Abstract
This cross-sectional study investigated the associations between urinary sodium (UNa) to potassium (UK) ratio, different phenotypes of elevated blood pressure (BP), and microalbuminuria (MAU) in a cohort of the Tehran Lipid and Glucose Study (TLGS). Adult participants (n = 1782, mean age of 43.0 ± 13.7 years and 46.0% were men) were recruited (2015–2017) for measurements of spot urinary metabolites, i.e., Na, K, creatinine (Cr), microalbumin, and BP. Multinomial logistic regression was used to estimate the relative risk ratios (RRR) of elevated BP phenotypes [i.e., isolated systolic (ISH), diastolic (IDH), and systolic-diastolic (SDH) hypertension], and binary logistic regression was used to estimate odds ratios (ORs) of MAU across quintile categories and per each SD-increment of UNa-K ratio. Mean UNa, UK, and its ratio was 137 ± 57.4, 72.1 ± 36.6 mmol/L, and 2.31 ± 1.41, respectively. Subjects with UNa-K > 3.14 had higher prevalence of ISH (3.4 vs. 1.1%), SDH (11.0 vs. 6.2%), and MAU (14.1 vs. 6.2%) (P for all < 0.05). Highest compared to the lowest UNa-K ratio values (> 3.14 vs. <1.23) was associated with an increased probability of SDH (RRR = 1.79, 95% CI 1.09–3.19) and MAU (OR = 2.53, 95% CI 1.23–5.20). Every 1 SD-increment of the UNa-K ratio was associated with a 29 and 38% increased chance of having SDH and MAU, respectively. Our findings imply that a high UNa-K ratio may be a potential risk factor for elevated BP and renal dysfunction.
Keywords: Sodium, Potassium, Blood pressure, Hypertension phenotypes, Microalbuminuria
Subject terms: Medical research, Epidemiology
Introduction
Hypertension (HTN), a progressive cardiovascular syndrome caused by complex and interrelated etiologies1, is a leading modifiable cause of premature death affecting over a quarter of the adult population worldwide2,3. Microalbuminuria (MAU), a marker of early nephropathy, primarily manifests as renal microvascular damage4,5. A direct and continuous association exists between MAU and elevated blood pressure (BP), making them detrimental due to cardiovascular disease (CVD) and all-cause mortality4,5.
Excess sodium (Na) intake is established as a strong risk factor for developing HTN and appears to be associated with different levels of albuminuria6–8. Dietary guidelines recommend reducing Na intake to less than 2000 mg/d and increasing K intake to more than 3500 mg/d9,10, to effectively reduce the risk of HTN and CVD11,12. High loading of Na along with a low potassium (K) diet may have a synergistic effect on the development of HTN13. Existing literature consistently reported a positive association between urinary Na-to-K (UNa-K) ratio and elevated BP14–16.
Although all forms of HTN have in common the finding of elevated blood pressure, it reveals heterogeneous pathophysiology and risk factors among different phenotypes, i.e., known as isolated systolic (ISH), diastolic (IDH), and systolic-diastolic (SDH) hypertension17,18. While existing literature supports the biological plausibility linking high Na intake to elevated BP19,20, the precise nature of this relationship remains complex and multifaceted. Existing studies have often focused on general population19,20, neglecting to explore phenotype-specific variations in response to Na exposure. The extent to which multi-nature distinction interact with dietary Na intake to affect HTN risk remains to be fully understood. On the other hand, the relationships of Na and K with albuminuria have not been fully elucidated, and the literature remains inconclusive21–23.
To address these knowledge gaps, this study investigates the associations between the UNa-K ratio and elevated BP phenotypes and MAU in a large, nationally representative adult population recruited from the cohort of Tehran Lipid and Glucose Study (TLGS).
Materials and methods
Study population
This cross-sectional sectional study was conducted in the framework of the Tehran Lipid and Glucose Study (TLGS), a population-based cohort study initiated in 1999 on a representative sample of males and females aged ≥ 3 years to investigate and prevent non-communicable diseases24. For this study, adult men and women (n = 2069, age ≥ 19 years) with completed measurements on spot urinary Na, K, creatinine, and microalbumin, as well as demographics, anthropometrics, and serum biochemical measurements were recruited from the sixth examination of the TLGS (2014–2017). The participants who reported regular (n = 258) or occasional (n = 27) use of antihypertensive medications (e.g., angiotensin converting enzyme inhibitors, angiotensin receptor blockers), or those with unknown information about antihypertensive treatment (n = 2) were excluded. The final sample for the cross-sectional analyses consisted of 1782 participants.
