Abstract
The management of eosinophilic esophagitis (EoE) has not been completely established yet. There is a controversy over the universal maintenance therapy for EoE to prevent esophageal fibrostenotic complications. Using an employer-based insurance claim database from January 2005 to September 2022, we investigated the treatment patterns of EoE and the occurrence of esophageal complications. The treatment patterns were analyzed at a 6-month interval from the diagnosis of EoE. The time to treatment discontinuation of proton pump inhibitor (PPI)/potassium-competitive acid blocker (P-CAB) was evaluated by the Kaplan–Meier method. Of 15,200,895 individuals, 615 patients with EoE were ultimately analyzed with the median follow-up time from the index date of 700 days. PPI/P-CAB and swallowed topical steroids accounted for 80% and 4.6% of the initial therapy, respectively. PPI/P-CAB use rapidly decreased by 40% in the first 6 months and afterwards reinitiation was rarely seen. The median time to treatment discontinuation were 172 days (95% CI 147–206 days) for PPI/P-CAB. Only 1 EoE patient developed esophageal fibrostenotic complications after the diagnosis. With the low incidence of esophageal complications, the universal maintenance therapy may not be necessary for mild EoE patients often seen in Japan.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-78868-4.
Keywords: Eosinophilic esophagitis, Proton pump inhibitors, Potassium-competitive acid blocker, Steroids, Esophageal dilation
Subject terms: Oesophageal diseases, Gastroenterology
Introduction
Eosinophilic esophagitis (EoE) is a chronic immune-mediated disorder where eosinophil-predominant inflammation causes esophageal dysfunction-related symptoms1. This relatively new disorder emerged from the 1990’s, and since then the number of EoE patients has continued to rise2. Food allergens and/or aeroallergens trigger the type 2-associated esophageal inflammation and cytokine secretion (i.e. IL-4, IL-13, IL-5 and transforming growth factor-beta (TGF-β)). IL-4 and IL-13 induce eotaxin-3 that attracts and activates eosinophils in the tissue1 whereas TGF-β contributes to tissue remodeling that results in subepithelial fibrosis3–5. EoE impairs quality of life (QOL) due to bothersome symptoms. In addition, esophageal remodeling and fibrosis can cause food impaction and esophageal stricture.
The main treatments of EoE include acid suppressants (i.e. proton pump inhibitor (PPI) and potassium-competitive acid blocker (P-CAB)), topical steroids, dietary therapy, and endoscopic dilation for esophageal stricture6–9. Other agents such as immune modulators, leukotriene antagonists, or antihistamines can also be used in clinical practice6,8. The Japanese eosinophilic gastrointestinal disorders (EGIDs) guidelines recommend PPIs for the first-line therapy and topical steroids and/or elimination diet for the second-line therapy6. However, the management strategy of EoE has not been globally established yet as other EoE guidelines equally recommend these therapeutic options as the first-line therapy.
Real world data demonstrated that PPI accounted for 50% of the first-line therapy in the US where more than half of the patients discontinued the treatment within two years10. In a European multi-center study, PPI was also most prescribed as the first-line therapy11. These studies indicate that PPI is the mainstream therapy of EoE in Western countries. The British guidelines recommend giving PPI at a double dose. However, there is a shortage of data about the appropriate dose and duration of the initial PPI treatment8. In addition, there is uncertainty over the universal maintenance therapy due to inadequate understanding of the natural history of EoE and the long-term cost-benefit balance2,6,12,13.
The management of EoE could differ between Japan and Western countries. In Japan, the mild phenotype is predominant, and P-CAB, the most powerful acid suppressant, is available besides PPI14. A previous single-academic-center study with more than 300 Japanese EoE patients reported that PPI/P-CAB accounted for 93.5% of the initial therapy, and 15% of which were later switched to swallowed topical steroid15. Only 1% of EoE patients required urgent endoscopy or endoscopic dilation during the observation period. This incidence is extremely low compared to the US where 20% of EoE patients required endoscopic dilation3. Thus, the Japanese real-world treatment pattern would provide an insight into the controversy about the management of EoE especially for mild phenotype. However, the current treatment patterns of EoE in Japan have not been widely evaluated to date.
Therefore, the aim of this study was to investigate the treatment patterns of EoE and their consequences related to esophageal complications in Japan using a large health care claim data.
