Abstract
Background and purpose
Guidelines help physicians to provide optimal care for stroke patients, but implementation is challenging due to the quantity of recommendations. Therefore a practical overview related to applicability of recommendations can be of assistance.
Methods
A systematic review was performed on ischaemic stroke guidelines published in scientific journals, covering the whole acute care process for patients with ischaemic stroke. After data extraction, experts rated the recommendations on dimensions of applicability, that is, actionability, feasibility and validity, on a 9‐point Likert scale. Agreement was defined as a score of ≥8 by ≥80% of the experts.
Results
Eighteen articles were identified and 48 recommendations were ultimately extracted. Papers were included only if they described the whole acute care process for patients with ischaemic stroke. Data extraction and analysis revealed variation in terms of both content and comprehensiveness of this description. Experts reached agreement on 34 of 48 (70.8%) recommendations in the dimension actionability, for 16 (33.3%) in feasibility and for 15 (31.3%) in validity. Agreement on all three dimensions was reached for seven (14.6%) recommendations: use of a stroke unit, exclusion of intracerebral haemorrhage as differential diagnosis, administration of intravenous thrombolysis, performance of electrocardiography/cardiac evaluation, non‐invasive vascular examination, deep venous thrombosis prophylaxis and administration of statins if needed.
Discussion and conclusion
Substantial variation in agreement was revealed on the three dimensions of the applicability of recommendations. This overview can guide stroke physicians in improving the care process and removing barriers where implementation may be hampered by validity and feasibility.
Keywords: expert testimony, guidelines, ischaemic stroke, quality improvement, systematic review
INTRODUCTION
The care for patients suffering from ischaemic stroke (IS) is rapidly evolving and treatment guidelines, provided by international stroke organizations, are continuously updated based on available evidence [1, 2, 3, 4]. However, a gap between daily clinical practice and guideline recommendations remains, emphasizing the need for improved translation of guideline recommendations into practice [5, 6, 7, 8]. Adequate and evidence‐based treatment of IS is crucial for reducing mortality and morbidity [9, 10, 11, 12]. Stroke care improvement projects have direct impacts on stroke care processes and patient outcomes [7, 8, 12, 13, 14].
In the context of these initiatives, adequate evaluations of quality measures play an important role. Quality measures are objective evaluations developed to support self‐assessment and improvement at the provider, hospital or healthcare system level [8, 15]. They are typically key components of the care process, actionable and aligned with evidence [8]. Currently, there are a multitude of guidelines on the care for IS patients as well as reviews of these guidelines [16]. However, for clinicians it is often not clear which recommendations have the greatest impact on quality of care and are easiest to implement in daily routine. For individual hospitals, care providers and multidisciplinary teams, it remains difficult to choose which actions to initiate first, and what the effort and effect will be in their specific context [17, 18]. Moreover, it is challenging to map and improve the entire care process all at once. As stated by Yu et al. [8] further work is needed toward the consideration around the dimensions of applicability, that is, actionability, feasibility and validity, of key measures. Evaluations of the recommendations on these dimensions could help to set improvement priorities.
Aims
To help healthcare professionals to set these improvement priorities the aim was to first give an overview of the existing recommendations in the literature for IS care, by conducting a systematic review. Second, the extracted recommendations were scored by experts on their applicability, that is, actionability, feasibility and validity, as a means to facilitate their implementation in the care process.
METHODS
Literature search
A systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) guidelines [19]. A search string was designed based on Medical Subject Heading terms, including ‘ischaemic stroke’ and ‘guideline’. All synonyms of these terms were included (Supporting Information). MEDLINE/PubMed libraries were searched. In addition, reference lists were screened to identify other possible eligible studies. The study was initiated as a narrative review for which a registration on PROSPERO or other similar databases was not required. During the conduct of the study with a focus on the expert review a systematic methodology became apparent. At that moment a registration was determined no longer to be appropriate according to the guidance by PROSPERO as the data extraction had already been initiated.
Study selection
The following inclusion criteria were identified: (i) papers on treatment guidelines concerning the whole care process for patients with acute IS, that is, from emergency department/stroke unit (SU) admission to discharge, (ii) published between January 2014 and May 2021, and (iii) written in English. Papers focusing exclusively on specific populations, acute (reperfusion) therapy, (secondary) prevention, rehabilitation, pre‐clinical studies, risk factors, pharmacological treatment, discussion of guidelines, development of guidelines and care processes for patients with transient ischaemic attack, haemorrhagic stroke or cerebral venous thrombosis were excluded. Additionally, papers focused on a narrower, specific aspect of the care process were excluded.
Data extraction
Two independent reviewers (CL and EC) screened all papers based on title and relevance of selected articles. Disagreements were resolved through discussion to reach consensus. One reviewer (CL) read abstracts and full texts to determine if papers fulfilled inclusion criteria. In case of doubt regarding inclusion based on abstract and full text, the second reviewer was consulted to collaboratively descide on in‐ or exclusion.
One reviewer (CL) performed data extraction to collect data on authors, publication year, country and publication type (Table S1). Each paper was categorized into one of the following publication types: guidelines, (systematic) review, papers describing the care process for patients with IS and studies evaluating care processes for IS patients via performance measures. For extraction of the content, the recommendations were categorized according to the following five clinical topics (Table S2): contextual, diagnostic, therapeutic, general supportive care and care transition interventions.
