risk of metastasis, while high expression indicated a combined oxidative phosphorylation and glycolysis phenotype [68]. The latter may confer a growth advantage thereby increasing metastatic potential [68]. MRPL44 expression positively correlated with the expression of proteins involved in electron transport, mitochondrial metabolism, and apoptosis, providing a potential mechanism for metabolic control. Similarly, one study observed a correlation between high MRPL44 expression and an increased risk of tumour recurrence in breast cancer [24]. These studies indicate a role for MRPL44 in the promotion of metastasis, however investigation into the effects of altered expression on metabolism and hormone resistance may elucidate its exact role.
MRPL49
Altered MRPL49 expression has been observed in breast and lung cancer, with a potential link to metastasis [27, 69]. In breast cancer, MRPL49 expression appears to support tumour development, but reduces metastatic capacity [69]. Specifically, a study observed upregulation of MRPL49 in non-invasive breast cancer cells, and downregulation in invasive cells compared to normal breast epithelial cells [69]. This suggests that MRPL49 modulates invasive capacity, perhaps through control of mitochondrial metabolism. Consistent with the Warburg effect, primary non-invasive cells may utilise oxidative phosphorylation to increase proliferation, with a switch towards glycolysis in invasive cells [82]. Interestingly, enrichment of the glycolysis pathway correlated with EMT in primary breast cancer, which could suggest that downregulation of MRPL49 in invasive cells is linked to EMT and metastatic progression [69, 83]. However, an alternate pattern of MRPL49 expression was observed in a study utilising TCGA lung cancer data [27]. Specifically, MRPL49 expression was increased in late-stage LUAD and LUSC tumours compared to normal lung samples [27]. These studies may indicate that cancer cells require varying metabolic needs during different stages of tumour progression and further research is needed to elicit the potential role of MRPL49 in mitochondrial metabolism and cancer.
MRPL54
MRPL54 expression has been used to predict outcomes in hepatocellular carcinoma and basal breast cancer [46, 70, 71]. MRPL54 was used in two separate survival risk prediction gene signatures in hepatocellular carcinoma patients [70, 71]. One of these studies additionally found that MRPL54 overexpression was a protective factor associated with better outcomes, with the authors suggesting it may function as a tumour suppressor gene [70]. In oestrogen receptor-negative basal breast cancer, MRPL54 was part of a six gene signature that could effectively predict distant metastasis, recurrence, and overall survival [46]. Further investigation to understand the function and mechanism by which MRPL54 may contribute or prevent cancer progression is warranted.
MRPL Summary
In summary, 12 MRPLs have been associated with lung cancer, 11 with breast, seven with colorectal, six with ovarian, two with prostate, two with liver (hepatocellular carcinoma), and only one with thyroid and one with gastric. Many of the MRPLs discussed throughout this review have conflicting evidence in the literature between cancer types. For example, in ovarian cancer, increased MRPL10 expression may have a protective response [26], whereas in lung cancer, high expression is associated with poorer outcomes and drug resistance [27, 78, 79]. Therefore, further investigation into the pathways effected by MRPL10 expression would assist in clarifying the protein’s role in cancer progression across the cancer spectrum. Likewise, for the other MRPLs that appear to have different functions between cancer types (e.g. MRPL19, MRPL37 and MRPL49). Studies suggest that differences in tumour microenvironment, metabolic phenotype, or cell type may alter their role in cancer progression. Conversely, MRPL genes with consistently established associations between expression and metastasis-related traits across a variety of cancer types (e.g. MRPL4, MRPL35 and MRPL42), are putative prognostic and therapeutic targets as they would have broad applicability across the cancer spectrum, thus directly benefit more patients. Although there are a number of MRPLs (and MRPSs) that have conflicting findings between cancers or have only been associated with one cancer type (as shown in the Supplementary Material), where strong evidence of therapeutic value has been demonstrated, these putative therapeutic targets remain worthy of investigation given the dearth of targeted options currently available for advanced disease.
Therapeutically targeting mitochondrial-associated ribosomal proteins in metastatic cancer
There is currently no cure for metastatic cancer and there are very few treatment strategies available for patients with advanced high-grade disease. Therefore, there is an urgent need to find a cure for this devastating condition. We believe that therapeutically targeting markers of metastasis, such as MRPs, is crucial to preventing disease progression. Although the use of MRPs as therapeutic targets in metastatic cancer has shown great promise, there are currently very few studies in the literature. This review has identified MRPL4, MRPL13 and MRPL36 as potential prognostic targets, and MRPS12, MRPL35 and MRPL42 as putative therapeutic targets from the current literature. Where there are direct links with specific MRPs with therapeutic options in the literature, we have provided further details in the previous sections, but there are additional promising avenues for targeting MRPs we would like to highlight.
