Abstract
The detection of Leishmania tarentolae in sympatric areas where Leishmania infantum is endemic raised questions regarding the protective effect exerted in dogs by L. tarentolae when in coinfection. This study aimed monitoring the in vitro gene expression of pro- (IFN- γ; TNF-α; IL-12) and anti-inflammatory (IL-4; IL-6; IL-10) cytokines in primary canine macrophages infected by L. tarentolae and L. infantum in single and in co-infections. Macrophages differentiated from dog blood mononuclear cells were infected with the L. tarentolae field-isolated (RI-325) and laboratory (LEM-124) strains, with L. infantum laboratory strain (IPT1), or both. Infection and the number of amastigotes per infected cell were evaluated microscopically by counting a total of 200 cells between 4 and 96 h. Cytokine gene expression was analyzed by real-time PCR from infected macrophages mRNA. Single infections presented higher expression of the cytokines IL-4 and IL-6, and lower of IL-12. Co-infections induced a lower gene expression of IL-4 and IL-6, and a higher gene expression of IL-12, correlating with the low amastigote burden despite the slight increase of infected cells. Data highlight the potential protective effect of L. tarentolae against L. infantum in co-infection by the reduced anti-inflammatory and increased pro-inflammatory cytokines gene expression, opening new perspectives for a canine vaccine development exploiting the non-pathogenic L. tarentolae.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-78451-x.
Subject terms: Immunology, Diseases
Introduction
Leishmaniases are neglected, vector-borne tropical diseases caused by Leishmania spp. (class of Kinetoplastida, family of Trypanosomatidae), with more than 350 million people at risk of infection worldwide1. The genus Leishmania includes more than 20 species capable to infect and replicate in dendritic cells and macrophages of vertebrate hosts2–6, causing different clinical manifestations. Leishmania infantum, the main etiological agent of visceral leishmaniasis in humans, is the most widespread species of zoonotic concern that finds its reservoir in dogs, as well as in other mammals7,8. This disease is prevalent in poor socio-economic contexts (e.g. East Africa, Southeast Asia, and Latin America), and in several Mediterranean countries9,10, with a distribution overlapping with that of its sand fly vectors, Phlebotomus spp. and Lutzomyia spp. in the Old and New Worlds, respectively11,12. In dogs, canine leishmaniasis (CanL) caused by L. infantum, is widespread also in high-income countries, in tropical and temperate areas. Other Leishmania species, such as those found in reptiles (subgenus Sauroleishmania), may also occur in the same geographical areas of L. infantum13. For example, Leishmania tarentolae has been firstly described in Tarentola mauritanica gecko14 and was later detected in other reptiles, including Podarcis filfolensis and Podarcis siculus lizards15–17. As indicated by molecular and serological evidence, other hosts such as dogs and humans are exposed to this non-pathogenic species, as indicated by its detection using molecular and serological methods17,18. This suggests that this species may adapt to different vertebrate organisms. Leishmania tarentolae is vectored by Sergentomyia minuta17, a sand fly that feeds preferentially on reptiles and is highly prevalent in Mediterranean countries19. Nonetheless, S. minuta has also been reported to feed on mammals19,20, which may account for the detection of L. tarentolae DNA in human and dog blood13,18,21,22.
In vitro studies showed that L. tarentolae is capable of infecting murine and human macrophages and dendritic cells2–5. Recently, this species has also been shown to infect and persist in primary cultures of canine macrophages23. The latter finding, coupled with the detection of L. tarentolae in sympatric areas where L. infantum is endemic, raised questions regarding the potential protective effect that the non-pathogenic species may exert towards CanL in dogs co-infected with the pathogenic L. infantum6,13. The outcomes of such mixed infections may depend on various factors, including the balance between the host Th1 and Th2 immune responses24–27. Indeed, macrophages can be polarized into activated M1 or alternatively activated M2 phenotypes depending on cytokines stimuli28. In particular, a Th1 subset mediated by IFN-γ, TNF-α and IL-12 is suggested to promote the macrophage activation against parasite infection, limiting the infection and therefore the severity of clinical presentation29. On the other hand, the Th2 immune response, characterized by the production of cytokines such as IL-4 and IL-10, shift macrophages towards the M2 phenotype, suppressing the M1 activation and allowing the parasite to survive and multiply within these cells, possibly causing severe clinical manifestations30. Although IL-6 is often associated with pro-inflammatory response, this cytokine may also contribute to anti-inflammatory processes by modulating macrophage polarization. Indeed, IL-6 may shift toward either M1 or M2 macrophages phenotype, depending on the presence of other immune cells or cytokines signaling31.
Here, we exploit our previously established experimental infection protocol using primary canine macrophages23 to compare the cytokine profiles in response to infection with either L. infantum, or L. tarentolae, or both. We monitored the gene expression of the pro-inflammatory cytokines IFN-gamma (IFN-γ), TNF-alpha (TNF-α) and IL-1232,33 that are associated with a protective Th1 response, as well as IL-4, IL-10 and IL-6 whose gene expression is generally linked to clinical disease34.
Results
Infection and parasite burden parameters
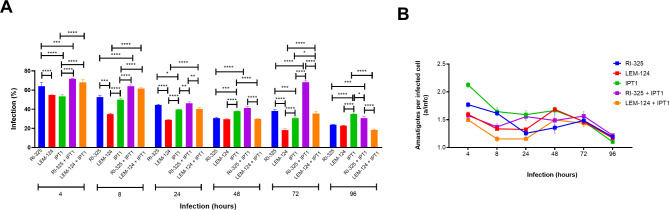
Changes in the percentage of infected dog macrophages and the number of intracellular parasites per infected cell (a/infc) were evaluated 96 h after the infection with L. infantum and/or L. tarentolae. For each Leishmania strain, the percentage of infected macrophages and a/infc is shown in Fig. 1.