Expert review
Recommendations were scored on three dimensions of applicability: (i) actionability, the recommendation can be acted upon to improve patient care [20]; (ii) feasibility, the performance of the recommendation can be measured, which means that data resources and collection, analysis and interpretation are possible (feasible); data are generally available and already routinely processed [21, 22, 23, 24, 25]; and (iii) validity, meaning the degree to which the indicator, derived from the recommendation, measures what it is intended to measure and/or has a direct effect on the quality of care [21, 26, 27, 28, 29]. These three dimensions were assessed using a 9‐point Likert scale. A score of 1 indicated that the recommendation was deemed not actionable/feasible/valid, whilst a score of 9 indicated that the recommendation was very actionable/feasible/valid. An expert review was designed based on the RAND/University of California, Los Angeles (UCLA) modified Delphi panel method [30, 31]. This consists of a formal group agreement process which combines evidence from a systematic review followed by expert opinion by querying panellists to rate different topics on various dimensions. Our expert panel consisted of 11 international stroke experts, which corresponds to the recommended panel size of 7–15 from the RAND/UCLA appropriateness method guidelines [31]. Experts were chosen based on their knowledge on evidence‐based medicine and guideline implementation.
Based on the results of the literature search the experts were provided with the definitions of the recommendations and three dimensions of applicability (Table S3) to be rated once on a 9‐point Likert scale. Agreement between experts was defined as a score of ≥8 for at least 80% of the experts. The 80% cut‐off point ensures strong intensity of agreement and enhances reproducibility of ratings with different experts [30, 32]. Data were collected online by using Qualtrics. Microsoft Excel was used for descriptive analyses.
RESULTS
Literature search
The electronic search yielded 2823 potentially relevant studies. After removal of non‐English papers and publications before 2014, 1618 studies were retained. Screening based on title relevance excluded 1532 studies. After abstract and full‐text screening, 15 articles met the inclusion criteria. Three extra papers were added via cross‐referencing. In total, 18 studies were included in the final analysis (Figure 1).
FIGURE 1.
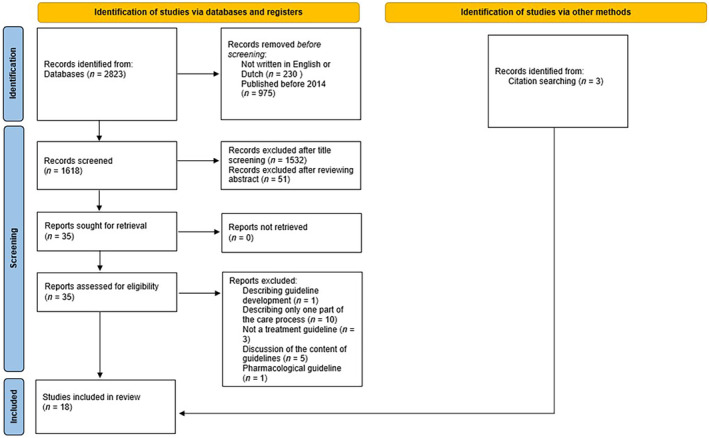
Study selection flowchart according to the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA). Flowchart designed based on Page et al. [19].
Characteristics of the studies reviewed
Papers were divided into four categories based on publication type: six guidelines and seven (systematic) reviews, three papers describing the care process for patients with IS and two papers evaluating the care process for IS patients via performance measures were included. Most papers were published on behalf of scientific organizations (Table S1).
Content of the studies reviewed
In total, 48 recommendations were extracted from the included articles (Table S3). The number of recommendations mentioned in each paper varied from six to 33. They were divided into five clinical topics: contextual (n = 5), diagnostic (n = 6), therapeutic (n = 6), general supportive care (n = 24) and care transition interventions (n = 7) (Table S2). Nineteen recommendations were identified that were present in half or more of the included papers. It was noticed that papers with a reduced number of recommendations provided less information on contextual elements, general supportive care and care transition interventions. This was most pronounced in papers describing performance measures and overviews; in both categories only one contextual recommendation was found. In the selected reviews, four out of seven papers mentioned a recommendation related to care transition interventions (Table S2). None of the selected publications described all 48 recommendations.
Expert review
A group of 11 European experts with at least eight years of experience in stroke care (median 10) agreed to participate. The response rate was 100%. The experts were representatives from 11 European countries: Belgium, Denmark, Finland, France, Germany, Greece, Ireland, Italy, Norway, Spain and Switzerland. Table 1 shows the results of the expert review. When evaluating all 48 recommendations extracted from the systematic review, agreement scores varied per dimension of applicability: agreement on actionability was identified for 34 (70.8%) recommendations, in the dimension feasibility for 16 (33.3%) and for 15 (31.3%) in the dimension of validity.
TABLE 1.
Recommendations for stroke care scored for their actionability, feasibility and validity (dimensions of applicability) by experts.
