Abstract
Tropical wetlands and freshwaters are major contributors to the growing atmospheric methane (CH4) burden. Extensive peatland drainage has lowered CH4 emissions from peat soils in Southeast Asia, but the canals draining these peatlands may be hotspots of CH4 emissions. Alternatively, CH4 oxidation (consumption) by methanotrophic microorganisms may attenuate emissions. Here, we used laboratory experiments and a synoptic survey of the isotopic composition of CH4 in 34 canals across West Kalimantan, Indonesia to quantify the proportion of CH4 that is consumed and therefore not emitted to the atmosphere. We find that CH4 oxidation mitigates 76.4 ± 12.0% of potential canal emissions, reducing emissions by ~70 mg CH4 m−2 d−1. Methane consumption also significantly impacts the stable isotopic fingerprint of canal CH4 emissions. As canals drain over 65% of peatlands in Southeast Asia, our results suggest that CH4 oxidation significantly influences landscape-scale CH4 emissions from these ecosystems.
Subject terms: Carbon cycle, Geochemistry, Limnology, Carbon cycle
Canals draining vast areas of peatland in Southeast Asia may be hotspots for methane emissions. Perryman et al. surveyed dozens of canals in Indonesia and found that methane-eating microbes reduce emissions from these waterways by over 50%.
Introduction
Wetlands and freshwaters contribute ~30–55% of global CH4 emissions1, with significant emissions from tropical ecosystems2–4. Rising CH4 emissions from tropical wetlands due to temperature and rainfall anomalies have contributed substantially to the growing atmospheric CH4 burden5–8. In addition to climate change, ongoing disturbances to tropical wetlands like deforestation, drainage, fertilizer application, and slash and burn agriculture, as well as rewetting and restoration efforts, stand to impact their contribution to the global CH4 budget9–14. However, the impact of tropical wetland disturbance on CH4 cycling is not well understood.
In Southeast Asia, wetland disturbance has heavily impacted peatlands, destabilizing the large pool of soil carbon stored in the peat soils of this region15,16. Peatlands in Southeast Asia have undergone extensive drainage for oil palm plantations, timber, and other agriculture through the construction of canals17,18 that lower the water table and therefore lower CH4 emissions from peat soils19–21. Instead, the CH4 produced in peat soils is transported into canals via lateral flow22,23, increasing the relative importance of drainage canals as a source of CH4 emissions24,25. Canals can represent over 50% of peatland CH4 emissions in Southeast Asia26, but estimates of the magnitude of drainage canal CH4 emissions vary by several orders of magnitude27,28. Given that drainage increases the importance of aquatic carbon fluxes from tropical peatlands29, and the large uncertainty around canal CH4 emissions, greater understanding of the key controls and mechanisms driving canal CH4 emissions is needed to constrain their role in tropical peatland CH4 budgets.
One process that strongly influences freshwater CH4 emissions is microbial oxidation of CH4 to carbon dioxide. In other tropical freshwaters (e.g., rivers, lakes) CH4 oxidation attenuates CH4 emissions by 40 to nearly 100%23,30–32. The fraction of CH4 transported into canals from drained peatlands that is oxidized instead of emitted is highly uncertain, as are the factors that mediate CH4 oxidation in drainage canals. For example, both aerobic and anaerobic methanotrophic microbiota are found in tropical freshwaters32–35. As variation in canal water depth and discharge can impact dissolved oxygen in canals36,37, examining the relationship between CH4 oxidation and dissolved oxygen could inform how CH4 oxidation in canal waters may vary over space and time. Constraining the importance of CH4 oxidation in canals draining tropical peatlands is a key step to improving our understanding of the processes controlling CH4 emissions from these ecosystems, especially in the densely drained peatlands of Southeast Asia where canals can have a disproportionate impact on landscape-scale CH4 emissions.
Here, we address how much CH4 transported from drained tropical peatlands into canals is oxidized instead of emitted to the atmosphere. We quantified the percent of CH4 oxidized in 34 canal reaches that drain peat soils under varying land uses across West Kalimantan, Indonesia (Fig. 1, Supplementary Data 1) through shifts in the δ13C composition of CH4 during incubation experiments of canal waters and from field observations of in situ canal CH4 concentration and δ13C-CH4 (Fig. S1). We find that 47.3-91.3% of CH4 transported into canals from drained peatlands is oxidized instead of emitted. The fraction of CH4 that is oxidized is influenced by factors including dissolved oxygen, vegetation, and canal water depth. Overall, our results suggest that CH4 oxidation substantially attenuates CH4 emissions from canals, and as a result, may be a significant control of landscape-level CH4 emissions from drained peatlands in Southeast Asia.
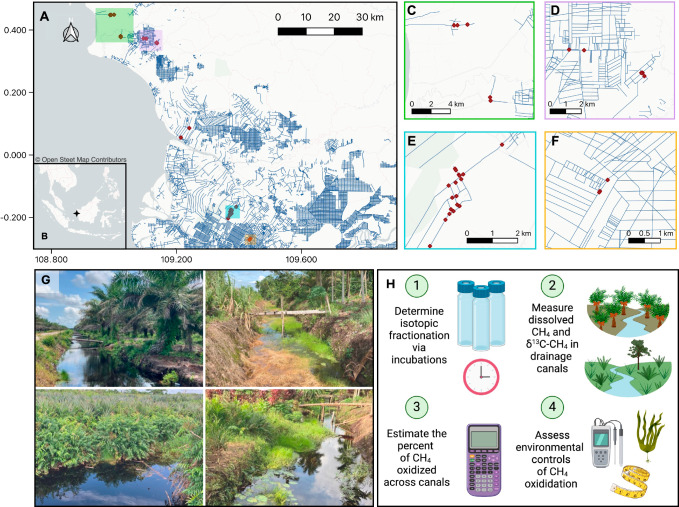
Fig. 1. Drainage canal waters were collected in West Kalimantan, Indonesia to measure methane oxidation.
A Study area with drainage canals shown as dark blue lines. B shows location of study within insular Southeast Asia. Canal sample locations marked in red points. C–F Zoomed in view of green, purple, teal, and orange boxes in panel A, respectively, showing sample locations. The base map layers in Panels A–F are available from OpenStreetMap (openstreetmap.org/copyright), available under the Open Database License. G Examples of canals in varying land use context and canals with and without aquatic vegetation. H Overview of study methods to estimate CH4 oxidation in drainage canals. Created in BioRender. Perryman, C. (2024) BioRender.com/c12r203.
