Abstract
Introduction:
COVID-19-associated cystitis (CAC) may arise following a COVID-19 infection and is characterized by the development of novel or worsening overactive bladder (OAB). CAC is possibly associated with bladder mucosal damage and the release of pro-inflammatory cytokines, resulting in inflammation and fibrosis of the bladder wall. Amniotic membrane (AM) has been shown to possess anti-inflammatory and anti-fibrotic properties and might potentially be beneficial for CAC. This study investigated the safety and efficacy of bladder injections of AM in CAC patients with resistant OAB symptoms.
Methods:
Five CAC patients, with an average age of 73 ± 1.0 years and a median disease duration of 2.4 years, received intra-detrusor injections of 100 mg micronized AM under general anesthesia and were followed for 20 weeks. Key urodynamic measures (involuntary detrusor contraction and maximum cystometric capacity) were determined to evaluate treatment response. Quality of life (QOL) was assessed using the OAB assessment tool, and safety was analyzed.
Results:
All five patients showed improved urodynamic bladder function and significantly improved QOL improvements. The improvement was evident from 4 weeks post-treatment and sustained until 12 weeks. Symptoms re-surged at 20 weeks. No safety concerns arose during the study.
Conclusion:
The observed improvements in symptom scores and bladder volume parameters highlighted the promise of AM bladder injections as a viable intervention for CAC patients with refractory OAB symptoms. Comprehensive studies are needed to validate its therapeutic potential, and treatment protocol refinement is warranted to address the observed reduction in efficacy over time.
Keywords: Amniotic bladder therapy, Bladder function, COVID-19, COVID-19-associated cystitis, Micronized amniotic membrane, Overactive bladder, Pro-inflammatory cytokines, Urodynamic study, Viral cystitis
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection led to the global COVID-19 pandemic. While we can say that the pandemic, although claiming a high death toll, has been satisfactorily controlled worldwide by effective clinical strategies, physicians are still encountering survivors with long-term consequences of COVID-19, with a significant impact on their general medical or oncological conditions [28,29]. It was estimated that 80% of patients who had recovered from COVID-19 had one or more long-lasting conditions associated with the disease, posing a highly significant clinical challenge at present [1]. COVID-19-associated cystitis (CAC) is a condition characterized by the emergence of new urinary symptoms or the exacerbation of pre-existing ones and is thought to be a clinical manifestation of long COVID [2].
This medical condition, thought not necessarily impairing the patients’ physical health, can exert a substantial impact on their quality of life and mental health, limiting their social life, travel capacity, and work activity. Stress, depression, and low self-esteem can result from CAC, with high costs imposed on the patients, the health system, and the work environment. Moreover, the cost of CAC treatments can be high, especially in the cases of prolonged therapies and failure or high drop-out rates of patients due to the side effects of available treatment options. Given the novelty of CAC, there are currently no published guidelines on the management of CAC. In a recent study, 270 of 310 (87%) CAC patients in our cohort reported improved scores on the Overactive Bladder (OAB) Assessment Tool, with conservative management whereas the remaining 40 patients (13%) continued to suffer from urinary frequency of over 13 episodes per day and nocturia >4 episodes a night [1].
The therapeutic approach to CAC is symptom-based, with the management of OAB symptoms being the primary goal. Treatment options for OAB typically begin with conservative approaches, such as lifestyle modifications, bladder retraining, and pelvic floor muscle exercises. If these measures are insufficient, they are followed by drug therapy, botulinum toxin injections, posterior tibial nerve stimulation, and sacral nerve stimulation. However, not all CAC patients achieved adequate relief with current therapies [30]. Of the 40 patients in our CAC cohort with ongoing OAB symptoms, five exhibited persistent symptoms despite undergoing behavioral modification, treatment with anticholinergics and beta-3 agonists, and intravesical botulinum toxin injections. Posterior tibial nerve stimulation and sacral nerve stimulation were not utilized, as they were not available at the time.
