Abstract
Introduction
The safety of antiviral agents in real-world clinical settings is crucial, as pre-marketing studies often do not capture all adverse events (AE). Active pharmacovigilance strategies are essential for detecting and characterising these AE comprehensively.
Objective
The aim of this study was to identify and characterise active pharmacovigilance strategies used in real-world clinical settings for patients under systemic antiviral agents, focusing on the frequency of AE and the clinical data sources used.
Methods
We conducted a systematic review by searching three electronic bibliographic databases targeting observational prospective active pharmacovigilance studies, phase IV clinical trials for post-marketing safety surveillance, and interventional studies assessing active pharmacovigilance strategies, focusing on individuals exposed to systemic antiviral agents.
Results
We included 36 primary studies, predominantly using Drug Event Monitoring (DEM), with a minority employing sentinel sites and registries. Human immunodeficiency virus (HIV) was the most common condition, with the majority using DEM. Within the DEM, there was a wide range of incidences of patients experiencing at least one AE, and most of these studies used one or two data sources. Sentinel site studies were less common, with two on hepatitis C virus (HCV) and one on HIV, each relying on one or two data sources. The single study using a registry focusing on HIV therapy reported using just one data source. Patient interviews were the most common data source, followed by medical records and laboratory tests. The quality of the studies was considered ‘good’ in 18/36, ‘fair’ in 1/36, and ‘poor’ in 17/36 studies.
Conclusion
DEM was the predominant pharmacovigilance strategy, employing multiple data sources, and appears to increase the likelihood of detecting higher AE incidence. Establishing such a framework would facilitate a more detailed and consistent approach across different studies and settings.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40264-024-01470-0.
Key Points
| Drug event monitoring is the predominant pharmacovigilance strategy for systemic antiviral treatments, especially for human immunodeficiency virus (HIV), followed by influenza, hepatitis C virus (HCV), and hepatitis B virus (HBV). |
| Sentinel sites and the use of registries in pharmacovigilance were less prevalent in our systematic review, with only a few studies employing these methods, primarily focusing on chronic conditions (HIV and HCV). |
| Patient interviews, medical records, and laboratory tests, despite their potential biases, are crucial in active pharmacovigilance for antivirals, offering valuable longitudinal data to aid in the understanding of adverse event patterns and risk factors, thus providing insights beyond conventional reporting methods. |
| Employing multiple data sources appears to increase the likelihood of detecting higher adverse event incidence. |
Introduction
No effective medicine is without risk, and a full understanding of a medicine’s safety profile is only achieved after wide clinical use. Given the inherent limitations of pre-marketing studies and randomised clinical trials (RCT), the safety of a new drug should be considered provisional at the time of its market introduction. As such, over time, there has been a significant shift towards demanding a comprehensive evaluation of the benefits and risks of interventions in real-life post-RCT conditions [1, 2]. In this context, adverse events (AE) monitoring in the post-marketing phase predominantly relies on passive surveillance methods, such as spontaneous reporting. This approach is particularly effective in identifying rare AE with low baseline incidence rates [3].
To address the inherent shortcomings of spontaneous reporting, the traditional passive approach of collecting voluntary reports has been supplemented with more dynamic and proactive strategies. Over time, active pharmacovigilance strategies (also known as ‘intensive monitoring’ or ‘active surveillance’) for the detection of safety signals have been used. Many of these methods are still in development, and their usefulness for identifying safety signals is being evaluated [4, 5]. According to the International Conference on Harmonisation (ICH) E2E Pharmacovigilance planning guideline [6], which serves as a fundamental framework for regulatory activities, active surveillance, in contrast to passive surveillance, aims to fully identify AE through an ongoing, structured process, such as monitoring patients under a specific drug as part of a risk management program. Generally, active surveillance strategies tend to provide more detailed and comprehensive information on individual AE reports compared with passive reporting systems [7–9].
One of the examples for which robust post-marketing safety surveillance is paramount is that of antiviral agents. These therapies often have complex safety profiles and long-term treatment regimens, increasing the likelihood of AE over time. Certain AE, such as liver toxicity and immune reconstitution inflammatory syndrome, can be particularly challenging to capture due to their overlap with symptoms of underlying viral infections or other co-morbid conditions [10]. Furthermore, AE may be underreported or misdiagnosed, especially in resource-limited settings, necessitating rigorous and systematic data collection. As for other medicines, active surveillance strategies on antiviral agents encompass various epidemiological designs like cross-sectional, case-control, and cohort studies. Ray et al. (2023) [11] utilised a drug event monitoring (DEM) strategy, actively enquiring about new events in patients on antiretroviral therapy (ART) during visits and encouraging reporting of symptoms via telephone or clinic visits outside scheduled appointments for the first 6 months after ART initiation. Similarly, Mann et al. (2016) [12] conducted a sentinel site study, focusing on the use of medical records at ART sites, which were found to contain high-quality information on therapy, clinical notes, and laboratory values. Along the same lines, the World Health Organization (WHO) recently provided tools to assist countries in implementing comprehensive, long-term monitoring of individuals with HIV [13]. Such longitudinal monitoring, using clinical data from patient records, helps identify patterns and risk factors of AE over time among those living with HIV [14–19].
Despite these considerations, there is insufficient systematised evidence to distinctly differentiate active pharmacovigilance strategies, particularly in their ability to detect AE in the context of monitoring antiviral agents. Although evidence suggests that active surveillance systems have advantages over passive surveillance in detecting AE [20, 21], the specific efficacy of these methods for antiviral agents remains underexplored. This gap in detailed understanding of how active pharmacovigilance strategies differ, particularly in aspects like specific study designs, data sources, and AE frequencies across various clinical settings, limits our ability to identify the most suitable strategy for each distinct clinical context. It is expected that new findings will significantly inform the choice of ideal monitoring methods for patients undergoing antiviral therapy, supporting future public health emergency management on this therapeutic approach.
The aim of this systematic review was to identify and characterise active pharmacovigilance strategies employed to detect AE in patients taking systemic antiviral agents in real-world clinical settings.
Methods
This study followed the current guidance of conducting and reporting systematic reviews, including guidance for undertaking reviews in health care on public health interventions by the Centre for Reviews and Dissemination of the University of York [22], as well as recommendations from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [23]. The protocol for this review was registered at PROSPERO (CRD42022337541).
Eligibility Criteria
We included studies with patients exposed to antiviral agents, focusing on active pharmacovigilance strategies. We aimed to measure the overall prevalence or incidence of AE, using (i) observational prospective active pharmacovigilance studies, (ii) phase IV clinical trials for post-marketing safety surveillance, and (iii) interventional studies assessing active pharmacovigilance strategies. We excluded articles that were solely protocol descriptions or methodological guides, lacking actual data or analysis relevant to active pharmacovigilance. Additionally, studies only reporting specific AE or restricted to certain disease severity levels were excluded. No restrictions were applied based on participants’ age, gender, or specific medical conditions.
For the purposes of this review, active pharmacovigilance was defined in line with the ICH E2E Pharmacovigilance planning guideline [6]. This encompasses various strategies, including but not limited to sentinel sites, DEM, and registries. AE were defined according to the Good Pharmacovigilance Practices (GVP) [24] of the European Medicines Agency (EMA). Lastly, the scope of antiviral agents was specified based on the Encyclopedia of Microbiology [25] and the Anatomical Therapeutic Chemical (ATC) classification system [26]. We limited our scope to antivirals for systemic use, as defined by the ATC classification code J05. This includes specific antiviral agents, while excluding vaccines, dermatological and ophthalmological antivirals, and amantadine when used as an antiparkinsonian drug. The description of these concepts is available in Table S1 (see electronic supplementary material [ESM]).
Information Sources and Search Strategy
We systematically searched MEDLINE (via PubMed), Web of Science, and SCOPUS to identify eligible studies, without setting any date restriction. Our search strategy is available in Table S2 (see ESM). We did not limit our search by the language of publication, performing translations or assessments by language-proficient individuals for non-English papers as necessary. In addition to database searches, we examined the reference list of all included studies to identify further potential studies, including unpublished or in-press citations. Expert consultations were conducted to discover additional unpublished materials, until nothing new was found.
Study Selection and Data Extraction
Study selection was carried out independently by two reviewers, firstly by title/abstract screening (R.F.S. and M.P.) and, in a second phase, by full-text reading (R.F.S. and J.R.P.).
Data extraction from the included studies was performed independently by two reviewers using a purposely built internal online form. Extracted variables included the year of publication, eligible antiviral-containing regimen, country, setting of the monitoring, number of study centres, period of inclusion, eligible population (age and sex), sample size, age (range, mean or median), clinical conditions, study design, clinical data sources, definitions of AE, severity and causality assessments, type of active pharmacovigilance strategy and its detailed description, as well as raw or pre-calculated data on the frequency of AE or the number of individuals who developed at least one AE. The clinical data sources reported (i.e., the resources where the researcher or healthcare professional obtained information for patient assessment) were categorised into nine labels: laboratory tests, physical examination, other complementary diagnostic and therapeutic procedures (CDTP), medical records, patient interview, patient self-report, healthcare professional interview, caregiver interview, and caregiver self-report (Table S3, see ESM).
Disagreements at any stage of the review process were resolved by a third reviewer, ensuring consistency and thoroughness in our approach. Agreement between reviewers on the stage selection was assessed by computation of the kappa coefficient.
Risk-of-Bias (Quality) Assessment
The risk of bias of each included primary study was independently assessed by two researchers (R.F.S. and J.R.P.) using the Newcastle-Ottawa Scale (NOS) [27] for evaluating the quality of nonrandomised studies. Three domains were considered to score the quality of included studies: (i) selection, including representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of exposure, and demonstration that at the start of the study the outcome of interest was not present; (ii) comparability, assessed on the basis of study design and analysis, and whether any confounding variables were adjusted for; and (iii) outcome, based on the follow-up period and cohort retention, and ascertained by independent blind assessment, record linkage, or self-report. Studies were awarded a maximum of one point for each numbered item within the selection and outcome categories. A maximum of two points were given for comparability. We rated the quality of the studies as good, fair and poor, by scoring in each domain following the guidelines of the NOS. Disagreements between reviewers were solved by consensus. We utilised the Robvis tool to generate traffic lights and summary plots for the assessment of risk of bias [28].
No scale was directly applied for clinical trials as none were included in our review.
