Abstract
Introduction
Atopic dermatitis (AD) is a frequent disease in infants with diverse clinical evolution. Although multiple studies have assessed inflammatory changes in chronic AD, little is known about the molecular transition from symptomatic stage to clinical remission without pharmacotherapy.
Objective
The aim of the study was to evaluate clinical and inflammatory factors and its relationship with AD clinical evolution.
Methods
Three groups of participants older than 10 years of age were recruited; 2 AD groups and 1 non-AD group. The AD-remission group (more than 1 year without AD symptoms and without pharmacotherapy), the AD-persistent group (AD symptoms and pharmacotherapy), and 1 non-AD group. We measured eosinophil peroxidase (EPX), eosinophil cationic protein (ECP), IgE autoantibodies against these antigens, and natural moisturizing factor (NMF).
Results
Different inflammatory profiles within each group were observed: AD-persistent group is characterized by a high frequency of IgE autoantibodies (55.5%), contrasting with the low occurrence in the non-AD group (2%) and a moderate frequency in the AD-remission group (21.4%). A similar distribution was observed for the other type 2 inflammatory biomarkers (Eosinophils, total IgE, EPX, ECP) and NMF.
Conclusion
Patients with AD-remission maintain a minimal T2 inflammation. We identified different potential biomarkers for prognosis of AD evolution. Further studies are necessary to evaluate the mechanisms that allow the coexistence of the inflammatory process without clinical symptoms.
Keywords: Autoantibodies, Autoimmunity, Hypersensitivity, Peroxidase, Remission, Spontaneous
Graphical abstract
Introduction
Atopic dermatitis (AD) is a prevalent skin disease during infancy with a significant impact in the quality of life of the patient and their family.1,2 AD affects 20%–40% of children under 6 years of age, and different studies suggest a remission rate of 20%–80% during the first decade of life.3, 4, 5, 6, 7 The wide range in remission rate across studies may be explained by environmental and genetic factors8 but also reflects differences in the design of the studies, for example, studies with tertiary care centers usually included AD patients with more severe symptoms and lower remission rate, and population-based studies included a more heterogeneous range of patients. Furthermore, a consensus definition of AD remission is not yet available. For some authors, AD remission refers to clinical control secondary to treatment (pharmacological remission).9 However, several studies support that a fraction of patients with AD in childhood achieve sustained clinical remission over time without pharmacological treatment.3, 4, 5, 6, 7 Characteristics of the patients who achieve AD remission without pharmacotherapy have been little studied.
AD pathogenesis involves different mechanisms; most patients exhibit type 2 inflammation with high IgE levels and serum eosinophilia.10,11 Several autoantigens have been associated with AD severity12, 13, 14, 15, 16 and recently IgE autoantibodies against eosinophil peroxidase (EPX), and eosinophil cationic protein (ECP) were detected.17 Natural moisturizing factor (NMF) has been associated with AD activity, and AD age of onset have been associated with AD severity.18, 19, 20, 21 Despite these associations, the impact of these biomarkers in AD evolution has not been explored thoroughly. Considering that prediction of AD evolution could have significant medical implications, the aim of this study was to investigate clinical and molecular biomarkers associated with the clinical evolution of AD. The identification of these biomarkers will help to a better understanding of the disease and contribute to the development of clinical diagnostic tools.
Methods
AD definitions and study populations groups
Atopic dermatitis (AD) was defined according to Hanifin and Rajka criteria, and AD patients with a SCORAD over 10 points were recruited. AD patients with diagnoses of other diseases that could cause eczema or similar skin injuries were excluded (eg, mastocytosis, contact dermatitis, and prurigo).
AD persistent group were patients with AD onset before 6 years of age in whom AD symptoms continued after 10 years of age.
AD-remissiongroup consisted of patients with AD onset before 6 years old but experiencing remission before 10 years old. Remission was defined as the absence of AD skin symptoms for more than 1 year, less than one point in the numerical rating scale (NRS) of pruritus (not pruritus at the begining of the phrase) and no need for pharmacotherapy. The occasional use of moisturizing creams was not considered pharmacotherapy as it could be used for other indications.
The non-AD group comprised healthy subjects without skin, autoimmune, or metabolic diseases.
Participants receiving therapies that could affect the interpretation of serum markers were excluded.
Study design
The main objective of the study was to evaluate the association between clinical and molecular factors and AD evolution. With a cross-sectional design, we evaluated different factors (Sex, AD age of onset, SCORAD, IgE anti-EPX, IgE anti-ECP, total IgE, serum eosinophils, NMF). Participants were recruited from 5 AD specialist centers from Colombia.
EPX and ECP blood levels
EPX and ECP were measure using EPX ELISA Kit (A79299) and ECP ELISA Kit (MBS700481) following the manufacturer's recommendations. Briefly, specific antibodies for each antigen were pre-coated onto a microtiter plate. Following incubation, the wells were washed and then incubated with Biotinylated Anti-EPX Antibody or Anti-ECP, which binds the captured EPX or ECP present in each well. Following incubation, unbound biotinylated detection antibody is removed by wash, and a streptavidin conjugate was added to the wells and the microtiter plate was incubated. Following incubation and washing, TMB substrate solution was then used to visualize the enzymatic reaction. The concentration of EPX and ECP can then be calculated by reading the OD (Optical density) absorbance at 450 nm in a microplate reader and referring to the standard curve.