Broad approaches to targeting MRP function, such as mitochondrial ribosome inhibition or disturbing MRP synthesis, have been shown to prevent drug resistance and metastasis in a variety of cancers [84, 85]. Mitoriboscins are a recently identified group of mitochondrial-related antibiotics that are capable of binding to, and inhibiting, the mitochondrial ribosome [84]. These drugs have been shown to preferentially target cancer stem cells and groups of cancer cells in breast cancer cell models, effectively inhibiting oxidative phosphorylation, cell viability, migration, and cancer stem cell propagation, while showing no effect on normal fibroblasts [84]. Another study showed that Mitoriboscins had little effect on tumour growth but did inhibit metastasis, with very low embryo toxicity [46], thus proving its potential as a metastatic cancer therapeutic option. Furthermore, MRP synthesis can be targeted by the HSP70 inhibitor, JG-98, which can disrupt MRP stability through misfolding in castration-resistant prostate cancer [85]. The study observed decreased expression of all five MRPs tested (MRPS27, MRPS23, MRPS17, MRPL44, MRPL19), reduced mitochondrial respiration, and found that JG-98 sensitised castration-resistant prostate cancer cells to androgen-deprivation therapy [85]. Whilst this study highlights how MRP synthesis can be effectively inhibited in prostate cancer, we suggest that perhaps further assessment of this mechanism in a more relevant cancer cell model would enable the efficacy of this treatment to be teased apart. Notably, most of the studies highlighted in this review were undertaken in lung, breast and ovarian cancer, which may provide a starting point for further studies examining the effect of HSP70 inhibitors.
Moreover, repurposing existing FDA approved therapies is a promising avenue for targeting MRPs in aggressive cancer. Repurposing FDA approved therapies has enabled the timely use of effective and well-studied therapeutics for a range of conditions, including cancer. For example, tetracycline analogues, such as doxycycline, COL-3 and tigecycline, are approved for use as broad-spectrum antibiotics to treat a wide variety of bacterial infections [86], however they have shown anti-tumour effects in several cancers in both in vitro studies and clinical trials [87–89]. These medications have also been shown to inhibit the process of mitochondrial translation, in which MRPs play a key role [87–89]. The possible effects of tetracycline analogues on MRP expression remains unclear and has not yet been studied, however, tigecycline is thought to inhibit the function of the mitochondrial ribosome, exhibiting selective inhibition of mitochondrial translation, biogenesis, and respiration to induce apoptosis in cancer cells [35, 90]. Given the known effect of tigecycline on MRPS12 expression, we suggest that studies assessing the interplay between tumour recurrence, tamoxifen resistance and MRPS12 expression is an ideal starting point. Additionally, pentamidine, a broad-spectrum antimicrobial drug used to treat several parasitic worms, protozoa, and fungi, has been found to inhibit prostate cancer progression in vitro by inducing mitochondrial DNA depletion and dysfunction [91]. Again, no study has assessed its effect on MRP expression and function, specifically, which is an obvious area of future research.
Conclusion
In addition to their normal function, MRPs play diverse roles in regulating the survival, development, and progression of cancers, particularly those that are advanced and metastatic. However, our understanding of how MRPs mechanistically contribute to the growth and progression of metastatic cancer is very limited. The majority of studies suggest that MRPs play key roles in driving proliferation and migration, as well as EMT, and propose that MRPs may promote the acquisition of metastatic properties in cancer cells. This review has explored studies which have assessed the expression of MRPs in cell lines and patient samples from a range of cancer types, and in some cases, associations with clinical characteristics and patient outcomes. Due to their expression profile in cancer cells and the role they are known to play in advanced disease, MRPs could provide therapeutically exploitable opportunities in a variety of advanced cancers, however there is limited literature in the area. Numerous studies have found conflicting data across cancer types and perhaps a systematic approach to unravelling the role MRPs play in metastasis would be more beneficial than studies assessing MRPs in individual cancers. MRPs associated with more than one cancer type hold the greatest potential as prognostic and therapeutic targets of metastasis given their broad application across the cancer spectrum. The authors conclude that targeting the mitochondria is a burgeoning field of cancer research and further investigations into the role of MRPs in cancer progression is warranted.