Data Analysis
Our main outcome consisted of the incidence of AE associated with antiviral agents, either directly obtained or calculated from raw data. We assessed the frequency of patients developing at least one AE (calculating both cumulative incidences and the incidence rates [IR]) and the IR for the occurrence of AE. IR were reported in events per person-years (PY). Where available, AE were collected in preference to adverse drug reactions, given that the former definition is more comprehensive than the latter. The estimated effect measures considered only the values reported for global AE, that is, values reported solely for specific groups of AE, severity levels, or other restrictive criteria were not included. For each measure, a 95% confidence interval (CI) was calculated to assess the precision of the estimates. The heterogeneity among studies of the same clinical condition, in terms of recorded strategies, prescribed therapeutic regimens and utilised clinical data sources, led us to decide against performing a meta-analysis. All analyses were performed using software R.
Results
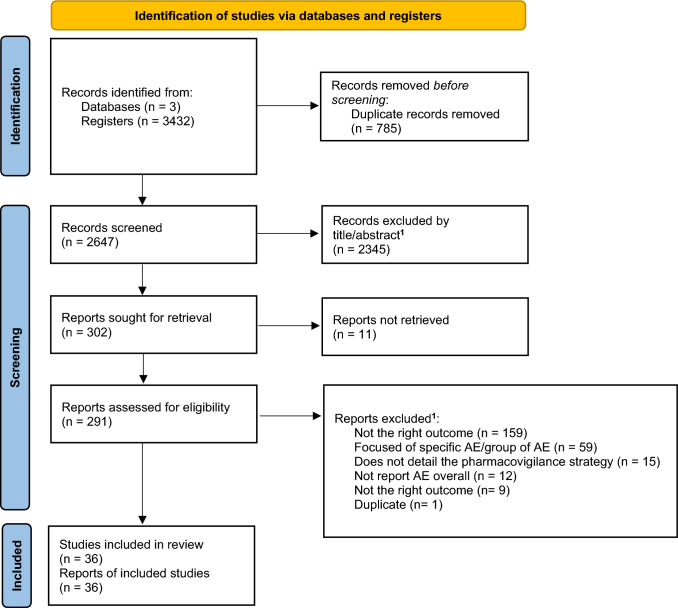
We retrieved 3432 records from three databases (Fig. 1). After duplicate removal, 2647 records were screened, of which 291 were assessed for eligibility. A total of 36 different primary studies (published in 36 publications/reports) were included in the systematic review [11, 12, 29–62]. At the end of the screening phase, the kappa coefficient was 0.715 (95% CI 0.670–0.760). Upon completion of the selection by full-text reading, the kappa coefficient reached 0.951 (95% CI 0.896–1).
Fig. 1.
PRISMA flow diagram for primary study selection
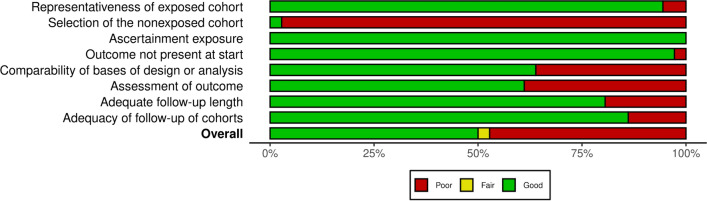
A comprehensive description of the included primary studies is presented in Table 1, and their risk-of-bias assessment is detailed in Table S4 (see ESM). A graphical representation of the risk-of-bias assessment has been illustrated in the summary and traffic-light plots, in Fig. 2 and Fig. S1 (see ESM), respectively.
Table 1.
Description of primary studies included in the systematic review, organised by clinical condition
| Study (first author, year) | Eligible antiviral-containing regimen | Studies’ characteristics | Patients’ characteristics | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Country | Setting of the monitoring; no. of study centres | Period of inclusion | Follow-up period | Eligible population age and sexa | Sample sizeb | Age range [mean (SD) or median (IQR)*] |
Male (n, %) | ||
| HIV | |||||||||
| Khalili et al. (2009) [29] | Any ART-containing regimen | Iran | Research centre/ University; 1 | 2005–2007 | At least 6 months | ≥18 years; both | 150 | 36.2 (10.6) | 123 (82%) |
| Modayil et al. (2010) [30] | India | Hospital; 1 | June 2008–February 2009 | 8 months | Any age; both |
400 (intensive monitoring group) |
NR | 223 (55.7%) | |
| Nagpal et al. (2010) [31] | India | ART centre; 1 | May 2006–April 2007 | 6 months | 18–60 years; both | 235 | NR | NR | |
| Abaissa et al. (2012) [32] | Ethiopia | Tertiary hospital outpatient clinic/ University teaching hospital; 1 | October 2008–December 2009 |
18 weeks 79 person-years |
≥18 years; both | 228 |
19–65 34.9 (8.3) |
82 (36%) | |
| Bernal et al. (2013) [33] | Chile | Hospital; 1 | May 2011–July 2011 | 1 month | NR; both | 92 | NR | 71 (77.2%) | |
| Bezabhe et al. (2015) [34] | Ethiopia | Hospital outpatient clinics/ University hospitals; 2 | December 2012–May 2014 |
12 months (2362 person-months) |
≥18 years; both | 211 | 32 (27–38)* | 84 (39.8%) | |
| Jha et al. (2015) [35] | India | Tertiary hospital; 1 | NR | 2 months | NR; both | 327 | NR | NR | |
| Mann et al. (2016) [12] | Namibia | Sentinel ART outpatient clinics; 2 | August 2012–April 2013 |
Up to 12 months (Mean: 6.6 [0.2] months) |
≥18 years; both | 413 | 37 (10.0) | 138 (41%) | |
| Gudina et al. (2017)c [36] | Ethiopia | University hospitals; 7 | September 2009–December 2013 | Treatment duration period (mean: 43.7 [28.7] months; median: 41.6 months) | ≥18 years; both | 3921 | 34.5 (9.2) | 1495 (38.1%) | |
| Isa et al. (2018) [37] | Nigeria | Outpatient clinic at a tertiary hospital; 1 | February 2017–July 2017 | 6 months | ≥12 years; both | 167 | 37.19 (9.704) | 63 (37.7%) | |
| Oumar et al. (2019) [38] | Mali | HIV clinic; 1 | June 2011–May 2012 | 6 months + 2 weeks | ≥18 years; both | 843 |
18–76 36.9 (10.1) 36.0 (29.0–44.0)* |
237 (28.1%) | |
| Sarraf et al. (2020) [39] | Nepal | ART clinics; 1 | April 2018–March 2019 | 1 year | ≥18 years; both | 496 |
18–70 37.23 (9.67) |
269 (54.2%) | |
| Omolo et al. (2020) [40] | South Africa | Hospital; 1 | 2009–2012 | 19 months |
All; both 268 female (78%) 75 male (22%) |
343 | NR | 75 (22%) | |
| Ray et al. (2023) [11] | India | Tertiary care children teaching hospital; 1 | March 2014–June 2019 | 6 months | ≤18 years; both | 174 | NR | 112 (64.4%) | |
| Bonfanti et al. (2000) [41] | Any PI-containing regimen | Italy | Hospitals; 10 | September 1997–April 1999 |
Treatment duration period |
≥18 years; both | 1207 | 37.1 (8) | 880 (72.9%) |
| Pujades-Rodríguez et al. (2011) [42] | Stavudine | Resource-limited countries | Hospitals and clinics with HIV programs (MSF); 23 | January 2005–December 2009 | Patient follow-up was censored at the earlier of the following events: date of last clinical visit with prescription of a stavudine-containing regimen, change in stavudine dose, death, transfer, toxicity diagnosis, or 4 years (62,505 person-years of follow-up; months on ART to event [IQR]: 13.0 [7.2–21.1]) | ≥15 years; both | 48,785 | 35.6 (30.0–43.0)* | 16,306 (33.4%) |
| Hongo et al. (2021) [43] | Dolutegravir | Japan | NR; 30 | From April 2014 on Tivicay tablets, and from April 2015 on Triumeq combination tablets until August 2020 | NR |
Trivicay tablets: children and ≥12 years; both Triumeq tablets: ≥18 years; both |
2292 |
13–83 41.1 (11.8) |
2181 (95.2%) |
| Ann et al. (2019) [44] | Kivexa® (lamivudine + abacavir sulfate)-containing regimen (Kivexa® + NNRTI or Kivexa® + PI + INI) | Korea | Hospitals; 23 | July 2011–July 2017 |
Treatment duration period (1,003.8 person-years) |
>12 years; both | 600 | 47.2 (12.4) | 551 (91.8%) |
| Tukei et al. (2012) [45] | 3-drug regimen comprising of 2 NRTI and 1 NNRTI | Uganda | Outpatient paediatric and family-centred clinic; 1 | July 2004–July 2009 |
Treatment duration period (median follow-up time: 170 [14–229] weeks) |
6 weeks–18 years; both | 378 | 7 (3–12)* | 181 (52.1%) |
| Tetteh et al. (2015) [46] |
Lamivudine + zidovudine or Lamivudine + zidovudine + lopinavir-ritonavir |
Ghana | Tertiary hospital; 1 | January 2005–December 2010 | Follow-up contact at 10 days for the 3-day lamivudine/zidovudine regimen, and additional follow-up at 35 days for the 28-day regimens (either lamivudine/ zidovudine or lamivudine/ zidovudine/ lopinavir-ritonavir) | NR; both | 228 | NR | 112 (40%) |
