Abstract
Background and study aims Patients with primary sclerosing cholangitis and inflammatory bowel disease (IBD) have a high risk of colorectal cancer. There is no agreement on the best technique for surveillance for colorectal neoplasia. We aimed to assess whether chromoendoscopy and/or high-definition endoscopy is associated with increased detection of neoplasia in patients with primary sclerosing cholangitis undergoing surveillance compared with when they were not used.
Patients and methods This was a single-center, retrospective, observational study designed to analyze differences in the detection of neoplasia (adenomatous and serrated) among patients with primary sclerosing cholangitis and IBD who underwent annual surveillance between 2010 and 2020. Multilevel logistic regression was used to adjust for confounders.
Results Ninety-one patients were identified, resulting in 359 colonoscopies with 360 person-years of follow up. Over the study period, 22 of 91 patients (24%) had at least one neoplastic lesion identified; however, the mean neoplastic lesion rate was 0.87 (54/63) for the primary sclerosing cholangitis-ulcerative colitis subgroup compared with 0.24 (4/17) for the primary sclerosing cholangitis-Crohn’s disease subgroup. Chromoendoscopy was associated with a significantly higher detection rate for neoplasia (odds ratio [OR] 5.58, 95% confidence interval [CI] 2.08–14.9, P =0.001), and this association remained after adjusting for confounders, including high-definition endoscopy. High-definition endoscopes had a higher rate of neoplasia detection, but the significance was lost after adjustment for confounders, including chromoendoscopy (OR 1.93, 95% CI 0.69–5.40, P =0.21).
Conclusions Chromoendoscopy is associated with a higher detection rate for neoplasia in patients with primary sclerosing cholangitis and IBD even with high-definition colonoscopes.
Keywords: Endoscopy Lower GI Tract; Inflammatory bowel disease; CRC screening; Diagnosis and imaging (inc chromoendoscopy, NBI, iSCAN, FICE, CLE...)
Introduction
Inflammatory bowel disease (IBD) is a risk factor for colorectal cancer (CRC) 1 . Early studies in patients with long-standing disease suggested a progressive risk of malignancies in the colon and rectum, with a cumulative incidence of 1% at 10 years, and 7% at 30 years 2 3 . In ulcerative colitis (UC), the incidence is higher, reaching 18% at 30 years 4 . Population-based studies, however, suggest this risk to be lower, particularly for patients who were diagnosed in the biological era 5 6 . On the other hand, patients with primary sclerosing cholangitis (PSC) and IBD concomitantly, PSC-IBD, have a higher risk of CRC. A Scandinavian study suggested that risk in those with PSC-UC may be as high as nine times that of the general population 7 . Moreover, a meta-analysis with more than 13,000 subjects observed that these patients have a 3-fold increase in risk of CRC compared with IBD-only patients 1 .
Incidence of PSC is about 1.0 per 10 5 person-years 6 , about 70% have IBD, of which 80% are UC and 20% Crohn’s disease (CD) 8 . Patients with PSC-IBD have a distinct phenotype of IBD that causes mildly symptomatic or even asymptomatic disease, often with rectal sparing, pancolitis, and backwash ileitis 9 . However, in a composite endpoint of cancer, liver transplantation, and death, patients with PSC-CD have a less severe course than those with PSC-UC, 9% vs 27% 10 . Moreover, patients with PSC-UC have a 3-fold increase in risk of dysplasia and cancer compared with patients with UC only, which has not been observed between patients with CD 11 12 . Recent studies have pointed to an increased risk of nonconventional neoplasia (i.e., different from the intestinal type and sporadic adenomas) in patients with PSC-IBD 13 14 . These changes can be macroscopically similar to the adjacent mucosa and result in suboptimal results of surveillance, with increased risk of progression to colectomy 15 16 . Among these, sessile serrated lesions (SSLs) represent an early stage of the serrated neoplasia pathway, marked by development of CRC with characteristically high levels of microsatellite instability 17 .
There is an unmet need for defining the best strategy for CRC surveillance in the IBD population. In conventional IBD, CRC screening is recommended after 6 to 10 years of IBD onset 18 19 20 . All patients with PSC should be offered an index colonoscopy at time of diagnosis with biopsies to detect subclinical IBD and repeat colonoscopy at 5-yearly intervals in the absence of clinically apparent IBD, or annual colonoscopic surveillance in the presence of IBD. Advanced endoscopic imaging may increase neoplasia detection rate and reduce unnecessary biopsies 21 . Chromoendoscopy (CE) has been recommended as the preferred method over white light endoscopy (WLE) 18 19 20 21 22 , despite the poor quality of evidence. With the advent of high-definition (HD) WLE, some have questioned the added value of CE with extra costs and time, with meta-analyses failing to show a statistically significant advantage of chromoendoscopy when HD-WLE is the comparator 23 . Thus, it is essential that we define the best surveillance technique for patients with PSC-IBD, more so for those with PSC-UC, who have a higher risk of CRC than others.
The aim of the present study was to use real-world data to analyze differences in detection of neoplasia using CE and HD-WLE in contrast to standard definition (SD) in the surveillance of CRC among patients with PSC-IBD of a tertiary referral center in the United Kingdom. We hypothesize that colonoscopy with CE and HD-WLE have higher neoplasia detection rates than SD-WLE.
Patients and methods
Study design
This was a single-center, retrospective, observational cohort study to analyze the difference in neoplasia detection between CE, HD-WLE, and SD-WLE among patients with PSC-IBD undergoing annual CRC surveillance with colonoscopy between January 2010 and March 2020 at the Oxford University Hospitals NHS Foundation Trust in the United Kingdom.
This study was approved by the Clinical Audit Division at Oxford University Hospitals Trust (reference number 8299). Patients in this study have consented to have their data collected and used in research developed by the biobank and the researchers from the Translational Gastroenterology Unit, Division of Experimental Medicine, Nuffield Department of Medicine, University of Oxford (REC 09/H1204/30, and 16/YH/0247).
