Abstract
The causes of variation in the success of laparoscopic artificial insemination (AI) in sheep are not well understood. As such, this study incorporated the contributions of multiple male and female factors relevant to the success of AI into a comprehensive prediction model for pregnancy success. Data from Merino ewes (N = 30 254) including age, uterine tone (1; pale/flaccid-5; turgid/pink), intra-abdominal fat (1; little to no fat present-5; high fat), time of insemination and sire used, were recorded during AI. A subset of semen per sire (N = 388) was thawed and assessed for volume, subjective motility, sperm concentration, and morphology. Sperm motility (CASA), viability and acrosome integrity (FITC-PNA/PI), membrane fluidity (M540/Yo-Pro), mitochondrial superoxide production (Mitosox Red/Sytox Green), lipid peroxidation (Bodipy C11), level of intracellular reactive oxygen species (H2DCFDA) and DNA fragmentation (Acridine Orange) were also assessed 0, 3 and 6 h post-thaw. Logistic binomial regression revealed sperm concentration (P < 0.001), CASA parameters at 0 h (PCA3; P = 0.03), viable acrosome intact sperm at 6 h (P = 0.02), abnormal morphology (P < 0.001), uterine tone (P < 0.001) and intra-abdominal fat (P = 0.03) of ewes influenced likelihood of pregnancy. Results generated will help standardise the pre-screening and selection of semen and ewes prior to artificial breeding programs, reducing variation in the success of sheep AI.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-79253-x.
Keywords: Sheep, Sperm, Morphology, Concentration, Acrosome, Viability, Uterine tone, Intra-abdominal fat, Motility, Laparoscopic
Subject terms: Animal biotechnology, Animal breeding
Introduction
The efficient and sustainable production of sheep requires consistent genetic improvement, most rapidly achieved through the application of assisted reproductive technologies, such as laparoscopic AI. It is generally accepted that 70% of ewes inseminated via laparoscopic AI should fall pregnant1, yet variation in success is apparent between geographical regions, across breeding seasons, sires and even ejaculates of the same sire2–5. This uncertainty surrounding the reliability of AI outcomes has contributed to waning adoption and subsequent negative flow on effects to the rate of genetic and production gains to the national flock. Identifying specific female and male fertility factors, particularly in vitro semen characteristics which are linked to pregnancy success following AI, would enable producers and breeding companies to screen sires and frozen samples prior to breeding programs, eliminating samples likely to give sub-optimal results. This would help reduce variability in program success and give the industry greater confidence in the application of AI.
The ability to predict the success of AI based on a sire’s semen characteristics or ewes’ condition has long been sought. While several factors are known to influence fertility both during natural and AI3,4,6–34, correlations to fertility outcomes in sheep have been largely contradictory and fail to consider multiple male and female factors in the same study, comparing within the individual ewe rather than flock average. Our previous work35 analysed data collected on ewes during AI and showed uterine tone (considered a proxy for the physiological response of ewes to the oestrous synchronisation protocol) to be an important indicator of AI success. Ewes, which scored a uterine tone of 4 or 5 at the time of AI, recorded a 12.41% increase in pregnancy rate compared to ewes, which scored a uterine tone of 1 or 2 (P < 0.05). Similarly, ewes that scored a uterine tone of 3 recorded notably lower pregnancy rates compared to ewes with a uterine tone score of 4 or 5 (P < 0.05). It is therefore now imperative to assess the influence of ewe factors, like uterine tone, on fertility in conjunction with the in vitro characteristics of the semen used for AI in the same model.
Today, with the advance of objective semen assessment techniques, there is a wide variety of semen parameters known to collectively define a ‘fertile’ spermatozoon36. Previous research on bulls37–39, rams12,40,41, stallions42,43, and boars44 have all identified a correlation between sperm motility and velocity parameters41,45–47, as well as sperm morphology48–50 with pregnancy success. Additional research has also looked at the concentration at which sperm is frozen prior to AI, as a proxy for insemination dose7,8 having an effect on pregnancy success. However, the use of flow cytometry and intracellular fluorochromes now enables us to study alterations in sperm membrane phospholipids51,52, cell viability, acrosome integrity13,53, DNA fragmentation54, and measures of excessive reactive oxygen species40,55 and mitochondrial function56. While certain studies have adeptly assessed the influence of these specific semen characteristics on ram fertility following AI13,15,53,57,58, limited standardisation in their application across studies has contributed to contradictions in their described effect on fertility. Notably, stemming from the multitude of tests available for a single trait, a lack of direct comparison between samples inseminated and analysed, as well as reduced sample sizes, have limited the likelihood of successful fertility prediction36.
As such, the present study sought to determine the influence of female data collected during AI and in vitro semen assessment characteristics post-thaw on the likelihood or probability of pregnancy occurring following laparoscopic AI in sheep. Results will lead to a better understanding of which in vitro semen traits correlate to fertility and, therefore, the fertility potential of a particular frozen-thawed sample. The design of a model to predict AI would also facilitate the identification of accurate standards for the sheep artificial breeding community. When optimal semen is combined with a fertile, well-conditioned ewe, the likelihood of pregnancy should increase, reducing the variability of AI programs and reproductive failure.
Methods
Ethics and animals
The data used in this research was generously donated by artificial breeding companies and stud breeders during routine commercial AI operations. Animals are not directly involved in this study, as such, no additional ethical approval was required. All procedures were conducted with consultation from the University of Sydney Animal Ethics Committee.
The management of ewes and rams adhered to the standard industry practices and requirements for each site, and all methods complied with relevant guidelines and regulations. All methods are reported in accordance with the ARRIVE guidelines.
Breeding season and location of the animals
AI data was collected in Australia between November and April during 2020-21 (N = 9 817 ewes, 123 sires, 10 sites), 2021-22 (N = 8 253 ewes, 116 rams, 9 sites) and 2022-23 (N = 12 184 ewes, 149 rams, 11 sites). Sites were located in the Central and South Wheat Belt of Western Australia (Mediterranean climate: hot, dry summers and mild, wet winters), Central North of Victoria (Temperate climate: cool to mild winters and warm to hot summers), Murray Land Yorke Peninsula of South Australia (Mediterranean climate: dry, hot summers and mild, rainy winters) and the Central West (Temperate climate: warm summers and cool winters), Tablelands (Oceanic climate: cooler with higher rainfall) and Northwest Slopes and plains (Subtropical climate: hot summers and mild winters) of NSW. Animals were selected and managed for artificial breeding programs as per individual commercial stud preferences.
Experimental design
Merino ewes (N = 30 254, split across three breeding seasons and 30 commercial AI programs conducted on farms located in NSW, VIC, SA and WA) were synchronised for oestrus and assessed for uterine tone, intra-abdominal fat, age, PMSG dose and time of insemination post-CIDR removal as part of routine AI protocols, as per the previous study35. Following industry standards, ejaculates from Merino sires (N = 388) were collected and immediately laparoscopically inseminated (fresh; N = 29 ejaculates) or frozen (N = 359) as either pellets (N = 239) or straws (N = 120), thawed and then laparoscopically inseminated into ewes. Parameters including season, day, site, sire and type of semen used, uterine tone, and intra-abdominal fat were recorded during AI. Synchronised ewes (0.3 g progesterone CIDR; Zoetis, Australia and eCG; Minitube, VIC, Australia) received approximately 0.2mL of semen per uterine horn. Each ewe underwent pregnancy scanning approximately 55 days post AI using standard industry practice. Approximately 2 pellets or 5 straws per batch per sire used for insemination were sent to The University of Sydney, for advanced in vitro semen assessment.
Assessment of ewe factors
Assessment of ewe age
The colour of the ear tag located in the left ear of each ewe indicated the year of birth. Each colour represents a year of drop (Supplementary File 1). This was then subtracted from the current year (2020, 2021, 2022, or 2023) of AI to determine the age of each ewe at AI.
Assessment of intra-abdominal fat score
During laparoscopic AI, the internal fat covering the abdominal organs was visualised and subjectively assessed by the technicians performing the insemination, scoring the ewe between one (little to no fat present) to five (high abundance of fat present) (Supplementary File 2) as per the pervious study35.
Assessment of uterine tone score
At the same time as the intra-abdominal fat assessment, the tone of the uterus was scored as a subjective observation by the technicians and recorded as a value between one (pale, flaccid uterus) to five (bright pink turgid uterus) (Supplementary File 3) as per the previous study35.
Assessment of AI time post-CIDR removal
As per the previous study35, during oestrous synchronisation, each ewe was assigned a CIDR pull group to ensure ewes were inseminated within the optimal time frame. At the time of insemination in the cradle, the eID tag of each ewe was scanned using a Tru-Test XRS2 (Tru-Test Datamars, Australia) giving a time stamp for data collection in the cradle. To determine the time of AI post-CIDR removal, this was subtracted from the end time of the CIDR pull group each ewe was allocated. This was standardised across each CIDR pull group across all programs. Data was presented as hh: mm: ss post CIDR pull.
Advanced in vitro Assessment of Semen characteristics post-thaw
A subset of the semen used from each sire was stored and thawed within the same breeding season and the AI program. Pellets (n = 2) were thawed in a glass thawing tube for 2 min in a 37ºC water bath with agitation, while straws (n = 4 straws) were thawed for 30 s in a 37⁰C water bath with agitation. The total volume per sample was recorded by suspending the sample in a pipette prior to being diluted 1:0.5 with PBS + 0.3% BSA (Phosphate Buffed Saline + 0.3% Bovine Serum Albumin; pH 7.4, osmolarity 297). This was then held at 37ºC over a 6 h incubation period. Following an initial assessment of sperm concentration (described below; 3.5.1), an aliquot of each sample was taken at 0, 3 and 6 h post-thaw and further diluted to 50 × 106 sperm/mL with PBS + 0.3% BSA.
Assessment of sperm concentration, subjective motility, and percentage of abnormal morphology post-thaw
The concentration of each frozen sample was determined using a NucleoCounter SP-100 (ChemoMetec) immediately post-thaw. 50 µl of semen was diluted with S100 reagent (ChemoMetec) and analysed according to the manufacturer’s instructions. Concentration recordings were taken twice (within 10%) and the average was used for further calculations.
Subjective motility was first assessed after the initial 1:0.5 dilution with PBS + 0.3% BSA as well as following dilution of the sample to 50 × 106 sperm/mL at each time point. The percentage of motile spermatozoa was subjectively assessed using a phase-contrast microscope (x100) described by Evans and Maxwell (1987). Samples (6 µL) were placed on slides and enclosed using a 22 × 22 mm coverslip warmed to 37⁰C. Values were obtained to the nearest 5% by examining five fields of each sample (kept on a heated slide and coverslip at 37⁰C).
After thawing, 10uL of semen was fixed with 190uL (1 + 10 dilution) in 3% NaCl. Within a 24 h timeframe, the percentage of abnormal spermatozoa was subjectively assessed using a phase-contrast microscope (x400). Capturing a minimum of 200 cells, the results were converted into the percentage of the sample containing spermatozoa with abnormal morphology. Morphological defects include head defects (detached heads, acrosomes reacted, amorphic heads), damaged midpieces (proximal droplets, bent midpieces) and tail abnormalities (distal reflex, coiled tails, broken tails). As such, this was a measure of the proportion of abnormal spermatozoa.
Assessment of sperm motility and kinetic analyses using a computer assisted sperm analysis (CASA)
Sperm motility was measured using the computer-assisted sperm analysis (HT CASA IVOS II (Animal Breeder) Version 1.13.7; Hamilton-Thorne, USA) using the appropriate settings for ram spermatozoa (this includes amongst others; head size 10–42 µm2, progressive motility thresholds of straightness 80% and average path velocity 75 μm/s). Samples were further diluted to a concentration of 25 × 106 sperm/mL with PBS + 0.3% BSA before 6 µL was placed on slides warmed to 37⁰C (Cell Vu; Millennium Sciences, Mulgrave, Victoria, Australia) and enclosed with a 22 × 22 mm coverslip. For each sample, eight fields of video recordings were recorded, capturing a minimum of 200 cells (frame rate 60 Hz). Motility and kinematic parameters were subsequently calculated including total and progressive motility, ALH, BCF, LIN, STR, VAP, VCL, VSL, and WOB.
