Abstract
Background and purpose
The purpose was to describe the use patterns of pharmacological and non‐pharmacological therapies and investigate potential determinants of riluzole use in patients newly diagnosed with amyotrophic lateral sclerosis (ALS) in three Italian regions.
Methods
Amyotrophic lateral sclerosis patients were selected from administrative healthcare databases of Latium, Tuscany and Umbria from 1 January 2014 to 31 December 2019 based on hospital‐ and disease‐specific co‐payment exemption data. The first trace of ALS was considered the index date. Incident ALS cases were those without a trace of ALS during the 3‐year look back. Patients were described in terms of demographics, clinical characteristics and drug use at baseline, and were classified into four categories based on riluzole use in the 2 years before and 1 year after the index date: prevalent, incident, former users and non‐users. Use of symptomatic pharmacological and non‐pharmacological therapies was described across these categories during 12 months after the index date. Determinants of riluzole use were also investigated.
Results and conclusions
A total of 1636 ALS incident subjects were detected in the three regions, mainly aged 65–74 years. Patients were generally fragile with a high prevalence of comorbidities at baseline. Riluzole was used by 27.4% of the overall study cohort at baseline and steeply increased in the first year after the index date differently between regions (Latium 61.2%, Tuscany 85.0%, Umbria 76.5%), with about half of the subjects being incident users. In the 12 months after the index date, also symptomatic therapies increased, in riluzole users and non‐users. Determinants analysis showed that higher patient severity and complexity were associated with a lower likelihood of being treated with riluzole.
Keywords: amyotrophic lateral sclerosis, determinants of use, pharmaco‐utilization, riluzole
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a progressive, heterogeneous neurodegenerative disorder characterized by the degeneration of upper and lower motor neurons, and associated frontotemporal spectrum dysfunction. Patients experience progressive muscular atrophy and weakness, typically leading to respiratory failure and death within 3–5 years [1]. People with ALS display an array of symptoms encompassing direct consequences of neuronal degeneration (e.g., muscle atrophy, dysphagia, respiratory failure) and also secondary to direct symptoms including mental disorders, hypoventilation symptoms and pain [2].
Amyotrophic lateral sclerosis, the most prevalent adult motor neuron disease, exhibits varying global incidence and prevalence [3]. A recent meta‐analysis with focus on the global population with ALS reported that both prevalence and incidence were lowest in South Asia (1.57 and 0.42 per 100,000 persons, respectively) and highest in West Europe (9.62 and 2.76 per 100,000 persons), with higher estimates amongst males compared to females [4]. A recent Italian epidemiological study aligns with the European estimates reported in the meta‐analysis, reporting ALS prevalence and incidence rates ranging between 9.90 and 12.31 per 100,000 persons and between 1.9 and 4.2 per 100,000 persons, respectively [5].
Although no known treatment can reverse the damage caused by ALS, certain medications can help slow the progression of the disease. To date, in Italy, riluzole is the only disease‐modifying drug reimbursed by the Italian public healthcare system for ALS treatment. As a glutamate antagonist, riluzole inhibits glutamate release, which may help prevent nerve cell damage [6, 7]. It delays the onset of ventilator dependence or tracheostomy without exerting therapeutic effect on motor and lung function [8]. Clinical trials have shown that, compared to placebo, riluzole may increase median survival by 2–3 months in patients with ALS [9, 10]. Nevertheless, real‐world evidence suggests that riluzole could potentially offer a considerably more substantial extension of survival, enhancing median survival times by 6–19 months [7, 8, 11]. Apart from riluzole, a series of symptomatic pharmacological and non‐pharmacological therapies are often employed to enhance the patient's quality of life by mitigating prevalent symptoms of the disease, such as muscle cramps, depression, insomnia and pain [12]. When ALS progresses to the point of impairing the ability to breathe, speak and move, tracheostomy and subsequent invasive mechanical ventilation can be performed. It has been suggested that invasive mechanical ventilation may prolong median survival time by 8 months to about 3 years amongst patients in the advanced stage of ALS [13]. In the later stages of ALS, recent guidelines recommend implementing percutaneous endoscopic gastrostomy (PEG) when weight loss surpasses 10% from baseline and prior to the forced vital capacity dropping below 50% of predicted levels. This helps maintain adequate oral intake and offers an alternative route for drug administration [14, 15].
Epidemiological studies on ALS, due to the rarity of the disease, often focus on limited geographical areas or specific clinical contexts, hindering the assessment of potential geographical variations in treatment. Current ALS epidemiological data derive from studies conducted up to 2014 which rely on small sample sizes [16, 17, 18, 19, 20, 21]. Moreover, there is limited evidence regarding the prevalence of the use of combined treatments (i.e., pharmacological and non‐pharmacological interventions) amongst ALS patients.
In light of this, the aim of the present real‐world study was to describe the use patterns of pharmacological and non‐pharmacological therapies and investigate potential determinants of riluzole use in patients newly diagnosed with ALS in three Italian regions, Latium, Tuscany and Umbria, between 2014 and 2019, leveraging the multicentre Italian project ‘Comparative Effectiveness and Safety of Drugs used in Rare Neuromuscular and Neurodegenerative Diseases—the CAESAR study’.
METHODS
Study design and setting
This is a retrospective cohort study on patients newly diagnosed with ALS, based on administrative healthcare data of three Italian regions, namely Latium, Tuscany and Umbria (about 10 million inhabitants). The study protocol was published on the ENCePP website (EUPAS37983).
Data sources
This study utilized administrative healthcare data comprising pseudonymized patient‐level information from various databases recording healthcare services reimbursed by the National Healthcare Service (NHS) and delivered to all residents enrolled with the Regional Healthcare Service (RHS). Enrolment is based on the registration with a general practitioner in Italy and is offered to all residents. For this study, the population registry, which contains demographic and vital status information on subjects enrolled with the RHS, was linked to the mortality registry, hospital discharge records, emergency room visits, disease‐specific co‐payment exemptions and drug dispensings from community and hospital pharmacies to outpatients.
Study population and cohort selection
Patients with ALS were selected amongst subjects registered in one of the three data sources between 1 January 2014 and 31 December 2019 according to the following algorithm: ≥1 hospital discharge record of ALS (primary diagnosis: International Classification of Diseases 9th Revision Clinical Modification [ICD‐9‐CM] 335.20) AND/OR ≥1 hospital discharge record of ALS (secondary diagnosis: ICD‐9‐CM 335.20 if discharged from neurological ward) AND/OR ≥1 discharge record from the emergency room of ALS (primary diagnosis: ICD‐9‐CM 335.20) AND/OR ≥1 record of new co‐payment exemption for ALS (code RF0100). The date of the patient's first trace of ALS in any of the data banks was considered as the index date and assumed as a proxy for the first clinical diagnosis of ALS in each patient. Patients aged less than 18 years, not enrolled in the RHS at the index date or with less than 3 years of look back were excluded. The study population was restricted to incident ALS cases, excluding subjects with a trace of ALS during the 3‐year look back.