| Recommendations | Actionability | Feasibility | Validity | |||
|---|---|---|---|---|---|---|
| Percentage in [8,9] | Median (Q1–Q3) | Percentage in [8,9] | Median (Q1–Q3) | Percentage in [8,9] | Median (Q1–Q3) | |
| Contextual | ||||||
| Transfer to an appropriate healthcare facility | 73% | 9 (7.5–9) | 55% | 8 (7–9) | 82% | 9 (8–9) |
| Stroke team activation | 82% | 9 (8–9) | 55% | 8 (7–8.5) | 91% | 9 (8–9) |
| Use of a stroke unit | 82% | 9 (9–9) | 82% | 9 (8–9) | 100% | 9 (9–9) |
| Advanced care planning | 36% | 7 (6.5–8) | 0% | 7 (6–7) | 55% | 8 (6.5–8) |
| Use of telemedicine | 64% | 8 (7–8.5) | 36% | 6 (6–8.5) | 55% | 8 (6–8) |
| Diagnostic | ||||||
| Initial evaluation: use of stroke severity scale | 82% | 9 (8–9) | 91% | 9 (8–9) | 73% | 9 (7.5–9) |
| Clinical (neurological) examination | 100% | 9 (9–9) | 91% | 9 (8–9) | 64% | 8 (7–9) |
| Exclusion of intracerebral haemorrhage as differential diagnosis | 100% | 9 (9–9) | 100% | 9 (9–9) | 100% | 9 (9–9) |
| Documentation of symptom onset | 100% | 9 (9–9) | 27% | 7 (7–8) | 73% | 8 (7.5–9) |
| Imaging: CT scan | 91% | 9 (9–9) | 82% | 9 (8.5–9) | 73% | 9 (7.5–9) |
| Imaging: MRI scan | 64% | 8 (6.5–9) | 36% | 7 (6–9) | 64% | 8 (7–9) |
| Therapeutic | ||||||
| Administration of IV thrombolysis | 91% | 9 (9–9) | 82% | 9 (8.5–9) | 100% | 9 (9–9) |
| Performing thrombectomy | 91% | 9 (9–9) | 73% | 9 (7.5–9) | 100% | 9 (9–9) |
| Administration of antithrombotics | 100% | 9 (9–9) | 91% | 9 (9–9) | 73% | 9 (8–9) |
| No administration of anticoagulation therapy | 100% | 9 (8.5–9) | 91% | 9 (9–9) | 64% | 8 (7–9) |
| Treatment of concomitant medical diseases | 82% | 9 (8–9) | 36% | 7 (7–8.5) | 36% | 7 (6–8.5) |
| Treatment and/or prevention of complications | 82% | 9 (8–9) | 36% | 7 (7–9) | 45% | 7 (7–9) |
| General supportive care | ||||||
| Airway management (ABCs) | 91% | 9 (9–9) | 64% | 9 (7–9) | 82% | 9 (8–9) |
| Glucose management | 82% | 9 (9–9) | 55% | 8 (7–9) | 82% | 8 (8–9) |
| New onset seizure assessment | 91% | 8 (8–9) | 45% | 7 (6.5–9) | 55% | 8 (6–9) |
| Oxygen administration | 73% | 9 (8–9) | 73% | 9 (7.5–9) | 55% | 9 (6–9) |
| Blood sampling | 100% | 9 (9–9) | 100% | 9 (9–9) | 73% | 9 (8–9) |
| Performance of ECG/cardiac evaluation | 100% | 9 (9–9) | 100% | 9 (9–9) | 91% | 9 (9–9) |
| Non‐invasive vascular examination | 91% | 9 (8–9) | 82% | 8 (8–9) | 82% | 8 (8–9) |
| Blood pressure management: acute phase | 100% | 9 (9–9) | 82% | 9 (8–9) | 64% | 8 (7–9) |
| Blood pressure management: follow‐up during hospital stay | 100% | 9 (8.5–9) | 55% | 8 (7–9) | 82% | 8 (8–9) |
| Temperature management | 73% | 9 (7.5–9) | 64% | 8 (7–9) | 64% | 8 (6.5–9) |
| First dysphagia screening | 91% | 9 (9–9) | 64% | 8 (7–9) | 73% | 9 (7.5–9) |
| Formal dysphagia screening by a speech pathologist | 73% | 8 (7,5–9) | 36% | 7 (5.5–8) | 73% | 8 (7.5–9) |
| Considering nutrition status; enteral feeding and nasogastric tube if necessary | 82% | 9 (8–9) | 64% | 8 (7–8) | 64% | 8 (7–9) |
| Deep venous thrombosis prophylaxis | 82% | 9 (8,5–9) | 91% | 8 (8–9) | 82% | 9 (8–9) |
| No administration of prophylactic antibiotics | 91% | 9 (8.5–9) | 82% | 9 (8–9) | 55% | 8 (7–8.5) |
| Administration of statins if needed | 100% | 9 (8.5–9) | 82% | 9 (8–9) | 82% | 9 (8–9) |
| Early carotid imaging | 100% | 9 (9–9) | 73% | 9 (7.5–9) | 91% | 9 (9–9) |
| No administration of urinary tract catheter | 64% | 9 (7–9) | 64% | 8 (7–8) | 73% | 8 (7.5–8.5) |
| Evaluation of pressure ulcer risk | 82% | 8 (8–9) | 45% | 7 (7–8.5) | 73% | 8 (7.5–9) |
| No administration of neuroprotection agents | 73% | 9 (7.5–9) | 82% | 9 (8.5–9) | 55% | 8 (7–9) |
| Evaluation of blood volume/fluid status | 73% | 9 (7.5–9) | 45% | 7 (6.5–9) | 45% | 7 (5–9) |
| Evaluation of incontinence and constipation | 73% | 8 (7.5–9) | 55% | 8 (6.5–8.5) | 45% | 7 (6.5–8.5) |
| Oral hygiene care | 82% | 8 (8–9) | 36% | 7 (6–8) | 55% | 8 (5.5–8.5) |
| Palliative care | 91% | 9 (8.5–9) | 55% | 8 (7–9) | 73% | 9 (7.5–9) |
| Care transition interventions | ||||||
| Performing depression screening | 45% | 7 (7–8.5) | 27% | 7 (6–7.5) | 36% | 7 (6.5–8) |
| Performing ADL screening/screening for rehabilitation needs | 91% | 9 (8–9) | 55% | 8 (7–8.5) | 64% | 8 (7–9) |
| Stroke education | 55% | 8 (7–8.5) | 18% | 7 (6–7) | 45% | 7 (6–8) |
| Patient mobilization | 91% | 9 (8–9) | 64% | 8 (7–8) | 64% | 8 (7–9) |
| Tobacco use counselling | 73% | 8 (7.5–9) | 27% | 7 (6.5–7) | 82% | 9 (8–9) |
| Discharge planning | 82% | 8 (8–8.5) | 45% | 7 (7–8) | 55% | 8 (7–8) |
| Providing (early) rehabilitation | 82% | 8 (8–9) | 27% | 7 (7–7.5) | 64% | 8 (7–9) |
Note: The recommendations extracted from the systematic review were scored on a 9‐point Likert scale on the three dimensions of applicability—actionability, feasibility and validity—by 11 international experts. The percentage of experts that give a score of 8 or 9 are given, as well as the median score for that recommendation. Recommendations for which consensus was reached are shown in bold.
Abbreviations: ABCs, Airway, Breathing, Circulation; ADL, activities of daily living; CT, computed tomography; ECG, electrocardiography; IV, intravenous; MRI, magnetic resonance imaging.