The study protocol was conducted based on the Declaration of Helsinki. The ethics committee of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences evaluated and approved the study protocol (Approval number: IR.SBMU.ENDOCRINE.REC.1402.116). Informed written consent form was completed by all the TLGS participants at the day of examination.
Assessments of covariates
Information on demographics, education, occupation, marital status, medical history, smoking habits, and medications was completed by trained interviewers at baseline and follow-up examinations25. Details of measuring anthropometric variables (i.e., body weight, height, and waist circumference)25, physical activity (PA)26, and serum biochemical variables [i.e., fasting (FSG) and 2 h-serum glucose (2 h-SG), triglycerides (TG), and high-density lipoprotein cholesterol (HDL-C)27) have been reported by the TLGS research group elsewhere.
Systolic (SBP) and diastolic (DBP) blood pressures were measured using a standard mercury sphygmomanometer calibrated by the Institute of Standards and Industrial Research of Iran28. Blood pressure was measured twice on the participants’ right arm, after a 15-minute rest in a sitting position, with at least a 30-second interval between two measurements. The two measurements’ mean was considered the participant’s BP.
Details of urine sampling and measurements have been described in detail elsewhere29,30. The second voiding of spot urine samples were collected from participants between 7:00 AM and 9:00 AM, following an overnight fast of 10–12 h. Aliquots (representative portions) of these casual urine samples were then frozen and shipped to the central laboratory of the TLGS for analysis. Urinary Na and K concentrations were determined using flame photometry (Screen lyte, Hospitex Diagnostics, Florence, Italy). This method ensures high accuracy, as evidenced by the intra-assay and inter-assay coefficients of variation (CVs) being ≤ 2.8% and ≤ 4.8% for UNa and UK, respectively. Spot urine (Cr) concentration was measured using the Jaffe method. This method also demonstrated good precision, with both inter- and intra-assay CVs being ≤ 5%. Urinary microalbumin concentration was measured using an ELISA kit (Padtan ELM Company, Tehran, Iran) and a microplate ELISA reader. Intra- and inter-assay coefficients of variations (CVs) were 9.3% and 9.8%, respectively.
Dietary assessment
The usual dietary intakes of the participants over the previous year were assessed using a validated semi-quantitative 147-item FFQ. Details of dietary assessment in the TLGS were described elsewhere31. Usual dietary intake of Na and K were also obtained from nutritional data and are reported as mg/d. The FFQ provided the mean intake of Na and K of the participants over the last year.
Definition of terms and outcomes
According to recent American College of Cardiology (ACC)/American Heart Association (AHA) guidelines, introducing the HTN threshold of at least 130/80 mm Hg33, HTN phenotypes were defined as: ISH (SBP ≥ 130 and DBP < 80 mm Hg), IDH (SBP < 130 and DBP ≥ 80 mm Hg), and combined systolic and diastolic hypertension (SDH; SBP ≥ 130 and DBP ≥ 80 mm Hg)33–35.
Microalbuminuria was defied using sex-specific classification of microalbumin-creatinine ratio MCR (mg/mmol)36,37 as: Normal, i.e., MCR < 3.5 in men and < 2.5 in women, and MAU as 3.5 < MCR < 35 in men 2.5 < MCR < 25 in women.
Types 2 diabetes (T2D) was defined as the FSG ≥ 126 mg/dL or 2 h-SG ≥ 200 mg/dL, or using glucose-lowering medications38.
The four BP index measures, including pulse pressure (PP), mid-blood pressure (MBP), mean arterial pressure (MAP), and mean proportional arterial pressure (MPAP) were calculated based on combining SBP and DBP into a single index, with the following formula:
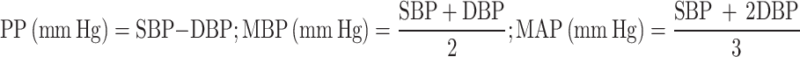 |
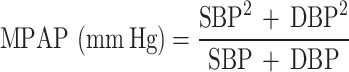 |
MBP and MAP are weighted means with constant weights, in which MBP gives equal weight to SBP and DBP, while MAP weights SBP one-third and DBP two-thirds. MPAP uses weights based on the relative contribution of each BP measurement to their sum; since SBP is typically higher, MPAP inherently assigns a greater weight to SBP, resulting in a generally higher value compared to MBP and MAP39. These BP indices are differentially associated with incidence of CVD and all-cause mortality39,40.