| Joseph et al. (2016) [47] | Tenofovir disoproxil fumarate-containing regimen (tenofovir + lamivudine + nevirapine regimen or tenofovir + lamivudine + efavirenz) | India | Outpatient clinic; 1 | January 2013–June 2013 | 12 months | NR; both | 198 | NR | 102 (51.5%) |
| Jena et al. (2009) [48] |
Lamivudine + stavudine + nevirapine or Lamivudine + zidovudine + nevirapine or Lamivudine + stavudine + efavirenz or Lamivudine + zidovudine + efavirenz |
India | Hospital outpatient clinic/Institute of Medical Education & Research; 1 | NR | 6 months | NR; both | 100 |
23–66 38.12 (9.8) |
67 (67%) |
| Sharma et al. (2008) [49] |
Zidovudine + lamivudine + nevirapine or Stavudine + lamivudine + nevirapine or Zidovudine + lamivudine + efavirenz or Stavudine + lamivudine + efavirenz |
India | HIV clinic; 1 | September 2004–October 2006 | 2 years | NR; both | 90 | NR | NR |
| Influenza | |||||||||
| Komeda et al. (2014) [50] | Peramivir hydrate | Japan | NR; 189 | October 2010–February 2012 | NR | Any age; both | 1174 |
2–100 40.3 (18.7) 38.0* |
560 (47.7%) |
| Komeda et al. (2015) [51] | Japan | NR; 161 | October 2010–February 2012 | NR | <15 years; both | 1199 |
0.0–14.0 6.9 (3.9) 7.0* |
654 (54.5%) | |
| Komeda et al. (2016) [52] | Japan | NR; 124 | January 2010–March 2013 | NR | Any age; both | 770 |
0–103 58.0 (32.2) 73.0 |
404 (52.5%) | |
| Kashiwagi et al. (2012) [53] | Laninamivir | Japan | NR; 787 | November 2010–April 2011 | 15 days | Any age; both | 3542 |
2–94 22.1 (16.5) |
1732 (48.9%) |
| Nakano et al. (2021) [54] | Japan | NR; 208 | November 2019–April 2020 | 15 days | <5 years; both | 1104 | NR | 564 (51.1%) | |
| Dalvi et al. (2011) [55] | Oseltamivir | India | Tertiary hospital; 1 | January 2010–March 2010 | 10 days | <12 years; both | 191 |
2 months–11 years 3 (1–6)* years |
124 (64.9%) |
| Tahara et al. (2013) [56] | Japan | Hospitals and clinics; 21 and 198, respectively | December 2004–March 2005 | 4 weeks | <1 year; both | 1284 | NR | 702 (54.7%) | |
| Nakazawa et al. (2020) [57] | Baloxavir | Japan | Hospitals and clinics; 688 | March 2018–March 2019 | 7 days | Any age; both | 3094 | NR | 1451 (47%) |
| HCV | |||||||||
| Tinè et al. (2010) [58] |
Pegylated interferon alfa-2a + ribavirin or Pegylated interferon alfa-2b + ribavirin |
Italy | Tertiary care; 1 |
Interferon alfa-2b + ribavirin: September 2000–January 2005 Pegylated interferon alfa-2b + ribavirin: January 2002–January 2005 |
180 days | ≥18 years; both | 312 | 47.5 (11) | 214 (68%) |
| Suzuki et al. (2018) [59] | Daclatasvir + asunaprevir | Japan | NR; 261 | September 2014–February 2017 | 24 weeks after the date of treatment completion or discontinuation; patients who discontinued early were followed up for 28 days for safety | NR; both | 2820 |
(21.0–92.0) 71.0* |
1239 (43.9%) |
| Ahmed et al. (2018) [60] |
Sofosbuvir + daclatasvir or Sofosbuvir + daclatasvir + ribavirin |
Egypt | Hospital outpatient clinic; 1 | January 2017–June 2017 | 6 months | 18–75 years; both | 345 | NR | 222 (64.3%) |
| Mizokami et al. (2021) [61] | Ledipasvir + sofosbuvir | Japan | Clinical sites; 169 | November 2015–January 2017 | 4 weeks | Any age; both | 3292 |
16.0–94.0 68.0 (60.0–75.0)* |
1348 (41%) |
| HBV | |||||||||
| Kim et al. (2018) [62] | Entecavir | Korea | NR; 132 | May 2006–May 2012 | Treatment duration period (mean: 258.34 [151.49] days; median: 204 days) | NR; both | 3367 |
16–85 43.74 (11.41) |
2375 (70.6%) |
ART antiretroviral therapy, DAA direct-acting antivirals, INI integrase inhibitors, MSF Médecins Sans Frontières, NRTI nucleoside reverse transcriptase inhibitors, NNRTI non-nucleoside reverse transcriptase inhibitors, PI protease inhibitors, HBV hepatitis B virus, HCV hepatitis C virus, HIV human immunodeficiency virus, NA not applicable, NR not reported
aF female, M male
bSample size used for safety analyses (which may differ from the sample size used for other outcomes analyses, e.g., effectiveness analyses) was considered
c Employed a mixed design, incorporating both retrospective and prospective designs. However, only the prospective approach was considered in our analysis
The primary studies were sequentially arranged in the table based on (i) clinical condition, (ii) antiviral-containing regimen, and (iii) year of publication, from oldest to most recent
Fig. 2.
Global summary plot for risk of bias based on the Newcastle-Ottawa Scale (NOS)
The included studies were performed in 14 different countries, mainly in Asia (n = 22; 61.1%) [11, 29–31, 35, 39, 43, 44, 47–57, 59, 61, 62] and Africa (n = 9; 25.0%) [12, 32, 34, 36–38, 40, 45, 46]. Additionally, there were studies from Europe (n = 2; 5.6%) [41, 58], South America (n = 1; 2.8%) [33], and Africa (n = 1) [60]. Based on the World Bank’s country classifications by income level for 2022–2023, 16 (44.4%) studies were reported in high-income countries, 12 (33.3%) in lower-middle-income countries, six (16.7%) in low-income countries, and two (5.6%) in upper-middle-income countries. One study was conducted in “resource-limited countries”, without specifying [42]. All studies were published post-2007, except for one from the year 2000 [41]. All included studies had a prospective cohort design, with one of them employing a mixed design that also incorporated a retrospective approach [36].
The studies were conducted in a diverse range of settings, including academic and research centres, hospitals, clinics, and other practice sites. Eight studies did not provide sufficient details regarding the monitoring setting [43, 50–54, 59, 62]. Most studies encompassed a wide variety of therapeutics regimens, reporting on at least one drug from the categories of ART, direct-acting antivirals (DAA), integrase inhibitors (INI), nucleoside reverse transcriptase inhibitors (NRTI), non-nucleoside reverse transcriptase inhibitors (NNRTI), and protease inhibitors (PI) [11, 12, 29–49, 60–62].
Most of the studies employed DEM as their active pharmacovigilance strategy, with only three utilising sentinel sites [12, 59, 61] and one a registry [42]. Twelve studies used one clinical data source [35, 40, 42–44, 50, 52, 54, 57–59, 62], 17 studies reported using two clinical data sources [11, 12, 29–33, 37, 38, 45, 46, 49, 51, 53, 55, 56, 61], four studies used three [36, 39, 41, 60], two studies used four [47, 48], and one study reported using five sources [34]. Among the 12 studies that reported using only one clinical data source for AE monitoring, nine relied solely on patient interviews [35, 40, 42–44, 50, 52, 58, 59]. Laboratory tests [29, 32, 33, 36, 41, 45, 47, 48, 60] and medical records [12, 30, 31, 34, 36–39, 61] were each reported in nine studies. Patients’ self-report appeared in eight studies [11, 34, 41, 49, 53, 57, 60, 62], while physical examinations were noted in five [39, 47–49, 56]. Caregivers’ interview [34, 51, 55] and caregivers’ self-report [54–56] were each used in three studies, while healthcare professionals’ interview [34, 46], and other CDTP [47, 48] were each used in two studies.