Results and Discussion
CH4 consumption and isotopic fractionation observed during incubations
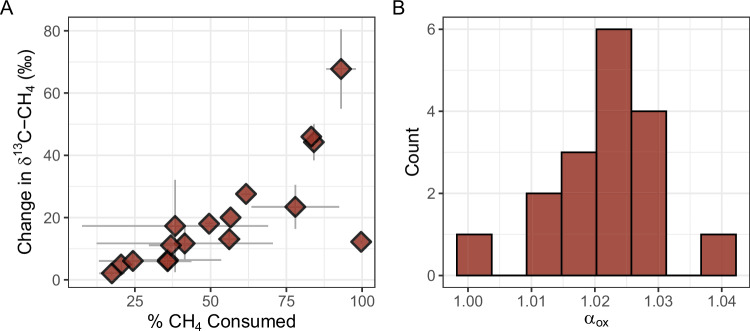
To confirm CH4 oxidation occurs in drainage canals, we incubated water from 13 canals at in situ dissolved CH4 and oxygen concentrations and measured the change in dissolved CH4 and δ13C-CH4 over time. On average, 53.8 ± 25.6% of the initial CH4 was consumed over the incubation period (17.6–99.7%) and δ13C-CH4 increased by 19.8 ± 17.7‰ (2.1–67.8‰, Fig. 2A, Table S1). The increase in δ13C-CH4 observed across incubated waters confirmed the loss of CH4 was from microbial oxidation, as CH4 oxidation leaves residual CH4 enriched in 13C38,39. Methane oxidation rates were variable across incubated waters (0.03–5.6 μmol CH4 L−1 d−1, Table S1) and were strongly influenced by initial CH4 concentration (Fig. S2). Neither CH4 production nor δ13C-CH4 depletion was observed in the canal waters during the incubation period.
Fig. 2. Methane consumption and resulting stable isotope fractionation in incubated canal waters.
A Across incubated waters, δ13C-CH4 increased as the percent of initial CH4 consumed increased. Each data point shows the mean change over ~50 hours of incubation ± standard error of replicates (Table S1). B Histogram of αox values calculated from incubation data. Source data are provided as a Source Data file.
From these data we calculated the first empirically derived isotopic fractionation factors for CH4 oxidation40 (αox) in peat-draining freshwaters. Ecosystem-specific values for αox are critical to estimating the percent of CH4 that is oxidized rather than emitted from the natural environment41,42. Mean αox was 1.022 ± 0.009 across the incubated canal waters (range: 1.002–1.039; Fig. 2B). The range of αox encompasses past observations from northern and temperate freshwaters incubated under in situ dissolved CH4 and oxygen concentrations and temperature42,43, as well as results from incubations of soil from subtropical rice paddies44 (αox of 1.025–1.033) that are often used in estimates of CH4 oxidation in tropical freshwaters30,32. While CH4 oxidation rates varied with initial CH4 concentration, we did not observe a correlation between αox and initial CH4 concentration, nor αox and CH4 oxidation rate (Fig. S2). Recent work in temperate lakes identified temperature, pH, and dissolved O2 as potential controls on αox43. Of these factors, αox was only weakly positively correlated with the initial dissolved O2 present in each of the incubated waters (p = 0.07). αox did not vary between surface and bottom waters of the subset of canals sampled at two depths for incubation experiments (1.024 ± 0.006 vs. 1.023 ± 0.012, n = 4 canals). As we did not find significant environmental correlates of αox, we used the mean value to estimate in situ CH4 oxidation as discussed below.
Oxidation mitigates the majority of drainage canal CH4 emissions
We find that the majority of CH4 transported into canals from drained tropical peatlands is oxidized instead of emitted to the atmosphere. Using the laboratory-derived αox values, measurements of in situ canal water δ13C-CH4 from 34 canal reaches (Supplementary Data 1), and measurements of source porewater δ13C-CH4 (Supplementary Data 2), we estimated that CH4 oxidation consumes 76.4 ± 12.0% of CH4 transported into canals (range: 47.3–91.3%). Considering the standard deviation of αox shifts the mean percent oxidized by ~10%, ranging from 65.5 ± 12.5% to 89.3 ± 8.9% (Figure. S3). Similarly, considering the standard deviation of the porewater source δ13C-CH4 measurements, the mean percent oxidized could range from 68.2 ± 16.1% to 82.4 ± 8.9% (Fig. S3). Our estimate of the fraction of CH4 transported into canals that is oxidized instead of emitted is consistent with Somers et al. (2023), who estimated that 70% of CH4 was oxidized in a canal draining a tropical peatland in Brunei using a reactive transport model. These results are also consistent with past work indicating that oxidation consumes ~80% of CH4 in blackwater rivers in the Amazon32 that have low pH and high concentrations of aromatic-rich, humic-like dissolved organic carbon like the canals in our study region29,45. Compared to previous work in lotic systems that use a similar approach as employed in our study, we find that oxidation mitigates a higher proportion of potential CH4 emissions in drainage canals than in headwater streams in temperate forests46 (55.6 ± 2.8%) or boreal peatlands47 (~60%).
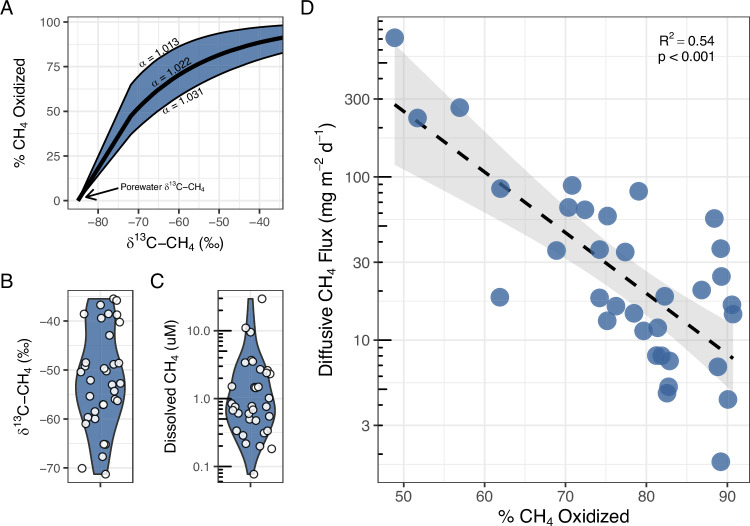
Canal water dissolved CH4 concentration and δ13C-CH4 across our study region supports our finding that CH4 oxidation limits CH4 release from canals. Dissolved CH4 concentration and δ13C-CH4 in canal waters ranged from 0.05 to 31.6 μM and −71.9 to −34.1‰, respectively (Fig. 3B), and dissolved CH4 decreased with increasing δ13C-CH4 (R2 = 0.43, p < 0.001, Fig. S4). Previous observations in tropical river networks32 also observed a negative relationship between the concentration of CH4 in river waters and δ13C-CH4. In these rivers δ13C-CH4 also had a positive relationship with gene markers for methanotrophic bacteria, indicating that variation in CH4 concentration and δ13C-CH4 is influenced by CH4 oxidation. The consistent relationship between CH4 concentration and δ13C-CH4 observed across the drainage canals in our study and these tropical rivers supports the idea that differences in dissolved CH4 concentrations between canal reaches are influenced by CH4 oxidation.
Fig. 3. Survey of drainage canal CH4 concentrations and δ13C-CH4 reveal the impact of CH4 oxidation on canal CH4 emissions.
A Curve showing the relationship between canal water δ13C-CH4 and estimated percent CH4 oxidized across the mean (black line) and ± 1 standard deviation (shaded region) of the laboratory derived αox value. B, C Surface water δ13C-CH4 and dissolved CH4 concentration across the studied canals (n = 34). D Estimates of the percent of CH4 oxidized versus estimated diffusive CH4 flux across the studied canals. For panels B–D each dot represents a canal. The shaded region of panel D represents the 95% confidence interval associated with the linear relationship. Dissolved CH4 concentration and estimated diffusive CH4 flux are shown on a log10 scale in panels C and D. Source data are provided as a Source Data file.