The exact pathophysiology of CAC remains under investigation, with several studies proposing different theories supported by scientific evidence. One hypothesis suggests that SARS-CoV-2 directly invades urothelial cells through the angiotensin-converting enzyme-2 (ACE2) receptor, as SARS-CoV-2 viral RNA has been detected in the urine of infected patients, and ACE2 is expressed on urothelial cells [2-4]. Another theory points to the role of pro-inflammatory cytokines, such as interleukin (IL)-6, IL-8, and IP-10, in the bladder mucosa as a cause of lower urinary tract symptoms (LUTS), since these cytokines were found to be elevated in the urine of COVID-19 patients compared to controls [3]. We hypothesized that these elevated cytokines in contact with the bladder mucosa may alter the sensitivity or function of these cells, leading to LUTS. Interestingly, studies have shown that the pathophysiological mechanism of CAC is similar to that observed in interstitial cystitis and chronic radiation-induced cystitis, where the response of the bladder wall to various insults is linked to the release of pro-inflammatory cytokines, eventually leading to chronic inflammation and fibrosis of the bladder wall. Therefore, treatments that aim to resolve inflammation, prevent fibrosis, and promote urothelial healing may help alleviate LUTS in refractory CAC patients [31,32].
Amniotic bladder therapy (ABT) may help overcome the limitations of the current treatment strategy for refractory CAC patients. Amniotic membranes (AMs) have been shown to possess anti-inflammatory and anti-scarring actions to promote wound healing in many clinical applications, including ocular surface and dermal wound healing [5,6,18]. ABT has been shown to improve LUTS in patients with interstitial cystitis/bladder pain syndrome (IC/BPS) and chronic radiation cystitis [7-9,32]. The aim of the present study was to evaluate the ability of intra-detrusor injections of amniotic fluid to reduce symptoms in patients with CAC refractory to conventional treatments.
2. MATERIALS AND METHODS
Refractory CAC patients were enrolled in this study when they had Idiopathic Detrusor Overactivity (IDO), and IDO patients were defined as those who experienced involuntary detrusor contractions (IDC) during the filling phase of baseline urodynamic assessment [10], and who had failed previous treatment modalities, including behavioral modification, treatment with anticholinergics and beta-3 agonists, and intravesical botulinum toxin injections. The study was approved by the Local Institutional Review Board and all patients gave their written informed consent.
Patients included in this study were initially admitted to Detroit Medical Center (Detroit, MI) between May and December 2020 for treatment of COVID-19. Following their discharge, these patients were referred to a specialized COVID clinic where they were surveyed regarding their recovery, including any new or worsening urological symptoms. Patients who reported such symptoms were subsequently referred to our urology clinic, where their symptoms were identified as CAC. By the time these patients received ABT in June 2023, they had been living with CAC symptoms for nearly 2 years. Before ABT, these patients had undergone a variety of treatments that ultimately did not resolve their symptoms. Importantly, all patients were confirmed to be COVID-negative through pre-operative testing before receiving ABT.
We excluded patients with concomitant bladder outlet obstruction, neurogenic detrusor overactivity, stress-predominant urinary incontinence, renal dysfunction, prior radiotherapy, intra-vesical stones, and a history of bladder and pelvic cancer. We also excluded the patients who had used anticholinergic agents, intravesical botulinum toxin (Botox), and neuromodulation within 6 months before the commencement of the study to avoid interference with the results.
Baseline evaluation included history, physical examination, serum chemistry profile, urinalyses, urine culture, urine cytology, post-void residuals (PVR), cystoscopy, urodynamic examination, including cystometry and pressure flow study (using a filling rate of 20 mL/min), and symptom assessment as measured by the OAB Assessment Tool. Urodynamic parameters analyzed were defined according to the standardization report of the International Continence Society. The assessments covered maximum cystometric capacity (MCC; mL), volume at first IDC (mL), and PVR urine volume (mL) through catheterization during urodynamic testing.