Supplementary information
Below is the link to the electronic supplementary material.
Author contributions
JMB, JLD and KR conceived the review topic. JMB and KR wrote the original draft. JMB formatted the figures and tables. JMB, JLJ, GL, JLD and KR reviewed and edited the manuscript. JLJ, GL, JLD and KR supervised and mentored JMB.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. JMB is supported by a College of Health and Medicine Graduate Research Scholarship Co-Funded Stipend and a Lillian Webb PhD Top-up Scholarship in Cancer Research. JLD is supported by a Select Foundation Cancer Research Fellowship. KR was supported by a Cancer Council Tasmania Joy & Robert Coghlan/ College of Health and Medicine Postdoctoral Research Fellowship and is now supported by a Cancer Council Tasmania/Evelyn Pedersen Fellowship.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pfanner, N., Warscheid, B., & Wiedemann, N. (2019). Mitochondrial proteins: From biogenesis to functional networks. Nature Review Molecular Cell Biology,20(5), 267–284. 10.1038/s41580-018-0092-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amunts, A., Brown, A., Toots, J., Scheres, S. H. W., & Ramakrishnan, V. (2015). The structure of the human mitochondrial ribosome. Science,348(6230), 95–98. 10.1126/science.aaa1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greber, B. J., & Ban, N. (2016). Structure and Function of the Mitochondrial Ribosome. Annual Review of Biochemistry,85(1), 103–132. 10.1146/annurev-biochem-060815-014343 [DOI] [PubMed] [Google Scholar]
- 4.Lopez Sanchez, M. I. G., Krüger, A., Shiriaev, D. I., Liu, Y., & Rorbach, J. (2021). Human Mitoribosome Biogenesis and Its Emerging Links to Disease. International Journal of Molecular Sciences,22(8), 3827. 10.3390/ijms22083827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sylvester, J. E., Fischel-Ghodsian, N., Mougey, E. B., & O’Brien, T. W. (2004). Mitochondrial ribosomal proteins: Candidate genes for mitochondrial disease. Genetics in Medicine,6(2), 73–80. 10.1097/01.gim.0000117333.21213.17 [DOI] [PubMed] [Google Scholar]
- 6.Koc, E. C., Ranasinghe, A., Burkhart, W., Blackburn, K., Koc, H., Moseley, A., & Spremulli, L. L. (2001). A new face on apoptosis: Death-associated protein 3 and PDCD9 are mitochondrial ribosomal proteins. FEBS Letters,492(1–2), 166–170. 10.1016/s0014-5793(01)02250-5 [DOI] [PubMed] [Google Scholar]
- 7.Wazir, U., Orakzai, M. M., Khanzada, Z. S., Jiang, W. G., Sharma, A. K., Kasem, A., & Mokbel, K. (2015). The role of death-associated protein 3 in apoptosis, anoikis and human cancer. Cancer Cell International,15, 39. 10.1186/s12935-015-0187-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyazaki, T., Shen, M., Fujikura, D., Tosa, N., Kim, H.-R., Kon, S., Uede, T., & Reed, J. C. (2004). Functional role of death-associated protein 3 (DAP3) in anoikis. Journal of Biological Chemistry,279(43), 44667–44672. 10.1074/jbc.m408101200 [DOI] [PubMed] [Google Scholar]
- 9.Yoo, Y. A., Kim, M. J., Park, J. K., Chung, Y. M., Lee, J. H., Chi, S.-G., Kim, J. S., & Yoo, Y. D. (2005). Mitochondrial ribosomal protein L41 suppresses cell growth in association with p53 and p27Kip1. Molecular and Cellular Biology,25(15), 6603–6616. 10.1128/mcb.25.15.6603-6616.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, Y.-C., Chang, M.-Y., Shiau, A.-L., Yo, Y.-T., & Wu, C.-L. (2007). Mitochondrial ribosomal protein S36 delays cell cycle progression in association with p53 modification and p21WAF1/CIP1 expression. Journal of Cellular Biochemistry,100(4), 981–990. 10.1002/jcb.21079 [DOI] [PubMed] [Google Scholar]
- 11.Zhang, X., Gao, X., Coots, R. A., Conn, C. S., Liu, B., & Qian, S.-B. (2015). Translational control of the cytosolic stress response by mitochondrial ribosomal protein L18. Nature Structural and Molecular Biology,22(5), 404–410. 10.1038/nsmb.3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li, H.-B., Wang, R.-X., Jiang, H.-B., Zhang, E.-D., Tan, J.-Q., Xu, H.-Z., Zhou, R.-R., & Xia, X.-B. (2016). Mitochondrial ribosomal protein L10 associates with cyclin B1/Cdk1 activity and mitochondrial function. DNA and Cell Biology,35(11), 680–690. 10.1089/dna.2016.3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Surovtseva, Y. V., Shutt, T. E., Cotney, J., Cimen, H., Chen, S. Y., Koc, E. C., & Shadel, G. S. (2011). Mitochondrial Ribosomal Protein L12 selectively associates with human mitochondrial RNA polymerase to activate transcription. Proceedings of the National Academy of Sciences,108(44), 17921–17926. 10.1073/pnas.1108852108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang, Z., Cotney, J., & Shadel, G. S. (2007). Human mitochondrial ribosomal protein MRPL12 interacts directly with mitochondrial RNA polymerase to modulate mitochondrial gene expression. Journal of Biological Chemistry,282(17), 12610–12618. 10.1074/jbc.m700461200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang, C.-W., Wei, Z., Durell, S. R., Ma, L., Forgues, M., & Wang, X. W. (2022). A compendium of co-regulated mitoribosomal proteins in pan-cancer uncovers collateral defective events in tumor malignancy. iScience,25(10), 105244. 10.1016/j.isci.2022.105244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu, J. (2019). The Warburg metabolism fuels tumor metastasis. Cancer and Metastasis Reviews,38(1–2), 157–164. 10.1007/s10555-019-09794-5 [DOI] [PubMed] [Google Scholar]