Description of the study cohort
Incident ALS patients were characterized in terms of demographic and clinical features, including major complications, comorbidities and recorded therapies in the 2 years preceding the index date, predefined by clinicians (Tables S1–S5). Incident ALS patients were divided into four categories, based on their riluzole use in the 2 years before and 1 year after the index date: (1) former users (≥1 dispensing in the 2 years before and no dispensing in the year after the index date), (2) prevalent users (≥1 dispensing in the 2 years before and ≥1 dispensing in the year after the index date), (3) incident users (nothing dispensed in the 2 years before and ≥1 dispensing in the year after the index date), (4) non‐users (nothing dispensed either in the 2 years before or in the year after the index date). For each of the above‐mentioned categories, use of symptomatic pharmacological and non‐pharmacological therapies was investigated during the 12 months after the index date. Drugs taken into consideration were predefined on the basis of clinical guidelines [22, 23] and revised by clinicians dropping drugs not refunded by the Italian NHS and therefore not traceable in our data. A detailed list of drugs and Anatomical Therapeutic Chemical (ATC) codes and non‐pharmacological treatment with ICD‐9‐CM codes is reported in Tables S4 and S5.
Statistical analysis
The descriptive analysis of the cohort at baseline was performed on age, sex, clinical characteristics and use of ALS‐related pharmacological and non‐pharmacological therapies, displaying only those variables observed in a minimum of 1% for ALS‐specific complications and therapies and 5% for other conditions. Categorical variables are reported as patient counts and percentages. The four categories of riluzole users are stratified by region and reported through a sunburst chart. Use of pharmacological and non‐pharmacological treatments at 12 months after the index date are presented through histograms. Differences between regions are represented by p values with a 5% threshold. A logistic regression analysis considering all characteristics mentioned above was computed to identify determinants of riluzole use, separately for each region. A forest plot was used to visualize model coefficients and their respective confidence intervals. The statistical software SAS and R version 4.0.3 were used for data analysis. All analyses presented in this paper were based on data routinely collected for administrative purposes. The data generated and analysed during the study are not publicly available in line with Italian privacy regulations.
RESULTS
A total of 2635 ALS patients were identified. After applying the exclusion criteria, the study population was made up of 1636 ALS incident subjects, distributed across the three regions as follows: 736 in Latium, 755 in Tuscany and 145 in Umbria (Figure 1). As reported in Table 1, the majority of subjects were men (53%) and in the age class 65–74 (36%). Amongst the complications recorded in the 2 years before the index date, fractures caused by injury were the most commonly observed overall (9.1%) followed by dysphagia (4.6%). Nervous system and sense organ diseases were the most frequent comorbidities (74.1%) with other motoneuron diseases excluding ALS accounting for 10%, followed by diseases of the circulatory system (32.5%). As reported in Table 2, the use of specific and symptomatic drug therapy was frequent in all the regions already at baseline. In detail, riluzole was used by about 20% of subjects in Latium and Umbria and by more than 35% of patients in Tuscany. Amongst symptomatic medications prescribed to more than 40% of the overall study population there were drugs for gastro‐oesophageal reflux, pain and thrombosis/embolism. Moreover, polytherapy with six or more other drugs was frequently observed ranging between 31.7% and 43.2% in the overall study population. Non‐invasive ventilation was detected in 7.5% of the subjects in Latium, compared to only 1.7% in Tuscany and 2.1% in Umbria, invasive ventilation in 5.2% versus 0.8% and 2.1%. Recourse to PEG and tracheostomy was more similar amongst regions, varying between 3.4% and 1.9% and between 0.3% and 1.8%, respectively. For both, pharmacological and non‐pharmacological therapy differences between regions were statistically significant for almost all treatments. Riluzole use steeply increased after the index date in all regions (Figure 2). Considering all users in Tuscany and Umbria, 85.0% and 76.5% received riluzole during the first year, whilst in Latium this percentage accounts for 61.2%. In all regions, about half of the subjects were newly prescribed with riluzole (Latium 42.7%, Tuscany 52.8% and Umbria 57.2%). Prevalent users were instead 32.3% in Tuscany and about 19% in the others. Latium registered a high portion of non‐users (37.1%), approximately double those registered elsewhere. Observed differences between regions in prescribing riluzole were statistically significant for all categories (p < 0.001), also after stratifying by sex and age classes (results not shown). During the first year after the index date more than 50% of both prevalent and incident riluzole users were prescribed with drugs to treat gastro‐oesophageal reflux, depression and cramps (Figure 3). Amongst former and non‐users of riluzole, drugs for thrombosis and gastro‐oesophageal reflux were most frequently prescribed, to over 50% of patients. Differences between user categories were statistically significant for some drug classes: psychoanaleptics, drugs for cramps, spasms, fasciculations, depression, anxiety, emotional fragility and opioids were more frequently prescribed to riluzole users, whilst drugs for thrombosis/embolism and gastro‐oesophageal reflux were more frequently used by non‐users. Regarding non‐pharmacological therapy, over 10% of patients underwent at least one amongst non‐invasive assisted ventilation, invasive assisted ventilation or PEG. Additionally, between 5% and 10% of patients needed tracheostomy. Invasive procedures were more frequent in former riluzole users. Results remained robust when stratifying by sex and age classes (results not shown). Region‐specific results are reported in Figure S1. Figure 4 reports the results of the analysis of potential determinants of being prescribed with riluzole during the first year after the index date by region. Due to small numbers, most results do not reach statistical significance. Significant associations for not using riluzole were detected in Latium for gender with a significant odds ratio (OR) of 0.63, older ages (75+) (OR = 0.49), presence of psychiatric disorders (OR = 0.21) and initiation of PEG (OR = 0.12) and invasive assisted ventilation (OR = 0.09), in Tuscany for use of respiratory insufficiency drugs (OR = 0.16) and in Umbria for the presence of tetraparesis or paraplegia (OR = 0.01) and the use of drugs for cramps and spasms (OR = 0.05).
FIGURE 1.