Fig. 1.
Leishmania infection efficiency (A) and intracellular parasite burden at 96 h post-infection (B) were assessed microscopically in primary dog macrophages performed in triplicate. RI-325 and LEM-124: Leishmania tarentolae; IPT1: Leishmania infantum. a/infc: average number of amastigotes in each infected cell. Asterisks indicate the statistical difference between groups (* P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001).
Specifically, among L. tarentolae strains, RI-325 presented a significatively higher number of infected macrophages in all time points when compared with LEM-124 (Fig. 1A), except at 96 h where the infection rate is the same for both strains. From 8 h time point, IPT1 showed a significantly (P < 0.0001) higher number of infected macrophages when compared with the other lab strain LEM-124. From 24 h, coinfections with RI-325 + IPT1 showed a significant (P < 0.01) higher percentage of cell infection in comparison with LEM-124 + IPT1. The medium value of a/infc was higher for IPT1 (1.549 a/infc) in comparison with L. tarentolae strains in single (up to 1.363 a/infc, P < 0.001) and in coinfection conditions (up to 1.364 a/infc, P < 0.0001). Significant differences in a/infc were observed mainly between LEM-124 in single and in coinfection with IPT1 (P < 0.001), and between IPT1 in coinfection with RI-325 (P < 0.0001) (Fig. 1B).
mRNA expression of anti- and pro-inflammatory cytokines
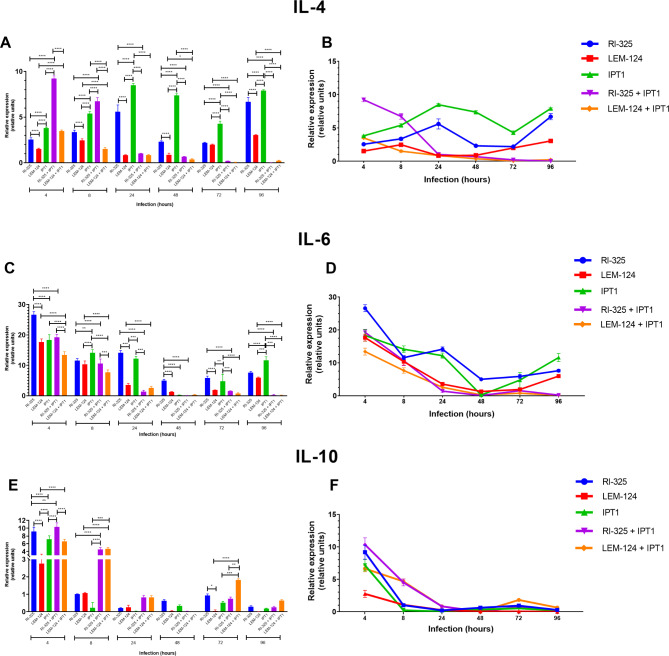
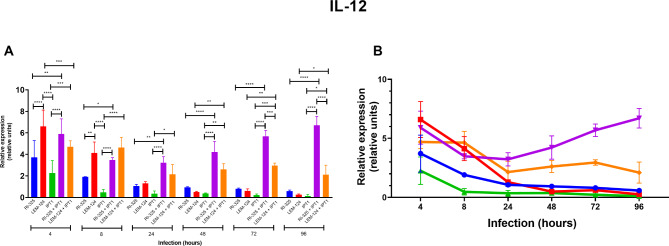
A lower relative gene expression of the anti-inflammatory cytokine IL-4 was observed for both L. tarentolae (i.e., up to 6.681 relative units for RI-325 and 3.041 for LEM-124 at 96 h) in comparison with IPT1 single infection (8.481 relative units at 24 h), along with a decrease in coinfections for both L. tarentolae strains until 96 h post-infection (i.e., 0.003 relative units for RI-325 + IPT1; 0.217 relative units for LEM-124 + IPT1, P < 0.0001) (Fig. 2A and B; Table 1). A reduction of IL-6 gene expression was detected in coinfections for all Leishmania strains (Fig. 2C and D; Table 1), from which the gene expression was significantly lower between coinfections (0.172 for RI-325 + IPT1, P < 0.001 at 96 h) and single infections (11.622 relative units for IPT1, P < 0.0001 at 96 h). In addition, the gene expression of IL-6 was statistically higher at 4 h (26.611 relative units, P < 0.0001) on RI-325 than both laboratory strains (17.644 relative units for LEM-124; 18.332 relative units for IPT1 at 4 h). The highest gene expression value for IL-10 was noticed for all Leishmania strains at 4 h in single and coinfected conditions, with a constant decrease over the assessed time points (Fig. 2E and F; Table 1). The highest gene expression values of the pro-inflammatory IL-12 was detected for dog macrophages infected with the LEM-124 strain (7.096 relative units) at 4 h and on RI-325 + IPT1 coinfection at 4 h (5.905 relative units) and 96 h (6.705 relative units) (Fig. 3; Table 2). Overall, the IL-12 gene expression values in coinfections conditions were significantly higher (P < 0.0001; up to 6.705 relative units for RI-325 + IPT1 at 96 h; up to 4.699 relative units for LEM 124 + IPT1 at 4 h) than those observed in single infection with IPT1 (2.261 relative units). The pro-inflammatory cytokines IFN-γ and TNF-α were not detected.
Fig. 2.