- 17.The Cancer Genome Atlas Research Network. (2014). Comprehensive molecular profiling of lung adenocarcinoma. Nature,511(7511), 543–550. 10.1038/nature13385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao, Y., Li, F., Zhou, H., Yang, Y., Wu, R., Chen, Y., Li, W., Li, Y., Xu, X., Ke, C., & Pei, Z. (2017). Down-regulation of MRPS23 inhibits rat breast cancer proliferation and metastasis. Oncotarget,8(42), 71772–71781. 10.18632/oncotarget.17888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klæstad, E., Opdahl, S., Engstrøm, M. J., Ytterhus, B., Wik, E., Bofin, A. M., & Valla, M. (2020). MRPS23 amplification and gene expression in breast cancer; association with proliferation and the non-basal subtypes. Breast Cancer Research and Treatment,180(1), 73–86. 10.1007/s10549-020-05532-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, L., Zhang, X., Ding, H., Liu, X., Cao, D., Liu, Y., Liu, J., Lin, C., Zhang, N., Wang, G., Hou, J., Huang, B., Zhang, Y., & Lu, J. (2021). Arginine and lysine methylation of MRPS23 promotes breast cancer metastasis through regulating OXPHOS. Oncogene,40(20), 3548–3563. 10.1038/s41388-021-01785-7 [DOI] [PubMed] [Google Scholar]
- 21.Lyng, H., Brøvig, R. S., Svendsrud, D. H., Holm, R., Kaalhus, O., Knutstad, K., Oksefjell, H., Sundfør, K., Kristensen, G. B., & Stokke, T. (2006). Gene expressions and copy numbers associated with metastatic phenotypes of uterine cervical cancer. BMC Genomics,7(1), 268. 10.1186/1471-2164-7-268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oviya, R. P., Gopal, G., Shirley, S. S., Sridevi, V., Jayavelu, S., & Rajkumar, T. (2021). Mitochondrial ribosomal small subunit proteins (MRPS) MRPS6 and MRPS23 show dysregulation in breast cancer affecting tumorigenic cellular processes. Gene,790, 145697. 10.1016/j.gene.2021.145697 [DOI] [PubMed] [Google Scholar]
- 23.Pu, M., Wang, J., Huang, Q., Zhao, G., Xia, C., Shang, R., Zhang, Z., Bian, Z., Yang, X., & Tao, K. (2017). High MRPS23 expression contributes to hepatocellular carcinoma proliferation and indicates poor survival outcomes. Tumor Biology,39(7), 101042831770912. 10.1177/1010428317709127 [DOI] [PubMed] [Google Scholar]
- 24.Sotgia, F., Fiorillo, M., & Lisanti, M. P. (2017). Mitochondrial markers predict recurrence, metastasis and tamoxifen-resistance in breast cancer patients: Early detection of treatment failure with companion diagnostics. Oncotarget,8(40), 68730–68745. 10.18632/oncotarget.19612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu, X., Guo, D., Du, J., Bai, Y., & Wang, F. (2021). A novel biomarker, MRPS12 functions as a potential oncogene in ovarian cancer and is a promising prognostic candidate. Medicine (Baltimore),100(8), e24898. 10.1097/md.0000000000024898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu, H., Zou, R., Li, F., Liu, J., Luan, N., Wang, S., & Zhu, L. (2021). MRPL15 is a novel prognostic biomarker and therapeutic target for epithelial ovarian cancer. Cancer Medicine,10(11), 3655–3673. 10.1002/cam4.3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hertweck, K. L., Vikramdeo, K. S., Galeas, J. N., Marbut, S. M., Pramanik, P., Yunus, F., Singh, S., Singh, A. P., & Dasgupta, S. (2023). Clinicopathological significance of unraveling mitochondrial pathway alterations in non‐small‐cell lung cancer. The FASEB Journal, 37(7). 10.1096/fj.202201724rr [DOI] [PubMed]
- 28.Mints, M., Mushtaq, M., Iurchenko, N., Kovalevska, L., Stip, M. C., Budnikova, D., Andersson, S., Polischuk, L., Buchynska, L., & Kashuba, E. (2016). Mitochondrial ribosomal protein S18–2 is highly expressed in endometrial cancers along with free E2F1. Oncotarget,7(16), 22150–22158. 10.18632/oncotarget.7905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mushtaq, M., Jensen, L., Davidsson, S., Grygoruk, O. V., Andrén, O., Kashuba, V., & Kashuba, E. (2018). The MRPS18–2 protein levels correlate with prostate tumor progression and it induces CXCR4-dependent migration of cancer cells. Scientific Reports, 8(1). 10.1038/s41598-018-20765-8 [DOI] [PMC free article] [PubMed]