Results
Characteristics of study population
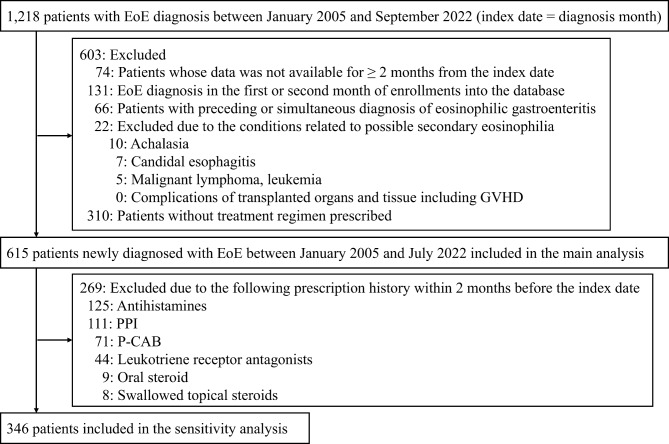
Of 15,200,895 individuals, 1,218 patients were diagnosed with EoE in the database (Fig. 1). Ultimately, 615 patients were analyzed in the main analysis according to the exclusion criteria. After the further exclusion of patients who had any treatment regimens for EoE within 2 months before the index date, 346 patients remained in the sensitivity analysis. The median (IQR) follow-up time from the index date was 700 days (335–1157) for the main analysis population and 730 days (365–1188) for the sensitivity analysis population. Table 1 shows the demographic and clinical characteristics of EoE patients. Most EoE patients were adult in both the main and sensitivity analysis. The sensitivity analysis population had a lower proportion of allergic conditions than the main analysis population.
Fig. 1.
Flowchart of the study population. Abbreviations: EoE, eosinophilic esophagitis; GVHD, graft-versus-host disease; PPI, proton pump inhibitor; P-CAB, potassium-competitive acid blocker.
Table 1.
Demographic and clinical characteristics of patients with EoE
| Variable | Main analysis (n = 615) | Sensitivity analysis (n = 346) |
|---|---|---|
| Age (years), Median [IQR] | 45.00 [36.00, 52.00] | 44.00 [37.00, 51.00] |
| Adult (≥ 20 years) | 592 (96.3%) | 336 (97.1%) |
| Female | 173 (28.1%) | 82 (23.7%) |
| Comorbidities | ||
| Asthma | 113 (18.4%) | 36 (10.4%) |
| Atopic dermatitis | 61 (9.9%) | 22 (6.4%) |
| Allergic rhinitis | 224 (36.4%) | 88 (25.4%) |
| Esophageal complications | ||
| Pre-EoE diagnosis | ||
| Esophageal foreign body | 4 (0.7 %) | 3 (0.9 %) |
| Esophageal stricture | 8 (1.3 %) | 6 (1.7 %) |
| Post-EoE diagnosis | ||
| Esophageal foreign body | 1 (0.1%) | 1 (0.3%) |
| Esophageal stricture | 0 (0.0%) | 0 (0.0%) |
Variables distributions are reported as n (%) unless otherwise specified. Abbreviations: IQR, interquartile range; EoE, eosinophilic esophagitis
Initial treatment for EoE
The initial treatment for patients with EoE is shown in Table 2 (all drug combinations in eTable 2). Overall, 33.6% (310/923) of EoE patients were not treated with any regimens. Out of 615 EoE patients with any of the five treatment regimens in the main analysis, PPI/P-CAB was prescribed predominantly (80%). In contrast, swallowed topical steroids accounted for 4.6% of the initial treatment, and 80% of which were combined with PPI/P-CAB. Oral steroid (i.e. prednisolone) was used for 5.7% of EoE patients with the average dose of 22.5 mg per day. Similar treatment patterns were observed in the sensitivity analysis.
Table 2.
Initial treatment for EoE in the main and sensitivity analyses
| Main analysis (n = 615) | Sensitivity analysis (n = 346) | |
|---|---|---|
| Medication | n (%) | n (%) |
| PPI/P-CAB | 492 (80.0%) | 296 (85.5%) |
| Swallowed topical steroids + PPI/P-CAB | 24 (3.9%) | 5 (1.4%) |
| Swallowed topical steroids | 4 (0.7%) | 1 (0.3%) |
| Oral steroid | 35 (5.7%) | 18 (5.2%) |
| Others | 60 (9.8%) | 26 (7.5%) |
Others include only antihistamines and/or leukotriene receptor antagonists. Abbreviations: EoE, eosinophilic esophagitis; PPI, proton pump inhibitor; P-CAB, potassium-competitive acid blocker
Treatment patterns for EoE
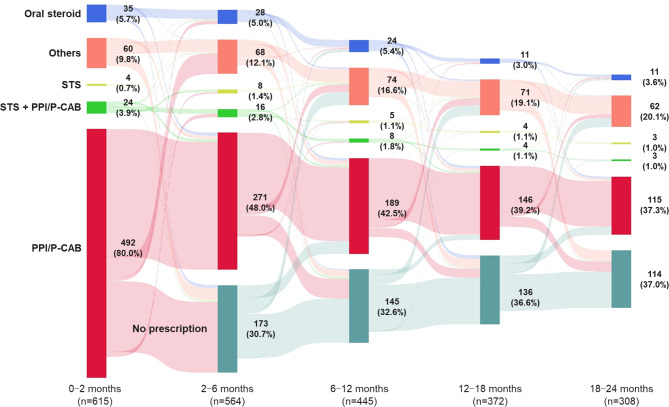
Figure 2 shows the treatment patterns of patients with EoE in the main analysis from the initial treatment. The proportion of PPI/P-CAB use rapidly decreased in the first 6 months from 80% to 48%, and a gradual downward trend continued in the following period. The proportion of swallowed topical steroids therapy also reduced over the course of time from 4.6% to 2% in 24 months. Out of 95 patients who discontinued PPI/P-CAB treatment in the first 6 months, reinitiation was only seen in 8 patients (8.4%) in the next 6 months. In addition, switch from PPI/P-CAB to swallowed topical steroids was rare (2% in the first 6 months, and 1.6% in the second 6 months). Overall, 30.7% of EoE patients who underwent the initial therapy discontinued pharmacological treatments at 6 months after the diagnosis, and their proportion increased to 37% at 24 months. The sensitivity analysis of the treatment patterns for EoE patients is shown in eFigure 1, which demonstrates similar patterns to the main analysis.