Relative mRNA gene expression (relative units) of anti-inflammatory cytokines IL-4 (A, B), IL-6 (C, D) and IL-10 (E, F) for each strain and its coinfections from 4 to 96 h in triplicate (standard deviation shown). RI-325 and LEM-124: Leishmania tarentolae; IPT1: Leishmania infantum. Asterisks indicate the statistical difference between species/strains (* P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001).
Table 1.
Relative gene mRNA expression (relative units) of anti-inflammatory cytokines normalized according to G3PDH housekeeping values.
| IL-4 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leishmania tarentolae | Leishmania infantum | L. tarentolae + L. infantum | |||||||||||||
| RI-325 | LEM-124 | IPT1 | RI-325 + IPT1 | LEM-124 + IPT1 | |||||||||||
| Infection (hours) | min | med | max | min | med | max | min | med | max | min | med | max | min | med | max |
| 4 | 2.305 | 2.540a | 2.706 | 1.477 | 1.519b | 1.605 | 3.502 | 3.804c | 4.023 | 8.985 | 9.224d | 9.562 | 3.400 | 3.493c | 3.590 |
| 8 | 3.200 | 3.354a | 3.605 | 2.350 | 2.468b | 2.653 | 5.170 | 5.395c | 5.610 | 6.540 | 6.720d | 7.201 | 1.331 | 1.505e | 1.652 |
| 24 | 4.750 | 5.589a | 6.205 | 0.784 | 0.830b | 0.905 | 8.342 | 8.481c | 8.702 | 0.998 | 1.017b | 1.032 | 0.799 | 0.848b | 0.905 |
| 48 | 2.210 | 2.310a | 2.420 | 0.705 | 0.882b | 1.038 | 7.305 | 7.365c | 7.590 | 0.650 | 0.677bd | 0.705 | 0.303 | 0.368d | 0.450 |
| 72 | 2.125 | 2.183a | 2.256 | 1.908 | 1.988a | 2.036 | 3.985 | 4.281b | 4.532 | 0.173 | 0.193c | 0.210 | 0.313 | 0.050c | 0.085 |
| 96 | 6.302 | 6.681a | 7.202 | 2.997 | 3.041b | 3.095 | 7.815 | 7.905c | 8.105 | 0.001 | 0.003d | 0.006 | 0.174 | 0.217d | 0.292 |
| IL-6 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leishmania tarentolae | Leishmania infantum | L. tarentolae + L. infantum | |||||||||||||
| RI-325 | LEM-124 | IPT1 | RI-325 + IPT1 | LEM-124 + IPT1 | |||||||||||
| Infection (hours) | min | med | max | min | med | max | min | med | max | min | med | max | min | med | max |
| 4 | 25.423 | 26.611a | 27.506 | 16.520 | 17.664b | 18.570 | 16.428 | 18.332bc | 19.878 | 18.228 | 19.273bd | 20.070 | 12.520 | 13.424e | 14.592 |
| 8 | 11.205 | 11.588a | 12.308 | 9.250 | 10.383a | 11.360 | 13.025 | 14.150b | 15.026 | 9.418 | 10.641a | 9.418 | 6.912 | 7.665c | 8.563 |
| 24 | 13.580 | 14.114a | 14.952 | 3.074 | 3.558b | 4.021 | 11.472 | 12.193a | 13.025 | 1.250 | 1.410c | 1.850 | 1.985 | 2.581bc | 2.908 |
| 48 | 5.134 | 5.011a | 5.320 | 1.210 | 1.296b | 1.376 | 0.149 | 0.231b | 0.302 | 0.045 | 0.075b | 0.120 | 0.063 | 0.285b | 0.440 |
| 72 | 5.205 | 5.883a | 6.235 | 1.890 | 1.915b | 1.950 | 2.770 | 4.772a | 7.311 | 1.410 | 1.546b | 1.647 | 0.560 | 0.767b | 0.985 |
| 96 | 7.205 | 7.625a | 8.012 | 5.808 | 5.972a | 6.204 | 10.629 | 11.622b | 12.985 | 0.026 | 0.192c | 0.465 | 0.123 | 0.172c | 0.252 |
| IL-10 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leishmania tarentolae | Leishmania infantum | L. tarentolae + L. infantum | |||||||||||||
| RI-325 | LEM-124 | IPT1 | RI-325 + IPT1 | LEM-124 + IPT1 | |||||||||||
| Infection (hours) | min | med | max | min | med | max | min | med | max | min | med | max | min | med | max |
| 4 | 8.125 | 9.174a | 10.260 | 2.140 | 2.766b | 3.142 | 6.251 | 7.173c | 8.011 | 9.205 | 10.345c | 11.250 | 6.091 | 6.651d | 7.020 |
| 8 | 0.980 | 1.006a | 1.036 | 1.036 | 1.069a | 1.125 | 0.049 | 0.233a | 0.586 | 4.175 | 4.515b | 5.120 | 4.264 | 4.676b | 5.012 |
| 24 | 0.188 | 0.211a | 0.250 | 0.126 | 0.256a | 0.365 | 0.021 | 0.032a | 0.042 | 0.710 | 0.833a | 0.914 | 0.755 | 0.835a | 0.930 |
| 48 | 0.536 | 0.620a | 0.680 | 0.036 | 0.049a | 0.069 | 0.293 | 0.336a | 0.410 | 0.014 | 0.022a | 0.041 | 0.008 | 0.009a | 0.010 |
| 72 | 0.845 | 0.937a | 1.021 | 0.009 | 0.047b | 0.121 | 0.453 | 0.525ab | 0.601 | 0.645 | 0.746ab | 0.841 | 1.758 | 1.823c | 1.906 |
| 96 | 0.241 | 0.289a | 0.360 | 0.010 | 0.008a | 0.011 | 0.174 | 0.189a | 0.210 | 0.267 | 0.259a | 0.312 | 0.602 | 0.644a | 0.702 |
Statistically differences (P < 0.05) between strains on each time point are indicated. Different letters indicate significative differences. Same letters indicate no significative difference.
min: minimal value of gene expression; med: medium value of gene expression; max: maximum value of gene expression.