Flow cytometric analysis
Samples were assessed for a range of membrane and metabolic indicators at a final concentration of 10 × 106 sperm/mL following staining with various fluorochromes. Using the CytoFLEX (CytoFLEX and CytExport 2.0 Software Beckman Coulter; USA), three lasers were employed; 50 mW 488 nm, 50 nW 638 nm and 80 mW 405 nm. All samples were stained with the DNA probe Hoechst 33,342 (final concentration of 1 µg/mL), which has a fluorescence detection filter of 450/45 BP, to gate any possible debris in the sample. Sperm cells were isolated from total events based on 488 nm forward and side scatter profiles. For each of the below variables, 10,000 sperm cells were analysed, and a minimum of 1000 Hoechst 33,342 positive events were required to obtain valid results.
Assessment of sperm acrosome integrity and viability
Sample preparation for sperm viability and acrosome integrity was performed as previously described59, by staining a combination of Propidium Iodine (PI, final concentration 6µM) and Fluorescein isothiocyanate peanut agglutinin (FITC-PNA, final concentration 0.4 µg/mL) for 10 min at 37⁰C. PI and FITC-PNA fluorescence detection was on 690/50, and 525/40 nm bandpass (BP) filters, respectively. Cells were considered viable with intact acrosomes if cells were both PI and FITC-PNA negative.
Assessment of sperm membrane lipid fluidity
Changes in lipid fluidity within the membrane of viable spermatozoa were assessed using a staining combination of both merocyanine 540 (M540, final concentration 0.83 µM) and Yo-Pro (final concentration 25nM) for 10 min at 37⁰C. The fluorescence of M540 and Yo-Pro was detected on a band-pass filter of 585/42 nm and 525/40 nm, respectively. A sperm population was considered viable if it was recorded as Yo-Pro negative. The median value for M540 fluorescence of this viable population was used to determine the relative membrane lipid fluidity. Results with a greater mean value corresponded to greater lipid destruction on the membrane, thus, greater membrane fluidity.
Assessment of mitochondrial superoxide production
Mitochondrial superoxide production was assessed using a dual stain combination of Mitosox Red (final concentration 2.5 µM) and Sytox green (final concentration 30 Nm) for 20 min at 37⁰C. Mitosox Red fluorescence was detected at 585/42 and Sytox Green fluorescence on 525/40 BP filters59. A sperm population of Sytox Green negative, “live”, was used to determine the median Mitsox Red fluorescence value relative to the amount of mitochondrial superoxide production. A positive control was created by combining each sample with 5 µM hydrogen peroxide to stimulate mitochondrial superoxide production. The positive control was used to assess the effectiveness of the stain and determine the appropriate gating of stained populations.
Assessment of lipid peroxidation
Lipid peroxidation of the sperm membrane was assessed using Bodipy C11 (581/591). Samples were aliquoted and stained with the Bodipy C11 probe (final concentration 10 µM) at 37⁰C for the entirety of the 6 h assessment. At each time point, the samples had a timed incubation for 30 min. Once staining was complete, samples were centrifuged for 10 min at 800 g. After removing the supernatant, each pellet was resuspended in a PBS + 0.3% BSA buffer and counterstained with PI (final concentration 6 µM) for 10 min at 37⁰C before running on the CytoFlex. The detection of lipid peroxidation was measured by both 585/42 and 525/40 bandpass filters. The live population was first gated and used to determine the percentage of cells with positive Bodipy C11 fluorescence. This indicated the relative change in lipid peroxidation of a given sample. A positive control was made up by combining aliquots of all samples and incubated with 5 µM hydrogen peroxide to induce greater lipid peroxidation. The positive control was used to assess the effectiveness of the stain and determine the appropriate gating of stained populations.
Assessment of intracellular reactive oxygen species (ROS)
To determine the relative amount of oxygen species (ROS) within a cell, a staining combination of dichlorodihydrofluorescein diacetate acetyl ester (H2DCFDA final concentration 5 µM) and PI (final concentration 6 µM) was used. Samples were aliquoted and stained with the H2DCFDA at 37⁰C for the entirety of the 6 h assessment. At each time point, the samples had a timed incubation of 1 h before being centrifuged for 10 min at 800 g. After removing the supernatant, pellets are resuspended in a PBS + 0.3% BSA buffer and counterstained with PI (final concentration 6 µM) for 10 min at 37⁰C before running on the CytoFlex. The H2DCFDA fluorescence was determined on the 525/40 bandpass filter. The H2DCFDA fluorescences in live cells (PI negative) was used to measure intracellular ROS production. A positive control was made up by combining aliquots of all samples and incubated with 5 µM hydrogen peroxide to induce greater ROS. The positive control was used to assess the effectiveness of the stain and determine the appropriate gating of stained populations.
Assessment of DNA integrity
At 0 and 6 h post thaw, 40 µL of each sample (50 × 106 sperm/mL) was aliquoted for DNA assessment. Each sample was washed by resuspending the sample in 2mL of PBS + 0.3% BSA and centrifuging for 10 min at 800 g. The supernatant was removed before resuspension and repeat centrifugation After the final spin, the supernatant was removed, and pellets (final concentration approx 2 × 106 sperm) were snap-frozen in liquid nitrogen for 30 s before being stored at -80⁰C until assessment.
DNA fragmentation was measured using flow cytometry on a (Cytek Aroura 3 L; Sydney Flow Cytometry) after staining with Acridine Orange (AO), as described by Evenson and Jost (2000), with some minor changes. In summary,, snap frozensamples were diluted to a concentration of 2 × 106 sperm/mL with a TNE buffer (0.15 M NaCl, 0.01 M Tris HCl, 1mM disodium EDTA pH 7.4). A 100uL aliquot of the sample was taken and diluted with 200uL of Acid Detergent Solution (0.08 NHCl, 0.15 M NaCl, 0.1% Triton X 100 pH 1.2), which was gently mixed by swirling in hand for 30 s. Once mixed, samples were stained with 600uL of Acridine Orange (final concentration 6 µg/mL) for 3 min before being assessed using flow cytometry. Green (B2) and Red (V11) fluorescence were detected using the 528/21 and 644/27 band pass filters, respectively. The flow rate was set to slow, and a minimum of 1000 cells were recorded per sample. DNA fragmentation was determined by the relative amount of single-stranded DNA (ssDNA) in proportion to the total amount of spermatozoa (dsDNA + ssDNA) and indicated by the amount of red fluorescence regarding the total amount of fluorescence.
Measure of Fertility determined by Ultrasound
Depending on the AI program and site, the pregnancy status per ewe was determined approximately 55 days post-insemination. Ewes were fasted 24 h prior to scanning. A real-time cutaneous ultrasound (Oviscan 6 with a 3.5 MHz probe) was used to scan each ewe to determine the presence of fetuses and their number. As per the previous study35, pregnancy from AI was recorded as either 1 (pregnant) or 0 (empty), while the number of fetuses observed was recorded as the exact number.
Statistical analysis
Ewe ID was matched between AI and pregnancy datasets while sire ID was matched between AI and in vitro semen analysis datasets to create one Masterfile. All data was therefore compared within the individual ewe. Data was then cleaned to remove ewes without pregnancy data. All statistical analyses were performed on R Studio (Version 2023.09.1 + 494).
In accordance with the previous study35, the overall pregnancy data was assessed to determine average pregnancy and reproductive rates. AI success was determined by calculating the number of ewes pregnant over the total number of ewes inseminated. The reproductive rate was determined by calculating the number of offspring (fetal number) over the total number of ewes inseminated. Descriptive statistics were performed to evaluate the number of ewes inseminated and the percentage pregnant for each categorical factor level recorded, as well as site, sire ID and breeding season. All values included mean ± standard error of the mean (SEM) and were de-identified for anonymity.
At 0, 3 and 6 h post-thaw, the relationships between in vitro semen traits were assessed by Spearman rank correlation. As the CASA measurements were highly correlated, measures of total motility, progressive motility, ALH, BCF, LIN, STR, VAP, VCL, VSL, and WOB were combined using a Principal Component Analysis (PCA, Table 1) to reduce multiple testing bias and collinearity. The first 3 principal components were significant (eigenvalues > 1), and together accounted for 92% of the variation in the data (Table 1). PCA1, accounted for 54.92% of the variation and was interpreted as a composite measure of sperm velocity, with positive loadings (< ±0.30) from WOB, VSL, VAP, STR, LIN and BCF (Table 1). PCA2, accounting for 24.73% of the variation, had a strong positive loading of VCL, VAP and ALH (Table 1), and again is interpreted as a measure of sperm velocity. PCA3 contributed 12.90% of the variation and had a strong negative loading from Total Motility and Progressive Motility (-0.70 and − 0.53, respectively), interpreted as a measure of sperm motility. The significant components were subsequently used in the regression analyses.
Table 1.
Eigenvalues and variances explained by the first 3 PCAs at 0 h post-thaw, along with the loading of each measurement within the PCA for CASA motility and velocity traits.
| PC1 | PC2 | PC3 | |
|---|---|---|---|
| Eigenvalue | 5.49 | 2.47 | 1.29 |
| Variance (%) | 54.92 | 24.73 | 12.90 |
| Total Motility | 0.21 | 0.21 | − 0.70 |
| Progressive Motility | 0.29 | 0.23 | − 0.53 |
| ALH | − 0.29 | 0.45 | 0.01 |
| BCF | 0.32 | 0.05 | 0.05 |
| LIN | 0.39 | − 0.21 | 0.09 |
| STR | 0.38 | − 0.19 | 0.11 |
| VAP | 0.32 | 0.37 | 0.26 |
| VCL | 0.03 | 0.60 | 0.26 |
| VSL | 0.36 | 0.28 | 0.26 |
| WOB | 0.39 | − 0.20 | 0.08 |
ALH, amplitude of lateral head displacement; BCF, beat-cross frequency; LIN, linearity; STR, straightness; VAP, average path velocity; VCL, curvilinear velocity; VSL, straight-line velocity; WOB, wobble; PCA, principal component.
A logistical binomial regression analysis was used to examine the influence of female and male fertility traits on the probability of pregnancy post-AI. Additionally, an intraclass correlation coefficient (ICC) analysis was conducted to explain the proportion of variance attributed to individual factors within the random model, encompassing Site, Sire ID including those that were Frozen-thawed, and Thaw Day. A preliminary univariate analysis was then run to determine the impact of each individual factor on pregnancy achievement. The logistical binominal regression model was refined by the backward selection process, eliminating non-significant fixed effect variables and interactions (P > 0.05). The final multivariable model included only significant factors and interactions (P < 0.05). For all variables within the model, an odds ratio was performed to determine the likelihood of change in pregnancy following a single unit change in the factor whilst keeping the model consistent. This included the odds ratio percentage change and 95% CL.
For any fixed categorical effects, an Emmeans pairwise comparison was performed to assess the significant difference between groups within the variable. Groups were considered significantly different to each other if the comparison returned a p-value of < 0.05. All values are reported with mean ±SEM.
Results
Overall descriptive statistics of the dataset
Data was collected from 30 254 ewes and 388 rams from 30 sites. Table 2 shows the total number of ewes and sires included in the dataset and the resultant fertility following laparoscopic AI across the 3 breeding seasons.
Table 2.
