Abstract
Background
Variant transthyretin amyloidosis (ATTRv) is a hereditary multisystem disorder with clinical spectrum ranging from predominant cardiomyopathy to polyneuropathy. In the Irish population, the T60A mutation has been previously recognised as the most common genotype.
Objectives
The aim of this study is to describe the diagnostic and phenotypic spectrum of patients with T60A ATTRv attending an Irish Expert Amyloidosis Network.
Methods
In this observational study design, the medical, laboratory and radiological records of patients enrolled in our amyloidosis registry with a confirmed genotype diagnosis of T60A ATTRv were reviewed.
Results
A cohort of 24 patients (12 female) met criteria for inclusion. The median age at diagnosis was 65 years (IQR 59.5–66.5) and median follow-up 44 months (IQR 31–58). Carpal tunnel syndrome was the initial manifestation in almost half (46%) of patients. Overall, a mixed cardioneuro phenotype was demonstrated including autonomic (75%), small (58%) and large fibre (46%) neuropathy largely predating a cardiac phenotype consisting of heart failure (63%), atrial arrhythmia (42%) and bradycardia (13%).
Conclusion
The contemporary clinical spectrum of T60A ATTRv in Ireland is one of patients typically presenting in the seventh decade with an already manifest neuropathy phenotype, largely predating a cardiac phenotype dominated by heart failure.
Keywords: Cardiomyopathies; Heart Failure; Heart Failure, Diastolic
WHAT IS ALREADY KNOWN ON THIS TOPIC
Despite being a major focus of research in recent years, the diagnosis of cardiac amyloidosis remains a clinical challenge due to a wide range of causative mutations with variable clinical spectrum. There are very few prior studies specifically targeting the T60A variant.
WHAT THIS STUDY ADDS
This study provides detailed phenotyping of the T60A variant across both cardiac and neurological systems.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study will aid in recognition of the condition in contemporary clinical practice.
Introduction
Variant transthyretin amyloidosis (ATTRv) is a progressive multisystem disorder with a wide clinical spectrum ranging from predominant polyneuropathy to cardiomyopathy. Over 100 amyloidogenic TTR mutations have been described, resulting in a variety of clinical syndromes and frequently presenting a diagnostic challenge.1 The recent approval of several novel agents with disease-modifying properties for the treatment of amyloidosis, in parallel with the contemporary availability of high-sensitivity non-invasive testing,2 has highlighted the importance of early diagnosis in limiting morbidity and mortality associated with ATTRv syndromes.3 Although ATTRv amyloidosis was initially thought to be a disease confined to endemic foci, studies have shown that late-onset cases with variable clinical features can be found in non-endemic areas.4 In the Irish population, the T60A (Thr60Ala, also referred to as p.Thr80Ala) mutation has been previously recognised as the most common genotype. Originally described as ‘Appalachian amyloid’,5 genotype studies have shown that this mutation has descended from a single founder in the Northwest of Ireland, where up to 1.1% of the population have previously been demonstrated to carry this genetic mutation.6 However, less is known about the phenotypic spectrum of disease in a contemporary cohort of patients with T60A ATTRv.
The aim of the study is to describe the diagnostic and phenotypic spectrum of patients with T60A ATTRv with confirmed organ amyloid deposition attending an Irish Expert Amyloidosis Network in the contemporary era.
Methods
Study population
All patients (n=34) attending the hereditary amyloidosis referral clinic under the joint care of subspecialist cardiac and neurology teams—the Irish Expert Amyloidosis Network—were assessed for inclusion in the study. Three patients had non-T60A pathogenic TTR mutations (p.Val142Ile (V221), p.Glu109Gln (glu89gln), p.Ser70Arg (S50R)) and were excluded. A further 4 patients were found to be genotype positive without any evidence of TTR amyloid organ deposition by time of study enrolment and were excluded. A total of 27 patients with confirmed ATTRv associated with the T60A variant were eligible for inclusion; of those n=24 were enrolled (3 patients died prior to enrolment). Data were collected retrospectively after patient enrolment.
