Abstract
In this multi-center, Phase-1 study (NCT03733717), we characterized the pharmacokinetics (PK) of the anti-CD38 antibody isatuximab (Isa) after IV administration (primary objective), and evaluated safety, immunogenicity, and preliminary anti-myeloma activity in Chinese patients with relapsed/refractory multiple myeloma (RRMM). Isa 20-mg/kg was administered weekly (QW) in cycle 1, then biweekly (Q2W). Twenty-one extensively pretreated RRMM patients (median 4 prior lines; 95.2% refractory to last regimen), received ≥ 1 dose of Isa. After first IV-infusion, mean maximum observed concentration was 402 μg/mL and mean area-under-the-concentration-versus-time curve (first 1-week dosing interval) 37,000 μg·h/mL. After repeated administration, exposure (Ctrough) increased 3.11-folds (day 1/cycle 2) versus first administration (day 8/cycle 1). Safety findings were consistent with the known Isa safety profile, with no new safety signals. Any-causality, grade ≥ 3 treatment-emergent adverse events (TEAEs) were reported in 47.6% of patients. Serious, treatment-related AEs occurred in 2 patients. Isa treatment was generally well tolerated; only 1 patient discontinued due to TEAE. Preliminary efficacy results showed a 19.0% overall response rate (clinical benefit, 33.3%). Our results demonstrate a PK profile for Isa comparable to prior findings in Western and other East-Asian populations, as well as safety and tolerability of treatment with IV Isa 20-mg/kg QW-Q2W in Chinese RRMM patients.
Trial registration: The trial was registered with ClinicalTrials.gov; NCT03733717. Date of first trial registration: 07/11/2018.
Subject terms: Drug development, Myeloma, Drug development, Cancer immunotherapy
Introduction
Treatment with an anti-CD38 monoclonal antibody, as monotherapy or in combination with an immunomodulatory drug (IMiD; eg, pomalidomide, lenalidomide) or a proteasome inhibitor (PI; eg, bortezomib, carfilzomib) and dexamethasone can provide benefit to patients with relapsed/refractory multiple myeloma (RRMM) or at an earlier disease stage1–13.
Isatuximab (Isa) is an anti-CD38 IgG1 monoclonal antibody able to mediate anti-myeloma activity through multiple mechanisms of action, including antibody-dependent cell-mediated cytotoxicity, complement-dependent cytotoxicity, antibody-dependent cellular phagocytosis, and direct induction of apoptosis without crosslinking14–16. Isa is approved in various countries in combination with pomalidomide and low-dose dexamethasone for adult patients with RRMM who have received 2 or more prior therapies, including lenalidomide and a PI, based on the results of the Phase 3 ICARIA-MM study10,17,18. Isa is also approved in combination with carfilzomib and low-dose dexamethasone in the United States for patients with RRMM who have received 1–3 prior lines of therapy, in the European Union for patients with MM who have received ≥ 1 prior therapy, and in Japan for patients with RRMM after 1 prior treatment, based on the results of the Phase 3 IKEMA study13,17–19. Furthermore, Isa monotherapy is approved in Japan by the Pharmaceutical and Medical Devices Agency (PMDA) for patients with RRMM, based on the Phase 1/2 Islands study7.
As limited information is available on the pharmacokinetics (PK) and safety of treatment with Isa in Chinese patients, we conducted a multi-center, Phase 1 study to characterize the PK profile of Isa after single-agent, intravenous (IV) administration (primary study objective), and to evaluate safety, immunogenicity, and preliminary anti-myeloma activity of Isa monotherapy in Chinese patients with RRMM.
Patients and methods
Study design and treatment
In this multi-center, open-label, single-arm, Phase 1 study (NCT03733717; date of first trial registration: 07/11/2018), Isa was administered to adult patients with RRMM by IV infusion at 20 mg/kg every week (QW) in cycle 1 (on days 1, 8, 15, and 22), followed by every 2 weeks (Q2W) in subsequent cycles (on days 1 and 15), until unacceptable adverse event (AE), disease progression, withdrawal of consent, or investigator's decision. Each cycle lasted 4 weeks. Selection of the Isa dosing regimen was based on the globally recommended dose (from the TED10893 [NCT01084252] and TED14154 [NCT02514668] studies), which was further confirmed in the Japanese, dose-escalation study TED14095 (NCT02812706)4–7. To reduce the risk of infusion reactions (IRs), the recommended premedications were: on the day of isatuximab infusion, methylprednisolone 100 mg (or equivalent) administered IV or PO along with diphenhydramine 25–50 mg IV (or equivalent), ranitidine 50 mg IV (or equivalent), and acetaminophen 650–1000 mg PO 15–30 min (but no longer than 60 min) prior to the start of the Isa infusion. The primary study objective was to characterize the PK profile of Isa in Chinese patients with RRMM. Secondary objectives included evaluation of safety and tolerability, immunogenicity, and preliminary anti-myeloma activity of Isa in this patient population.