Statistical methods
Statistical analyses were conducted using the SPSS for Windows version 20 (SPSS Inc., Chicago, IL, USA). Descriptive statistics are reported as means (SD), median (inter-quartile range), and percentages for continuous normal- and non-normal distributed variables, and categorical variables, respectively. Analysis of variance with a Bonferroni post hoc test (for continues normal-distributed variables) was used; Chi-square test (for categorical variables) or independent-sample median test (for nonparametric variables) were used to compare subject’s study characteristics.
Multinomial logistic regression was used to estimate the relative risk ratio (RRR) of HTN phenotypes [isolated systolic (ISH), diastolic (IDH), and systolic-diastolic (SDH) hypertension], and binary logistic regression was used to estimate odds ratio (OR) of MAU across quintile categories (i.e., quintile 1, quintile 2–4, and quintile 5) and per each SD-increment of UNa-K ratio. Unadjusted- and multivariable-adjusted multinomial logistic regression coefficients are expressed in terms of relative risk ratios (RRRs) and 95% confidence intervals (95% CIs), which compare the relative probabilities of the outcomes occurring between the exposure groups41,42. Potential confounding variables were selected from the literature43, and confirmed by the statistical evidence44. A univariate analysis was performed for potential confounding variables, and those with PE<0.2 were selected for the final multivariable model; PE (P-value for entry) determines which variables should be included in the multivariable model44.
Finally, two models were conducted for multinomial and binary logistic regressions: Model 1 was adjusted for age, sex, BMI, eGFR and urinary creatinine (just for HTN outcomes), and SBP and T2D (just for MAU); Model 2 (additionally adjusted for physical activity level and smoking).
Result
Mean age of the study participants was 43.0 ± 13.7 y and 46.0% were men. Mean UNa, UK, and its ratio was 137 ± 57.4, 72.1 ± 36.6 mmol/L, and 2.31 ± 1.41, respectively. The prevalence of ISH, IDH, and SDH was 2.5, 27.9, and 8.0%, respectively. The prevalence of MAU was 10.0%. Table 1 shows a comparison of characteristics of the study population across quintile categories of UNa-K ratio. Participants in the highest compared to lowest quintiles of UNa-K ratio were more likely to be men with a lower BMI, and had a higher levels of UNa, UK, microalbumin and MCR. A higher prevalence of ISH (3.4 vs. 1.1%), SDH (11.0 vs. 6.2%), and MAU (14.1 vs. 6.2%) was observed among subjects with UNa-K > 3.14 (P for all < 0.05).
Table 1.
The baseline characteristics of the study participants across quintiles of UNa-K ratio (n = 1782).
| Total | Q1 < 1.23 |
Q2-Q4 1.23–3.14 |
Q5 ≥ 3.14 |
|
|---|---|---|---|---|
| Age (years) | 43.0 ± 13.7 | 43.1 ± 13.0 | 43.2 ± 13.4 | 42.5 ± 15.6 |
| Men (%) | 46.0 | 37.6 | 46.6 | 53.1a |
