Abstract
Background: As fat grafting is commonly used as a filler, Adipose-derived stem/stromal cells (ASC) have been reported to be key player in retention rate. Paracrine and differentiation potential of those cells confer them strong pro-angiogenic capacities. However, a full characterization of the influence of aging on ASC has not been reported yet. Here we’ve investigated the effect of age on paracrine function, stemness and angiogenic potential of ASC. Methods: ASC were extracted from young and old adult donors. We assessed stromal vascular fraction cell populations repartition, ASC stemness potential, capability to differentiate into mesenchymal lineages as well as their secretome. Angiogenic potential was assessed using a sprouting assay, an indirect co-culture of ASC and dermal microvascular endothelial cells (EC). Total vascular sprout length was measured, and co-culture soluble factors were quantified. Pro-angiogenic factors alone or in combination as well as ASC-conditioned medium (CM) were added to EC to assess sprouting induction. Results: Decrease of endothelial cells yield and percentage is observed in cells extracted from adipose tissue of older patients, whereas ASC percentage increased with age. Clonogenic potential of ASC is stable with age. ASC can differentiate into adipocytes, chondrocytes and osteoblasts, and aging does not alter this potential. Among the 25 analytes quantified, high levels of pro-angiogenic factors were found, but none is significantly modulated with age. ASC induce a significantly longer vascular sprouts compared to fibroblasts, and no difference was found between young and old ASC donors on that parameter. Higher concentrations of FGF-2, G-CSF, HGF and IL-8, and lower concentrations of VEGF-C were quantified in EC/ASC co-cultures compared to EC/fibroblasts co-cultures. EC/ASC from young donors secrete higher levels of VEGF-A compared to old ones. Neither soluble factor nor CM without cells are able to induce organized sprouts, highlighting the requirement of cell communication for sprouting. CM produced by ASC supporting development of long vascular sprouts promote sprouting in co-cultures that establish shorter sprouts. Conclusion: Our results show cells from young and old donors exhibit no difference in all assessed parameters, suggesting all patients could be included in clinical applications. We emphasized the leading role of ASC in angiogenesis, without impairment with age, where secretome is a key but not sufficient actor.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-73875-x.
Keywords: Adipose-derived stem/stromal cells, Angiogenesis, Aging, Paracrine, Fat graft
Subject terms: Mesenchymal stem cells, Cell biology, Ageing
Introduction
Autologous fat grafting is widely used in surgery for both regenerative and aesthetic applications1. This procedure has been reported to improve skin quality and facial aging2–4 even if some authors stated the limited effect on healthy skin5,6. However, the variable and unpredictable resorption rate remains the major limitation in therapeutic use of fat grafting7,8. Many factors are described to alter fat graft survival, such as surgeon gesture, fat process or adipose tissue biology9,10. Donor age could influence graft retention, as graft survival rate is reported to be higher in patients below 55 yo (27,8%) compared to patients above 55 yo (15,4%)8. Moreover, vascularization is an important parameter, as Peer demonstrate the anastomosis between graft and recipient site11,12. Furthermore, Allen et al. showed that high density fraction of fat, containing a higher number of vascular progenitors and vascular endothelial growth factor (VEGF), has a better survival rate than low density fraction of fat13. In order to improve fat graft survival, Yoshimura’s team developed a new method called cell-assisted lipotransfer, which consist of graft enrichment with either stromal vascular fraction (SVF) or Adipose-derived Stem Cells (ASC)14.
ASC are mesenchymal stem/stromal cells highly studied since their identification in 2001 and are often described as key player in adipose regeneration potential15 and fat graft survival14,16. Due to their paracrine function, immunomodulatory potential and pro-angiogenic capacities17,18, those cells have been extensively used in regenerative medicine19,20. Those cells have been reported to play a critical role in regulating skin regeneration and facial rejuvenation21–23. ASC promote angiogenesis by their capacity to differentiate into endothelial cells24, increase vascular network density25 and secrete many pro-angiogenic factor such as VEGF or HGF26,27. ASC also bear hallmarks of pericytes, and provide vascular stability through functional interaction with endothelial cells28. The pro-angiogenic effects of ASC are mostly described to be mediated by their paracrine function. ASC secretome has been widely studied and cells are known to secrete pro-angiogenic factors such as VEGF, HGF, Angiopoietin-1, Angiogenin or PlGF29–31.
Aging is a progressive decrease in the regenerative abilities to maintain tissue homeostasis, such as vascularization or stem cell potential. Although researchers have studied effect of age on ASC functionality, results are still controversial. Aging has been described to have no or negative effect on cell proliferation32–34. ASC from old donors seem to be more senescent compared to young ones, with a decreased capacity to form colonies34, a shortening of telomeres35 or increase of β-galactosidase activity36. Pro-angiogenic potential of ASC seems impaired with aging, as ASC have a decreased potential to differentiate into endothelial cells37, to promote vascular network38 and to secrete angiogenic factors37,39. Intriguingly, the effect of age on a wider panel of soluble factors secreted by ASC has not been investigated yet.
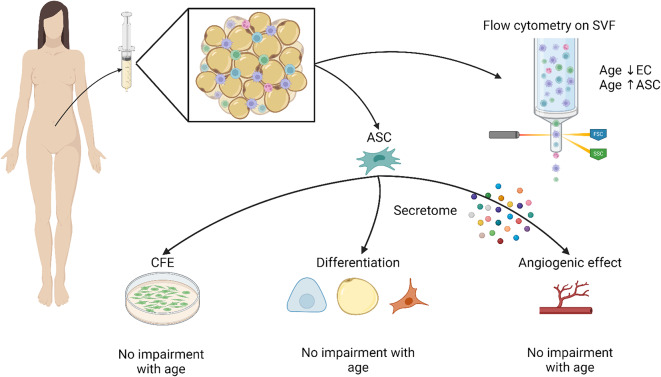
As ASC are often used in older patients, the effect of age on their functionality is a hot topic in clinical use. Indeed, identification of parameters modifying ASC capacities could be linked to a better choice of donors in clinical studies. We hypothesized that age alter ASC paracrine function and their capacity to promote angiogenesis, leading to a decrease in fat graft survival of old donors. In this study we investigate how aging can affect ASC functionality, especially their capacity to mediate angiogenic processes (Fig. 1).
Fig. 1.
Stromal vascular fraction was analyzed with flow cytometry and then ASC were cultured in order to assess their clonogenic, chondrogenic, osteogenic, adipogenic and pro-angiogenic potentials as well as their secretome. (Created with BioRender)
Methods
Tissue harvest
Human subcutaneous adipose tissues were obtained from liposuction procedures under general anesthesia from anonymous healthy donors. Donors were not recruited to fit this study but were selected to have no obesity history nor metabolic diseases. Surgical residues were anonymized, and written informed consent was obtained from the patients in accordance with the ethical guidelines from Lyon University Hospital (Hospices Civils de Lyon) and approved by ethical committee of the Hospices Civils de Lyon according to the principles of the Declaration of Helsinki and Article L. 1243-4 of the French Public Health Code. All the samples used in this study belong to a collection of human skin samples declared to the French research ministry (Declaration no. DC-2008-162 delivered to the Bank of Tissues and Cells of the Hospices Civils de Lyon). Tissues were obtained from 12 female patients, classified as young (< 30 years old, 6 donors, mean 24,0 ± 4,1 years) or old (> 50 years old, 6 donors, mean 59,8 ± 8,3 years). Average BMI was 22,8 ± 2,6 kg/m² and did not differ significantly between the young (23,2 ± 1,6 kg/m2) and old groups (22,5 ± 3,5 kg/m2).
ASC isolation
To isolate the stromal-vascular fraction (SVF), adipose tissue was centrifuged (1452 g for 3 min), then the oil (upper phase) and tumescent phase (lower phase) were removed. Adipose tissue was then digested with collagenase (1 mg/ml in PBS, Roche, Indianapolis, USA) at a collagenase/adipose tissue ratio 1:1 (vol/vol) at 37 °C for 45 min under constant shaking. Collagenase activity was neutralized with an equal volume of Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Invitrogen, Carlsbad, USA) containing 10% fetal bovine serum (FBS) (HyClone, Logan, USA) and the suspension was centrifuged at 232 g for 5 min. The stromal vascular fraction (SVF) was filtered through a 70 μm-strainer then red blood cells were removed by dilution (1/10) in ACK lysis buffer (Gibco, Invitrogen, Carlsbad, USA) and incubation for 15 min at 37 °C. At the end, same volume of PBS was added and the suspension of stromal vascular fraction was centrifuged at 232 g for 5 min.
Flow cytometry analysis
Freshly isolated cells from the SVF were maintained on ice and stained for analytical flow cytometry. Cell suspensions (100 000 cells per condition) were centrifuged, and the cell pellet was incubated with FC-Block (Miltenyi Biotec, Bergisch Gladbach, Germany) to minimize non-specific antibody binding. Cells were simultaneously stained with monoclonal mouse anti-human antibodies: CD31-BB515, CD34-PE-Cy7, CD163-AF647, CD45-PerCP-Cy5.5, CD90-BV510, CD146-BV421 (BD Biosciences, San Jose, USA). In addition, cells were stained with live/dead fixable dye FVS780 (BD Biosciences, San Jose, USA) to identify live cells. Spectral overlap compensation was performed using single-stained CompBead PLUS (BD Biosciences, San Jose, USA). Multicolor flow cytometry was performed with an Aria II (BD Biosciences, San Jose, USA) and cell composition percentages were calculated according to data of surface marker expression profiles with FlowJo Software version 10.7.2 (BD Biosciences, San Jose, USA) (Table 1). Gates were defined with fluorescence minus one.