Total IgE, and specific IgE autoantibodies
Total IgE levels and specific IgE againt mites (Dermatophagoides pteronyssinus, Dermatophagoides farinae, Blomia tropicalis), pet dander (Felis domesticus, Canis familiaris) in the serum samples were determined using a flouroenzyme immunoassay (ImmunoCap System, Uppsala, Sweden). A limitation of the InmmunoCap system is that the reading range of the equipment is up to 100 KUA/mL. When the levels were above the reading range of the equipment, the sample was diluted, depending on the case, 1:5 or 1:10, and the total concentration was calculated by conversion. Each patient serum sample was evaluated individually.17,22,23
Antigens ECP and EPX were obtained as recombinant proteins and we quantified IgE autoantibodies against these human antigens using a protocol previously described17,22 (Supplementary material S1). The sera used for the quantification of IgE were depleted of IgG by immunoaffinity depletion. The cut-off value for serum anti-EPX IgE and anti-ECP IgE were previously defined in 60 healthy controls. The results were expressed in OD. The absorbance at 405 nm was determined using a spectrophotometer.
Evaluation of allergenicity
The CD203c-based basophil activation assay was used for ex vivo evaluation of the allergenic activity of autoantibodies as described previously.17 Basophils of each patient were stimulated with different concentrations of EPX or ECP antigen (0.01, 0.1, or 1 μg/mL). The basophil activation assay was quantified as the percentage of basophils expressing more CD203c than the critical point, which was 10.0% of the basophils incubated with buffer only.17,24
Natural moisturizing factors
Natural moisturizing factors (NMF) was assessed using a non-invasive tape-stripping technique.18,20,21,25 In brief, stratum corneum was recollected through 10 consecutive tape strips from the same area. The selected skin was left untreated for at least 5 days before tape strip collection. The 4 first tape-stripe was discarded to improve sample homogeneity and limit contamination error.20 Small marks were made on the skin using a pen, after placing the first tape-strip to ensure consistent placement of each subsequent tape-strip. In AD patients tape strips were recollect from an injured skin, and for a non-lesioned sample located between 10 and 20 cm from injured skin sample. Each tape was pressed against the skin with a roller for 5 s (approx. 200 g/cm3). After applying and releasing the pressure, tape-strips were removed and immediately frozen on dry ice, then stored in a −70 C freezer until the time of shipment.
NMF was defined as the sum of the concentrations from the components histidine, pyrrolidone-5- carboxylic acid (PCA), and cis and trans isomers of urocanic acid (cis-UCA and trans-UCA).21,25 These components were measure using high-performance liquid chromatography (HPLC) analysis. The concentrations of NMF were corrected for total protein and expressed as nmol/ug protein.
Ethical considerations
The study received approval from the Ethics committees (Code F-CBI-023 act 029). Written informed consent was obtained from all participants and, for minor patients, from their parents. We also request assent from minors.
Statistical analysis
Statistical analyses were conducted using JAMOVI, (version 2.3 Sidney, Australia) and GraphPad Prism 10 (La Jolla, CA). Considering the data distribution, Pearson's χ2 test was used to evaluate the differences in categorical variables between groups. Student's t-test was used to test intergroup differences between 2 samples normally distributed. Kruskal-Wallis's test and Mann-Whitney test were employed for non-normally distributed data. Correlations were assessed with the Spearman coefficient (r). Multivariate regression was performed using AD evolution (remission or persistence) as the outcome (dependent variable). A “p” value of <0.05 was considered statistically significant and clinically relevant based on dispersion measures (standard deviation, confidence interval, interquartile range). Considering previous studies,20,23,26 and taking into consideration the potential of AD-remission (20–60%), we considered a sample size of at least 80 patients per group to be adequate.
We performed multiple regression as an exploratory analysis to identify potential predictor variables of the evolution of AD (AD remission or persistent AD). Those variables with a p < 0.1 were considered potential predictors following statistical recommendations.27 Collinearity between the model variables was assessed using the variance inflation factor (VIF) where a value greater than 5 indicates collinearity.
Results
Patients’ characteristics
Sociodemographic and clinical characteristics are presented in Table 1. AD groups had higher frequency of atopy and allergic comorbidities compared to the non-AD group. The AD-persistent group had higher levels of total IgE and eosinophils. These biomarkers were similar between the AD-remission group and control group. Atopy was evaluated by sIgE against house dust mites or pet dander, and it was similar between AD groups and lower in the control group.
Table 1.