Overall pregnancy data across all 3 breeding seasons. Calculated as the proportion of ewes pregnant compared to the total number of ewes inseminated per breeding seasons.
| Breeding Season | Number of sites | Total ewes in program | Total sires used in the program | Ewes pregnant to AI (%) | Total Reproductive Rate (%) |
|---|---|---|---|---|---|
| 2020-21 | 10 | 9817 | 123 | 68.07 | 105.35 |
| 2021-22 | 9 | 9253 | 116 | 69.78 | 119.27 |
| 2022-23 | 11 | 12 184 | 149 | 63.05 | 90.03 |
| Total | 30 | 30 254 | 388 | 66.51 | 102.97 |
Table 3 displays the mean ± SEM and range for each fertility factor recorded. For each in vitro semen parameter, it tracks the change of each trait 0, 3, and 6 h post-thaw. Additionally, Supplementary File 4 describes the percentage of ewes’ pregnancy for each level within the categorical factors recorded in the data set.
Table 3.
Descriptive statistics of factors recorded at AI, as well as in vitro semen parameters recorded 0, 3 and 6 h post-thaw, including mean (± SEM) and range for each factor.
| Factor | Variables | 0 h | 3 h | 6 h | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (± SEM) | Min | Max | Mean (± SEM) | Min | Max | Mean (± SEM) | Min | Max | ||
| Initial Assessment | Volume (µL) | 509.14 ± 0.80 | 160 | 800 | ||||||
| Motility (%) | 57.37 ± 0.08 | 20 | 85 | |||||||
| Concentration (×106) | 569.75 ± 2.06 | 81.29 | 2283.75 | |||||||
| Morphology (%) | 15.46 ± 0.06 | 2.5 | 70 | |||||||
| Package | ||||||||||
| Subjective Motility | 50 × 106 sperm/mL (%) | 48.99 ± 0.10 | 10 | 80 | 40.03 ± 0.10 | 0 | 70 | 33.47 ± 0.10 | 0.1 | 78.4 |
| 25 × 106 sperm/mL (%) | 41.43 ± 0.12 | 0 | 85 | 32.17 ± 0.11 | 0 | 75 | 26.36 ± 0.12 | 0 | 67.7 | |
| CASA | Total motility (%) | 40.77 ± 0.12 | 5.8 | 89.5 | 32.75 ± 0.11 | 1.8 | 87.1 | 29.35 ± 0.12 | 0 | 75 |
| Progressive motility (%) | 30.21 ± 0.10 | 2.3 | 79.8 | 22.11 ± 0.10 | 0.3 | 73.3 | 20.26 ± 0.10 | 0 | 70 | |
| ALH (µm) | 6.01 ± 0.01 | 3.29 | 9.58 | 8.41 ± 0.07 | 0.40 | 73.19 | 6.79 ± 0.04 | 0.50 | 43.75 | |
| BCF (Hz) | 38.78 ± 0.03 | 20.97 | 45.67 | 39.62 ± 0.07 | 5.81 | 95.51 | 38.92 ± 0.05 | 6.24 | 74.28 | |
| LIN (%) | 63.23 ± 0.06 | 34.58 | 87.14 | 60.46 ± 0.08 | 30.49 | 150.09 | 63.26 ± 0.07 | 19.03 | 94.47 | |
| STR (%) | 90.23 ± 0.03 | 70.47 | 97.79 | 89.52 ± 0.10 | 36.97 | 209.53 | 88.99 ± 0.06 | 39.60 | 125.64 | |
| VAP (µm/s) | 117.08 ± 0.13 | 64.74 | 188.15 | 114.11 ± 0.12 | 58.56 | 205.17 | 107.11 ± 0.20 | 8.82 | 192.69 | |
| VCL (µm/s) | 179.00 ± 0.16 | 114.55 | 262.26 | 180.74 ± 0.24 | 14.42 | 268.76 | 161.05 ± 0.31 | 11.05 | 271.42 | |
| VSL (µm/s) | 106.68 ± 0.14 | 44.87 | 180.25 | 100.75 ± 0.17 | 0.8 | 205.29 | 96.75 ± 0.21 | 8.47 | 254.15 | |
| WOB (%) | 68.59 ± 0.05 | 47.94 | 89.89 | 66.50 ± 0.06 | 0.3 | 112.60 | 67.68 ± 0.08 | 3.4 | 116.16 | |
| Flow Cytometry | Acrosome Integrity and Viability (%) | 20.35 ± 0.06 | 0.20 | 60.00 | 15.58 ± 0.05 | 0.20 | 50.1 | 11.74 ± 0.04 | 0 | 34.64 |
| Membrane Fluidity | 56685.55 ± 380.32 | 8035.60 | 186186.70 | 58271.32 ± 382.78 | 4647.7 | 246915.6 | 60655.29 ± 389.77 | 10338.1 | 192183.6 | |
| Mitochondrial Superoxide | 4616.44 ± 23.24 | 1450.30 | 19,392 | 6168.49 ± 46.86 | 1351.2 | 55,111 | 9223.27 ± 80.17 | 1671.1 | 71570.9 | |
| Lipid Peroxidation | 398.23 ± 15.43 | − 4049.3 | 8905.5 | 3494.14 ± 55.25 | − 3525.8 | 55870.6 | 3733.28 ± 39.93 | − 5114.1 | 31011.3 | |
| Intracellular Reactive Oxygen Species | 983.23 ± 2.07 | 101.8 | 2234.1 | 1149.75 ± 2.87 | 123.9 | 3956.8 | 1289.26 ± 3.35 | 477.3 | 4496.3 | |
| DNA Integrity (%) | 3.16 ± 0.02 | 0.53 | 18.16 | 6.56 ± 0.05 | 0.29 | 51.69 | ||||
| Factors collected at AI | Tone | 3.22 ± 0.005 | 1 | 5 | ||||||
| Fat | 3.06 ± 0.005 | 1 | 5 | |||||||
| Age | 3.18 ± 0.009 | 1 | 13 | |||||||
| Time of AI Post-CIDR Pull | 51:48:46 ± 0:00:42 | 43:49:26 | 59:30:45 | |||||||
ALH, amplitude of lateral head displacement; BCF, beat-cross frequency; LIN, linearity; STR, straightness; VAP, average path velocity; VCL, curvilinear velocity; VSL, straight-line velocity; WOB, wobble; PCA, principal component.
Contribution of Variation caused by Random terms
The ICC analysis was performed on the random model to assess the proportion of total variance contributed by random terms. The proportion of variation in pregnancy success attributed to Site, Sire frozen-thawed, and Thaw Day was 53.57%, 22.02%, and 24.42%, respectively. The level of significance was not assessed on these variations.
The proportion of variation in pregnancy success contributed by site
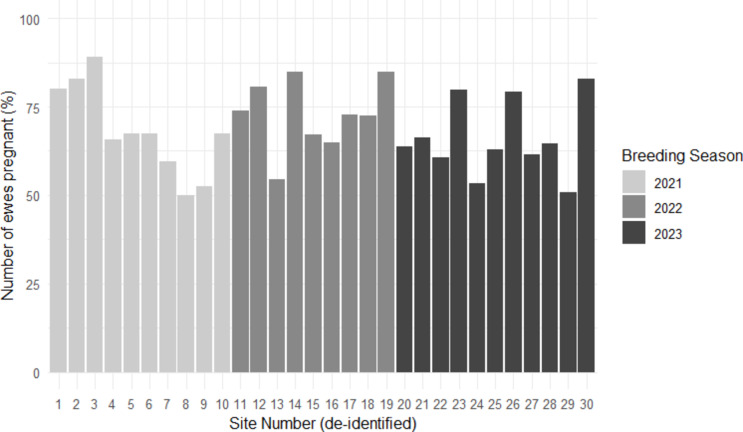
From the multivariable model, the site where data was collected contributed 53.57% of the variation detected between the random terms. With 30 sites across the 3 breeding seasons, the pregnancy rate ranged from 49.89 to 89.02% (Fig. 1).
Fig. 1.
Variation in pregnancy rates for each site (deidentified, N = 30) recorded during the 2021, 2022 and 2023 breeding seasons. Calculated as the proportion of ewes pregnant compared to the total number of ewes inseminated per site.
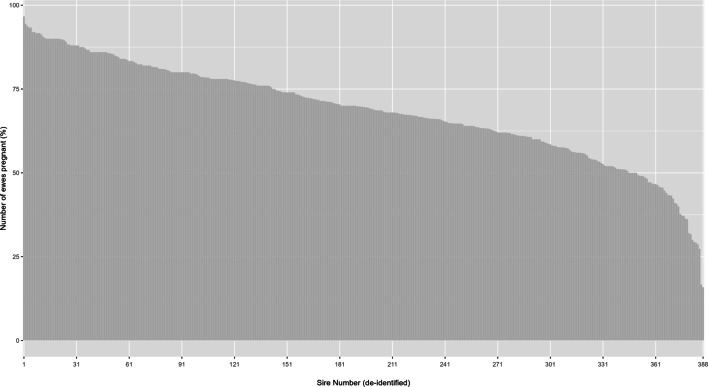
The proportion of variation in pregnancy success contributed by sire
From the multivariable model, 24.42% of the variation detected between the random terms was contributed by the sires with frozen-thawed semen used for AI. Across the 388 sires, the pregnancy rate ranged from 15.88 to 96.67%, averaging 68.54% (Fig. 2).
Fig. 2.
Pregnancy rate for each sire (deidentified) throughout the three breeding seasons, n = 388. Calculated as the proportion of ewes pregnant compared to the total number of ewes inseminated per sire. The number of ewes inseminated per sire ranged from 7 to 361, with an average of 69.74 ± 2.15 ewes.
Factors within the model found to influence pregnancy following laparoscopic AI of sheep
Despite 33 factors returning significant p values when considered in a univariate logistic model (data not shown), only 7 remained significant when included in the same binomial logistic regression model. Sperm freezing concentration (×106 sperm/mL), the percent of morphologically abnormal spermatozoa, the proportion of viable spermatozoa with intact acrosomes at 6 h post-thaw, CASA at 0 h post-thaw, uterine tone and intra-abdominal Fat of ewes, were found to significantly (P < 0.001, P < 0.001, P = 0.021, P = 0.033, P < 0.001, P = 0.047, respectively), to influence the likelihood of pregnancy. There were no significant interactions between variables (P > 0.05).
The impact of the number of sperm frozen on the probability of successful pregnancy
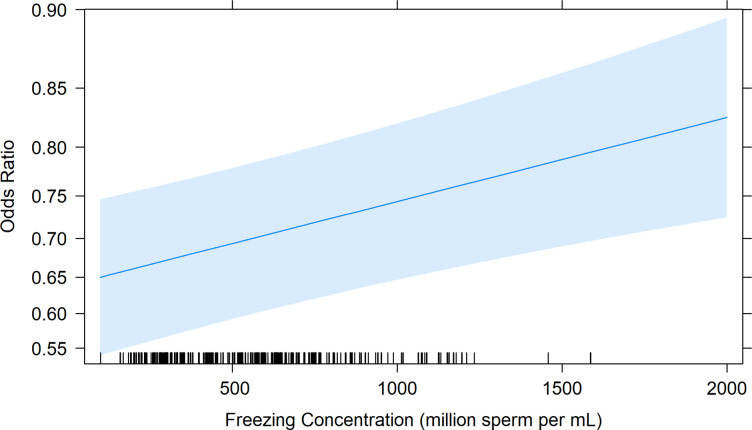
The average freezing concentration for a pellet and straw was 722 × 106 ± 15.27 sperm/mL, and 270.75 × 106 ± 16.47 sperm/mL, respectively. Freezing concentration ranged from 81.29 to 2283.75 × 106 sperm/mL and averaged 569.75 ± 2.06 sperm/mL. Notably, there was no significant interaction observed between freezing concentration and package type within the model (p > 0.05). Therefore, freezing concentration was considered across package types. Following an odds ratio calculation, it was determined that an additional 100 × 106 sperm/mL frozen in either a pellet or straw corresponded to a 5.09% increase in pregnancy probability (OR = 1.05, 95% CI: 1.05 to 1.05, Fig. 3).