Follow-up
Patients were followed in both cardiac and neurology clinics at regular 6-monthly intervals and/or as dictated by clinical need.
Laboratory analysis
At the time of diagnosis, patients were screened for light- chain amyloidosis with analysis of serum immunoglobins, serum immunofixation and serum-free light-chain quantification. In the absence of any abnormal haematological markers, a non-invasive pathway for diagnosis based on myocardial scintigraphy scanning was used in the majority of cases.2 In the presence of abnormal haematological markers or based on era (prior to the accepted non-invasive pathway guideline) and/or centre preference, tissue confirmation of amyloid subtype was obtained. Genetic testing was subsequently performed to investigate the presence of a pathogenic TTR variant.
N-terminal pro B-type natriuretic peptide (NTpro-BNP), serum troponin and renal function including estimated glomerular filtration rate (eGFR) were quantified at each clinical review. NTpro-BNP (cut-off 3000 ng/L) and eGFR (cut-off 45 mL/min) were used to determine the cardiac ATTR stage.7
Specialty investigations
Cardiac: a 12-lead ECG was recorded and analysed at each visit. Transthoracic echocardiography was performed on diagnosis and at approximately 1-year intervals during follow-up by certified echocardiographers in accordance with British Society of Echocardiography imaging guidelines. Left ventricular ejection fraction (LVEF) was calculated using Simpson’s biplane assessment and global longitudinal strain (GLS) was quantified where possible (based on image quality and adequate frame rate) and was calculated as the average regional longitudinal strain of 17 segments.
Radiological: technitium-99m, 3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy (TC-DPD) was performed according to defined protocols.2 Cardiac uptake was identified and graded according to the Perugini scale.
Neurological: presence of carpal tunnel syndrome was documented if patients had consistent symptoms and signs (sensory symptoms predominantly affecting median nerve territory, positive Phalen’s and/or Tinel’s test, thenar atrophy/weakness) with or without compatible nerve conduction studies (NCS) findings.8 Neurophysiology testing included assessment of large fibre, small fibre and autonomic function. Motor and sensory NCS were performed using standardised techniques. Departmental normal limits were used. Quantitative sensory testing (QST) assessed perception of cold and heat pain and thermal thresholds. Autonomic tests included R–R interval testing to Valsalva, posture and deep breathing as well as sympathetic skin response. Patients were documented as having large fibre neuropathy if they had consistent symptoms (numbness, weakness or non-painful paraesthesia) and/or signs (sensory loss, weakness, reduced or absent reflexes) along with compatible NCS. Patients were documented as having small fibre neuropathy if they had consistent symptoms (spontaneous paraesthesia or dysaesthesia, abnormal burning or cold sensation, electric-shock like pain, allodynia or hyperaesthesia) along with reproducible abnormalities on QST. All neurophysiological investigations were analysed by a single consultant neurophysiologist.
Statistical analysis
Categorical variables were expressed as numbers and percentages. Continuous variables were expressed as mean±SD or median and IQR.
Results
Study population and diagnosis
Initial mode of presentation was due to a suggestive clinical syndrome in 16 patients (67%) while the remaining eight individuals were referred in the setting of screening of family members of an affected individual. On detailed initial review at the Amyloid Network Clinic, all included patients reported symptoms attributable to the condition. Regarding family history, 18 patients (75%) had a family history suggestive of amyloidosis and 14 patients (58%) had a first degree relative with a confirmed diagnosis of T60A ATTRv. Two-thirds (67%) of patients reported familial origins in the Northwest of Ireland.
Median age at diagnosis was 65 years (IQR=59.5–66.5) and median follow-up was 44 months (IQR=31–58). A total of 50% (n=12) of the study population were female, in whom median age at diagnosis trended towards being older (65 vs 60 years) but did not reach significance (p=0.07). No significant differences according to sex were seen in other clinical or diagnostic parameters.