Patient characteristics
Patients in the study were between 18 and 85 years of age, with a diagnosis of PSC and IBD, who were seen at specialist IBD and PSC clinics, and had at least one colonoscopy for CRC surveillance. Patients with a history of neoplasia in previous screenings were excluded from subsequent analysis.
Data collection
Data were collected from electronic patient records and included gender, age in years at PSC and IBD diagnosis, subtype of IBD, date of first colonoscopy and subsequent scopes, use of dye spray, use of HD scope, bowel preparation, if random or targeted biopsies were collected, and histology of lesion (adenomatous polyps, serrated lesions, hyperplastic polyps).
Endoscopic techniques
Procedures were performed under conscious sedation using intravenous benzodiazepines and opiates as needed. Carbon dioxide insufflation was used for all colonoscopies. Patients received bowel preparation with Citramag and senna, or Moviprep, scored as: inadequate; adequate; good; excellent. Olympus SD colonoscopes (CF-260DL) or HD colonoscopes (CF-H290DL) were used for procedures. Dye spray was applied with a spray catheter (Olympus PW-205V) in a segmental fashion with 0.2% indigo carmine. Targeted biopsies were taken of suspected lesions, or they were resected. Pairs of segmental biopsies were taken for activity and extent, without the intent to detect dysplasia. Random biopsies could also be taken at the discretion of the endoscopist but were not mandated. Use of chromoendoscopy and HD endoscopes was encouraged through the study period via mentorship, departmental presentations, and highlighting new guidelines (e.g., SCENIC 2015). Narrow band imaging was not used.
Pathology
Pathology was reported according to the Vienna criteria. Neoplastic lesions were defined as: all adenomatous polyps plus all serrated lesions (sessile serrated lesion and hyperplastic polyps) proximal to the rectum and serrated polyps in the rectum > 5 mm in size. Rectal hyperplastic polyps ≤5 mm were not considered neoplastic. This is in line with the British Society of Gastroenterology 2020 post-polypectomy guidelines which uses this definition for premalignant polyps 24 .
Outcomes
The primary outcome measure was the odds ratio (OR) for neoplasia detection with use of chromoendoscopy or HD colonoscope, adjusted for other confounding factors.
Preplanned secondary outcomes included: primary outcomes subdivided into HD versus chromoendoscopy; adenomatous lesions and serrated lesions; and rate of adoption of both HD and chromoendoscopy over the study period. We performed a sensitivity analysis to look at the primary outcome subdivided into PSC-UC and PSC-CD groups.
Statistical analysis
Patients were categorized according to use of CE and HD-WLE or SD-WLE at each colonoscopy. Data were censored after the first diagnosis of neoplasia, last follow-up, or at the end of the study period (31 March 2020). Continuous data are presented as median and interquartile range. Categorical data are described using number and percentage. Patient-level demographic measures were compared using the Chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables. Multilevel logistic regression examined the association between each technique and occurrence of neoplasia in the entire cohort, and it was also used to perform subgroup analyses according to IBD subtype. For each technique, three different analyses were carried out (unadjusted; adjusted for demographic factors associated with first analysis; adjusted for factors associated with the first analysis and for the other technique). Two-side P =0.05 was considered statistically significant (Stata 15.1, Stata Corp.). All comparisons were done on a base of complete-case analysis. Patients were censored at the time that neoplasia was detected during annual surveillance or at the end of the study. There were no strategies to prevent loss to follow-up, given the nature of a retrospective analysis. Percentages are rounded to the nearest integer. This cohort study is reported according to STROBE guidelines for reporting observational studies 25 .
Confounders and bias
The long follow-up of these patients exposes this cohort to confounders due to new developments in CRC surveillance that happened throughout the study period. Furthermore, there are several factors that might affect occurrence of neoplasia among these patients, such as age at diagnosis of PSC, age at diagnosis of IBD, use of CE and/or HD-WLE, bowel preparation, use of aminosalicylates, and use of biologicals. The statistical analysis adjusted for possible confounding factors, which were found to vary between groups. Finally, collection of data from electronic patient records is effective against recollection bias.
Results
Demographics
Ninety-one patients with PSC-IBD met the eligibility criteria for this study, resulting in an average follow-up of 3.95 person-years and a total follow-up of 360 person-years ( Fig. 1 ). There were 58 men (64%), median age at diagnosis of PSC was 39.2 years, and median age at diagnosis of IBD was 29.4 years. Sixty-three patients (70%) had UC, of whom 56 (90%) had pan-colonic inflammation, six (9%) had active disease only in the left side, and one (1%) had no inflammation detected. There were 17 patients with CD, 16 (94%) had pan-colonic inflammation, six (35%) had active disease in the ileum, and two (12%) had perianal disease. Among the 10 patients (11%) with unclassified IBD, all of them had pan-colonic inflammation and one (10%) had ileal disease. Data on IBD type were unavailable for one patient.
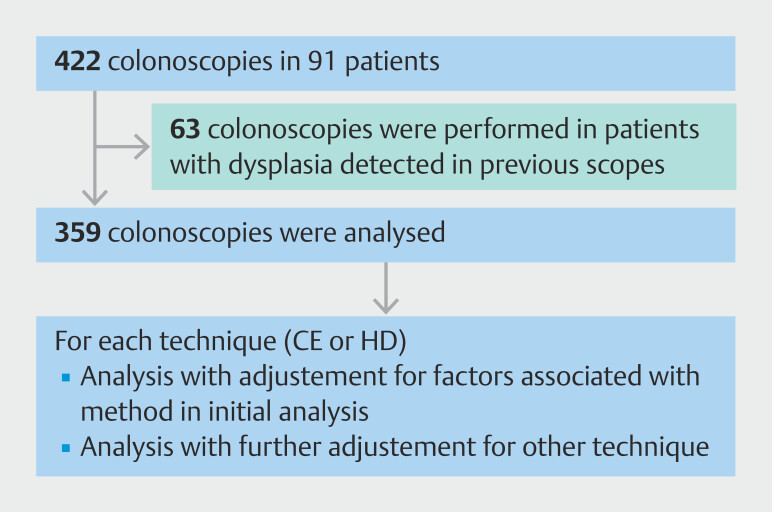
Fig. 1.