Fig. 2.
Treatment patterns of EoE in the main analysis. PPI/P-CAB, STS+PPI/P-CAB, STS, and oral steroids could include concomitant use of antihistamines and/or leukotriene receptor antagonists. Others included only antihistamines and/or leukotriene receptor antagonists. Abbreviations: EoE, eosinophilic esophagitis; PPI, proton pump inhibitor; P-CAB, potassium-competitive acid blocker; STS, swallowed topical steroids.
Duration and dose of the initial EoE treatment using PPI/P-CAB or swallowed topical steroids
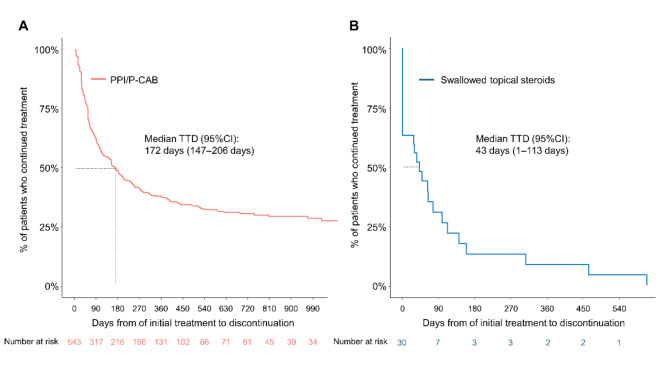
Figure 3A and B show the time to discontinuation of the initial therapy using PPI/P-CAB or swallowed topical steroids. The median time to treatment discontinuation were 172 days (95% CI 147–206 days) for PPI/P-CAB and 43 days (95% CI 1–113 days) for swallowed topical steroids. PPI/P-CAB was continuously prescribed for at least 990 days in 28.9% of EoE patients. Whilst most swallowed topical steroids were terminated within a year as the retention rate was 13.2% (95% CI 4.7–37.3) at 6 months and 8.8% (95% CI 2.4–32.7) at 12 months.
Fig. 3.
Time to treatment discontinuation of the initial therapy for EoE in the main analysis. (A) PPI/P-CAB. (B) Swallowed topical steroids. Abbreviations: EoE, eosinophilic esophagitis; PPI, proton pump inhibitor; P-CAB, potassium-competitive acid blocker; TTD, time to treatment discontinuation; CI, confidence interval
In PPI/P-CAB treatment, PPIs (322/543, 59.3%) were more frequently prescribed than P-CAB (Table 3). PPIs were administered at half, standard, or double doses, in which the most common dose was standard (i.e. esomeprazole 20mg/day, rabeprazole 10mg/day, lansoprazole 30mg/day, or omeprazole 20mg/day) (270/322, 83.9%). As for P-CAB, vonoprazan was more frequently prescribed at 20mg per day (150/221, 67.9%) than 10mg/day.
Table 3.
Dose of PPIs and P-CAB for the initial treatment of EoE
| Main analysis (n = 615) | |
|---|---|
| Medication | n (%) |
| PPI (n=322) | |
| Esomeprazole | |
| 20mg/day | 198 (32.2%) |
| 10mg/day | 24 (3.9%) |
| Rabeprazole | |
| 20mg/day | 4 (0.7%) |
| 10mg/day | 58 (9.4%) |
| Lansoprazole | |
| 30mg/day | 12 (2.0%) |
| 15mg/day | 22 (3.6%) |
| Omeprazole | |
| 20mg/day | 2 (0.3%) |
| 10mg/day | 2 (0.3%) |
| P-CAB (n=221) | |
| Vonoprazan | |
| 20mg/day | 150 (24.4%) |
| 10mg/day | 71 (11.5%) |
Abbreviations EoE, eosinophilic esophagitis; PPI, proton pump inhibitor; P-CAB, potassium-competitive acid blocker
Occurrence of esophageal complications
Out of 615 patients with EoE, 12 patients (2.0%) had a history of esophageal foreign body or stricture (Table 1). The esophageal complications mostly occurred before the index date of EoE diagnosis (11/12, 88.3%). One EoE case developed esophageal foreign body within a month after the index date. The patient received PPI/P-CAB treatment for less than 6 months and did not subsequently develop esophageal stricture. Regarding 310 patients with no treatment, only one case developed esophageal foreign body after the diagnosis of EoE.