Under general anesthesia, patients were given intra-detrusor injections of 100 mg of commercially available micronized AM (Clarix Flo) diluted in 10 mL 0.9% preservative-free sodium chloride. A laryngeal mask was used during intubation with IV sedation to reduce invasiveness. Injections were performed through a cystoscope using a 25-gauge Williams needle, into the lateral and posterior bladder wall, sparing the dome (to avoid intraperitoneal injection) and the trigone (due to the possible risk of reflux). No bladder catheter was left in place following the procedure, and all patients were discharged the same day without receiving neuropathic or narcotic pain medications. Clinical evaluation and questionnaire investigation were repeated at 4 weeks, 8 weeks, 12 weeks, and 20 weeks. Urodynamic studies were repeated at 12 and 20 weeks. Local or systemic side effects were noted during and after treatment.
Descriptive statistics for continuous variables are presented as the mean ± SD, and statistical analyses were conducted using MiniTab (Minitab Inc., State College, PA, USA). The Wilcoxon signed-rank test, a non-parametric method, was used to analyze differences between parameters before and after treatment. P < 0.05 was considered statistically significant.
3. RESULTS
Five consecutive female patients fulfilling all criteria were included in the study and were observed between May and December 2020. The patients had an average age of 72.5 ± 1.0 years (range 70–73 years) and a median disease duration of 2.4 years (range 2.1–2.6 years), with the condition being refractory to multiple therapies, including anticholinergics (n = 5), beta-3 adrenergic agonists (n = 5), tricyclic antidepressants (n = 5), hydrodistension (n = 5), and Botox injection (n = 5). Before treatment, patients had severe symptoms of OAB, as suggested by an average score of 23.2 ± 0.8 on the OAB Assessment Tool. All patients experienced urinary urgency, frequency, and incontinence in the absence of infection or other obvious pathologies. None of the patients had received any COVID-19 vaccinations, as vaccines were not available at that time. In addition, this was the first and only recorded COVID-19 infection experienced by each patient in the cohort. The average severity of urinary urgency, frequency, and incontinence was 5 ± 0 (out of 5), 4.8 ± 0.4, and 4.0 ± 0.7, respectively, based on an assessment using the OAB Assessment Tool, suggesting they suffered from these symptoms almost persistently (as indicated by a score of 5) or for about half the time (as indicated by a score of 4). After ABT therapy, the OAB score decreased from 23.2 ± 0.8 at baseline to 17.2 ± 0.8 at 4 weeks (P < 0.05), 14.0 ± 0.7 at 8 weeks (P < 0.05), 10.0 ± 0 at 12 weeks (P < 0.05), and then increased to 16.4 ± 0.8 at 16 weeks (P < 0.05) and to 22.2 ± 0.7 at 20 weeks (P = 0.56) (Figure 1).
Figure 1.
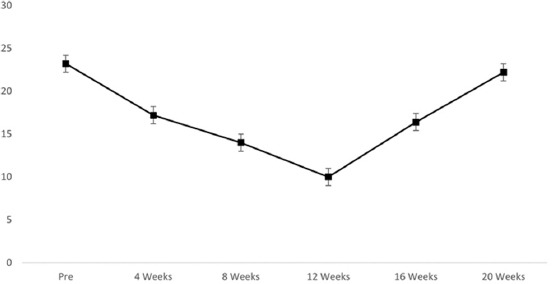
Average scores before and after amniotic bladder therapy on the overactive bladder assessment tool.