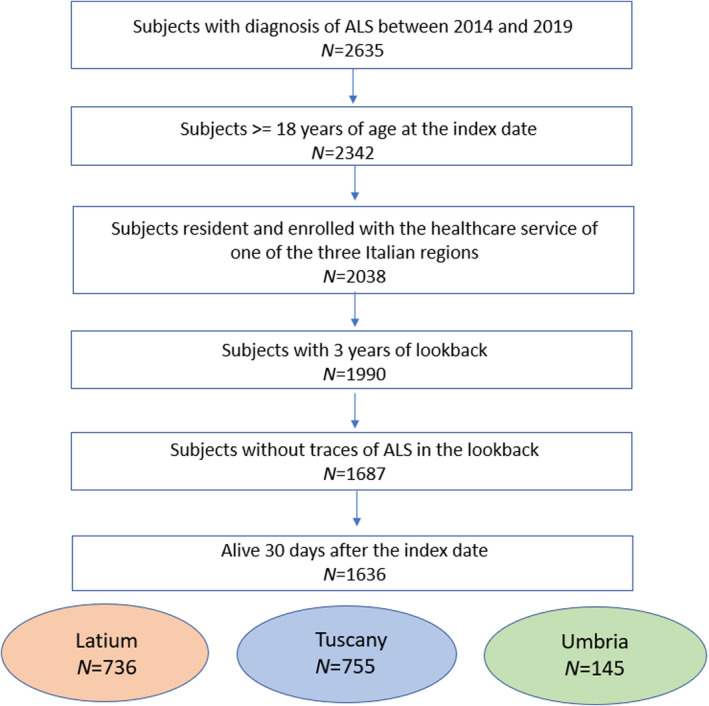
Flowchart of the cohort selection.
TABLE 1.
Characteristics of the amyotrophic lateral sclerosis (ALS) patients at baseline by region and overall.
| Latium | Tuscany | Umbria | Total | |||||
|---|---|---|---|---|---|---|---|---|
| 736 | 755 | 145 | 1636 | |||||
| n | % | n | % | n | % | n | % | |
| Characteristics of the patients at index date | ||||||||
| Gender | ||||||||
| Male | 400 | 54.3% | 389 | 51.5% | 81 | 55.9% | 870 | 53.2% |
| Female | 336 | 45.7% | 366 | 48.5% | 64 | 44.1% | 766 | 46.8% |
| Age in classes | ||||||||
| 18–64 | 274 | 37.2% | 236 | 31.3% | 46 | 31.7% | 556 | 34.0% |
| 65–74 | 255 | 34.6% | 283 | 37.5% | 58 | 40.0% | 596 | 36.4% |
| 75+ | 207 | 28.1% | 236 | 31.3% | 41 | 28.3% | 484 | 29.6% |
| Clinical information in the 2 years before the index date | ||||||||
| Major complications a | ||||||||
| Fractures caused by injury | 51 | 6.9% | 87 | 11.5% | 11 | 7.6% | 149 | 9.1% |
| Dysphagia | 31 | 4.2% | 39 | 5.2% | 6 | 4.1% | 76 | 4.6% |
| Staggering gait | 7 | 1.0% | 25 | 3.3% | 1 | 0.7% | 33 | 2.0% |
| Paraplegia, superior diplegia | 6 | 0.8% | 18 | 2.4% | 3 | 2.1% | 27 | 1.7% |
| Problems related to nutrition | 18 | 2.4% | 8 | 1.1% | 1 | 0.7% | 27 | 1.7% |
| Comorbidities a | ||||||||
| Other motoneuron disease b | 78 | 10.6% | 85 | 11.3% | 8 | 5.5% | 171 | 10.5% |
| Chronic respiratory failure | 64 | 8.7% | 9 | 1.2% | 1 | 0.7% | 74 | 4.5% |
| Acute respiratory failure | 33 | 4.5% | 31 | 4.1% | 4 | 2.8% | 68 | 4.2% |
| Acute and chronic respiratory failure | 40 | 5.4% | 17 | 2.3% | 2 | 1.4% | 59 | 3.6% |
| Depression‐related disorders | 10 | 1.4% | 24 | 3.2% | 3 | 2.1% | 37 | 2.3% |
| Comorbidities in groups c | ||||||||
| Nervous system and sense organ diseases | 495 | 67.3% | 600 | 79.5% | 117 | 80.7% | 1212 | 74.1% |
| Central nervous system diseases | 469 | 63.7% | 564 | 74.7% | 113 | 77.9% | 1146 | 70.0% |
| Diseases of the circulatory system | 228 | 31.0% | 248 | 32.8% | 55 | 37.9% | 531 | 32.5% |
| Injuries and poisonings | 191 | 26.0% | 229 | 30.3% | 31 | 21.4% | 451 | 27.6% |
| Symptoms, signs and undefined morbid states | 181 | 24.6% | 197 | 26.1% | 37 | 25.5% | 415 | 25.4% |
| Endocrine, nutritional, metabolic and immune disorders | 118 | 16.0% | 139 | 18.4% | 27 | 18.6% | 284 | 17.4% |
| Musculoskeletal system and connective tissue diseases | 109 | 14.8% | 140 | 18.5% | 30 | 20.7% | 279 | 17.1% |
| Diseases of the respiratory system | 161 | 21.9% | 95 | 12.6% | 21 | 14.5% | 277 | 16.9% |
| Diseases of the digestive system | 77 | 10.5% | 73 | 9.7% | 14 | 9.7% | 164 | 10.0% |
| Peripheral nervous system diseases | 56 | 7.6% | 91 | 12.1% | 14 | 9.7% | 161 | 9.8% |
| Genitourinary system diseases | 53 | 7.2% | 55 | 7.3% | 16 | 11.0% | 124 | 7.6% |
| Mental disorders | 34 | 4.6% | 54 | 7.2% | 20 | 13.8% | 108 | 6.6% |
| Tumours | 45 | 6.1% | 41 | 5.4% | 14 | 9.7% | 100 | 6.1% |
Only observations occurring in >1% in the total group are reported.
ICD‐9 codes starting with 335.2 (excluding 335.20, corresponding to ALS).
Only observations occurring in >5% in the total group are reported.
TABLE 2.
Pharmacological and non‐pharmacological therapy at baseline by region and overall.