Table 1.
Cell population definition according to surface marker.
Monolayer cell culture
ASC
SVF was resuspended in ASC complete culture medium consisting of DMEM/Ham’s F-12 (ratio 1:1) supplemented with 10% FBS, 1% penicillin/gentamycin solution (Gibco, Invitrogen, Carlsbad, USA), 1 µg/ml amphotericin B (Gibco, Invitrogen, Carlsbad, USA) and 10 ng/ml FGF-2. Cells were either used for flow cytometry analysis or plated for cell culture. As demonstrated previously by our team, in our experimental condition, subculture ASC at passage 0 are positive for CD90 and CD73, and negative for CD14, CD45 and HLA-DR49, reaching definition of ASC by ISCT and IFATS50. Cultured cells were then considered as ASC and this initial culture was referred to as passage 0 (P0). Medium was replaced every 2–3 days with fresh complete medium until reaching 80–90% confluence.
Endothelial cells
Human Dermal Endothelial Cells (EC) from juvenile donor (3yo) were purchased from Promocell and cultivated in EGM-2 (PromoCell, Heidelberg, Germany). Cells were cultured to passage 4 and medium was changed every three days until confluence was reached. At confluency, cells were trypsinized with trypsin-EDTA 0,01% (Gibco, Invitrogen, Carlsbad, USA) and used for sprouting assay.
Fibroblasts
Human dermal fibroblasts were extracted from mammary skin from an adult caucasian women (23 y/o), after plastic surgery. Informed consent was obtained before tissue collection, according to European guidelines. After removing the subcutaneous tissue, skin samples were sliced according to depth using a dermatome. The upper part of the skin dermis (from the surface to a 0,3 mm depth) contained the epidermis and the papillary dermis. The epidermis was separated from papillary dermis by dissection, after treatment with 0.25% trypsin for 1.5 h at 37 °C (Gibco, Invitrogen, Carlsbad, USA). For the preparation of fibroblasts, pieces of superficial dermis were placed in culture in MEM supplemented with 10% FBS, penicillin-streptomycin (Gibco, Invitrogen, Carlsbad, USA), and glutamine (2 mM) (Gibco, Invitrogen, Carlsbad, USA), and maintained at 37 °C in a 90% humidified atmosphere containing 5% CO2. After migration of fibroblasts outward dermal explants, these cells were amplified in culture and stored in liquid nitrogen as frozen aliquots until use.
Cell numeration and viability
At each step (extraction, trypsinization, thawing) and for each cell type, viable and nonviable cells were counted in a Malassez chamber (hemocytometer) using a light microscope. The number of viable cells was recorded using a dye exclusion test with 4% trypan blue solution (Thermo Fischer Scientific, USA).
CFE (colony forming efficiency) to assess clonogenic potential of the cells
P0 ASC were seeded in triplicate in a 6-wells plate at a density of 200 cells/well in complete medium. Colonies were grown for 10 to 14 days, depending on the growth rate of the cells. Cells were rinsed with PBS then fixed with absolute ethanol for 10 min at room temperature before staining with 20% GIEMSA (Sigma Aldrich, Saint Quentin Fallavier, France). Cell colonies were counted under optical microscopy. All three wells were counted for each donor. The final colony forming efficiency (CFE) value was determined by dividing the number of colonies counted ×100 by the number of cells seeded. Results are expressed as mean for the 3 replicates.
Adipogenic, osteogenic and chondrogenic assay
ASC at passage 4 were seeded in a 48-wells plate at a density of 15 000 cells/well (for adipogenic and osteogenic assay) or 40 000 cells/well (for chondrogenic assay) and grown in complete medium. Once cells reached confluence (around 1 week), positive control wells were switched to adipogenic, osteogenic or chondrogenic Stempro kit, following the manufacturer’s instructions (Gibco, Invitrogen, Carlsbad, USA) and grown for 14 days (adipogenic) or 21 days (osteogenic and chondrogenic) with media change every 2–3 days. Cells were then stained with Bodipy, Alizarin red, alkaline phosphatase or Alcian blue according to manufacturer protocol. Briefly, after 14 or 21 days of culture, cells were rinsed with PBS then fixed with 4% methanol-free paraformaldehyde for 20 min (RT). Cells were rinsed twice with PBS then stained with specific probe for each lineage.
Adipocyte-differentiated cells were stained with Bodipy (20 µg/ml) (Invitrogen, Carlsbad, USA) /Hoechst (1/2000) (Invitrogen, Carlsbad, USA) solution for 45 min then rinsed with PBS and imaged with epifluorescence microscope to visualize neutral lipids. Mean intensity of Bodipy per cell was quantified with High Content Screening Array Scan VTI (Cellomics, Thermo Scientific, Waltham, USA). Osteoblast- differentiated cells were stained with either BCIP/NBT (Sigma Aldrich, Saint Quentin Fallavier, France) or Alizarin Red S (ScienCell, San Diego, USA) solution (2%, w/w) for 10 and 20 min respectively prior to visualization of alkaline phosphatase (early differentiation) or mineralization (late differentiation) respectively. Alizarin Red S staining was eluted using acetic acid (10%) and calcium deposition was quantified with optical density determined at 405 nm using a plate reader (SpectraMax M5, Molecular Devices, Sunnyvale, USA). Chondrocyte-differentiated cells were stained with Alcian blue solution (1% w/w) (ScienCell, San Diego, USA) for 45 min prior to visualization of proteoglycans.
ASC secretome P0
P0 ASC were seeded in a 6-wells plate at a density of 300 000 cells/well in complete medium. Once cells reach confluence (around 1 week), complete medium (DMEM/Ham’s F-12 supplemented with 10% FBS, 1% penicillin/gentamycin solution, 1 µg/ml amphotericin B and 10 ng/ml FGF-2), was replaced by serum-free medium without FGF-2. After 24 H, supernatant samples were harvested and stored at -80 °C and cells were counted as described previously. Quantitative Luminex® (Merck Millipore, Darmstadt, Germany; R&D Systems, Minneapolis, USA) assays were performed using 25 cytokines: Adiponectin, Angiogenin, Angiopoietin-1 (Ang-1), Epithelial Growth Factor (EGF), acid Fibroblast Growth Factor (FGF-1), basic FGF (FGF-2), Heparin-binding EGF (HB-EGF), Hepatocyte Growth Factor (HGF), Interleukin (IL)-6, -8, Leptin, Matrix Metalloproteinase (MMP)-1, -2, -3, -8, -13, Platelet Derived Growth Factor (PDGF)-AA, -AB, -BB, -CC, -DD, Placental Growth Factor (PlGF), Stem Cell Factor (SCF), Vascular Endothelial Growth Factor (VEGF)-A, -C. Supernatants were centrifuged at 300 g for 5 min at + 4 °C to remove cell debris then Luminex® assays were performed following manufacturer’s instructions. Serum-free medium was used as blank and reading was done using Bio-Plex 200 system (Bio-Rad, Hercules, USA). Protein levels were expressed in pg/ml for 106 cells.
Angiogenesis assay
Conditioned media production and quantification
P4 ASC were seeded in a 75 cm² flask at a density of 475 000 cells/flask in EGM-2 (PromoCell, Heidelberg, Germany). Once cells reached confluence (around 1 week), EGM-2 was replaced by serum and growth factors-free EGM-2 (EBM). After 72 H, supernatants samples were harvested and stored at -20 °C. A quantitative Luminex® (R&D Systems, Minneapolis, USA) assay was performed using cytokines: Angiopoietin-1, Angiopoietin-2, bone morphogenic protein 9 (BMP-9), EGF, Endoglin, FGF-1, FGF-2, granulocyte colony stimulating factor (G-CSF), HB-EGF, HGF, IL-1α, IL-8, MMP-2, PDGF-AA, PDGF-BB, PlGF, VEGF-A, VEGF-C. Luminex® assay was performed following manufacturer’s instructions. EBM was used as blank and reading was done using Bio-Plex 200 system (Bio-Rad, Hercules, USA). Protein levels were expressed in pg/ml.
Sprouting assay
ASC and fibroblasts were cultured in endothelial growth medium at least 24 h prior to starting the sprouting assay. The procedure is based on a sprouting assay previously described51, the experimental design can be found in Supplementary Fig. 3. Briefly, dextran-coated Cytodex-3© microcarrier beads (Amersham Pharmacia Biotech, Piscataway, USA) were incubated with EC in EGM-2 (PromoCell, Heidelberg, Germany) overnight. Cell-coated beads were then embedded into fibrin gels consisting of EBM with 2,5 mg/ml fibrinogen (Sigma Aldrich, Saint Quentin Fallavier, France) and 0,625 U/ml thrombin (Sigma Aldrich, Saint Quentin Fallavier, France). 200 µl of fibrin gel containing cell-coated beads were added per well into a 48-well plate. After gelification, ASC or fibroblasts were added on the top of the gels (10 000 cells per well) in EGM-2 medium. Negative control consists of adding medium without any cell on the top of the gel. 24 h after seeding stimulating cells, EGM-2 medium was replaced by EBM. Medium was harvested every 2–3 days and replaced with fresh medium (EBM) for a week. For each cell donor, 4 wells were seeded and experiment was conducted 4 times. Co-culture of EC and ASC from young donors are mentioned as EC/ASCy, co-culture of EC and ASC from old donors are mentioned as EC/ASCo and co-culture of EC and fibroblasts are mentioned as EC/Fb.