Patients
Adult patients (≥ 18 years of age) with symptomatic MM and measurable disease (defined as serum M-protein ≥ 5 g/L and/or urine M-protein ≥ 200 mg/24 h)20 were eligible for enrollment if they had received at least 2 prior lines of anti-myeloma treatment, including one or more IMiD drug or PI and prior therapy with an IMiD drug or PI for ≥ 2 cycles or ≥ 2 months of treatment. Furthermore, patients should have responded to at least 1 prior line of therapy (with minimal response [MR] or better) and be refractory to the most recently received IMiD drug- or PI-based therapy (eg, with disease progression during or within 60 days of IMiD or PI treatment completion). For patients who had received more than 1 type of IMiD or PI, their disease had to be refractory to the most recently received one.
Patients were not eligible if they had any of the following: Eastern Cooperative Oncology Group performance status (ECOG PS) > 2, a life expectancy < 3 months, concurrent plasma cell leukemia, known amyloidosis, disease measurable only by serum free-light chain analysis, Waldenstrom’s macroglobulinemia, an absolute neutrophil count < 1.0 × 109/L, platelets < 50 × 109/L (if < 50% of bone marrow nucleated cells were plasma cells) or < 30 × 109/L (if ≥ 50% of bone marrow nucleated cells were plasma cells), hemoglobin < 8 g/dL, inadequate liver or renal function (estimated glomerular filtration rate [eGFR] < 15 mL/min/1.73 m2), grade ≥ 3 neuropathy, and/or grade ≥ 2 peripheral neuropathic pain. Patients were also excluded if they had received prior treatment with any anti-CD38 agent, prior anti-cancer therapy (chemotherapy, targeted agents, or immunotherapy) within 21 days, systemic radiation therapy within 4 weeks, or a major surgical procedure within 4 weeks, prior to first study drug infusion.
The study protocol was approved by the Institutional Review Board of the Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College and by the Ethics Committee of the Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. The study was conducted following the Declaration of Helsinki and the International Council for Harmonisation (ICH) Guidelines for Good Clinical Practice. All patients provided written informed consent.
Assessments
Pharmacokinetics
Blood samples for PK analyses were collected from patients at protocol-specified time points: on days 1, 2, 3, 4, 8, 15, and 22 of cycle 1 and then mainly prior to and/or at the end of infusion (EOI) or just after the EOI in subsequent cycles until cycle 10. Gyrolab Platform, a quantitative sandwich immunoassay using biotinylated anti-Isa antibodies bound by streptavidin beads, within the Gyrolab Bioaffy CD microstructure, for capture and Alexa Fluor® 647-conjugated CD38 antibody for detection, was used to measure levels of functional Isa (Isa with ≥ 1 site available to bind target) in plasma, with a lower limit of quantitation (LLOQ) of 5.0 µg/mL and an upper limit of quantitation of 500 µg/mL21,22. Concentrations and actual sampling times, actual dose values, and infusion duration were used to perform Isa PK analyses over the first week of administration, by non-compartmental analysis (NCA) using Phoenix WinNonlin® v8.2 (Pharsight, Cary, NC). The PK parameters included Ceoi (concentration observed at the end of IV infusion), Cmax (maximum observed concentration), tmax (time to reach Cmax), Clast (last concentration observed above the LLOQ), tlast (time to Clast), and AUCtau (area under the curve of plasma concentrations versus time calculated using the trapezoidal method over the dosing interval; ie, 168 h).
Immunogenicity
Blood samples for the assessment of Isa immunogenicity were collected pre-infusion on day 1 of each cycle up to cycle 10 (or cut-off date, whichever came first) and then 3 months after the last Isa administration. Samples were analyzed for anti-drug antibodies using a validated PandA (polyethylene glycol precipitation and acid dissociation) method23,24.
Safety and efficacy
AEs and laboratory abnormalities were monitored and graded by the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03. Best overall responses were evaluated according to the International Myeloma Working Group (IMWG) uniform response criteria25. Overall response rate (ORR) was defined as the proportion of patients with stringent complete response (sCR), CR, very good partial response (VGPR), or partial response (PR) as best overall response.