| BMI (kg/m2) | 27.4 ± 5.1 | 27.5 ± 4.7 | 27.6 ± 5.3 | 26.8 ± 4.9a, b |
| WC (cm) | 92.3 ± 12.3 | 91.9 ± 11.8 | 92.7 ± 12.4 | 91.4 ± 12.2 |
| SBP (mmHg) | 111 ± 14.7 | 110 ± 13.8 | 111 ± 14.5 | 112 ± 15.9 |
| DBP (mmHg) | 75.1 ± 9.4 | 74.8 ± 9.1 | 75.2 ± 9.3 | 75.3 ± 10.1 |
| PP (mmHg) | 35.9 ± 11.0 | 35.4 ± 10.7 | 35.8 ± 10.8 | 37.0 ± 12.1 |
| MAP (mmHg) | 87.1 ± 10.2 | 86.0 ± 9.7 | 87.1 ± 10.0 | 88.0 ± 10.9 |
| MBP (mmHg) | 93.1 ± 11.0 | 92.4 ± 10.4 | 93.2 ± 10.9 | 94.0 ± 11.9 |
| MPAP (mmHg) | 237 ± 28.0 | 236 ± 26.9 | 238 ± 27.7 | 239 ± 30.2 |
| TG-to-HDL-C | 3.36 ± 3.16 | 3.44 ± 3.79 | 3.37 ± 3.12 | 3.24 ± 2.5 |
| Serum Cr (mg/dL) | 1.08 ± 0.16 | 1.08 ± 0.14 | 1.07 ± 0.14 | 1.09 ± 0.21 |
| Urine Cr (mmol/L) | 13.9 ± 6.1 | 13.4 ± 5.8 | 14.0 ± 6.1 | 14.6 ± 6.2 |
| Urine microalbumin (mg/L) | 7.9 (3.9–13.7) | 5.9 (2.1–9.7) | 8.0 (4.1–13.1) | 9.7 (5.4–16.4)a |
| MCR (mg/mmol) | 0.70 (0.53–2.02) | 0.48 (0.20–1.05) | 0.72 (0.34–1.39) | 1.01 (0.53–2.02)a |
| Urine Na (mmol/L) | 137 ± 57.4 | 90.1 ± 42.4 | 143 ± 52.6 | 167 ± 56.2a, b |
| Urine K (mmol/L) | 72.1 ± 36.6 | 105 ± 57.4 | 71.9 ± 28.3 | 39.5 ± 16.2a, b |
| T2D (%) | 10.6 | 11.0 | 10.0 | 12.0 |
| HTN phenotypes (%) | ||||
| ISH | 2.5 | 1.1 | 2.6 | 3.4a |
| IDH | 27.9 | 31.7 | 27.5 | 25.6 |
| SDH | 8.0 | 6.2 | 7.6 | 11.0a, b |
| Microalbuminuria (%) | 10.0 | 6.2 | 9.7 | 14.1a |
| Current smokers (%) | 11.9 | 11.9 | 11.1 | 14.4 |
| Low-PA levels, % | 67.2 | 71.0 | 66.7 | 64.6 |
Data are mean ± SD, percent or median (inter-quartile range).
aSignificant difference with Q1.
bSignificant difference with Q2–Q4.
Analysis of variance with a Bonferroni post hoc test (for continues normal-distributed variables) was used; Chi-square test (for categorical variables) or independent-sample median test (for nonparametric variables) was used.
BMI body mass index, Cr creatinine, DBP diastolic blood pressure, Na sodium, K potassium, SBP systolic blood pressure, PP pulse pressure, MAP mean arterial pressure, MBP mean blood pressure, MPAP mean proportional arterial pressure, PA physical activity, WC waist circumference.
HTN phenotypes were defined as: ISH (SBP ≥ 130 and DBP < 80 mmHg), IDH (SBP < 130 and DBP ≥ 80 mmHg), and SDH (SBP ≥ 130 and DBP ≥ 80 mmHg).
Microalbuminuria was defied using sex-specific classification of microalbumin-creatinine ratio MCR (mg/mmol)36,37 as: Normal: MCR < 3.5 in men and < 2.5 in women; Microalbuminuria: 3.5 < MCR < 35 in men 2.5 < MCR < 25 in women.
Dietary intakes of the participants across quintiles of UNa-K ratio are presented in Table 2. Participants in the highest compared to lowest quintiles of UNa-K ratio had significantly lower intake of protein, fruits and whole grains while their intakes of sodium and ultra-processed foods were significantly higher. There was no significant difference in dietary intakes of other food groups across quintiles of UNa-K ratio.
Table 2.
Dietary intakes of the study participants across quintiles of UNa-K ratio (n = 1782).