Diagnosis of the enrolled cohort occurred between 2012 and end 2022; 50% were diagnosed from 2020 onwards. Endomyocardial biopsy was the primary mode of diagnosis in four patients. One patient had abnormal haematological markers requiring tissue confirmation of ATTR amyloid subtype and a further three patients underwent tissue confirmation of ATTR subtype based on era and centre preference at time of presentation. The remaining patients (n=20) had normal haematological markers and subsequently were diagnosed with ATTR amyloidosis via the non-invasive diagnostic pathway. Of these, TC-DPD imaging confirmed Perugini grade 2 in 8 (40%) patients and grade 3 in 12 (60%) patients.Table 1
Table 1. Clinical characteristics of the study population.
| Median | |
| Age at diagnosis, years | 65 (IQR 59.5–66.5) |
| Male (n, %) | 12 (50%) |
| Female (n, %) | 12 (50%) |
| Comorbidities | |
| Hypertension | 9 (38%) |
| Coronary artery disease | 3 (13%) |
| Chronic kidney disease | 4 (17%) |
| Diagnosis based on symptoms | 16 (67%) |
| Family history T60A variant | 14 (58%) |
Neurological manifestations
Neurological manifestations largely predated cardiac manifestations by median of 7 (IQR 3.8–9) years and were present in all patients during follow-up. A remote history of carpal tunnel decompression or symptoms consistent with carpal tunnel syndrome was reported as the first symptom by 46% of patients, with 71% of patients affected by the time of diagnosis. A majority (75%) of patients had clinical features of autonomic neuropathy, including erectile dysfunction, symptomatic postural hypotension, diarrhoea or constipation, nausea and vomiting and/or weight loss. Large (46%) and small (58%) fibre peripheral neuropathy were also commonly present at time of diagnosis. Neurological findings at presentation are further characterised in table 2.
Table 2. Neurological manifestations.
| Neurological manifestations | |
| Carpal tunnel syndrome | 17 (71%) |
| Spinal stenosis | 4 (17%) |
| Neurological symptoms at diagnosis | |
| Autonomic | 18 (75%) |
| Altered bowel habit | 14 (58%) |
| Orthostatic hypotension | 13 (54%) |
| Erectile dysfunction | 6 (50%) |
| Small fibre neuropathy | 14 (58%) |
| Large fibre neuropathy | 11 (46%) |
| First neurological symptom | |
| Carpal tunnel syndrome | 11 (46%) |
| Spinal stenosis | 1 (4%) |
| Autonomic neuropathy | 8 (33%) |
| Peripheral neuropathy | 4 (17%) |
Cardiac manifestations
Clinical manifestations of cardiac disease were frequent at diagnosis and present in 67% of patients. During the study period, 16 patients (63%) had heart failure (HF) as defined by the universal definition of HF9; 21% of patients had at least one HF hospitalisation. Atrial arrhythmias were present in 42%, while 13% of patients required a pacemaker. Three patients died during the study period (cardiac cause n=2, cause unrelated to ATTRv syndrome n=1). Table 3 summarises the cardiac findings of the population at presentation and during the study period.
Table 3. Cardiac manifestations.