General characteristics
| General characteristics | AD-remission n = 90 | AD-persistence n = 84 | Non-AD n = 100 | p |
|---|---|---|---|---|
| Sex: Female, n (%) | 48 (53.4) | 42 (50) | 63 (63) | 0.3 |
| Age median ± SD | 15 (SD 2.8) | 15 (SD 2.8) | 22 (SD 10.2) | 0.2 |
| AD onset age median ± SD | 3 (SD 1.39) | 2 (SD 1.49) | N/A | 0.5 |
| AD remission age median ± SD | 8 (SD 3.4) | N/A | N/A | N/A |
| Asthma, n (%) | 14 (15.5) | 23 (27.3) | 6 (6) | 0.03 |
| Rhinitis, n (%) | 26 (30.9) | 42 (50) | 15 (15) | 0.04 |
| Food IgE sensitization, n (%) | 4 (4.4) | 8 (9.5) | 2 (2) | 0.3 |
| Atopy, n (%) | 88 (97.7%) | 78 (92.8%) | 18 (18%) | 0.03 |
| Total IgE (IU/ml), median ± SD | 177 (SD 287) | 404 (SD 574) | 122 (SD 87) | 0.05 |
| Eosinophil serum (cells/mL), median ± SD | 138 (SD 234) | 294 (SD 248) | 119 (SD 54) | 0.06 |
| SCORAD index, median ± SD | N/A | 32 (SD 15) | N/A | N/A |
Atopy evaluated by sIgE against house dust mites or pet dander was similar between AD groups and lower in control group. Abbreviations: Atopic dermatitis (AD), severe score for atopic dermatitis (SCORAD), N/A: Not apply. Median, SD: standard difference, “p” value according to Pearson's χ2 test, Kruskal-Wallis test and Mann-Whitney test
EPX and ECP levels and IgE autoantibodies
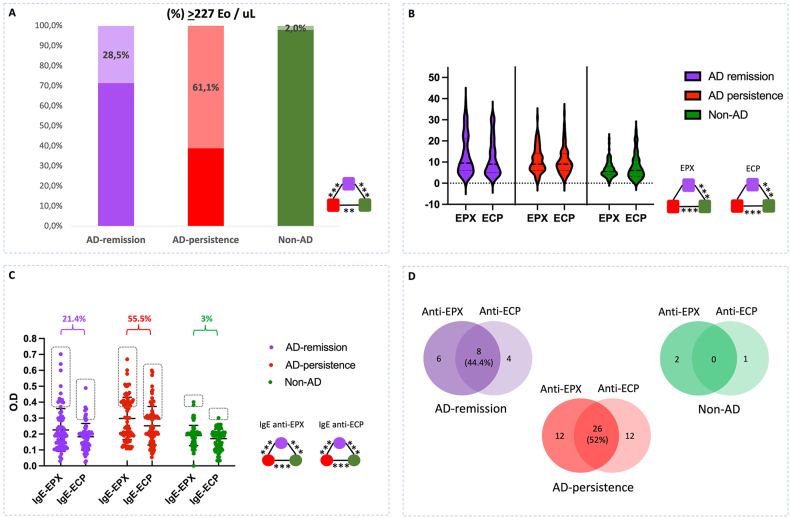
Eosinophil levels in the non-AD group were lower compared with the AD-persistent group or the AD-remission group (Table 1). In the non-AD group, 227 eosinophils/uL represented the high normal level (median + 2SD); 61% of patients in the AD-persistent group had eosinophils above this cutoff, and 28.5% in the AD-remission group (Fig. 1A). Levels of EPX and ECP were lower in the non-AD group and there were no differences between the AD groups (Fig. 1B). Patients with anti-EPX IgE, anti-ECP IgE, were higher in the AD-persistent group (Fig. 1C). Patients in AD groups had both IgE autoantibodies (Fig. 1D). In non-AD patients only 3 participants had IgE autoantibodies.
Fig. 1.
EPX and ECP levels and IgE autoantibodies. A: levels of eosinophils. In non-AD group, the normal high range of eosinophil levels was 227 cells/uL (median + 2SD). B: EPX and ECP levels. C: ant-EPX IgE and anti-ECP levels. D: patients with 1 or both IgE autoantibodies. Eosinophil peroxidase (EPX), and eosinophil cationic protein (ECP). ∗p < 0.05, ∗∗0.01, ∗∗∗<0.001, according to Pearson's χ2 test, Kruskal-Wallis test and Mann-Whitney test
Significant association was observed between EPX and ECP levels in AD groups (Fig. 2A). IgE autoantibodies were associated with SCORAD in the AD persistent group (Fig. 2B).
Fig. 2.
Interaction between levels of eosinophil biomarkers. A: Spearman correlation between eosinophils (Eo), EPX, ECP, anti-EPX IgE, anti-ECP IgE, in AD-remission group (ADr), AD-persistence group (ADp) and non-AD group (nonAD). B: SCORAD According to the presence or not of anti-EPX IgE, anti-ECP IgE. ∗p < 0.05, ∗∗0.01, ∗∗∗<0.001
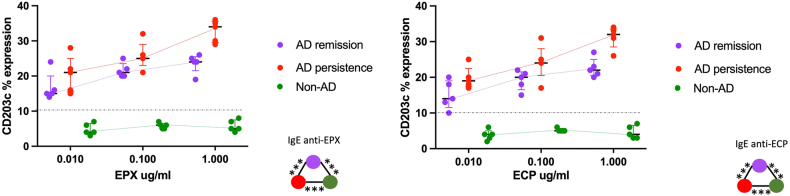
EPX and ECP allergenicity
IgE autoantibodies against EPX and ECP induced BAT reaction in both AD groups (Fig. 3). In the AD-persistent group CD203c expression was higher than in the AD-remission group in the 3 EPX and ECP concentrations. Non-AD patients had not CD203c expression.
Fig. 3.