| Q1 < 1.23 |
Q2-Q4 1.23–3.14 |
Q5 ≥ 3.14 |
P-value | |
|---|---|---|---|---|
| Energy (kcal/day) | 2236 ± 697 | 2208 ± 678 | 2276 ± 662 | 0.209 |
| Protein (g/day) | 92.4 ± 43.0 | 87.8 ± 39.5 | 87.3 ± 36.7 | 0.271 |
| Na (mg/day) | 3510 ± 1180 | 3533 ± 1169 | 3740 ± 1459a | 0.021 |
| K (mg/day) | 4774 ± 1816 | 4458 ± 1798 | 4640 ± 2161 | 0.060 |
| Fruits (g/day) | 489 ± 381 | 409 ± 338a | 338 ± 258a, b | 0.001 |
| Starchy-vegetables (g/day) | 25.6 ± 20.3 | 25.8 ± 22.5 | 28.0 ± 25.3 | 0.418 |
| Non-starchy vegetables (g/day) | 309 ± 189 | 299 ± 189 | 284 ± 169 | 0.328 |
| Whole grains (g/day) | 158 ± 112 | 135 ± 101a | 133 ± 104a | 0.005 |
| Poultry (g/day) | 32.8 ± 34.6 | 30.4 ± 28.3 | 29.4 ± 31.2 | 0.436 |
| Red meat (g/day) | 23.1 ± 25.3 | 20.6 ± 18.4 | 21.4 ± 19.3 | 0.437 |
| Low-fat dairy (g/day) | 260 ± 179 | 231 ± 164 | 236 ± 172 | 0.087 |
| High-fat dairy (g/day) | 110 ± 144 | 107 ± 117 | 122 ± 133 | 0.221 |
| Nuts and seeds (g/day) | 14.2 ± 14.9 | 12.9 ± 15.6 | 11.1 ± 12.5 | 0.049 |
| Legumes (g/day) | 43.1 ± 33.4 | 42.8 ± 34.9 | 44.3 ± 35.5 | 0.835 |
| Ultra-processed foods (g/day) | 378 ± 214 | 415 ± 222 | 443 ± 213a | 0.005 |
| Tea and coffee (ml/day) | 598 ± 428 | 586 ± 466 | 545 ± 391 | 0.329 |
Data are mean ± SD.
Analysis of variance with a Bonferroni post hoc test.
aSignificant difference with Q1.
bSignificant difference with Q2–Q4.
The associations of UNa-K ratio and phenotypes of HTN are shown Table 3. In the full-adjusted model of multinomial regression, highest compared to the lowest UNa-K ratio (> 3.14 vs. <1.23) was associated with an increased probability of SDH (RRR = 1.79, 95% CI = 1.09–3.19). The UNa-K ratio had a borderline-significant positive association with an increased chance of ISH (RRR = 3.00, 95% CI = 0.93–9.69), in the highest compared to the lowest quintile. Every 1 SD-increment of UNa-K ratio was associated with 29% increased chance of having SDH (RRR = 1.29, 95% CI = 1.00-1.70). The associations of UNa-K ratio and phenotypes of HTN are shown Table 4. Highest compared to the lowest UNa-K ratio (> 3.14 vs. <1.23) was associated with an elevated chance of having MAU (OR = 2.53, 95% CI = 1.23–5.20). Every 1 SD-increment of UNa-K ratio was associated with 38% increased chance of having MAU (OR = 1.38, 95% CI = 1.03–1.84).
Table 3.
The cross-sectional association of UNa-K ratio and phenotypes of elevated BP.
| HTN phenotypes | UNa-K ratio | ||
|---|---|---|---|
| Q2-Q4 1.23–3.14 |
Q5 ≥ 3.14 |
Per 1 SD | |
| ISH | |||
| Crude | 2.27 (0.79–6.56) | 3.04 (0.96–9.58) | 1.23 (0.78–1.95) |
| Model 1 | 2.37 (0.81–6.95) | 3.08 (0.96–9.93) | 1.24 (0.78–1.97) |
| Model 2 | 2.33 (0.79–6.86) | 3.00 (0.93–9.69) | 1.21 (0.76–1.93) |
| IDH | |||
| Crude | 0.85 (0.65–1.10) | 0.82 (0.58–1.14) | 0.86 (0.71–1.04) |
| Model 1 | 0.83 (0.64–1.09) | 0.82 (0.58–1.14) | 0.87 (0.72–1.05) |
| Model 2 | 0.83 (0.63–1.07) | 0.81 (0.58–1.14) | 0.86 (0.71–1.05) |
| SDH | |||
| Crude | 1.19 (0.73–1.96) | 1.79 (1.03–3.13) | 1.32 (1.01–1.71) |
| Model 1 | 1.24 (0.75–2.07) | 1.86 (1.05–3.31) | 1.33 (1.01–1.74) |
| Model 2 | 1.21 (0.73–2.03) | 1.79 (1.09–3.19) | 1.29 (1.00-1.70) |
Data are RRRs (95% CI); Model 1 was adjusted for age, sex, BMI, eGFR, and urinary creatinine; Model 2 was additionally adjusted for physical activity level and smoking. Multinomial logistic regression models were used (Q1 was considered as reference category).