Before ABT, the average volume at the first IDC was 128 ± 11.7 mL. After injection, an increase in the mean volume of the first IDC and MCC from baseline was observed in all 5 patients (Table 1), which coincided with the improvement in symptoms (Figure 1). The average volume of the first IDC improved from 128 ± 11.7 to 190 ± 21.0 ml at 12 weeks (P < 0.05) and then worsened to 140 ± 11.0 at 20 weeks (P = 0.17). The average MCC improved from 206 ± 4.9 to 350 ± 43.4 mL at 12 weeks (P < 0.05) and then worsened to 204 ± 10.2 at 20 weeks (P = 0.7). Pressure flow parameters did not change pre- and post-injection in all five patients (Table 1). One patient developed an acute urinary tract infection 3 weeks after injection and was successfully treated with oral antibiotics. Importantly, no major complications were observed, and in no case did the symptom profile of the patients deteriorate after the procedure, either initially or after 12 weeks. The procedure demonstrated an excellent safety profile, as all patients were discharged the same day without the need for bladder catheterization, neuropathic or narcotic pain medications. Patients were advised to use acetaminophen or phenazopyridine for any post-injection discomfort, underscoring the overall mild nature of post-procedure symptoms.
Table 1.
Average volume at first IDC and MCC before and 12 weeks post-ABT
| CAC patient | Volume of the first IDC (mL) | MCC (mL) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Before ABT | 12 Weeks | 20 Weeks | Before ABT | 12 Weeks | 20 Weeks | |
| Patient 1 | 150 | 190 | 160 | 200 | 310 | 190 |
| Patient 2 | 120 | 150 | 130 | 210 | 290 | 220 |
| Patient 3 | 120 | 200 | 140 | 210 | 360 | 200 |
| Patient 4 | 120 | 210 | 130 | 210 | 400 | 200 |
| Patient 5 | 130 | 200 | 140 | 200 | 390 | 210 |
IDC: Involuntary detrusor contractions; MCC: Maximum cystometric capacity; ABT: Amniotic bladder therapy; CAC: COVID-19-associated cystitis.
4. DISCUSSION
The COVID-19 pandemic significantly impacted all medical specialties, including urology. Increased urinary frequency associated with COVID-19 was first reported in seven men by Mumm et al. [11] CAC is manifested as the increased urinary frequency of ≥13 episodes/24 h and nocturia ≥4 episodes/night, without any specific preponderance but with a higher incidence in patients with comorbidities [12]. The exact mechanism by which COVID-19 causes CAC is relatively unknown, but is potentially through direct replication of SARS-CoV-2 in the urinary tract and/or due to local and systemic inflammation. Cystitis can arise due to various causes, including bacterial or viral infections, medications, chemical irritants, radiation, or idiopathic factors. However, when the bladder mucosa is compromised, it can initiate a series of events that lead to the release of pro-inflammatory cytokines [13]. Once released, these cytokines can produce bothersome LUTS. Inflammation of the bladder wall exerts a direct effect on the function of the bladder [14]. These changes inevitably lead to destruction and dysfunction of the urothelium, manifesting as fibrotic changes, decreased bladder compliance, and overactive detrusor. Chronic inflammation stiffens the bladder wall, making it difficult for the bladder to fully expand when storing urine [17].
The evidence that the symptoms observed in our cohort were caused by COVID-19 is primarily based on temporal association, as these symptoms developed following COVID-19 infection and aligned with what we have defined as CAC. At the time of our study, there was limited research on CAC, and our work was among the first to systematically investigate this condition. Consequently, while our findings strongly suggest a link between COVID-19 and the development of CAC, direct causation has not been conclusively established. The exact mechanisms by which COVID-19 may lead to CAC are still under investigation, and current theories – such as direct viral invasion of the urothelium or the role of systemic inflammation – remain hypotheses that require further research.