Considering all 48 recommendations included in this study, seven of the 48 recommendations (14.6%) were identified with consensus for all three dimensions of applicability. For contextual recommendations this was ‘use of a SU’; for diagnostic ‘exclusion of intracerebral haemorrhage as differential diagnosis’; and for therapeutic ‘administration of intravenous thrombolysis’. There were four recommendations relating to general supportive care: ‘performance of electrocardiography (ECG)/cardiac evaluation’, ‘non‐invasive vascular examination’, ‘deep venous thrombosis prophylaxis’ and ‘administration of statins if needed’. Agreement on all three dimensions of applicability was not present for any of the recommendations on care transition interventions. For 14 of the 48 recommendations (29.2%), agreement was reached in two of the three dimensions of applicability. One of the two dimensions was always actionability, in combination with feasibility for eight (16.7%) and with validity for five (10.4%). For 16 recommendations (33.3%) agreement was found in only one of the three dimensions of applicability: 13 (27.1%) for actionability, one (2%) for feasibility and two (4%) for validity. The remaining 11 of the 48 (22.9%) did not achieve scores qualifying for agreement in any of the dimensions of applicability. The recommendation on advanced care planning was the only one for which none of the experts gave a score of ≥8.
DISCUSSION
A systematic review was performed and 48 stroke care recommendations were identified which were rated by 11 European stroke experts on actionability, feasibility and validity. In this study, an overview is given of existing guidelines and their recommendations for the care of patients with IS and guidance is provided to clinicians in selecting appropriate measures for care process improvements (Figure 2).
FIGURE 2.
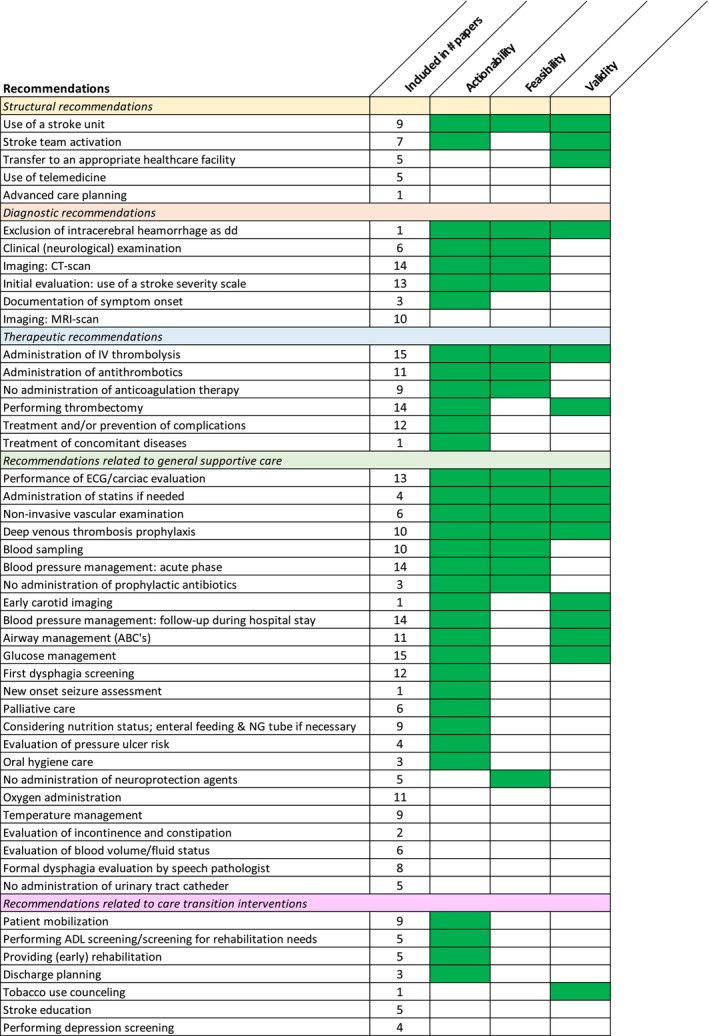
Research overview. This figure provides an overview of the systematic review and expert opinion. The recommendations are listed in the first column. In the second column, the frequency with which each recommendation was mentioned in the included papers is displayed. The following columns indicate whether there was expert consensus (green) or not (blank) for the three domains of applicability. Within each clinical topic category, the recommendations are ranked based on the following priority criteria, from highest to lowest: the recommendations with consensus in the three domains of applicability; the recommendations with consensus for actionability and feasibility; the recommendations with consensus for actionability and validity; the recommendations with consensus for actionability alone, with the highest consensus percentage first; the recommendations with consensus for feasibility alone and the recommendations with consensus for validity alone; recommendations for which no consensus was reached. If the criteria described above were the same for multiple recommendations, their ranking was further refined by the frequency of their mention in the papers included in the systematic review.
Variation in comprehensiveness of the IS care process description was observed in the 18 papers evaluated. This is reflected in differences in the number of topics discussed, although the scope of most papers was similar. No included publication described all 48 extracted recommendations. A recent report from the World Stroke Organization [16] also stated that only a minority of stroke guidelines covered the entire care process. Another level of detail can be obtained depending on the document consulted by a healthcare provider seeking to improve the care process. Guidelines aim to provide easy access to evidence‐based IS care recommendations. Variability may affect development of comprehensive care processes in an individual hospital, especially if only a single guideline is considered [33]. It is conceivable that this will impact the choices in an implementation plan for improvement. Here the aim was to give an overall oversight and understanding of the current literature. The description of diagnostic and therapeutic recommendations was notably extensive, indicating a focus on acute treatment for improving outcomes [34]. In comparison, general supportive care and care transition interventions were less comprehensively described in the included papers. It is believed that a similar focus, in both literature and daily clinical practice, is required on the subacute and chronic phase as many complications (e.g., for dysphagia, post‐stroke depression and fatigue) can be prevented with appropriate screenings [9, 35, 36, 37].