Discussion
EoE is characterized by a chronic allergic condition with eosinophilic infiltration. As is the case with other conditions, several issues need to be taken into consideration for the treatment decision (e.g. disease course, treatment durability, cost-effectiveness, etc.). However, the natural course of EoE has not been completely understood because of few long-term observational studies. In addition, there have been no head-to-head clinical trials pertaining to pharmacological therapies. Thus, there are still controversy over the management of EoE. Many guidelines nonhierarchically recommend either PPIs, topical steroids, or diet therapy for the initial and maintenance therapy6–9. Since transcription profiles revealed three different EoE phenotypes associated with endoscopic findings (e.g. fibrostenosis) and treatment response, it is likely that the management of EoE should be tailored accordingly16. For instance, mild EoE phenotype may not necessarily require long-term therapy owing to the low likelihood of developing stricture or food impaction as such EoE patients tend to poorly adhere to treatments17,18. Given the difficulty in examining transcription profiles in all EoE patients, the real-world data can contribute to getting an insight into the pragmatic approach to EoE. Therefore, we analyzed a large health care claim database to find out the treatment patterns of EoE and the occurrence of esophageal complications in Japan. This study found that 1) PPI/P-CAB and swallowed topical steroids accounted for 80% and 4.6% in the initial pharmacological treatment for EoE, respectively, 2) half of the initial PPI/P-CAB treatment was short-term (i.e. < 6 months) and afterwards rarely reinitiated, 3) the long-term PPI/P-CAB treatment (i.e. for at least 3 years) were provided to 25% of EoE patients, 4) switch from PPI/P-CAB to swallowed topical steroids were rare, and 5) esophageal foreign body or stricture rarely occurred in EoE patients after the diagnosis.
In the initial EoE therapy, PPI/P-CAB was the most common option. This treatment pattern is consistent with the Japanese guidelines as well as the previous observational study by a single academic center in Japan6,15. A large European cohort similarly demonstrated a high frequency of PPI use (76.5%)11, whereas the US showed a much lower proportion of PPI (50%) in the initial therapy10. It might reflect the inconsistency between guidelines where American Gastroenterological Association makes a strong recommendation only for topical steroids7. Also, over-the-counter PPIs are prevalent in the US. The topical steroids use further highlights the different EoE management between Japan and Western countries. The ratio of swallowed topical steroids to PPI/P-CAB was 6% in this study whereas 17% in Europe11 and 90% in the US10. The high efficacy of PPI/P-CAB for Japanese EoE could result in the infrequent use of topical steroids as symptom and histological improvement can be achieved in 81% and 75% of Japanese EoE patients, respectively19,20. This study demonstrated less frequent switch from PPI/P-CAB to swallowed topical steroids compared to the previous Japanese academic center study (15%)15. It might be attributed to the inclusion of primary and secondary centers that see relatively mild EoE patients in this database. Interestingly, 33.6% of EoE patients did not undergo any pharmacological treatment. EoE patients with very mild symptom might have been willing to wait and watch the disease activity on their own. In addition, EoE patients can reduce symptoms substantially by modifying their eating habits (chew more, avoid tough meat, drink more liquids)12.
Currently there is a paucity of evidence pertaining to the appropriate dose of PPI/P-CAB for EoE despite the latest guidelines’ recommendations (i.e. omeprazole at a dose of 20-40 mg two times per day)8,9. In this study, standard-dose PPIs were predominant, followed by P-CAB 20mg per day. Step-down approach can be pragmatic given dose-dependent efficacy of PPIs for EoE in Western countries as well as the influence of CYP2C19 genotype on the loss of response to the long-term PPI therapy21,22. However, standard or half dose of PPI may be enough for the initial therapy in Japan as the strength of acid suppression does not correlate to their efficacy in Japanese EoE19.