Number of patients and colonoscopies that met the eligibility criteria and how the outcome was analyzed.
Aminosalicylates (5-ASA) were used as monotherapy in 36 patients (40%) and as dual therapy with immunosuppressants and with biologics in 24 (26%) and 17 (19%), respectively. Intrahepatic strictures were the only manifestation of PSC in 55 patients (60%), while 23 (25%) had both intrahepatic and extrahepatic strictures. Fifty-seven patients (63%) used ursodeoxycholic acid for PSC. The 91 PSC-IBD patients had 422 colonoscopies in total, but 63 were excluded from analysis because there was evidence of neoplasia in a previous exam, leaving 359 colonoscopies to be analyzed.
Identification of neoplasia
Twenty-two patients (24%) had at least one neoplastic lesion identified. Random biopsies were used in 340 colonoscopies and in one occasion, identified dysplasia; however, targeted biopsies also identified dysplasia in this patient. The mean neoplastic lesion rate was much higher for the PSC-UC patients 0.87 (54/63) than for the PSC-CD patients 0.24 (4/17); however, the PSC-UC group includes one patient who had 20 hyperplastic polyps resected in the right colon, a potential case of PSC-UC with serrated polyposis syndrome. Removing this outlier gives a revised rate of PSC-UC mean neoplastic lesion rate of 0.55 (34/62). No SSLs with dysplasia were observed in this cohort. There was one case of moderately differentiated adenocarcinoma identified in the cecum 25 × 50 mm pT3 pN0 pM0 R0, managed by subtotal colectomy with ileorectal anastomosis; the patient is alive 8 years post-operatively ( Table 1 ).
Table 1 Distribution of lesions among patients and IBD subtype.
| Category | All PSC-IBD (n = 91) | PSC-UC (n = 63) |
PSC-CD
(n = 17)
|
PSC-IBDU (n = 10) |
| *Data about IBD subtype were unavailable for one patient. †This includes one patient who had 20 hyperplastic polyps in the right colon. IBD, inflammatory bowel disease; HP, hyperplastic polyp; IBDU, IBD unclassified; PSC, primary sclerosing cholangitis; SSL, sessile serrated lesion; UC, ulcerative colitis. | ||||
| Per patient with neoplasia | 22/91* (24%) | 18/63 (29%) | 3/17 (17%) | 1/10 (10%) |
| Dysplasia (all adenomas) | 10/91 (11%) | 8/63 (13%) | 2/17 (12%) | 0/10 (0%) |
| Serrated lesions (SSL and HP) | 14/91 (15%) | 11/63 (17%) | 2/17 (12%) | 1/10 (10%) |
| SSL | 5/91 (5 %) | 5/63 (8%) | 0/17 (0%) | 0/10 (0%) |
| Hyperplastic | 11/91 (12%) | 8/63 (13%) | 2/17 (12%) | 1 (10%) |
| Adenocarcinoma | 1/91 (1%) | 1/63 (2%) | 0/17 (0%) | 0/10 (0%) |
| Total neoplastic lesions | 59 | 54 | 4 | 1 |
| Dysplasia (all adenomas) | 13 | 11 | 2 | 0 |
| Serrated lesions (SSL and HP) | 45 | 42 | 2 | 1 |
| SSL | 8 | 8 | 0 | 0 |
| Hyperplastic | 37 † | 34 † | 2 | 1 |
| Adenocarcinoma | 1 | 1 | 0 | 0 |
Choice of endoscopic technique
Chromoendoscopy (CE)
PSC-IBD patients were divided into three categories: those where CE was never used “never”; those where CE was used for some surveillance procedures “sometimes”; and those patients where CE was used for all surveillance procedures “always” ( Table 2 ). There was a significant difference between year of first colonoscopy and use of CE; patients who had a mix of techniques were recruited earlier (median 2012) than those who had their surveillance with CE or with SD-WLE only (median 2015) ( P =0.002). Age at PSC diagnosis, age at IBD diagnosis, gender, type of IBD, and adequacy of bowel preparation were not significantly associated with endoscopic technique used ( Table 2 and Table 3 ). Although we recommended that CE should not be performed in patients with fair or inadequate bowel preparation, at the discretion of the endoscopist, in three patients with inadequate preparation, CE was still used.
Table 2 Patient-level demographics between dye spray groups.
| Variable | Category | Never (n = 19) | Sometimes (n = 54) | Always (n = 18) | P value |
| Figures are median interquartile range or number (percentage). IBD, inflammatory bowel disease; IBDU, IBD unclassified; PSC, primary sclerosing cholangitis: UC, ulcerative colitis. | |||||
| Year of first procedure | – | 2015 (2010–2018) | 2012 (2010–2014) | 2015 (2013–2018) | 0.002 |
| Age PSC diagnosed | – | 23 (16–55) | 40 (26–55) | 33 (26–48) | 0.21 |
| Age IBD diagnosed | – | 26 (15–37) | 34 (22–52) | 27 (21–41) | 0.14 |
| Gender | Female | 8 (42%) | 19 (35%) | 6 (33%) | 0.83 |
| Male | 11 (58%) | 35 (65%) | 12 (67%) | ||
| Type IBD | UC | 13 (72%) | 35 (65%) | 15 (83%) | 0.68 |
| Crohn’s | 3 (17%) | 12 (22%) | 2 (11%) | ||
| IBDU | 2 (11%) | 7 (13%) | 1 (6%) | ||
Table 3 Procedure-level demographics between dye spray groups.
| Variable | Category | No dye spray (n = 178) n (%) | Dye spray (n = 180) n (%) | P value |
| Bowel preparation | Inadequate | 19 (11%) | 3 (2%) | 0.21 |
| Adequate | 66 (37%) | 81 (45%) | ||
| Good | 52 (29%) | 38 (21%) | ||
| Excellent | 41 (23%) | 58 (32%) |
High-definition and standard definition white light endoscopy
PSC-IBD patients were divided into three categories: never used, sometimes done, all surveillance was done using HD-WLE or SD-WLE ( Table 4 ). Patients who had all procedures performed with SD-WLE were recruited to the screening program earlier (median 2010) than those who had all surveillance investigations done with HD-WLE (median 2017) ( P <0.001).