Fig. 3.
The Odds Ratio plot shows the relationship between an increase in freezing concentration per pellet or straw on the predicted probability of a ewe falling pregnant if she was laparoscopically inseminated with that sample. The predicted probability was generated from the model in RStudio, with 95% Confidence Interval (shaded blue area). Black markers along the x-axis indicate the spread of raw data per individual sire within the model. The blue line indicates the odds ratio of the sample at the given freezing concentration.
The impact of abnormal sperm morphology on the probability of successful pregnancy following laparoscopic AI of sheep
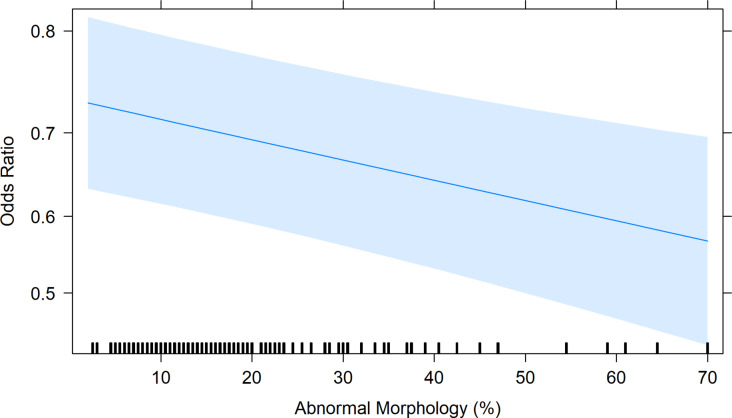
The average number of morphologically abnormal spermatozoa per frozen-thawed sires sample was 15.46 ± 0.06%, ranging from 2.5 to 70% abnormal spermatozoa. The odds of a 1% increase in abnormal spermatozoa corresponded to a 1.07% decrease (OR = 0.99, 95% CL: 0.98 to 1.00, Fig. 4) in the probability of a ewe being pregnant.
Fig. 4.
The Odds Ratio plot shows the relationship between an increase in the number of abnormal spermatozoa on the predicted probability of an ewe falling pregnant if she was laparoscopically inseminated with that sample. The predicted probability was generated from the model in RStudio, with 95% Confidence Interval (shaded blue area). Black markers along the x-axis indicate the spread of raw data per individual sire within the model. The blue line indicates the odds ratio of the sample at the given abnormal morphology percent.
The impact of sperm viability and acrosome integrity on the probability of successful pregnancy following laparoscopic AI of sheep
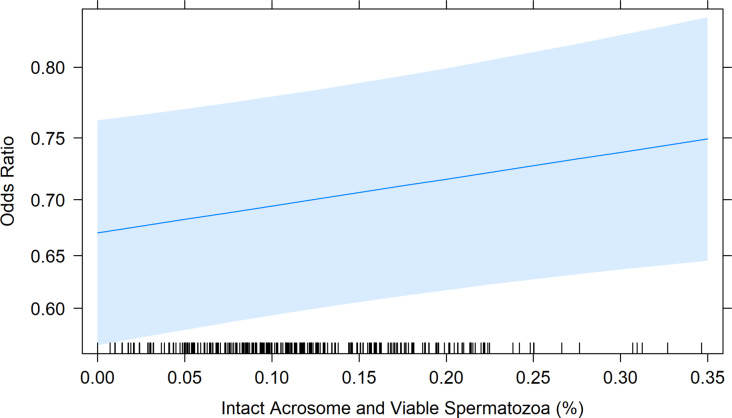
At 6 h post-thaw, the average percentage of acrosome intact and viable spermatozoa for frozen-thawed sires was 11.74% ± 0.04%, ranging from 0 to 34.64%. An analysis of the data revealed that the odds of a 1% increase in the number of viable sperm with intact acrosomes corresponded to a 1.01% increase (OR = 1.01, 95% CI: 0.34 to 2.56) in the probability of ewes being pregnancy (Fig. 5).
Fig. 5.
The Odds Ratio plot showing the effect of the percentage of Acrosome Intact and Viable spermatozoa on the predicted probability of an ewe falling pregnant if she was laparoscopically inseminated with that sample. The predicted probability was generated from the model in RStudio, with 95% Confidence Interval (shaded blue area). Black markers along the x-axis indicate the spread of raw data per individual sire within the model. The blue line indicates the odds ratio of the sample at the given percentage of viable spermatozoa with intact acrosomes.
The impact of CASA motility and velocity traits (CASA PCA3) on the probability of successful pregnancy following laparoscopic AI of sheep
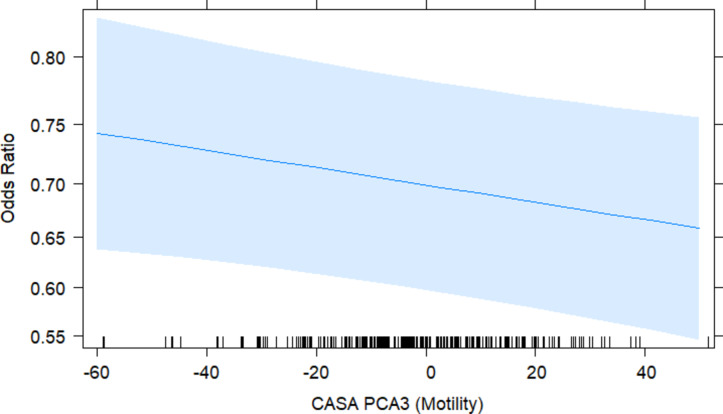
As seen in Supplementary File 5, there is a clear and strong correlation between CASA traits at 0, 3 and 6 h post-thaw. Following the formation of the PCA variable (Table 1), although PCA1 and PCA2 explained a significant amount of variation, only PCA3 remained significant in the final model (P = 0.033). PCA3 had a strong negative loading from Total Motility and Progressive Motility (-0.70 and − 0.53, respectively), which is interpreted as a measure of sperm motility.
At 0 h post-thaw, the average total motility for frozen-thawed sires was 40.77% ± 0.12%, ranging from 5.8 to 89.5%. The average CASA progressive motility for frozen-thawed sires was 30.21 ± 0.10%, ranging from 2.3 to 79.8%. The odds ratio analysis of CASA PCA3 indicated an inverse association with pregnancy outcomes, with the odds of pregnancy decreasing by 0.37% for every standard deviation away from the average for motility (OR = 1.00, 95% Cl: 1.00 to 0.99, Fig. 6). This infers that reduced sperm motility characteristics, as represented by higher CASA PCA3 scores, are associated with reduced odds of pregnancy.
Fig. 6.
The Odds Ratio plot shows the relationship between the CASA PC3 at 0 h post-thaw on the predicted probability of an ewe falling pregnant if she was laparoscopically inseminated with that sample. The PC3 is centred around the mean value of 0, and each individual point is represented by its deviation (distance) from this mean, measured in standard deviations. The predicted probability was generated from the model in RStudio, with 95% Confidence Interval (shaded blue area). Black markers along the x-axis indicate the spread of each PC3 point calculated by the PCA. The blue line indicates the odds ratio of the sample at the given abnormal morphology percent.
The impact of uterine tone on the probability of successful pregnancy
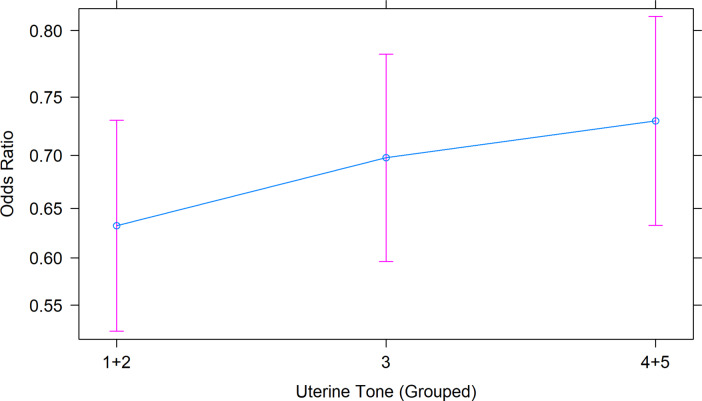
Within the model, an increase in uterine tone had a positive effect on the probability of pregnancy rate. The average uterine tone score was 3.22 ± 0.0005, ranging from 1 to 5. As seen in Supplementary File 4, a uterine tone score of 1 + 2 (57.88%, N = 2780 ewes) scored a significantly lower AI success rate than a uterine tone score of 3 (65.20%, N = 12387 ewes) and 4 + 5 (70.29%, N = 7086 ewes) (P < 0.0001, < 0.001, respectively). Additionally, ewes with a uterine tone score of 3 recorded significantly lower probability of pregnancy than ewes which scored a uterine tone score of 4 + 5 (P = 0.0091).
The odds ratio of achieving a successful pregnancy was 5.55% higher for ewes that scored a uterine tone score of “3” compared to a uterine tone score of “1 + 2” (OR = 1.06, 95% Cl: 0.59 to 1.88, Fig. 7). Similarly, the odds of AI pregnancy were 7.21% higher for the category “4 + 5” compared to “1 + 2” (OR = 1.07, 95% Cl: 0.44 to 2.60, Fig. 7).
Fig. 7.
The Odds Ratio plot shows the relationship between the uterine tone groups on the predicted probability of an ewe falling pregnant if she was laparoscopically inseminated. The predicted probability was generated from the model in RStudio, with 95% Confidence Interval (pink error bars). The blue dots indicate the odds ratio of the ewe with the given uterine tone score.
The impact of intra-abdominal fat on the probability of successful pregnancy following laparoscopic AI of sheep
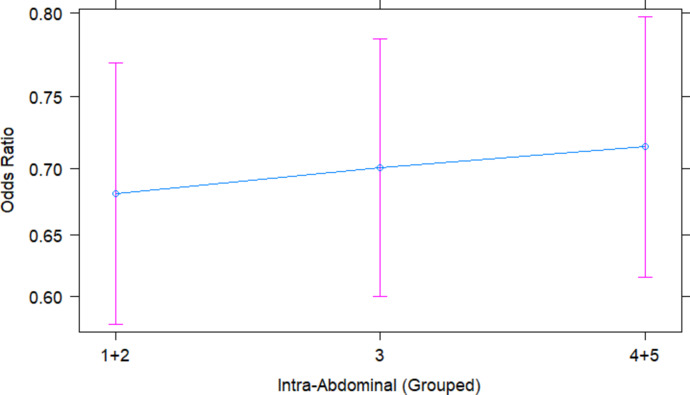
The model also calculated that an increase in intra-abdominal fat had a positive effect on the probability of pregnancy rate. The average intra-abdominal fat score was 3.06 ± 0.005, ranging from 1 to 5. As seen in Supplementary File 4, an intra-abdominal fat score of 1 + 2 (59.36%, N = 3895 ewes) scored a significantly lower AI success rate than an intra-abdominal fat score of 4 + 5 (71.94%, N = 4748 ewes, P = 0.038,). There was no significant difference in pregnancy rate between ewes which scored an intra-abdominal fat score of 3 (65.60%, N = 13385 ewes) and 1 + 2 or 3 to 4 + 5 (P > 0.05).
The odds ratio of achieving a successful pregnancy was 5.37% higher for ewes, with an intra-abdominal fat score of “3” compared to an intra-abdominal fat score of “1 + 2” (OR = 1.05, 95% Cl: 0.89 to 1.25, Fig. 8). Similarly, the odds of successful pregnancy were 6.81% higher for the intra-abdominal fat category “4 + 5” compared to “1 + 2” (OR = 1.07, 95% Cl: 0.78 to 1.47, Fig. 8).
Fig. 8.