Human antigens allergenicity. CD203c expression was measure in each group according to Median and interquartile range. Statistical comparison (∗p < 0.05), was done between AD remission group and AD persistence group. ∗p < 0.05, Kruskal-Wallis test and Mann-Whitney test
NMF status
NMF level was different in each group; higher in the non-AD group and lower in the AD-persistence group (Fig. 4). In the AD-persistence group, patients with low NMF had higher SCORAD (p = 0.008). There was a tendency to correlation between NMF and levels of IgE autoantibodies, but it was not statistically significant.
Fig. 4.
Natural moisturizing factor (NMF) was the sum of 4 components (histidine, pyrrolidone-5-carbolix acid, cis and trans isomers of urocanic acid) expressed as mmol/g protein. ∗∗∗p < 0.001, according to, Kruskal-Wallis test and Mann-Whitney test
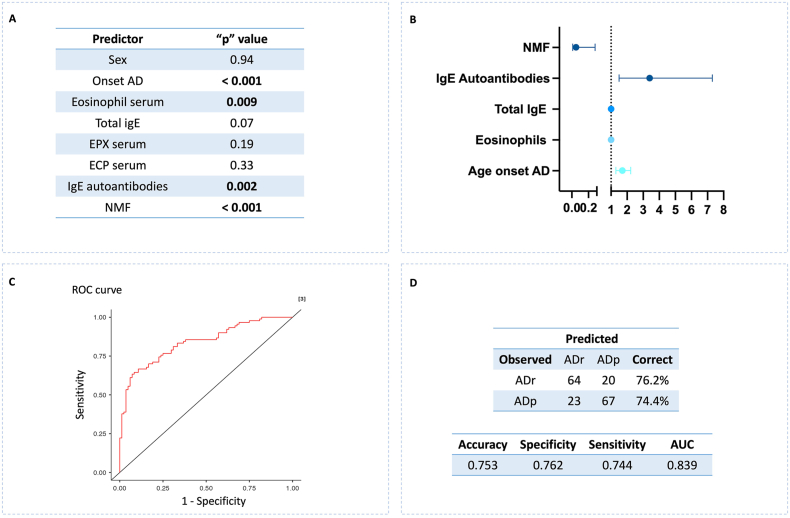
Factors associated with AD evolution
To identify prognosis biomarkers for AD remission or AD persistence, we performed a multivariable regression model (Fig. 5). Among the variables explored (Fig. 5A), those that had a p < 0.1 were evaluated as potential prognosis biomarkers; NMF, AD age of onset, IgE autoantibodies, total IgE and serum eosinophils (Fig. 5A). NMF had a protective effect and had an inverse association with the outcome AD persistence. Anti-EPX IgE, and anti-ECP IgE, were included together as IgE autoantibodies to improve prediction performance. There was not collinearity between variables (VIF <5), which means that each one makes their own contribution to the model. The predictive performance of the model had an accuracy greater than 75% (Fig. 5B, C, D).
Fig. 5.
Potential predictors of AD evolution. A: Variables evaluated as potential biomarkers in AD groups. B; risk effect of potential biomarkers with p < 0.1 C: ROC curve of the regression model including potential biomarkers with p < 0.1. D: Accuracy evaluation of the regression model. AD-remission group (ADr), AD-persistence group (ADp), AUC: Area under the curve
Discussion
Atopic dermatitis is a frequent disease in infants. Some AD patients achieve clinical remission during childhood, while other patients persist with symptoms into adolescence and adulthood. Over the last 2 decades numerous studies have suggested that biomarkers could be associated with AD severity; however, it is unclear which factors determine the clinical AD evolution. Some studies have suggested changes in the inflammatory and proteomic profile of both symptomatic and non-symptomatic skin in AD patients or following AD therapies.9,28 However, little has been studied on the immunological and skin profiles of those patients who achieved AD remission without pharmacological treatment.
This study provided interesting information about the AD pathogenesis and its evolution:
-
(i)
Patients with AD remission exhibits distinctive features than AD patients or non-AD subjects.
-
(ii)
The presence of autoantibodies is an important factor in AD evolution.
-
(iii)
Although epithelial barrier damage and type 2 inflammation participate in AD evolution, both are independent mechanisms.
-
(iv)
Different biomarkers could be useful in a prediction model for AD evolution.
Patients with AD-remission presented a particular immunological response compared to that observed in AD patients and non-AD subjects. In AD-remission, clinical control was achieved without pharmacotherapy, however subclinical inflammation persists, similar to what occurs in other type 2 inflammatory diseases, and it has been called “minimal persistent inflammation” (MPI) in asthma and rhinitis.29,30 From a technical point of view, we define MPI in AD as the presence of inflammatory markers above the levels observed in the control group. To our knowledge, this is the first study demonstrating MPI in AD patients with clinical remission without pharmacotherapy for an extended period. Our finding supports previous observations reported by Tsoi et al,28 based in global transcriptome suggesting that the AD evolution is associated with quantitative rather than qualitative molecular characteristics. AD clinical control without pharmacotherapy despite MPI may be attributed to an increase in the tolerance threshold secondary to the development of immune or skin regulatory mechanisms. Theoretically the increase in the expression of anti-inflammatory mediators and the formation of regulatory T lymphocytes could reduce the intensity of type 2 inflammation to a level where it is clinically not sufficient for the development of symptoms.31,32 This hypothesis is supported by the immunological changes of allergic rhinitis patients with immunotherapy with allergens, where type 2 inflammation persist but regulatory response is stimulated. Another example is food allergy where some patients reach a tolerance to foods like milk or eggs but sIgE response persist.33,34 Furthermore, the formation of other skin moisturizing components due to hormonal changes or microbiota changes could compensate the lack of NMF components.35,36 The evaluation of these patients over a longer period is necessary to define whether they present relapses or clinical control continues indefinitely.