For growth factors or conditioned media (CM) assessment, no cell was added on the top of the gel. CM or EBM containing Angiopoietin-2 (1; 5; 10ng/ml) (Preprotech, Rocky Hill, USA), HGF (0,2; 0,5; 1ng/ml) (Preprotech, Rocky Hill, USA), VEGF-A (0,02; 0,1; 0,5ng/ml) (Preprotech, Rocky Hill, USA) or a combination of those 3 growth factors were added on top of the gel and replaced every 2–3 days for a week. Experiment was conducted once for pro-angiogenic factor and twice for CM assays. Within an experiment, 4 wells per condition were performed.
At the end of the assay, fibrin gels were washed twice then fixed 1 h using 4% paraformaldehyde. Gels were washed twice and then permeabilized with PBS containing 0,5% Triton X-100 and 3% BSA for 45 min (RT). Subsequently, gels were stained with Alexa Fluor-488-conjugated phalloidin (dilution 1/40) and Hoechst 33342 (dilution 1/2500) for 1 h in dark. Gels were washed twice and then imaged using Leica SP8 confocal microscope (405/488nm diode lasers, 10x/0.30 dry HC PL Fluotar objective). The Alexa Fluor-488-conjugated phalloidin was acquired on the first detector with an (494–676 nm) emission filter and Hoechst 33342 was acquired on the second detector with an (410–483 nm) emission filter. A total of 10–20 beads were acquired per well. For each bead, Z-stack including all sprouts with z-step of 20 μm was acquired. Image analysis was performed using the Sprout Morphology plugin previously described by Eglinger et al.52 using Fiji software version 1.53c; Java 1.8.0_172. Total sprouts length of each condition was assessed.
Soluble factors quantification
Media from the angiogenesis assay co-cultures were harvested and frozen at -20 °C every 2 days until end of the assay. A quantitative Luminex® (Merck Millipore, Darmstadt, Germany) assay was performed using 16 cytokines: Angiopoietin-2, bone morphogenic protein 9 (BMP-9), EGF, Endoglin, Endothelin-1, FGF-1, FGF-2, granulocyte colony stimulating factor (G-CSF), HB-EGF, HGF, IL-8, Leptin, PlGF, VEGF-A, VEGF-C, VEGF-D. Supernatant was centrifuged at 300 g for 5 min at + 4 °C to remove cell debris then Luminex® assay was performed following manufacturer’s instructions. EBM was used as blank and reading was done using Bio-Plex 200 system (Bio-Rad, Hercules, USA) and protein levels were expressed in pg/ml.
Statistical analysis
Data analysis was performed using R software (version 4.2.2). Comparisons between two groups were conducted using Student’s t-test for normally distributed data and the Wilcoxon test for non-normally distributed data. Comparisons between multiple groups were performed using analysis of variance (ANOVA) followed by Benjamini-Hochberg correction for multiple comparisons.
Results were considered statistically significant when the p-value was less than 0,05. A statistical trend was considered to be present when the p-value was between 0,05 and 0,1. Statistical tests are specified for each figure.
Results
Age does not affect SVF cell yield
Total nucleated cells extracted from adipose tissues are not statistically different from young (mean 107 130 ± 27 903 cells/ml) and old donors (mean 86 693 ± 42 668 cells/ml) (Supplementary Fig. 2).
Endothelial cells population decreases with age but no other SVF cells population
Yield of each immune as resident/M2 macrophages (CD45+ / CD163+) or other immune cells (CD45+ / CD163-) and non-immune cell type, i.e. ASC (CD45- / CD34+ / CD31-), endothelial cells (CD45- / CD34+ / CD31+) and pericytes (CD45- / CD34- / CD31- / CD146+) from young and old donors were compared (gating strategy is described in Supplementary Fig. 3). ASC were CD146- and mainly expressed CD90 (> 90%) (data not shown). Endothelial cells were found to express CD146 and differentially expressed CD90. Pericytes populations were composed of CD90 + and CD90- populations (data not shown).
The amount of each population among either CD45 + or CD45- population is expressed in percentage. Yield of each cell population, expressed in cell/ml, gives an overview of the expression of each cell population among the whole adipose tissue (Table 2). M2/resident macrophages, other immune cells, ASC and pericytes yields show no statistical difference between age categories. Endothelial cells yield significantly decreases with age (young 8 847 ± 4 375 c/ml; old 2 782 ± 2 374 c/ml; p value 0,014). While ASC yield is stable with age, percentage of ASC within CD45- population increases significantly with age (young 52 ± 18%; old 77 ± 13%; p value 0,009). Consistently with yield, there is a statistical trend (0,05 < p value < 0,1) for a decrease of endothelial cells proportion with age (young 23 ± 16%; old 7 ± 5%; p value 0,054).
Table 2.
Quantification of cell populations in the SVF and their repartition among CD45- and CD45 + populations. n = 6 donors were used in each age category. * p value < 0,05 indicated in bold. Unpaired Wilcoxon test was used to test the significance of ASC and pericytes populations. Unpaired student t-test was used to test the significance of other cell populations.
| Cell type | Phenotype | Age category | Mean | SD | p value | |
|---|---|---|---|---|---|---|
| Immune cells | CD45+ | cell/ml | Young | 63 550 | 23 890 | 0,262 |
| Old | 47 120 | 23 939 | ||||
| % (of all cells) | Young | 58,1% | 14% | 0,648 | ||
| Old | 54,5% | 13% | ||||
| Resident & M2 macrophages | CD45 + CD163+ | cell/ml | Young | 11 725 | 6 502 | 0,677 |
| Old | 10 099 | 6 614 | ||||
| % (of CD45+) | Young | 19,8% | 10% | 0,643 | ||
| Old | 22,2% | 8% | ||||
| Other immune cells | CD45 + CD163- | cell/ml | Young | 50 790 | 21 744 | 0,235 |
| Old | 35 922 | 18 950 | ||||
| % (of CD45+) | Young | 78,4% | 10% | 0,613 | ||
| Old | 75,6% | 8% | ||||
| Non-immune cells | CD45- | cell/ml | Young | 43 165 | 16 393 | 0,714 |
| Old | 38 827 | 22 869 | ||||
| % (of CD45-) | Young | 41,5% | 14% | 0,704 | ||
| Old | 44,5% | 13% | ||||
| ASC |
CD45- CD31- CD34+ |
cell/ml | Young | 22 815 | 10 455 | 0,818 |
| Old | 30 001 | 18 495 | ||||
| % (of CD45-) | Young | 52,5% | 18% | 0,009* | ||
| Old | 77,5% | 13% | ||||
| Endothelial cells |
CD45- CD31 + CD34+ |
cell/ml | Young | 8 847 | 4 375 | 0,014* |
| Old | 2 782 | 2 374 | ||||
| % (of CD45-) | Young | 23,5% | 16% | 0,054 | ||
| Old | 7,2% | 5% | ||||
| Pericytes |
CD45- CD31- CD34- CD146+ |
cell/ml | Young | 4 439 | 2 242 | 0,240 |
| Old | 2 763 | 3 481 | ||||
| % (of CD45-) | Young | 9,9% | 4% | 0,132 | ||
| Old | 7,6% | 13% |
Age does not affect the clonogenic potential of ASC
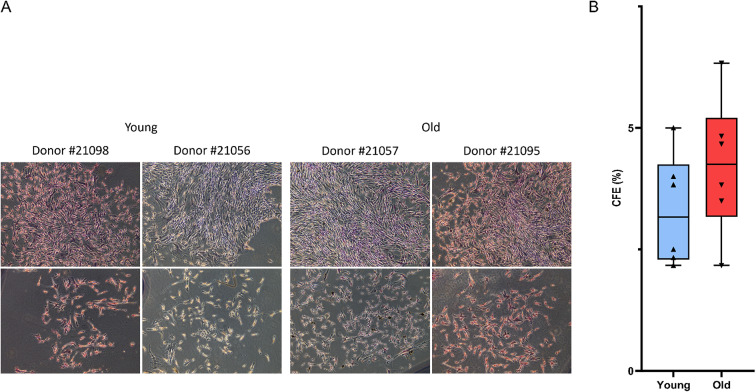
ASC adhered to plastic and display a phenotype consistent with fibroblast-like morphology. Diverse types of colonies are observed independently of the donor and age category (Fig. 2A). ASC CFE mean from young donors was 3,3 ± 1,1%. No significant difference was found when compared to ASC CFE mean from old donors (4,2 ± 1,4%) (Fig. 2B). Furthermore, we did not see colony size differences in from a donor to another (data not shown).
Fig. 2.
Colony forming efficiency of ASC from young and old donors. (A) photography of CFE from young and old donors, 2 pictures of the same well are presented for each donor. Original magnification 10x. (B) quantification of colonies formed by ASC after 15 days of culture. n = 6 donors were used in each age category. No significant difference was found between CFE of ASC from young and old donors. Unpaired Student t-test was used to test the significance.