Table 4 Patient-level demographics between HD scope groups.
| Variable | Category | Never (n = 22) | Sometimes (n = 54) | Always (n = 14) | P value |
| Figures are median interquartile range or number (percentage). HD, high definition; IBD, inflammatory bowel disease; IBDU, IBD unclassified; UC, ulcerative colitis. | |||||
| Year of first procedure | – | 2010 2010, 2014 | 2013 2010, 2014 | 2017 2015, 2019 | <0.001 |
| Age PSC diagnosed | – | 39 23, 54 | 41 26, 54 | 25 15, 37 | 0.10 |
| Age IBD diagnosed | – | 26 21, 51 | 36 24, 50 | 18 13, 28 | 0.02 |
| Gender | Female | 5 (23%) | 20 (37%) | 7 (50%) | 0.23 |
| Male | 17 (77%) | 34 (63%) | 7 (50%) | ||
| Type IBD | UC | 15 (68%) | 35 (66%) | 12 (86%) | 0.51 |
| Crohn’s | 5 (23%) | 10 (19%) | 2 (14%) | ||
| IBDU | 2 (9%) | 8 (15%) | 0 (0%) | ||
Those who had a mix of HD and SD-WLE received the IBD diagnosis at an older age (median 36 years) than those who always were screened with HD-WLE (median 18 years) ( P =0.02).
Quality of bowel preparation was associated with use of SD or HD-WLE. Twenty percent of patients with excellent bowel preparation had colonoscopies with SD-WLE, while 37% of those with excellent bowel preparation used HD-WLE technology ( P <0.001), as seen in Table 5 .
Table 5 Procedure-level demographics between HD scope groups.
| Variable | Category | No HD scope (n = 198) n (%) | HD scope (n = 132) n (%) | P value |
| HD, high definition. | ||||
| Bowel prep | Inadequate | 16 (8%) | 2 (2%) | <0.001 |
| Adequate | 87 (44%) | 51 (39%) | ||
| Good | 56 (28%) | 30 (23%) | ||
| Excellent | 39 (20%) | 49 (37%) | ||
Logistic regression of endoscopic technique and neoplasia detection
Unadjusted multilevel logistic regression suggests that CE detected more neoplasia than just WLE (13.9% vs. 2.8%, OR 5.58, 95% CI 2.08–14.9, P =0.001) ( Table 6 ). When the analysis factored year of colonoscopy and age at diagnosis of IBD, CE maintained a significant difference (OR 5.95, 95% CI 1.55–22.8, P =0.009). After adjusting for previous confounders and for use of HD colonoscopes, CE was still significantly better at detecting neoplasia (OR 5.02, 95% CI 1.43–17.7, P =0.01).
Table 6 Association between endoscopic technique and neoplasia.
| Factor | Analysis | All neoplasia-n/N (%) | Odds ratio * | P | |
| *Odds ratio expressed as odds of dysplasia when dye spray/HD scope used
relative to odds when it was not used. † Adjusted for year of procedure and age at IBD diagnosis. ‡ As Adjusted Analysis 1, plus additional adjustment for use of HD scope. § Adjusted for year of procedure, age of IBD diagnosis, age of PSC diagnosis and bowel prep quality. ¶ As Adjusted Analysis 1, plus additional adjustment for use of dye spray. CI, confidence interval; HD, high definition; IBD, inflammatory bowel disease; PSC, primary sclerosing cholangitis. | |||||
| Not used | Used | (95% CI) | value | ||
| Dye spray | Unadjusted | 5/178 (2.8%) | 25/180 (13.9%) | 5.58 (2.08–14.9) | 0.001 |
| Adjusted 1 † | – | – | 5.95 (1.55–22.8) | 0.009 | |
| Adjusted 2 ‡ | – | – | 5.02 (1.43–17.7) | 0.01 | |
| HD scope | Unadjusted | 12/199 (6.0%) | 18/132 (13.6%) | 2.46 (1.14–5.30) | 0.02 |
| Adjusted 1 § | - | - | 1.87 (0.72–4.83) | 0.20 | |
| Adjusted 2 ¶ | - | - | 1.93 (0.69–5.40) | 0.21 | |
Unadjusted multilevel logistic regression suggests that HD-WLE detected more neoplasia than SD (OR 2.46, 95% CI 1.14–5.30, P =0.02). Nevertheless, the differences were no longer significant when adjusted for year of procedure, age of IBD diagnosis, age of PSC diagnosis, and bowel preparation quality (OR 1.87, 95% CI 0.72–4.83, P =0.20), and for use of dye spray on top of the previous confounders (OR 1.93, 95% CI 0.69–5.40, P =0.21).
Logistic regression of endoscopic technique and type of neoplasia
Unadjusted multilevel logistic regression suggests that CE (8.9%) identified more serrated neoplasia than WLE (1.7%) (OR 5.69, 95% CI 1.62–19.8, P =0.006), and HD-WLE detected more serrated neoplasia (9.1%) than SD (3.5%) (OR 2.74, 95% CI 1.05–7.16, P =0.04) ( Table 7 ). However, when adjusting for confounders including year of procedure, age at IBD and PSC diagnosis, and quality of bowel preparation, there was no significant difference.