Maintenance therapy for EoE is generally recommended in the guidelines for prevention of esophageal subepithelial fibrosis resulting in esophageal complications6–9,23,24. Indeed, EoE can recur clinically, endoscopically, and histologically after treatment discontinuation5,25. However, there is a shortage of evidence to support the universal maintenance therapy12. In addition, there are concerns about potential adverse events of the long-time use of PPI/P-CAB and topical steroids26–28. We found that PPI/P-CAB and swallowed topical steroids treatment discontinued in half of EoE patients within 6 and 2 months, respectively. The much shorter treatment period with topical steroids suggests that most of their treatment may not have been substantial due to mild EoE activity in Japan. This is further supported by approximately 30% of EoE patients having no pharmacological treatment from 6 months after the index date onwards. This pattern matches the previous hospital-based study by Yamamoto, et al. pertaining to the clinical course of Japanese EoE where 66% of EoE patients were continuous type (i.e. symptom or treatment duration > 6 months)29. Interestingly, the American population data also showed that 46% of pharmacological treatment was discontinued within approximately 16 months30. These real-world data suggest two possibilities. Firstly, the maintenance therapy is not required for all EoE patients. Secondly, patients are not willing to continue the treatment due to mild symptoms and the cost incurred. Straumann, et al. demonstrated that dysphagia and eosinophilic infiltration persisted but not exacerbated for over 7 years in approximately 80% of EoE patients without pharmacological treatment4. In addition, nearly 80% of young EoE patients reported symptom improvement during 8-year follow-up despite 67% of the patients not receiving active treatment31. Furthermore, Abe, et al. showed very mild or asymptomatic EoE patients did not worsen endoscopically and histologically in approximately 7 years without any treatment32. Since this study also demonstrated rare esophageal complications in Japanese EoE as previously15,29,32,33, maintenance therapy may not be necessary for mild EoE patients. Along with genetic and environmental factors, health check-ups could contribute to a relatively high proportion of mild EoE in Japan. Further study is warranted to evaluate the long-term data.
Oral steroid used to be prescribed for EoE patients29,34 as the previous EoE guidelines from American College of Gastroenterology suggested using systemic steroid for topical steroids-refractory cases. Although the latest guidelines do not recommend systemic steroid for EoE7,8, we confirmed that approximately half of prednisolone was prescribed after 2020. Due to similarity of oral steroids prescription between main and sensitivity analysis, oral steroids were likely to be prescribed for EoE in this study. Systemic steroid could be replaced with novel treatments including biologics in the future12. The prescription of antihistamines and/or leukotriene receptor antagonists solely increased from 9.8% to 20.1% in this study. These drugs might be used as the maintenance therapy, or alternatively for concomitant allergic conditions.
This study has some limitations. All the treatment regimens analyzed in this study are off-label for EoE in Japan, and for this reason the use of these drugs might not capture the treatment for EoE. For instance, steroid inhalers could have been prescribed for concurrent asthma although short-period prescriptions are uncommon for managing the condition. On the other hand, a large health claim data enabled us to analyze the large sample size, and the similar treatment patterns to the previous hospital-based study validate this study’s data15. Moreover, the sensitivity analysis excluded the prescription of treatment regimens for other conditions than EoE as much as possible. It is possible that some patients underwent diet therapy with or without simultaneous pharmacological treatment, which cannot be identified in the claim database. Nonetheless, diet therapy for EoE is unusual in Japan because of limited accessibility to dietitians15,29,34. Follow-up period was relatively short in this study for the assessment of esophageal complication. However, esophageal food impaction and stenosis have rarely occurred in the previous Japanese EoE study with over 7-year follow-up period32. Along with genetic and environmental factors, health check-ups could contribute to a relatively high proportion of mild EoE in Japan. Apart from esophageal complications, the database could not evaluate treatment outcomes including symptoms, endoscopic findings and histology. Regarding EoE patients with no prescription, on-demand treatment could be classified as no prescription in the sanky diagrams.
In conclusion, a large health care claim database demonstrated that PPI/P-CAB accounted for 80% of the initial EoE treatment whereas swallowed topical steroids use was rare in Japan. Half of the initial PPI/P-CAB treatment was discontinued in the first 6 months and 30% of EoE patients were constantly off pharmacological treatments after the initial treatment. Given the very low incidence of esophageal complications, universal maintenance therapy may not be required for mild EoE patients often seen in Japan.
Methods
Data source
This real-world retrospective cohort study used the Japanese employer–based health insurance database from January 2005 to September 2022 provided by a commercial database vendor (JMDC inc., Tokyo, Japan)35. The JMDC database consists of insurance claims issued from health care providers to health insurance societies that insured relatively large company employees and their dependents in Japan. The database covered approximately 10% of the Japanese population36. The insurance claims contain medical conditions and prescription on the basis of the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) and Anatomical Therapeutic Chemical Classification System, respectively. In addition, medical procedures coded according to the Japanese claim codes were included. In the JMDC database, all claims of the patients can be followed longitudinally regardless of medical facilities they visited and inpatient or outpatient care unless they withdraw from their health insurance. This study was performed in accordance with the Declaration of Helsinki. The ethical board review was waived by the Osaka Metropolitan University ethics committee because all data provided by JMDC were already anonymized. Thus, the need to obtain informed consent was waived by the Osaka Metropolitan University ethics committee.