It is unlikely that CH4 concentration in canal waters is dictated only by the amount of CH4 originally transported into canals from the surrounding landscape, including CH4 produced in peat soils and canal sediments. Methane produced in ombrotrophic tropical peat soils is highly depleted in 13C23,48. Unlike in lakes where δ13C-CH4 in littoral sediments and adjacent groundwater can differ by more than 10‰49, porewater δ13C-CH4 has not been shown to differ between canal bottoms and adjacent peat soils22. Porewater δ13C-CH4 collected from 6 profiles (40 to 150 cm depth) located alongside canal waters in our study region had a mean δ13C-CH4 of −85.0 ± 5.9‰, which was consistently more depleted than any observed canal δ13C-CH4 value (Supplementary Data 1, Supplementary Data 2). Porewater δ13C-CH4 varied more between sample depths within each profile than between profiles collected across the landscape, suggesting source δ13C-CH4 is similarly depleted in 13C throughout the study region. Methane production in the water column could also influence canal water CH4 concentration and δ13C-CH4. However, this is unlikely to explain our results because we did not observe net CH4 production in any of the laboratory incubations of canal waters, as CH4 concentration decreased and δ13C-CH4 increased in all incubated waters (Fig. 2A, Table S1). If canal water CH4 concentration were influenced solely by the total amount of CH4 produced and then transported into canal waters, we would expect canal water δ13C-CH4 to be similarly depleted across canals and not vary systematically with dissolved CH4 concentration. Given that CH4 concentrations varied ~600-fold alongside a ~40‰ range in δ13C-CH4, our results indicate that CH4 oxidation has a significant influence on canal water CH4 concentration and δ13C-CH4.
As CH4 oxidation was a major control of canal water CH4 concentration, diffusive CH4 emissions were also strongly influenced by the percent of CH4 oxidized. Diffusive CH4 emissions estimated from dissolved CH4 concentration (Supplemental Text 1) ranged from 1.0 to 761.8 mg CH4 m−2 d−1 (mean = 72.2 ± 151.2; median = 18.0) and decreased as the percent of CH4 oxidized increased (R2 = 0.54, p < 0.001; Fig. 3D). Measurements of CH4 emissions from floating chamber deployments at a subset of study sites (n = 12 canals, mean = 94.9 ± 142.3 CH4 m−2 d−1, median = 33.0, Supplementary Data 1) also indicated a negative relationship between CH4 emissions and the percent of CH4 oxidized (Fig. S5). By back-calculating what diffusive CH4 flux would be in the absence of oxidation, we estimate that CH4 oxidation reduces drainage canal CH4 emissions by a mean of 136.8 ± 154.1 mg CH4 m−2 d−1 (range: 9.9–684.2). Given the skewed distribution of dissolved CH4 concentrations that underlie this estimate, the median value of 72.1 mg CH4 m−2 d−1 (IQR: 36.2–173.6) may be a more robust estimate of the emissions attenuated by CH4 oxidation. Overall, our results provide evidence suggesting that CH4 oxidation mitigates the majority of potential CH4 emissions from canals on the landscape.
Controls on CH4 oxidation in drainage canals
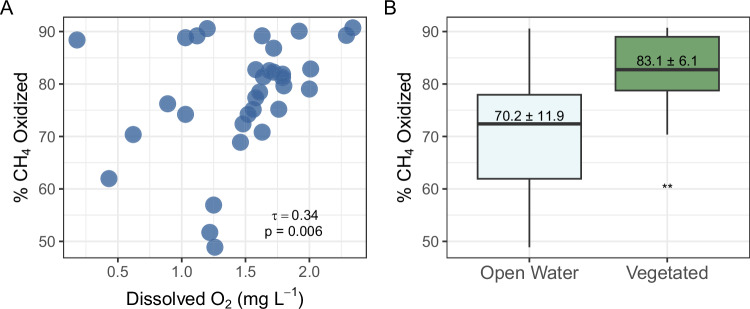
Of the studied controls on CH4 oxidation, dissolved oxygen and aquatic vegetation had the most significant influence on the percent of CH4 oxidized in canals as determined by canal water δ13C-CH4. We found that the percent of CH4 oxidized increased and dissolved CH4 concentration decreased with the concentration of dissolved oxygen at the canal water surface (0-10 cm; p < 0.05, Fig. 4A, Table S2). The relationship between dissolved oxygen and CH4 oxidation is consistent with oxidation mediated by aerobic methanotrophic bacteria, as has been observed in other stream and river networks32,46. While all canals had low dissolved oxygen (0.2 to 2.3 mg L-1), methanotrophic bacteria of the order Methylococcales have been shown to have the genetic potential for survival and methanotrophic activity in low oxygen environments50. Abundant Methylococcales have been identified in hypoxic tropical freshwaters where paired measurements of dissolved CH4 concentration and δ13C-CH4 indicate ongoing CH4 oxidation34,35. Our results further support the idea that aerobic CH4 oxidation occurs in tropical freshwaters with low dissolved oxygen.
Fig. 4. Controls on CH4 oxidation in drainage canals.
A Dissolved oxygen in the surface waters (0–10 cm) of drainage canals versus the percent of CH4 oxidized. Each point represents a canal (n = 34). B Boxplot of the percent of CH4 oxidized in open water (light blue, n = 19) and vegetated (green, n = 15) canals. Within each box the black lines represent median values and the height of the boxes represent the interquartile range. Error bars extend up to 1.5 times the interquartile range. The number in each box represents the mean ± 1 standard deviation of the percent of CH4 oxidized for each group. Source data are provided as a Source Data file.
Furthermore, the percent of CH4 oxidized was higher in vegetated canals than those with open water (p = 0.01, Fig. 4B). Vegetation may enhance CH4 oxidation via radial oxygen loss from roots51,52 or via oxidation by epiphytic methanotrophs in submersed plants53. Although we did not observe a significant difference in dissolved oxygen based on the presence of aquatic vegetation (p > 0.05, Table S3), oxygen delivered to the water column by aquatic vegetation is likely rapidly consumed by methanotrophs or by competing aerobic heterotrophs as deposition of more labile organic carbon by aquatic vegetation could stimulate heterotrophic respiration in canal waters29. Lower CH4 concentrations and more enriched δ13C-CH4 in vegetated canals could alternatively be explained by plant-mediated emissions54, which reduce CH4 concentration and enrich the δ13C of residual CH4 due to the isotopic fractionation of plant-mediated transport55. The deposition of labile organic matter from vegetation could also stimulate acetoclastic methanogenesis, which like CH4 oxidation would contribute towards larger δ13C-CH4 in vegetated canals39. However, acetoclastic methanogenesis likely contributes little to the δ13C-CH4 in vegetated canals because hydrogenotrophic methanogenesis has been identified as the dominant pathway in the ombrotrophic tropical peatlands of Southeast Asia23 and the Americas48,56. Disturbance in peatlands in Southeast Asia has been observed to increase the abundance of plant functional types associated with acetoclastic methanogenesis, like graminoids, but this shift does not appear to increase the abundance of acetoclastic methanogens57. While we cannot rule out the possible influence of acetoclastic methanogenesis on canal water δ13C-CH4, the lower dissolved CH4 concentration in vegetated canals (p = 0.02, Table S3) lends more support to the idea that vegetation enhances CH4 oxidation rather than acetoclastic CH4 production in canals.