Age does not affect adipogenic, osteogenic and chondrogenic potential of ASC
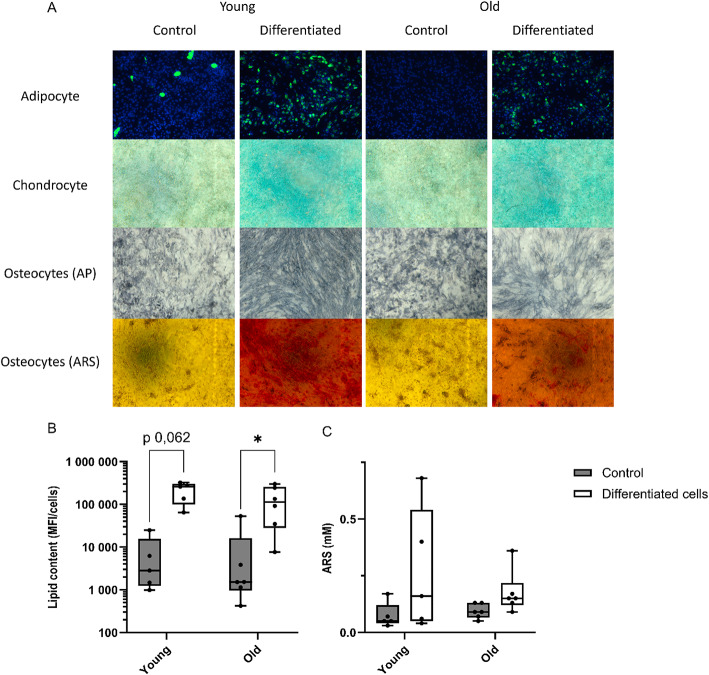
The differentiation potential of ASC was evaluated by culturing them in adipogenic, osteogenic, and chondrogenic media to stimulate multilineage differentiation. All donors of ASC show specific staining for lipid droplets, calcification and proteoglycan, highlighting their ability to differentiate into adipocytes, osteoblasts and chondrocytes respectively (Fig. 3A). Quantification of neutral lipids with Bodipy probe shows a statistical trend increase of fluorescence in differentiated cells relative to untreated controls for young (differentiated cells 212 237 ± 107 590 UA; control group 7 266 ± 10 014 UA, p value 0,062) and aged ASC (differentiated cells 135 700 ± 116 162 UA; control group 10 244 ± 20 985 UA, p value 0,031). No difference was found between young and aged ASC in untreated or differentiated cells (Fig. 3B). Alizarin red S staining quantification shows no change on mineralization quantification between differentiated and untreated cells for old donors (differentiated cells 0,17 ± 0,09 mM; control group 0,09 ± 0,03 mM) and young ones (differentiated cells 0,27 ± 0,27 mM; control group 0,07 ± 0,06 mM). There is no statistical difference between age categories either in untreated or in differentiated cells (Fig. 3C).
Fig. 3.
ASC differentiation into lineages. (A) Microscopic images of ASC at P4 in proliferation medium (control) or differentiation medium (differentiated) for 3 lineages. Adipocytes were stained with Bodipy probe and Hoechst to visualize neutral lipid droplets and nucleus, chondrocytes were stained with Alcian blue to visualize proteoglycans and osteoblasts were stained with either BCIP/NBT or Alizarin Red S to visualize alkaline phosphatase (early differentiation) or mineralization (late differentiation) respectively (original magnification 10x). Bodipy (B) or ARS (C) quantification of control or differentiated ASC according to age category. n = 6 donors were used in each age category. Paired Wilcoxon test was used to test the significance. * P value < 0,05.
Age does not affect ASC Secretome
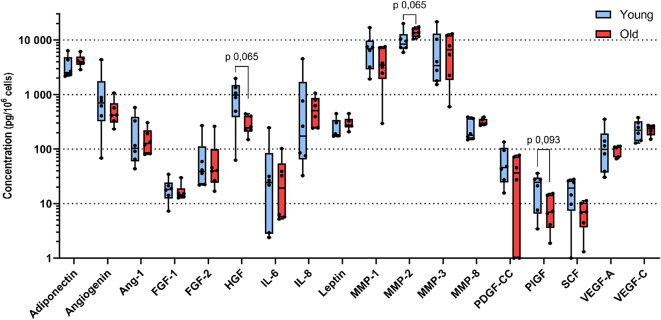
ASC secrete numerous analytes involved in immunomodulation, angiogenesis and proliferation. We selected a set of 25 analytes described or not yet to be synthetized by ASC. Seven analytes were synthetized below the detection threshold by ASC at P0 in our conditions. Six analytes were found to be secreted at concentrations below 100 pg/ml and 12 analytes were found to be secreted at concentrations above 100 pg/ml (Table 3). As donors do not have the same proliferation rate, concentrations were normalized to cell number. There is a statistical trend for a decrease of HGF, PlGF and SCF with age (p value < 0,10), however no statistical difference has been found. Despite normalization, there is a high donor-to-donor variability for some analytes concentrations. Median concentrations of HGF, PlGF and SCF from young donors (975 pg/106 cells; 24 pg/106 cells; 19 pg/106 cells, respectively) are at least 2fold higher compared to old ones (244 pg/106 cells, 7 pg/106 cells; 7 pg/106 cells, respectively). Median concentration of IL-8 in young donors (174 pg/ml) is 2,9 times lower compared to old donors (508 pg/ml). However, no significant difference on analyte concentration between age categories has been found (Fig. 4).
Table 3.
Quantification range of analytes assessed into ASC secretome.
| [Analytes] < threshold | [Analytes] < 100 pg/ml | [Analytes] > 100 pg/ml |
|---|---|---|
| EGF | FGF-1 | Adiponectin |
| HB-EGF | FGF-2 | Angiogenin |
| MMP-13 | IL-6 | Angiopoietin-1 |
| PDGF-AA | PDGF-CC | HGF |
| PDGF-AB/BB | PlGF | IL-8 |
| PDGF-BB | SCF | Leptin |
| PDGF-DD | MMP-1 | |
| MMP-2 | ||
| MMP-3 | ||
| MMP-8 | ||
| VEGF-A | ||
| VEGF-C |
Fig. 4.
Secretome of ASC according to age category. n = 6 donors were used in each age category. Unpaired Wilcoxon test was used to test the significance. * P value < 0,05.
Age does not affect sprout length induced by ASC
ASC are described to be involved in angiogenesis due to their ability to secrete pro-angiogenic factors and their capacity to differentiate into endothelial cells. Sprouting assay has been described to assess anti-angiogenic components. This anti-vascularization in vitro assay has been described with human umbilical vein endothelial cells (HUVEC) in co-culture with skin fibroblasts. In our assay, we used dermal microvascular EC in co-culture with dermal fibroblast (EC/Fb) or ASC (EC/ASC), to be closer as possible to fat graft physiology, and to assess pro-angiogenic effects. In our conditions, EC/ASC and EC/Fb are able to induce vascular sprouts in a similar way that previously described with HUVEC (Fig. 5A).
Fig. 5.
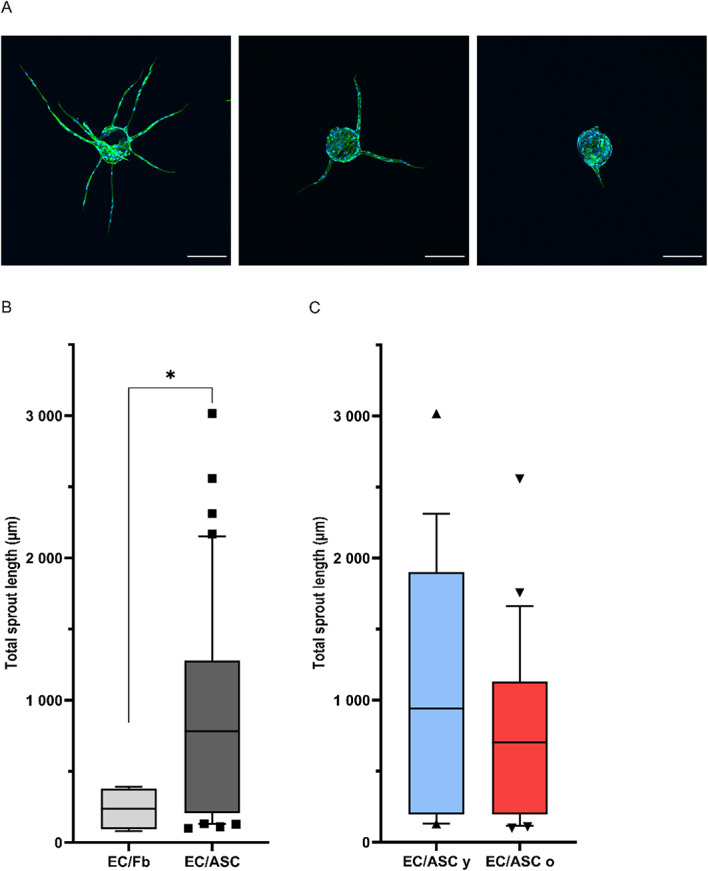
(A) Diversity of sprouting of endothelial cells from dextran beads stained with phalloidin and Hoechst to visualize actin and nucleus, respectively (original magnification 10x, bar, 200 μm). (B) Total sprouts length with co-culture of EC with ASC (EC/ASC; dark grey) and fibroblasts (EC/Fb; light grey) as stimulating cells. (B) Total sprouts length with co-culture of EC with either ASC (EC/ASC; dark grey) and fibroblasts (EC/Fb; light grey) as stimulating cells. (C) Total sprouts length according to co-culture of EC with ASC from young (EC/ASC y; blue) or old (EC/ASC o; red) donors as stimulating cells. n = 5 donors for ASC young, n = 6 donors for ASC old, n = 1 donor for fibroblasts, experiments were conducted in 4 replicates. Unpaired Wilcoxon test was used to test the significance. * P value < 0.05.