Study population and follow-up
Patients newly diagnosed as EoE between January 2005 to July 2022 were included in the analysis. EoE was identified on the basis of the ICD-10 diagnostic code of K20.0 in the database. EoE diagnosis in the database was previously validated37. To adequately capture the new onset of EoE, patients with EoE diagnosis in the first or second month of enrollment into the database were excluded. In addition, patients with EoE were excluded if they had the preceding or simultaneous diagnosis of eosinophilic gastroenteritis (EGE), or conditions related to secondary esophageal eosinophilia including candida esophagitis, achalasia, malignant lymphoma or leukemia, or complications of transplanted organs and tissues including graft–versus–host disease (GVHD)38,39. Lists of ICD-10 codes for these conditions of exclusion criteria are shown in eTable 1–1. The month of the first diagnosis with EoE was set as the index date, and the follow-up period was from the index date to the end of record. EoE patients who had no prescription within 2 months from the index date or had less than a 2-month follow-up period were also excluded.
Therapeutic options for EoE
To investigate the treatment patterns of EoE, we analyzed the prescription of the following drugs according to the Japanese EGIDs guidelines6. PPI/P-CAB, swallowed topical steroids (inhaled budesonide or fluticasone), oral steroid (prednisolone), and others (antihistamines and/or leukotriene receptor antagonists). Lists of medication codes for these drugs are shown in eTable 1–2. The Japanese guidelines recommend PPI/P-CAB and swallowed topical steroids as the mainstream treatment similar to guidelines from other countries7–9. This study categorized these options into the following five regimens:
PPI/P-CAB
swallowed topical steroids
a combination of swallowed topical steroids and PPI/P-CAB
oral steroid
others (antihistamines and/or leukotriene receptor antagonists)
The regimes (1) to (4) could include concomitant use of antihistamines and/or leukotriene receptor antagonists.
Study outcomes
Treatment patterns of EoE
The treatment patterns for EoE were visualized using Sankey diagrams. The initial therapy for EoE was identified as a treatment regimen prescribed for patients with EoE within 2 months from the index date. Due to the great heterogeneity in the duration of the initial and following treatments, we analyzed the treatment patterns in every 6-month period after the index date of EoE except the first 6 months that divided into the first 2 months for identification of the initial therapy and the following 4 months. There were two treatment patterns in each period, 1) switch to other drugs, or 2) no prescription. Switch to other drugs was defined when the treatment in the last period switched to any of other regimens in a period. No prescription was defined when any treatment regimens had not been prescribed for at least 6 months since the end of the last treatment.
To eliminate the possibility of the regimens being prescribed for other pre-existing disorders, the sensitivity analysis excluded patients who had prescriptions of any regimens within 2 months before the index date of EoE from the main analysis.
Duration of the initial EoE treatment using PPI/P-CAB or swallowed topical steroids
The time to treatment discontinuation evaluated the duration of the initial treatment with each PPI/P-CAB and swallowed topical steroids, the most established medications for the treatment of EoE. The time to treatment discontinuation was defined as the time from the first prescription of the treatment to any of the following events that determined the end of the treatment: (a) no prescription of each regimen following an over 90-day gap for the initial treatment, or (b) the last date of the observable period.
Occurrence of esophageal complications
The number of EoE patients with esophageal foreign body or esophageal stricture was investigated. Lists of ICD-10 codes for these conditions are shown in eTable 1–3.
Statistical analysis
Demographic and clinical characteristics of patients were summarized using median and interquartile range (IQR) for continuous variables and numbers (percentage) for categorical variables. Treatment patterns were analyzed descriptively and illustrated using Sankey diagrams. The Kaplan–Meier method was used to estimate medians and 95% confidence intervals (CIs) for the time to treatment discontinuation. Data handling and statistical analyses were conducted using R version 4.3.1 (R Core Team). Study design was summarized in eFigure 2 according to a structured template for planning and reporting on the implementation of real world evidence studies (STaRT-RWE)40.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by a research grant from Bristol Myers Squibb (#75267729).
Author contributions
AS contributed to study concept and design, data analysis, data interpretation, funding, drafting the manuscript, and approval of the final version. YI and TI contributed to study concept and design, data analysis, data interpretation, drafting the manuscript, and approval of the final version. FT contributed to editing the manuscript critically and approval of the final version. YF contributed to study concept and design, data interpretation, and editing the manuscript critically and approval of the final version.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
AS receives a research grant from Bristol Myers Squibb. YI is an employee of Daiichi Sankyo and has worked for the company since March 2024. TI received lecture fees from Kyowa Kirin. The remaining authors declare no conflicts of interest with this study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Akinari Sawada and Yasutaka Ihara.