Fig. 3.
Relative mRNA gene expression (relative units) of pro-inflammatory cytokines IL-12 (A, B) for each strain and its coinfections from 4 to 96 h in triplicate (standard deviation shown). RI-325 and LEM-124: Leishmania tarentolae; IPT1: Leishmania infantum. Asterisks indicate the statistical difference between groups (* P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001).
Table 2.
Relative gene mRNA expression (relative units) of pro-inflammatory cytokines normalized according to G3PDH housekeeping values.
| IL-12 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leishmania tarentolae | Leishmania infantum | L. tarentolae + L. infantum | |||||||||||||
| RI-325 | LEM-124 | IPT1 | RI-325 + IPT1 | LEM-124 + IPT1 | |||||||||||
| Infection (hours) | min | med | max | min | med | max | min | med | max | min | med | max | min | med | max |
| 4 | 2.445 | 3.714ab | 5.464 | 4.377 | 7.096c | 9.781 | 1.080 | 2.261a | 3.402 | 4.816 | 5.905cd | 7.708 | 4.102 | 4.699bd | 5.169 |
| 8 | 1.922 | 1.908ab | 1.923 | 3.092 | 4.132c | 5.123 | 0.200 | 0.474a | 0.737 | 3.325 | 3.490bc | 3.796 | 3.239 | 4.643c | 5.230 |
| 24 | 0.938 | 1.066ab | 1.210 | 1.162 | 1.297ab | 1.490 | 0.165 | 0.352b | 0.495 | 2.784 | 3.228c | 4.023 | 1.021 | 2.146ac | 3.259 |
| 48 | 0.907 | 0.939abc | 1.002 | 0.425 | 0.495ab | 0.567 | 0.394 | 0.373b | 0.401 | 3.232 | 4.225d | 5.212 | 2.321 | 2.619cd | 3.212 |
| 72 | 0.792 | 0.809a | 0.875 | 0.423 | 0.616a | 0.782 | 0.123 | 0.223a | 0.312 | 5.092 | 5.694b | 6.063 | 2.734 | 2.959c | 3.214 |
| 96 | 0.482 | 0.580ab | 0.685 | 0.223 | 0.262ab | 0.324 | 0.002 | 0.115b | 0.320 | 6.032 | 6.704c | 7.623 | 1.240 | 2.104a | 3.023 |
Statistically differences (P < 0.05) between strains on each time point are indicated. Different letters indicate significative differences. Same letters indicate no significative difference.
min: minimum value of gene expression; med: medium value of gene expression; max: maximum value of gene expression.
Discussion
The results herein reported suggest that L. tarentolae may elicit the production of a cytokine profile that could ultimately exert a protective effect against L. infantum infection. This is highlighted by the reduced expression of anti-inflammatory cytokines (i.e., IL-4 and IL-6) coupled with an increased expression of the pro-inflammatory cytokine IL-12 after 96 h of coinfection in primary canine monocyte-derived macrophages.
The lowest expression of both IL-4 and IL-6 anti-inflammatory cytokines in coinfections (RI-325 + IPT1), in relation to single infection by IPT1 at 24 h, is suggestive of a protective role of L. tarentolae. Accordingly, the higher gene expression of pro-inflammatory IL-12 observed in coinfections than in single IPT1 infection throughout all time points may suggest that L. tarentolae effectively contributes to an increase in pro-inflammatory response probably due to a sum effect between both species, further increasing the percentage of infected cells. This possibility is further supported by the lower mean value of a/infc observed in coinfection than in single IPT1 infection (with both RI-325 + IPT1 and LEM-124 + IPT1, P < 0.0001). Indeed, although a strong competitiveness between these two Leishmania spp. in infected cells has been previously suggested35,36, a similar infection efficiency of L. infantum and L. tarentolae was inferred by the percentual of infection mainly at 72 h. This data also highlights the potential of the RI-325 to induce an anti-inflammatory response by IL-4 and IL-6 cytokines gene expression. In addition, the general higher infectivity of RI-325 compared to L. infantum in single infections (i.e., at 4, 8, 24 and 72 h) confirms previous studies23, ultimately explaining the difference in higher infection observed in coinfected cells (up to 69.2% for RI-325 + IPT1 at 72 h) than in L. infantum single infection, even if the number of a/infc is higher on IPT1 than RI-325. This increase of the number of infected cells at 72 h was previously reported23, which could be mainly by a persistent uptake of amastigotes released in the medium by the macrophage cells despite a decrease in the number of infected cells. Furthermore, the overall decrease in IL-10 gene expression after 8 h post infection among all Leishmania strains is not surprising, considering that the role of this cytokine in the disease progression is controversial24,27,37, at least in in vitro models.