Co-culture of EC/ASC induces a 3,1fold longer total vascular sprouts (783 μm) compared to EC/Fb (238 μm) (Fig. 5B). EC/ASC from young donors induce a median total sprouts length of 941 μm when EC/ASC from old donors induce a median total sprouts length of 704 μm (Fig. 5C). Differences in total sprouts length between age categories are not significative, meaning that ASC sprouting induction is not altered with age (Fig. 5C).
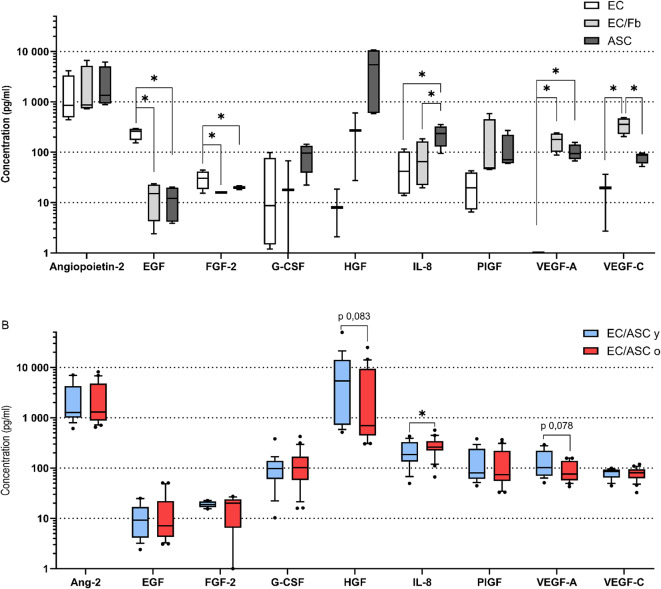
G-CSF, HGF and IL-8 are more expressed by EC/ASC than EC/Fb
In order to elucidate which factors could be responsible for the pro-angiogenic effect observed, a total of 16 soluble factors were quantified in co-culture supernatants: Angiopoietin-2, bone morphogenic protein 9 (BMP-9), EGF, Endoglin, Endothelin-1, FGF-1, FGF-2, granulocyte colony stimulating factor (G-CSF), HB-EGF, HGF, IL-8, Leptin, PlGF, VEGF-A, VEGF-C, VEGF-D.
Some analytes concentrations were found below threshold: BMP-9, Endoglin, FGF-1, HB-EGF, Leptin, and VEGF-D (Supplementary data 1). The vasoconstrictor Endothelin-1 was detected only into negative controls (no stimulating cell), and EGF and FGF-2 were detected with significantly higher concentration in negative controls compared to other conditions (Fig. 6A). On the contrary, HGF, VEGF-A and -C were barely found into negative controls (mean 7,2 ± 8,3 pg/ml; 0,2 ± 0,5 pg/ml; 9,8 ± 17,9 pg/ml respectively). EC/ASC secrete significantly higher concentrations of IL-8 (230,4 ± 106,7 pg/ml) and lower level of VEGF-C (80,5 ± 19,7 pg/ml) compared to EC/Fb (83,8 ± 76,6 pg/ml; 352 ± 124,3 pg/ml respectively) (Fig. 6A). G-CSF and HGF are secreted at higher concentration in EC/ASC co-cultures (89,7 ± 50,6 pg/ml; 5555,8 ± 5701,2 pg/ml respectively) compared to EC/Fb (21,5 ± 31,7 pg/ml; 224,8 ± 278,9 pg/ml respectively) co-cultures but results are not statistically significant, probably due to high variability.
Fig. 6.
(A) Secretome of EC without stimulating cells (EC; clear box), or EC co-culture with either ASC (EC/ASC; dark grey) (independently of donor age) or fibroblasts (EC/Fb; light grey). (B) Secretome of EC co-culture with ASC from young (EC/ASC y; blue) or old (EC/ASC o; red) donor. n = 5 donors for ASC young, n = 6 donors for ASC old, n = 1 donor for fibroblasts, experiments were conducted in 4 replicates. ANOVA test was used to test the significance in (A). Unpaired Wilcoxon test was used to test the significance in (B). * P value < 0,05.
Among co-culture of EC with ASC, IL-8 is secreted in significantly higher levels in EC/ASCo (mean 280 ± 116 pg/ml) compared to EC/ASCy (mean 211 ± 109 pg/ml) (Fig. 6B). Lower levels of VEGF-A have been found in EC/ASCo (mean 96,6 ± 42,1 pg/ml) compared to EC/ASCy (mean 142 ± 81,1 pg/ml), but it is unsignificant (p value 0,078). No difference was found between age categories for other analytes.
Soluble factors or secretome are required but not sufficient for inducing EC sprouting
Mechanism of action of the pro-angiogenic properties of ASC has not been completely elucidated yet. In this in vitro test, pro-angiogenic environment is supposed to be mostly linked to soluble factors. Two ASC donors were identified to induce longer total sprout length compared to others. As those donors seem to secrete more Ang-2, HGF and VEGF-A than other ASC donors, we’ve tried to induce angiogenesis with those pro-angiogenic factors (at concentrations assessed previously) without stimulating cells on top of the gel. Sprourting assay was performed without stimulating cell (on top of the gel) and with dose effect of HGF, Ang-2 and VEGF-A as well as a combination of those 3 factors. Absence of stimulating cell leads to deficiency of organized sprout, with or without angiogenic factors (data not shown). Without stimulating cell, EC seem to die into fibrin gel.
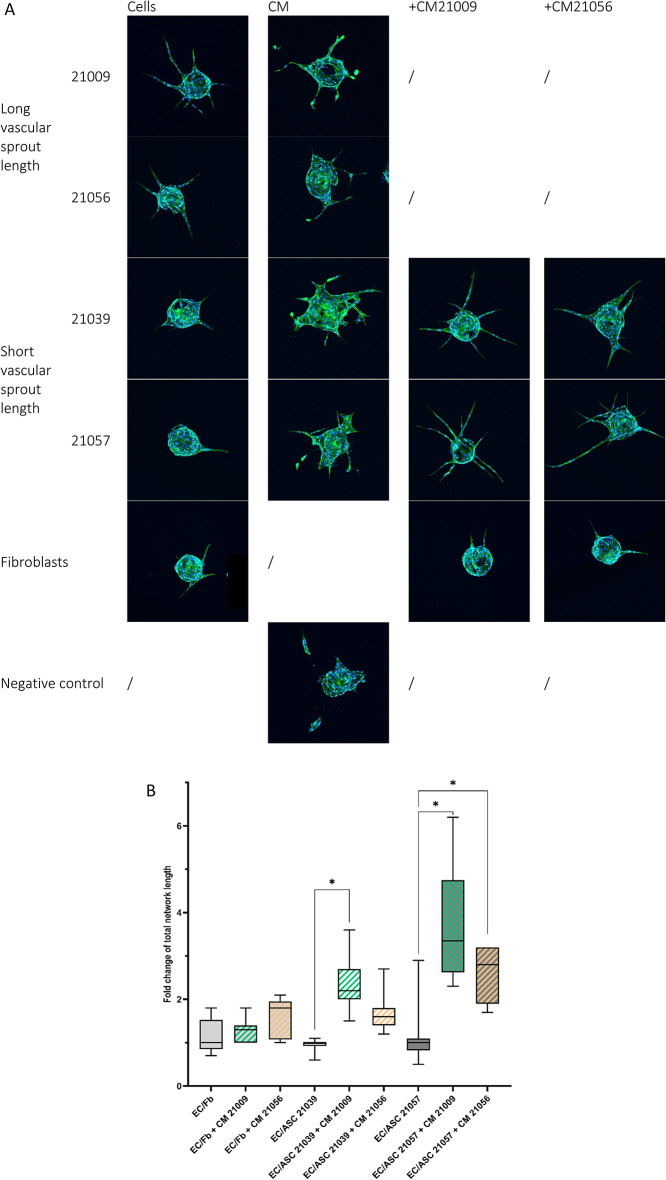
As in vitro angiogenesis test is not induced by soluble factors tested themselves, sprouting mechanism of action could be linked to a specific dosage of soluble factors that lacks in our previous experiment. Conditioned media (ASC-CM) produced by ASC were tested on sprouting assay without stimulating cells. ASC-CM from the two donors identified to induce the longest vascular total sprout length (#21009 and #21056), annotated ASClong-CM, and two donors identified to induce the shortest vascular sprout length (#21039 and #21057), annotated ASCshort-CM, were tested on this in vitro angiogenesis assay.
Addition of ASC-CM without stimulating cell leads to deficiency of organized sprout (Fig. 7A). In ASC-CM condition, only tiny sprouts were observed and some EC seem enter in a necrotic phase. No difference was observed between CM from different ASC donors in absence of stimulating cells. As CM condition do not induce an organized sprout, it was not relevant to measure it.
Fig. 7.