For seven recommendations agreement, defined as a score of ≥8, was identified for at least 80% of the experts, for three dimensions of applicability. (i) Admitting patients to SUs is of clear benefit, but their presence varies across Europe [10, 38, 39]. To improve quality and to reduce variability in stroke care, the European Stroke Organization initiated activities to install certification processes for SUs [40, 41]. (ii) Agreement on exclusion of intracerebral haemorrhage as a differential diagnosis illustrates the importance of neuroimaging to direct treatment plans for IS [42]. (iii) Despite clear benefit of intravenous thrombolysis for selected patients with acute IS, implementation can still be hampered by various factors (e.g., institutional hurdles, patient characteristics) [43, 44]. (iv) ECG changes are independent risk factors for 1‐year mortality in IS patients, underlining the importance of performing ECG to, for example, document atrial fibrillation [45]. (v) Non‐invasive vascular examination is essential to reveal the presence of a large vessel occlusion amenable for mechanical thrombectomy. In primary stroke centres this will necessitate the transfer to comprehensive stroke centres where mechanical thrombectomy can be initiated [46]. (vi) Deep venous thrombosis is a frequent complication, as many of the patients are immobile, which negatively impacts outcome. Simple interventions can reduce risks of developing deep venous thrombosis [47]. (vii) Administration of statins for secondary stroke prevention has been robustly validated and should be part of clinical SU routine [48, 49]. These seven recommendations can provide a starting point for healthcare professionals seeking easily implementable quality measures. As this can remain challenging, the following actions can be considered to facilitate the implementation process: ensure enough knowledge and education chances for the multidisciplinary team to update their knowledge about newly implemented recommendations; provide detailed (online) protocols and feedback mechanisms (e.g., by using and visualizing quality indicators); and stimulate leadership engagement [18, 50].
Although agreement for other recommendations was less robust, as there was no consensus for the three dimensions of applicability, they may have an important role in the care process. Therefore, it is of interest to understand the limitations with regard to their applicability. The presence of implementation barriers can cause absence of consensus regarding feasibility and explain the gap between evidence and daily clinical practice [5, 6, 7]. The literature addresses limited workforce, lack of equipment and education as barriers for implementation of protocols related to general supportive care [51]. Several recommendations were evaluated as less feasible. Advanced care planning may seem understandable from the perspective that patients are admitted in the setting of an acute illness. It may be more appropriate to talk about such a sensitive topic in a more chronic phase where this can be timely discussed with patients and relatives. The evaluation of telemedicine may depend on the country and area where experts have their clinical practice. For physicians working in urban areas, where the nearest hospital with expertise in acute stroke care may be nearby, the need to develop a telemedicine programme may be limited. However, in rural, less densely populated areas telehealth may be critical and hurdles should be addressed [52].
The diagnostic pathway for most patients is clear, but documentation of symptom onset can be challenging. A substantial proportion of patients wake up with stroke symptoms or are alone at the moment of onset. In the context of aphasia healthcare professionals rely on others to obtain exact stroke onset times which may be difficult or take time. The typical neuroimaging planned in the acute stroke remains computed tomography as the first choice as magnetic resonance imaging is logistically not always feasible resulting in longer door‐to‐treatment times, although exceptions do exist.
Therapeutic interventions related to reperfusion and antithrombotics were judged feasible but treating concomitant medical disease and prevention of complications revealed lower ratings. A possible explanation could be that these treatments are beyond the expertise of stroke physicians. Decreased scores were noticed for most care transition interventions. This may be an illustration of the focus on acute care over the last years as a result of great progress in reperfusion strategies. Similar attention is required towards for instance depression screening and proper education on stroke prevention for which communication skills are essential to reach out and connect with patients. Limited capacity for organizational change is a known hurdle for evidence‐based stroke care [5]. Continued efforts to identify and develop actions to remove barriers are needed especially when recommendations are actionable and valid but perceived as less feasible in the existing environment.
Lack of agreement on validity suggests that experts deem the impact of those recommendations on improvement of clinical outcome to be less certain. For most items no clear lack of agreement on validity was noticed with the exception of the treatment of concomitant medical disease and prevention of complications and several items for care transition. This evaluation of reduced validity may reflect less robust evidence for certain of these recommendations [1].
Overall, higher agreement was found on actionability than feasibility and validity. One recommendation with low actionability agreement was advanced care planning, which may reflect a taboo or reduced familiarity surrounding communication about end‐of‐life decisions. Low actionability agreement was also found on depression screening, potentially due to the lack of evidence for optimal screening tools in IS patients [36]. Depression screening had lower ratings for all dimensions of applicability. This is one of the more recent recommendations as awareness on the importance of mental wellbeing is increasing. Based on the interpretation of the experts, implementation is currently hampered and needs further exploration.
When trying to improve the IS care process, it is important to identify possible barriers and response to them in advance [5]. The lack of agreement found in this study may provide assistance to healthcare professionals to predict potential problems with the initiation of quality improvement projects OR when initiating quality improvement projects.
In the current study applicability was assessed according to three vectors: actionability, feasibility and validity. It is believed that the used framework has important value but it is acknowledged that others exist, such as the Clinical Practice Guidelines Applicability Evaluation (CPGAE‐V1.0) scale [53] which focuses on four domains: technical, coordination of support, structure and content, and the role of the guidelines. Linan et al. [54] developed the ‘Instrument for evaluating applicability of clinical practice guidelines’, highlighting the domains availability, readability, acceptability and feasibility. There are also frameworks available for assessing the quality of clinical practice guidelines like the AGREE tool [55], which is beyond the scope of this research. It would be conceivable that analysing application and implementation with another framework would have resulted in different findings.