Among studies using the DEM strategy (n = 32), 15 (47%) reported using two clinical data sources, ten (31%) reported a single source, four (13%) three sources, two (6%) four sources, and one (3%) five sources. For the sentinel sites strategy (n = 3), two (67%) reported two data sources, and one (33%) reported a single source. Finally, a single data source was used for the sole study employing a registry (Fig. S2, see ESM).
A full description of the active pharmacovigilance strategy, along with categorisation by strategy type and clinical data sources, is provided in Table S5 (see ESM). A summary of the results for the different outcomes assessed in each primary study can be found in Table 2 and Figs. 3, 4, 5.
Table 2.
Summary of the results obtained for the different outcomes assessed for each primary study
| Study (first author, year) | Number of patients developing AEa | Number of AE | Total number of patients | Total person-years observed | Incidence of individuals with AE, % (95% CI) | Incidence rate of individuals with AE per 100 person-years (95% CI) | Incidence rate of AE per 100 person-years (95% CI) |
|---|---|---|---|---|---|---|---|
| HIV | |||||||
| Khalili et al. (2009) [29] | 131 | * | 150 | * |
87.33 (82.17–92.82) |
* | * |
| Modayil et al. (2010) [30] | * | 159 | 400 | 266.80 | * | * | 59.60 (51.02–69.62) |
| Nagpal et al. (2010) [31] | 213 | 618 | 235 | 117.50 |
90.64 (86.99–94.44) |
181.28 (158.50–207.33) | 525.96 (486.08–569.10) |
| Abaissa et al. (2012) [32] | 116 | 575 | 392 | 79.00 |
29.59 (25.4–34.47) |
146.84 (122.40–176.14) | 727.85 (670.72–789.84) |
| Bernal et al. (2013) [33] | 62 | 76 | 92 | 7.66 |
67.39 (58.46–77.68) |
809.02 (630.75–1037.68) | 991.70 (792.03–1241.72) |
| Bezabhe et al. (2015) [34] | 181 | * | 211 | 196.83 |
85.78 (81.2–90.63) |
91.96 (79.49–106.38) | * |
| Jha et al. (2015) [35] | 43 | 53 | 327 | 54.61 |
13.15 (9.95–17.37) |
78.74 (58.40–106.17) | 97.05 (74.15–127.04) |
| Mann et al. (2016) [12] | 66 | 102 | 413 | 227.15 |
15.98 (12.81–19.94) |
29.06 (22.83–36.98) | 44.90 (36.98–54.52) |
| Gudina et al. (2017) [36] | 867 | 1253 | 3921 | 14,280.28 |
22.11 (20.85–23.45) |
6.07 (5.68–6.49) | 8.77 (8.30–9.27) |
| Isa et al. (2018) [37] | 98 | 313 | 167 | 83.50 |
58.68 (51.67–66.65) |
117.37 (96.28–143.06) | 374.85 (335.54–418.77) |
| Oumar et al. (2019) [38] | 357 | * | 843 | 45.69 |
42.35 (39.14–45.82) |
781.34 (704.35–866.75) | * |
| Sarraf et al. (2020) [39] | 172 | 240 | 496 | 496.00 |
34.68 (30.73–39.13) |
34.68 (29.86–40.27) | 48.39 (42.64–54.91) |
| Omolo et al. (2020) [40] | * | 406 | 343 | 541.94 | * | * | 74.92 (67.97–82.57) |
| Ray et al. (2023) [11] | 78 | 110 | 174 | 87.00 |
44.83 (38.02–52.86) |
89.66 (71.81–111.93) | 126.44 (104.88–152.42) |
| Bonfanti et al. (2000) [41] | 433 | * | 1207 | 1076.64 |
35.87 (33.27–38.68) |
40.22 (36.60–44.19) | * |
| Pujades-Rodríguez et al. (2011) [42] | * | 4878 | 48,785 | 62,505.00 | * | * | 7.80 (7.59–8.03) |
| Hongo et al. (2021) [43] | 565 | * | 2292 | * |
24.65 (22.95–26.48) |
* | * |
| Ann et al. (2019) [44] | 310 | 674 | 600 | 1003.80 |
51.67 (47.82–55.82) |
30.88 (27.63–34.52) | 67.14 (62.26–72.41) |
| Tukei et al. (2012) [45] | 107 | 126 | 378 | 1236.06 |
28.31 (24.11–33.23) |
8.66 (7.16–10.46) | 10.19 (8.56–12.14) |
| Tetteh et al. (2015) [46] | |||||||
| 3TC/AZT for 3 days | 146b | 62 | 101 | 2.77 |
64.04 (58.10–70.58)2 |
5275.71 (4485.74–6204.81) | 2240.37 (1746.69–2873.59) |
| 3TC/AZT for 28 days | 197 | 75 | 7.19 | 2029.89 (1725.94–2387.37) | 2738.96 (2381.99–3149.44) | ||
| 3TC/AZT/LPV-RTV for 28 days | 169 | 52 | 4.99 | 2927.73 (2489.34–3443.33) | 3388.95 (2914.65–3940.42) | ||
| Joseph et al. (2016) [47] | * | 178 | 198 | 198.00 | * | * | 89.90 (77.62–104.13) |
| Jena et al. (2009) [48] | 46 | * | 100 | 50.00 |
46.0 (37.20–56.88) |
92.00 (68.91–122.83) | * |
| Sharma et al. (2008) [49] | 64 | 143 | 90 | 180.00 |
71.11 (62.34–81.12) |
35.56 (27.83–45.43) | 79.44 (67.43–93.59) |
| Influenza | |||||||
| Komeda et al. (2014) [50] | 86 | 143 | 1174 | * |
7.33 (5.98–8.98) |
* | * |
| Komeda et al. (2015) [51] | 245 | 168 | 1199 | * |
20.43 (18.27–22.85) |
* | * |
| Komeda et al. (2016) [52] | 219 | 412 | 770 | * |
28.44 (25.43–31.81) |
* | * |
| Kashiwagi et al. (2012) [53] | 50 | 59 | 3542 | 145.58 |
1.41 (1.07–1.86) |
34.35 (26.03–45.32) | 40.53 (31.4–52.31) |
| Nakano et al. (2021) [54] | 11 | 15 | 1104 | 45.37 |
1.00 (0.55–1.79) |
24.24 (13.43–43.78) | 33.06 (19.93–54.84) |
| Dalvi et al. (2011) [55] | 69 | 146 | 191 | 5.23 |
36.13 (29.92–43.62) |
1318.45 (1041.34–1669.32) | 2789.77 (2372.04–3281.08) |
| Tahara et al. (2013) [56] | 385 | * | 1284 | 98.87 |
29.98 (27.58–32.6) |
389.41 (352.39–430.32) | * |
| Nakazawa et al. (2020) [57] | 345 | * | 3094 | 59.40 |
11.15 (10.09–12.32) |
580.76 (522.60–645.39) | * |
| HCV | |||||||
| Tinè et al. (2010) [58] | 84 | 853 | 312 | 153.82 |
26.92 (22.42–32.32) |
54.61 (44.1–67.63) | 554.56 (518.56–593.05) |
| Suzuki et al. (2018) [59] | 726 | 1063 | 2820 | 1302.84 |
25.74 (24.18–27.41) |
55.72 (51.81–59.93) | 81.59 (76.83–86.65) |
| Ahmed et al. (2018) [60] | 261 | * | 345 | 172.40 |
75.65 (71.26–80.32) |
151.39 (134.10–170.92) | * |
| Mizokami et al. (2021) [61] | 197 | 265 | 3292 | 253.49 |
5.98 (5.23–6.85) |
77.72 (67.59–89.36) | 104.54 (92.68–117.92) |
| HBV | |||||||
| Kim et al. (2018) [62] | 255 | 380 | 3367 | 2383.84 |
7.57 (6.73–8.52) |
10.70 (9.46–12.09) | 15.94 (14.42–17.63) |
AE adverse events, CI confidence interval, HBV hepatitis B virus, HCV hepatitis C virus, HIV human immunodeficiency virus
*Fields left blank indicate that the data were unavailable in the original studies, or that estimating a measure of effect was not feasible due to insufficient data
a Corresponds to the number of people who developed at least one adverse event. Where available, adverse events were collected rather than adverse drug reactions
b The n = 146 corresponds to the overall number of people who developed at least one adverse event (i.e., including all therapeutic regimens). The respective effect measure—cumulative incidence—was calculated based on this value
The estimated effect measures—both cumulative incidence and incidence rates—considered only the values reported for global adverse events, i.e., values reported solely for specific groups of adverse events (e.g., only gastrointestinal disorders), severity levels (e.g., only severe cases), or other restrictive criteria were not included
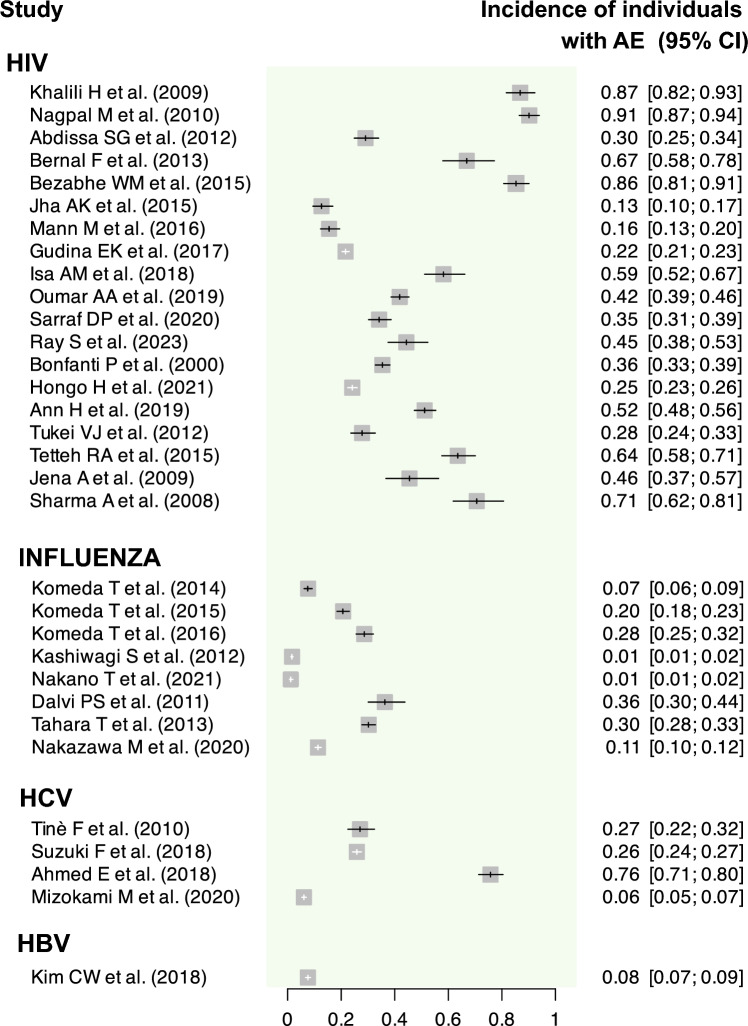
Fig. 3.
Graphical summary of the incidence of individuals with at least one AE assessed for each primary study. AE adverse events, CI confidence interval, HBV hepatitis B virus, HCV hepatitis C virus, HIV human immunodeficiency virus
Fig. 4.
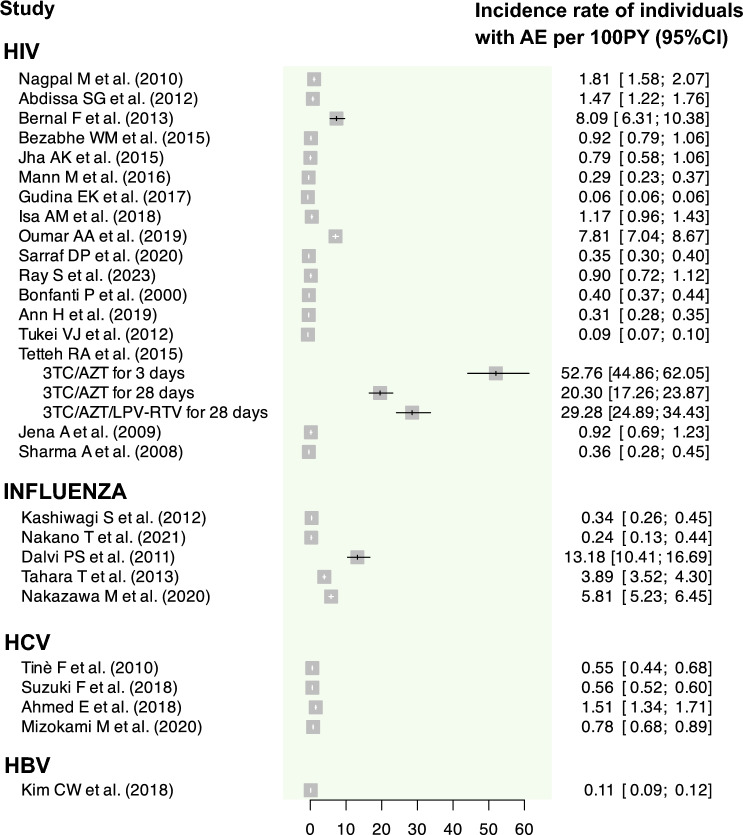
Graphical summary of the incidence rate of individuals with at least one AE assessed for each primary study. AE adverse events, CI confidence interval, HBV hepatitis B virus, HCV hepatitis C virus, HIV human immunodeficiency virus, PY person-years
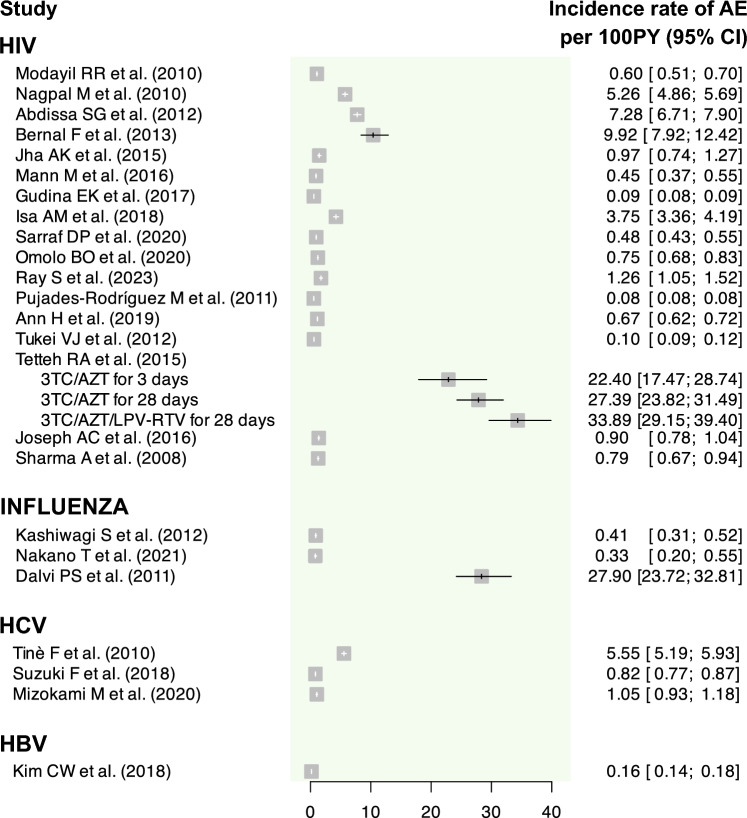
Fig. 5.