Statistical analyses
A total of 20 evaluable Chinese patients was considered a reasonable sample size for the planned PK analyses, based on the drug registration requirements of the China National Medical Products Administration. The PK population included patients who had received at least 1 dose of Isa, even if incomplete, and had at least 1 PK sample concentration post-dosing, with adequate documentation of sampling and dosing. The safety population included patients who had received at least 1 dose of Isa. Categorical and ordinal data were summarized using the number and percentage of patients. Continuous data were summarized using the number of available observations, mean, median, standard deviation (SD), minimum, and maximum. PK parameters were summarized by descriptive statistics (such as mean, geometric mean, median, SD, standard error of the mean [SEM], coefficient of variation [CV], minimum, and maximum). SAS 9.4 software (SAS, Cary, NC) was used for all the analyses, except for the PK parameters which were analyzed using Phoenix WinNonlin® version 8.2.
Results
Patients
This study was conducted in adult, Chinese patients with RRMM at 3 investigational sites in China. A total of 21 enrolled patients received at least 1 dose of study treatment. Nineteen (90.5%) patients discontinued treatment, owing to progressive disease (71.4%), patient withdrawal (14.3%), or AE (4.8%). Two (9.5%) patients were still receiving treatment at the data cut-off date (September 6, 2020). One (4.8%) of these 2 patients was still on treatment as of October 14, 2022.
The patients on study had a median age of 58 (46–72) years (Table 1). Approximately half (57.1%) of the patients had ECOG PS 1 and 14.3% had ECOG PS 2. The majority (76.2%) of the patients had International Staging System (ISS) stage II or III at study entry (42.9% at stage III) and bone lesions. Median bone marrow plasma cells at baseline was 24% (range, 0–97%). This patient population had been extensively pretreated for multiple myeloma, with a median of 4 (range, 2–11) prior lines. Twelve (57.1%) patients had received 4 or more prior treatment lines. Most patients were refractory to an IMiD drug (90.5%), a PI (66.7%), or both (57.1%). Twenty patients (95.2%) were refractory to the last treatment regimen received (Table 1).
Table 1.
Patient demographics and baseline characteristics.
| Isa (N = 21) | |
|---|---|
| Age, median (range), yrs | 58 (46–72) |
| < 65 years, n (%) | 18 (85.7) |
| 65 to < 75 years, n (%) | 3 (14.3) |
| Female, n (%) | 11 (52.4) |
| Male, n (%) | 10 (47.6) |
| ECOG PS, n (%) | |
| 0 | 6 (28.6) |
| 1 | 12 (57.1) |
| 2 | 3 (14.3) |
| Median time from initial diagnosis (range), yrs | 2.61 (0.7–10.9) |
| ISS stage at study entry, n (%) | |
| Stage I | 4 (19.0) |
| Stage II | 7 (33.3) |
| Stage III | 9 (42.9) |
| Not known | 1 (4.8) |
| Median BMPC (range) at study entry, % | 24.0 (0–97.0) |
| Patients with bone lesions at study entry, n (%) | 16 (76.2) |
| Number of prior lines, median (range) | 4 (2–11) |
| Number of prior lines, n (%) | |
| 1 | 0 |
| 2 | 7 (33.3) |
| 3 | 2 (9.5) |
| ≥ 4 | 12 (57.1) |
| Refractory to, n (%) | |
| IMiD | 19 (90.5) |
| PI | 14 (66.7) |
| IMiD and PI | 12 (57.1) |
| Refractory to last treatment regimen, n (%) | 20 (95.2) |
BMPC, bone marrow plasma cells; ECOG PS, Eastern Cooperative Oncology Group performance status; IMiD, immunomodulatory drug; Isa, isatuximab; ISS, international staging system; PI, proteasome inhibitor.
Treatment exposure
The median duration of first infusion was 6.1 h and 4.1 h for subsequent infusions. The overall median number of cycles started with Isa in these patients was 3 (range, 1–18), with a median duration of exposure of 11.14 (range, 1.0–71.9) weeks and a median total cumulative dose of 142 (range, 3.0–738.8) mg/kg. Delays of at least 1 cycle occurred in 35.0% of patients. The median relative dose intensity for Isa was ~ 101.0% in cycle 1 and ~ 99.2% in subsequent cycles.
Pharmacokinetics
Twenty patients were evaluable for PK. The mean concentration–time profile after first IV infusion of Isa at 20 mg/kg in cycle 1, in Chinese patients (n = 20), is shown in Fig. 1. Isa given at 20 mg/kg was quantifiable in plasma over the dosing period of 1 week in all patients (LLOQ, 5 µg/mL). The observed inter-patient variability was moderate (CV% = 27–45%).
Figure 1.