The Odds Ratio plot shows the relationship between the intra-abdominal fat groups on the predicted probability of an ewe falling pregnant if she was laparoscopically inseminated. The predicted probability was generated from the model in RStudio, with 95% Confidence Interval (pink error bars). The blue dots indicate the odds ratio of the ewe with the given intra-abdominal fat score.
Discussion
This study investigated the impact of female factors recorded during AI and male in vitro semen traits assessed post-thaw on the probability of pregnancy occurring in sheep. This study has considered male and female fertility traits simultaneously, comparing the pregnancy success of the individual ewe rather than across flock averages. The resultant fertility model can now be used to explain the variation detected in pregnancy success following laparoscopic AI. An increase in freezing concentration (and thus sperm per insemination dose), percentage of viable, acrosome intact spermatozoa and motility kinematics will result in a positive linear increase in the probability of pregnancy occurring in a ewe. In addition, ewes with a uterine tone and intra-abdominal fat score of 4 or 5 are more likely to result in pregnancy than ewes with a score of 1 or 2. Finally, a decrease in the percentage of spermatozoa in a sample with morphological abnormalities will increase pregnancy probability. However, it is still important to consider the overarching variation contributed by the environment, site or location where AI occurred, as these factors will further modify the influence of the predictive factors mentioned above. The results of the above study demonstrate that the fertility of sheep following laparoscopic AI cannot be predicted off a single parameter and further shows the importance of considering multiple fertility factors with confounding effects in concert. While it is useful to critically analyse previous studies which have referred to the role or impact of these factors individually on sheep fertility (discussed below), the model will only successfully predict fertility if all are considered in unison. Nevertheless, the identification of predictive in vitro semen traits can now be used to pre-screen and select sires or frozen semen samples prior to use in a breeding program. This will help to improve the success rates of artificial breeding programs, offering producers an effective tool to increase genetic and production gains in a challenging industry.
The impact of the number of sperm frozen on the probability of successful pregnancy.
The concentration at which sperm is frozen has the potential to impact cryosurvival post-thaw as well as subsequent insemination dose. In the current study, the number of sperm frozen in either a pellet or straw was found to influence the likelihood of successful pregnancy following laparoscopic AI. For every additional 100 × 106 sperm/mL frozen, the probability of achieving pregnancy increased by 5.09% (Fig. 3). However, there was no interaction with package type. While there is currently no agreed-upon standard for the industry, it is generally assumed that a pellet should be frozen between 600–800 × 106 sperm/mL and used to inseminate approximately 3 ewes. A straw should be frozen at 200–300 × 106 sperm/mL and equate to 1 dose per ewe60. Pleasingly, in the current study, the average concentration of pellets and straws was 722 × 106 ± 15.27 and 270.75 × 106 ± 16.47 sperm/mL, respectively, suggesting the data collected accurately reflected current protocols used throughout the sheep artificial breeding industry in Australia.
The authors interpret the above result as the number of spermatozoa frozen as a proxy for the insemination dose. For laparoscopic AI, it is recommended that each ewe should receive approximately 25 × 106 motile sperm or 12.5 × 106 motile sperm/horn61. Assessing the sperm concentration post-thaw ensures a more accurate insemination dose. The recommended dose in sheep has remained largely unchanged since the mid-80s, when it first emerged as a reproductive tool for sheep62. Previous studies have demonstrated that an increase in motile sperm dose from 0.5 to 50 × 106 sperm resulted in lambing rates of 27–62%61. Which then later, a sperm dose of 20 × 106 sperm/mL achieved a rate of 76.8%32. In contrast, other studies have also found no difference in conception rates when doses were reduced from 52.2 × 106 to 13 × 106 sperm/mL31,63. The lack of significant difference in these studies may be related to the number of ewes used per treatment structure, which limits statistical power, or the compounding effects of sperm type (fresh, liquid stored, or frozen) or diluents used, ultimately making it difficult to compare results across studies.
It’s important to emphasise that while higher numbers of sperm frozen theoretically offer increased insemination doses, it’s important to find the balance between optimal freezing conditions to ensure sperm survival and effective insemination doses. This concept has been studied abundantly in previous literature7,64–66 across livestock species. Studies7,66 found that freezing at concentrations above 600 × 106 sperm/mL reduced sperm viability, acrosome integrity and motility. Attributed to the excessive build-up of free radicals, it’s proposed to cause changes in the sperm: cryoprotective agents67. The higher the amount of cryoprotectant per sperm cell, the higher the percentage of microdomains (unfrozen water channels), leading to better quality post-thaw65. Alternate studies66 reported lambing rates of 57.1% when sperm was frozen at 800 × 106 sperm/mL and inseminated at 160 × 106 spermatozoa. This was compared to 81.2% when sperm was frozen at 200 × 106 sperm/mL and inseminated at 40 × 106 spermatozoa. Standardising doses prior to insemination recorded similar results to 25 million sperm7. Sperm frozen at 200 and 400 × 106 sperm/ml recorded a higher lambing rate of 57.5% compared to sperm frozen at 800 × 106 sperm/mL, which returned a lambing rate of 45.5%.
In any event, the concentration at which sperm is frozen directly impacts insemination dose, which can then further alter pregnancy results. Semen must be frozen at an appropriate concentration to mitigate the impacts of freeze-thaw damage and optimise the number of sperm per ewe per insemination dose. With an increase in the accuracy and number of technologies currently available on the market that can objectively measure sperm concentration, it should be easier to ensure samples are frozen at accurate concentrations, regardless of package type. Further studies must now focus on establishing thresholds that could be used as standards in industry, increasing the chances of pregnancy success.
The impact of the percentage of abnormal spermatozoa on the probability of successful pregnancy.
The assessment of sperm morphology is common practice during routine basic semen assessment for a number of species, including stallions43,68, bulls69 and boars70, yet its correlation with the fertility of frozen-thawed ram spermatozoa has been contradictory11,13,71. In the current study, results reported that for every 1% increase in the percentage of abnormal spermatozoa within a frozen sample, a 1.07% decrease in the probability of a ewe falling pregnant would be observed (Fig. 4). In our study, sperm morphology was classified as either abnormal or normal with this approach aligning with the methods and results reported by previous ram studies52. In this study, a significant difference in the percentage of abnormal ram spermatozoa was reported between groups that exhibited high and low fertility (4.46 ± 0.30% compared to 13.46 ± 1.37%, respectively). Our current study builds upon these results by directly comparing the morphology of samples inseminated per ewe rather than the fertility average of a group of individuals.
As reviewed36, the morphology of an individual spermatozoon is an important indicator of its fertilising potential and has been proven in a number of species including; deer72, bulls69,73 and stallions43,68 and rams10,11. At least in the cattle industry, standards to measure bull sperm morphology are frequently used to assess and grade the quality of bull samples50. To date, nothing of this detail exists for the sheep industry. Thus, the results of the current study are a positive step forward for the industry, providing a comprehensive data set that accurately reflects a negative relationship between increasing morphology abnormalities and sheep fertility following AI. Even more so, the results provide evidence that even basic morphology assessment is a key parameter that should be considered when assessing the quality of ram samples during fertility assessment.
The influence of the percentage of viable, acrosome intact spermatozoa on the probability of successful pregnancy.
The current model revealed that for every 1% increase in the number of viable sperm with intact acrosomes at 6 h post-thaw, a 1.01% increase in the probability of pregnancy occurred (Fig. 5). A viable, acrosome intact spermatozoon is essential for the normal functioning of the cell, implying that sperm are capable of transitioning to the site of fertilisation, fusing with the zona pellucida, undergoing the acrosome reaction38,74 and achieving successful fertilisation38.
The relationship between sperm viability and fertility following insemination has been extensively proven across multiple species, recording a correlation ‘r’ score of r = 0.32, 0.64, 0.64, 0.05, 0.68 and 0.28 in bulls37,38,69, dairy bulls75, stallions76 and boars77, respectively. These studies agreed with the current study, that the greater the proportion of viable sperm and acrosomal integrity, the greater the probability of pregnancy. Of the literature above, only37 successfully measured the viability of bull sperm at 0 and 4 h post-thaw. However, as the current study measured viability at 6 h, this could more closely imitate the environment sperm are exposed to following deposition within the female tract. In general, sperm are inseminated just prior to a ewe ovulating; therefore, they are required to survive for up to 6–12 h before interacting with an oocyte. For laparoscopic AI, measuring the level of viability or live: dead with intact acrosomes at 6 h would ensure the population of sperm deposited in the uterus was capable of achieving fertilisation after incubation at 37ºC (artificially post-thaw in a water bath or in vivo within the reproductive tract).
Opposing this trend13, measured no importance or significance of ram sperm viability to pregnancy rates. In contrast to the current study, however, this study compared sperm viability to previously recorded fertility following cervical AI with a very low number of samples13.Nonetheless, these results have the potential to establish industry standards, which would be crucial for enhancing reproductive outcomes.
The impact of motility and velocity traits assessed using CASA (PCA3) on the probability of successful pregnancy.
The use of a principal component analysis (PCA) for CASA variables underscores the positive impact of sperm motility and kinetic traits on pregnancy likelihood while considering the extreme correlation between factors. Of all the PCAs considered within the current dataset, PCA3 exhibited the greatest influence on pregnancy, primarily driven by total and progressive motility (-0.70 and − 0.53, respectively). Analysis of the odds ratios revealed a 0.37% increase in the odds of pregnancy occurring for every negative standard deviation away from the average (Fig. 6). This indicates that as total and progressive motility values increase, the PCA3 loadings or value decreases. Thus, as PCA3 values decline, the likelihood of pregnancy occurring increases. In other words, the model makes biological sense given that a sample with high total and progressive motility is also likely to exhibit efficient metabolism of substrates and be more capable of achieving fertilisation78,79 so the probability of achieving pregnancy increases.
Extensive research in several species has focused on the relationship between motility as determined by CASA and fertility. Studies across livestock species, including bulls38,69,80, stallions43, deer72, rats81, humans82, salmon83 and rams4, have all shown similar results to the current study where, as total sperm motility and average path velocity increase, the likelihood of pregnancy also increases38,43,72,80. Previous research4 has demonstrated the relationship between motility assessed by CASA and fertility of cryopreserved ram sperm. They saw a direct relationship between an increase in the average-path velocity (VAP), the curvilinear velocity (VCL) and the head beat-cross frequency (BCF), correlated with the percentage of ewes pregnant following AI (R2 = 0.678, 0.745, 0.852, respectively). This is not always the case in all literature, and some studies13,84, have reported a lack of correlation between kinematic parameters and fertility following laparoscopic AI in sheep. The contradictory results in previous ram studies are unsurprising, given the different protocols and diluents used for assessment, making it difficult to compare across studies. Suggesting the need for standardisation across the industry.
The Impact of Uterine Tone on the Probability of Successful Pregnancy.
The present study revealed a clear linear relationship between the uterine tone of ewes at the time of AI and the resultant pregnancy. Pregnancy rates increased from groups 1 + 2 (58.13%), 3 (65.30%) and 4 + 5 (71.04%; p < 0.05, Fig. 7). Changes in uterine tone have been previously studied in mares85–87 and dairy cows88, and more recently in ewes35. The relationship between uterine tone, sperm quality and fertility is hypothesised to be related to the response of the ewe to oestrous synchronisation27, coinciding the deposition of semen artificially with subsequent ovulation in the ewe89. An increase in uterine tone corresponds to a surge in oestrogen levels, causing epithelial uterine gland cells and rough endoplasmic reticulum to expand90 increasing blood flow to the area35. This results in an increase in hypertrophy and contractions, aiding sperm transport from the cervix to the oviduct91,92. It also implies ovulation is imminent, signposting optimal insemination time and increased chances of fertilisation35 should sperm quality be appropriate. Further studies could track changes in the hormone profile of ewes corresponding to alterations of uterine tone and follicular development via laparoscope to pinpoint the exact time between a uterine tone score 4 or 5 and ovulation. Overlaid with the ability of different sperm types to survive incubation, this would enable us to accurately predict the optimum time of insemination in relation to uterine tone, increasing pregnancy success after AI. For now, the results above suggest that uterine tone could be a useful tool for screening ewes prior to insemination. Observing the tone of ewes subjectively (once standardised) and excluding those ewes with a uterine tone lower than 3.5 prior to AI could contribute to less variable pregnancy rates across the industry.