Following ABT in refractory CAC patients, improvements in bladder function, as evaluated by UDS, were seen for a maximum of 12 weeks, and, afterward, all patients rebounded by 20 weeks. From baseline to 12 weeks, the average OAB score gradually improved. However, it began to decline after 12 weeks, and by 20 weeks, all patients had rebounded. Importantly, in no case were the symptoms at the rebound worse than before the treatment, suggesting that while the benefits of ABT might not be long-lasting, they did not exacerbate the condition. Our research indicated that ABT appeared to be safe and effective in improving OAB symptoms in CAC patients for up to 12 weeks but the treatment benefits did not seem to be durable beyond 12 weeks. This pattern of initial improvement, followed by a decline in therapeutic effect, was similar to the rebound of symptoms observed in the cases of OAB treated with botulinum toxin, where repeated injections were necessary to maintain the benefit [25-27]. This similarity suggests that continued or repeated ABT injections might be beneficial, and further studies should explore the efficacy of a second or even a third course of ABT.
The benefits of micronized AM in treating CAC may be attributed to its known anti-inflammatory and anti-scarring properties, which facilitate regenerative healing [5,6,18]. AM is composed of various cytokines, growth factors, and proteins, including heavy chain-hyaluronic acid/pentraxin 3 (HC-HA/PTX3), which has been shown, in other contexts, to modulate inflammatory responses [15,16]. By potentially reducing inflammation, AM might help protect neurons in the bladder wall and support the healing of the urothelium. Although these properties could theoretically benefit patients with CAC by mitigating bladder inflammation and fibrosis, it is important to note that this mechanistic explanation remains speculative in the absence of direct evidence from biopsies or cytokine measurements in our study. Despite these potential benefits, ABT did not demonstrate a durable response in our cohort of refractory CAC patients, highlighting the need for further research to confirm the specific pathways through which AM may exert its effects on this condition and to improve the durability of the therapeutic response.
ABT may not be durable in refractory CAC patients for several reasons. First, HC-HA/PTX3 has only been studied in the ophthalmological model, and it is therefore possible that this molecule alone may not have any effect in the bladder or other non-ophthalmological applications. Future research on the mechanism of action should be directed at validating that HC-HA/PTX3 or, potentially, another component modulates bladder tissue. At this time, it is unknown which specific protein(s) contained in AM might work on bladder tissue.
Second, the treatment protocol may have been suboptimal. A treatment protocol includes product selection, dosing, product preparation, reconstitution, timing and frequency of treatment, injection technique, and aftercare. Changes to the injection technique, however, are unlikely to result in a considerable improvement in durability because a subpar technique would have most likely caused either no response to ABT or a relapse after 2–4 weeks [19]. After 12 weeks, a diminished response resulting from inadequate injection technique was highly unusual because the bladder would have undergone several regenerative cycles by that point [20]. Product selection, however, is one part of the therapy regimen that may have an impact. It should be noted that our treatment strategy was restricted to the amnion monolayer. In addition to offering potential advantages, a monolayer chorion product or a bilayer chorion and amnion product may also increase the durability of the treatment. AM consists of both amnion and chorion layers, with the amnion being thinner and the chorion being 4 times thicker, containing a higher concentration of cytokines and growth factors. The greater thickness of the chorion accounts for its higher content of these bioactive molecules compared to the amnion. Studies have shown that both fresh and dehydrated chorion layers possess a greater load of growth factors and cytokines than their amnion counterparts [21]. Thus, the chorion membrane may better aid in inflammation reduction and thereby urothelial healing [22].