- 30.Revathi Paramasivam, O., Gopisetty, G., Subramani, J., & Thangarajan, R. (2021). Expression and affinity purification of recombinant mammalian mitochondrial ribosomal small subunit (MRPS) proteins and protein-protein interaction analysis indicate putative role in tumourigenic cellular processes. Journal of Biochemistry,169(6), 675–692. 10.1093/jb/mvab004 [DOI] [PubMed] [Google Scholar]
- 31.Lin, X., Guo, L., Lin, X., Wang, Y., & Zhang, G. (2022). Expression and prognosis analysis of mitochondrial ribosomal protein family in breast cancer. Scientific Reports,12(1), 10658. 10.1038/s41598-022-14724-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan, X., Liu, L., Shi, Y., Guo, F., Wang, H., Zhao, X., Zhong, D., & Li, G. (2020). Integrated analysis of RNA-binding proteins in human colorectal cancer. World Journal of Surgical Oncology, 18(1). 10.1186/s12957-020-01995-5 [DOI] [PMC free article] [PubMed]
- 33.Yu, X., Zhang, Y., Xiong, S., McDaniel, J. M., Sun, C., Chau, G. P., Gencel-Augusto, J., Chachad, D., Morrissey, R. L., Rao, X., Wang, J., & Lozano, G. (2022). Omics analyses of a somatic Trp53R245W/+ breast cancer model identify cooperating driver events activating PI3K/AKT/mTOR signaling. Proceedings of the National Academy of Sciences, 119(45). 10.1073/pnas.2210618119 [DOI] [PMC free article] [PubMed]
- 34.Huang, S.-W., Sun, M.-T., Lee, W.-S., Su, Y.-s, Lee, Y.-T., Chiang, M.-H., Wang, Y.-C., Yang, Y.-S., Tzeng, S.-C., Wu, T.-Y., Sun, J.-S., & Lin, F.-H. (2022). Cancer as an infectious disease: A different treatment alternative using a combination of tigecycline and pyrvinium pamoate – An example of breast cancer. Journal of Microbiology, Immunology and Infection,55(1), 51–59. 10.1016/j.jmii.2020.12.008 [DOI] [PubMed] [Google Scholar]
- 35.Hu, B., & Guo, Y. (2019). Inhibition of mitochondrial translation as a therapeutic strategy for human ovarian cancer to overcome chemoresistance. Biochemical and Biophysical Research Communications,509(2), 373–378. 10.1016/j.bbrc.2018.12.127 [DOI] [PubMed] [Google Scholar]
- 36.O’Brien, T. W. (2003). Properties of human mitochondrial ribosomes. IUBMB Life,55(9), 505–513. 10.1080/15216540310001626610 [DOI] [PubMed] [Google Scholar]
- 37.Yang, X., Cheng, Y., Li, X., Zhou, J., Dong, Y., Shen, B., Zhao, L., & Wang, J. (2021). A novel transcription factor-based prognostic signature in endometrial cancer: Establishment and validation. OncoTargets and Therapy,14, 2579–2598. 10.2147/ott.S293085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Contreras-Zárate, M. J., Day, N. L., Ormond, D. R., Borges, V. F., Tobet, S., Gril, B., Steeg, P. S., & Cittelly, D. M. (2019). Estradiol induces BDNF/TrkB signaling in triple-negative breast cancer to promote brain metastases. Oncogene,38(24), 4685–4699. 10.1038/s41388-019-0756-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai, Y.-F., Tseng, L.-M., Hsu, C.-Y., Yang, M.-H., Chiu, J.-H., & Shyr, Y.-M. (2017). Brain-derived neurotrophic factor (BDNF) -TrKB signaling modulates cancer-endothelial cells interaction and affects the outcomes of triple negative breast cancer. PLoS ONE,12(6), e0178173. 10.1371/journal.pone.0178173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huth, L., Rose, M., Kloubert, V., Winkens, W., Schlensog, M., Hartmann, A., Knüchel, R., & Dahl, E. (2014). BDNF is associated with SFRP1 expression in luminal and basal-like breast cancer cell lines and primary breast cancer tissues: A novel role in tumor suppression? PLoS ONE,9(7), e102558. 10.1371/journal.pone.0102558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serafim Junior, V., Fernandes, GMd. M., Oliveira-Cucolo, JGd., Pavarino, E. C., & Goloni-Bertollo, E. M. (2020). Role of Tropomyosin-related kinase B receptor and brain-derived neurotrophic factor in cancer. Cytokine,136, 155270. 10.1016/j.cyto.2020.155270 [DOI] [PubMed] [Google Scholar]
- 42.Paplomata, E., & O’Regan, R. (2014). The PI3K/AKT/mTOR pathway in breast cancer: Targets, trials and biomarkers. Therapeutic Advances in Medical Oncology,6(4), 154–166. 10.1177/1758834014530023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riobo-Del Galdo, N., Lara Montero, Á., & Wertheimer, E. (2019). Role of hedgehog signaling in breast cancer: Pathogenesis and therapeutics. Cells,8(4), 375. 10.3390/cells8040375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel, D. K., Kesharwani, R., Verma, A., Al-Abbasi, F. A., Anwar, F., & Kumar, V. (2023). Scope of Wnt signaling in the precise diagnosis and treatment of breast cancer. Drug Discovery Today,28(7), 103597. 10.1016/j.drudis.2023.103597 [DOI] [PubMed] [Google Scholar]
- 45.Fu, Q., Hong, R., Zhou, H., Li, Y., Liu, X., Gong, J., Wang, X., Chen, J., Ran, H., Wang, L., Li, F., & Yuan, J. (2022). Proteomics reveals MRPL4 as a high-risk factor and a potential diagnostic biomarker for prostate cancer. Proteomics,22(21), 2200081. 10.1002/pmic.202200081 [DOI] [PubMed] [Google Scholar]