Graphical summary of the incidence rate of AE assessed for each primary study. AE adverse events, CI confidence interval, HBV hepatitis B virus, HCV hepatitis C virus, HIV human immunodeficiency virus, PY person-years
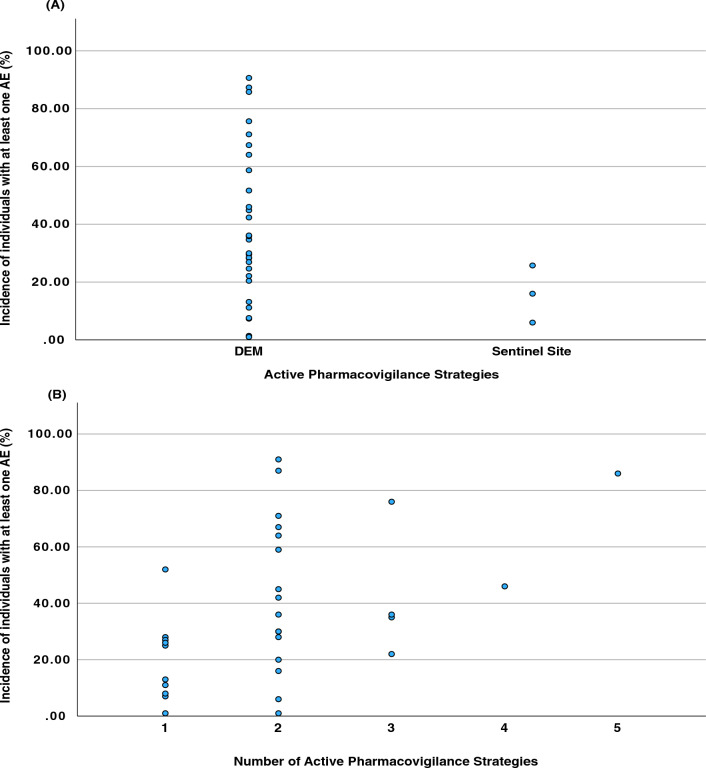
While a significant variation in the incidence of AE was observed in relation to the number of clinical data sources (Fig. 6), a noteworthy pattern emerges where studies relying solely on one data source typically report lower AE incidences. Incidences exceeding 30% are predominantly found in studies that employ at least two different data sources. Furthermore, incidences exceeding 60% were documented in seven studies (19%), with five utilising two data sources, one utilising three sources, and one involving five different sources.
Fig. 6.
Scatter plots of the incidence of individuals with at least one AE. (A) Dispersion of incidences in relation to the active pharmacovigilance strategy. (B) Dispersion of incidences in relation to the number of active pharmacovigilance strategies used. AE adverse events, DEM drug event monitoring
Human Immunodeficiency Virus
We identified 23 monitoring studies related to ART for HIV [11, 12, 29–35, 37–49]. Of these, 14 studies covered any ART-containing regimen [11, 12, 29–35, 37–40], one focused on any PI-containing regimen [41], one on stavudine [42], one on dolutegravir [43], and six on mixed regimens for HIV treatment [44–49]. Twenty-one studies were classified as DEM [11, 29–41, 43–49], one as a sentinel site [12], and one as a registry [42]. As for clinical data sources, patient interviews were reported in 22 studies [11, 12, 29–48], laboratory tests [29, 32, 33, 36, 41, 45, 47, 48] and medical records [12, 30, 31, 34, 36–39] in eight each, physical examinations [39, 47–49] and patient self-report [11, 34, 41, 49] in four each, HCP interviews [34, 46] and other CDTP [47, 48] in two each, and caregiver interviews [34] in one.
Only four studies did not report the incidence of patients with AE or provide raw data for such estimations [30, 40, 42, 47].
The incidence of patients developing at least one AE varied widely, ranging from 13.15% (95% CI 9.95–17.37%) [35] to 90.64% (95% CI 86.99–94.44) [31] (range of IR of patients developing at least one AE per 100 PY: 6.07 (95% CI 5.68–6.49) [36] to 5275.71 (95% CI 4485.74–6204.81) [46], the latter specifically observed in one subgroup of patients who underwent the 3TC/AZT regimen for 3 days). The IR of AE per 100 PY also showed considerable variation, with a range from 7.80 (95% CI 7.59–8.03) [42] to 3388.95 (95% CI 2914.65–3940.42) [46], the latter specifically observed in one subgroup of patients who underwent the 3TC/AZT/LPV-RTV regimen for 28 days. Only two studies [29, 43] failed to report either the IR of AE or the IR of patients with at least one AE, while eight studies [30, 34, 38, 40–42, 47, 48] reported only one of these effect measures.
The report [12] using sentinel sites as a pharmacovigilance strategy documented an incidence of 15.98% (95% CI 12.81–19.94) of HIV patients developing at least one AE, representing the second lowest incidence among the HIV studies. For this study, the IR of AE per 100 PY was 44.90 (95% CI 36.98–54.42), which was also among the lowest observed IR. For the report employing a registry-based pharmacovigilance strategy [42], only the IR of AE per 100 PY was reported, which was 7.80 (95% CI 7.59–8.03). This was the lowest reported rate for HIV.
Regarding the risk of bias, 14 studies were classified as of good quality [11, 12, 30, 32–34, 36, 37, 41–45, 48], eight as poor quality [29, 31, 35, 38–40, 47, 49], and one as fair quality [46]. All studies scored zero points in the ‘selection of the non-exposed cohort’ domain and one point for ‘ascertainment of exposure’. The only study classified as fair quality did not achieve the minimum of three points in the ‘selection' dimension to be classified as good quality, as it scored zero points in the ‘selection of the non-exposed cohort’ domain.
Influenza
Eight studies focused on patients with influenza [50–57]. Of these, three monitored the therapeutic regimen of peramivir hydrate [50–52], two laninamivir [53, 54], two oseltamivir [55, 56], and one baloxavir [57]. All studies were conducted in Japanese study centres, with the exception of one which was carried out in India [55].
All studies were classified as DEM. Regarding clinical data sources, patient interviews were reported in four studies [50–52], caregiver self-report in three [54–56], caregiver interview in two [51, 55], patient self-report in two [53, 57], and physical examination in one [56].
All studies reported the incidence of patients developing at least one AE. Only three [53–55] of these studies reported both IR for patients with AE and the IR of AE, while two [56, 57] reported solely the IR of patients with AE. The highest incidence of patients developing at least one AE was observed with oseltamivir (incidences of 36.13% [95% CI 29.92–43.62] [55] and 29.98% [95% CI 27.58–32.60 [56]), while the lowest incidences were for laninamivir (incidences of 1.00% [95% CI 0.55–1.79] [54] and 1.41% [95% CI 1.07–1.86] [53]). Regarding the IR of AE, although reported in only three studies [53–55], its values ranged from 33.06 (95% CI 19.93–54.94) to 2789.77 (95% CI 2372.04–3281.08) events per 100 PY.
Only one study [56] was deemed of good quality in the risk-of-bias assessment, while the remaining were classified as poor quality. All studies received one point in the ‘ascertainment of exposure’ and ‘outcome does not present at start’ domains. However, no study scored any points in the ‘assessment of outcome’ domain. The only study classified as good quality achieved maximum scores in all domains, except in ‘assessment of outcome’, which was affected by its use of self-reporting.
Hepatitis C Virus
We identified four studies [58–61] for HCV, all of which involved mixed therapeutic regimens. Two of these studies were classified as DEM [58, 60] and two as sentinel sites [59, 61]. All studies utilised patient interviews as clinical data sources, with one also including laboratory tests [60], another using medical records [61], and another incorporating patient self-report [60].
Incidence and both IR were computed for all studies, except for the IR for AE in one of the studies [60]. The incidence of patients developing at least one AE ranged between 5.98% (95% CI 5.23–6.85) [61] and 75.65% (95% CI 71.26–80.32) [60] (range of IR of patients developing at least one AE per 100 PY: 54.61 [95% CI 44.1–67.63] [58] to 151.39 [95% CI 134.10–170.92] [60]). The IR of AE ranged from 81.59 (95% CI 76.83–86.65) [59] to 554.56 (95% CI 518.56–593.05) [58] events per 100 PY.
Regarding the risk-of-bias assessment, two studies were classified as good quality [58, 60], and two as poor quality [59, 61]. All four studies had identical scores, except the two of good quality with an extra point in the ‘comparability of basis of design or analysis’ for controlling concomitant drug exposure. Also, among the poor quality studies, only one [61] achieved a point for ‘adequate follow-up length’.
Hepatitis B Virus
For the hepatitis B virus (HBV), we only identified one report, focusing on entecavir [62]. This report employed a DEM pharmacovigilance strategy and relied on patient self-reporting as the clinical data source. It estimated an incidence of patients developing at least one AE at 7.57% (95% CI 6.73–8.52) (IR of patients developing at least one AE per 100 PY: 10.70 [95% CI 9.46–12.09]). The IR of AE was 15.94 (95% CI 14.42–17.63) per 100 PY.
This study demonstrated good quality in the risk-of-bias assessment, with all domains scoring one point each, except for ‘selection of the non-exposed cohort’.
Discussion
To our knowledge, this is the first systematic review to provide a comprehensive characterisation of active pharmacovigilance strategies employed in patients undergoing systemic antiviral treatment in real-world clinical settings. Our analysis, based on the strategies identified in the ICH guidelines, also considers a wide range of clinical data sources used alongside these strategies, serving as pivotal guidance in planning pharmacovigilance activities within the regulatory framework of medication.
Our systematic review reveals that DEM is the most used active pharmacovigilance strategy for patients on systemic antiviral agents, with a notable focus on HIV, followed by influenza, HCV, and HBV. Interestingly, more than three-quarters of these DEM studies used one or two data sources, though some reported employing up to five different sources. In contrast, studies based on sentinel sites were less common, with only two conducted on HCV and one on HIV, each relying on one or two data sources. We only identified one study that utilised a registry, (specifically in the context of HIV) and which reported using just one data source. The most effective active pharmacovigilance strategy for a given scenario can vary widely, particularly in the context of systemic antiviral agents. This variability depends on factors such as the specific antiviral agent, the indication, the population receiving the treatment and the safety issue being addressed [6, 63, 64]. When selecting a pharmacovigilance method, it is crucial to consider the nature of the safety concern, whether it's a confirmed risk, a potential risk, or an information gap [6]. The aim of the investigation—whether for signal detection, thorough evaluation, or proving the safety of the drug—also plays a critical role in this decision [19]. Given the prolonged exposure of patients in chronic conditions to these antivirals, sponsors must choose a method that not only suits the study design but also adequately addresses the long-term safety concerns associated with sustained antiviral therapy [7, 16, 65, 66].