The literature review was performed systematically and international experts were consulted to ensure generalizability of the results. However, some limitations must be acknowledged. First, exact comparison between 18 included papers was difficult, as papers were included that describe the care process in different ways (e.g., guideline papers vs. systematic review). Secondly, papers were included only if they described the complete care process for patients with IS from emergency department admission to neurology ward discharge. Papers describing only the acute phase or one part of the care process were excluded, which may cause selection bias. In recent years the European Stroke Organization has published and expedited focused guidelines on specific components of the care process. These were beyond the scope of this review as the intention was to evaluate the guidelines professionals would consult when considering updating the entire IS care process. It is acknowledged that these specific guidelines have value in quality improvement projects related to specific parts of the care process. Thirdly, although the expert panel was conducted according to the RAND/UCLA method guidelines, the influence of the opinion of experts is a limitation, as the degree of applicability can be subjective, depending on the resources available within their specific healthcare context. Fourthly, the findings need external validation to show that the appreciation by the experts is reflected in clinical practice. Ideally an implementation study of the various recommendations should be performed followed by a structured evaluation of applicability by the team members involved.
CONCLUSION
In this systematic review on the complete care process for patients with acute IS, 48 different guideline recommendations were extracted. Agreement on applicability between experts was present for seven recommendations. These may provide a first and solid foundation when updating the care process for patients with IS. When initiating quality improvement projects, it is important to understand and target barriers that may hamper implementation. This may alter the applicability of various recommendations on which full agreement was not present.
AUTHOR CONTRIBUTIONS
Charlotte Lens: Conceptualization; investigation; writing – original draft; methodology; visualization. Jelle Demeestere: Writing – review and editing. Barbara Casolla: Writing – review and editing. Hanne Christensen: Writing – review and editing. Urs Fischer: Writing – review and editing. Peter Kelly: Writing – review and editing. Carlos Molina: Writing – review and editing. Simona Sacco: Writing – review and editing. Else Charlotte Sandset: Writing – review and editing. Daniel Strbian: Writing – review and editing. Götz Thomalla: Writing – review and editing. Georgios Tsivgoulis: Writing – review and editing. Kris Vanhaecht: Conceptualization; writing – review and editing. Caroline Weltens: Conceptualization; writing – review and editing; supervision. Ellen Coeckelberghs: Conceptualization; supervision; methodology; investigation; writing – review and editing. Robin Lemmens: Conceptualization; supervision; methodology; writing – review and editing.
CONFLICT OF INTEREST STATEMENT
Dr. Sandset reports other from Bayer, other from AstraZeneca, other from BMS, outside the submitted work; Dr. Thomalla reports personal fees from Acandis, personal fees from Alexion, personal fees from Amarin, personal fees from Astra Zeneca, personal fees from Bayer, personal fees from Boeringer Ingelheim, personal fees from Daiichi Sankyo, personal fees from Stryker,outside the submitted work; Dr. Sacco reports personal fees from Abbvie, personal fees from Novartis, personal fees from Lundbeck, personal fees from Pfizer, personal fees from Boheringer, personal fees from Teva, personal fees from Lilly,outside the submitted work; Dr. Fischer participates in an advisory board for AstraZeneca (former Alexion/Portola), Boehringer Ingelheim, Biogen, AbbVie and Acthera (fees paid to institution); member of a clinical event committee (CEC) of the COATING study (Phenox) and member of the data and safety monitoring committee (DSMB) of the TITAN, LATE_MT and IN EXTREMIS trials; president of the Swiss Neurological Society, president‐elect of the European Stroke Organisation. The other authors have nothing to disclose.
Supporting information
Appendix S1.
Lens C, Demeestere J, Casolla B, et al. From guidelines to clinical practice in care for ischaemic stroke patients: A systematic review and expert opinion. Eur J Neurol. 2024;31:e16417. doi: 10.1111/ene.16417
DATA AVAILABILITY STATEMENT
Research materials will be made available by contacting the corresponding author (RL).
REFERENCES
- 1. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early Management of Patients with Acute Ischemic Stroke: 2019 update to the 2018 guidelines for the early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344‐e418. doi: 10.1161/str.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 2. Berge E, Whiteley W, Audebert H, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021;6(1):I‐lxii. doi: 10.1177/2396987321989865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sandset EC, Anderson CS, Bath PM, et al. European Stroke Organisation (ESO) guidelines on blood pressure management in acute ischaemic stroke and intracerebral haemorrhage. Eur Stroke J. 2021;6(2):Xlviii‐lxxxix. doi: 10.1177/23969873211012133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lindsay P, Furie KL, Davis SM, Donnan GA, Norrving B. World Stroke Organization global stroke services guidelines and action plan. Int J Stroke. 2014;9(Suppl A100):4‐13. doi: 10.1111/ijs.12371 [DOI] [PubMed] [Google Scholar]