The absence of the gene expression of pro- inflammatory IFN-γ and TNF-α, which are considered key cytokines in the host’s protective immune response against Leishmania infection38, could be due to the inhibitory effect mediated by IL-4, IL-6 and IL-1025,27,29,39,40. Furthermore, the absence of gene expression of IFN-γ is expected, once the macrophages are not the main producers of this cytokine, and its transcription may require more than 96 h41. Conversely, even if the protective effect may be partially due to the TNF-α, this cytokine could not be detected probably due to the rapid turnover and quick degradation of its mRNA, being not always considered as a reliable marker in in vitro model27,42. Indeed, the growth factor-free process of macrophage differentiation did not seem to influence the cytokine gene expression herein reported, as highlighted also in previous studies25.
In the in vitro study design herein conducted, the potential protective effect of L. tarentolae, when in coinfection with L. infantum, is suggested by the decrease of IL-4 and IL-6 and the increase of IL-12 in coinfections. The partial protection against infection when two species of Leishmania coinfect the same individual host was already demonstrated, where a primary infection with Leishmania tropica induces partial protection against Leishmania major when coinfecting mice43. Furthermore, cross-reacting immune responses and the potential protection between taxonomically different Leishmania species was also previously observed in an experimental study with Rhesus monkeys, where L. major induces significant protection for Leishmania amazonensis and Leishmania guyanensis44.
The in vitro mechanisms underlying the low anti-inflammatory and a high pro-inflammatory response to L. tarentolae when in coinfection with L. infantum needs further investigations and may explain hindrances in interpreting cytokine gene expression profile in canine leishmaniasis under natural conditions45, where mixed Th1/Th2 responses induced by L. infantum have been reported in both asymptomatic and symptomatic dogs, with high mRNA levels observed for IL-4, IL-10 and TNF-alpha in dogs with clinical manifestation of CanL. Ultimately, these results may explain the recorded cases of asymptomatic dogs coinfected with L. tarentolae and L. infantum13, suggesting the possibility of genetic exchange and hybridization in sympatric areas, and opening new perspectives for vaccine development against CanL and other leishmaniases.
Materials and methods
Parasites and cultures
Leishmania tarentolae strain isolated from T. mauritanica gecko (RTAR/IT/21/RI-325) in Italy, and the laboratory strains of L. tarentolae (RTAR/IT/81/ISS21-G.6c/LEM-124) and L. infantum zymodeme MON-1 (MHOM/TN/80/IPT1) at 6th passage were cultivated in 1 mL of blood agar and Tobie-Evans, supplemented with Fluorocytosine (250 µg/mL), Gentamicin (250 µg/mL), and Fetal Bovine Serum (FBS, 5%). The cultures were maintained at 26 ºC for 5 days before cell infection.
Dog blood sample and clinical examination
Given the aims of the study, in order to avoid further interference caused by biological factors (e.g., age, nutrition, gender) only one dog was used as blood source. The dog was seven-year-old male German Shepherd, after a written informed consent from the owner (one of the authors of the study, D.O.) and he was included being assessed as negative for Leishmania spp. infection by indirect immunofluorescent antibody test (IFAT) and by duplex real-time PCR (dqPCR)18,46. The study protocol was approved by the ethical committee of the Department of Veterinary Medicine of the University of Bari, Italy (Prot. Uniba 21/2022). The methods to handle animal cells were carried out in accordance with the ARRIVE guidelines and with international, national, and/or institutional regulations.
Generation of macrophages from canine peripheral blood monocytes
Mononuclear cells were obtained according to procedures previously described23. Briefly, a total of 10 mL of canine blood was collected in Ethylene Diamine Tetra Acetic acid (EDTA) tubes (BD Vacutainer, San Diego CA, USA) and mixed in equal proportions with RPMI-1640 medium (Euroclone, Milan, Italy). The mixture was subsequently centrifugated with double the volume of Lympholyte®-H (Cedarlane, Italy) at 400 × g for 45 min at room temperature (RT). The resulting buffy coat layer was subjected to two successive washes using 15 mL of warm RPMI-1640 medium, with centrifugation steps performed at 310 × g and 210 × g for 10 min at RT, respectively. The obtained peripheral blood mononuclear cells (PBMC) were suspended in warm RPMI-1640 medium supplemented with FBS (10%) and a mix of antibiotics (100× of Penicillin-Streptomycin). Cells were seeded at a concentration of 2 × 106 cells/mL into 24-well plates containing sterile coverslips. Approximately 10% of the cells differentiated into monocyte-macrophages during the incubation period, and were confirmed by the morphology (e.g., larger and irregular size), cytoplasmic features (i.e., amount of cytoplasm, phagocytic activity) and nuclear form (e.g., larger and irregularly shaped)47. Furthermore, cells were also plated in wells without coverslips for subsequent scraped cells analyses. The plates were incubated at 37 ºC with 5% CO2 for 24 h (h) for the adhesion of the mononuclear cells. Subsequently, the medium was replaced to remove non-adherent cells, and 1 mL of fresh RPMI-1640 supplemented with 10% of FBS and 10% of antibiotics mix was added. The plate was incubated for 120 h at 37 ºC with 5% CO2 for complete macrophage maturation. No stimulation factor was used as the cells exhibit adequate differentiation in culture for this short period.
Infection of primary canine monocyte-derived macrophages by Leishmania spp.
Leishmania tarentolae (LEM-124 and RI-325 strains) and L. infantum (IPT1 strain) promastigotes at the stationary phase of growth were centrifuged at 3000 × g for 10 min at RT, followed by three washes with sterile PBS (1×). The resulting pellet was resuspended in RPMI-1640 and counted in a Burker chamber at a dilution ratio of 1:100. Subsequently, promastigotes at a concentration of 2 × 106 parasites/ml (parasite/macrophage ratio 10:1) were seeded on the plate after the removal of the growth medium. The cells were infected by each individual strain or by mixed strains (i.e., L. infantum IPT1 + L. tarentolae LEM-124; L. infantum IPT1 + L. tarentolae RI-325), maintaining the same proportion of promastigotes/macrophage on all infection conditions, and the plate was incubated at 37ºC with 5% CO2. After 4 h of infection, each well was washed twice with sterile PBS to remove non-internalized parasites. The plates were then maintained to monitor the infection progression during post-infection time intervals (4–96 h). All experiments were conducted in triplicate. A negative non-infected control was included in each experimental assay.