| Latium | Tuscany | Umbria | Total | p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 736 | 755 | 145 | 1636 | ||||||
| n | % | n | % | n | % | n | % | ||
| Drug therapy | |||||||||
| Riluzole a | 149 | 20.2% | 265 | 35.1% | 34 | 23.4% | 448 | 27.4% | <0.05 |
| Psychoanaleptics | 15 | 2.0% | 105 | 13.9% | 2 | 1.4% | 122 | 7.5% | <0.05 |
| Bronchial hypersecretion | 176 | 23.9% | 128 | 17.0% | 22 | 15.2% | 326 | 19.9% | < 0.05 |
| Constipation | 6 | 0.8% | 9 | 1.2% | 4 | 2.8% | 19 | 1.2% | 0.136 |
| Gastro‐oesophageal reflux | 448 | 60.9% | 354 | 46.9% | 71 | 49.0% | 873 | 53.4% | <0.05 |
| Cramps, spasms, fasciculation | 189 | 25.7% | 258 | 34.2% | 16 | 11.0% | 463 | 28.3% | <0.05 |
| Baclofen | 50 | 6.8% | 41 | 5.4% | 3 | 2.1% | 94 | 5.7% | 0.072 |
| Gabapentin | 61 | 8.3% | 70 | 9.3% | 2 | 1.4% | 133 | 8.1% | < 0.05 |
| Pregabalin | 88 | 12.0% | 53 | 7.0% | 5 | 3.4% | 146 | 8.9% | <0.05 |
| Anxiety/depression/emotional frailty | 195 | 26.5% | 251 | 33.2% | 41 | 28.3% | 487 | 29.8% | <0.05 |
| Duloxetine | 29 | 3.9% | 21 | 2.8% | 9 | 6.2% | 59 | 3.6% | 0.104 |
| SSRI | 141 | 19.2% | 216 | 28.6% | 30 | 20.7% | 387 | 23.7% | <0.05 |
| Amitriptyline | 33 | 4.5% | 35 | 4.6% | 6 | 4.1% | 74 | 4.5% | 0.963 |
| Dementia‐related psychiatric disorders | 29 | 3.9% | 24 | 3.2% | 5 | 3.4% | 58 | 3.5% | 0.728 |
| Pain | 329 | 44.7% | 312 | 41.3% | 42 | 29.0% | 683 | 41.7% | <0.05 |
| NSAIDs | 307 | 41.7% | 230 | 30.5% | 35 | 24.1% | 572 | 35.0% | <0.05 |
| Opioids | 80 | 10.9% | 151 | 20.0% | 10 | 6.9% | 241 | 14.7% | <0.05 |
| Thrombosis/embolism | 368 | 50.0% | 335 | 44.4% | 61 | 42.1% | 764 | 46.7% | <0.05 |
| Heparinics | 122 | 16.6% | 131 | 17.4% | 17 | 11.7% | 270 | 16.5% | 0.247 |
| Other drugs (atc 4th level) b | |||||||||
| ≤1 | 100 | 13.6% | 147 | 19.5% | 48 | 33.1% | 295 | 18.0% | |
| 2–5 | 318 | 43.2% | 346 | 45.8% | 51 | 35.2% | 715 | 43.7% | <0.05 |
| 6+ | 318 | 43.2% | 262 | 34.7% | 46 | 31.7% | 626 | 38.3% | |
| Non‐pharmacological treatments a | |||||||||
| Non‐invasive ventilation | 55 | 7.5% | 13 | 1.7% | 3 | 2.1% | 71 | 4.3% | <0.05 |
| Invasive ventilation | 38 | 5.2% | 6 | 0.8% | 3 | 2.1% | 47 | 2.9% | <0.05 |
| Percutaneous endoscopic gastrostomy (PEG) | 25 | 3.4% | 14 | 1.9% | 4 | 2.8% | 43 | 2.6% | 0.176 |
| Tracheostomy | 13 | 1.8% | 2 | 0.3% | 2 | 1.4% | 17 | 1.0% | <0.05 |
Abbreviations: NSAIDs, non‐steroidal anti‐inflammatory drugs; SSRI, selective serotonin reuptake inhibitor.
In the 2 years before the index date.
Any fourth level ATC drug recorded in the 12 months before the index date (excluding the other drugs mentioned above).
FIGURE 2.
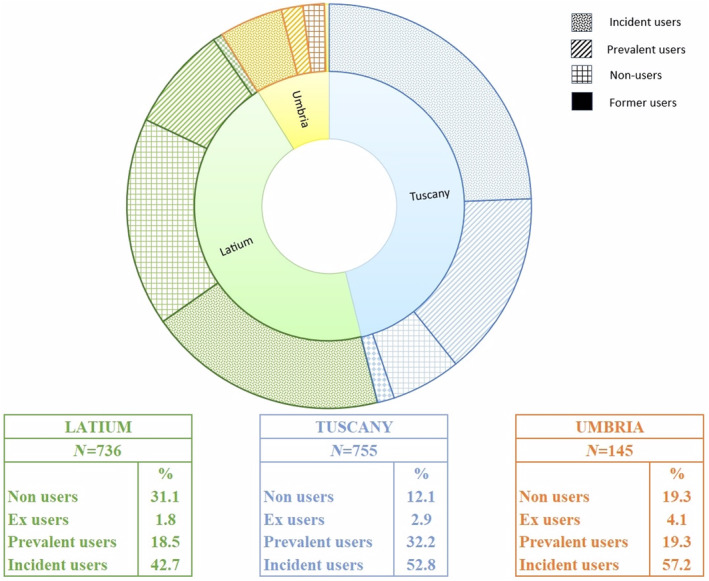
Riluzole use in the first year after the index date (ALS diagnosis) by region and by category of riluzole use.
FIGURE 3.
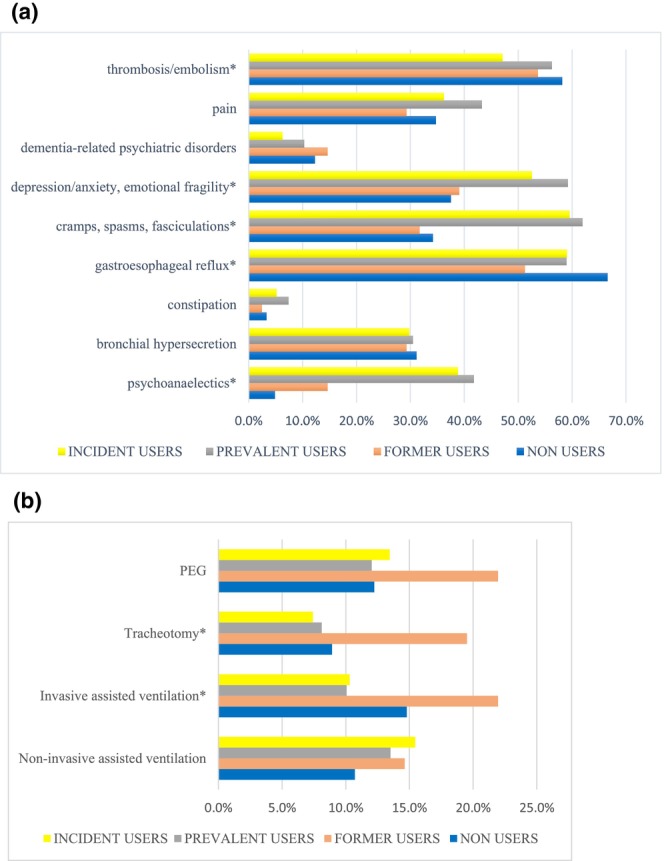
(a) Pharmacological therapy use during the first year after the index date, grouped for all the three regions and stratified by category of riluzole users. *Drug class for which the difference between the four categories of riluzole users was statistically significant. (b) Non‐pharmacological therapy during the first year after the index date, grouped for all the three regions and stratified by the different category of riluzole users. *Procedure for which the difference between the four categories of riluzole users was statistically significant.
FIGURE 4.