- 5. Baatiema L, Otim ME, Mnatzaganian G, de‐Graft Aikins A, Coombes J, Somerset S. Health professionals' views on the barriers and enablers to evidence‐based practice for acute stroke care: a systematic review. Implement Sci. 2017;12(1):74. doi: 10.1186/s13012-017-0599-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lens C, Coeckelberghs E, Seys D, et al. Variation in stroke care at the hospital level: a cross‐sectional multicenter study. Front Neurol. 2022;13:1004901. doi: 10.3389/fneur.2022.1004901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen Y, Gong X, Zhong W, et al. Evaluation of a multilevel program to improve clinician adherence to management guidelines for acute ischemic stroke. JAMA Netw Open. 2022;5(5):e2210596. doi: 10.1001/jamanetworkopen.2022.10596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu AYX, Bravata DM, Norrving B, Reeves MJ, Liu L, Kilkenny MF. Measuring stroke quality: methodological considerations in selecting, defining, and analyzing quality measures. Stroke. 2022;53:3214‐3221. doi: 10.1161/strokeaha.122.036485 [DOI] [PubMed] [Google Scholar]
- 9. Herpich F, Rincon F. Management of acute ischemic stroke. Crit Care Med. 2020;48(11):1654‐1663. doi: 10.1097/ccm.0000000000004597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Langhorne P, Ramachandra S. Organised inpatient (stroke unit) care for stroke: network meta‐analysis. Cochrane Database Syst Rev. 2020;4(4):Cd000197. doi: 10.1002/14651858.CD000197.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Belvis AG, Lohmeyer FM, Barbara A, et al. Ischemic stroke: clinical pathway impact. Int J Health Care Qual Assur. 2019;32(3):588‐598. doi: 10.1108/ijhcqa-05-2018-0111 [DOI] [PubMed] [Google Scholar]
- 12. Bravata DM, Purvis T, Kilkenny MF. Advances in stroke: quality improvement. Stroke. 2022;53(5):1767‐1771. doi: 10.1161/strokeaha.122.037450 [DOI] [PubMed] [Google Scholar]
- 13. Middleton S, McElduff P, Ward J, et al. Implementation of evidence‐based treatment protocols to manage fever, hyperglycaemia, and swallowing dysfunction in acute stroke (QASC): a cluster randomised controlled trial. Lancet. 2011;378(9804):1699‐1706. doi: 10.1016/s0140-6736(11)61485-2 [DOI] [PubMed] [Google Scholar]
- 14. Cadilhac DA, Grimley R, Kilkenny MF, et al. Multicenter, prospective, controlled, before‐and‐after, quality improvement study (Stroke123) of acute stroke care. Stroke. 2019;50(6):1525‐1530. doi: 10.1161/strokeaha.118.023075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonow RO, Masoudi FA, Rumsfeld JS, et al. ACC/AHA classification of care metrics: performance measures and quality metrics: a report of the American College of Cardiology/American Heart Association task force on performance measures. J Am Coll Cardiol. 2008;52(24):2113‐2117. doi: 10.1016/j.jacc.2008.10.014 [DOI] [PubMed] [Google Scholar]
- 16. Mead GE, Sposato LA, Sampaio Silva G, et al. A systematic review and synthesis of global stroke guidelines on behalf of the World Stroke Organization. Int J Stroke. 2023;18(5):499‐531. doi: 10.1177/17474930231156753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Torab‐Miandoab A, Samad‐Soltani T, Shams‐Vahdati S, Rezaei‐Hachesu P. An intelligent system for improving adherence to guidelines on acute stroke. Turk J Emerg Med. 2020;20(3):118‐134. doi: 10.4103/2452-2473.290062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lowther HJ, Harrison J, Hill JE, et al. The effectiveness of quality improvement collaboratives in improving stroke care and the facilitators and barriers to their implementation: a systematic review. Implement Sci. 2021;16(1):95. doi: 10.1186/s13012-021-01162-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arias AV, Garza M, Murthy S, et al. Quality and capacity indicators for hospitalized pediatric oncology patients with critical illness: a modified Delphi consensus. Cancer Med. 2020;9(19):6984‐6995. doi: 10.1002/cam4.3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Noltes ME, Cottrell J, Madani A, et al. Quality indicators for the diagnosis and management of primary hyperparathyroidism. JAMA Otolaryngol Head Neck Surg. 2022;148(3):209‐219. doi: 10.1001/jamaoto.2021.3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hassan A, Ouzounian M, Dagenais F, et al. Development of quality indicators for the management of acute type A aortic dissection. Can J Cardiol. 2021;37(10):1635‐1638. doi: 10.1016/j.cjca.2021.05.015 [DOI] [PubMed] [Google Scholar]
- 23. Pap R, Lockwood C, Stephenson M, Simpson P. Development and testing of Australian prehospital care quality indicators: study protocol. BMJ Open. 2020;10(7):e038310. doi: 10.1136/bmjopen-2020-038310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Henson LA, Edmonds P, Johnston A, et al. Population‐based quality indicators for end‐of‐life cancer care: a systematic review. JAMA Oncol. 2020;6(1):142‐150. doi: 10.1001/jamaoncol.2019.3388 [DOI] [PubMed] [Google Scholar]
- 25. Koch D, Kutz A, Conca A, Wenke J, Schuetz P, Mueller B. The relevance, feasibility and benchmarking of nursing quality indicators: a Delphi study. J Adv Nurs. 2020;76(12):3483‐3494. doi: 10.1111/jan.14560 [DOI] [PubMed] [Google Scholar]
- 26. Leighton JA, Brock AS, Semrad CE, et al. Quality indicators for capsule endoscopy and deep enteroscopy. Gastrointest Endosc. 2022;96(5):693‐711. doi: 10.1016/j.gie.2022.08.039 [DOI] [PubMed] [Google Scholar]
- 27. Santos JV, Martins FS, Vidal‐Castro J, et al. Indicators for local health plan monitoring and evaluation: a modified Delphi consensus. Public Health Nurs. 2022;39(4):752‐759. doi: 10.1111/phn.13036 [DOI] [PubMed] [Google Scholar]
- 28. Lee KC, Walling AM, Senglaub SS, et al. Improving serious illness care for surgical patients: quality indicators for surgical palliative care. Ann Surg. 2022;275(1):196‐202. doi: 10.1097/sla.0000000000003894 [DOI] [PubMed] [Google Scholar]
- 29. Mazzone PJ, White CS, Kazerooni EA, Smith RA, Thomson CC. Proposed quality metrics for lung cancer screening programs: a National Lung Cancer Roundtable Project. Chest. 2021;160(1):368‐378. doi: 10.1016/j.chest.2021.01.063 [DOI] [PubMed] [Google Scholar]
- 30. Rodríguez‐Mañas L, Féart C, Mann G, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition‐consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68(1):62‐67. doi: 10.1093/gerona/gls119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kathryn Fitch SJB, Aguilar MD, Burnand B, et al. The RAND/UCLA Appropriateness Method User's Manual. 2001.