Given that higher dissolved oxygen and the presence of aquatic vegetation were observed in canals with a shallower water depth (Fig. S6), canal water depth may indirectly mediate CH4 oxidation in drainage canal waters. Overall, dissolved oxygen in the surface water of canals (0–10 cm) decreased with the depth of water present in the canal (Kendall’s τ = −0.41, p < 0.05, Fig. S6). Dissolved CH4 concentration, and therefore estimated diffusive emissions, also had a weak but significant positive correlation with canal water depth (τ = 0.26, p = 0.03, Table S2). This result contradicts previous findings in drainage ditches in temperate peatlands where CH4 emissions had a weak negative correlation with depth58, but these differing results may be explained by how well canal waters are mixed and aerated. For example, while we observed CH4 oxidation in canals where dissolved oxygen is low (<2.5 mg L−1) at the surface, dissolved oxygen may become depleted at depth29,45 to below the concentration needed for aerobic methanotrophs with high oxygen affinity. As such, CH4 oxidation may be limited to the surface waters of deeper canals, while in shallower canals oxidation may occur throughout the water column. Our study also only explicitly considered diffusive emissions. Measurements of CH4 ebullition from canals could further clarify the role of water depth in shaping net canal CH4 emissions, as ebullitive emissions vary with water depth59. Altogether, our results suggest that shallower, vegetated canals may attenuate a higher percentage of CH4 emissions through CH4 oxidation.
Land use and seasonal precipitation cycles can both influence canal water depth and therefore dissolved oxygen. While we did not observe a significant impact of peatland land use on CH4 oxidation nor other parameters including dissolved oxygen (Table S4), peatland water table, which directly influences canal water levels, has been shown to vary significantly between land use types60. Canal water depth also varies 2- to 5-fold throughout the year in response to precipitation (Fig. S7), and reduced precipitation and flow during drier months may facilitate oxygen depletion by limiting turbulent mixing and re-aeration of canal waters27,61. Accordingly, past studies have reported higher canal CH4 emissions during dry periods27,28. While our study was not conducted during pronounced wet or dry periods, the dissolved CH4 and oxygen concentrations measured in our study fall within the range observed across Southeast Asia under varying land uses and seasons22,28,45,62,63 (Table S5). As such, we anticipate that water column CH4 oxidation is prevalent across canals draining degraded peatlands in Southeast Asia.
Influence of oxidation on CH4 emissions and their 13C in drained tropical peatlands
Our observations of canal CH4 emissions estimated from dissolved CH4 concentration (72.2 ± 151.2 mg CH4 m-2 d-1) and collected using floating chambers (94.9 ± 142.3 mg CH4 m-2 d-1) are within range of past observations from Indonesia27,28,64 and Malaysia26 where mean emissions range from 2.8 to 1073 mg CH4 m-2 d-1 (Table S6). The IPCC CH4 Emissions Factor for canals in tropical peatlands of 618.9 mg CH4 m-2 d-1 (2259 kg CH4 ha-1 y-1) was based on the only reported data27 at the time of the 2013 Wetlands Supplement65 This emission factor now represents the high end of field estimates to date among a still small number of existing studies and should be reconsidered to more accurately inventory the anthropogenic (e.g., from land use change) component66 of CH4 emissions from degraded tropical peatlands.
Despite high oxidation efficiencies, drainage canals can still emit large amounts of CH4. For example, in canals where ~50% of the CH4 transported from peatlands is oxidized we observe emissions >200 mg CH4 m-2 d-1 (Figs. 3, S5). The canals in this study were primarily situated in smallholder agricultural systems (Supplementary Data 1), and the mean estimated diffusive CH4 emissions from canals presented here are 30x larger on a per area basis than mean peat soil CH4 emissions from smallholder agriculture fields in West Kalimantan67. Thus, while CH4 oxidation plays a critical role in attenuating canal CH4 emissions, canals can still contribute significantly to landscape-level CH4 emissions from drained peatlands in Southeast Asia.
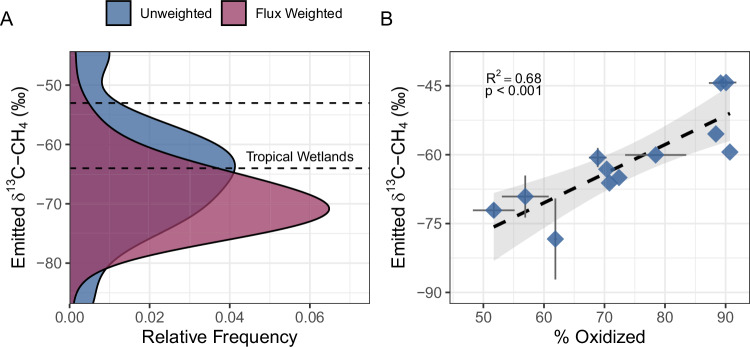
Beyond the rate of emissions, the δ13C signature of CH4 emitted from tropical wetlands and freshwaters are critical for constraining their contribution to the global CH4 budget, as δ13C-CH4 values underpin source partitioning by atmospheric inversion models. Using a floating chamber to capture CH4 emitted from a subset of the studied canals, we found that the mean δ13C-CH4 was -64.7 ± 10.5‰ (Fig. 5A). Canal CH4 emissions generally decreased as emitted δ13C-CH4 increased (Fig. S8), therefore the flux-weighted mean δ13C-CH4 was more negative at -69.0 ± 5.7‰ (Fig. 5A). Past observations of the δ13C signature of tropical wetland CH4 emissions68 indicate a range of -64‰ to -53‰. Our results suggest that the δ13C signature of CH4 emissions from drainage canals, and potentially drained peatlands in Southeast Asia as whole due to the contribution of canals to landscape-scale CH4 emissions, is more negative than prior measurements from tropical wetlands. As such, implementing a distinct δ13C-CH4 source signature for Southeast Asian peatlands may improve top-down estimates of their CH4 emissions.
Fig. 5. The isotopic composition of CH4 emissions from tropical peatland drainage canals.
A Probability density estimates of the δ13C of CH4 emitted from canal waters showing the unweighted (blue) and flux-weighted (purple) distributions of emitted δ13C-CH4. The dashed black lines show the range of δ13C of tropical wetland CH4 emissions reported in ref. 68. B The percent of CH4 oxidized in drainage canal waters (estimated from dissolved δ13C-CH4 using αox = 1.022) versus the δ13C of CH4 emitted from the corresponding canal. Each point represents a canal (n = 12) and error bars show the mean ± 1 standard deviation if replicates were collected at a canal. The shaded regions represent the 95% confidence interval associated with the linear relationship shown in panel B. Source data are provided as a Source Data file.
Furthermore, we find that the variation in δ13C of CH4 emissions from the canal water surface (−86.9 to -44.3‰) was largely explained by the percent of CH4 oxidized in canal waters (R2 = 0.68, p < 0.05; Fig. 5B). Previous studies have identified oxidation, alongside variation in methanogenic pathways and wetland vegetation, as one potential explanation for latitudinal differences in the δ13C of wetland CH4 emissions68–70. Our results indicate that once CH4 produced in peat soils is transported into canals, both the magnitude and the isotopic signature of canal CH4 emissions are strongly influenced by CH4 oxidation.