HTN phenotypes were defined as: ISH (SBP ≥ 130 and DBP < 80 mmHg), IDH (SBP < 130 and DBP ≥ 80 mmHg), and SDH (SBP ≥ 130 and DBP ≥ 80 mmHg).
UNa-K ratio: Q1 < 1.23, 1.23 ≤ Q2–Q4 < 3.14, Q5 ≥ 3.14.
Median of UNa-K ratio was 0.91, 2.01, and 4.05 in Q1, Q2–Q4, and Q5, respectively.
HTN hypertension, ISH isolated systolic hypertension, IDH isolated diastolic hypertension, SDH combined systolic and diastolic hypertension.
Table 4.
The association of UNa-K ratio and microalbuminuria.
| Microalbuminuria | UNa-K ratio | ||
|---|---|---|---|
| Q2–Q4 1.23–3.14 |
Q5 ≥ 3.14 |
Per 1 SD | |
| Crude | 1.62 (0.89–2.92) | 2.46 (1.30–4.68) | 1.21 (0.92–1.59) |
| Model 1 | 1.64 (0.84–3.21) | 2.53 (1.23–5.21) | 1.38 (1.03–1.85) |
| Model 2 | 1.65 (0.84–3.22) | 2.53 (1.23–5.20) | 1.38 (1.03–1.84) |
Data are ORs (95% CI); Model 1 was adjusted for age, sex, BMI, SBP and T2D; Model 2 was additionally adjusted for physical activity level and smoking. Multinomial logistic regression models were used (Q1 was considered as reference category).
UNa-K ratio: Q1 < 1.23, 1.23 ≤ Q2-Q4 < 3.14, Q5 ≥ 3.14.
Median of UNa-K ratio was 0.91, 2.01, and 4.05 in Q1, Q2–Q4, and Q5, respectively.
Microalbuminuria was defied using sex-specific classification of microalbumin-creatinine ratio MCR (mg/mmol)36,37 as: Normal: MCR < 3.5 in men and < 2.5 in women; Microalbuminuria: 3.5 < MCR < 35 in men 2.5 < MCR < 25 in women.
Discussion
Our findings from a nationally representative cross-sectional study showed that a high UNa-K ratio was associated with a greater likelihood of SDH phenotype by about 80% and MA, by 2.5-fold, respectively. The UNa-K ratio of more than 3.14 was associated with a higher prevalence of ISH, however, the small number of subjects with ISH resulted in a borderline-significant probability. These findings imply that a high UNa-K ratio may be a potential risk factor for BP dysregulation and impaired renal function. Furthermore, the phenotype-specific association of the UNa-K ratio and elevated BP may call for precision dietary intervention approaches for the prevention and management of HTN.
The median UNa-K ratio in our population was 2 with an inter-quartile range of 1.36–2.85. A wide range of urinary UNa-K ratio were reported among different populations. A mean value of 2.88 and 2.97 was reported for men and women, respectively, with a higher value in black compared to white people (3.43 vs. 2.83)45. The mean 24-h UNa-K ratio ranged from 1 in Brazil to 7.58 in China; in Asian and Western populations this value was reported approximately in a range of 3–5 46,47. Long-term high-Na exposure may alter the renin-angiotensin-aldosterone system (RAAS) system resulting in the development of HTN, transitively from a high cardiac output [extracellular volume (ECV) expansion] and normal systemic vascular resistance at an early stage to a normal cardiac output (normal ECV) and increased systemic vascular resistance at a later stage48,49. In contrast, dietary K contributes to BP regulation through its vasodilatory effects and modulation of renal Na excretion50,51.