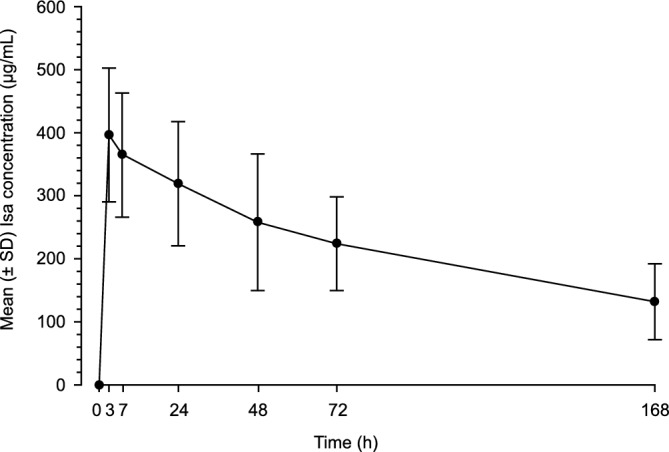
Mean (± SD) Isa concentration–time profile after first IV infusion in Chinese patients (20 mg/kg, n = 20). h, hours; Isa, isatuximab; IV, intravenous; n, number of patients; SD, standard deviation.
PK parameters for Isa after first IV infusion at 20 mg/kg in this population are listed in Table 2. The mean Cmax was 402 ± 113 μg/mL, close to the mean Ceoi (396 ± 106 µg/mL). The mean AUCtau over the first 1-week dosing interval was 37 000 ± 12 000 μg·h/mL, with low to moderate variability (CV% for Cmax and AUCtau: 28% and 33%, respectively). Cmax was observed at the EOI in the majority of patients (15 out of 20). tmax was observed ~ 4 h after the EOI in 4 patients and at 48 h in 1 patient. The median Tmax was 7.6 h (~ 3 h post EOI).
Table 2.
Isa PK parameters observed over the first week after IV administration in Chinese patients.
| Mean ± SD [geometric mean] | Isa 20 mg/kg (n = 20) |
|---|---|
| Dose (mg/kg) | 19.8 ± 0.455 [19.8] |
| Infusion durationa (h) | 6.05 (3.85–9.47) |
| Ceoi (µg/mL) | 396 ± 106 [384] |
| Cmax (µg/mL) | 402 ± 113 [388] |
| tmaxa (h) | 7.63 (3.93–46.7) |
| Clast (µg/mL) | 132 ± 59.8 [113] |
| tlasta (h) | 167 (144–195) |
| AUCtau (µg·h/mL) | 37 000 ± 12 000 [35 100] |
aMedian (min–max) values.
AUCtau, area under the curve of plasma concentrations versus time over the dosing interval of 0–168 h; Ceoi, concentration observed at the end of IV infusion; Clast, last observed concentration above the LLOQ (5 µg/mL); Cmax, maximum observed concentration; h, hours; Isa, isatuximab; IV, intravenous; LLOQ, lower limit of quantitation; SD, standard deviation; Tlast, time to last concentration observed above the LLOQ; Tmax, time to reach Cmax.
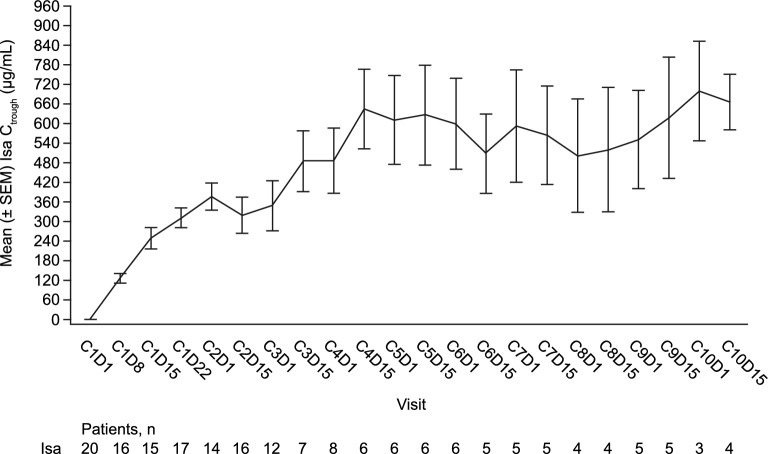
Evaluation of the Isa plasma trough concentrations (prior to start of infusion) observed during repeated administration QW for 4 weeks then Q2W (Fig. 2) showed a 3.11-fold increase in exposure (Ctrough) at the end of weekly administration (cycle 2/day 1) compared with the first administration (cycle 1/day 8), based on the geometric mean ratio. Taking into account the low number of Ctrough values per cycle from cycle 3/day 15 (less than 8/20), the exposure was considered to remain within the same magnitude during Q2W administration (cycle 4/day 1 to cycle 1/day 8 geometric mean ratio = 3.68).