The Impact of Intra-abdominal Fat on the Probability of Successful Pregnancy.
Similar trends were also observed in the intra-abdominal fat level of ewes undergoing AI. The study revealed a clear linear relationship between intra-abdominal fat and AI success in sheep, showing an increase in pregnancy rate from groups 1 + 2 (59.77%), 3 (65.70%) and 4 + 5 (72.56%, Fig. 8). From these findings, the probability of a ewe with an intra-abdominal fat score of 4 or 5 was 6.81% higher than ewes’, which scored an intra-abdominal fat score of “1 + 2”. The inclusion of intra-abdominal fat in a fertility model is a novel parameter yet to be fully explored in livestock species. Previous research has focused heavily on the use of a ewes’ body condition score (BCS) as the gold standard for pre-breeding soundness or fertility assessments36 given it identifies a ewes’ nutritional status, health, and reproductive potential.
Studies in sheep have successfully linked BCS scores to fertility success93–95, yet limited studies have linked internal fat levels with fertility96. A small proportion of papers have demonstrated a significant correlation between BCS, internal fat deposits97 and ultrasound subcutaneous fat depth within the abdominal region of the ewe94. Studies have recommended maintaining a BCS 3–3.5 will help optimise reproductive performance (conception rate, little size, weaning rate and oestrus cycles to conception) and flock profitability98. The current study saw an increase in the likelihood of pregnancy when ewes had an intra-abdominal fat score of 4 or 5. This suggests that while BCS and internal fat score are likely correlated to some degree, the scale used to assess internal fat score means at the upper limits, intra-abdominal fat score should still be considered individually and likely acts as a more accurate predictor for pregnancy following AI. To maximise fertility potential, its recommended that ewes with an intra-abdominal score below 3 at the time of AI should be excluded from insemination, when matched with the correct sperm type of adequate quality.
Conclusion
This study revealed the link between in vitro semen parameters, ewe traits, and pregnancy following laparoscopic AI, increasing the potential of artificial breeding programs to reduce breeding inefficiencies and improve sheep reproductive potential. This model must now be validated with unseen data to establish thresholds for each variable, allowing the pre-screening of rams, their semen and ewes prior to an AI breeding program. The application of these results in the industry could result in a reduction in variable results, restoring industry confidence and increasing the adoption of artificial reproductive technologies within the sheep industry.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge the ongoing support, time and donation of data from Merino stud breeders and artificial breeding companies around Australia including Livestock Breeding Services (NSW), Central West Genetics (NSW), Westbreed (WA), Genstock Australia (NSW), Genstock (WA) and Brecon Breeders (SA). They also acknowledge Dr Michelle Humphries, Livestock Breeding Services for her assistance in obtaining imaging for the standardisation of uterine tone and intra-abdominal fat scores. Staff and students within the Animal Reproduction Group, The University of Sydney are also thanked for their dedication, time and assistance towards data collection and advanced semen assessment. Sydney Informatics Hub (SIH), University of Sydney are also acknowledged for their guidance and support on the statistical analysis of data contained within this project. The Australian Wool Innovation (AWI) invests in research, development, innovation and marketing activities along the global supply chain for Australian wool. AWI is grateful for its funding, which is primarily provided by Australian wool-growers through a wool levy and by the Australian Government which provides a matching contribution for eligible R&D activities.
Author contributions
This is the original work of the authors. E.A. Spanner: Conceptualisation, Data collection, Statistical analysis, Writing – original draft, Writing – review and editing. S.P. de Graaf: Conceptualisation, Writing – review and editing. J.P. Rickard: Supervision, Conceptualisation, Data collection, Project administration, Writing – review and editing.
Data availability
The dataset generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Financial Disclosure Statement
Dr J.P. Rickard and Miss E.A. Spanner were supported by funding from the McCaughey Memorial Institute. This work was supported by the Australian Wool Innovation [ON-00837] and NSW Merino Breeders’ Association Trust.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Geenty, K. G. et al. Reproductive performance in the Sheep CRC Information Nucleus using artificial insemination across different sheep-production environments in southern Australia. Anim. Reprod. Sci.54 (6), 715–726 (2014). [Google Scholar]
- 2.Eppleston, J. & Maxwell, W. M. C. Sources of variation in the reproductive performance of ewes inseminated with frozen-thawed ram semen by laparoscopy, Theriogenology, vol. 43, no. 4, pp. 777–788, 1995/03/01/ (1995). [DOI] [PubMed]
- 3.Hill, J. R., Thompson, J. A. & Perkins, N. R. Factors affecting pregnancy rates following laparoscopic insemination of 28,447 merino ewes under commercial conditions: A survey, Theriogenology, vol. 49, no. 4, pp. 697–709, 1998/03/01/ 1998, 10.1016/S0093-691X(98)00019-3 [DOI] [PubMed]
- 4.Del Olmo, E. et al. Fertility of cryopreserved ovine semen is determined by sperm velocity. Anim. Reprod. Sci.138 (1), 102–109. 10.1016/j.anireprosci.2013.02.007 (2013). 04/01/ 2013, doi. [DOI] [PubMed] [Google Scholar]
- 5.Gibbons, A. E., Fernandez, J., Bruno-Galarraga, M. M., Spinelli, M. V. & Cueto, M. I. Technical recommendations for artificial insemination in sheep. Anim. Reprod., 16, 4, pp. 803–809 10.21451/1984-3143-ar2018-0129 [DOI] [PMC free article] [PubMed]
- 6.Macías, A. et al. Cervical artificial insemination in sheep: sperm volume and concentration using an antiretrograde flow device. Anim. Reprod. Sci., 221, p. 106551, 2020/10/01/ 2020, doi: 10.1016/j.anireprosci.2020.106551 [DOI] [PubMed]
- 7.Alvarez, M. et al. Sperm concentration at freezing affects post-thaw quality and fertility of ram semen, Theriogenology, vol. 77, no. 6, pp. 1111–1118, /04/01/ 2012, doi: (2012). 10.1016/j.theriogenology.2011.10.013 [DOI] [PubMed]
- 8.Visser, D. S. & Salamon, S. Fertility following inseminations with frozen-thawed reconcentrated and unconcentrated ram semen. Aust J. Biol. Sci.27 (4), 423–425 (1974). [DOI] [PubMed] [Google Scholar]
- 9.Visser, D. & Salamon, S. The effect of freezing method on the survival of ram spermatozoa. S Afr. J. Anim. Sci.4, 157–163 (1974). [Google Scholar]
- 10.Almadaly, E. A., Ashour, M. A., El-Kon, I. I. & Heleil, B. A. Traditional and non-traditional methods used for discrimination among Ossimi rams with different field fertility. Small Rumin Res., 179, pp. 30–38, 2019/10/01/ 2019, doi: 10.1016/j.smallrumres.2019.09.003
- 11.Almadaly, E. A., Farrag, F. A., Saadeldin, I. M., El-Magd, M. A. & El-Razek, I. M. A. Relationship between total protein concentration of seminal plasma and sperm characteristics of highly fertile, fertile and subfertile Barki ram semen collected by electroejaculation. Small Rumin Res., 144, pp. 90–99, 2016/11/01/ 2016, doi: 10.1016/j.smallrumres.2016.07.023
- 12.Santolaria, P. et al. Predictive capacity of sperm quality parameters and sperm subpopulations on field fertility after artificial insemination in sheep. Anim. Reprod. Sci., 163, pp. 82–88, 2015/12/01/ 2015, doi: 10.1016/j.anireprosci.2015.10.001 [DOI] [PubMed]
- 13.O’ Meara, C. M. et al. Relationship between in vitro sperm functional tests and in vivo fertility of rams following cervical artificial insemination of ewes with frozen-thawed semen, (in eng), Theriogenology, vol. 69, no. 4, pp. 513 – 22, Mar 1 2008, 10.1016/j.theriogenology.2007.12.003 [DOI] [PubMed]
- 14.Nordstoga, A. B., Krogenæs, A., Nødtvedt, A., Farstad, W. & Waterhouse, K. The relationship between Post-thaw sperm DNA Integrity and Non-return Rate among Norwegian Cross-bred rams. Reprod. Domest. Anim.48 (2), 207–212. 10.1111/j.1439-0531.2012.02132.x (2013). /04/01 2013, doi. [DOI] [PubMed] [Google Scholar]
- 15.Pérez-Pé, R. et al. Prediction of fertility by centrifugal countercurrent distribution (CCCD) analysis: Correlation between viability and heterogeneity of ram semen and field fertility, Reproduction, vol. 123, pp. 869 – 75, 07/01 2002, 10.1530/rep.0.1230869 [DOI] [PubMed]
- 16.Hitit, M. et al. Proteomic fertility markers in ram sperm. Anim. Reprod. Sci.235, 106882. 10.1016/j.anireprosci.2021.106882 (2021). 12/01/ 2021, doi. [DOI] [PubMed] [Google Scholar]
- 17.Riesco, M. et al. ProAKAP4 as Novel Molecular Marker of Sperm Quality in Ram: An Integrative Study in Fresh, Cooled and Cryopreserved Sperm, Biomolecules, vol. 10, (2020). [DOI] [PMC free article] [PubMed]
- 18.Swelum, A., Alowaimer, A. & Abouheif, M. Use of fluorogestone acetate sponges or controlled internal drug release for estrus synchronization in ewes: Effects of hormonal profiles and reproductive performance, Theriogenology, vol. 84, no. 4, pp. 498–503, 2015/09/01/ doi: (2015). 10.1016/j.theriogenology.2015.03.018 [DOI] [PubMed]
- 19.Vilariño, M., Rubianes, E., van Lier, E. & Menchaca, A. Serum progesterone concentrations, follicular development and time of ovulation using a new progesterone releasing device (DICO®) in sheep, Small Rumin Res, vol. 91, no. 2, pp. 219–224, /07/01/ 2010. (2010).