DEM typically involves identifying patients via electronic prescription data or health insurance claims, followed by administering follow-up questionnaires to physicians or patients at specified intervals [6]. These methods resonate with the well-established Prescription Event Monitoring (PEM) studies developed in New Zealand in the 1970s [67] and England in the 1980s [68, 69]. Such studies, known to target large sample sizes, historically aimed to enhance sensitivity in detecting rare AE [70]. However, later findings suggest a shift from this conventional approach, with a more tailored sample size in modern Modified-PEM (M-PEM) to meet specific research questions. This evolution is evident in the predominant application of DEM in chronic conditions like HIV and viral hepatitis in our study, highlighting its utility in long-term monitoring in outpatient settings [71]. As reflected in our primary studies, the extensive use of DEM in Japan underscores the global adaptation of this strategy [72]. With similar monitoring schemes under different names, these methods have been instrumental in complementing spontaneous reporting, particularly for chronic diseases [73]. The Japanese adaptation, J-PEM, which focuses on pharmacists and physicians for gathering information, mirrors this trend [74]. DEM methodology necessitates tailored follow-up strategies, adapted to each specific therapeutic regimen and clinical context. In particular for HIV treatments involving ART-containing regimens, the highest incidences of AE were observed in studies with a minimum follow-up of 6 months post-exposure. These factors, including the length and nature of follow-up, likely contribute to the observed diversity in the incidence of AE.
Sentinel site and registry strategies were less frequently used than DEM. The sentinel site approach enables the longitudinal collection and analysis of patient data at institutions chosen for their geographic location, characteristics of medical practice, and capacity to record and report high-quality data during routine clinical care [6]. In our systematic review, the reported incidences of AE were lower in sentinel site studies than expected, particularly given the nature of the strategy, which would typically anticipate higher values compared with those observed in DEM studies [6]. The limited number of sentinel site studies, along with varying incidences reported in high-income and limited-resource countries, adds complexity to interpreting these results. Furthermore, only one of the sentinel site studies reported monitoring during the treatment duration period, potentially limiting the detection of late-occurring AE. The diverse geographical and resource settings of these studies highlight the need for a nuanced understanding of how different contexts can impact the reporting and frequency of AE in active pharmacovigilance strategies. This suggests that while sentinel sites provide valuable insights in certain settings, their focused approach might limit their utility, requiring a broader range of data sources to capture a more comprehensive safety profile of antiviral agents across varied healthcare environments [75].
Registry strategies gather information about patients with specific characteristics, like a particular disease or drug exposure [6]. Though not primarily intended for safety event recording, drug exposure registries are vital for evaluating the effects of specific drugs on targeted groups, such as pregnant women, rheumatoid arthritis patients, or those with severe immune deficiencies [76]. We identified only one study employing a registry strategy, which relied solely on patient interviews as its data source and reported a notably low IR. This could be attributed to the fact that the study was conducted in resource-limited countries. Additionally, the follow-up of patients was censored at the occurrence of any of several AE, including the last clinical visit with a stavudine-containing regimen, change in stavudine dose, death, transfer, diagnosis of toxicity, or after 4 years. These factors likely contributed to the low IR observed. Furthermore, a global limitation of the registry approach is its focus on single cohorts, which restricts the ability to compare exposed versus unexposed groups. While registries are effective in measuring incidences, they are less useful for establishing associations. This highlights the inherent limitations of registries in pharmacovigilance, particularly when applied in settings with limited resources, and underscores the need for a diverse range of data sources to comprehensively assess the safety of antiviral agents [77]. The medical documentation in these cases should be straightforward, easily integrated into a physician’s routine visit notes, and regularly updated [75]. Approximately one-third of the medications approved in Europe (2007–2010) required the establishment of a registry, primarily for the purpose of gathering extra safety data. In September 2015, the EMA launched an initiative to better utilise existing registries and facilitate the creation of high-quality new registries where there are none that adequately provide post-authorisation data for regulatory decision-making [78].
Patient interviews have been identified as the predominant method for collecting AE reports. It should be noted that interviews are subject to bias by human memory, context, and experience so their reliability can be variable [79, 80]. For our purposes, we defined interviews as structured or unstructured, conducted in-person or remotely, and aimed at gathering AE reports from patients. This broad definition was crucial to accommodate the diverse interview methods encountered, particularly due to the deficient data reporting in the primary studies of our systematic review. These findings highlight the need for future studies to assess the quality of interviews in primary studies, an aspect not covered in our current analysis [81].
Medical records and laboratory tests were the next most commonly reported data sources after patient interviews. Additionally, it is noteworthy that employing multiple data sources appears to be associated with a higher likelihood of detecting increased AE incidence. The longitudinal monitoring using these sources provides a valuable complement to AE reporting. Using clinical data from patient records allows the identification of risk factors for AE, particularly in individuals with chronic infections [73, 82, 83]. Collecting longitudinal data from the first day of medication use is rarely provided by post-authorisation methods but enables tracking over time (latency and duration), outcomes, and management (to assist clinicians and patients in adequately anticipating AE management, improving adherence, and preventing early discontinuation) [4, 84, 85].
This study has some limitations, mostly arising from the included primary studies. Firstly, due to the lack of detail provided about the therapeutic regimens, we were unable to perform more comprehensive analyses of specific subgroups (e.g., subgroup analyses based on prescribed drugs, the severity of AE, or the degree of causality). Although several articles described the groups of antiviral agents used, they often lacked specific information on the exact drugs involved. Secondly, the variability among studies (even if focused on the same clinical condition) in terms of recorded strategies, prescribed therapeutic regimens and clinical data sources, led us to decide against performing a meta-analysis. Thirdly, regardless of causality assessment, terms such as ‘adverse event’ and ‘adverse drug reaction’ were often used interchangeably, lacking explicit definitions for consistent usage. In cases where both AE and ADR were reported, particularly when causality assessment algorithms were used, we opted to retrieve AE data due to their broader scope (as AE encompass ADR). Lastly, nearly half of the included studies were classified as having poor or fair quality. Future studies should follow observational study reporting guidelines, with a specific focus on addressing methodological weaknesses identified in our review. These weaknesses include aspects such as improving the selection and detailed description of the non-exposed cohort, enhancing comparability of cohorts based on study design or analysis, and rigorously assessing outcomes by considering factors like blinding and data reporting methods. Particularly, the lack of a well-defined non-exposed cohort and insufficient details in outcome definition and assessment could impede drawing causal conclusions and risk underreporting AE.
This systematic review also has important strengths. Firstly, this is the first systematic review to provide an extensive overview of active pharmacovigilance strategies in patients undergoing systemic antiviral treatments in real-world clinical settings. This review is grounded in the official ICH guidelines, which describe various pharmacovigilance methods for regulatory purposes. Secondly, despite the absence of a concurrent control group (single cohort design) in most of the included studies, it was still possible to calculate at least one incidence measure. Thirdly, to minimise the impact of publication bias, we conducted a comprehensive bibliographic search across three databases and did not exclude studies based on language, publication date, or status. These strategies could be driven either by clinical interest in ongoing patient follow-up or by regulatory requirements to collect additional data for market authorisation revalidation or to clarify safety signals. Our findings offer insights that can help guide the selection of strategies and data sources in different contexts, although the choice may depend on additional factors not covered in our study. This adaptability is essential for guiding healthcare professionals in choosing the best monitoring methods for patients on antivirals and proves to be crucial in managing future public health emergencies that require antiviral therapy.
Conclusion
DEM was the predominant pharmacovigilance strategy used and employing multiple data sources appears to increase the likelihood of detecting higher AE incidence. Although the ICH offers clear definitions for regulatory purposes, there remains a critical need to establish a common framework with a higher level of detail for defining active pharmacovigilance strategies. Currently, these strategies are referred to by a range of terms and typically involve a combination of different methodologies and clinical data sources. This variability poses significant challenges in the distinct identification and analysis of these strategies. We suggest that stakeholders in the pharmacovigilance field collaborate to establish a common framework for characterising active pharmacovigilance strategies based on study design, clinical data sources, target populations, clinical conditions, and types of therapeutic regimens.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors of this systematic review extend their sincere appreciation to all the authors of the primary studies for their invaluable contributions, including data provision and clarifications that greatly enriched the quality of this research.
Funding
Open access funding provided by FCT|FCCN (b-on).
Declarations
Funding
Renato Ferreira da Silva is grateful for the PhD scholarship 2020.10231.BD (DOCTORATES 4 COVID-19), funded by Portuguese national funds and community funds from the European Social Fund (ESF) through FCT – Fundação para a Ciência e a Tecnologia (Portugal). The Porto Pharmacovigilance Centre is a regional centre of the Portuguese Pharmacovigilance System, funded by INFARMED – National Authority for Medicines and Health Products of Portugal, I.P.
Ethical Approval
This article includes a review of studies involving human participants. However, as the review does not contain identifiable information and relies on previously published data, it is exempt from ethical approval.
Availability of data and material
All data generated and analysed during this study are included in this published article (and its electronic supplementary material).
Competing interests
The authors declare that they have no conflicts of interest that could potentially influence the work.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
The online form used for data extraction was created internally and is not publicly available. Upon reasonable request, the form can be provided by the corresponding author.
Author contributions
R.F.S. wrote the manuscript; R.F.S., M.M.S., M.M., J.J.P., and I.R.V. designed the research; R.F.S., J.R.P., M.P. performed the research; R.F.S., R.F.S., J.R.P., M.P., M.M.S., B.S.P., M.M., J.J.P., and I.R.V. analysed the data. All authors read and approved the final version of the manuscript.