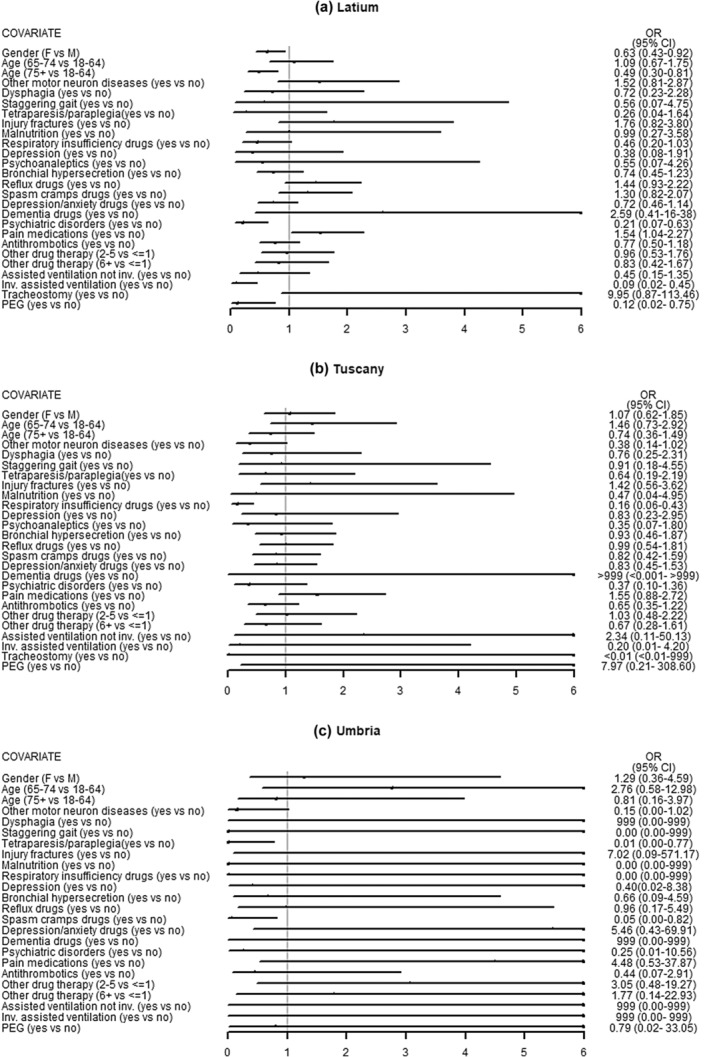
Potential determinants of incident ALS cases being prescribed with riluzole therapy during the first year after the index date by region. *All covariates without explicit categories specified in parentheses refer to the following comparison: yes versus no.
DISCUSSION
This is the first pharmacoepidemiological study based on real‐world data which provides insights into treatment patterns of a large sample of ALS patients particularly with respect to the use of riluzole, symptomatic drugs and non‐pharmacological therapies. Furthermore, determinants associated with riluzole use were investigated. In this study, the index date was the best proxy of the clinical ALS diagnosis, considered as the disease incidence.
All three regional cohorts comprised slightly more male patients, the majority being over 65 years, many of whom were affected by comorbidities and complications typically associated with ALS. Fractures resulting from injuries were the most common major complication observed amongst newly diagnosed patients with ALS. The relationship between ALS and fractures has been extensively studied for several decades, with variations in study design and reported results [24, 25, 26]. Despite such variability, many of these studies reported a positive association between history of fractures and onset of ALS.
In Italy to date, riluzole remains the only disease‐modifying drug approved for the treatment of ALS and fully reimbursed by the NHS for that indication. A similar percentage of ALS patients used riluzole in Latium and Umbria, whilst in Tuscany this percentage was higher. This finding may be an indicator of prescribing riluzole to patients during the complex diagnostic pathway and before a final clinical diagnosis. The differences observed amongst regions may be partly attributed to drug policies implemented at regional level, which regulate access to medicines and their reimbursement, or differences in clinical practice. Even within a single region, therapeutic choices may differ amongst health districts and hospitals [27]. In this regard, it should be noted that, although Italy has a national healthcare system, individual regions have autonomy in managing healthcare delivery. This regional autonomy allows each region to make decisions on the organization and management of health services, leading to variations in treatment protocols [28]. Observed differences may also depend on individual clinician's choices. Clinical trials have demonstrated that riluzole may extend both survival time and time to tracheostomy by approximately 2–3 months compared to placebo. However, its overall impact on other functional measures and muscle strength has been limited, leading some clinicians to opt against prescribing it due to the modest benefits considering also the risk of and potential side effects [9]. Apart from gastro‐oesophageal reflux, pain and thrombosis/embolism medicines, observed in more than 40% of patients affected by ALS, the highest‐ranking symptomatic drugs encompassed also agents for treating anxiety, depression, hypersalivation, cramps as well as bronchial hypersecretion, reflecting the complex diagnostic pathway typical of degenerative diseases.
This study focused on quantifying different categories of riluzole users (prevalent, incident, former users and non‐users) amongst incident ALS patients within the three Italian regions participating in this study. Overall, incident users accounted for more than half of riluzole users with a 2:1 incident/prevalent user ratio in Latium and Tuscany and 3:1 in Umbria. Notably, Latium exhibited lower riluzole prescription rates, with a three‐fold prevalence of non‐users. Despite the European Federation of Neurological Societies (EFNS) recommends starting riluzole as early as possible after diagnosis, a notable proportion of patients received riluzole treatment already before the index date [22], with variations between regions. Part of these patients may have received off‐label riluzole prescriptions for other motor neuron diseases than ALS mainly due to the lack of specific therapies [8] or may have been diagnosed with and treated for ALS before our index date; in this case the diagnosis was not performed within the public healthcare service or in the ambulatory setting and therefore was not traced in administrative healthcare data. In other cases, clinicians may have prescribed riluzole even before making the definite final diagnosis, as reported from a French cross‐sectional study which revealed that riluzole was initiated before confirming the diagnosis in 13.0% of cases [29].
As expected, in the first year after the index date, riluzole and symptomatic drugs steeply increased. Patterns of riluzole treatment differ between regions, and in general the proportions of patients treated were highest in Tuscany. Common pharmacological therapies include antithrombotics, antidepressants and drugs for pain, spasticity, reflux and bronchial hypersecretion. Non‐pharmacological interventions, like PEG, invasive and non‐invasive ventilation, were administered to over 10% of various riluzole user categories. Regardless of the category of riluzole users, such findings align with the natural clinical progression of ALS [30, 31, 32, 33, 34, 35] and comply with EFNS and National Institute for Health and Care Excellence (NICE) guidelines [22, 23]. Notably, approximately 20% of former riluzole users underwent procedures such as PEG placement, tracheotomy and invasive assisted ventilation, which is in line with riluzole contraindications.