- 20.Walker, S. K., Smith, D. H., Godfrey, B. & Seamark, R. F. Time of ovulation in the South Australian Merino ewe following synchronization of estrus. 1. Variation within and between flocks, Theriogenology, vol. 31, no. 3, pp. 545–553, /03/01/ 1989. (1989). [DOI] [PubMed]
- 21.Maxwell, W. M. C. & Barnes, D. R. Induction of oestrus in ewes using a controlled internal drug release device and PMSG. J. Agric. Sci.106 (1), 201–203. 10.1017/S0021859600061931 (1986). [Google Scholar]
- 22.Fukui, Y. et al. Comparison of fertility of estrous synchronized ewes with four different Intravaginal devices during the breeding season. J. Reprod. Dev.45 (5), 337–343. 10.1262/jrd.45.337 (1999). [Google Scholar]
- 23.Walker, S. K. et al. Ovarian follicle dynamics in ewes treated with intra-vaginal progesterone pessaries. 2. Factors affecting timing of estrus and reproductive outcomes following artificial insemination, Theriogenology, vol. 202, pp. 103–109, (2023). 2023/05/01/ doi: https://doi.org/10.1016/j.theriogenology.2023.03.008. [DOI] [PubMed]
- 24.Ainsworth, L. & Downey, B. R. A controlled internal drug-release dispenser containing progesterone for control of the estrous cycle of ewes, Theriogenology, vol. 26, no. 6, pp. 847–856, 1986/12/01/ 1986, 10.1016/0093-691X(86)90014-2
- 25.Walker, S. K., Smith, D. H. & Seamark, R. F. Timing of multiple ovulations in the ewe after treatment with FSH or PMSG with and without GnRH, (in eng). J. Reprod. Fertil.77 (1), 135–142. 10.1530/jrf.0.0770135 (May 1986). [DOI] [PubMed]
- 26.Naqvi, S. M. & Gulyani, R. The effect of gonadotrophin releasing hormone and follicle stimulating hormone in conjunction with pregnant mare serum gonadotrophin on the superovulatory response in crossbred sheep in India, (in eng), Trop Anim Health Prod, vol. 30, no. 6, pp. 369 – 76, Dec doi: (1998). 10.1023/a:1005196705369 [DOI] [PubMed]
- 27.Langford, G. A. Influence of PMSG and Time of Aritificial Insemination on Fertility of Progestogen-treated Sheep in Confinement. J. Anim. Sci.54, 1205–1211. 10.2527/jas1982.5461205x (1982). [DOI] [PubMed] [Google Scholar]
- 28.Olivera-Muzante, J., Fierro, S., López, V. & Gil, J. Comparison of prostaglandin- and progesterone-based protocols for timed artificial insemination in sheep, Theriogenology, vol. 75, no. 7, pp. 1232–1238, 2011/04/15/ 2011, 10.1016/j.theriogenology.2010.11.036 [DOI] [PubMed]
- 29.Bruno-Galarraga, M. et al. The Use of hCG for Inducing Ovulation in Sheep Estrus Synchronization Impairs Ovulatory Follicle Growth and Fertility, Animals, vol. 11, no. 4, p. 984, [Online]. Available: (2021). https://www.mdpi.com/2076-2615/11/4/984 [DOI] [PMC free article] [PubMed]
- 30.Menchaca, A. et al. Prostaglandin F2alpha treatment associated with timed artificial insemination in ewes, (in eng), Reprod Domest Anim, vol. 39, no. 5, pp. 352-5, Oct doi: (2004). 10.1111/j.1439-0531.2004.00527.x [DOI] [PubMed]
- 31.Findlater, R. C. F., Haresign, W., Curnock, R. M. & Beck, N. F. G. Evaluation of intrauterine insemination of sheep with frozen semen: effects of time of insemination and semen dose on conception rates. Anim. Prod.53 (1), 89–96. 10.1017/S0003356100006012 (1991). [Google Scholar]
- 32.Maxwell, W. M. C. Artificial insemination of ewes with frozen-thawed semen at a synchronized oestrus. 1. Effect of time of onset of oestrus, ovulation and insemination on fertility. Anim. Reprod. Sci.10 (4), 301–308. 10.1016/0378-4320(86)90005-9 (1986). 1986/04/01/. [Google Scholar]
- 33.King, M. E. et al. Lambing rates and litter sizes following intrauterine or cervical insemination of frozen/thawed semen with or without oxytocin administration, Theriogenology, vol. 62, no. 7, pp. 1236–1244, 2004/10/01/ 2004, 10.1016/j.theriogenology.2004.01.009 [DOI] [PubMed]
- 34.Scudamore, C. L., Robinson, J. J., Aitken, R. P. & Robertson, I. S. The effect of method of oestrous synchronisation on the response of ewes to superovulation with porcine follicle stimulating hormone. Anim. Reprod. Sci., 34, 2, pp. 127–133, 1993/12/01/ 1993, doi: 10.1016/0378-4320(93)90071-X
- 35.Spanner, E. A., de Graaf, S. P. & Rickard, J. P. Uterine tone influences fertility of Merino ewes following laparoscopic artificial insemination, Theriogenology, 2024/04/10/ 2024, 10.1016/j.theriogenology.2024.04.002 [DOI] [PubMed]
- 36.Spanner, E. A., de Graaf, S. P. & Rickard, J. P. Factors affecting the success of laparoscopic artificial insemination in sheep. Anim. Reprod. Sci., p. 107453, 2024/03/14/ 2024, doi: 10.1016/j.anireprosci.2024.107453 [DOI] [PubMed]
- 37.Sellem, E. et al. Use of combinations of in vitro quality assessments to predict fertility of bovine semen, Theriogenology, vol. 84, no. 9, pp. 1447–1454.e5, 2015/12/01/ 2015, 10.1016/j.theriogenology.2015.07.035 [DOI] [PubMed]
- 38.Januskauskas, A., Johannisson, A., Söderquist, L. & Rodriguez-Martinez, H. Assessment of sperm characteristics post-thaw and response to calcium ionophore in relation to fertility in Swedish dairy AI bulls, (in eng), Theriogenology, vol. 53, no. 4, pp. 859 – 75, Mar 1 2000, 10.1016/s0093-691x(00)00235-1 [DOI] [PubMed]
- 39.Gillan, L., Kroetsch, T., Chis, W. M., Maxwell & Evans, G. Assessment of in vitro sperm characteristics in relation to fertility in dairy bulls. Anim. Reprod. Sci.103 (3), 201–214 (2008). 2008/01/30/. [DOI] [PubMed] [Google Scholar]
- 40.Vašíček, J. et al. Comprehensive Flow-Cytometric Quality Assessment of Ram sperm intended for Gene Banking Using Standard and Novel Fertility Biomarkers, (in eng). Int. J. Mol. Sci.23 (11). 10.3390/ijms23115920 (May 25 2022). [DOI] [PMC free article] [PubMed]
- 41.Palacín, I., Vicente-Fiel, S., Santolaria, P. & Yániz, J. L. Standardization of CASA sperm motility assessment in the ram. Small Rumin Res., 112, 1, pp. 128–135, 2013/05/01/ 2013, doi: 10.1016/j.smallrumres.2012.12.014
- 42.Morrell, J. M. et al. Sperm morphology and chromatin integrity in Swedish warmblood stallions and their relationship to pregnancy rates. Acta Vet. Scand., 50, 1, p. 2, 2008/01/07 (2008). [DOI] [PMC free article] [PubMed]
- 43.Love, C. C. Relationship between Sperm Motility, Morphology and the Fertility of Stallions, (in eng), Theriogenologyvol. 76 (pp. 547 – 57, Aug 2011). no. 310.1016/j.theriogenology.2011.03.007 [DOI] [PubMed]
- 44.Didion, B. A., Kasperson, K. M., Wixon, R. L. & Evenson, D. P. Boar fertility and sperm chromatin structure status: a retrospective report. J. Androl., 10.2164/jandrol.108.00625430, 6, pp. 655–660, 2009/11/12 2009. [DOI] [PubMed]
- 45.Bailey, J. L., Buhr, M. M. & Robertson, L. Relationships among in vivo fertility, computer-analysed motility and in vitro Ca2 + flux in bovine spermatozoa. Can. J. Anim. Sci.74 (1), 53–58. 10.4141/cjas94-008 (1994). [Google Scholar]
- 46.Januskauskas, A., Johannisson, A. & Rodriguez-Martinez, H. Subtle membrane changes in cryopreserved bull semen in relation with sperm viability, chromatin structure, and field fertility, Theriogenology, vol. 60, no. 4, pp. 743–758, 2003/09/01/ doi: (2003). 10.1016/S0093-691X(03)00050-5 [DOI] [PubMed]
- 47.Holt, C., Holt, W. V., Moore, H. D., Reed, H. C. & Curnock, R. M. Objectively measured boar sperm motility parameters correlate with the outcomes of on-farm inseminations: results of two fertility trials, (in eng). J. Androl., 18, 3, pp. 312 – 23, May-Jun (1997). [PubMed]
- 48.Phillips, N. J., McGowan, M. R., Johnston, S. D. & Mayer, D. G. Relationship between thirty post-thaw spermatozoal characteristics and the field fertility of 11 high-use Australian dairy AI sires, (in eng), Anim Reprod Sci, vol. 81, no. 1–2, pp. 47–61, Mar doi: (2004). 10.1016/j.anireprosci.2003.10.003 [DOI] [PubMed]
- 49.Kruger, T. F. et al. Predictive value of abnormal sperm morphology in in vitro fertilization, (in eng), Fertil Steril, vol. 49, no. 1, pp. 112-7, Jan doi: (1988). 10.1016/s0015-0282(16)59660-5 [DOI] [PubMed]
- 50.Perry, V. E. A. The Role of Sperm Morphology Standards in the Laboratory Assessment of Bull Fertility in Australia, (in eng), Front Vet Sci, vol. 8, p. 672058, doi: (2021). 10.3389/fvets.2021.672058 [DOI] [PMC free article] [PubMed]
- 51.Thundathil, J. et al. Relationship between the proportion of capacitated spermatozoa present in frozen-thawed semen and fertility with artifcial insemination, Int J Androl, vol. 22, pp. 366 – 73, 01/01 2000, 10.1046/j.1365-2605.1999.00194.x [DOI] [PubMed]
- 52.Almadaly, E. A. et al. Seminal plasma and serum fertility biomarkers in Ossimi rams and their relationship with functional membrane integrity and morphology of spermatozoa. Small Rumin Res., 196, p. 106318, 2021/03/01/ 2021, doi: 10.1016/j.smallrumres.2021.106318
- 53.Vicente-Fiel, S. et al. In vitro assessment of sperm quality from rams of high and low field fertility. Anim. Reprod. Sci., 146, 1, pp. 15–20, 2014/04/01/ 2014, doi: 10.1016/j.anireprosci.2014.02.005 [DOI] [PubMed]
- 54.Evenson, D. & Jost, L. Sperm chromatin structure assay for Fertility Assessment. Curr. Protoc. Cytom, 13, 1, pp. 7.13.1–7.13.27, 2000, 10.1002/0471142956.cy0713s13 [DOI] [PubMed]
- 55.Peris, S. I., Bilodeau, J. F., Dufour, M. & Bailey, J. L. Impact of cryopreservation and reactive oxygen species on DNA integrity, lipid peroxidation, and functional parameters in ram sperm, (in eng), Mol Reprod Dev, vol. 74, no. 7, pp. 878 – 92, Jul doi: (2007). 10.1002/mrd.20686 [DOI] [PubMed]
- 56.Kumaresan, A., Johannisson, A., Al-Essawe, E. M. & Morrell, J. M. Sperm viability, reactive oxygen species, and DNA fragmentation index combined can discriminate between above- and below-average fertility bulls. J. Dairy. Sci., 100, 7, pp. 5824–5836, 2017/07/01/ 2017, doi: 10.3168/jds.2016-12484 [DOI] [PubMed]
- 57.Papadopoulos, S. et al. In vitro fertilization as a predictor of fertility from cervical insemination of sheep, Theriogenology, vol. 63, no. 1, pp. 150–159, 2005/01/01/ doi: (2005). 10.1016/j.theriogenology.2004.04.015 [DOI] [PubMed]
- 58.Sánchez-Partida, L. G. W. et al. Fertility and its relationship to Motility Characteristics of Spermatozoa in ewes after Cervical, Transcervical, and Intrauterine Insemination with Frozen-Thawed Ram Semen. J. Androl.20 (2), 280–288. 10.1002/j.1939-4640.1999.tb02519.x (1999). [PubMed] [Google Scholar]
- 59.Pool, K. R., Rickard, J. P., Tumeth, E. & de Graaf, S. P. Treatment of rams with melatonin implants in the non-breeding season improves post-thaw sperm progressive motility and DNA integrity. Anim. Reprod. Sci., 221, p. 106579, 2020/10/01/ 2020, doi: 10.1016/j.anireprosci.2020.106579 [DOI] [PubMed]
- 60.Evans, G. & Maxwell, W. M. C. Salamon’s Artificial Insemination of Sheep and Goats (Butterworth-Heinemann, 1987).