Evaluation of Leishmania spp. intracellular parasite burden
For each time point, coverslips containing infected macrophages were subjected to Diff-Quick® staining (Merck, Darmstadt, Germany) following the established protocol previously described48. Intracellular parasites were monitored at different times post-infection (i.e. 4, 8, 24, 48, 72, and 96 h). Stained cells on coverslips were observed under bright-field microscopy at 100× magnification (LEICA DM LB2, Germany). The internalization capacity of parasites was determined by counting a total of 200 cells to estimate the percentage of infection and the number of amastigotes per infected cell (a/infc).
RNA extraction and quantitative reverse transcription PCR (RT-qPCR)
The mechanically scraped cells from each infection time point were collected and immediately subjected to centrifugation at 12,000 × g for 10 min at RT. The resulting pellet was resuspended in 800 µL of Trizol (Invitrogen, USA) and stored at – 80 °C until RNA extraction. Scraped cells were submitted to RNA extraction by Trizol (Invitrogen, USA) accordingly to the manufacturer guidelines. The final pellet was eluted in 15 µL of DEPC (Diethyl pyrocarbonate)-treated water. The synthesis of cDNA was performed in 10 µL reverse transcription reactions using 3 µL of total RNA in 1× SuperScript IV VILO Mastermix (Thermo Fisher Scientific, Whaltam, USA). The RT-PCR synthesis was carried out at 25 °C for 10 min, followed by 50 °C for 10 min, and at 85 °C for 5 min. The cDNA concentration was quantified by Qubit dsDNA HS Assay Kit and stored at – 20 °C. The quantitative PCR (qPCR) for cytokine gene expression of and anti- IL-6, IL-10, IL-4 and pro-inflammatory TNF-α, IL-12 were carried out with 10 µL of SsoAdvanced™ Universal Supermix (Bio-Rad, CA, USA) and 0.7 µL of PrimerPCR Costum Assay of primers (400 nM) and probes (250 nM) previously reported with modifications49, (S1 Table). Similarly, gene expression of IFN- γ was also evaluated, being macrophages able to produce this cytokine, as elsewhere described50. The qPCR reactions were performed at 55 °C for 2 min, 95 °C for 10 min, followed by 45 cycles of 95 °C for 15 s and 60 °C for 1 min for each cytokine. We monitored the expression of the control genes G3PDH49 and OAZ151 for normalization purposes. Cytokine gene expression was assessed using the 2−ΔΔCq value52, by computing the ΔCq from the reference gene and the target gene in each sample. Subsequently, ΔΔCq was calculated by subtracting the ΔCq of the control group from the ΔCq of the experimental group. Gene expression levels were expressed as mRNA relative units corresponding to the ratio between the gene expression of infected and uninfected macrophages.
Statistical data
Statistical analyses were performed using GraphPad Prism v. 8.0.0 (GraphPad Software, San Diego, CA, USA). Shapiro–Wilk test was used to assess normal data distribution. Two-way ANOVA followed by Tukey’s post hoc test was performed for the triplicates of each variable. Values were considered statistically significant when P < 0.05.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to Gerald F Späth (Institut Pasteur, Université Paris Cité, Unité de Parasitologie moléculaire et Signalisation, France; Visiting professor, University of Bari, Italy 2023/2024) for his suggestions and comments to the manuscript.
Author contributions
V.N.L.F., M.S.L. and D.O. performed the conceptualization, investigation, supervision, project administration, visualization, and wrote the main manuscript text. V.N.L.F. performed the formal analysis, validation and software analysis. V.N.L.F. and M.S.L. performed data curation. V.N.L.F. and M.S.L. performed the methodology. D.O. was responsible for the funding acquisition. J.A.M.R., S.E., I.V.B. and C.B. reviewed and edited the manuscript. All authors reviewed the manuscript.
Data availability
All data analyzed during the study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alvar, J. et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One7, e35671. 10.1371/journal.pone.0035671 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breton, M., Tremblay, M. J., Ouellette, M. & Papadopoulou, B. Live nonpathogenic parasitic vector as a candidate vaccine against visceral leishmaniasis. Infect. Immun.73, 6372–6382. 10.1128/IAI.73.10.6372-6382.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breton, M., Zhao, C., Ouellette, M., Tremblay, M. J. & Papadopoulou, B. A recombinant non-pathogenic Leishmania vaccine expressing human immunodeficiency virus 1 (HIV-1) gag elicits cell-mediated immunity in mice and decreases HIV-1 replication in human tonsillar tissue following exposure to HIV-1 infection. J. Gen. Virol.88, 217–225. 10.1099/vir.0.81995-0 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Taylor, V. M. et al. Leishmania tarentolae: utility as an in vitro model for screening of antileishmanial agents. Exp. Parasitol.126, 471–475. 10.1016/j.exppara.2010.05.016 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Varotto-Boccazzi, I. et al. Leishmania tarentolae as an antigen delivery platform: dendritic cell maturation after infection with a clone engineered to express the SARS-CoV-2 spike protein. Vaccines (Basel)10, 803. 10.3390/vaccines10050803 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bandi, C. et al. Leishmania tarentolae: a vaccine platform to target dendritic cells and a surrogate pathogen for next generation vaccine research in leishmaniases and viral infections. Parasit. Vectors16, 35. 10.1186/s13071-023-05651-1 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otranto, D. et al. The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part I: Protozoa and tick-borne agents. Vet. Parasitol.213, 12–23. 10.1016/j.vetpar.2015.04.022 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Morales-Yuste, M., Martín-Sánchez, J. & Corpas-Lopez, V. Canine leishmaniasis: update on epidemiology, diagnosis, treatment, and prevention. Vet Sci9, 387. 10.3390/vetsci9080387 (2022). [DOI] [PMC free article] [PubMed]
- 9.Pace, D. Leishmaniasis. J. Infect.69, S10–S18. 10.1016/j.jinf.2014.07.016 (2014).