A recent meta-analysis of population-based studies reported a pooled estimated relative risk of stroke by 1.22 (95% CI = 1.04, 1.41) per 1-unit increment in dietary Na-K ratio (mmol/mmol)52. A UNa-K ratio of ≤ 1 was associated with a clinically relevant reducing risk of stroke53. The Shandong Ministry of Health Action on Salt and Hypertension (SMASH) recently reported a non-linear positive association between UNa-K ratio and risk of HTN (OR = 1.09, 95%CI = 1.08–1.11) and a linear association between UNa-K ratio and SBP, DBP and MAP15. In our previous study, we reported a strong association between a higher UNa-K ratio (> 2.37 vs. <1.49) and lower dietary intakes of vegetables, low-fat dairy, and fruit intakes54. Furthermore, a Western dietary pattern was associated with a higher UNa-K ratio, while the Mediterranean and DASH pattern scores demonstrated an inverse association with UNa-K ratio54. Higher dietary the Na-K ratio increased 6.3-y and 10.6-y risk of CVD (HR = 2.19, 95% C = 1.16–4.14, and (HR = 1.99, 95% CI = 1.13–3.52)55,56. However, we failed to show any significant association between dietary Na-K ratio and 3.6-y incidence of HTN (i.e., defined as SBP/DBP of 140/70 mm Hg)55. In the current study, estimation of Na-K ratio using urine- rather food frequency questionnaire (FFQ)-based method, definition of HTN phenotypes according to the thresholds of ACC/AHA for SBP/DBP, helped us to distinguish phenotype-specific association of UNa-K ratio and BP. There is a disagreements on the optimal cut-off values for defining HTN57, leading to outcome misclassifications in population-based studies, may contribute to the contradictory state of knowledge on modifiable risk factors of HTN. Because early and advanced stages of HTN might be differentially affected by the risk factors and distinctively contribute to CVD and all-cause mortality58–60, BP classification based on the 2017 ACC/ AHA guideline33 might improve risk stratification for identifying HTN risk factors.
In the current study, we observed a strong independent association between high UNa-K ratio and MAU. Although, excessive Na exposure is suggested as an independent risk factor of MAU, an early renal dysfunction primarily caused by increased glomerular capillary pressure5, the association of urinary or dietary Na-K ratio with MAU is not clear21. A recent cross-sectional study among Chinese population (with a 9.0% prevalence of MAU), failed to show significant association between 24 h-urinary K and Na-K ratio and MAU, while the highest quartile of 24-h UNa was associated with increased chance of MAU (OR = 2.20, 95% CI = 1.26–3.84)21. Similarly, higher quintile of UNa-to-Cr ratio was associated with odds ratio of 1.62 (95% CI = 1.35–1.9) for high-MCR (≥ 30 mg/g) in an Italian population22. In normotensive subjects, a higher level of urinary microalbumin was reported in those who had higher 24 h-UNa excretion23. High vs. low 24-hUNa excretion (263 vs. 111 mmol/d) was significantly associated with MAU in hypertensive individuals61.
Our study findings indicated a significant association between UNa-K ratio and SDH phenotype, and a borderline significant association with ISH. This observation may suggest that imbalanced Na-K intake might influence both SBP and DBP, however, SBP seems to be affected primarily. ISH, i.e., characterized by increased aortic stiffness, occurs when the peripheral vascular resistance is normal or even reduced while the SBP is increased17. ISH in young adults is usually caused by high amplification of the central pressure wave, whereas ISH in the elderly (> age 60) is associated with aortic stiffening62. ISH is commonly associated with a normal central aortic SBP due to excessive peripheral systolic pressure amplification, while IDH manifests as elevated peripheral vascular resistance and mean arterial pressure63. While a substantial overlap in CVD risks between ISH and SDH has been reported64, subjects with SDH may have a higher risk of CVD morbidity and mortality, compared with those who suffering from the isolated phenotypes of HTN65.
While 24-hours UNa and UK excretion remains the gold standard for quantifying dietary Na and K intake, methodological challenges and high incompletion rates (up to 40%) of 24 h urine collections limit its utility in large epidemiological studies66. A growing interest exist in utilizing spot urine samples for Na and K intake estimation due to the flexibility in collection times67–69. Several validated formulas exist to estimate the 24 h UNa-K ratio from spot urine Na and K concentrations, with some incorporating creatinine measurements for enhanced accuracy66,67. Studies report strong correlation coefficients (r = 0.88 to 0.96) between 24-hours and spot UNa-K ratio in Western and Asian populations47. This suggests that the spot UNa-K ratio may serve as a valuable, less burdensome alternative for estimating population-level 24-hours urinary Na and K excretion47,66. An additional advantage of the spot UNa-K ratio, compared to measuring individual urinary sodium or potassium levels, is that it bypasses the need for conversion to 24-hours excretion values, a step prone to introducing inaccuracies70. Collection time (overnight, morning, afternoon, or evening) has minimal impact on the accuracy of Na and K excretion estimates in spot samples; this time-independence, combined with the convenience and reduced participant burden compared to 24-h urine collection, positions spot urine as a potentially superior method for large-scale population studies investigating association of UNa-K ratio with diseases. Although spot urinary Na and K concentration may have diurnal and day-to-day variations, the second voiding of casual urine is suggested being suitable for estimating daily Na and K excretion, i.e., fairly correlated with 24-h urine sample and BP measurements71,72. Furthermore, 24-h urine collection is the gold standard for measuring MAU, studies have demonstrated that spot urine samples exhibit high sensitivity and specificity for detecting MAU when compared to 24-hour urine collections73. Therefore, the use of spot urine samples in our study is likely to have minimal impact on the overall findings.