References
- 1.Avorn J. In defense of pharmacoepidemiology—embracing the yin and yang of drug research. N Engl J Med. 2007;357(22):2219–21. 10.1056/NEJMp0706892. [DOI] [PubMed] [Google Scholar]
- 2.Garattini S, Chalmers I. Patients and the public deserve big changes in evaluation of drugs. BMJ. 2009;338:1025. 10.1136/bmj.b1025. [DOI] [PubMed] [Google Scholar]
- 3.Alomar M, Tawfiq AM, Hassan N, Palaian S. Post marketing surveillance of suspected adverse drug reactions through spontaneous reporting: current status, challenges and the future. Ther Adv Drug Saf. 2020;11:2042098620938595. 10.1177/2042098620938595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torre C, Cary M, Borges FC, et al. Intensive monitoring studies for assessing medicines: a systematic review. Front Med (Lausanne). 2019;6:147. 10.3389/fmed.2019.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vázquez-Alvarez AO, Brennan-Bourdon LM, Rincón-Sánchez AR, Islas-Carbajal MC, Huerta-Olvera SG. Improved drug safety through intensive pharmacovigilance in hospitalized pediatric patients. BMC Pharmacol Toxicol. 2017;18(1):79. 10.1186/s40360-017-0186-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agency EM. ICH Topic E 2 E Pharmacovigilance Planning (Pvp). 2005. CPMP/ICH/5716/03. June 2005. https://www.ema.europa.eu/en/ich-e2e-pharmacovigilance-planning-pvp-scientific-guideline
- 7.Härmark L, van Grootheest K. Web-based intensive monitoring: from passive to active drug surveillance. Expert Opin Drug Saf. 2012;11(1):45–51. 10.1517/14740338.2012.629184. [DOI] [PubMed] [Google Scholar]
- 8.Jha AK, Kuperman GJ, Teich JM, et al. Identifying adverse drug events: development of a computer-based monitor and comparison with chart review and stimulated voluntary report. J Am Med Inform Assoc. 1998;5(3):305–14. 10.1136/jamia.1998.0050305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bansal A, Agrawal AK, Sharma L, Jain S. A comparative study of active and passive adverse drug reaction reporting systems in terms of false reporting rate. 2020.
- 10.Otto AO, Rivera CG, Zeuli JD, Temesgen Z. Hepatotoxicity of contemporary antiretroviral drugs: a review and evaluation of published clinical data. Cells. 2021;10:5. 10.3390/cells10051263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ray S, Seth A, Singh S, et al. Short-term adverse drug reactions to antiretroviral therapy in children with HIV: a cohort study. Indian J Pediatr. 2023;90(1):9–15. 10.1007/s12098-021-04045-4. [DOI] [PubMed] [Google Scholar]
- 12.Mann M, Mengistu A, Gaeseb J, et al. Sentinel site active surveillance of safety of first-line antiretroviral medicines in Namibia. Pharmacoepidemiol Drug Saf. 2016;25(9):1052–60. 10.1002/pds.4022. [DOI] [PubMed] [Google Scholar]
- 13.Organization WH. WHO implementation tool for monitoring the toxicity of new antiretroviral and antiviral medicines in HIV and viral hepatitis programmes. Accessed December 10, 2023. https://iris.who.int/handle/10665/273053
- 14.Organization WH. Surveillance of antiretroviral toxicity: global HIV, hepatitis and STIs programme: what's new in person-centred HIV patient and antiretroviral drug toxicity monitoring: technical brief. Accessed December 10, 2023. https://iris.who.int/handle/10665/333000
- 15.Star K, Watson S, Sandberg L, Johansson J, Edwards IR. Longitudinal medical records as a complement to routine drug safety signal analysis. Pharmacoepidemiol Drug Saf. 2015;24(5):486–94. 10.1002/pds.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauben M, Bate A. Decision support methods for the detection of adverse events in post-marketing data. Drug Discov Today. 2009;14(7–8):343–57. 10.1016/j.drudis.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Lao KS, Chui CS, Man KK, Lau WC, Chan EW, Wong IC. Medication safety research by observational study design. Int J Clin Pharm. 2016;38(3):676–84. 10.1007/s11096-016-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahman M, Dal Pan G, Stein P, et al. When can real-world data generate real-world evidence? Pharmacoepidemiol Drug Saf. 2023. 10.1002/pds.5715. [DOI] [PubMed] [Google Scholar]
- 19.da Ferreira SR, Silva AM, Morato M, Ribeiro-Vaz I, Polónia JJ. Embracing uncertainties over the evidence of new oral antivirals for COVID-19: challenges in pharmacoepidemiologic research. J Clin Pharmacol. 2023;63(5):521–5. 10.1002/jcph.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y-L, Moon J, Segal JB. A comparison of active adverse event surveillance systems worldwide. Drug Saf. 2014;37(8):581–96. 10.1007/s40264-014-0194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chatterjee S, Aparasu RR. Pharmacovigilance to inform drug safety: challenges and opportunities. Encyclopedia of evidence in pharmaceutical public health and health services research in pharmacy. Springer International Publishing; 2020:1-12:chap Chapter 33-1.
- 22.Akers J, Aguiar-Ibáñez R, Baba-Akbari A. Systematic reviews: CRD’s guidance for undertaking reviews in health care. Centre for Reviews and Dissemination: University of York; 2009. [Google Scholar]
- 23.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agency EM. Guideline on Good Pharmacovigilance Practices (GVP) - Annex I - Definitions (Rev 4). 2017. EMA/876333/2011 Rev 4. 9 October 2017. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-annex-i-definitions-rev-4_en.pdf
- 25.Paintsil E, Cheng Y-C. Antiviral agents. In: Schaechter M, editor. Encyclopedia of microbiology (Third Edition). Academic Press; 2009. p. 223–57. [Google Scholar]
- 26.Mehtodology WCCfDS. ATC/DDD Index 2023. Accessed November 15, 2023. https://www.whocc.no/atc_ddd_index/
- 27.Wells GA, Wells G, Shea B, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Accessed November 15, 2023. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 28.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2020;2:55–61. 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 29.Khalili H, Dashti-Khavidaki S, Mohraz M, Etghani A, Almasi F. Antiretroviral induced adverse drug reactions in Iranian human immunodeficiency virus positive patients. Pharmacoepidemiol Drug Saf. 2009;18(9):848–57. 10.1002/pds.1793. [DOI] [PubMed] [Google Scholar]
- 30.Modayil RR, Harugeri A, Parthasarathi G, et al. Adverse drug reactions to antiretroviral therapy (ART): an experience of spontaneous reporting and intensive monitoring from ART centre in India. Pharmacoepidemiol Drug Saf. 2010;19(3):247–55. 10.1002/pds.1907. [DOI] [PubMed] [Google Scholar]
- 31.Nagpal M, Tayal V, Kumar S, Gupta U. Adverse drug reactions to antiretroviral therapy in aids patients at a tertiary care hospital in India: a prospective observational study. Indian J Med Sci. 2010;64(6):245–52. 10.4103/0019-5359.99597. [PubMed] [Google Scholar]
- 32.Abaissa SG, Fekade D, Feleke Y, Seboxa T, Diro E. Adverse drug reactions associated with antiretroviral treatment among adult Ethiopian patients in a tertiary hospital. Ethiop Med J. 2012;50(2):107–13. [PubMed] [Google Scholar]
- 33.Bernal F, Vasquez P, Giadalah C, Rodriguez L, Villagran A. Incidence of adverse drug reactions in patients initiating or changing antiretroviral therapy. Rev Chilena Infectol. 2013;30(5):507–12. 10.4067/S0716-10182013000500007. [DOI] [PubMed] [Google Scholar]
- 34.Bezabhe WM, Bereznicki LR, Chalmers L, et al. Adverse drug reactions and clinical outcomes in patients initiated on antiretroviral therapy: a prospective cohort study from Ethiopia. Drug Saf. 2015;38(7):629–39. 10.1007/s40264-015-0295-7. [DOI] [PubMed] [Google Scholar]
- 35.Jha AK, Gadgade A, Shenoy AK, Chowta MN, Ramapuram JT. Evaluation of adverse drug reactions in HIV positive patients in a tertiary care hospital. Perspect Clin Res. 2015;6(1):34–8. 10.4103/2229-3485.148808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gudina EK, Teklu AM, Berhan A, et al. Magnitude of antiretroviral drug toxicity in adult HIV patients in Ethiopia: a cohort study at seven teaching hospitals. Ethiop J Health Sci. 2017;2017–2(27):39–52. 10.4314/ejhs.v27i1.5s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isa AM, Abubakar IJ, Chedi BAZ. Adverse drug reactions to antiretroviral drugs and impact on treatment adherence among HIV patients in northwestern Nigeria. Drugs Therapy Perspect. 2018;34(10):488–95. 10.1007/s40267-018-0546-7. [Google Scholar]
- 38.Oumar AA, Dakouo M, Tchibozo A, et al. Antiretroviral-induced adverse drug reactions in HIV-infected patients in Mali: a resource-limited setting experience. Int J Basic Clin Pharmacol. 2019;8(5):831–6. 10.18203/2319-2003.ijbcp20191565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarraf DP, Rauniar GP, Chhetri R, et al. Pharmacovigilance of antiretroviral drugs at B.P. Koirala institute of health sciences. J Nepal Health Res Counc. 2020;18(4):596–603. 10.33314/jnhrc.v18i4.2634. [DOI] [PubMed] [Google Scholar]
- 40.Omolo BO, Njuho PM. Adverse event risk assessment on patients receiving combination antiretroviral therapy in South Africa. Int J Stat Med Res. 2020;2020(9):10–9. 10.6000/1929-6029.2020.09.02. [Google Scholar]
- 41.Bonfanti P, Valsecchi L, Parazzini F, et al. Incidence of adverse reactions in HIV patients treated with protease inhibitors: a cohort study. J Acquir Immune Defic Syndr. 2000;23(3):236–45. 10.1097/00126334-200003010-00004. [DOI] [PubMed] [Google Scholar]
- 42.Pujades-Rodriguez M, Dantony E, Pinoges L, et al. Toxicity associated with stavudine dose reduction from 40 to 30 mg in first-line antiretroviral therapy. PLoS ONE. 2011;6(11): e28112. 10.1371/journal.pone.0028112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hongo H, Nagao T, Nakamura K, et al. Safety and effectiveness analysis of dolutegravir in patients with HIV-1: interim report of post-marketing surveillance in Japan. Adv Ther. 2021;38(8):4480–504. 10.1007/s12325-021-01842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ann H, Lee YS, Kim YS, et al. Safety and effectiveness analysis of Kivexa(R) (lamivudine/abacavir sulfate) in human immunodeficiency virus infected Korean patients. Infect Chemother. 2019;51(2):150–60. 10.3947/ic.2019.51.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tukei VJ, Asiimwe A, et al. Safety and tolerability of antiretroviral therapy among HIV-infected children and adolescents in Uganda. J Acquir Immune Defic Syndr. 2012;59(3):274–80. 10.1097/QAI.0b013e3182423668. [DOI] [PubMed] [Google Scholar]