- 10.World Health Organization (WHO). Leishmaniasis (2024, accessed 20 Jan 2024). https://www.who.int/news-room/fact-sheets/detail/leishmaniasis.
- 11.Akhoundi, M. et al. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Negl. Trop. Dis.10, e0004349. 10.1371/journal.pntd.0004349 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dantas-Torres, F. et al. Canine leishmaniosis in the Old and New worlds: unveiled similarities and differences. Trends Parasitol.28, 531–538. 10.1016/j.pt.2012.08.007 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Mendoza-Roldan, J. A. et al. Detection of Leishmania tarentolae in lizards, sand flies and dogs in southern Italy, where Leishmania infantum is endemic: hindrances and opportunities. Parasit. Vectors14, 461. 10.1186/s13071-021-04973-2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wenyon, C. M. Observations on the intestinal protozoa of three Egyptian lizards, with a note on a cell-invading fungus. Parasitology12, 350–365. 10.1017/S0031182000014347 (1920). [Google Scholar]
- 15.Elwasila, M. Leishmania tarentolae Wenyon, 1921 from the gecko Tarentola Annularis in the Sudan. Parasitol. Res.74, 591–592. 10.1007/BF00531640 (1988). [DOI] [PubMed] [Google Scholar]
- 16.Mendoza-Roldan, J. A. et al. Leishmania spp. in Squamata reptiles from the Mediterranean basin. Transbound. Emerg. Dis.69, 2856–2866. 10.1111/tbed.14438 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Mendoza-Roldan, J. A. et al. Leishmania tarentalae: A new frontier in the epidemiology and control of the leishmaniases. Transbound. Emerg. Dis.69, e1326–e1337. 10.1111/tbed.14660 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iatta, R. et al. Leishmania tarentolae and Leishmania infantum in humans, dogs and cats in the Pelagie archipelago, southern Italy. PLoS Negl. Trop. Dis.15, e0009817. 10.1371/journal.pntd.0009817 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maia, C. & Depaquit, J. Can Sergentomyia (Diptera, Psychodidae) play a role in the transmission of mammal-infecting Leishmania? Parasite23, 55. 10.1051/parasite/2016062 (2016). [DOI] [PMC free article] [PubMed]
- 20.Abbate, J. M. et al. Identification of trypanosomatids and blood feeding preferences of phlebotomine sand fly species common in Sicily, Southern Italy. PLoS One15, e0229536. 10.1371/journal.pone.0229536 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otranto, D. et al. Efficacy of a combination of 10% imidacloprid/50% permethrin for the prevention of leishmaniasis in kennelled dogs in an endemic area. Vet. Parasitol.144, 270–278. 10.1016/j.vetpar.2006.09.012 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Pombi, M. et al. Molecular detection of Leishmania (Sauroleishmania) tarentolae in human blood and Leishmania (Leishmania) infantum in Sergentomyia minuta: unexpected host-parasite contacts. Med. Vet. Entomol.34, 470–475. 10.1111/mve.12464 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Louzada-Flores, V. N. et al. Intracellular persistence of Leishmania tarentolae in primary canine macrophage cells. Acta Trop.243, 106935. 10.1016/j.actatropica.2023.106935 (2023). [DOI] [PubMed] [Google Scholar]
- 24.Solbach, W. & Laskay, T. The host response to Leishmania infection. Adv. Immunol.74, 275–317. 10.1016/s0065-2776(08)60912-8 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Chamizo, C., Moreno, J. & Alvar, J. Semi-quantitative analysis of cytokine expression in asymptomatic canine leishmaniasis. Vet. Immunol. Immunopathol.103, 67–75. 10.1016/j.vetimm.2004.08.010 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Elmahallawy, E. K., Alkhaldi, A. A. M. & Saleh, A. A. Host immune response against leishmaniasis and parasite persistence strategies: a review and assessment of recent research. Biomed. Pharmacother.139, 111671. 10.1016/j.biopha.2021.111671 (2021). [DOI] [PubMed] [Google Scholar]
- 27.Dayakar, A., Chandrasekaran, S., Kuchipudi, S. V. & Kalangi, S. K. Cytokines: key determinants of resistance or disease progression in visceral leishmaniasis: opportunities for novel diagnostics and immunotherapy. Front. Immunol.10, 670. 10.3389/fimmu.2019.00670 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez, F. O. & Gordon, S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep.6, 13. 10.12703/P6-13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonçalves, A. et al. An overview of immunotherapeutic approaches against Canine Visceral Leishmaniasis: what has been tested on dogs and a new perspective on improving treatment efficacy. Front. Cell. Infect. Microbiol.9, 427. 10.3389/fcimb.2019.00427 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samant, M., Sahu, U., Pandey, S. C. & Khare, P. Role of cytokines in experimental and human visceral leishmaniasis. Front. Cell. Infect. Microbiol.11, 624009. 10.3389/fcimb.2021.624009 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aliyu, M. et al. Interleukin-6 cytokine: an overview of the immune regulation, immune dysregulation, and therapeutic approach. Int. Immunopharmacol.111, 109130. 10.1016/j.intimp.2022.109130 (2022). [DOI] [PubMed] [Google Scholar]