Finally, it is possible that the observed OAB symptom improvements lasting up to 12 weeks and the subsequent decline in the therapeutic effect could have been ascribed to a placebo effect. Existing evidence from clinical trial data suggests that a positive placebo effect occurs in patients receiving treatment for OAB [23]. In a meta-analysis of the placebo arms from randomized controlled trials (RCTs) assessing medical treatments for OAB, multiple statistically significant improvements were reported in clinically relevant outcomes [24]. Several randomized, double-blind, and placebo-controlled trials have assessed the safety and efficacy of botulinum toxin-A (Botn-A) in the management of patients with OAB. For instance, Brubaker et al. reported a median duration of benefit of 62 days for the placebo group compared to 373 days for the Botn-A group [26]. Similarly, Dowson et al. found that the placebo group had a median benefit duration of 12 weeks, which was the same as the Botn-A group at a dose of 100 U [25]. Finally, Sievert et al. observed a median duration of benefit of 13 weeks in the placebo group [27]. The duration of treatment effect (median time to qualification for re-treatment) was, in general, 24 weeks following treatment with botulinum toxin A compared with 8.8–13 weeks with placebo [25-27]. In the studies in which re-treatment could occur from 12 weeks onward, the placebo-treated patients requested/received re-treatment shortly after it was permitted. To ascertain whether our 12-week improvements resulted from ABT or a placebo effect, a randomized placebo-controlled trial with a larger sample size is necessary. A prospective, double-blinded, and RCT evaluating ABT for IC/BPS patients has started recruiting subjects from January 2024 (ClinicalTrials.gov ID NCT06096597). Minimally invasive therapies, such as repeatable cycles of amniotic fluid injections into the bladder wall, have been proven to be relatively safe and to have good rates of patients’ satisfaction. At this time, ABT should not be offered outside of a clinical trial.
This study had several limitations. These included the small sample size, the lack of a control/placebo arm, and the non-randomization design. In addition, a potential placebo bias could have altered the outcome. One significant limitation was the descriptive approach of this study. More quantitative assessments, particularly objective measurements at the molecular level, should be performed on tissue samples to evaluate the inflammatory reaction more precisely. Recent studies have shown distinctive inflammation-related molecular changes in response to treatments in other benign urological conditions [33]. Incorporating such quantitative assessments into future studies could provide more in-depth insights into the underlying mechanisms.
Moreover, the study’s relatively short time frame and follow-up period limited the ability to assess the long-term effects of ABT. A longer follow-up is necessary to evaluate whether any long-term benefits or responses are sustained over time. In addition, it will be beneficial to analyze patients undergoing multiple rounds of ABT treatment to observe changes over time and to verify if subsequent treatments can enhance or diminish the initial effects.
Future research should aim to address these limitations by utilizing larger-sized, RCTs with longer follow-up periods, and incorporate molecular-level analyses to more reliably assess the effects of ABT on CAC patients.
5. CONCLUSION
Our study demonstrated that using ABT by endoscopic injections in patients with CAC seemed to be a safe and effective option, with good results achieved in all five cases in the short term. Despite the patients showing symptom rebound at 12 weeks, we believe that larger-sized controlled studies can add more data to this novel topic and confirm the effectiveness of ABT as a strategy in selected cases of severe bladder involvement in CAC refractory to other currently available treatments. Finally, more evidence on the effects of ABT at the cellular level on the bladder wall is needed in the future to confirm our results.
ACKNOWLEDGMENTS
None.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflicts of interest. The product was not donated by the Company.