Table 7 Association between endoscopic technique and serrated neoplasia.
| Factor | Analysis | Serrated neoplasia-n/N (%) | Odds ratio * | P | |
| *Odds ratio expressed as odds of dysplasia when dye spray/HD scope used
relative to odds when it was not used. † Adjusted for year of procedure and age of IBD diagnosis. ‡ As Adjusted Analysis 1, plus additional adjustment for use of HD scope. § Adjusted for year of procedure, age of IBD diagnosis, age of PSC diagnosis and bowel prep quality. ¶ As Adjusted Analysis 1, plus additional adjustment for use of dye spray. HD, high definition; IBD, inflammatory bowel disease; PSC, primary sclerosing cholangitis. | |||||
| Not used | Used | (95% CI) | value | ||
| Dye spray | Unadjusted | 3/178 (1.7%) | 16/180 (8.9%) | 5.69 (1.62–19.8) | 0.006 |
| Adjusted 1 † | – | – | 3.67 (0.79–17.0) | 0.10 | |
| Adjusted 2 ‡ | – | – | 3.50 (0.87–14.1) | 0.08 | |
| HD scope | Unadjusted | 7/199 (3.5%) | 12/132 (9.1%) | 2.74 (1.05–7.16) | 0.04 |
| Adjusted § | - | - | 2.09 (0.65–6.72) | 0.22 | |
| Adjusted 2 ¶ | - | - | 2.01 (0.62–6.57) | 0.24 | |
Unadjusted multilevel logistic regression suggests that CE (6.1%) detected more adenomatous polyps than WLE (1.1%) (OR 12.8, 95% CI 1.24–132, P =0.03) ( Table 8 ). This difference remained significant after accounting for year of procedure and age at IBD diagnosis (OR 14.7 (95% CI 1.41–153, P =0.02) and use of HD-WLE (OR 12.0, 95% CI 1.08–134, P =0.04).
Table 8 Association between endoscopic technique and adenomatous neoplasia.
| Factor | Analysis | Adenomatous neoplasia-n/N (%) | Odds ratio * | P | |
| *Odds ratio expressed as odds of dysplasia when dye spray/HD scope used
relative to odds when it was not used. † Adjusted for year of procedure and age of IBD diagnosis. ‡ Adjusted Analysis 1, plus additional adjustment for use of HD scope. § Adjusted for year of procedure, age of IBD diagnosis, age of PSC diagnosis and bowel prep quality. ¶ As Adjusted Analysis 1, plus additional adjustment for use of dye spray. HD, high definition; IBD, inflammatory bowel disease; PSC, primary sclerosing cholangitis. | |||||
| Not used | Used | (95% CI) | value | ||
| Dye spray | Unadjusted | 2/178 (1.1%) | 11/180 (6.1%) | 12.8 (1.24, 132) | 0.03 |
| Adjusted 1† | – | – | 14.7 (1.41, 153) | 0.02 | |
| Adjusted 2‡ | – | – | 12.0 (1.08, 134) | 0.04 | |
| HD scope | Unadjusted | 5/199 (2.5%) | 8/132 (6.1%) | 2.50 (0.80, 7.82) | 0.12 |
| Adjusted 1 § | – | – | 2.26 (0.63, 8.09) | 0.21 | |
| Adjusted 2 ¶ | – | – | 2.34 (0.66, 8.38) | 0.19 | |
Unadjusted multilevel logistic regression suggests that HD-WLE found more adenomatous neoplasia (6.1%) than SD (2.5%) (OR 2.50, 95% CI 0.80–7.82, P =0.12), but it was not significant when adjusting for confounders including year of procedure, age at IBD and PSC diagnosis, and quality of bowel preparation (OR 2.26, 95% CI 0.65–8.09, P =0.21), and use of dye spray (OR 2.34, 95% CI 0.66–8.38, P =0.19).
Logistic regression of endoscopic technique and neoplasia detection according to IBD subtype
Ulcerative colitis
Unadjusted multilevel logistic regression identified that CE detected more neoplasia (16%) than WLE (3%) (OR 8.10, 95% CI 1.72–38.2, P =0.008). When year of procedure and age of IBD diagnosis were factored into the analysis, the association remained (OR10.7, 95% CI 1.44–79.4, P =0.02), as well as when further adjustment was made for the previous variables and use of an HD scope (OR 8.86, 95% CI 1.33–59.2, P = 0.02) ( Supplementary Table 1 ).
Unadjusted multilevel logistic regression showed that HD-WLE detected more neoplasia (15%) than SD (8%) although it was not significant (OR 2.14, 95% 0.92–4.94, P =0.08).
Crohn’s disease
Unadjusted multilevel logistic regression showed that CE identified less neoplasia (4.4%) than WLE (5.1%) (OR 0.82, 95% CI 0.06–11.2, P =0.88) although it was not significant ( Supplementary Table 2 ). It was not possible to fit use of HD scopes into the regression because no cases of neoplasia were detected when HD scopes were not used. This also impacted the analysis of the association between use of HD scopes and detection of neoplasia. However, using Fisher’s exact test, the association was not statistically significant ( P =0.07).
Discussion
This study demonstrated a higher frequency of colorectal neoplasia detection in patients with PSC-IBD using chromoendoscopy compared with WLE, even when adjusting for use of HD colonoscopes. Use of chromoendoscopy increased detection of both SSLs and adenomatous polyps, although only adenomatous lesions remained significant once adjusted for confounders.