Multiple autoantigens have been reported in AD and their recognition by immunoglobulin E has been associated with AD severity.12,37,38 The release of EPX and ECP by eosinophils appears to play an important role in type 2 inflammatory process,39 and they have been used as in vitro parameters of inflammation in asthma and skin diseases.39, 40, 41, 42 The reason for the reaction to self-proteins is unknown. One hypothesis is that, after a chronic recognition and production of IgE to environmental allergens, these immunoglobulins by molecular mimicry could recognize similar epitopes presents in human proteins and epitope spreading could enhance autoreactivity. This hypothesis is supported in AD for some autoantigens.13,23,43 Another hypothesis suggest that the IgE response against autoantigens may be due to their greater expression in AD, which facilitates their recognition by the polyclonal IgE that occurs in this disease.44 This hypothesis is supported in this study by the fact that about half of the subjects in the AD groups presented a response with EPX and ECP, maybe because both are expressed in eosinophil grains. Additionally, IgE autoantibodies were more frequent in patients with high SCORAD, and its inflammatory capacity was demonstrated with BAT, suggesting an association between these autoantibodies and the intensity of the inflammatory process.
Like what was reported by Basu et al,20 we observed differences between patients with AD-remission, AD-persistence and non-AD in relation to NMF. According to the statistical analyses, immunological and NMF changes were independent factors, suggesting that both contribute to disease activity by they have different origins. Similarly, although eosinophils and the IgE response are part of type 2 inflammation, their production responds to different cytokines, which could explain the low correlation found between the levels of EPX, ECP, autoantibodies and eosinophils in the blood.45 As has been described in other studies, some populations located in tropic region like ours, present high levels of eosinophils and IgE, even among individuals without allergic diseases, which makes it difficult to find correlations.45, 46, 47
From a clinical perspective, it would be useful to know whether AD in children will persist over time requiring anti-inflammatory treatment or if it will have a sustained remission that allows the future suspension of pharmacotherapy. Different molecular and clinical biomarkers for AD severity and prognosis have been described21,25,39,48,49 and our results support the usefulness of some of them especially when they are evaluated together for example in a regression model. Although the magnitude of the effect for the level of serum eosinophils and total IgE was low, in the regression analysis they demonstrated a significant contribution. The AD age of onset was also a factor associated with AD evolution, with remission being more common among patients with onset of the disease before 3 years of age. The highest prediction coefficient in the model was provided by the presence of anti-EPX and anti-ECP autoantibodies, and by NMF levels. Unfortunately, these variables are not easily accessible in clinical practice, which highlights the need to implement the use of tape-stripping technique in clinical practice.
Our study includes some limitations. A longitudinal study presents several advantages over our cross-sectional design to study AD progression. However, considering that the main objective of the study was to establish the immunological and clinical patterns related to the persistence or remission of AD, the cross-sectional design used allows us to identify those variables with the highest probability of being useful as predictors of the disease. Because AD is a disease where multiple mechanisms intervene, it is difficult to establish the role of IgE in the pathogenesis of the disease. In fact, some previous studies suggest that most AD patients treated with omalizumab have not a good clinical response. However, regardless of IgE autoantibodies is and epiphenomenon or no, our results suggest that it could be a useful biomarker to predict AD evolution.
In conclusion, AD remission is a particular endophenotype where the absence of symptoms and the presence of subclinical inflammation coexist. It is necessary to confirm our results through prospective studies and evaluates potential prognostic biomarkers for example, the role of IgE autoantibodies in AD evolution. It is also necessary to explore the possible factors that increase the tolerance threshold in AD-remission patients.
Abbreviations
AD: Atopic Dermatitis, BAT: Basophil Activation Test, EPX: Eosinophil Peroxidase, ECP: Eosinophil Cationic Protein, MPI: minimal persistent inflammation, NMF: Natural Moisturizing Factor, IgE: Immunoglobulin E, SCORAD: Score Atopic Dermatitis, VIF: Variance Inflation Factor.
Availability of data and materials
Considering that the information collected includes personal data of the patients, the study data can be shared upon request to the corresponding author with prior authorization from the ethics committee.
Author contributions
JS, LA, and AS contributed the central idea of the article.
JS, LA, AS, AC, DA OV, MNR, and JRU contributed equally to the collection of information and the writing of the manuscript.
Ethics approval
The study received approval from the Ethics committees of the University of Antioquia and the Hospital “Alma Mater de Antioquia” (Code F-CBI-023 act 029 from University of Antioquia).
Authors’ consent for publication
All authors agreed with the final version of the manuscript and its publication.