| Cardiac manifestations | N (%) |
| Heart failure | |
| Heart failure symptoms at presentation | |
| NYHA I | 12 (50%) |
| NYHA II | 10 (42%) |
| NYHA III | 2 (8%) |
| NYHA IV | 0 |
| Hospitalisation for heart failure | 5 (21%) |
| Diuretic use at presentation | 9 (38%) |
| ECG at presentation | |
| Sinus rhythm | 17 (71%) |
| Atrial fibrillation | 6 (25%) |
| Atrial flutter | 1 (4%) |
| First degree heart block | 5 (21%) |
| Right bundle branch block | 3 (13%) |
| Left bundle branch block | 4 (17%) |
| Non-specific interventricular conduction delay | 1 (4%) |
| Arrhythmia during follow-up | |
| Atrial fibrillation | 9 (38%) |
| Atrial flutter | 1 (4%) |
| Bradycardia requiring PPM | 3 (13%) |
| Echo (n=23) | |
| LV EF (%) | 50.5±10.4 |
| LV IVSd (cm) | 1.5±0.4 |
| RV-free wall thickness (cm) | 0.6±0.25 |
| GLS (%) | −15.4±3.7 |
| Left ventricular hypertrophy (n, %) | 19 (83%) |
| Right ventricular hypertrophy | 15 (65%) |
| Apical sparing pattern GLS pattern | 9 (39%) |
| Pericardial effusion | 4 (17%) |
| Biomarkers | |
| NT-pro BNP | n=22 |
| <3000 ng/L | 18 (82%) |
| >3000 ng/L | 4 (18%) |
| Renal function (eGFR) | n=24 |
| >60 mL/min | 20 (83%) |
| 45–60 mL/min | 3 (13%) |
| <45 mL/min | 1 (4%) |
| NAC stage | n=22 |
| Stage I | 18 (82%) |
| Stage II | 3 (14%) |
| Stage III | 1 (4%) |
eGFRestimated glomerular filtration rateGLSglobal longitudinal strainLVEFleft ventricular ejection fractionNACNational Amyloidosis CentreNTpro-BNPN-terminal pro B-type natriuretic peptideNYHANew York Heart AssociationPPMpermanent pacemakerRVright ventricle
Almost all patients (n=23) had transthoracic echocardiography performed at least once in our hospital. At first assessment, mean LVEF was 50 (±10.4%) and n=6 patients had an LVEF <50%. Mean GLS was −15.4 (±3.7%) and mean interventricular septum diameter in diastole was 1.5 (±0.4 cm). Other echocardiographic and ECG findings at the time of presentation are summarised in table 3.
Treatment
Disease-targeting treatments for ATTRv were approved in the Republic of Ireland in 2021 (patisiran) and 2022 (tafamidis), respectively. During the study period, all patients were treated with evidence-based disease-modifying therapies according to national eligibility criteria and treatment availability at that time (diflunisal 33%; tafamidis 25%, patisiran 46%; therapies pending approval—per clinical trial/compassionate use as appropriate—25%).
Discussion
This study highlights the phenotypic spectrum of ATTRv amyloidosis in a contemporary cohort of Irish patients. T60A ATTRv remains the predominant genetic mutation identified in Irish patients attending our Irish Expert Amyloidosis Network. Our report details that these patients typically present with an already manifest neuropathy phenotype, often initially carpal tunnel syndrome and frequently autonomic as well as small and large fibre neuropathy. This neuropathic syndrome largely predates their cardiac phenotype, which is dominated by HF. Family history and origin in the Northwest of Ireland remain important demographic factors; however, a quarter of the cohort had no suggestive family history, highlighting the importance of clinical suspicion and recognition of the clinical syndrome. Importantly, despite only two-thirds of patients showing an overt cardiac clinical syndrome at the time of diagnosis at a median age of 65, a significant proportion of patients had echocardiographic evidence of cardiac disease at first assessment alongside evidence of cardiac amyloid deposition by TC-DPD or cardiac biopsy. Finally, a signal towards older age at diagnosis was seen for females versus males.
First described in 1986,5 T60A variant amyloidosis is reported to have descended from a single founder in the Northwest region of Ireland where carrier status of the condition in a classic 1995 epidemiological study was estimated to be as high as 1.1%.6 Although this variant has subsequently been described in families across the world, family origin in the Northwest of Ireland remains an important demographic factor with the majority of our cohort tracing their origins to Donegal or its surrounding counties.
Prior studies specifically studying the T60A ATTRv amyloidosis population are rare. A prior UK study evaluating the neurological phenotype in patients with ATTR amyloidosis including 17 patients with T60A variant highlighted the relatively early presentation of carpal tunnel syndrome in T60A compared with other variants.10 Notably, our study found that carpal tunnel syndrome is frequently the first symptom in this cohort and its onset precedes diagnosis by a median of 7 years. Sperry et al11 published an observational study of n=98 patients undergoing carpal tunnel release surgery where analysis of the flexor retinaculum in men ≥50 years and women ≥60 years detected amyloid deposits (mixed picture of ATTRv, wtATTR and light chain amyloidosis) in 10.2%. Based on this and correlating with our findings, routine analysis of biopsy samples from carpal tunnel surgery in patients with or without a family history of T60A ATTRv >50 years could support presymptomatic diagnosis and/or targeted screening.