Elderly patients, those with invasive assisted ventilation, PEG, respiratory medications, cramps, spasms, and those on more than six medications were less likely to use riluzole. Patients with psychiatric disorders, contrary to recent theories, were also more prone not to use riluzole, challenging the idea of dementia as an ALS symptom rather than a comorbidity [1, 36]. In the latter case, riluzole treatment might positively affect these symptoms, potentially reducing the need for symptomatic therapies. Based on these findings, patients less likely to be prescribed with riluzole fall into two groups. First, the more severe and advanced the disease (e.g., use of invasive assisted ventilation or PEG), the lower is the probability of using riluzole, suggesting that in the advanced stage of the disease the potential benefits of riluzole are limited, and physicians may consider alternative treatment options. This is in line with EFNS guidelines [22] and findings from several open‐label non‐randomized trials supporting that the most significant benefits of riluzole occur during the early stages of the disease [37, 38, 39]. Second, some patients, due to demographic characteristics, pre‐existing medical conditions, contraindications or medications interacting with riluzole, may face increased vulnerability to adverse effects, prompting physicians to refrain from prescribing riluzole [40, 41, 42].
This study has several strengths. To the best of our knowledge, this is the first study that investigates, through an observational study, real‐world drug utilization in ALS patients encompassing disease‐modifying, symptomatic and non‐pharmacological therapies. Moreover, this is a multicentre study based on administrative healthcare data extensively used for pharmacoepidemiological research over the past two decades [43, 44] providing evidence from three Italian regions accounting for about 17% of the overall Italian population. The three regions represent different settings characterized by different healthcare organizations. These regions feature distinct healthcare structures, fostering valuable insights into treatment variations. The observed differences offer a foundation for cross‐disciplinary discussions amongst clinicians in various healthcare settings, promoting knowledge exchange and professional growth. However, some study limitations deserve to be mentioned, the first of which stems from the observational and administrative nature of the data. First, our algorithm may have missed patients not diagnosed or treated in public healthcare facilities, affecting the identification of the date of diagnosis, which was approximated through the index date. For instance, some of those prescribed with riluzole before the index date may have been diagnosed outside the public healthcare service in the early stage of the disease, or treated in an outpatient specialist ambulatory, for which our data do not provide diagnosis. This potential postponement of the real disease onset in our data may have contributed to the findings of complications typically present in ALS patients and use of non‐pharmacological therapies already at baseline. In these cases, the index date may not represent the real disease onset. Second, drugs administered during hospitalization are not tracked at the patient level leading to potential underestimation of drug therapy utilization both at baseline and during the first year after ALS diagnosis. Moreover, data completeness can vary across the three Italian regions participating in this study due to changes in healthcare management and coding practices, which might be a stimulus for improvement. Finally, compliance with data protection regulations limit central‐level processing and pooled analysis, resulting in inadequate sample sizes for robust regional comparisons of the determinants of riluzole use.
In conclusion, the present study showed regional variations in riluzole prescribing for ALS patients. Many patients started pharmacological therapy even before diagnosis and, in the first year after ALS diagnosis, use of both riluzole and symptomatic drugs steeply increased. Greater patient severity and complexity may lead to a lower likelihood of being treated with riluzole. This demonstrates the challenging nature of ALS therapeutic management, emphasizing the need for a multidisciplinary comprehensive approach. The observed differences between regions prompt discussions and knowledge exchange amongst professionals, aiding in clinical audits for appropriate riluzole prescribing and alignment between settings. The present data may also provide insights for future development and evaluation of alternative disease‐modifying therapies for ALS. Future research should comprise a wider range of geographical areas, ideally going beyond the Italian context. This would allow for bigger numbers resulting in higher statistical power, thus allowing more robust results to be obtained. Furthermore, it would be interesting to compare patterns across regions and enlarge the external validity of the findings. In addition, the establishment of ALS registries with a nationwide coverage would offer an important opportunity to study this rare disease, both in terms of case ascertainment and collecting clinical details for each patient. In the context of the CAESAR project, studies regarding adherence to riluzole through trajectory analysis and riluzole use in the presence of contraindications in ALS and off‐label use in other motor neuron diseases have been investigated and results have been published in open access [8, 45].
AUTHOR CONTRIBUTIONS
Olga Paoletti: Formal analysis; data curation; supervision; writing – original draft; writing – review and editing; conceptualization. Giulia Hyeraci: Writing – original draft; writing – review and editing; supervision; conceptualization. Marco Finochietti: Formal analysis; writing – review and editing; methodology; conceptualization. Maria Grazia Celani: Writing – review and editing; supervision; conceptualization. Ilaria Bacigalupo: Conceptualization; writing – review and editing. Niccolò Lombardi: Conceptualization; writing – review and editing. Giada Crescioli: Conceptualization; writing – review and editing. Marco Tuccori: Conceptualization; writing – review and editing. Silvia Cascini: Conceptualization; writing – review and editing. Rosa Gini: Conceptualization; writing – review and editing. Antonio Addis: Conceptualization; writing – review and editing. Ursula Kirchmayer: Conceptualization; investigation; funding acquisition; writing – original draft; methodology; writing – review and editing; project administration; data curation; supervision; resources.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article. Giulia Hyeraci and Olga Paoletti are employed by/consultants of ARS, a public health agency that conducts or participates in pharmacoepidemiology studies compliant with the ENCePP Code of Conduct. The budget of ARS is partially sustained by such studies.