In summary, we demonstrate that CH4 oxidation can substantially attenuate CH4 emissions from canals draining peatlands in Southeast Asia. We estimate that CH4 oxidation mitigates >50% of potential CH4 emissions from canals across West Kalimantan, Indonesia. As landscape-scale measurements of CH4 exchange in drained tropical peatlands indicate that canal networks contribute disproportionately to emissions from these ecosystems20, our results suggest that CH4 oxidation influences emissions not only from drainage canals but from degraded peatlands in Southeast Asia as a whole. Our results also have implications for peatland CH4 emissions in response to land use change, including peatland restoration efforts. For example, we find that oxidation attenuates more CH4 emissions from shallower canals that have higher dissolved oxygen concentrations. As such, efforts to rewet drained peatlands in Southeast Asia through canal blocking may impact CH4 oxidation and therefore canal CH4 emissions through changing canal water depth. Given the extensive networks of drainage canals in Southeast Asia and their substantial contribution to peatland CH4 emissions, land use changes impacting CH4 oxidation in canals will be reflected in the contribution of peatlands in Southeast Asia to the global CH4 budget.
Methods
Field sampling
Drainage canals in lowland peatlands were sampled in Kubu Raya and Mempawah Districts, West Kalimantan, Indonesia. Canals were sampled in Kubu Raya in May 2023 and Mempawah in April 2024. This region has an equatorial rainfall pattern with no clear wet and dry season71. There is heavy rainfall year-round, but the driest months of the year usually occur in July or August. We sampled waters from canals of different sizes (5 to 90 cm water depths, 0.5 to 6 m canal widths), canals with (n = 15) and without aquatic vegetation (n = 19), and canals situated on peatlands under a variety of land uses. Smallholder mixed agriculture is the most represented land use in this study, but the sampled canals also include areas in smallholder plantations (pineapple and oil palm), industrial oil palm plantations, and open undeveloped land (i.e., deforested and/or burned areas), as well as 1 canal in a degraded forest, to capture the heterogeneity of drainage canals in the region. At each canal, we measured the canal dimensions as well as water temperature (°C), pH, dissolved oxygen (mg L-1), conductivity (μS cm-1), and redox potential (Eh, in mV) using a Hanna Instruments HI9829 multiparameter meter. A summary of the canals included in this study is available in Supplementary Data 1.
To measure the isotopic composition of source CH4, we collected porewater profiles at 6 locations adjacent to a subset of the sampled canals. As shallow porewater is the primary source of discharge to drainage canals22, porewater was collected from 4-5 depths between 40 cm and 150 cm pending water table depth. Porewater was collected using a portable piezometer made of 3/8” stainless steel tubing housing 1/4” polyethylene tubing equipped with a coarse polypropylene screen to prevent collection of coarse debris (SedPoints, M.H.E. Products). Porewater samples were stored in 12 mL glass ExetainerTM vials (Labco Ltd.) without headspace and acidified in the field to a pH of less than 2 using 1.5 M HCl.
Canal CH4 concentration and δ13C
We collected surface water samples for analysis of dissolved CH4 concentration and δ13C-CH4 at all canals. Canal water samples were collected approximately 5 cm below the water surface and stored in 12 mL glass ExetainerTM vials (Labco Ltd.) without headspace. Canal waters collected for assessment of in situ dissolved CH4 concentration and δ13C-CH4 were acidified in the field to a pH of less than 2 using 1.5 M HCl. Canal water samples collected in 2023 (including incubations described below) and all porewater samples were analyzed at the Stable Isotope Facility at UC Davis via a Delta V Plus IRMS following headspace equilibration. Samples collected in 2024 were analyzed at Stanford University via a Picarro G2210-i cavity ring down spectrometer following headspace equilibration. Reference standards with CH4 mixing ratios and δ13C-CH4 of 10 ppm/-45.5‰ and 30 ppm/-69.0‰ were run before and after sample analysis on the Picarro G2210-i to check for accuracy and instrument drift. Dissolved CH4 concentrations were calculated considering the mixing ratio of CH4 in the equilibrated headspace, using the ideal gas law, and in solution, following Henry’s Law, using the neonDissGas package72.
Incubations
We collected canal waters at a subset (n = 13) of the drainage canals for incubation experiments. Surface waters (~5 cm) were collected for all canals included in the incubation experiments, and at 5 of the canals we collected water from ~10 cm above the canal bottom using gas-tight tubing and a hand pump. Collecting these deeper canal waters for incubation experiments enabled us to account for any variability in isotopic fractionation of CH4 oxidation with depth in the water column that could impact our estimates of oxidation efficiency. For canal waters collected for incubation experiments, we collected waters as described above but only field acidified samples for the initial incubation time point. Incubations occurred in the dark at room temperature (~25 °C) for 3 days. Duplicate samples for each canal (and depth, if applicable) were acidified every ~24 hours to pH <2 using 1.5 M HCl to stop CH4 oxidation. All incubated waters were analyzed at the Stable Isotope Facility at UC Davis. Dissolved CH4 concentration was calculated as described above.
Dissolved CH4 concentration fell below the limit of quantification within 72 hours, as such incubation results only consider data from the first 2 days. One of the 5 deeper canal waters was omitted as CH4 was not detectable after 24 hours. We calculated potential oxidation rates as the change in CH4 concentration over the total incubation time. We also calculated the fractionation factor of CH4 oxidation, or αox, from the CH4 mixing ratios (in ppm) and δ13C-CH4 of the incubated waters using a simplified Rayleigh model40:
| 1 |
Plotting Eq. (1) with ln(1000 + δ13C-CH4) on the x-axis and ln(CH4) on the y-axis produces a line with a slope of (αox/1-αox). As such, we calculated the slope as the difference in ln(CH4) between the initial and final time points over the difference in ln(1000 + δ13C-CH4) over the same time and then solved for αox.
Canal CH4 emissions
We used a floating chamber to manually collect chamber headspace gasses at 12 of the sampled canals to assess CH4 emissions and emitted δ13C-CH4. A 20 cm diameter/2.1 L floating chamber was deployed on the canal water surfaces for 6 minutes in 2023 and 12 minutes in 2024. Chamber deployment time was increased in 2024 to ensure sufficient CH4 accumulation for analysis via Picarro CRDS. The floating chamber was not held in place, but due to low canal water flow (stagnant to ~0.1 m s-1) that chamber did not travel during flux measurement. Three 15 mL gas samples were collected from the chamber headspace over the deployment time via a sampling syringe and injected into a pre-evacuated 12 mL glass ExetainerTM vial (Labco Ltd.). Floating chamber headspace gas samples from 2023 were analyzed at the Stable Isotope Facility at UC Davis and from 2024 at Stanford University, as described above. Methane emissions were calculated as the linear increase in chamber headspace CH4 mixing ratio over the measurement period and converted from ppm CH4 min-1 to mg CH4 m-2 d-1 using the Ideal Gas Law and the floating chamber dimensions. Fluxes were accepted if the linear increase in CH4 over time met the standards of R2 > 0.9 and p < 0.05. Emitted δ13C-CH4 was determined via a Keeling plot approach, in which the δ13C of CH4 emissions is the y-intercept of a linear regression of the inverse mixing ratio of CH4 versus the δ13C-CH4 of the corresponding sample73,74.