References
- 1.O’Shea, K. M. et al. Pathophysiology of eosinophilic esophagitis. Gastroenterology154, 333–345. 10.1053/j.gastro.2017.06.065 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellon, E. S. & Hirano, I. Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology154, 319-332.e313. 10.1053/j.gastro.2017.06.067 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dellon, E. S. et al. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest. Endosc.79, 577–585. 10.1016/j.gie.2013.10.027 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Straumann, A. et al. Natural history of primary eosinophilic esophagitis: A follow-up of 30 adult patients for up to 11.5 years. Gastroenterology125, 1660–1669. 10.1053/j.gastro.2003.09.024 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Straumann, A. et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol/9, 400–409. 10.1016/j.cgh.2011.01.017 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Ministry of Health, Labor and Welfare EGIDs Research Group. Japanese Guidelines on the Management of Eosinophilic Gastrointestinal Disorders. https://mhlw-grants.niph.go.jp/system/files/2019/192051/201911035B_upload/201911035B202006121223304940004.pdf. Accessed 3 Jan 2024 (2020). (in Japanese).
- 7.Hirano, I. et al. AGA institute and the joint task force on allergy-immunology practice parameters clinical guidelines for the management of eosinophilic esophagitis. Gastroenterology158, 1776–1786. 10.1053/j.gastro.2020.02.038 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhar, A. et al. British society of gastroenterology (BSG) and British society of paediatric gastroenterology, hepatology and nutrition (BSPGHAN) joint consensus guidelines on the diagnosis and management of eosinophilic oesophagitis in children and adults. Gut71, 1459–1487. 10.1136/gutjnl-2022-327326 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucendo, A. J. et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur. Gastroenterol. J.5, 335–358. 10.1177/2050640616689525 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dellon, E. S., Xia, Q., Qian, E. W., Kumari, S. & Tencer, T. Real-world treatment patterns and persistence in patients with eosinophilic esophagitis. Gastroenterology162, S-529. 10.1016/S0016-5085(22)61260-5 (2022). [Google Scholar]
- 11.Laserna-Mendieta, E. J. et al. Efficacy of therapy for eosinophilic esophagitis in real-world practice. Clin. Gastroenterol. Hepatol.18, 2903–2911. 10.1016/j.cgh.2020.01.024 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Hirano, I. & Furuta, G. T. Approaches and challenges to management of pediatric and adult patients with eosinophilic esophagitis. Gastroenterology158, 840–851. 10.1053/j.gastro.2019.09.052 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Philpott, H. & Dellon, E. S. The role of maintenance therapy in eosinophilic esophagitis: Who, why, and how?. J. Gastroenterol.53, 165–171. 10.1007/s00535-017-1397-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishimura, N., Okimoto, E., Shibagaki, K., Nagano, N. & Ishihara, S. Similarity and difference in the characteristics of eosinophilic esophagitis between Western countries and Japan. Dig. Endosc.33, 708–719. 10.1111/den.13786 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Fujiwara, Y. et al. Current real-world treatments for Japanese patients with eosinophilic esophagitis. Nihon Shokakibyo Gakkai Zasshi119, 929–936. 10.11405/nisshoshi.119.929 (2022). [DOI] [PubMed] [Google Scholar]
- 16.Shoda, T. et al. Eosinophilic oesophagitis endotype classification by molecular, clinical, and histopathological analyses: A cross-sectional study. Lancet Gastroenterol. Hepatol.3, 477–488. 10.1016/S2468-1253(18)30096-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang, R. et al. Assessing adherence and barriers to long-term elimination diet therapy in adults with eosinophilic esophagitis. Dig. Dis. Sci.63, 1756–1762. 10.1007/s10620-018-5045-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haasnoot, M. L., Safi, S. & Bredenoord, A. J. Poor adherence to medical and dietary treatments in adult patients with eosinophilic esophagitis. Am. J. Gastroenterol.117, 1412–1418. 10.14309/ajg.0000000000001850 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Kuzumoto, T. et al. Vonoprazan shows efficacy similar to that of proton pump inhibitors with respect to symptomatic, endoscopic, and histological responses in patients with eosinophilic esophagitis. Esophagus18, 372–379. 10.1007/s10388-020-00783-0 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Fujiwara, Y. et al. Responses of proton pump inhibitors and potassium-competitive acid blockers according to outcomes of symptom, endoscopy, and histology in patients with eosinophilic esophagitis. J. Clin. Gastroenterol.10.1097/MCG.0000000000001869 (2023). [DOI] [PubMed] [Google Scholar]