Supporting information
Data S1:
ACKNOWLEDGEMENTS
The current analysis was performed in collaboration with the CAESAR study group. Members of the study group and their affiliations are listed in alphabetical order: Antonio Addis (Department of Epidemiology, ASL Roma 1, SSR Lazio, Rome, Italy), Antonio Ancidoni (National Centre for Disease Prevention and Health Promotion, National Institute of Health, Rome, Italy), Ilaria Bacigalupo (National Centre for Disease Prevention and Health Promotion, National Institute of Health, Rome, Italy), Anna Maria Bargagli (Department of Epidemiology, ASL Roma 1, Lazio Regional Health Service, Rome, Italy), Valeria Belleudi (Department of Epidemiology, ASL Roma 1, Lazio Regional Health Service, Rome, Italy), Roberto Bonaiuti (Department of Neurosciences, Psychology, Drug Research and Child Health, Section of Pharmacology and Toxicology, University of Florence, Italy; Tuscan Regional Centre of Pharmacovigilance, Florence, Italy), Paola Brunori (Neurophysiopathology, Perugia Hospital, Perugia, Italy), Giampaolo Bucaneve (Regional Centre of Pharmacovigilance, Health Authority of Umbria, Perugia, Italy), Teresa Anna Cantisani (Neurophysiopathology, Perugia Hospital, Perugia, Italy), Silvia Cascini (Department of Epidemiology, ASL Roma 1, SSR Lazio, Rome, Italy), Maria Grazia Celani (Neurophysiopathology, Perugia Hospital, Perugia, Italy), Livia Convertino (Area Data Analytics, PuntoZero scarl, Perugia, Italy), Giada Crescioli (Department of Neurosciences, Psychology, Drug Research and Child Health, Section of Pharmacology and Toxicology, University of Florence, Italy; Tuscan Regional Centre of Pharmacovigilance, Florence, Italy), Marco Finocchietti (Department of Epidemiology, ASL Roma 1, SSR Lazio, Rome, Italy), Rosa Gini (Tuscan Regional Centre of Pharmacovigilance, Florence, Italy), Giulia Hyeraci (Tuscan Regional Centre of Pharmacovigilance, Florence, Italy), Ursula Kirchmayer (Department of Epidemiology, ASL Roma 1, SSR Lazio, Rome, Italy), Niccolò Lombardi (Department of Neurosciences, Psychology, Drug Research and Child Health, Section of Pharmacology and Toxicology, University of Florence, Italy; Tuscan Regional Centre of Pharmacovigilance, Florence, Italy), Olga Paoletti (Tuscan Regional Centre of Pharmacovigilance, Florence, Italy), Rosalba Elisabetta Rocchi (Regional Centre of Pharmacovigilance, Health Authority of Umbria, Perugia, Italy), Mariangela Rossi (Regional Centre of Pharmacovigilance, Health Authority of Umbria, Perugia, Italy), Francesco Sciancalepore (National Centre for Disease Prevention and Health Promotion, National Institute of Health, Rome, Italy), Marco Tuccori (Department of Clinical and Experimental Medicine, Unit of Pharmacology and Pharmacovigilance, University of Pisa, Pisa, Italy; Unit of Adverse Drug Reactions Monitoring, University Hospital of Pisa, Pisa, Italy), Nicola Vanacore (National Centre for Disease Prevention and Health Promotion, National Institute of Health, Rome, Italy), Alfredo Vannacci (Department of Neurosciences, Psychology, Drug Research and Child Health, Section of Pharmacology and Toxicology, University of Florence, Italy; Tuscan Regional Centre of Pharmacovigilance, Florence, Italy).
Paoletti O, Hyeraci G, Finochietti M, et al. Pharmacological and non‐pharmacological treatments in amyotrophic lateral sclerosis: an Italian real‐world data study. Eur J Neurol. 2024;31:e16470. doi: 10.1111/ene.16470
DATA AVAILABILITY STATEMENT
The datasets generated and/or analysed during the current study are not publicly available because of privacy reasons.
REFERENCES
- 1. Strong MJ, Abrahams S, Goldstein LH, et al. Amyotrophic lateral sclerosis—frontotemporal spectrum disorder (ALS‐FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(3–4):153‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mitchell JD, Borasio GD. Amyotrophic lateral sclerosis. Lancet. 2007;369(9578):2031‐2041. [DOI] [PubMed] [Google Scholar]
- 3. Talbott EO, Malek AM, Lacomis D. The epidemiology of amyotrophic lateral sclerosis. Handb Clin Neurol. 2016;138:225‐238. [DOI] [PubMed] [Google Scholar]
- 4. Xu L, Liu T, Liu L, et al. Global variation in prevalence and incidence of amyotrophic lateral sclerosis: a systematic review and meta‐analysis. J Neurol. 2020;267(4):944‐953. [DOI] [PubMed] [Google Scholar]
- 5. Bacigalupo I, Finocchietti M, Paoletti O, et al. Incidence and prevalence of amyotrophic lateral sclerosis in three Italian regions: a study based on health administrative databases. Epidemiol Prev. 2024;48(3):201‐209. [DOI] [PubMed] [Google Scholar]
- 6. Gaber TAZK, Mehmood Z, Siringwani H. Riluzole. Prog Neurol Psychiatry. 2016;20(5):32‐33. [Google Scholar]
- 7. Hinchcliffe M, Smith A. Riluzole: real‐world evidence supports significant extension of median survival times in patients with amyotrophic lateral sclerosis. Degener Neurol Neuromuscul Dis. 2017;7:61‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crescioli G, Finocchietti M, Cascini S, et al. Riluzole use in presence of contraindications in adults affected by amyotrophic lateral sclerosis and its off‐label use in other motor neuron diseases: findings from an Italian multicentre study (the CAESAR project). Front Drug Safe Regul. 2022;2:2. doi: 10.3389/fdsfr.2022.1041275 [DOI] [Google Scholar]
- 9. Miller RG, Mitchell JD, Lyon M, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev. 2002;(2):CD001447. [DOI] [PubMed] [Google Scholar]
- 10. Stewart A, Sandercock J, Bryan S, et al. The clinical effectiveness and cost‐effectiveness of riluzole for motor neurone disease: a rapid and systematic review. Health Technol Assess. 2001;5(2):1‐97. [DOI] [PubMed] [Google Scholar]
- 11. Andrews JA, Jackson CE, Heiman‐Patterson TD, Bettica P, Brooks BR, Pioro EP. Real‐world evidence of riluzole effectiveness in treating amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21(7–8):509‐518. [DOI] [PubMed] [Google Scholar]
- 12. Dorst J, Ludolph AC, Huebers A. Disease‐modifying and symptomatic treatment of amyotrophic lateral sclerosis. Ther Adv Neurol Disord. 2017;11:1756285617734734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Atsuta N, Watanabe H, Ito M, et al. Age at onset influences on wide‐ranged clinical features of sporadic amyotrophic lateral sclerosis. J Neurol Sci. 2009;276(1):163‐169. [DOI] [PubMed] [Google Scholar]
- 14. Andersen PM, Borasio GD, Dengler R, et al. Good practice in the management of amyotrophic lateral sclerosis: clinical guidelines. An evidence‐based review with good practice points. EALSC Working Group. Amyotroph Lateral Scler. 2007;8(4):195‐213. [DOI] [PubMed] [Google Scholar]
- 15. Radunović A, Mitsumoto H, Leigh PN. Clinical care of patients with amyotrophic lateral sclerosis. Lancet Neurol. 2007;6(10):913‐925. [DOI] [PubMed] [Google Scholar]
- 16. D'Ovidio F, d'Errico A, Farina E, Calvo A, Costa G, Chiò A. Amyotrophic lateral sclerosis incidence and previous prescriptions of drugs for the nervous system. Neuroepidemiology. 2016;47(1):59‐66. [DOI] [PubMed] [Google Scholar]