- 46.Ózsvári, B., Sotgia, F., & Lisanti, M. P. (2020). First-in-class candidate therapeutics that target mitochondria and effectively prevent cancer cell metastasis: Mitoriboscins and TPP compounds. Aging,12(11), 10162–10179. 10.18632/aging.103336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu, Z., Xu, S., Li, L., Zhong, X., Chen, C., Fan, Y., Shen, W., Zu, L., Xue, F., Wang, M., & Zhou, Q. (2019). Comparative mitochondrial proteomic analysis of human large cell lung cancer cell lines with different metastasis potential. Thoracic Cancer,10(5), 1111–1128. 10.1111/1759-7714.13052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qi, L., & Ding, Y. (2018). Analysis of metastasis associated signal regulatory network in colorectal cancer. Biochemical and Biophysical Research Communications,501(1), 113–118. 10.1016/j.bbrc.2018.04.186 [DOI] [PubMed] [Google Scholar]
- 49.Li, X.-Y., He, X.-Y., Zhao, H., Qi, L., & Lu, J.-J. (2023). Identification of a novel therapeutic target for lung cancer: Mitochondrial ribosome protein L9. Pathology - Research and Practice,248, 154625. 10.1016/j.prp.2023.154625 [DOI] [PubMed] [Google Scholar]
- 50.Tang, B., Zhu, J., Zhao, Z., Lu, C., Liu, S., Fang, S., Zheng, L., Zhang, N., Chen, M., Xu, M., Yu, R., & Ji, J. (2021). Diagnosis and prognosis models for hepatocellular carcinoma patient’s management based on tumor mutation burden. Journal of Advanced Research,33, 153–165. 10.1016/j.jare.2021.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu, Y., Liu, Y., Ma, C., & Ai, K. (2023). MRPL12 acts as a novel prognostic biomarker involved in immune cell infiltration and tumor progression of lung adenocarcinoma. International Journal of Molecular Sciences, 24(3). 10.3390/ijms24032762 [DOI] [PMC free article] [PubMed]
- 52.Liu, Y., Sun, H., Li, X., Liu, Q., Zhao, Y., Li, L., Xu, B., Hou, Y., & Jin, W. (2021). Identification of a three-RNA binding proteins (RBPs) signature predicting prognosis for breast cancer. Frontiers in Oncology, 11. 10.3389/fonc.2021.663556 [DOI] [PMC free article] [PubMed]
- 53.Tao, Z., Suo, H., Zhang, L., Jin, Z., Wang, Z., Wang, D., Wu, M., Peng, N., Zhao, Y., & Chen, B. (2020). MRPL13 is a prognostic cancer biomarker and correlates with immune infiltrates in breast cancer. OncoTargets and Therapy,13, 12255–12268. 10.2147/ott.s263998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ye, H., & Zhang, N. (2021). Identification of the upregulation of MRPL13 as a novel prognostic marker associated with overall survival time and immunotherapy response in breast cancer. Computational and Mathematical Methods in Medicine,2021, 1–14. 10.1155/2021/1498924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cai, M., Li, H., Chen, R., & Zhou, X. (2021). MRPL13 promotes tumor cell proliferation, migration and EMT process in breast cancer through the PI3K-AKT-mTOR pathway. Cancer Management and Research,13, 2009–2024. 10.2147/cmar.s296038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jing, C., Fu, R., Wang, C., Li, X., & Zhang, W. (2021). MRPL13 act as a novel therapeutic target and could promote cell proliferation in non-small cell lung cancer. Cancer Management and Research,13, 5535–5545. 10.2147/cmar.s316428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhong, X., He, Z., Fan, Y., Yin, L., Hong, Z., Tong, Y., Bi, Q., & Zhu, S. (2023). Multi-omics analysis of MRPL-13 as a tumor-promoting marker from pan-cancer to lung adenocarcinoma. Aging,15(19), 10640–10680. 10.18632/aging.205104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei, D., Sun, D., Sirera, R., Afzal, M. Z., Leong, T. L., Li, X., & Wang, Y. (2023). Overexpression of MRPL19 in predicting poor prognosis and promoting the development of lung adenocarcinoma. Translational Lung Cancer Research,12(7), 1517–1538. 10.21037/tlcr-23-306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Best, C. J. M., Gillespie, J. W., Yi, Y., Chandramouli, G. V. R., Perlmutter, M. A., Gathright, Y., Erickson, H. S., Georgevich, L., Tangrea, M. A., Duray, P. H., GonzáLez, S., Velasco, A., Linehan, W. M., Matusik, R. J., Price, D. K., Figg, W. D., Emmert-Buck, M. R., & Chuaqui, R. F. (2005). Molecular alterations in primary prostate cancer after androgen ablation therapy. Clinical Cancer Research,11(19), 6823–6834. 10.1158/1078-0432.ccr-05-0585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hou, W., Chen, J., & Wang, Y. (2024). MRPL35 Induces Proliferation, Invasion, and Glutamine Metabolism in NSCLC Cells by Upregulating SLC7A5 Expression. The Clinical Respiratory Journal, 18(7). 10.1111/crj.13799 [DOI] [PMC free article] [PubMed]