EC required cells and specific dosage of soluble factors to form organized. (A) Confocal images of sprouting beads according to conditions. (B) Total sprout length quantification (normalized cells alone) supplemented with ASC-CM. ASC donor is specified in the graph. Experiments were conducted in duplicate. ANOVA test was used to test the significance. * P value < 0,05.
As stimulating cells are mandatory to induce an organized sprout, we’ve tested if ASC-CM from a specific donor could influence sprout length induced by EC/ASC co-culture with another ASC donor. ASClong-CM (from donors #21009 and #21056) were added to EC/ASCshort (from donors #21039 and #21057) co-cultures. Total sprout length is significantly increased in co-cultures of EC/ ASCshort cultivated with ASClong-CM in comparison to basal medium (Fig. 7B). This result is valid for both ASCshort in culture with EC. However, ASClong-CM do not have any effect on EC/fibroblasts co-culture.
Discussion
As ASC are stem/stromal cells obtained through minimally invasive procedures, they are widely involved in cell-based and cell-free therapeutic strategies. ASC have been extensively described for their beneficial outcomes on skin quality by surgeons2,53–55. Although mechanism of action is still unknown, differentiation and paracrine function as well as pro-angiogenic effects are pointed out21,22,56. ASC therapy is often used in older patients, raising the question of the sustainability of ASC potential through age. Our results show that age does not impair ASC yield, neither their capacity to differentiate into adipocytes, chondrocytes or osteoblasts. At P0, ASC secretome is not altered by age. Aging has no effect on ASC capacity to induce EC sprouting in our experimental conditions.
Composition of SVF cells were described and ASC stemness and pro-angiogenic potential were investigated. SVF is divided into hematopoietic and non-hematopoietic cells, categorized thanks to CD45. Immune cells count for half of the SVF, and this proportion is stable with age, highlighting the pivotal role of adipose tissue immunomodulation. Among non-immune cells, percentages are consistent with those found in the literature41,46,47 as CD45- SVF is mainly composed of ASC (CD45- CD31- CD34+) (31–91%), endothelial cells (CD45- CD31 + CD34+) (3–48%) and pericytes (CD45- CD31- CD34- CD146+) (1–20%).
The non-significant increase in ASC yield with age could be an explanation of the non-significant increased proportion of CFE observed with age. However, as sample size is small, further study including more donors is required to complete these results. ASC maintain the capacity to differentiate into adipocytes, chondrocytes and osteoblasts through age. Some donors show lipid droplets into control wells, indicating that cells are able to spontaneously differentiate into adipocytes. This observation is made independently of donor age. Those results are consistent with previously published data57–59 showing cell yield and adipogenic potential are unrelated to donor age, while they are contradictory with other studies exhibiting a decrease of osteogenic potential with age59. Specific lineages gene expression (PPARγ, ACAN, RUNX2) could be investigated to see more slight differences between age categories.
Many studies exist on ASC secretome29–31, as cell-free therapies seem promising in clinical applications29. Nevertheless, most of those studies are in the context of wound healing60, senescence61 or disease such as diabetes27,62. Here we assessed 25 analytes described in the literature, covering angiogenic and mitogenic activity of ASC. Yet, due to a lack of uniformity in the method of secretome preparation, quality and quantity of the secreted analytes vary from a study to another. Lower concentrations of soluble factors were found in this study compared to others27. However, cells in other experiments were either cultured into 5% serum media27 or stimulated with LPS31. Even if no statistical significance was found, many analyte concentrations were lowered in aged category compared to young one (angiogenin, FGF-1, HGF, IL-6, MMP-1, PDGF-CC, PlGF, SCF). Park et al. found that age affects ASC secretome with a decrease of VEGF and HGF39. Differences could be explained as panel used for those experiments was older than ours (old panel > 70 yo) and a mix of male and female patients. ASC-CM has been extensively studied and authors conclude that due to their paracrine capacity, ASC are a promising tool in the field of skin diseases or disorders such as wound healing, photo-aging or atopic dermatitis63–65. Our results highlight that paracrine function is maintained through age. Some authors have investigated the ASC secretome under environmental pressure such as hypoxia or cytokines to increase factors secretion31,66,67. Stimulation of ASC with hypoxia could be a way to assess reactivity of ASC from young donors compared to old ones.
ASC are well described for their role in angiogenesis as they differentiate into endothelial cells24,68 and secrete numerous pro-angiogenic factors69,70, however, age has been described to impair this pro-angiogenic potential1,37. A large number of in vitro assays have been developed for studying some aspects of angiogenesis such as endothelial cells migration or proliferation. Yet, Matrigel- or collagen gel-based assays fail to model sprouting in 3 dimensions. Nakatsu et al.51 developed a simple and quick 3D assay allowing to assess multiple parameters of sprouting. This assay has been mostly used to evaluate the activity of angiogenesis inhibitors71 and is widely used with HUVECs. In our assay, we demonstrated this assay could be adapted with dermal microvascular EC, and ASC could be used as stimulating cells instead of fibroblasts. To our knowledge, this is the first time ASC are used in this sprouting in vitro assay with dermal microvascular EC. As ASC are perivascular cells46, well described as potent pro-angiogenic regulators72, it is not astonishing that they induce a longer total sprout compared to fibroblasts. Our first results highlight a trend of ASC having a higher pro-angiogenic potential compared to fibroblasts. However, as these data have been produced on only one donor of fibroblasts, a dedicated study is mandatory to validate this trend. In our study, total sprout length and average sprout length are stable with ASC donor age. Those results are consistent with the absence of alteration with age of the ASC secretome in monolayer. Studies showing that age decreases ASC pro-angiogenic activity also point a decrease of pro-angiogenic secretion37. Indeed, co-culture supernatants exhibit stable analyte secretion through age. Interestingly, there is a trend for a decrease of HGF and VEGF-A in EC/ASCo in comparison to EC/ASCy, but no change in sprout length was observed between EC/ASCo and EC/ASCy.
Surprisingly, while co-cultures with fibroblasts induce a statistically shorter sprout length compared to ASC, they also express significantly higher concentrations of VEGF-C and similar concentrations of VEGF-A. As VEGF members are pivotal players in vascularization73, it is interesting to notice that high levels of those well-known pro-angiogenic factors are not sufficient for sprouting induction. ASC capacity to induce sprouting and secrete pro-angiogenic factors is stable with age. Yet, the two donors inducing the longest sprout length are those secreting highest quantity of VEGF-A, highlighting the importance of secretome in angiogenesis. Furthermore, those donors are both under 30yo.
As some publications have used pro-angiogenic factors to stimulate EC sprouting without using stimulating cells, we investigated the soluble factors that could be responsible for sprouting. It is to notice that HUVEC are EC utilized in almost all the studies using this sprouting assay. To our knowledge, the only publication using dermal microvascular EC in this sprouting assay did not demonstrate the formation of a vascular sprout in absence of stimulating cells or pro-angiogenic factors embedded in fibrin gel74. As ASC do not penetrate the gel, we found more relevant to add pro-angiogenic factors and CM on the top instead of embedded inside the gel.
Absence of stimulating cells seems to induce EC death (observations), and sprout length is not relevant to measure. In CM tested conditions, few numbers of beads were able to sprout, those conditions do not lead to formation of an organized sprouting. Intriguingly, conditioned media from ASClong induce the same outcomes compared to conditioned media from ASCshort. Those results highlight that pro-angiogenic factors themselves are not sufficient and a cell communication is required to induce first step of angiogenesis.
Interestingly, addition of ASClong-CM to EC/ASCshort lead to an increase of the sprout length in comparison with basal media. As those results are not observed on EC/fibroblasts co-culture, ASC-CM components seem to stimulate ASC to induce sprouting from EC. Further studies are required to assess angiogenic factor receptors on ASC in order to elucidate those results. In the future, there is also a necessity to identify CM components (soluble factors, exosomes, miRNAs…) required for sprouting development.
Although one of the strengths of this study consists of wide characterization of young and old healthy populations, one limitation of this research is the small sample size (N = 12), which was all female. We’ve excluded patients with BMI over 30 and with obesity history as it was demonstrated that obesity and weight loss alter cellular composition of subcutaneous adipose tissue and ASC inflammatory profile75,76. Further study including more donors is required to complete these in vitro results. Moreover, the hypothesis that age does not affect ASC potential, including pro-angiogenic properties, has to be validated through in vivo experiments. It is not excluded that other environmental parameters could affect adipose tissue metabolism and ASC behavior such as tobacco use or pollution.