Figure 2.
Mean (± SEM) Isa Ctrough over time during repeated administration in Chinese patients (20 mg/kg, QW for 4 weeks then Q2W). C, cycle; Ctrough, pre-dose trough concentration during repeated dosing; D, day, Isa, isatuximab; n, number of patients; QW, once weekly; Q2W, once every 2 weeks; SEM, standard error of the mean.
None of the 20 patients evaluable for immunogenicity developed an anti-drug antibody response to Isa during treatment.
Safety
All patients (N = 21) had a TEAE of any grade and 10 (47.6%) patients experienced a grade ≥ 3 TEAE of any causality, as summarized in Table 3. Four (19%) of patients died within 30 days of last study treatment (2 due to progressive disease, 1 to an AE of grade ≥ 3 pneumonia, and 1 to other, unknown reason). Treatment-related, grade ≥ 3 TEAEs occurred in 4 (19.0%) patients and serious TEAEs in 2 (9.5%) patients (grade ≥ 3 pneumonia and bacterial sepsis, n = 1 each).
Table 3.
Overview of TEAEs and treatment discontinuations.
| n (%) | Isa (N = 21) |
|---|---|
| Any TEAE (all grades) | 21 (100) |
| Any grade ≥ 3 TEAE | 10 (47.6) |
| Any serious TEAE | 7 (33.3) |
| Any drug-related grade ≥ 3 TEAE | 4 (19.0) |
| Any drug-related serious TEAE | 2 (9.5) |
| Any grade 5 TEAE | 4 (19) |
| Any TEAE leading to definitive treatment discontinuation | 1 (4.8) |
| Any AESI (all grades) | 0 |
| Any IR (all grades) | 13 (61.9) |
| Any IR grade ≥ 3 | 0 |
AESI, adverse event of special interest; IR, infusion reaction; Isa, isatuximab; TEAE, treatment-emergent adverse event.
Treatment was generally well tolerated, as only 1 (4.8%) patient definitively discontinued treatment due to a TEAE (fatal pneumonia). Infusion reactions occurred in 13 (61.9%) patients. All patients with IR had only 1 episode, of grade 1–2, occurring on infusion day in cycle 1. All infusion reactions resolved within 1 day.
Other most common, any-grade, all-causality, non-hematologic TEAEs were upper respiratory infection (42.9% of patients), decreased weight (28.6%), pneumonia (28.6%), bone pain (23.8%), constipation (23.8%), and diarrhea (23.8%) (Table 4). Grade ≥ 3, all-causality TEAEs reported in more than 1 patient included pneumonia (19.0%), upper respiratory infection (14.3%), bone pain (9.5%), and gingivitis (9.5%).
Table 4.
Most common, all-causality, non-hematologic TEAEs (in > 20% of patients).
| TEAE, n (%) | Isa (N = 21) | |
|---|---|---|
| All grades | Grade ≥ 3 | |
| Infusion reaction | 13 (61.9) | 0 |
| Upper respiratory infection | 9 (42.9) | 3 (14.3) |
| Decreased weight | 6 (28.6) | 1 (4.8) |
| Pneumonia | 6 (28.6) | 4 (19.0) |
| Bone pain | 5 (23.8) | 2 (9.5) |
| Constipation | 5 (23.8) | 0 |
| Diarrhea | 5 (23.8) | 0 |
Isa, isatuximab; TEAE, treatment-emergent adverse event.
For hematologic laboratory abnormalities (n = 20), grade 3 anemia and neutropenia were reported in 45% and 20% of patients, respectively, with no grade 4 events. Grade 3–4 thrombocytopenia was observed in 35% of patients (grade 4 in 20%) (Supplementary Table S1 online).
Anti-myeloma activity
Evaluation of best overall responses, per investigator’s assessment (N = 21), showed limited anti-myeloma activity in this group of RRMM patients with treatment-refractory disease after multiple, prior lines of therapy. Three patients achieved a PR and 1 patient a CR, for an ORR of 19.0% (95% CI 0.05–0.42). Clinical benefit (MR or better) was observed in 7 (33.3%) patients (95% CI 0.15–0.57) (Supplementary Table S2 online). The Kaplan–Meier estimate of the median duration of response in the 4 responders was 12.5 (95% CI 5.52–12.52) months.