- 61.Zhao, C., Chen, L., Jin, Z., Liu, H., Ma, C., Zhou, H., Xu, L., Zhou, S., Shi, Y., Li, W., Chen, Y., Dou, C., & Wang. X. (2023). Knockdown of MRPL35 promotes cell apoptosis and inhibits cell proliferation in non-small-cell lung cancer. BMC Pulmonary Medicine, 23(1). 10.1186/s12890-023-02677-0 [DOI] [PMC free article] [PubMed]
- 62.Zhang, L., Lu, P., Yan, L., Yang, L., Wang, Y., Chen, J., Dai, J., Li, Y., Kang, Z., Bai, T., Xi, Y., Xu, J., Sun, G., & Yang, T. (2019). MRPL35 is up-regulated in colorectal cancer and regulates colorectal cancer cell growth and apoptosis. The American Journal of Pathology,189(5), 1105–1120. 10.1016/j.ajpath.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 63.Yuan, L., Li, J. X., Yang, Y., Chen, Y., Ma, T. T., Liang, S., Bu, Y., Yu, L., & Nan, Y. (2021). Depletion of MRPL35 inhibits gastric carcinoma cell proliferation by regulating downstream signaling proteins. World Journal of Gastroenterology,27(16), 1785–1804. 10.3748/wjg.v27.i16.1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuan, L., Yang, Y., Li, X., Zhou, X., Du, Y. H., Liu, W. J., Zhang, L., Yu, L., Ma, T. T., Li, J. X., Chen, Y., & Nan, Y. (2022). 18β-glycyrrhetinic acid regulates mitochondrial ribosomal protein L35-associated apoptosis signaling pathways to inhibit proliferation of gastric carcinoma cells. World Journal of Gastroenterology,28(22), 2437–2456. 10.3748/wjg.v28.i22.2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luo, W., Han, Y., Li, X., Liu, Z., Meng, P., & Wang, Y. (2022). Breast cancer prognosis prediction and immune pathway molecular analysis based on mitochondria-related genes. Genetics Research,2022, 1–13. 10.1155/2022/2249909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang, Y., Dong, L., Cui, H., Shen, D.-H., Wang, Y., Chang, X.-H., Fu, T.-Y., Ye, X., & Yao, Y.-Y. (2011). Up-regulation of mitochondrial antioxidation signals in ovarian cancer cells with aggressive biologic behavior. Journal of Zhejiang University SCIENCE B,12(5), 346–356. 10.1631/jzus.b1000192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang, W., Zhang, C., Kang, Y., Yu, X., Pang, P., Li, G., & Feng, Y. (2021). MRPL42 is activated by YY1 to promote lung adenocarcinoma progression. Journal of Cancer,12(8), 2403–2411. 10.7150/jca.52277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee, J., Seol, M.-Y., Jeong, S., Lee, C. R., Ku, C. R., Kang, S.-W., Jeong, J. J., Shin, D. Y., Nam, K.-H., Lee, E. J., Chung, W. Y., & Jo, Y. S. (2015). A metabolic phenotype based on mitochondrial ribosomal protein expression as a predictor of lymph node metastasis in papillary thyroid carcinoma. Medicine, 94(2). 10.1097/MD.0000000000000380 [DOI] [PMC free article] [PubMed]
- 69.Chen, Y.-W., Chou, H.-C., Lyu, P.-C., Yin, H.-S., Huang, F.-L., Chang, W.-S.W., Fan, C.-Y., Tu, I. F., Lai, T.-C., Lin, S.-T., Lu, Y.-C., Wu, C.-L., Huang, S.-H., & Chan, H.-L. (2011). Mitochondrial proteomics analysis of tumorigenic and metastatic breast cancer markers. Functional and Integrative Genomics,11(2), 225–239. 10.1007/s10142-011-0210-y [DOI] [PubMed] [Google Scholar]
- 70.Huang, Y., Chen, S., Qin, W., Wang, Y., Li, L., Li, Q., & Yuan, X. (2020). A novel RNA binding protein-related prognostic signature for hepatocellular carcinoma. Frontiers in Oncology, 10. 10.3389/fonc.2020.580513 [DOI] [PMC free article] [PubMed]
- 71.Wang, M., Jiang, F., Wei, K., Wang, J., Zhou, G., Wu, C., & Yin, G. (2021). Development and validation of a RNA binding protein-associated prognostic model for hepatocellular carcinoma. Technology in Cancer Research & Treatment,20, 153303382110049. 10.1177/15330338211004936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang, N., Zhu, S., Chen, J., Niu, Y., & Zhou, L. (2013). A-methylacyl-CoA racemase (AMACR) and prostate-cancer risk: A meta-analysis of 4,385 participants. PLoS ONE,8(10), e74386. 10.1371/journal.pone.0074386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seth, P. P., Tanowitz, M., & Bennett, C. F. (2019). Selective tissue targeting of synthetic nucleic acid drugs. Journal of Clinical Investigation,129(3), 915–925. 10.1172/jci125228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kulkarni, J. A., Witzigmann, D., Thomson, S. B., Chen, S., Leavitt, B. R., Cullis, P. R., & Van Der Meel, R. (2021). The current landscape of nucleic acid therapeutics. Nature Nanotechnology,16(6), 630–643. 10.1038/s41565-021-00898-0 [DOI] [PubMed] [Google Scholar]
- 75.Liu, Y., Ma, G., Liu, J., Zheng, H., Huang, G., Song, Q., Pang, Z., & Du, J. (2022). SLC7A5 is a lung adenocarcinoma-specific prognostic biomarker and participates in forming immunosuppressive tumor microenvironment. Heliyon,8(10), e10866. 10.1016/j.heliyon.2022.e10866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou, B., Mai, Z., Ye, Y., Song, Y., Zhang, M., Yang, X., Xia, W., & Qiu, X. (2022). The role of PYCR1 in inhibiting 5-fluorouracil-induced ferroptosis and apoptosis through SLC25A10 in colorectal cancer. Human Cell,35(6), 1900–1911. 10.1007/s13577-022-00775-5 [DOI] [PubMed] [Google Scholar]