A second study investigating cardiac phenotype and clinical outcome in 60 patients diagnosed with T60A ATTRv attending UK and Canadian centres demonstrates congruent findings to the current study in a number of areas.12 Although more patients in our cohort were symptomatic with cardiac disease at presentation, there was similar prevalence of cardiac structural abnormalities on initial echocardiography regardless of symptoms. Further, a similar proportion of patients presenting with autonomic and peripheral neuropathy was noted in both cohorts.
Meanwhile, in the 10 years since this study was published, the landscape of both diagnosis and treatment of ATTR amyloidosis has changed dramatically. The contemporary availability of TC-DPD imaging as a non-invasive diagnostic tool allowed 83% of our patients to be diagnosed without requiring invasive confirmation of ATTR subtype. This study also highlights how treatment options have evolved with two alternate mechanism guideline-approved therapies becoming available to patients during the study period, where previously liver transplantation was considered the only treatment option. Prospective, longitudinal follow-up of these patients on disease-targeted treatments to determine outcomes in this contemporary cohort is ongoing.
The present study is limited by its modest numbers and descriptive design. However, it represents a national cohort from a country where this particular mutation is predominant and is additionally a frequent mutation encountered in many other countries worldwide, including the UK and USA, through historical migration. Moreover, detailed and systematic phenotyping across both major organ syndromes affected is provided in a modern era of ATTR amyloidosis diagnosis and treatment.
Conclusion
This report highlights the current clinical spectrum of T60A ATTRv in an Irish Amyloidosis quaternary referral network in the modern era. Patients typically present in the seventh decade with an already manifest neuropathy phenotype, largely predating their cardiac phenotype, which is dominated by HF.
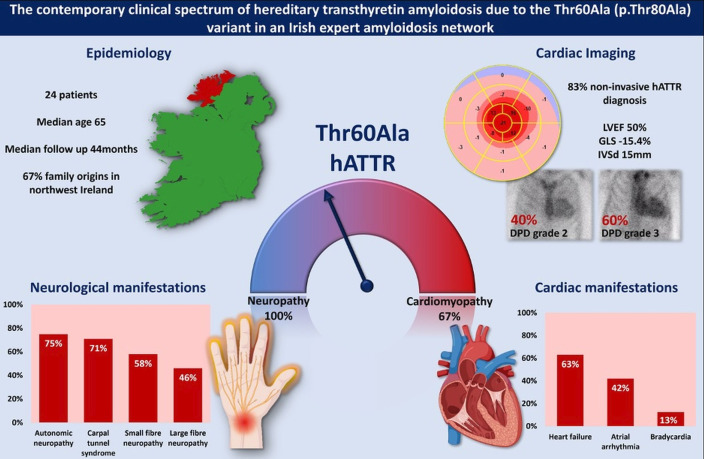
Figure 1. The contemporary clinical spectrum of T60A (p.Thr80Ala) variant transthyretin amyloidosis (ATTR) in Ireland. A total of 24 patients were included. The median age at diagnosis was 65 years and median follow-up 44 months. 67% of patients had family origins in the North West of Ireland. These patients presented with a mixed phenotype of carpal tunnel syndrome (71%), autonomic (75%), small (58%) and large (46%) fibre neuropathy. The cardiac phenotype included heart failure (63%), atrial arrhythmia (42%) and bradycardia (13%). Regardless of clinical presentation, all patients had cardiac involvement by 3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy (DPD) imaging or tissue analysis. GLS, global longitudinal strain; LVEF, left ventricular ejection fraction.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Data availability free text: Deidentified participant data can be made available upon reasonable request. Requests should be made to the corresponding author.
Patient consent for publication: Not applicable.
Ethics approval: Institutional ethical approval was granted by the Institutional Review Board at the Mater Misericordiae University Hospital (Ref: 1/378/2261) and all participants provided informed written consent.