Author Contributions
Conceptualization: Jack A. Considine, Kyle O’Hollaren, Aron Liaw, Nivedita Dhar
Data analysis: Jack A. Considine, Codrut Radoiu, Nivedita Dhar
Methodology: Aron Liaw, Nivedita Dhar
Writing – original draft: Jack A. Considine
Writing – review & editing: Jack A. Considine, Kyle O’Hollaren, John Knapp
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the local institutional review board. Informed consent was obtained from participants included in the study.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Raveendran AV, Jayadevan R, Sashidharan S. Long COVID: An overview. Diabetes Metab Syndr 2021;15(3)869–875. doi: 10.1016/j.dsx.2021.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhar N, Dhar S, Timar R, Lucas S, Lamb LE, Chancellor MB. De novo urinary symptoms associated with COVID-19: COVID-19-associated cystitis. J Clin Med Res 2020;12(10)681–682. doi: 10.14740/jocmr4294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamb LE, Dhar N, Timar R, Wills M, Dhar S, Chancellor MB. COVID-19 inflammation results in urine cytokine elevation and causes COVID-19 associated cystitis (CAC). Med Hypotheses 2020;145:110375. doi: 10.1016/j.mehy.2020.110375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med 2020;14(2)185–192. doi: 10.1007/s11684-020-0754-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tseng SCG. HC-HA/PTX3 purified from amniotic membrane as novel regenerative matrix: Insight into relationship between inflammation and regeneration. Invest Ophthalmol Vis Sci 2016;57(5)ORSFh1–ORSFh8. doi: 10.1167/iovs.15-17637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tighe S, Mead OG, Lee A, Tseng SCG. Basic science review of birth tissue uses in ophthalmology. Taiwan J Ophthalmol 2020;10(1)3–12. doi: 10.4103/tjo.tjo_4_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madan R, Radoiu C, Liaw A, Lucas S, Hamada A, Dhar N. Early three-month report of amniotic bladder therapy in patients with interstitial cystitis/bladder pain syndrome. Int Urol Nephrol 2023;55(8)1937–1942. doi: 10.1007/s11255-023-03652-8 [DOI] [PubMed] [Google Scholar]
- 8.Radoiu C, Jeberaeel J, Madan R, et al. A preliminary report assessing the feasibility and effectiveness of amniotic bladder therapy in patients with chronic radiation cystitis. Can J Urol 2023;30(4)11607–11612 [PubMed] [Google Scholar]
- 9.Wittenberg S, Madan R, Liaw A, Lucas S, Hamada A, Dhar N. Amniotic bladder therapy in patients with recalcitrant interstitial cystitis and bladder pain syndrome. Can Urol Assoc J 2023;17(11)E402–E404. doi: 10.5489/cuaj.8441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: Report from the standardisation sub-committee of the International Continence Society. Urology 2003;61(1)37–49. doi: 10.1016/s0090-4295(02)02243-4 [DOI] [PubMed] [Google Scholar]
- 11.Mumm JN, Osterman A, Ruzicka M, et al. Urinary frequency as a possibly overlooked symptom in COVID-19 patients: Does SARS-CoV-2 cause viral cystitis?. Eur Urol 2020;78(4)624–628. doi: 10.1016/j.eururo.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamb LE, Timar R, Wills M, et al. Long COVID and COVID-19-associated cystitis (CAC). Int Urol Nephrol 2022;54(1)17–21. doi: 10.1007/s11255-021-03030-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grover S, Srivastava A, Lee R, Tewari AK, Te AE. Role of inflammation in bladder function and interstitial cystitis. Ther Adv Urol 2011;3(1)19–33. doi: 10.1177/1756287211398255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fry CH, Vahabi B. The role of the mucosa in normal and abnormal bladder function. Basic Clin Pharmacol Toxicol 2016;119(Suppl 3)57–62. doi: 10.1111/bcpt.12626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He H, Li W, Tseng DY, et al. Biochemical characterization and function of complexes formed by hyaluronan and the heavy chains of inter-alpha-inhibitor (HC*HA) purified from extracts of human amniotic membrane. J Biol Chem 2009;284(30)20136–20146. doi: 10.1074/jbc.M109.021881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He H, Zhang S, Tighe S, Son J, Tseng SCG. Immobilized heavy chain-hyaluronic acid polarizes lipopolysaccharide-activated macrophages toward M2 phenotype. J Biol Chem 2013;288(36)25792–25803. doi: 10.1074/jbc.M113.479584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moretti L, Stalfort J, Barker TH, Abebayehu D. The interplay of fibroblasts, the extracellular matrix, and inflammation in scar formation. J Biol Chem 2022;298(2)101530. doi: 10.1016/j.jbc.2021.101530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Sheha H, Fu Y, Liang L, Tseng SC. Update on amniotic membrane transplantation. Expert Rev Ophthalmol 2010;5(5)645–661. doi: 10.1586/eop.10.