- 77.Xiao, D., & He, J. (2010). Epithelial mesenchymal transition and lung cancer. Journal of Thoracic Disease,2(3), 154–159. 10.3978/j.issn.2072-1439.2010.02.03.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ho, K.-H., Huang, T.-W., Liu, A.-J., Shih, C.-M., & Chen, K.-C. (2021). Cancer essential genes stratified lung adenocarcinoma patients with distinct survival outcomes and identified a subgroup from the terminal respiratory unit type with different proliferative signatures in multiple cohorts. Cancers,13(9), 2128. 10.3390/cancers13092128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Halvorsen, A. R., Ragle Aure, M., Õjlert, Å. K., Brustugun, O. T., Solberg, S., Nebdal, D., & Helland, Å. (2019). Identification of microRNAs involved in pathways which characterize the expression subtypes of NSCLC. Molecular Oncology,13(12), 2604–2615. 10.1002/1878-0261.12571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wilkerson, M. D., Yin, X., Hoadley, K. A., Liu, Y., Hayward, M. C., Cabanski, C. R., Muldrew, K., Miller, C. R., Randell, S. H., Socinski, M. A., Parsons, A. M., Funkhouser, W. K., Lee, C. B., Roberts, P. J., Thorne, L., Bernard, P. S., Perou, C. M., & Hayes, D. N. (2010). Lung squamous cell carcinoma mRNA expression subtypes are reproducible, clinically important, and correspond to normal cell types. Clinical Cancer Research,16(19), 4864–4875. 10.1158/1078-0432.ccr-10-0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu, D., Hu, J., Agorreta, J., Cesario, A., Zhang, Y., Harris, A. L., Gatter, K., & Pezzella, F. (2010). Tumor necrosis factor receptor-associated protein 1 (TRAP1) regulates genes involved in cell cycle and metastases. Cancer Letters,296(2), 194–205. 10.1016/j.canlet.2010.04.017 [DOI] [PubMed] [Google Scholar]
- 82.Liberti, M. V., & Locasale, J. W. (2016). The Warburg effect: How does it benefit cancer cells? Trends in Biochemical Sciences,41(3), 211–218. 10.1016/j.tibs.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roshanzamir, F., Robinson, J. L., Cook, D., Karimi-Jafari M. H., & Nielsen, J. (2022). Metastatic triple negative breast cancer adapts its metabolism to destination tissues while retaining key metabolic signatures. Proceedings of the National Academy of Sciences, 119(35). 10.1073/pnas.2205456119 [DOI] [PMC free article] [PubMed]
- 84.Ózsvári, B., Fiorillo, M., Bonuccelli, G., Cappello, A. R., Frattaruolo, L., Sotgia, F., Trowbridge, R., Foster, R., & Lisanti, M. P. (2017). Mitoriboscins: Mitochondrial-based therapeutics targeting cancer stem cells (CSCs), bacteria and pathogenic yeast. Oncotarget,8(40), 67457–67472. 10.18632/oncotarget.19084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Echtenkamp, F. J., Ishida, R., Rivera-Marquez, G. M., Maisiak, M., Johnson, O. T., Shrimp, J. H., Sinha, A., Ralph, S. J., Nisbet, I., Cherukuri, M. K., Gestwicki, J. E., & Neckers, L. M. (2023). Mitoribosome sensitivity to HSP70 inhibition uncovers metabolic liabilities of castration-resistant prostate cancer. PNAS Nexus, 2(4). 10.1093/pnasnexus/pgad115 [DOI] [PMC free article] [PubMed]
- 86.Nelson, M. L., & Levy, S. B. (2011). The history of the tetracyclines. Annals of the New York Academy of Sciences,1241(1), 17–32. 10.1111/j.1749-6632.2011.06354.x [DOI] [PubMed] [Google Scholar]
- 87.Skrtić, M., Sriskanthadevan, S., Jhas, B., Gebbia, M., Wang, X., Wang, Z., Hurren, R., Jitkova, Y., Gronda, M., Maclean, N., Lai, C. K., Eberhard, Y., Bartoszko, J., Spagnuolo, P., Rutledge, A. C., Datti, A., Ketela, T., Moffat, J., Robinson, B. H., . . . Schimmer, A. D. (2011). Inhibition of mitochondrial translation as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell, 20(5), 674–688. 10.1016/j.ccr.2011.10.015 [DOI] [PMC free article] [PubMed]
- 88.Protasoni, M., Kroon, A. M., & Taanman, J.-W. (2018). Mitochondria as oncotarget: A comparison between the tetracycline analogs doxycycline and COL-3. Oncotarget,9(73), 33818–33831. 10.18632/oncotarget.26107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Richards, C., Pantanowitz, L., & Dezube, B. J. (2011). Antimicrobial and non-antimicrobial tetracyclines in human cancer trials. Pharmacological Research,63(2), 151–156. 10.1016/j.phrs.2010.10.008 [DOI] [PubMed] [Google Scholar]
- 90.Li, J., Qin, Y., Zhao, C., Zhang, Z., & Zhou, Z. (2023). Tetracycline antibiotics: Potential anticancer drugs. European Journal of Pharmacology,956, 175949. 10.1016/j.ejphar.2023.175949 [DOI] [PubMed] [Google Scholar]
- 91.Liu, L., Wang, F., Tong, Y., Li, L. F., Liu, Y., & Gao, W. Q. (2020). Pentamidine inhibits prostate cancer progression via selectively inducing mitochondrial DNA depletion and dysfunction. Cell Proliferation, 53(1). 10.1111/cpr.12718 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.