Funding
This article was founding by Group of Experimental and Clinical Allergy, Hospital “Alma Mater de Antioquia”, University of Antioquia (Medellín, Colombia) and by Colombian Association of Allergy, Asthma and Immunology (ACAAI). ACAAI is a non-profit scientific association that received funding from the Pfizer and Sanofi laboratories for the development of academic initiatives about atopic dermatitis in Colombia. The funders did not influence the development of the proposal or its execution, nor did they participate in the debates and decisions regarding this article.
Declaration of competing interest
JS, have been advisors and speakers for Novartis, Sanofi, FAES, Galderma, Nettle, Glaxon, Astrazeneca, Thermofhiser. DA has been a consultant and speaker for Novartis and Glaxon. MV-L have been advisor and speaker for Abbvie, Boehringer ingelheim, Galderma, Janssen, Novartis, Pfizer, and Sanofi, RG have been advisors and speakers for Novartis, Sanofi. RG have been advisors and speakers for Novartis, Sanofi. AS, AC, LA, OV, MNR, JRU have not conflict of interest to declare.
Acknowledgments
We extend our gratitude to the “Alma Mater de Antioquia” Hospital, “Unidad Alergologica” Clinic, “San Vicente de Paul” Hospital, the “Asociación Colombiana de Alergia Asma e Inmunología” Organization, and the University of Antioquia, for their financial and material support for this project.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2024.100983.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Lloyd-Lavery A., Solman L., Grindlay D.J.C., Rogers N.K., Thomas K.S., Harman K.E. What’s new in atopic eczema? An analysis of systematic reviews published in 2016. Part 3: nomenclature and outcome assessment. Clin Exp Dermatol. 2019;44(4):376–380. doi: 10.1111/ced.13886. [DOI] [PubMed] [Google Scholar]
- 2.Sánchez-Pérez J., Daudén-Tello E., Mora A.M., Lara Surinyac N. Impact of atopic dermatitis on health-related quality of life in Spanish children and adults: the PSEDA study. Actas Dermosifiliogr. 2013;104(1):44–52. doi: 10.1016/j.ad.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 3.von Kobyletzki L., Svensson Å., Apfelbacher C., Schmitt J. Factors that predict remission of infant atopic dermatitis: a systematic review. Acta Derm Venereol. 2015;95(4):389–394. doi: 10.2340/00015555-1941. [DOI] [PubMed] [Google Scholar]
- 4.Wananukul S., Chatproedprai S., Tempark T., Phuthongkamt W., Chatchatee P. The natural course of childhood atopic dermatitis: a retrospective cohort study. Asian Pac J Allergy Immunol. 2015;33(2):161–168. doi: 10.12932/AP0498.33.2.2015. [DOI] [PubMed] [Google Scholar]
- 5.Somanunt S., Chinratanapisit S., Pacharn P., Visitsunthorn N., Jirapongsananuruk O. The natural history of atopic dermatitis and its association with Atopic March. Asian Pac J Allergy Immunol. 2017;35(3):137–143. doi: 10.12932/AP0825. [DOI] [PubMed] [Google Scholar]
- 6.Berna R., Mitra N., Hoffstad O., Wubbenhorst B., Nathanson K.L., Margolis D.J. Using a machine learning approach to identify low-frequency and rare. JID Innov. 2021;1(4) doi: 10.1016/j.xjidi.2021.100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berdyshev E., Kim J., Kim B.E., et al. Stratum corneum lipid and cytokine biomarkers at age 2 months predict the future onset of atopic dermatitis. J Allergy Clin Immunol. 2023;151(5):1307–1316. doi: 10.1016/j.jaci.2023.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Garmhausen D., Hagemann T., Bieber T., et al. Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy. 2013;68(4):498–506. doi: 10.1111/all.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang T.S., Bieber T., Williams H.C. Are the concepts of induction of remission and treatment of subclinical inflammation in atopic dermatitis clinically useful? J Allergy Clin Immunol. 2014;133(6):1615–16125.e1. doi: 10.1016/j.jaci.2013.12.1079. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z.Y., Zheng Y.X., Xu F., et al. Epidermal keratinocyte-specific STAT3 deficiency aggravated atopic dermatitis-like skin inflammation in mice through TSLP upregulation. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1273182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fassett M.S., Braz J.M., Castellanos C.A., et al. IL-31-dependent neurogenic inflammation restrains cutaneous type 2 immune response in allergic dermatitis. Sci Immunol. 2023;8(88) doi: 10.1126/sciimmunol.abi6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valenta R., Natter S., Seiberler S., et al. Molecular characterization of an autoallergen, Hom s 1, identified by serum IgE from atopic dermatitis patients. J Invest Dermatol. 1998;111(6):1178–1183. doi: 10.1046/j.1523-1747.1998.00413.x. [DOI] [PubMed] [Google Scholar]
- 13.Valenta R., Duchene M., Pettenburger K., et al. Identification of profilin as a novel pollen allergen; IgE autoreactivity in sensitized individuals. Science. 1991;253(5019):557–560. doi: 10.1126/science.1857985. [DOI] [PubMed] [Google Scholar]