- 32. Giltenane M, Sheridan A, Kroll T, Frazer K. Identification of quality indicators of public health nursing practice: ‘modified Delphi’ approach. Public Health Nurs. 2022;39(1):214‐228. doi: 10.1111/phn.13000 [DOI] [PubMed] [Google Scholar]
- 33. Feigin VL, Brainin M, Norrving B, et al. World Stroke Organization (WSO): global stroke fact sheet 2022. Int J Stroke. 2022;17(1):18‐29. doi: 10.1177/17474930211065917 [DOI] [PubMed] [Google Scholar]
- 34. Feske SK. Ischemic stroke. Am J Med. 2021;134(12):1457‐1464. doi: 10.1016/j.amjmed.2021.07.027 [DOI] [PubMed] [Google Scholar]
- 35. Joundi RA, Martino R, Saposnik G, Giannakeas V, Fang J, Kapral MK. Predictors and outcomes of dysphagia screening after acute ischemic stroke. Stroke. 2017;48(4):900‐906. doi: 10.1161/strokeaha.116.015332 [DOI] [PubMed] [Google Scholar]
- 36. Medeiros GC, Roy D, Kontos N, Beach SR. Post‐stroke depression: a 2020 updated review. Gen Hosp Psychiatry. 2020;66:70‐80. doi: 10.1016/j.genhosppsych.2020.06.011 [DOI] [PubMed] [Google Scholar]
- 37. Cumming TB, Packer M, Kramer SF, English C. The prevalence of fatigue after stroke: a systematic review and meta‐analysis. Int J Stroke. 2016;11(9):968‐977. doi: 10.1177/1747493016669861 [DOI] [PubMed] [Google Scholar]
- 38. Leys D. Another benefit of stroke unit care. Eur J Neurol. 2022;29(9):2557‐2558. doi: 10.1111/ene.15480 [DOI] [PubMed] [Google Scholar]
- 39. Aguiar de Sousa D, von Martial R, Abilleira S, et al. Access to and delivery of acute ischaemic stroke treatments: a survey of national scientific societies and stroke experts in 44 European countries. Eur Stroke J. 2019;4(1):13‐28. doi: 10.1177/2396987318786023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Waje‐Andreassen U, Nabavi DG, Engelter ST, et al. European Stroke Organisation certification of stroke units and stroke centres. Eur Stroke J. 2018;3(3):220‐226. doi: 10.1177/2396987318778971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. European Stroke Organization . ESO Certification Map. https://eso‐certification.org/european‐database‐by‐country
- 42. Choi EY, Nieves GA, Jones DE. Acute stroke diagnosis. Am Fam Physician. 2022;105(6):616‐624. [PubMed] [Google Scholar]
- 43. Donnellan C, Sweetman S, Shelley E. Health professionals' adherence to stroke clinical guidelines: a review of the literature. Health Policy. 2013;111(3):245‐263. doi: 10.1016/j.healthpol.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 44. Mikulik R, Bar M, Cernik D, et al. Stroke 20 20: implementation goals for intravenous thrombolysis. Eur Stroke J. 2021;6(2):151‐159. doi: 10.1177/23969873211007684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Asadi P, Zia Ziabari SM, Naghshe Jahan D, Jafarian YA. Electrocardiogram changes as an independent predictive factor of mortality in patients with acute ischemic stroke; a cohort study. Arch Acad Emerg Med. 2019;7(1):e27. [PMC free article] [PubMed] [Google Scholar]
- 46. Maas WJ, van der Zee DJ, Buskens E, Uyttenboogaart M, Lahr MM. Simulation modelling to study the impact of adding comprehensive stroke centres. Can we deliver endovascular thrombectomy sooner? BMJ Open. 2023;13(7):e068749. doi: 10.1136/bmjopen-2022-068749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dennis M, Caso V, Kappelle LJ, Pavlovic A, Sandercock P. European Stroke Organisation (ESO) guidelines for prophylaxis for venous thromboembolism in immobile patients with acute ischaemic stroke. Eur Stroke J. 2016;1(1):6‐19. doi: 10.1177/2396987316628384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tramacere I, Boncoraglio GB, Banzi R, et al. Comparison of statins for secondary prevention in patients with ischemic stroke or transient ischemic attack: a systematic review and network meta‐analysis. BMC Med. 2019;17(1):67. doi: 10.1186/s12916-019-1298-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dawson J, Béjot Y, Christensen LM, et al. European Stroke Organisation (ESO) guideline on pharmacological interventions for long‐term secondary prevention after ischaemic stroke or transient ischaemic attack. Eur Stroke J. 2022;7(3):I‐XLI. doi: 10.1177/23969873221100032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McCluskey A, Vratsistas‐Curto A, Schurr K. Barriers and enablers to implementing multiple stroke guideline recommendations: a qualitative study. BMC Health Serv Res. 2013;13:323. doi: 10.1186/1472-6963-13-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dale S, Levi C, Ward J, et al. Barriers and enablers to implementing clinical treatment protocols for fever, hyperglycaemia, and swallowing dysfunction in the quality in acute stroke care (QASC) project—a mixed methods study. Worldviews Evid Based Nurs. 2015;12(1):41‐50. doi: 10.1111/wvn.12078 [DOI] [PubMed] [Google Scholar]
- 52. Takahashi EA, Schwamm LH, Adeoye OM, et al. An overview of telehealth in the management of cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2022;146(25):e558‐e568. doi: 10.1161/cir.0000000000001107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li H, Xie R, Wang Y, Xie X, Deng J, Lu C. A new scale for the evaluation of clinical practice guidelines applicability: development and appraisal. Implement Sci. 2018;13(1):61. doi: 10.1186/s13012-018-0746-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Linan Z, Qiusha Y, Chuan Z, et al. An instrument for evaluating the clinical applicability of guidelines. J Evid Based Med. 2020;14(1):75‐81. doi: 10.1111/jebm.12416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003;12(1):18‐23. doi: 10.1136/qhc.12.1.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Data Availability Statement
Research materials will be made available by contacting the corresponding author (RL).