We calculated gas transfer velocity (k, m d-1) using data from the subset of canals where paired floating chamber CH4 fluxes and canal water CH4 concentrations were collected using Eq. (2):
| 2 |
Where CH4-canal is the concentration of CH4 in canal water, CH4-eq is the CH4 concentration at equilibrium the atmosphere (CH4-eq), and flux is the rate of CH4 emissions measured using the floating chamber. We used the median k value from the floating chamber deployments to estimate diffusive fluxes across all sampled (n = 34) canals. While applying a uniform value introduces uncertainty into the estimates of diffusive fluxes, conditions across the study region are characterized by high canal water temperature, low canal flow velocity (~0.1 m s-1), and low windspeed. As such, factors that strongly influence CH4 degassing (e.g., solubility and turbulence) should have minimal variation relative to the ~600-fold variation in canal water CH4 concentration across study sites. Values were normalized to k600 for literature comparison. See Supplementary Text 1 for further discussion of approaches to estimate k.
Estimating percent oxidation
We used a simple box model to estimate the percent of CH4 transported from drained peatlands into canals that is oxidized and therefore not emitted to the atmosphere. The model calculated the percent oxidized based on the difference in δ13C between the source CH4 (e.g., peat porewater) and CH4 after oxidation (e.g., in the canal waters) as well as the isotopic fractionation of CH4 oxidation (αox), which we determined via incubations as described above. Oxidation efficiency (fox) was calculated using a Rayleigh model for closed systems42,75:
| 3 |
Where δsource and δcanal are the δ13C-CH4 of peat porewater and drainage canal waters, respectively, and fox is the fraction of CH4 oxidized. Values of fox were multiplied by 100 to convert to the percent of CH4 oxidized. The closed system approach represents a lower bound on oxidation, as open system models often result in estimates of the percent oxidized >100%.
The results presented in the main analyses and figures are estimates of the percent oxidized based on mean observed values of αox (1.022 ± 0.009, from incubations) and δsource (-85.0 ± 5.9‰, n = 27 measurements from 6 porewater profiles, Supplementary Data 2). To characterize the uncertainty of our estimates due to variability in αox and δsource, we also report how our estimate varies when using ± 1 standard deviation of αox or δsource in Eq. (3). Varying αox or δsource by ± 1 standard deviation changes our estimate of the mean percent oxidized by ~10%.
To estimate the amount of CH4 emissions attenuated by CH4 oxidation, we back-calculated the concentration of CH4 in canal waters based on the fox value for each canal:
| 4 |
Using this predicted concentration, we calculated predicted diffusive CH4 fluxes as described above. We then subtracted the diffusive CH4 fluxes calculated from observed CH4 concentrations from the predicted CH4 fluxes based on the back-calculated concentrations to estimate the CH4 emissions mitigated by CH4 oxidation.
Statistical analysis
Statistical analysis and data visualization were performed in R v4.0.3. Data preparation was conducted using the dplyr package76. Data and analyses were visualized using the ggplot277 and patchwork78 packages. Statistical analyses were performed using the R Core Team stats package. Dissolved CH4 concentration and estimated diffusive emissions were log10-transformed prior to all statistical analysis to improve normality. If there were replicate measurements taken in a canal, the mean value of replicates was used in statistical analysis and data visualization. Summary statistics were calculated using all observations, including spatial replicates, to report the full range of observations. The level of significance for all analyses was 0.05.
We tested relationships between canal water CH4 concentration and δ13C-CH4 and between estimated diffusive CH4 fluxes and the percent of CH4 oxidized using least squares regression. We used Kendall’s rank correlation to assess the strength and direction of monotonic relationships between dissolved oxygen or canal depth and canal water dissolved CH4 concentration, δ13C-CH4, and percent CH4 oxidized. We used a non-parametric correlation for these analyses as relationships between CH4 oxidation and dissolved oxygen are often non-linear due to substrate saturation and potential inhibitory effects of oxygen above the optimal levels for CH4 oxidation in freshwater environments79. We used one-way ANOVA to assess the impact of vegetation and land use on canal properties and CH4 cycling.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
The research was supported by the NSF Earth Sciences Postdoctoral Research Fellowship (Award ID 2305578, CRP) and by the Precourt Institute for Energy (CRP, JCB, AMH). This research was conducted under permit #37/SIP/IV/FR/1/2024 from the Indonesian National Research and Innovation Agency (BRIN). We thank Insen Amri and Anggit Djoko Wibowo for their assistance with fieldwork and Rob Jackson (Stanford University) for access to the Picarro CRDS used to analyze samples collected in 2024.
Author contributions
C.R.P., J.C.B., and A.M.H. designed the study. C.R.P., J.C.B., J.S., D.S.P.A.B., E.D., and Y.A. performed the research. C.R.P. analyzed the data and wrote the manuscript. N.N. and G.Z.A. provided field site and laboratory access. J.C.B., J.S., D.S.P.A.B., E.D., Y.A., A.A., A.G., N.N., G.Z.A., and A.M.H. reviewed the manuscript and contributed to manuscript revisions.
Peer review
Peer review information
Nature Communications thanks Michael Peacock, Paula Reis and the other, anonymous, reviewer for their contribution to the peer review of this work. A peer review file is available.
Data availability
All data are presented in the manuscript and/or the Supplementary Information. The data used in this study are available at the Zenodo repository under ‘Tropical Peatland Drainage Canal Methane Concentrations, Fluxes, and Isotopic Composition’ (10.5281/zenodo.11155160). Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-54063-x.