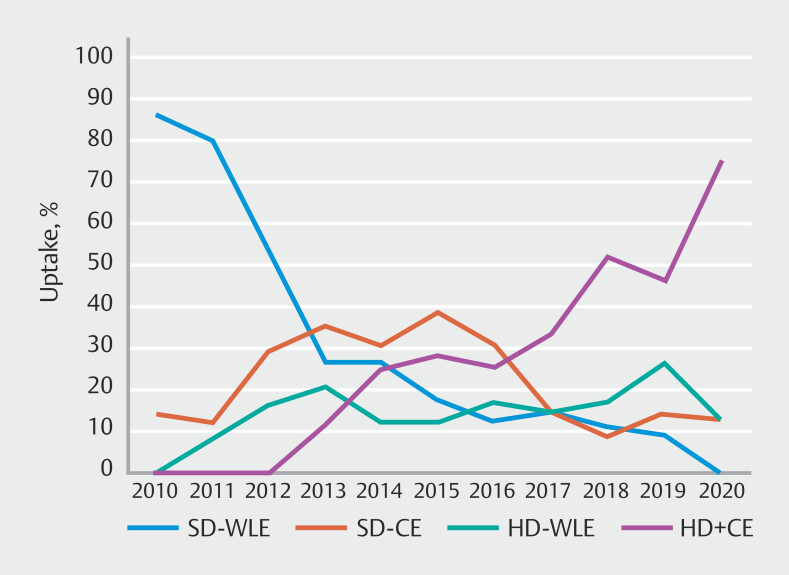
We found that adoption of both CE and HD colonoscopes in PSC-IBD was slow over the 10 years and was not universal by 2020. Similarly, rates of adoption for CE in IBD surveillance have been poor. Even in expert centers, rates of CE use only reached 76%, equivalent to our results 26 . Slow implementation and lack of systematic guidance for training in CE are factors that need consideration. Despite the British Society of Gastroenterology recommendation since 2010 22 , CE was less used than SD-WLE among our cohort until 2013 and from 2015 onward, it was used in about 60% of colonoscopies in these patients ( Fig. 2 ). Furthermore, training endoscopists in use of CE takes time, more so given increased detection of lesions and need to determine whether to act on the findings. This gap was resolved only recently when the European Society of Gastrointestinal Endoscopy (ESGE) released guidance on competence standards for optical diagnosis of diminutive colorectal polyps 27 .
Fig. 2.
Uptake of colonoscopies (percentage) using standard-definition colonoscopes and chromoendoscopy (CE), high-definition colonoscopes only (HD), a combination of CE and high-definition colonoscopes (CE + HD), and standard-definition colonoscopes only (SD) throughout the study duration.
There has been considerable debate about the additional value of chromoendoscopy in the HD era. In a network meta-analysis in patients with IBD alone, dye-based CE was not superior to HD-WLE alone to detect polyps 28 . The combined introduction of HD-WLE and CE may explain why in our cohort the median year of first procedure in the “never” chromoendoscopy category was later than the “sometimes” category. A cohort study using virtual and dye-based CE compared to HD-WLE in patients with IBD detected roughly twice more serrated lesions with CE compared with HD-WLE, 17% vs 8% 29 . Another cohort study identified that CE use increased the likelihood of detection of serrated neoplasia (Adjusted OR 1.99, 95% CI 1.13–3.51) 30 . The increased detection of serrated neoplasia, usually subtle and non-polypoid, may have similarly made CE more effective in our series. Furthermore, “invisible” dysplasia is more frequently reported in PSC-IBD cohorts than IBD-only cohorts, 66% vs 21% in one large study, which might also be revealed by CE for targeted biopsy 14 . The rate of progression from low-grade dysplasia to high-grade dysplasia and finally CRC has not been elucidated in patients with PSC-IBD 9 .
In our PSC-IBD cohort, both SSL and adenomatous polyps were detected at surveillance colonoscopy, with higher numbers of serrated than adenomatous lesions. This may be due to an underappreciated understanding that PSC-related colonic lesion progress via the serrated pathway, or those techniques used heighted SSL detection. A possible role for the serrated carcinogenesis pathway in contributing to the increased risk of colon cancer in PSC is not well understood, but warrants further investigation, especially following the high frequency of serrated lesions detected here. PSC cancers and serrated lesions both have a strong predilection for the right side of the colon 31 , and serrated nonconventional dysplasia was associated with higher CRC progression risk in IBD 14 , . Moreover, serrated polyps with dysplasia are associated with advanced neoplasia risk in IBD patients 32 . Given that PSC is characterized by low-grade, right-sided inflammation, dysbiosis and possible immune dysregulation, it is possible that the serrated carcinogenesis pathway predominates and contributes to the disproportionate cancer risk in this condition. Further research will be required to establish this.
The differences in colonic neoplasia between PSC-UC and PSC-CD were evident in our cohort and confirm previous reports of increased chance of neoplasia in those with PSC-UC 11 12 . This subgroup had the majority of lesions identified in this cohort, including the only case of CRC among our patients. They also had 90% of all sessile serrated polyps identified, further supporting the argument that PSC-UC patients might have an increased risk of nonconventional neoplasia compared to PSC-CD and IBD-only populations.
There are some limitations in our study. As a retrospective cohort, there is possible historical bias. Our service is a national reference center for the management of IBD and PSC, hence our cohort of patients may not be representative of the overall population with PSC-IBD. The pathology reporting for this study follows the Vienna criteria and included serrated lesions; however recent publications have highlighted the role of nonconventional dysplasia which were mainly published after the study period and are not reported here 33 . We did not consider the use of virtual chromoendoscopy (e.g., NBI) as the ESGE guidelines that proposed this were only published in 2019 21 . Finally, the sample size of this study is relatively small, which may underpower the subgroup analyses, and the findings ideally should be replicated in larger randomized controlled trials. However, it is unlikely that resources would be devoted to a major trial in this niche area.
Dye-based CE enhanced detection of all neoplastic lesions (adenomatous and serrated) at surveillance colonoscopy in PSC-IBD, with results suggesting that it detected more neoplasia than WLE even when adjusting for confounding factors, including use of HD endoscopes. These results highlight that implementing recommendations and recognizing their results takes time. It has been almost a decade since the first SCENIC guidelines on use of chromoendoscopy for CRC surveillance in IBD, and we are finally identifying the impact of the technique in real-life patient care. Furthermore, these results support recommendations by international societies, including the most recent SCENIC update from 2021 34 , that CE positively impacts neoplasia screening in patients with IBD. They also suggest a benefit of this technique to this high-risk group with PSC-IBD.
The role of virtual chromoendoscopy in identifying neoplasia in PSC-IBD patients has yet to be clarified, and guideline recommendations to consider its use in general IBD surveillance as equivalent to dye-based CE post-date our cohort 34 35 36 . Artificial intelligence-based neoplasia detection (computer-aided detection [CADe]) for IBD neoplasia is being developed (IBD-CADe) 37 ; however, it is unclear if CADe algorithms developed on non-PSC-IBD datasets will be generalizable to PSC-IBD surveillance 38 .