- 14.Schmid-Grendelmeier P., Fluckiger S., Disch R., et al. IgE-mediated and T cell-mediated autoimmunity against manganese superoxide dismutase in atopic dermatitis. J Allergy Clin Immunol. 2005;115(5):1068–1075. doi: 10.1016/j.jaci.2005.01.065. [DOI] [PubMed] [Google Scholar]
- 15.Mossabeb R., Seiberler S., Mittermann I., et al. Characterization of a novel isoform of alpha-nascent polypeptide-associated complex as IgE-defined autoantigen. J Invest Dermatol. 2002;119(4):820–829. doi: 10.1046/j.1523-1747.2002.00518.x. [DOI] [PubMed] [Google Scholar]
- 16.Zeller S., Rhyner C., Meyer N., Schmid-Grendelmeier P., Akdis C.A., Crameri R. Exploring the repertoire of IgE-binding self-antigens associated with atopic eczema. J Allergy Clin Immunol. 2009;124(2):278–285. doi: 10.1016/j.jaci.2009.05.015. 85.e1-7. [DOI] [PubMed] [Google Scholar]
- 17.Sánchez J., Sánchez A., Munera M., et al. Presence of IgE autoantibodies against eosinophil peroxidase and eosinophil cationic protein in severe chronic spontaneous urticaria and atopic dermatitis. Allergy Asthma Immunol Res. 2021;13(5):746–761. doi: 10.4168/aair.2021.13.5.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koppes S.A., Kemperman P., Van Tilburg I., et al. Determination of natural moisturizing factors in the skin: Raman microspectroscopy versus HPLC. Biomarkers. 2017;22(6):502–507. doi: 10.1080/1354750X.2016.1256428. [DOI] [PubMed] [Google Scholar]
- 19.Wärnberg Gerdin S., Lie A., Asarnoj A., et al. Impaired skin barrier and allergic sensitization in early infancy. Allergy. 2022;77(5):1464–1476. doi: 10.1111/all.15170. [DOI] [PubMed] [Google Scholar]
- 20.Basu M.N., Mortz C.G., Jensen T.K., Barington T., Halken S. Natural moisturizing factors in children with and without eczema: associations with lifestyle and genetic factors. J Eur Acad Dermatol Venereol. 2022;36(2):255–262. doi: 10.1111/jdv.17787. [DOI] [PubMed] [Google Scholar]
- 21.Rinnov M.R., Halling A.S., Gerner T., et al. Skin biomarkers predict development of atopic dermatitis in infancy. Allergy. 2023;78(3):791–802. doi: 10.1111/all.15518. [DOI] [PubMed] [Google Scholar]
- 22.Sánchez J., Sánchez Biol A., Múnera Biol M., García E., López J.F. Immunoglobulin E and G autoantibodies against eosinophil proteins in children and adults with asthma and healthy subjects. World Allergy Organ J. 2023;16(2) doi: 10.1016/j.waojou.2023.100742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sánchez J., Munera M., Arango J., Cardona R., Puerta L. IgE auto-antibodies to human fatty acid-binding proteins in atopic dermatitis patients. Curr Trends Immunol. 2020;21:29–39. [Google Scholar]
- 24.Ye Y.M., Yang E.M., Yoo H.S., Shin Y.S., Kim S.H., Park H.S. Increased level of basophil CD203c expression predicts severe chronic urticaria. J Kor Med Sci. 2014;29(1):43–47. doi: 10.3346/jkms.2014.29.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersson A.M., Sølberg J., Koch A., et al. Assessment of biomarkers in pediatric atopic dermatitis by tape strips and skin biopsies. Allergy. 2022;77(5):1499–1509. doi: 10.1111/all.15153. [DOI] [PubMed] [Google Scholar]
- 26.Sánchez J., Sánchez A., Cardona R. Clinical characterization of patients with chronic spontaneous urticaria according to anti-TPO IgE levels. J Immunol Res. 2019;2019 doi: 10.1155/2019/4202145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heus P., Damen J.A.A.G., Pajouheshnia R., et al. Poor reporting of multivariable prediction model studies: towards a targeted implementation strategy of the TRIPOD statement. BMC Med. 2018;16(1):120. doi: 10.1186/s12916-018-1099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsoi L.C., Rodriguez E., Stölzl D., et al. Progression of acute-to-chronic atopic dermatitis is associated with quantitative rather than qualitative changes in cytokine responses. J Allergy Clin Immunol. 2020;145(5):1406–1415. doi: 10.1016/j.jaci.2019.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X.J., Liu C., Zhang S., et al. ILC3-like ILC2 subset increases in minimal persistent inflammation after acute type II inflammation of allergic rhinitis and inhibited by Biminkang: plasticity of ILC2 in minimal persistent inflammation. J Leukoc Biol. 2022;112(6):1445–1455. doi: 10.1002/JLB.3MA0822-436RR. [DOI] [PubMed] [Google Scholar]