Provenance and peer review: Not commissioned; externally peer reviewed.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Contributor Information
Katie Hewitt, Email: katierosehewitt@gmail.com.
Neasa Starr, Email: starrn@tcd.ie.
Zara Togher, Email: zara.togher1@gmail.com.
Saadah Sulong, Email: saadahsulong@gmail.com.
Joseph P Morris, Email: jpo.morris22@gmail.com.
Michael Alexander, Email: Michael.Alexander@tuh.ie.
Mark Coyne, Email: mark.coyne@ucd.ie.
Katie Murphy, Email: katiemurphy@mater.ie.
Gerard Giblin, Email: gerardgiblin@mater.ie.
Sinéad M Murphy, Email: Sinead.Murphy@tuh.ie.
Emer Joyce, Email: emerjoyce@mater.ie.
Data availability statement
Data are available upon reasonable request.
References
- 1.Arruda-Olson AM, Zeldenrust SR, Dispenzieri A, et al. Genotype, echocardiography, and survival in familial transthyretin amyloidosis. Amyloid. 2013;20:263–8. doi: 10.3109/13506129.2013.845745. [DOI] [PubMed] [Google Scholar]
- 2.Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation. 2016;133:2404–12. doi: 10.1161/CIRCULATIONAHA.116.021612. [DOI] [PubMed] [Google Scholar]
- 3.Maurer MS, Bokhari S, Damy T, et al. Expert Consensus Recommendations for the Suspicion and Diagnosis of Transthyretin Cardiac Amyloidosis. Circ Heart Fail. 2019;12:e006075. doi: 10.1161/CIRCHEARTFAILURE.119.006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koike H, Misu K, Ikeda S, et al. Type I (transthyretin Met30) familial amyloid polyneuropathy in Japan: early- vs late-onset form. Arch Neurol. 2002;59:1771–6. doi: 10.1001/archneur.59.11.1771. [DOI] [PubMed] [Google Scholar]
- 5.Benson MD, Wallace MR, Tejada E, et al. Hereditary amyloidosis: description of a new American kindred with late onset cardiomyopathy. Appalachian amyloid. Arthritis Rheum. 1987;30:195–200. doi: 10.1002/art.1780300210. [DOI] [PubMed] [Google Scholar]
- 6.Reilly MM, Staunton H, Harding AE. Familial amyloid polyneuropathy (TTR ala 60) in north west Ireland: a clinical, genetic, and epidemiological study. J Neurol Neurosurg Psychiatry . 1995;59:45–9. doi: 10.1136/jnnp.59.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillmore JD, Damy T, Fontana M, et al. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018;39:2799–806. doi: 10.1093/eurheartj/ehx589. [DOI] [PubMed] [Google Scholar]
- 8.Bland JD. A neurophysiological grading scale for carpal tunnel syndrome. Muscle Nerve. 2000;23:1280–3. doi: 10.1002/1097-4598(200008)23:8<1280::aid-mus20>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 9.Bozkurt B, Coats AJ, Tsutsui H, et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. 2021 doi: 10.1016/j.cardfail.2021.01.022. [DOI] [Google Scholar]
- 10.Carr AS, Pelayo-Negro AL, Evans MR, et al. A study of the neuropathy associated with transthyretin amyloidosis (ATTR) in the UK. J Neurol Neurosurg Psychiatry . 2016;87:620–7. doi: 10.1136/jnnp-2015-310907. [DOI] [PubMed] [Google Scholar]
- 11.Sperry BW, Reyes BA, Ikram A, et al. Tenosynovial and Cardiac Amyloidosis in Patients Undergoing Carpal Tunnel Release. J Am Coll Cardiol. 2018;72:2040–50. doi: 10.1016/j.jacc.2018.07.092. [DOI] [PubMed] [Google Scholar]
- 12.Sattianayagam PT, Hahn AF, Whelan CJ, et al. Cardiac phenotype and clinical outcome of familial amyloid polyneuropathy associated with transthyretin alanine 60 variant. Eur Heart J. 2012;33:1120–7. doi: 10.1093/eurheartj/ehr383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.