Conclusions
In conclusion we found that dye-based chromoendoscopy increased neoplasia detection even when HD colonoscopes were used in a PSC-IBD cohort over a 10-year period. The majority of neoplasia was detected in the PSC-UC subgroup.
Acknowledgement
The authors would like to thank the Oxford IBD Cohort Study Investigators: Philip Allan, Tim Ambrose, Carolina Arancibia-Cárcamo, Adam Bailey, Eleanor Barnes, Elizabeth Bird-Lieberman, Jan Bornschein, Barbara Braden, Oliver Brain, Jane Collier, Alessandra Geremia, Bruce George, Lucy Howarth, Kelsey Jones, Paul Klenerman, Rebecca Palmer, Fiona Powrie, Astor Rodrigues, Jack Satsangi, Alison Simmons, Holm Uhlig, Alissa Walsh, Kate Lynch, the National Institute for Health and Care Research Oxford Biomedical Research Centre, and the Hepatology Clinical Research Network Thames Valley and South Midlands.
Conflict of Interest Simon PL Travis has served as a paid consultant to AbbVie, Allergan, Amgen, Asahi, Bioclinica, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, ChemoCentryx, Cosmo, Enterome, Equillium, Ferring, GSK, Genentech, Genzyme, Giuliani SpA, Immunocore, Immunometabolism, Janssen, Lilly, MSD, Merck, Mestag, Neovacs, Novo Nordisk, NPS Pharmaceuticals, Pfizer, Proximagen, Receptos, Roche, Satisfai Health, Sensyne Health, Shire, Sigmoid Pharma, Sorriso, Takeda, Topivert, UCB, VHsquared, Vifor, and Zeria. He has received grants and/or has grants pending from AbbVie, ECCO, Helmsley Trust, IOIBD, Janssen, Lilly, Norman Collisson Foundation, Pfizer, UCB, UKIERI, and Vifor. He has received honoraria from AbbVie, Amgen, Biogen, Ferring, Lilly, Pfizer, and Takeda. He has had travel/accommodation expenses covered or reimbursed by AbbVie, Amgen, Biogen, Ferring, Lilly, JNJ, Pfizer, and Takeda. Emma Culver: Speaking Fees: Horizon Therapeutics, Advanz (Intercept), Albireo, Dr Falk Pharma, Gilead, GSK; Consulting Fees: Advanz (Intercept), Horizon Therapeutics, Ipsen, Mirum, Moderna, Sanofi, Zenus Pharma; Grant Support: Jansen, Innovate UK, PSC Support, Wellcome Trust; Institutional Funding Support: BRC Oxford NIHR (UK), Oxford Charitable Fund, Research Capability Fund. James E East has served on clinical advisory boards for Lumendi, Boston Scientific, and Paion; has served on the clinical advisory board and has share options in Satisfai Health; and reports speaker fees from Falk, Janssen and Medtronic. The remaining authors declare no conflict of interests.
These authors share senior authorship.
Supplementary Material
References
- 1.Zheng H-H, Jiang X-L. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a meta-analysis of 16 observational studies. Eur J Gastroenterol Hepatol. 2016;28:383–390. doi: 10.1097/MEG.0000000000000576. [DOI] [PubMed] [Google Scholar]
- 2.Selinger CP, Andrews JM, Titman A et al. Long-term follow-up reveals low incidence of colorectal cancer, but frequent need for resection, among Australian patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;12:644–650. doi: 10.1016/j.cgh.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Lutgens MWMD, van Oijen MGH, van der Heijden GJMG et al. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis. 2013;19:789–799. doi: 10.1097/MIB.0b013e31828029c0. [DOI] [PubMed] [Google Scholar]
- 4.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jess T, Simonsen J, Jørgensen KTet al. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years Gastroenterology 2012143375–810.quiz e13–4 [DOI] [PubMed] [Google Scholar]
- 6.Molodecky NA, Kareemi H, Parab R et al. Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology. 2011;53:1590–1599. doi: 10.1002/hep.24247. [DOI] [PubMed] [Google Scholar]
- 7.Olén O, Erichsen R, Sachs MC et al. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet. 2020;395:123–131. doi: 10.1016/S0140-6736(19)32545-0. [DOI] [PubMed] [Google Scholar]
- 8.de Vries AB, Janse M, Blokzijl H et al. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J Gastroenterol. 2015;21:1956–1971. doi: 10.3748/wjg.v21.i6.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmela C, Peerani F, Castaneda D et al. Inflammatory bowel disease and primary sclerosing cholangitis: A review of the phenotype and associated specific features. Gut Liver. 2018;12:17–29. doi: 10.5009/gnl16510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halliday JS, Djordjevic J, Lust M et al. A unique clinical phenotype of primary sclerosing cholangitis associated with Crohn’s disease. J Crohns Colitis. 2012;6:174–181. doi: 10.1016/j.crohns.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Braden B, Halliday J, Aryasingha S et al. Risk for colorectal neoplasia in patients with colonic Crohn’s disease and concomitant primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2012;10:303–308. doi: 10.1016/j.cgh.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Zheng H-H, Jiang X-L. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a meta-analysis of 16 observational studies. Eur J Gastroenterol Hepatol. 2016;28:383–390. doi: 10.1097/MEG.0000000000000576. [DOI] [PubMed] [Google Scholar]
- 13.Choi W-T, Yozu M, Miller GC et al. Nonconventional dysplasia in patients with inflammatory bowel disease and colorectal carcinoma: a multicenter clinicopathologic study. Mod Pathol. 2020;33:933–943. doi: 10.1038/s41379-019-0419-1. [DOI] [PubMed] [Google Scholar]
- 14.Zhang R, Lauwers GY, Choi W-T. Increased risk of non-conventional and invisible dysplasias in patients with primary sclerosing cholangitis and inflammatory bowel disease. J Crohns Colitis. 2022;16:1825–1834. doi: 10.1093/ecco-jcc/jjac090. [DOI] [PubMed] [Google Scholar]