Discussion
At the time this Phase 1 trial was designed in 2017, no PK data were published on anti-CD38 antibody therapy in Chinese patients. Results from this first study of Isa monotherapy in Chinese patients with RRMM, administered intravenously at 20 mg/kg once weekly for 4 weeks then every 2 weeks (QW–Q2W), have shown a PK profile (the primary study objective) consistent with prior findings from clinical studies of Isa administered as a single agent and in combination with dexamethasone, pomalidomide-dexamethasone, or carfilzomib-dexamethasone in Western and other East Asian patients5–7,17,18. Exposure to Isa, as measured by mean Cmax (402 ± 113 µg/mL) and mean AUCtau (37 000 ± 12 000 µg·h/mL), was within the range previously described in other studies3,5,7, with a median Tmax of ~ 7.6 h. A comparable 3.02 ratio in the geometric mean of the trough concentration observed between day 1 of cycle 2 and day 8 of cycle 1, which is a PK predictor of efficacy26, was reported in the Isa arm of the Phase 2 study by Dimopoulos et al., conducted in Western patients treated with Isa 20 mg/kg QW–Q2W at European and North American clinical sites5. Hence, our results with Isa in a Chinese patient population add to the current evidence indicating that race and ethnicity are covariates that generally do not appear to significantly influence the PK profile of therapeutic monoclonal antibodies7,27–30.
Safety findings, observed in this study in Chinese patients, were consistent with the known safety profile of Isa, with no new safety signals3–7,10,13. All-causality, grade ≥ 3 TEAEs were reported in 47.6% of patients and serious AEs in 33.3%. Serious, treatment-related TEAEs occurred in 2 (9.5%) patients. Respiratory tract infections were among the most common TEAEs, as reported with anti-CD38 antibody therapy in other populations of patients with RRMM3–9. Laboratory grade 3 neutropenia was observed infrequently in this study (20% of patients), with no grade 4 events.
Treatment with Isa was generally well tolerated in most patients, although they had received multiple lines of prior treatment, as only 1 patient discontinued due to a TEAE. As previously observed in other patient populations, Chinese patients experienced IRs at the beginning of treatment (infusion day in cycle 1, only 1 event per patient), with all events of grade 1–23–7. IRs resolved in all patients, with temporary dose interruptions in 57.1% of them, at first infusion. Overall, the patients received a median relative dose intensity for Isa of 99.2–101% across treatment cycles.
Differently from the Phase 1/2 trial conducted in Japanese patients treated with Isa 20 mg/kg7, in our study there was a greater proportion of patients with more advanced disease (eg, ISS stage III 42.9% vs 18%), bone lesions (76.2% vs 61%), ECOG PS ≥ 1 (71.4 vs 48%), higher median proportion of bone marrow plasma cells at study entry (24.0% vs 15.6%), and patients with plasmacytoma (47.6% vs 18%). Results of the pooled analysis of the GEN501 and SIRIUS Phase 1/2 studies of anti-CD38 monotherapy with the monoclonal antibody daratumumab in patients with RRMM and ≥ 2–3 prior lines of therapy have previously shown a lower ORR in patients with ISS stage III (22.5%) than in the overall study population (31.1%)8.
Preliminary efficacy results showed limited benefit in our group of patients with RRMM and treatment-refractory disease to last prior treatment regimen, after multiple prior lines of therapy, with an ORR of 19.0% and clinical benefit (MR or better) in 33.3% of the patients. Nonetheless, the observed responses lasted a median of 12.5 months. Our results are in line with the Phase 2 study by Dimopoulos et al. who reported an ORR of 19.6% (10/51) in RRMM patients with ≥ 4 prior lines of therapy and disease refractory to ≥ 2 PIs and ≥ 2 IMiDs following treatment with Isa 20 mg/kg QW–Q2W5. Earlier treatment in the course of the disease and combination of Isa with dexamethasone, as well as a PI or an IMiD, such as bortezomib, carfilzomib, pomalidomide, or lenalidomide, may further increase efficacy and improve overall treatment outcomes in Chinese patients with RRMM, as previously achieved with anti-CD38 antibody therapy in other patient populations5,10–13,31–34.
While conducted at multiple centers in China, with central review, limitations of this single-arm study include the relatively small number of patients as well as its open-label design. However, in addition to this study, there are currently three randomized, Phase 3 clinical trials and one real-world study of Isa combination therapy ongoing in China, which include Chinese patients with smoldering, newly diagnosed, or relapsed/refractory multiple myeloma. Results from these trials will contribute to overcome the limitations of this first, Phase 1 study.
In conclusion, taken together, our findings demonstrate a PK profile for Isa comparable to prior findings in Western and other East Asian patient populations, as well the safety and tolerability of treatment with IV Isa at 20 mg/kg QW–Q2W in Chinese patients with RRMM.