References
- 1.Saunois, M. et al. The Global Methane Budget 2000–2017. Earth Syst. Sci. Data12, 1561–1623 (2020). [Google Scholar]
- 2.Johnson, M. S., Matthews, E., Du, J., Genovese, V. & Bastviken, D. Methane emission from global lakes: new spatiotemporal data and observation‐driven modeling of methane dynamics indicates lower emissions. J. Geophys. Res. Biogeosci.127, e2022JG006793 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stavert, A. R. et al. Regional trends and drivers of the global methane budget. Glob. Change Biol.28, 182–200 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocher-Ros, G. et al. Global methane emissions from rivers and streams. Nature621, 530–535 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng, L., Palmer, P. I., Zhu, S., Parker, R. J. & Liu, Y. Tropical methane emissions explain large fraction of recent changes in global atmospheric methane growth rate. Nat. Commun.13, 1378 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nisbet, E. G. et al. Rising atmospheric methane: 2007–2014 growth and isotopic shift. Glob. Biogeochem. Cycles30, 1356–1370 (2016). [Google Scholar]
- 7.Yin, Y. et al. Accelerating methane growth rate from 2010 to 2017: leading contributions from the tropics and East Asia. Atmos. Chem. Phys.21, 12631–12647 (2021). [Google Scholar]
- 8.Qu, Z. et al. Inverse modeling of 2010–2022 satellite observations shows that inundation of the wet tropics drove the 2020–2022 methane surge. Proc. Natl Acad. Sci.121, e2402730121 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhandapani, S. & Evers, S. Oil palm ‘slash-and-burn’ practice increases post-fire greenhouse gas emissions and nutrient concentrations in burnt regions of an agricultural tropical peatland. Sci. Total Environ.742, 140648 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Hergoualc’h, K. et al. Spatial and temporal variability of soil N2O and CH4 fluxes along a degradation gradient in a palm swamp peat forest in the Peruvian Amazon. Glob. Change Biol.26, 7198–7216 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swails, E., Frolking, S., Deng, J. & Hergoualc’h, K. Degradation increases peat greenhouse gas emissions in undrained tropical peat swamp forests. Biogeochemistry167, 59–74 (2024). [Google Scholar]
- 12.Darusman, T., Murdiyarso, D., Impron & Anas, I. Effect of rewetting degraded peatlands on carbon fluxes: a meta-analysis. Mitig. Adapt. Strateg. Glob. Change28, 10 (2023). [Google Scholar]
- 13.Novita, N. et al. Strong climate mitigation potential of rewetting oil palm plantations on tropical peatlands. Sci. Total Environ.952, 175829 (2024). [DOI] [PubMed] [Google Scholar]
- 14.Watanabe, A., Purwanto, B. H., Ando, H., Kakuda, K. & Jong, F.-S. Methane and CO2 fluxes from an Indonesian peatland used for sago palm (Metroxylon sagu Rottb.) cultivation: Effects of fertilizer and groundwater level management. Agric. Ecosyst. Environ.134, 14–18 (2009). [Google Scholar]
- 15.Hoyt, A. M., Chaussard, E., Seppalainen, S. S. & Harvey, C. F. Widespread subsidence and carbon emissions across Southeast Asian peatlands. Nat. Geosci.13, 435–440 (2020). [Google Scholar]
- 16.Miettinen, J., Hooijer, A., Vernimmen, R., Liew, S. C. & Page, S. E. From carbon sink to carbon source: extensive peat oxidation in insular Southeast Asia since 1990. Environ. Res. Lett.12, 024014 (2017). [Google Scholar]
- 17.Dadap, N. C. et al. Drainage canals in Southeast Asian Peatlands increase carbon emissions. AGU Adv.2, e2020AV000321 (2021). [Google Scholar]
- 18.Miettinen, J., Shi, C. & Liew, S. C. Land cover distribution in the peatlands of Peninsular Malaysia, Sumatra and Borneo in 2015 with changes since 1990. Glob. Ecol. Conserv.6, 67–78 (2016). [Google Scholar]
- 19.Cooper, H. V. et al. Greenhouse gas emissions resulting from conversion of peat swamp forest to oil palm plantation. Nat. Commun.11, 407 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deshmukh, C. S. et al. Impact of forest plantation on methane emissions from tropical peatland. Glob. Change Biol.26, 2477–2495 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhandapani, S., Ritz, K., Evers, S., Yule, C. M. & Sjögersten, S. Are secondary forests second-rate? Comparing peatland greenhouse gas emissions, chemical and microbial community properties between primary and secondary forests in Peninsular Malaysia. Sci. Total Environ.655, 220–231 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Somers, L. D. et al. Processes Controlling Methane Emissions From a Tropical Peatland Drainage Canal. J. Geophys. Res. Biogeosci.128, e2022JG007194 (2023). [Google Scholar]
- 23.Hoyt, A. M. Carbon Fluxes from Tropical Peatlands: Methane, Carbon Dioxide, and Peatland Subsidence. (Massachusetts Institute of Technology, 2017).
- 24.Peacock, M. et al. Global importance of methane emissions from drainage ditches and canals. Environ. Res. Lett.16, 044010 (2021). [Google Scholar]
- 25.Roulet, N. T. & Moore, T. R. The effect of forestry draining practices on the emission of methane from northern peatlands. Can. J. Res.25, 491–499 (1995). [Google Scholar]
- 26.Manning, F. C., Kho, L. K., Hill, T. C., Cornulier, T. & Teh, Y. A. Carbon emissions from oil palm plantations on peat soil. Front. Glob. Change2, 37 (2019). [Google Scholar]
- 27.Jauhiainen, J. & Silvennoinen, H. Diffusion GHG fluxes at tropical peatland drainage canal water surfaces. Suoseura63, 93–105 (2012). [Google Scholar]
- 28.Kent, M. S. Greenhouse gas emissions from channels draining intact and degraded tropical peat swamp forest. (The Open University, 2019).
- 29.Bowen, J. C., Wahyudio, P. J., Anshari, G. Z., Aluwihare, L. I. & Hoyt, A. M. Canal networks regulate aquatic losses of carbon from degraded tropical peatlands. Nat. Geosci.17, 213–218 (2024). [Google Scholar]
- 30.Barbosa, P. M. et al. High rates of methane oxidation in an Amazon floodplain lake. Biogeochemistry137, 351–365 (2018). [Google Scholar]
- 31.Guérin, F. & Abril, G. Significance of pelagic aerobic methane oxidation in the methane and carbon budget of a tropical reservoir. J. Geophys. Res. Biogeosci.112, 2006JG000393 (2007). [Google Scholar]
- 32.Sawakuchi, H. O. et al. Oxidative mitigation of aquatic methane emissions in large Amazonian rivers. Glob. Change Biol.22, 1075–1085 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Pierangeli, G. M. F. et al. Higher abundance of sediment methanogens and methanotrophs do not predict the atmospheric methane and carbon dioxide flows in eutrophic tropical freshwater reservoirs. Front. Microbiol.12, 647921 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reis, P. C. J., Ruiz-González, C., Crevecoeur, S., Soued, C. & Prairie, Y. T. Rapid shifts in methanotrophic bacterial communities mitigate methane emissions from a tropical hydropower reservoir and its downstream river. Sci. Total Environ.748, 141374 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Zigah, P. K. et al. Methane oxidation pathways and associated methanotrophic communities in the water column of a tropical lake: Lake Kivu methane oxidation pathways. Limnol. Oceanogr.60, 553–572 (2015). [Google Scholar]
- 36.Moore, S. et al. Deep instability of deforested tropical peatlands revealed by fluvial organic carbon fluxes. Nature493, 660–663 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Yupi, H. M., Inoue, T. & Bathgate, J. Concentrations, loads and yields of organic carbon from two tropical peat swamp forest streams in Riau Province, Sumatra, Indonesia. Mires Peat 1–15 (2016) 10.19189/MaP.2015.OMB.181.