Conclusion
ASC have been extensively described for their beneficial outcomes on skin quality by surgeons2,53–55. Although mechanism of action is still unknown, differentiation and paracrine function as well as pro-angiogenic effects are pointed out21,22,56. ASC therapy is often used in older patients, raising the question of the sustainability of ASC potential through age. Our results suggest donor age might not influence and ASC differentiation, but this hypothesis has to be validated in vivo. In the context of fat grafting, some authors display that age could decrease graft survival77,78. However, our experimental results suggest there is no alteration with age on our in vitro assessment. Still no consensus on predictability of fat graft retention and effect of fat and ASC on skin rejuvenation has been found, researchers have to deep dive into SVF cells interactions between them and their environment. This statement is supported by our results showing that induction of EC sprouting required not only pro-angiogenic factors but also a dynamic cellular communication.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- ASC
Adipose-derived stem/stromal cells
- CFE
Colony forming efficiency
- CM
Conditioned medium
- EC
Endothelial cells
- HUVEC
Human umbilical vein endothelial cells
- SVF
Stromal vascular fraction
Author contributions
CT, AM and CA contributed to conception and design of the study. AM provided adipose tissue samples. CT performed and analyzed extraction, flow cytometry, secretome and angiogenesis assays. CB performed angiogenesis assays. HA and PD performed and analyzed differentiation assays. CT and CA wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was financially supported by L’Oréal Research & Innovation.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Written informed consent was obtained from the patient and surgical residue was collected according to French regulation and declared to research ministry.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Trotzier, C., Sequeira, I., Auxenfans, C. & Mojallal, A. A. Fat graft retention: Adipose tissue, adipose-derived stem cells, and aging. Plast. Reconstr .Surg. 151(3), 420e-31e (2023). 10.1097/PRS.0000000000009918 [DOI] [PubMed]
- 2.Charles-de-Sa, L. et al. Antiaging treatment of the facial skin by fat graft and adipose-derived stem cells. Plast. Reconstr. Surg. 135 (4), 999–1009. 10.1097/PRS.0000000000001123 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Coleman, S. R. & Katzel, E. B. Fat grafting for facial filling and regeneration. Clin. Plast. Surg. 42(3), 289–300 vii (2015). 10.1016/j.cps.2015.04.001 [DOI] [PubMed]
- 4.Del Papa, N. et al. Autologous fat grafting in the treatment of fibrotic perioral changes in patients with systemic sclerosis. Cell. Transpl. 24 (1), 63–72. 10.3727/096368914X674062 (2015). [DOI] [PubMed] [Google Scholar]
- 5.van Dongen, J. A. et al. The effects of facial lipografting on skin quality: A systematic review. Plast. Reconstr. Surg. 144 (5), 784e–97e. 10.1097/PRS.0000000000006147 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Walocko, F. M., Eber, A. E., Kirsner, R. S., Badiavas, E. & Nouri, K. Systematic review of the therapeutic roles of adipose tissue in dermatology. J. Am. Acad. Dermatol. 79 (5), 935–944. 10.1016/j.jaad.2018.06.010 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Fontdevila, J. et al. Assessing the long-term viability of facial fat grafts: An objective measure using computed tomography. Aesthet. Surg. J. 28 (4), 380–386. 10.1016/j.asj.2008.05.002 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Gerth, D. J., King, B., Rabach, L., Glasgold, R. A. & Glasgold, M. J. Long-term volumetric retention of autologous fat grafting processed with closed-membrane filtration. Aesthet. Surg. J. 34 (7), 985–994. 10.1177/1090820X14542649 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Gause, T. M. et al. Particle size in fat graft retention: A review on the impact of harvesting technique in lipofilling surgical outcomes. Adipocyte. 77. 3 (4), 273–279. 10.4161/21623945.2014.957987 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conde-Green, A. et al. Comparison of 3 techniques of fat grafting and cell-supplemented lipotransfer in athymic rats: A pilot study. Aesthet. Surg. J. 33 (5), 713–721. 10.1177/1090820X13487371 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Peer, L. A. Loss of weight and volume in human fat graft, with postulation of a cell survival theory. Plast. Reconstr. Surg. 5 (3), 217–230 (1950). [Google Scholar]
- 12.Peer, L. A. Cell survival theory versus replacement theory. Plast. Reconstr. Surg. 16 (3), 161–168. 10.1097/00006534-195509000-00001 (1955). [DOI] [PubMed] [Google Scholar]
- 13.Allen, R. J. J. et al. Spinning into control: Centrifugation creates an optimal density for fat grafting. Plast. Reconstr. Surg. 124 (4S), 35–36. 10.1097/01.prs.0000364045.45819.d1 (2009). [Google Scholar]
- 14.Matsumoto, D. et al. Cell-assisted lipotransfer: Supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 12 (12), 3375–3382. 10.1089/ten.2006.12.3375 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Zuk, P. A. et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 7 (2), 211–228. 10.1089/107632701300062859 (2001). [DOI] [PubMed] [Google Scholar]
- 16.Laloze, J. et al. Cell-assisted lipotransfer: Friend or foe in fat grafting? Systematic review and meta-analysis. J. Tissue Eng. Regen Med. 12 (2), e1237–e50. 10.1002/term.2524 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Shukla, L., Yuan, Y., Shayan, R., Greening, D. W. & Karnezis, T. Fat therapeutics: The clinical capacity of adipose-derived stem cells and exosomes for human disease and tissue regeneration. Front. Pharmacol. 11, 158. 10.3389/fphar.2020.00158 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Ghadban, S., Artiles, M. & Bunnell, B. A. Adipose stem cells in regenerative medicine: Looking forward. Front. Bioeng. Biotechnol. 9, 837464. 10.3389/fbioe.2021.837464 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizuno, H., Tobita, M. & Uysal, A. C. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 30 (5), 804–810. 10.1002/stem.1076 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Lim, M. H., Ong, W. K. & Sugii, S. The current landscape of adipose-derived stem cells in clinical applications. Expert Rev. Mol. Med. 16, e8. 10.1017/erm.2014.8 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Mazini, L., Rochette, L., Admou, B., Amal, S. & Malka, G. Hopes and limits of adipose-derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 21 (4). 10.3390/ijms21041306 (2020). [DOI] [PMC free article] [PubMed]
- 22.Surowiecka, A. & Struzyna, J. Adipose-derived stem cells for facial rejuvenation. J. Pers. Med.12 (1). 10.3390/jpm12010117 (2022). [DOI] [PMC free article] [PubMed]
- 23.Metral, E. et al. Adipose-derived stem cells promote skin homeostasis and prevent its senescence in an in vitro skin model. J. Stem Cell. Res. Therapy. 04 (04). 10.4172/2157-7633.1000194 (2014).
- 24.Planat-Benard, V. et al. Plasticity of human adipose lineage cells toward endothelial cells: Physiological and therapeutic perspectives. Circulation. 109 (5), 656–663. 10.1161/01.CIR.0000114522.38265.61 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Lee, H. C. et al. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia. Circ. J. 76 (7), 1750–1760. 10.1253/circj.CJ-11-1135 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Nakagami, H. et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler. Thromb. Vasc Biol. 25 (12), 2542–2547. 10.1161/01.ATV.0000190701.92007.6d (2005). [DOI] [PubMed] [Google Scholar]
- 27.Rehman, J. et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 109 (10), 1292–1298. 10.1161/01.CIR.0000121425.42966.F1 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Traktuev, D. O. et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ. Res. 102 (1), 77–85. 10.1161/CIRCRESAHA.107.159475 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Kapur, S. K. & Katz, A. J. Review of the adipose derived stem cell secretome. Biochimie. 95 (12), 2222–2228. 10.1016/j.biochi.2013.06.001 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Salgado, A. J., Reis, R. L., Sousa, N. J. & Gimble, J. M. Adipose tissue derived stem cells secretome: Soluble factors and their roles in regenerative medicine. Curr. Stem Cell Res. Ther. 5 (2), 103–110 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Kilroy, G. E. et al. Cytokine profile of human adipose-derived stem cells: Expression of angiogenic, hematopoietic, and pro-inflammatory factors. J. Cell. Physiol. 212 (3), 702–709. 10.1002/jcp.21068 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Mojallal, A. et al. Influence of age and body mass index on the yield and proliferation capacity of adipose-derived stem cells. Aesthetic Plast. Surg. 35 (6), 1097–1105. 10.1007/s00266-011-9743-7 (2011). [DOI] [PubMed] [Google Scholar]
- 33.van Harmelen, V. et al. Effect of BMI and age on adipose tissue cellularity and differentiation capacity in women. Int. J. Obes. Relat. Metab. Disord. 27 (8), 889–895. 10.1038/sj.ijo.0802314 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Choudhery, M. S., Badowski, M., Muise, A., Pierce, J. & Harris, D. T. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J. Transl Med. 12, 8. 10.1186/1479-5876-12-8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu, W., Niklason, L. & Steinbacher, D. M. The effect of age on human adipose-derived stem cells. Plast. Reconstr. Surg. 131 (1), 27–37. 10.1097/PRS.0b013e3182729cfc (2013). [DOI] [PubMed] [Google Scholar]