- 46.Tetteh RA, Nartey ET, Lartey M, et al. Adverse events and adherence to HIV post-exposure prophylaxis: a cohort study at the Korle-Bu Teaching Hospital in Accra, Ghana. BMC Public Health. 2015;15(1):573. 10.1186/s12889-015-1928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joseph AC, Sanalkumar KB, Bharathan CS, Andrews MA, Ajithkumar K. Adverse effect profile of tenofovir disoproxil fumarate in hiv positive patients—a prospective study. J Evol Med Dent Sci. 2016;5(31):1659–62. 10.14260/jemds/2016/391. [Google Scholar]
- 48.Jena A, Sachdeva RK, Sharma A, Wanchu A. Adverse drug reactions to nonnucleoside reverse transcriptase inhibitor-based antiretroviral regimen: a 24-week prospective study. J Int Assoc Physicians AIDS Care. 2009;8(5):318–22. 10.1177/1545109709343967. [DOI] [PubMed] [Google Scholar]
- 49.Sharma A, Vora R, Modi M, Sharma A, Marfatia Y. Adverse effects of antiretroviral treatment. Indian J Dermatol Venereol Leprol. 2008;74(3):234–7. 10.4103/0378-6323.41368. [PubMed] [Google Scholar]
- 50.Komeda T, Ishii S, Itoh Y, et al. Post-marketing safety and effectiveness evaluation of the intravenous anti-influenza neuraminidase inhibitor peramivir (I): a drug use investigation. J Infect Chemother. 2014;20(11):689–95. 10.1016/j.jiac.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 51.Komeda T, Ishii S, Itoh Y, et al. Post-marketing safety and effectiveness evaluation of the intravenous anti-influenza neuraminidase inhibitor peramivir. II: a pediatric drug use investigation. J Infect Chemother. 2015;21(3):194–201. 10.1016/j.jiac.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 52.Komeda T, Ishii S, Itoh Y, Sanekata M, Yoshikawa T, Shimada J. Post-marketing safety evaluation of the intravenous anti-influenza neuraminidase inhibitor peramivir: a drug-use investigation in patients with high risk factors. J Infect Chemother. 2016;22(10):677–84. 10.1016/j.jiac.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Kashiwagi S, Yoshida S, Yamaguchi H, et al. Safety of the long-acting neuraminidase inhibitor laninamivir octanoate hydrate in post-marketing surveillance. Int J Antimicrob Agents. 2012;40(5):381–8. 10.1016/j.ijantimicag.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 54.Nakano T, Yamaguchi H, Chiba T, Shiosakai K, Chikada S, Matsuoka Y. The safety and efficacy of the long-acting neuraminidase inhibitor laninamivir octanoate hydrate for Inhalation Suspension Set in children under the age of 5 in a post-marketing surveillance. J Infect Chemother. 2021;27(10):1436–46. 10.1016/j.jiac.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Dalvi PS, Singh A, Trivedi HR, Mistry SD, Vyas BR. Adverse drug reaction profile of oseltamivir in children. J Pharmacol Pharmacother. 2011;2(2):100–3. 10.4103/0976-500X.81901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tahara T, Asano Y, Mitamura K, Nakamura H, Itoh S. Safety of oseltamivir in infants less than one year old: Prospective surveillance during the 2004–2005 influenza season in Japan. J Pediatr Infect Dis. 2013;8(2):071–81. 10.3233/jpi-130381. [Google Scholar]
- 57.Nakazawa M, Hara K, Komeda T, Ogura E. Safety and effectiveness of baloxavir marboxil for the treatment of influenza in Japanese clinical practice: a postmarketing surveillance of more than 3000 patients. J Infect Chemother. 2020;26(7):729–35. 10.1016/j.jiac.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 58.Tinè F, Graviano D, Giannuoli G, et al. An open-safety study of dual antiviral therapy in real-world patients with chronic hepatitis C. Pharmacoepidemiol Drug Saf. 2010;19(11):1113–23. 10.1002/pds.2025. [DOI] [PubMed] [Google Scholar]
- 59.Suzuki F, Hatanaka N, Nakamura K, Komoto A. Safety and effectiveness of daclatasvir and asunaprevir dual therapy in patients with genotype 1 chronic hepatitis C: results from postmarketing surveillance in Japan. Hepatol Int. 2018;12(3):244–53. 10.1007/s12072-018-9872-z. [DOI] [PubMed] [Google Scholar]
- 60.Ahmed EI, Wahed WYA, Hassan EA, Ahmed TI. Study of adverse drug effects of direct-acting antivirals for chronic HCV infection at fayoum governorate, Egypt—a pharmacovigilance study. Curr Drug Saf. 2018;13(3):187–95. 10.2174/1574886313666180716111529. [DOI] [PubMed] [Google Scholar]
- 61.Mizokami M, Liu LJ, Fujiyama N, et al. Real-world safety and effectiveness of ledipasvir/sofosbuvir for the treatment of chronic hepatitis C virus genotype 1 in Japan. J Viral Hepat. 2021;28(1):129–41. 10.1111/jvh.13395. [DOI] [PubMed] [Google Scholar]
- 62.Kim CW, Kim CS, Kim HY, et al. Large-scale surveillance study of the safety and effectiveness of entecavir in Korean patients with chronic hepatitis B. Korean J Intern Med. 2018;33(1):91–101. 10.3904/kjim.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nadkarni PM. Drug safety surveillance using de-identified EMR and claims data: issues and challenges. J Am Med Inform Assoc. 2010;17(6):671–4. 10.1136/jamia.2010.008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Platt R, Madre L, Reynolds R, Tilson H. Active drug safety surveillance: a tool to improve public health. Pharmacoepidemiol Drug Saf. 2008;17(12):1175–82. 10.1002/pds.1668. [DOI] [PubMed] [Google Scholar]
- 65.Younossi ZM, Stepanova M, Younossi I, et al. Long-term effects of treatment for chronic HBV infection on patient-reported outcomes. Clin Gastroenterol Hepatol. 2019;17(8):1641-1642.e1. 10.1016/j.cgh.2018.09.041. [DOI] [PubMed] [Google Scholar]
- 66.Quinn TC. HIV epidemiology and the effects of antiviral therapy on long-term consequences. AIDS. 2008;22(3):S7-12. 10.1097/01.aids.0000327510.68503.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coulter DM. The New Zealand Intensive Medicines Monitoring Programme. Pharmacoepidemiol Drug Saf. 1998;7(2):79–90. 10.1002/(sici)1099-1557(199803/04)7:2%3c79::Aid-pds330%3e3.0.Co;2-1. [DOI] [PubMed] [Google Scholar]
- 68.Inman WH. Postmarketing surveillance of adverse drug reactions in general practice. II: Prescription-event monitoring at the University of Southampton. Br Med J. 1981;282(6271):1216–7. 10.1136/bmj.282.6271.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Inman WH. Prescription-event monitoring. Br Med J (Clin Res Ed). 1982;285(6344):809–10. 10.1136/bmj.285.6344.809-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Waller PC, Evans SJ. A model for the future conduct of pharmacovigilance. Pharmacoepidemiol Drug Saf. 2003;12(1):17–29. 10.1002/pds.773. [DOI] [PubMed] [Google Scholar]
- 71.Layton D, Hazell L, Shakir SA. Modified prescription-event monitoring studies: a tool for pharmacovigilance and risk management. Drug Saf. 2011;34(12):e1-9. 10.2165/11593830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 72.Kubota K, Kawabe E, Hinotsu S, Hamada C, Ohashi Y, Kurokawa K. Pilot study of prescription-event monitoring in Japan comparing troglitazone with alternative oral hypoglycemics. Eur J Clin Pharmacol. 2001;56(11):831–8. 10.1007/s002280000232. [DOI] [PubMed] [Google Scholar]
- 73.Wang L, Rastegar-Mojarad M, Ji Z, et al. Detecting pharmacovigilance signals combining electronic medical records with spontaneous reports: a case study of conventional disease-modifying antirheumatic drugs for rheumatoid arthritis. Front Pharmacol. 2018;9:875. 10.3389/fphar.2018.00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kubota K. A design for prescription-event monitoring in Japan (J-PEM). Pharmacoepidemiol Drug Saf. 1999;8(6):447–56. 10.1002/(SICI)1099-1557(199910/11)8:6%3c447::AID-PDS446%3e3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 75.Simbrich A, Thibaut J, Khil L, Maximov S, Wiendl H, Berger K. Chances and challenges of registry-based pharmacovigilance in multiple sclerosis: lessons learnt from the implementation of the multicenter REGIMS registry. Drug Saf. 2021;44(1):7–15. 10.1007/s40264-020-01007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gelperin K, Hammad H, Leishear K, et al. A systematic review of pregnancy exposure registries: examination of protocol-specified pregnancy outcomes, target sample size, and comparator selection. Pharmacoepidemiol Drug Saf. 2017;26(2):208–14. 10.1002/pds.4150. [DOI] [PubMed] [Google Scholar]
- 77.Miroshnychenko A, Zeraatkar D, Phillips MR, et al. Cohort studies investigating the effects of exposures: key principles that impact the credibility of the results. Eye. 2022;36(5):905–6. 10.1038/s41433-021-01897-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Agency EM. Patient registries. Accessed December 12, 2023. https://www.ema.europa.eu/en/human-regulatory-overview/post-authorisation/patient-registries
- 79.Mitchell AS, Henry DA, Sanson-Fisher R, O’Connell DL. Patients as a direct source of information on adverse drug reactions. BMJ. 1988;297(6653):891–3. 10.1136/bmj.297.6653.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sienkiewicz K, Burzyńska M, Rydlewska-Liszkowska I, Sienkiewicz J, Gaszyńska E. The importance of direct patient reporting of adverse drug reactions in the safety monitoring process. Int J Environ Res Public Health. 2021;19:1. 10.3390/ijerph19010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Organization WH. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Accessed December 11, 2023. https://www.who.int/publications/i/item/9789240031593 [PubMed]
- 82.Liu M, McPeek Hinz ER, Matheny ME, et al. Comparative analysis of pharmacovigilance methods in the detection of adverse drug reactions using electronic medical records. J Am Med Inform Assoc. 2013;20(3):420–6. 10.1136/amiajnl-2012-001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee S, Choi J, Kim HS, et al. Standard-based comprehensive detection of adverse drug reaction signals from nursing statements and laboratory results in electronic health records. J Am Med Inform Assoc. 2017;24(4):697–708. 10.1093/jamia/ocw168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Andrews EB, Moore N. Mann’s pharmacovigilance. New York: Wiley; 2014. [Google Scholar]
- 85.Satwika MV, Sushma DS, Jaiswal V, Asha S, Pal T. The role of advanced technologies supplemented with traditional methods in pharmacovigilance sciences. Recent Pat Biotechnol. 2021;15(1):34–50. 10.2174/1872208314666201021162704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.