- 38.Coleman, D. D., Risatti, J. B. & Schoell, M. Fractionation of carbon and hydrogen isotopes by methane-oxidizing bacteria. Geochim. Cosmochim. Acta45, 1033–1037 (1981). [Google Scholar]
- 39.Whiticar, M. J. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem. Geol.161, 291–314 (1999). [Google Scholar]
- 40.Mahieu, K., Visscher, A. D., Vanrolleghem, P. A. & Cleemput, O. V. Carbon and hydrogen isotope fractionation by microbial methane oxidation: Improved determination. Waste Manag26, 389–398 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Liptay, K., Chanton, J., Czepiel, P. & Mosher, B. Use of stable isotopes to determine methane oxidation in landfill cover soils. J. Geophys. Res. Atmos.103, 8243–8250 (1998). [Google Scholar]
- 42.Bastviken, D., Ejlertsson, J. & Tranvik, L. Measurement of methane oxidation in lakes: a comparison of methods. Environ. Sci. Technol.36, 3354–3361 (2002). [DOI] [PubMed] [Google Scholar]
- 43.Thottathil, S. D., Reis, P. C. J. & Prairie, Y. T. Variability and controls of stable carbon isotopic fractionation during aerobic methane oxidation in temperate lakes. Front. Environ. Sci.10, 833688 (2022). [Google Scholar]
- 44.Zhang, G. B. et al. Pathway of CH4 production, fraction of CH4 oxidized, and 13C isotope fractionation in a straw-incorporated rice field. Biogeosciences10, 3375–3389 (2013). [Google Scholar]
- 45.Gandois, L. et al. From canals to the coast: dissolved organic matter and trace metal composition in rivers draining degraded tropical peatlands in Indonesia. Biogeosciences17, 1897–1909 (2020). [Google Scholar]
- 46.Robison, A. L. et al. Dominance of diffusive methane emissions from lowland headwater streams promotes oxidation and isotopic enrichment. Front. Environ. Sci.9, 791305 (2022). [Google Scholar]
- 47.Taillardat, P. et al. Carbon dioxide and methane dynamics in a peatland headwater stream: origins, processes and implications. J. Geophys. Res. Biogeosci.127, e2022JG006855 (2022). [Google Scholar]
- 48.Holmes, M. E., Chanton, J. P., Tfaily, M. M. & Ogram, A. CO2 and CH4 isotope compositions and production pathways in a tropical peatland. Glob. Biogeochem. Cycles29, 1–18 (2015). [Google Scholar]
- 49.Schenk, J. et al. Methane in lakes: variability in stable carbon isotopic composition and the potential importance of groundwater input. Front. Earth Sci.9, 722215 (2021). [Google Scholar]
- 50.Reis, P. C. J. et al. Enigmatic persistence of aerobic methanotrophs in oxygen-limiting freshwater habitats. ISME J.18, wrae041 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Girkin, N. T., Vane, C. H., Turner, B. L., Ostle, N. J. & Sjögersten, S. Root oxygen mitigates methane fluxes in tropical peatlands. Environ. Res. Lett.15, 064013 (2020). [Google Scholar]
- 52.Määttä, T. & Malhotra, A. The hidden roots of wetland methane emissions. Glob. Change Biol.30, e17127 (2024). [DOI] [PubMed] [Google Scholar]
- 53.Heilman, M. A. & Carlton, R. G. Methane oxidation associated with submersed vascular macrophytes and its impact on plant diffusive methane flux. Biogeochemistry52, 207–224 (2001). [Google Scholar]
- 54.Akhtar, H. et al. Significant sedge-mediated methane emissions from degraded tropical peatlands. Environ. Res. Lett. (2021) 10.1088/1748-9326/abc7dc.
- 55.Chanton, J. P. The effect of gas transport on the isotope signature of methane in wetlands. Org. Geochem.36, 753–768 (2005). [Google Scholar]
- 56.Buessecker, S. et al. Microbial communities and interactions of nitrogen oxides with methanogenesis in diverse peatlands of the Amazon basin. Front. Microbiol.12, 659079 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bandla, A., Akhtar, H., Lupascu, M., Sukri, R. S. & Swarup, S. Elevated methane flux in a tropical peatland post-fire is linked to depth-dependent changes in peat microbiome assembly. Npj Biofilms Microbiomes10, 8 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vermaat, J. E. et al. Greenhouse gas fluxes from Dutch peatland water bodies: importance of the surrounding landscape. Wetlands31, 493–498 (2011). [Google Scholar]
- 59.Sawakuchi, H. O. et al. Methane emissions from Amazonian Rivers and their contribution to the global methane budget. Glob. Change Biol.20, 2829–2840 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Hirano, T., Kusin, K., Limin, S. & Osaki, M. Evapotranspiration of tropical peat swamp forests. Glob. Change Biol.21, 1914–1927 (2015). [DOI] [PubMed] [Google Scholar]
- 61.Liu, M. et al. Effects of rainfall on thermal stratification and dissolved oxygen in a deep drinking water reservoir. Hydrol. Process.34, 3387–3399 (2020). [Google Scholar]
- 62.Waldron, S. et al. C mobilisation in disturbed tropical peat swamps: old DOC can fuel the fluvial efflux of old carbon dioxide, but site recovery can occur. Sci. Rep.9, 11429 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thornton, S. A., Dudin,., Page, S. E., Upton, C. & Harrison, M. E. Peatland fish of Sebangau, Borneo: diversity, monitoring and conservation. Mires Peat. 1–25 (2018) 10.19189/MaP.2017.OMB.313.
- 64.Swails, E. et al. Soil nitrous oxide and methane fluxes from a land-use change transition of primary forest to oil palm in an Indonesian peatland. Biogeochemistry167, 363–381 (2023). [Google Scholar]
- 65.Hiraishi, T. et al. 2013 Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories: Wetlands: Methodological Guidance on Lands with Wet and Drained Soils, and Constructed Wetlands for Wastewater Treatment. (IPCC, Intergovernmental Panel on Climate Change, Hayama, Japan, 2014).
- 66.Jackson, R. B. et al. Human activities now fuel two-thirds of global methane emissions. Environ. Res. Lett.19, 101002 (2024). [Google Scholar]
- 67.Jovani‐Sancho, A. J. et al. CH4 and N2O emissions from smallholder agricultural systems on tropical peatlands in Southeast Asia. Glob. Change Biol.29, 4279–4297 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brownlow, R. et al. Isotopic ratios of tropical methane emissions by atmospheric measurement. Glob. Biogeochem. Cycles31, 1408–1419 (2017). [Google Scholar]
- 69.Feinberg, A. I., Coulon, A., Stenke, A., Schwietzke, S. & Peter, T. Isotopic source signatures: Impact of regional variability on the δ 13 CH 4 trend and spatial distribution. Atmos. Environ.174, 99–111 (2018). [Google Scholar]
- 70.Oh, Y. et al. Improved global wetland carbon isotopic signatures support post-2006 microbial methane emission increase. Commun. Earth Environ.3, 159 (2022). [Google Scholar]
- 71.Aldrian, E. & Dwi Susanto, R. Identification of three dominant rainfall regions within Indonesia and their relationship to sea surface temperature. Int. J. Climatol.23, 1435–1452 (2003). [Google Scholar]
- 72.Cawley, K., Goodman, K., Weintraub, S. & Parker, S. Neon user guide to dissolved gases in surface water (DP1.20097.001). neonDissGas package. (2020).
- 73.Keeling, C. D. The concentration and isotopic abundances of atmospheric carbon dioxide in rural areas. Geochim. Cosmochim. Acta13, 322–334 (1958). [Google Scholar]
- 74.Pataki, D. E. et al. The application and interpretation of Keeling plots in terrestrial carbon cycle research. Glob. Biogeochem. Cycles17, 2001GB001850 (2003). [Google Scholar]
- 75.Happell, J. D., Chanton, J. & Showers, W. S. The influence of methane oxidation on the stable isotopic composition of methane emitted from Florida swamp forests. Geochim. Cosmochim. Acta58, 4377–4388 (1994). [Google Scholar]
- 76.Wickham, H., Francois, R., Henry, L. & Muller, K. dplyr: a grammar of data manipulation. (2021).
- 77.Wickham, H. Ggplot2: Elegant Graphics for Data Analysis. (Springer-Verlag, New York, 2016).
- 78.Pedersen, T. L. Patchwork: the composer of plots. (2020).
- 79.Thottathil, S. D., Reis, P. C. J. & Prairie, Y. T. Methane oxidation kinetics in northern freshwater lakes. Biogeochemistry143, 105–116 (2019). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data are presented in the manuscript and/or the Supplementary Information. The data used in this study are available at the Zenodo repository under ‘Tropical Peatland Drainage Canal Methane Concentrations, Fluxes, and Isotopic Composition’ (10.5281/zenodo.11155160). Source data are provided with this paper.