- 61.Maxwell, W. M. C. Artificial insemination of ewes with frozen-thawed semen at a synchronised oestrus. 2. Effect of dose of spermatozoa and site of intrauterine insemination on fertility. Anim. Reprod. Sci.10 (4), 309–316. 10.1016/0378-4320(86)90006-0 (1986). 1986/04/01/. [Google Scholar]
- 62.Killen, I. D. & Caffery, G. J. Uterine insemination of ewes with the aid of a laparoscope, (in eng). Aust Vet. J.59 (3), 95. 10.1111/j.1751-0813.1982.tb02737.x (Sep 1982). [DOI] [PubMed]
- 63.Eppleston, J., Salamon, S., Moore, N. W. & Evans, G. The depth of cervical insemination and site of intrauterine insemination and their relationship to the fertility of frozen-thawed ram semen. Anim. Reprod. Sci.36, 211–225 (1994). [Google Scholar]
- 64.Crockett, E. C., Graham, J. K., Bruemmer, J. E. & Squires, E. L. Effect of cooling of equine spermatozoa before freezing on post-thaw motility: Preliminary results, Theriogenology, vol. 55, no. 3, pp. 793–803, 2001/02/01/ doi: (2001). 10.1016/S0093-691X(01)00444-7 [DOI] [PubMed]
- 65.Nascimento, J. et al. Effects of sperm concentration and straw volume on Motion Characteristics and Plasma, Acrosomal, and mitochondrial membranes of Equine Cryopreserved Spermatozoa. J. Equine Vet. Sci.28 (6), 351–358. 2008/06/01/ (2008). [Google Scholar]
- 66.D’Alessandro, A. G., Martemucci, G., Colonna, M. A. & Bellitti, A. Post-thaw survival of ram spermatozoa and fertility after insemination as affected by prefreezing sperm concentration and extender composition, Theriogenology, vol. 55, no. 5, pp. 1159–1170, 2001/03/15/ doi: (2001). 10.1016/S0093-691X(01)00474-5 [DOI] [PubMed]
- 67.Palacios Angola, A., Valencia, J., Méndez & Zarco Quintero, L. Effect of packaging system and sperm concentration on acrosomal damage and post-thaw motility of equine semen. Vet. Mex. 23 (4), 315–318 (1992). [Google Scholar]
- 68.Morrell, J. M. et al. Sperm morphology and chromatin integrity in Swedish warmblood stallions and their relationship to pregnancy rates, Acta Vet Scand, vol. 50, no. 1, p. 2, 2008/01/07 2008, 10.1186/1751-0147-50-2 [DOI] [PMC free article] [PubMed]
- 69.Gillan, L., Kroetsch, T., Chis, W. M., Maxwell & Evans, G. Assessment of in vitro sperm characteristics in relation to fertility in dairy bulls. Anim. Reprod. Sci., 103, 3, pp. 201–214, 2008/01/30/ 2008, doi: 10.1016/j.anireprosci.2006.12.010 [DOI] [PubMed]
- 70.Kondracki, S., Górski, K. & Iwanina, M. Impact of sperm concentration on sperm morphology of large white and landrace boars. Livest. Sci., 241, p. 104214, 2020/11/01/ 2020, doi: 10.1016/j.livsci.2020.104214
- 71.Mickelsen, W. D., Paisley, L. G. & Dahmen, J. J. The effect of scrotal circumference, sperm motility and morphology in the ram on conception rates and lambing percentage in the ewe, Theriogenology, vol. 16, no. 1, pp. 53–59, /07/01/ 1981. (1981). [DOI] [PubMed]
- 72.Malo, A. F. et al. Male fertility in natural populations of red deer is determined by sperm velocity and the proportion of normal spermatozoa. (in eng) Biol. Reprod.72 (4), 822–829. 10.1095/biolreprod.104.036368 (Apr 2005). [DOI] [PubMed]
- 73.McGowan, M. R. et al. Bull selection and use in northern Australia: 1. Physical traits. Anim. Reprod. Sci.71 (1), 25–37. 10.1016/S0378-4320(02)00023-4 (2002). 05/15/ 2002, doi. [DOI] [PubMed] [Google Scholar]
- 74.Abou-Haila, A. & Tulsiani, D. R. P. Mammalian sperm acrosome: formation, contents, and function. Arch. Biochem. Biophys.379 (2), 173–182. 10.1006/abbi.2000.1880 (2000). 2000/07/15/. [DOI] [PubMed] [Google Scholar]
- 75.Alm, K. et al. A novel automated fluorometric assay to evaluate sperm viability and fertility in dairy bulls, Theriogenology, vol. 56, no. 4, pp. 677–684, 2001/09/01/ doi: (2001). 10.1016/S0093-691X(01)00599-4 [DOI] [PubMed]
- 76.Wilhelm, K. M., Graham, J. K. & Squires, E. L. Comparison of the fertility of cryopreserved stallion spermatozoa with sperm motion analyses, flow cytometric evaluation, and zona-free hamster oocyte penetration, (in eng), Theriogenology, vol. 46, no. 4, pp. 559 – 78, Sep doi: (1996). 10.1016/0093-691x(96)00209-9 [DOI] [PubMed]
- 77.Christensen, P., Knudsen, D. B., Wachmann, H. & Madsen, M. T. Quality control in boar semen production by use of the FACSCount AF system, Theriogenology, vol. 62, no. 7, pp. 1218–1228, 2004/10/01/ 2004, 10.1016/j.theriogenology.2004.01.015 [DOI] [PubMed]
- 78.Aitken, R. et al. Sperm motility is lost in Vitro as a consequence of mitochondrial free radical production and the generation of Electrophilic aldehydes but can be significantly rescued by the Presence of Nucleophilic Thiols. Biol. Reprod.87 (29). 10.1095/biolreprod.112.102020 (2012). [DOI] [PubMed]
- 79.Martínez-Pastor, F. et al. Reactive oxygen species generators affect quality parameters and apoptosis markers differently in redFeb deer spermatozoa, (in eng), Reproduction, vol. 137, no. 2, pp. 225 – 35, doi: (2009). 10.1530/rep-08-0357 [DOI] [PubMed]
- 80.Kumar, P. et al. Quantification of leptin in seminal plasma of buffalo bulls and its correlation with antioxidant status, conventional and computer-assisted sperm analysis (CASA) semen variables. Anim. Reprod. Sci.166, 122–127. 10.1016/j.anireprosci.2016.01.011 (2016). /03/01/ 2016, doi. [DOI] [PubMed] [Google Scholar]
- 81.Gaddum-Rosse, P. Some observations on sperm transport through the uterotubal junction of the rat, (in eng), Am J Anat, vol. 160, no. 3, pp. 333 – 41, Mar doi: (1981). 10.1002/aja.1001600309 [DOI] [PubMed]
- 82.Holt, W. V. et al. The value of sperm swimming speed measurements in assessing the fertility of human frozen semen, (in eng). Hum. Reprod.4 (3), 292–297. 10.1093/oxfordjournals.humrep.a136891 (Apr 1989). [DOI] [PubMed]
- 83.Gage, M. J. et al. Spermatozoal traits and sperm competition in Atlantic salmon: relative sperm velocity is the primary determinant of fertilization success, (in eng), Curr Biol, vol. 14, no. 1, pp. 44 – 7, Jan 6 2004. [PubMed]
- 84.García-Álvarez, O. et al. Analysis of selected sperm by density gradient centrifugation might aid in the estimation of in vivo fertility of thawed ram spermatozoa, Theriogenology, vol. 74, no. 6, pp. 979–988, 2010/10/01/ 2010, 10.1016/j.theriogenology.2010.04.027 [DOI] [PubMed]
- 85.Hayes, K. E. N. & Ginther, O. J. Role of progesterone and estrogen in development of uterine tone in mares, Theriogenology, vol. 25, no. 4, pp. 581–590, 1986/04/01/ 1986, 10.1016/0093-691X(86)90142-1 [DOI] [PubMed]
- 86.Griffin, P. G., Hermenet, M. J. & Ginther, O. J. A transient increase in uterine tone during early diestrus in mares, Theriogenology, vol. 37, no. 6, pp. 1185–1190, 1992/06/01/ 1992, 10.1016/0093-691X(92)90174-P
- 87.Bonafos, L. D., Carnevale, E. M., Smith, C. A. & Ginther, O. J. Development of uterine tone in nonbred and pregnant mares, Theriogenology, vol. 42, no. 8, pp. 1247–1255, 1994/12/01/ 1994, 10.1016/0093-691X(94)90244-D
- 88.Loeffler, S. H. et al. Use of ai technician scores for body condition, uterine tone and uterine discharge in a model with disease and milk production parameters to predict pregnancy risk at first ai in holstein dairy cows, Theriogenology, vol. 51, no. 7, pp. 1267–1284, /05/01/ 1999, doi: (1999). 10.1016/S0093-691X(99)00071-0 [DOI] [PubMed]
- 89.Evans, G. Current topics in artificial insemination of sheep, (in eng), Aust J Biol Sci, vol. 41, no. 1, pp. 103 – 16, (1988). [PubMed]
- 90.Akbulut, N. K. & Çelik, H. A. Differences in mean grey levels of uterine ultrasonographic images between non-pregnant and pregnant ewes may serve as a tool for early pregnancy diagnosis. Anim. Reprod. Sci.226, 106716. 10.1016/j.anireprosci.2021.106716 (2021). 2021/03/01/. [DOI] [PubMed] [Google Scholar]
- 91.Hawk, H. W. & Conley, H. H. Altered motility of myometrium from estrous ewes after the regulation of estrus with progestagen or prostaglandin, Theriogenology, vol. 2, no. 3, pp. 37–46, 1974/09/01/ 1974, 10.1016/0093-691X(74)90011-9 [DOI] [PubMed]
- 92.Hawk, H. W. & Cooper, B. S. Sperm Transport into the cervix of the Ewe after Regulation of Estrus with Prostaglandin or Progestogen. J. Anim. Sci.44 (4), 638–644. 10.2527/jas1977.444638x (1977). [DOI] [PubMed] [Google Scholar]
- 93.Kenyon, P. R., Morel, P. C. H. & Morris, S. T. The effect of individual liveweight and condition scores of ewes at mating on reproductive and scanning performance. N. Z. Vet. J., 52, 5, pp. 230–235, 2004/10/01 2004, 10.1080/00480169.2004.36433 [DOI] [PubMed]
- 94.Silva, S. R., Payan-Carreira, R., Quaresma, M., Guedes, C. M. & Santos, A. S. Relationships between body condition score and ultrasound skin-associated subcutaneous fat depth in equids, (in eng), Acta Vet Scand, vol. 58, no. Suppl 1, p. 62, Oct 20 doi: (2016). 10.1186/s13028-016-0243-2 [DOI] [PMC free article] [PubMed]
- 95.Corner-Thomas, R. A. et al. Effects of body condition score and nutrition in lactation on twin-bearing ewe and lamb performance to weaning. New. Z. J. Agricultural Res., 58, 2, pp. 156–169, 2015/04/03 2015, 10.1080/00288233.2014.987401
- 96.Kenyon, P. R., Maloney, S. K. & Blache, D. Review of sheep body condition score in relation to production characteristics. New. Z. J. Agricultural Res.57 (1), 38–64. 10.1080/00288233.2013.857698 (2014). /01/02 2014. [Google Scholar]
- 97.Bravo, M. V., Gabiña, D., Oregui, L. M., Treacher, T. & Vicente, M. S. Relationships between body condition score, body weight and internal fat deposits in Latxa ewes. Anim. Sci.65 (1), 63–69. 10.1017/S1357729800016301 (1997). [Google Scholar]
- 98.Vatankhah, M., Talebi, M. A. & Zamani, F. Relationship between ewe body condition score (BCS) at mating and reproductive and productive traits in Lori-Bakhtiari sheep. Small Ruminant Res., 106, 2, pp. 105–109, 2012/08/01/ 2012, doi: 10.1016/j.smallrumres.2012.02.004
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset generated during and/or analysed during the current study are available from the corresponding author on reasonable request.