Supplementary Information
Acknowledgements
The authors thank the participating patients and their caregivers, the study centers, and the investigators for their contributions to the study. This study was funded by Sanofi. Medical writing support was provided by S. Mariani, MD, PhD of Envision Pharma Group, funded by Sanofi.
Abbreviations
- AE
Adverse event
- AUClast
Area under the curve of plasma concentrations versus time from time zero to tlast
- AUCtau
Area under the curve of plasma concentrations versus time over the dosing interval
- Ceoi
Concentration observed at the end of IV infusion
- CI
Confidence interval
- Clast
Last concentration observed above the LLOQ
- Cmax
Maximum observed concentration
- CR
Complete response
- CV
Coefficient of variation
- ECOG
Eastern cooperative oncology group
- IMiD
Immunomodulatory drug
- IMWG
International myeloma working group
- Isa
Isatuximab
- ISS
International staging system
- IV
Intravenous
- LLOQ
Lower limit of quantitation
- MM
Multiple myeloma
- MR
Minimal response
- ORR
Overall response rate
- PI
Proteasome inhibitor
- PK
Pharmacokinetics
- PR
Partial response
- PS
Performance status
- QW
Every week
- Q2W
Every 2 weeks
- RRMM
Relapsed/refractory multiple myeloma
- sCR
Stringent complete response
- SD
Standard deviation
- SEM
Standard error of the mean
- TEAE
Treatment-emergent adverse event
- tlast
Time to reach Clast
- tmax
Time to reach Cmax
- VGPR
Very good partial response
Author contributions
M.S., H.J., X.Q., Z.F., J.Q., and L.Q. designed the study, analyzed the data, wrote/critically revised the manuscript, and approved final version. F.D., Y.L., S.Z.-L., D.S., and L.L. analyzed the data, critically revised the manuscript, and approved final version.
Funding
The study was funded by Sanofi.
Data availability
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.vivli.org/. Contact for study data requests: Zhaoyi Feng; email: roy.feng@sanofi.com.
Competing interests
MS, HJ, XQ, FD, and JQ: nothing to disclose. YL, ZF, SZ-L, DS, and LL are employed by Sanofi and may hold stock and/or stock options. LQ: consulting for AstraZeneca, Beigene, Xi'an Janssen, Pfizer; Speaker Bureau participation for AstraZeneca, Beigene, Xi'an Janssen, Pfizer, Roche, and Sanofi.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Mingyuan Sun and Hongmei Jing.
Contributor Information
Junyuan Qi, Email: qijy@ihcams.ac.cn.
Lugui Qiu, Email: qiulg@ihcams.ac.cn.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-59186-1.
References
- 1.Cowan, A. J. et al. Diagnosis and management of multiple myeloma: A review. JAMA.327, 464–477 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Jullien, M., Touzeau, C. & Moreau, P. Monoclonal antibodies as an addition to current myeloma therapy strategies. Expert Rev. Anticancer Ther.21, 33–43 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Martin, T. et al. Phase I trial of isatuximab monotherapy in the treatment of refractory multiple myeloma. Blood Cancer J.9, 41 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mikhael, J. et al. A dose-finding Phase 2 study of single agent isatuximab (anti-CD38 mAb) in relapsed/refractory multiple myeloma. Leukemia34, 3298–3309 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimopoulos, M. et al. Isatuximab as monotherapy and combined with dexamethasone in patients with relapsed/refractory multiple myeloma. Blood137, 1154–1165 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikhael, J. et al. A phase 2 study of isatuximab monotherapy in patients with multiple myeloma who are refractory to daratumumab. Blood Cancer J.11, 89 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sunami, K. et al. Isatuximab monotherapy in relapsed/refractory multiple myeloma: A Japanese, multicenter, phase 1/2, safety and efficacy study. Cancer Sci.111, 4526–4539 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Usmani, S. Z. et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood.128, 37–44 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lonial, S. et al. Daratumumab monotherapy in patients with treatment refractory multiple myeloma (SIRIUS): An open-label, randomised, phase 2 trial. Lancet.387, 1551–1560 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Attal, M. et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): A randomised, multicentre, open-label, phase 3 study. Lancet.394, 2096–2107 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Dimopoulos, M. A. et al. Daratumumab plus pomalidomide and dexamethasone versus pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO): An open-label, randomised, phase 3 trial. Lancet Oncol.22, 801–812 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Dimopoulos, M. et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): Results from a randomised, multicentre, open-label, phase 3 study. Lancet.396, 186–197 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Moreau, P. et al. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): A multicentre, open-label, randomised phase 3 trial. Lancet.397, 2361–2371 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Deckert, J. et al. SAR650984, a novel humanized CD38-targeting antibody, demonstrates potent antitumor activity in models of multiple myeloma and other CD38+ hematologic malignancies. Clin Cancer Res.20, 4574–4583 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Cai, T., Wetzel, M., Nicolazzi, C., Vallee, F. & Deckert, J. Preclinical characterisation of SAR650984, a humanized anti-CD38 antibody, for the treatment of multiple myeloma. Clin. Lymphoma Myeloma Leuk.13, S180 (abstr P-288) (2013). [Google Scholar]
- 16.Jiang, H. et al. SAR650984 directly induces multiple myeloma cell death via lysosomal-associated and apoptotic pathways, which is further enhanced by pomalidomide. Leukemia.30, 399–408 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Sarclisa. Prescribing information. Sanofi; July 2022. Accessed 15 May 2023. https://products.sanofi.us/Sarclisa/sarclisa.pdf (2022).