- 15.Shah SC, Ten Hove JR, Castaneda D et al. High risk of advanced colorectal neoplasia in patients with primary sclerosing cholangitis associated with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2018;16:1106–1.113E6. doi: 10.1016/j.cgh.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 16.Hu AB, Burke KE, Kochar B et al. Yield of random biopsies during colonoscopies in inflammatory bowel disease patients undergoing dysplasia surveillance. Inflamm Bowel Dis. 2021;27:779–786. doi: 10.1093/ibd/izaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Brien MJ, Yang S, Mack C et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol. 2006;30:1491–1501. doi: 10.1097/01.pas.0000213313.36306.85. [DOI] [PubMed] [Google Scholar]
- 18.Maaser C, Sturm A, Vavricka SR et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144–164. doi: 10.1093/ecco-jcc/jjy113. [DOI] [PubMed] [Google Scholar]
- 19.Lichtenstein GR, Loftus E V, Isaacs KL et al. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am J Gastroenterol. 2018;113:481–517. doi: 10.1038/ajg.2018.27. [DOI] [PubMed] [Google Scholar]
- 20.Rubin DT, Ananthakrishnan AN, Siegel CA et al. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterol. 2019;114:384–413. doi: 10.14309/ajg.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 21.Bisschops R, East JE, Hassan C et al. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline-Update 2019. Endoscopy. 2019;51:1155–1179. doi: 10.1055/a-1031-7657. [DOI] [PubMed] [Google Scholar]
- 22.Cairns SR, Scholefield JH, Steele RJ et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–689. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 23.Feuerstein JD, Rakowsky S, Sattler L et al. Meta-analysis of dye-based chromoendoscopy compared with standard- and high-definition white-light endoscopy in patients with inflammatory bowel disease at increased risk of colon cancer. Gastrointest Endosc. 2019;90:186–1950. doi: 10.1016/j.gie.2019.04.219. [DOI] [PubMed] [Google Scholar]
- 24.Rutter MD, East J, Rees CJ et al. British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/Public Health England post-polypectomy and post-colorectal cancer resection surveillance guidelines. Gut. 2020;69:201–223. doi: 10.1136/gutjnl-2019-319858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Elm E, Altman DG, Egger M et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 26.Smith SCL, Cannatelli R, Bazarova A et al. Performance measures in inflammatory bowel disease surveillance colonoscopy: Implementing changes to practice improves performance. Dig Endosc. 2020;32:592–599. doi: 10.1111/den.13521. [DOI] [PubMed] [Google Scholar]
- 27.Houwen BBSL, Hassan C, Coupé VMH et al. Definition of competence standards for optical diagnosis of diminutive colorectal polyps: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2022;54:88–99. doi: 10.1055/a-1689-5130. [DOI] [PubMed] [Google Scholar]
- 28.Imperatore N, Castiglione F, Testa A et al. Augmented Endoscopy for Surveillance of Colonic Inflammatory Bowel Disease: Systematic Review With Network Meta-analysis. J Crohns Colitis. 2019;13:714–724. doi: 10.1093/ecco-jcc/jjy218. [DOI] [PubMed] [Google Scholar]
- 29.Iacucci M, Hassan C, Fort Gasia M et al. Serrated adenoma prevalence in inflammatory bowel disease surveillance colonoscopy, and characteristics revealed by chromoendoscopy and virtual chromoendoscopy. Can J Gastroenterol Hepatol. 2014;28:589–594. doi: 10.1155/2014/386540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeaman F, Thin L. The yield of dysplasia and serrated lesions in a single-centre tertiary inflammatory bowel disease cohort. Therap Adv Gastroenterol 2023; 16: 17562848231167280. [DOI] [PMC free article] [PubMed]
- 31.Fung BM, Lindor KD, Tabibian JH. Cancer risk in primary sclerosing cholangitis: Epidemiology, prevention, and surveillance strategies. World J Gastroenterol. 2019;25:659–671. doi: 10.3748/wjg.v25.i6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Jong ME, Nagtegaal ID, Vos S et al. Increased colorectal neoplasia risk in patients with inflammatory bowel disease and serrated polyps with dysplasia. Dig Dis Sci. 2022;67:5647–5656. doi: 10.1007/s10620-022-07485-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harpaz N, Goldblum JR, Shepherd NA et al. Colorectal dysplasia in chronic inflammatory bowel disease: A contemporary consensus classification and interobserver study. Hum Pathol. 2023;138:49–61. doi: 10.1016/j.humpath.2023.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Rabinowitz LG, Kumta NA, Marion JF. Beyond the SCENIC route: updates in chromoendoscopy and dysplasia screening in patients with inflammatory bowel disease. Gastrointest Endosc. 2022;95:30–37. doi: 10.1016/j.gie.2021.07.024. [DOI] [PubMed] [Google Scholar]
- 35.Murthy SK, Feuerstein JD, Nguyen GC et al. AGA Clinical Practice Update on Endoscopic Surveillance and Management of Colorectal Dysplasia in Inflammatory Bowel Diseases: Expert Review. Gastroenterology. 2021;161:1043–1.051E7. doi: 10.1053/j.gastro.2021.05.063. [DOI] [PubMed] [Google Scholar]
- 36.Bisschops R, East JE, Hassan C et al. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline-Update 2019. Endoscopy. 2019;51:1155–1179. doi: 10.1055/a-1031-7657. [DOI] [PubMed] [Google Scholar]
- 37.Guerrero Vinsard D, Fetzer JR, Agrawal U et al. Development of an artificial intelligence tool for detecting colorectal lesions in inflammatory bowel disease. iGIE. 2023;2:91–1.01E8. [Google Scholar]
- 38.Ahmad HA, East JE, Panaccione R et al. Artificial intelligence in inflammatory bowel disease: implications for clinical practice and future directions. Intest Res. 2023;21:283–294. doi: 10.5217/ir.2023.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.