- 17. Scialò C, Novi G, Bandettini di Poggio M, et al. Clinical epidemiology of amyotrophic lateral sclerosis in Liguria, Italy: an update of LIGALS register. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17(7–8):535‐542. [DOI] [PubMed] [Google Scholar]
- 18. Tesauro M, Consonni M, Filippini T, et al. Incidence of amyotrophic lateral sclerosis in the province of Novara, Italy, and possible role of environmental pollution. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(3–4):284‐290. [DOI] [PubMed] [Google Scholar]
- 19. Weil C, Zach N, Rishoni S, Shalev V, Chodick G. Epidemiology of amyotrophic lateral sclerosis: a population‐based study in Israel. Neuroepidemiology. 2016;47(2):76‐81. [DOI] [PubMed] [Google Scholar]
- 20. Demetriou CA, Hadjivasiliou PM, Kleopa KA, et al. Epidemiology of amyotrophic lateral sclerosis in the Republic of Cyprus: a 25‐year retrospective study. Neuroepidemiology. 2017;48(1–2):79‐85. [DOI] [PubMed] [Google Scholar]
- 21. Govoni V, Cesnik E, Casetta I, Tugnoli V, Tola MR, Granieri E. Temporal trend of amyotrophic lateral sclerosis incidence in southern Europe: a population study in the health district of Ferrara. Italy J Neurol. 2012;259(8):1623‐1631. [DOI] [PubMed] [Google Scholar]
- 22. EFNS Task Force on Diagnosis and Management of Amyotrophic Lateral Sclerosis , Andersen PM, Abrahams S, et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)—revised report of an EFNS task force. Eur J Neurol. 2012;19(3):360‐375. [DOI] [PubMed] [Google Scholar]
- 23. National Institute for Health and Care Excellence (NICE) guideline . Motor Neurone Disease: Assessment and Management. 2019. https://www.nice.org.uk/guidance/ng42 [PubMed] [Google Scholar]
- 24. Pupillo E, Messina P, Logroscino G, et al. Trauma and amyotrophic lateral sclerosis: a case–control study from a population‐based registry. Eur J Neurol. 2012;19(12):1509‐1517. [DOI] [PubMed] [Google Scholar]
- 25. Chancellor AM, Slattery JM, Fraser H, Warlow CP. Risk factors for motor neuron disease: a case–control study based on patients from the Scottish motor neuron disease register. J Neurol Neurosurg Psychiatry. 1993;56(11):1200‐1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Campbell AM, Williams ER, Barltrop D. Motor neurone disease and exposure to lead. J Neurol Neurosurg Psychiatry. 1970;33(6):877‐885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prada Mariangela, Ruggeri Matteo, Sansone Carmen, De Fazio Dalila, Tettamanti Alessia, Mantovani Matteo, Timeline of authorization and reimbursement for oncology drugs in Italy in the last 3 years, The Journal of Medicine Access. 2017. 1. https://journals.sagepub.com/doi/10.5301/maapoc.0000007 [Google Scholar]
- 28. Garattini L, Badinella Martini M, Zanetti M. The Italian NHS at regional level: same in theory, different in practice. Eur J Health Econ. 2022;23(1):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marin B, Beghi E, Vial C, et al. Evaluation of the application of the European guidelines for the diagnosis and clinical care of amyotrophic lateral sclerosis (ALS) patients in six French ALS centres. Eur J Neurol. 2016;23(4):787‐795. [DOI] [PubMed] [Google Scholar]
- 30. Heidari ME, Nadali J, Parouhan A, et al. Prevalence of depression among amyotrophic lateral sclerosis (ALS) patients: a systematic review and meta‐analysis. J Affect Disord. 2021;287:182‐190. [DOI] [PubMed] [Google Scholar]
- 31. Vender RL, Mauger D, Walsh S, Alam S, Simmons Z. Respiratory systems abnormalities and clinical milestones for patients with amyotrophic lateral sclerosis with emphasis upon survival. Amyotroph Lateral Scler. 2007;8(1):36‐41. [DOI] [PubMed] [Google Scholar]
- 32. Verschueren A, Grapperon AM, Delmont E, Attarian S. Prevalence of spasticity and spasticity‐related pain among patients with amyotrophic lateral sclerosis. Rev Neurol. 2021;177(6):694‐698. [DOI] [PubMed] [Google Scholar]
- 33. Vitacca M, Vianello A. Respiratory outcomes of patients with amyotrophic lateral sclerosis: an Italian nationwide survey. Respir Care. 2013;58(9):1433‐1441. [DOI] [PubMed] [Google Scholar]
- 34. Braun AT, Caballero‐Eraso C, Lechtzin N. Amyotrophic lateral sclerosis and the respiratory system. Clin Chest Med. 2018;39(2):391‐400. [DOI] [PubMed] [Google Scholar]
- 35. Muscaritoli M, Kushta I, Molfino A, Inghilleri M, Sabatelli M, Rossi FF. Nutritional and metabolic support in patients with amyotrophic lateral sclerosis. Nutrition. 2012;28(10):959‐966. [DOI] [PubMed] [Google Scholar]
- 36. McHutchison CA, Wuu J, McMillan CT, et al. Temporal course of cognitive and behavioural changes in motor neuron diseases. J Neurol Neurosurg Psychiatry. 2023;95:331697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Traynor BJ, Alexander M, Corr B, Frost E, Hardiman O. An outcome study of riluzole in amyotrophic lateral sclerosis—a population‐based study in Ireland, 1996–2000. J Neurol. 2003;250(4):473‐479. [DOI] [PubMed] [Google Scholar]
- 38. Zoing MC, Burke D, Pamphlett R, Kiernan MC. Riluzole therapy for motor neurone disease: an early Australian experience (1996–2002). J Clin Neurosci. 2006;13(1):78‐83. [DOI] [PubMed] [Google Scholar]
- 39. Riviere M, Meininger V, Zeisser P, Munsat T. An analysis of extended survival in patients with amyotrophic lateral sclerosis treated with riluzole. Arch Neurol. 1998;55(4):526‐528. [DOI] [PubMed] [Google Scholar]
- 40. Bensimon G, Doble A. The tolerability of riluzole in the treatment of patients with amyotrophic lateral sclerosis. Expert Opin Drug Saf. 2004;3(6):525‐534. [DOI] [PubMed] [Google Scholar]
- 41. Kakuta T, Hirata H, Soda S, et al. Riluzole‐induced lung injury in two patients with amyotrophic lateral sclerosis. Intern Med. 2012;51(14):1903‐1907. [DOI] [PubMed] [Google Scholar]
- 42. Jayaprakash K, Glasmacher SA, Pang B, et al. Riluzole prescribing, uptake and treatment discontinuation in people with amyotrophic lateral sclerosis in Scotland. J Neurol. 2020;267(8):2459‐2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roberto G, Barone‐Adesi F, Giorgianni F, et al. Patterns and trends of utilization of incretin‐based medicines between 2008 and 2014 in three Italian geographic areas. BMC Endocr Disord. 2019;19(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Trifirò G, Gini R, Barone‐Adesi F, et al. The role of European healthcare databases for post‐marketing drug effectiveness, safety and value evaluation: where does Italy stand? Drug Saf. 2019;42(3):347‐363. [DOI] [PubMed] [Google Scholar]
- 45. Giometto S, Finocchietti M, Paoletti O, et al. Adherence to riluzole therapy in patients with amyotrophic lateral sclerosis in three Italian regions—the CAESAR study. Pharmacoepidemiol Drug Saf. 2024;33(1):e5736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1:
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available because of privacy reasons.