- 32.Maia, C. & Campino, L. Cytokine and phenotypic cell profiles of Leishmania infantum infection in the dog. J Trop Med2012, 541571. 10.1155/2012/541571 (2012). [DOI] [PMC free article] [PubMed]
- 33.Varotto-Boccazzi, I. et al. Boosting immunity to treat parasitic infections: Asaia bacteria expressing a protein from Wolbachia determine M1 macrophage activation and killing of Leishmania protozoans. Pharmacol. Res.161, 105288. 10.1016/j.phrs.2020.105288 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Quinnell, R. J. et al. Tissue cytokine responses in canine visceral leishmaniasis. J. Infect. Dis.183, 1421–1424. 10.1086/319869 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Kinnula, H., Mappes, J. & Sundberg, L. R. Coinfection outcome in an opportunistic pathogen depends on the inter-strain interactions. BMC Evol. Biol.17, 77. 10.1186/s12862-017-0922-2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.das Chagas, B. D. et al. Interspecies and intrastrain interplay among Leishmania spp. parasites. Microorganisms10, 1883. 10.3390/microorganisms10101883 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Latrofa, M. S. et al. Interaction between Wolbachia pipientis and Leishmania infantum in heartworm infected dogs. Parasit. Vectors16, 77. 10.1186/s13071-023-05662-y (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barbosa, M. A. et al. Cytokine gene expression in the tissues of dogs infected by Leishmania Infantum. J. Comp. Pathol.145, 336–344. 10.1016/j.jcpa.2011.03.001 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Santos-Gomes, G. M. et al. Cytokine expression during the outcome of canine experimental infection by Leishmania Infantum. Vet. Immunol. Immunopathol.88, 21–30. 10.1016/s0165-2427(02)00134-4 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Pessoa-E-Silva, R. et al. Immunoprophylactic potential of a new recombinant leishmania infantum antigen for canine visceral leishmaniasis: an in vitro finding. Front. Immunol.11, 605044. 10.3389/fimmu.2020.605044 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darwich, L. et al. Secretion of interferon-gamma by human macrophages demonstrated at the single-cell level after costimulation with interleukin (IL)-12 plus IL-18. Immunology. 126, 386–393. 10.1111/j.1365-2567.2008.02905.x (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DE Lima Celeste, J. L. et al. Experimental mixed infection of Leishmania (Leishmania) amazonensis and Leishmania (L.) infantum in hamsters (Mesocricetus auratus). Parasitology144, 1191–1202. 10.1017/S0031182017000464 (2017). [DOI] [PubMed]
- 43.Mahmoudzadeh-Niknam, H., Kiaei, S. S. & Iravani, D. LeishTropicaropica infection, in comparison to Leishmania major, induces lower delayed type hypersensitivity in BALB/c mice. Korean J. Parasitol.45, 103–109. 10.3347/kjp.2007.45.2.103 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porrozzi, R., Teva, A., Amaral, V. F., Santos da Costa, M. V. & Grimaldi, G. Jr cross-immunity experiments between different species or strains of Leishmania in rhesus macaques (Macaca mulatta). Am. J. Trop. Med. Hyg.71, 297–305. 10.4269/ajtmh.2004.71.297 (2004). [PubMed] [Google Scholar]
- 45.Panaro, M. A. et al. Canine leishmaniasis in Southern Italy: a role for nitric oxide released from activated macrophages in asymptomatic infection? Parasit. Vectors1, 10. 10.1186/1756-3305-1-10 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Latrofa, M. S. et al. A duplex real-time PCR assay for the detection and differentiation of Leishmania infantum and Leishmania tarentolae in vectors and potential reservoir hosts. Entomol. Generalis41, 543–551. 10.1127/entomologia/2021/1178 (2021). [Google Scholar]
- 47.Italiani, P. & Boraschi, D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front. Immunol.5, 514. 10.3389/fimmu.2014.00514 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skipper, R. & De Stephano, D. B. A rapid stain for Campylobacter pylori in gastrointestinal tissue sections using Diff-Quik®. J. Histotechnol.12, 303–304 (1989). [Google Scholar]
- 49.Peeters, D., Peters, I. R., Farnir, F., Clercx, C. & Day, M. J. Real-time RT-PCR quantification of mRNA encoding cytokines and chemokines in histologically normal canine nasal, bronchial and pulmonary tissue. Vet. Immunol. Immunopathol.104, 195–204. 10.1016/j.vetimm.2004.11.007 (2005). [DOI] [PubMed] [Google Scholar]
- 50.Siebeler, R., de Winther, M. P. J. & Hoeksema, M. A. The regulatory landscape of macrophage interferon signaling in inflammation. J. Allergy Clin. Immunol.152, 326–337. 10.1016/j.jaci.2023.04.022 (2023). [DOI] [PubMed] [Google Scholar]
- 51.Herrmann, I. et al. Canine macrophages can like human macrophages be in vitro activated toward the M2a subtype relevant in allergy. Dev. Comp. Immunol.82, 118–127. 10.1016/j.dci.2018.01.005 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25, 402–408. 10.1006/meth.2001.1262 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analyzed during the study are included in this published article.