- 18.European Medicines Agency. Sarclisa, INN-Ixatuximab. Summary of product characteristics. Accessed 15 May 2023. https://www.ema.europa.eu/en/documents/product-information/sarclisa-epar-product-information_en.pdf (2021).
- 19.SARCLISA® (isatuximab). Prescribing Information. Nishi Shinjuku, Tokyo, 2021. Accessed 15 May 2023. https://www.pmda.go.jp/PmdaSearch/iyakuDetail/ResultDataSetPDF/780069_4291454A1021_1_02 (2021).
- 20.Rajkumar, S. V. et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol.15, e538–e548 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Fraley, K. J. et al. The Gyrolab immunoassay system: A platform for automated bioanalysis and rapid sample turnaround. Bioanalysis.5, 1765–1774 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Dudal, S. et al. Assay formats: Recommendation for best practices and harmonization from the global bioanalysis consortium harmonization team. AAPS J.16, 194–205 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zoghbi, J., Xu, Y., Grabert, R., Theobald, V. & Richards, S. A breakthrough novel method to resolve the drug and target interference problem in immunogenicity assays. J. Immunol. Methods426, 62–69 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Myler, H. et al. Anti-drug antibody validation testing and reporting harmonization. AAPS J.24, 4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar, S. et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol.17, e328–e346 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Rachedi, F. et al. Exposure-response analyses for selection/confirmation of optimal isatuximab dosing regimen in combination with pomalidomide/dexamethasone treatment in patients with multiple myeloma. CPT Pharmacomet. Syst. Pharmacol.11, 766–777 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Othman, A. A., Tran, J. Q., Tang, M. T. & Dutta, S. Population pharmacokinetics of daclizumab high-yield process in healthy volunteers: Integrated analysis of intravenous and subcutaneous, single-and multiple-dose administration. Clin. Pharmacokinet.53, 907–918 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Honma, W., Gautier, A., Paule, I., Yamaguchi, M. & Lowe, P. J. Ethnic sensitivity assessment of pharmacokinetics and pharmacodynamics of omalizumab with dosing table expansion. Drug Metab Pharmacokinet.31, 173–184 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Xu, X. S. et al. Pharmacokinetics and exposure-response analyses of daratumumab in combination therapy regimens for patients with multiple myeloma. Adv. Ther.35, 1859–1872 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas, V. A. & Balthasar, J. P. Understanding inter-individual variability in monoclonal antibody disposition. Antibodies (Basel)8, 56 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leleu, X. et al. Anti-CD38 antibody therapy for patients with relapsed/refractory multiple myeloma: Differential mechanisms of action and recent clinical trial outcomes. Ann. Hematol.101, 2123–2137 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu, J. et al. Daratumumab, bortezomib, and dexamethasone versus bortezomib and dexamethasone in Chinese patients with relapsed or refractory multiple myeloma: Phase 3 LEPUS (MMY3009) study. Clin. Lymphoma Myeloma Leuk.21, e699–e709 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Sunami, K. et al. Isatuximab-pomalidomide-dexamethasone versus pomalidomide-dexamethasone in East Asian patients with relapsed/refractory multiple myeloma: ICARIA-MM subgroup analysis. Clin. Lymphoma Myeloma Leuk.22, 751–761 (2022). [DOI] [PubMed] [Google Scholar]
- 34.Kim, K. et al. Isatuximab plus carfilzomib and dexamethasone in East Asian patients with relapsed multiple myeloma: IKEMA subgroup analysis. Int. J. Hematol.116, 553–562 (2022). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.vivli.org/. Contact for study data requests: Zhaoyi Feng; email: roy.feng@sanofi.com.