- 21.Laserna-Mendieta, E. J. et al. Efficacy of proton pump inhibitor therapy for eosinophilic oesophagitis in 630 patients: Results from the EoE connect registry. Aliment. Pharmacol. Ther.52, 798–807. 10.1111/apt.15957 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Molina-Infante, J. et al. Long-term loss of response in proton pump inhibitor-responsive esophageal eosinophilia is uncommon and influenced by CYP2C19 genotype and rhinoconjunctivitis. Am. J. Gastroenterol.110, 1567–1575. 10.1038/ajg.2015.314 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Straumann, A. et al. Budesonide orodispersible tablets maintain remission in a randomized, placebo-controlled trial of patients with eosinophilic esophagitis. Gastroenterology159, 1672–1685. 10.1053/j.gastro.2020.07.039 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Schupack, D. A. et al. Effect of maintenance therapy for eosinophilic esophagitis on need for recurrent dilation. Dig. Dis. Sci.66, 503–510. 10.1007/s10620-020-06192-8 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Dellon, E. S. et al. Rapid recurrence of eosinophilic esophagitis activity after successful treatment in the observation phase of a randomized, double-blind, double-dummy trial. Clin. Gastroenterol. Hepatol.18, 1483–1492. 10.1016/j.cgh.2019.08.050 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yadlapati, R. & Kahrilas, P. J. The, “dangers” of chronic proton pump inhibitor use. J. Allergy Clin. Immunol.141, 79–81. 10.1016/j.jaci.2017.06.017 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Henderson, A. F., Khan, S. M., Hornung, L. N., Mukkada, V. A. & Kalkwarf, H. J. Prevalence and predictors of compromised bone mineral density in pediatric eosinophilic esophagitis. J. Pediatr. Gastroenterol. Nutr.71, 764–770. 10.1097/MPG.0000000000002866 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bose, P. et al. Adrenal insufficiency in children with eosinophilic esophagitis treated with topical corticosteroids. J. Pediatr. Gastroenterol. Nutr.70, 324–329. 10.1097/MPG.0000000000002537 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto, M. et al. Comparison of nonesophageal eosinophilic gastrointestinal disorders with eosinophilic esophagitis: A nationwide survey. J. Allergy Clin. Immunol. Pract.9, 3339–3349. 10.1016/j.jaip.2021.06.026 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Dellon, E. S., Xia, Q., Qian, E. W., Kumari, S. & Tencer, T. Su1171: Real-world treatment patterns and persistence in patients with eosinophilic esophagitis. Gastroenterology162, S-529. 10.1016/S0016-5085(22)61260-5 (2022). [Google Scholar]
- 31.Bohm, M., Jacobs, J. W. Jr., Gupta, A., Gupta, S. & Wo, J. M. Most children with eosinophilic esophagitis have a favorable outcome as young adults. Dis. Esophagus30, 1–6. 10.1111/dote.12454 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Abe, Y. et al. Long-term course of untreated asymptomatic esophageal eosinophilia and minimally symptomatic eosinophilic esophagitis. Endosc. Int. Open.12, E545–E553. 10.1055/a-2280-8277 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujiwara, Y. et al. A multicenter study on the prevalence of eosinophilic esophagitis and PPI-responsive esophageal eosinophilic infiltration. Intern. Med.51, 3235–3239 (2012). [DOI] [PubMed] [Google Scholar]
- 34.Kinoshita, Y. et al. Clinical characteristics of Japanese patients with eosinophilic esophagitis and eosinophilic gastroenteritis. J. Gastroenterol.48, 333–339. 10.1007/s00535-012-0640-x (2013). [DOI] [PubMed] [Google Scholar]
- 35.Nagai, K. et al. Data resource profile: JMDC claims database sourced from health insurance societies. J. Gen. Fam. Med.22, 118–127. 10.1002/jgf2.422 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.JMDC. About JMDC Claims Database. https://www.jmdc.co.jp/en/jmdc-claims-database/ (2023).
- 37.Sawada, A. et al. Epidemiology and risk factors of eosinophilic esophagitis in Japan: A population-based study. Clin. Gastroenterol. Hepatol.10.1016/j.cgh.2024.04.035 (2024). [DOI] [PubMed] [Google Scholar]
- 38.Dellon, E. S., Jensen, E. T., Martin, C. F., Shaheen, N. J. & Kappelman, M. D. Prevalence of eosinophilic esophagitis in the United States. Clin. Gastroenterol. Hepatol.12, 589–596. 10.1016/j.cgh.2013.09.008 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Low, E. E. et al. Development and validation of the veterans affairs eosinophilic esophagitis cohort. Clin. Gastroenterol. Hepatol.21, 3030–3040. 10.1016/j.cgh.2023.03.033 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang, S. V. et al. STaRT-RWE: Structured template for planning and reporting on the implementation of real world evidence studies. BMJ372, m4856. 10.1136/bmj.m4856 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.