- 30.Canonica G.W., Compalati E. Minimal persistent inflammation in allergic rhinitis: implications for current treatment strategies. Clin Exp Immunol. 2009;158(3):260–271. doi: 10.1111/j.1365-2249.2009.04017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Victor J.R., Nahm D.H. Mechanism underlying polyvalent IgG-induced regulatory T cell activation and its clinical application: anti-idiotypic regulatory T cell theory for immune tolerance. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1242860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuji G., Yamamura K., Kawamura K., Kido-Nakahara M., Ito T., Nakahara T. Regulatory mechanism of the IL-33-IL-37 Axis via aryl hydrocarbon receptor in atopic dermatitis and psoriasis. Int J Mol Sci. 2023;24(19) doi: 10.3390/ijms241914633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penagos M., Durham S.R. Allergen immunotherapy for long-term tolerance and prevention. J Allergy Clin Immunol. 2022;149(3):802–811. doi: 10.1016/j.jaci.2022.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Kubota K., Nagakura K.I., Ejiri Y., Sato S., Ebisawa M., Yanagida N. Natural history of cow's milk allergy in children aged 6-12 years. Pediatr Allergy Immunol. 2023;34(12) doi: 10.1111/pai.14064. [DOI] [PubMed] [Google Scholar]
- 35.Nouwen A.E.M., Karadavut D., Pasmans S.G.M.A., et al. Natural moisturizing factor as a clinical marker in atopic dermatitis. Allergy. 2020;75(1):188–190. doi: 10.1111/all.13942. [DOI] [PubMed] [Google Scholar]
- 36.Jurakic Toncic R., Jakasa I., Sun Y., et al. Stratum corneum markers of innate and T helper cell-related immunity and their relation to the disease severity in Croatian patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2021;35(5):1186–1196. doi: 10.1111/jdv.17132. [DOI] [PubMed] [Google Scholar]
- 37.Natter S., Seiberler S., Hufnagl P., et al. Isolation of cDNA clones coding for IgE autoantigens with serum IgE from atopic dermatitis patients. Faseb J. 1998;12(14):1559–1569. doi: 10.1096/fasebj.12.14.1559. [DOI] [PubMed] [Google Scholar]
- 38.Seiberler S., Natter S., Hufnagl P., Binder B.R., Valenta R. Characterization of IgE-reactive autoantigens in atopic dermatitis. 2. A pilot study on IgE versus IgG subclass response and seasonal variation of IgE autoreactivity. Int Arch Allergy Immunol. 1999;120(2):117–125. doi: 10.1159/000024229. [DOI] [PubMed] [Google Scholar]
- 39.Breuer K., Kapp A., Werfel T. Urine eosinophil protein X (EPX) is an in vitro parameter of inflammation in atopic dermatitis of the adult age. Allergy. 2001;56(8):780–784. doi: 10.1034/j.1398-9995.2001.056008780.x. [DOI] [PubMed] [Google Scholar]
- 40.Remes S., Korppi M., Remes K., Savolainen K., Mononen I., Pekkanen J. Serum eosinophil cationic protein (ECP) and eosinophil protein X (EPX) in childhood asthma: the influence of atopy. Pediatr Pulmonol. 1998;25(3):167–174. doi: 10.1002/(sici)1099-0496(199803)25:3<167::aid-ppul6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 41.Kim T.Y., Park H.J., Kim C.W. Eosinophil cationic protein (ECP) level and its correlation with eosinophil number or IgE level of peripheral blood in patients with various skin diseases. J Dermatol Sci. 1997;15(2):89–94. doi: 10.1016/s0923-1811(97)00614-2. [DOI] [PubMed] [Google Scholar]
- 42.Haas N., Motel K., Czarnetzki B.M. Comparative immunoreactivity of the eosinophil constituents MBP and ECP in different types of urticaria. Arch Dermatol Res. 1995;287(2):180–185. doi: 10.1007/BF01262329. [DOI] [PubMed] [Google Scholar]
- 43.Fedorov A.A., Ball T., Mahoney N.M., Valenta R., Almo S.C. The molecular basis for allergen cross-reactivity: crystal structure and IgE-epitope mapping of birch pollen profilin. Structure. 1997;5(1):33–45. doi: 10.1016/s0969-2126(97)00164-0. [DOI] [PubMed] [Google Scholar]
- 44.Stern L.J., Clement C., Galluzzi L., Santambrogio L. Non-mutational neoantigens in disease. Nat Immunol. 2024;25(1):29–40. doi: 10.1038/s41590-023-01664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maurer M., Altrichter S., Schmetzer O., Scheffel J., Church M.K., Metz M. Immunoglobulin E-mediated autoimmunity. Front Immunol. 2018;9:689. doi: 10.3389/fimmu.2018.00689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zakzuk J., Mercado D., Bornacelly A., et al. Hygienic conditions influence sensitization to Blomia tropicalis allergenic components: results from the FRAAT birth cohort. Pediatr Allergy Immunol. 2019;30(2):172–178. doi: 10.1111/pai.13004. [DOI] [PubMed] [Google Scholar]
- 47.Caraballo L., Zakzuk J., Lee B.W., et al. Particularities of allergy in the tropics. World Allergy Organ J. 2016;9:20. doi: 10.1186/s40413-016-0110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pucci N., Lombardi E., Novembre E., et al. Urinary eosinophil protein X and serum eosinophil cationic protein in infants and young children with atopic dermatitis: correlation with disease activity. J Allergy Clin Immunol. 2000;105(2 Pt 1):353–357. doi: 10.1016/s0091-6749(00)90087-3. [DOI] [PubMed] [Google Scholar]
- 49.Jenerowicz D., Czarnecka-Operacz M., Silny W. Selected eosinophil proteins as markers of inflammation in atopic dermatitis patients. Acta Dermatovenerol Croat. 2006;14(2):73–80. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Considering that the information collected includes personal data of the patients, the study data can be shared upon request to the corresponding author with prior authorization from the ethics committee.