- 36.Liu, M. et al. Adipose-derived mesenchymal stem cells from the elderly exhibit decreased migration and differentiation abilities with senescent properties. Cell. Transpl. 26 (9), 1505–1519. 10.1177/0963689717721221 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Barros, S. et al. Aging-related decrease of human ASC angiogenic potential is reversed by hypoxia preconditioning through ROS production. Mol. Ther. 21 (2), 399–408. 10.1038/mt.2012.213 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Efimenko, A. et al. Adipose-derived mesenchymal stromal cells from aged patients with coronary artery disease keep mesenchymal stromal cell properties but exhibit characteristics of aging and have impaired angiogenic potential. Stem Cells Transl. Med. 3 (1), 32–41. 10.5966/sctm.2013-0014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park, J., Park, G. & Hong, H. Age affects the paracrine activity and differentiation potential of human adipose-derived stem cells. Mol. Med. Rep. 23 (2). 10.3892/mmr.2020.11799 (2020). [DOI] [PMC free article] [PubMed]
- 40.Eto, H. et al. Characterization of structure and cellular components of aspirated and excised adipose tissue. Plast. Reconstr. Surg. 124 (4), 1087–1097. 10.1097/PRS.0b013e3181b5a3f1 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Yoshimura, K. et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J. Cell. Physiol. 208 (1), 64–76. 10.1002/jcp.20636 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Viola, A., Munari, F., Sanchez-Rodriguez, R., Scolaro, T. & Castegna, A. The metabolic signature of macrophage responses. Front. Immunol. 10, 1462. 10.3389/fimmu.2019.01462 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russo, L. & Lumeng, C. N. Properties and functions of adipose tissue macrophages in obesity. Immunology. 155 (4), 407–417. 10.1111/imm.13002 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manferdini, C. et al. Adipose stromal cells mediated switching of the pro-inflammatory profile of M1-like macrophages is facilitated by PGE2: In vitro evaluation. Osteoarthr. Cartil. 25 (7), 1161–1171. 10.1016/j.joca.2017.01.011 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Eto, H. et al. The fate of adipocytes after nonvascularized fat grafting: Evidence of early death and replacement of adipocytes. Plast. Reconstr. Surg. 129 (5), 1081–1092. 10.1097/PRS.0b013e31824a2b19 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Zimmerlin, L., Donnenberg, V. S., Rubin, J. P. & Donnenberg, A. D. Mesenchymal markers on human adipose stem/progenitor cells. Cytometry A. 83 (1), 134–140. 10.1002/cyto.a.22227 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feisst, V., Brooks, A. E., Chen, C. J. & Dunbar, P. R. Characterization of mesenchymal progenitor cell populations directly derived from human dermis. Stem Cells Dev. 23 (6), 631–642. 10.1089/scd.2013.0207 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Silva, K. R. et al. Characterization of stromal vascular fraction and adipose stem cells from subcutaneous, preperitoneal and visceral morbidly obese human adipose tissue depots. PLoS One. 12 (3), e0174115. 10.1371/journal.pone.0174115 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez, J. et al. Evaluation of three devices for the isolation of the stromal vascular fraction from adipose tissue and for ASC culture: A comparative study. Stem Cells Int. 2017, 9289213. 10.1155/2017/9289213 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bourin, P. et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 15 (6), 641–648. 10.1016/j.jcyt.2013.02.006 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakatsu, M. N. et al. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: The role of fibroblasts and Angiopoietin-1. Microvasc. Res. 66 (2), 102–112. 10.1016/s0026-2862(03)00045-1 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Eglinger, J., Karsjens, H. & Lammert, E. Quantitative assessment of angiogenesis and pericyte coverage in human cell-derived vascular sprouts. Inflamm. Regen. 37, 2. 10.1186/s41232-016-0033-2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coleman, S. R. Structural fat grafting: More than a permanent filler. Plast. Reconstr. Surg. 118 (3 Suppl), :108S-20S. 10.1097/01.prs.0000234610.81672.e7 (2006). [DOI] [PubMed]
- 54.Charles-de-Sa, L., Gontijo-de-Amorim, N. F., Coleman, S. & Rigotti, G. Regen fat code: A standardized protocol for facial volumetry and rejuvenation. Aesthet. Surg. J. 41 (11), NP1394–NP404. 10.1093/asj/sjab016 (2021). [DOI] [PubMed] [Google Scholar]
- 55.Charles-de-Sa, L. et al. Photoaged skin therapy with adipose-derived stem cells. Plast. Reconstr. Surg. 145 (6), 1037e–49e. 10.1097/PRS.0000000000006867 (2020). [DOI] [PubMed] [Google Scholar]
- 56.Mou, S. et al. Extracellular vesicles from human adipose-derived stem cells for the improvement of angiogenesis and fat-grafting application. Plast. Reconstr. Surg. 144 (4), 869–880. 10.1097/prs.0000000000006046 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Dufrane, D. Impact of age on human adipose stem cells for bone tissue engineering. Cell. Transpl. 26 (9), 1496–1504. 10.1177/0963689717721203 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Devitt, S. M. et al. Successful isolation of viable adipose-derived stem cells from human adipose tissue subject to long-term cryopreservation: Positive implications for adult stem cell-based therapeutics in patients of advanced age. Stem Cells Int. 2015, 146421. 10.1155/2015/146421 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu, M. et al. The effect of age on osteogenic, adipogenic and proliferative potential of female adipose-derived stem cells. J. Tissue Eng. Regen. Med. 3 (4), 290–301. 10.1002/term.165 (2009). [DOI] [PubMed] [Google Scholar]
- 60.Lombardi, F. et al. Secretome of adipose tissue-derived stem cells (ASCs) as a novel trend in chronic non-healing wounds: An overview of experimental in vitro and in vivo studies and methodological variables. Int. J. Mol. Sci. 20 (15). 10.3390/ijms20153721 (2019). [DOI] [PMC free article] [PubMed]
- 61.Ratushnyy, A., Ezdakova, M. & Buravkova, L. Secretome of senescent adipose-derived mesenchymal stem cells negatively regulates angiogenesis. Int. J. Mol. Sci.21 (5). 10.3390/ijms21051802 (2020). [DOI] [PMC free article] [PubMed]
- 62.Alicka, M., Major, P., Wysocki, M. & Marycz, K. Adipose-derived mesenchymal stem cells isolated from patients with type 2 diabetes show reduced stemness through an altered secretome profile, impaired anti-oxidative protection, and mitochondrial dynamics deterioration. J. Clin. Med. 8 (6). 10.3390/jcm8060765 (2019). [DOI] [PMC free article] [PubMed]
- 63.An, Y. et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell. Prolif. e12993. 10.1111/cpr.12993 (2021). [DOI] [PMC free article] [PubMed]
- 64.Schneider, I., Calcagni, M. & Buschmann, J. Adipose-derived stem cells applied in skin diseases, wound healing and skin defects: A review. Cytotherapy. 25 (2), 105–119. 10.1016/j.jcyt.2022.08.005 (2023). [DOI] [PubMed] [Google Scholar]
- 65.Chen, S., He, Z. & Xu, J. Application of adipose-derived stem cells in photoaging: Basic science and literature review. Stem Cell. Res. Ther. 11 (1), 491. 10.1186/s13287-020-01994-z (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seo, Y., Shin, T. H. & Kim, H. S. Current strategies to enhance adipose stem cell function: an update. Int. J. Mol. Sci. 20(15). 10.3390/ijms20153827 (2019). [DOI] [PMC free article] [PubMed]
- 67.Efimenko, A., Starostina, E., Kalinina, N. & Stolzing, A. Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J. Transl. Med. 9, 10. 10.1186/1479-5876-9-10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fischer, L. J. et al. Endothelial differentiation of adipose-derived stem cells: Effects of endothelial cell growth supplement and shear force. J. Surg. Res. 152 (1), 157–166. 10.1016/j.jss.2008.06.029 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsuda, K. et al. Adipose-derived stem cells promote angiogenesis and tissue formation for in vivo tissue engineering. Tissue Eng. Part. A. 19 (11–12), 1327–1335. 10.1089/ten.TEA.2012.0391 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rubina, K. et al. Adipose stromal cells stimulate angiogenesis via promoting progenitor cell differentiation, secretion of angiogenic factors, and enhancing vessel maturation. Tissue Eng. Part. A. 15 (8), 2039–2050. 10.1089/ten.tea.2008.0359 (2009). [DOI] [PubMed] [Google Scholar]
- 71.Winters, L., Thambi, N., Andreev, J. & Kuhnert, F. Evaluation of angiogenesis inhibitors using the HUVEC fibrin bead sprouting assay. Bio-Protoc. 6 (19). 10.21769/BioProtoc.1947 (2016).
- 72.Wang, X. et al. Adipose-derived stem cell-secreted exosomes enhance angiogenesis by promoting macrophage M2 polarization in type 2 diabetic mice with limb ischemia via the JAK/STAT6 pathway. Heliyon. 8 (11), e11495. 10.1016/j.heliyon.2022.e11495 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Melincovici, C. S. et al. Vascular endothelial growth factor (VEGF) - Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 59 (2), 455–467 (2018). [PubMed] [Google Scholar]
- 74.Feng, X., Tonnesen, M. G., Mousa, S. A. & Clark, R. A. Fibrin and collagen differentially but synergistically regulate sprout angiogenesis of human dermal microvascular endothelial cells in 3-dimensional matrix. Int. J. Cell. Biol. 2013, 231279. 10.1155/2013/231279 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Silva, K. R. et al. Stromal-vascular fraction content and adipose stem cell behavior are altered in morbid obese and post bariatric surgery ex-obese women. Stem Cell. Res. Ther. 6 (1), 72. 10.1186/s13287-015-0029-x (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baptista, L. S., Silva, K. R. & Borojevic, R. Obesity and weight loss could alter the properties of adipose stem cells? World J. Stem Cells. 7 (1), 165–173. 10.4252/wjsc.v7.i1.165 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cohen, S. R. et al. Standardized anatomic and regenerative facial fat grafting: Objective photometric evaluation 1 to 19 months after injectable tissue replacement and regeneration. Aesthet. Surg. J. 42 (4), 327–339. 10.1093/asj/sjab379 (2022). [DOI] [PubMed] [Google Scholar]
- 78.Montenegro, C. F. et al. Fat graft retention decreases with recipient age. J. Am. Coll. Surg. 225 (4), e144–e5. 10.1016/j.jamcollsurg.2017.07.917 (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.