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burmeister D, Aboushwareb T, Tan J, Link K, Andersson KE, Christ G. Early stages of in situ bladder regeneration in a rodent model. Tissue Eng Part A 2010;16(8)2541–2451. doi: 10.1089/ten.TEA.2009.0697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hastings JC, Van Winkle W, Barker E, Hines D, Nichols W. The effect of suture materials on healing wounds of the bladder. Surg Gynecol Obstet 1975;140(6)933–937 [PubMed] [Google Scholar]
- 21.McQuilling JP, Vines JB, Kimmerling KA, Mowry KC. Proteomic comparison of amnion and chorion and evaluation of the effects of processing on placental membranes. Wounds 2017;29(6)E36–E40 [PMC free article] [PubMed] [Google Scholar]
- 22.Baskin LS, Sutherland RS, Thomson AA, et al. Growth factors in bladder wound healing. J Urol 1997;157(6)2388–2395 [PubMed] [Google Scholar]
- 23.Mangera A, Chapple CR, Kopp ZS, Plested M. The placebo effect in overactive bladder syndrome. Nat Rev Urol 2011;8(9)495–503. doi: 10.1038/nrurol.2011.99 [DOI] [PubMed] [Google Scholar]
- 24.Mostafaei H, Janisch F, Mori K, et al. Placebo response in patients with oral therapy for overactive bladder: A systematic review and meta-analysis. Eur Urol Focus 2022;8(1)239–252. doi: 10.1016/j.euf.2021.02.005 [DOI] [PubMed] [Google Scholar]
- 25.Dowson C, Sahai A, Watkins J, Dasgupta P, Khan MS. The safety and efficacy of botulinum toxin-A in the management of bladder oversensitivity: A randomised double-blind placebo-controlled trial. Int J Clin Pract 2011;65(6)698–704. doi: 10.1111/j.1742-1241.2011.02663.x [DOI] [PubMed] [Google Scholar]
- 26.Brubaker L, Richter HE, Visco A, et al. Refractory idiopathic urge urinary incontinence and botulinum A injection. J Urol 2008;180(1)217–222. doi: 10.1016/j.juro.2008.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sievert KD, Chapple C, Herschorn S, et al. OnabotulinumtoxinA 100U provides significant improvements in overactive bladder symptoms in patients with urinary incontinence regardless of the number of anticholinergic therapies used or reason for inadequate management of overactive bladder. Int J Clin Pract 2014;68(10)1246–1256. doi: 10.1111/ijcp.12443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cakir OO, Castiglione F, Tandogdu Z, et al. Management of penile cancer patients during the COVID-19 pandemic: An eUROGEN accelerated Delphi consensus study. Urol Oncol 2021;39(3. 197.e9.e17 doi: 10.1016/j.urolonc.2020.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mancini M, Randazzo G, Piazza G, Dal Moro F. Arterial thrombotic complications in COVID-19: A case of renal infarction. Biomedicines 2022;10(10)2354. doi: 10.3390/biomedicines10102354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wittenberg S, Vercnocke J, Chancellor M, et al. Prolonged impacts of COVID-19-associated cystitis: A study on long-term consequences. World J Clin Cases 2023;11(33)7987–7993. doi: 10.12998/wjcc.v11.i33.7987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morlacco A, Mancini M, Soligo M, et al. Relevance of the endoscopic evaluation in the diagnosis of bladder pain syndrome/interstitial cystitis. Urology 2020;144:106–110. doi: 10.1016/j.urology.2020.06.032 [DOI] [PubMed] [Google Scholar]
- 32.Lutchka J, Vercnocke J, Fisher E, et al. Treatment of chronic post-radiation cystitis with trans-urethral amniotic bladder therapy appears durable at 9 months: A clinical study. Urologia 2024;91(3)623–627. doi: 10.1177/03915603241248014 [DOI] [PubMed] [Google Scholar]
- 33.Saponaro M, Giacomini I, Morandin G, et al. Serenoa repens and Urtica dioica fixed combination: In-vitro validation of a therapy for benign prostatic hyperplasia (BPH). Int J Mol Sci 2020;21(23)9178. doi: 10.3390/ijms21239178 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