To our knowledge, this population-based study represents a novel investigation into the association between UNa-K ratio and BP, specifically accounting for multi-pathophysiological nature of HTN. In conclusion, our study findings may imply that subjects at early stage of HTN and those with SDH phenotype (based on the thresholds of ACC/AHA for SBP/DBP) might take more advantage from dietary intervention focusing on reducing Na-to-K intake. These findings, however, should be interpreted in the context of study limitations. First, due the cross-sectional setting the observed relationship between UNa-K ratio and elevated BP phenotypes and MAU does not necessarily imply causation. Further research, including randomized controlled trials, is warranted to establish a more definitive causal relationship and inform clinical practice. Second, the study was conducted at a single time point. Repeated measurements over time would help to assess the stability of the associations and potential changes in the UNa-K ratio and health outcomes. Third, our relatively low prevalence of ISH resulted in a wide 95% CIs and a borderline P value (0.056) being small enough to justify rejection of the null hypothesis, despite of a large effect size. Our stratification was based on BP measured on a single occasion (office but not home BP measurements), so we cannot exclude a possible white-coat effect among the participants, despite the standardized measurement conditions and considering mean of twice BP measurements. Fourth, there may be unmeasured confounders (e.g., genetic backgrounds, lifestyle behaviors) that influence the relationship between UNa-K ratio and the study outcomes. Finally, the study was conducted in a specific cohort (i.e., Tehran Lipid and Glucose Study), so the study findings may not be generalizable to other populations with different demographic, genetic, or lifestyle characteristics.
In conclusion, findings suggest that keeping the balance between dietary Na and K intake may be particularly effective in mitigating the early onset of renal dysfunction and specific phenotypes of elevated BP, warranting further investigation in larger, prospective studies.
Acknowledgements
We thank the Tehran Lipid and Glucose Study participants and the field investigators of the Tehran Lipid and Glucose Study for their cooperation and assistance in physical examinations, biochemical evaluation and database management. This study, was supported by Shahid Beheshti University of Medical Sciences (Grant number: 43008251).
Author contributions
Zahra Bahadoran: Conceptualization, Methodology, Formal analysis, Writing - original draft. Parvin Mirmiran: Investigation, Supervision. Fereidoun Azizi: Methodology, Investigation, Supervision, Writing- Reviewing and Editing. All authors reviewed the manuscript.
Funding
This work was not supported by any funding agency.
Data availability
Data will be available upon forwarding the request to the corresponding author (z.bahadoran@sbmu.ac.ir) and confirmation of the director of RIES (azizi@sbmu.ac.ir).
Declarations
Ethics approval and consent to participate
Written informed consent was obtained from all participants. The ethics research committee of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran, approved the study protocol. The study protocol was carried out according to the relevant guidelines expressed in the Declaration of Helsinki.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zahra Bahadoran, Email: zahrabahadoran@yahoo.com, Email: z.bahadoran@sbmu.ac.ir.
Parvin Mirmiran, Email: mirmiran@sbum.ac.ir.
References
- 1.Giles, T. D., Materson, B. J., Cohn, J. N. & Kostis, J. B. Definition and classification of hypertension: an update. J. Clin. Hypertens. (Greenwich Conn). 11, 611–614. 10.1111/j.1751-7176.2009.00179.x (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen, T. N. & Chow, C. K. Global and national high blood pressure burden and control. Lancet (London England). 398, 932–933. 10.1016/s0140-6736(21)01688-3 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (London, England) 396, 1223–1249 (2020). 10.1016/s0140-6736(20)30752-2 [DOI] [PMC free article] [PubMed]
- 4.Weir, M. R. Microalbuminuria and cardiovascular disease. Clin. J. Am. Soc. Nephrology: CJASN. 2, 581–590. 10.2215/cjn.03190906 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Verdecchia, P. & Reboldi, G. P. Hypertension and microalbuminuria: the new detrimental duo. Blood Press.13, 198–211. 10.1080/08037050410016456 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Whelton, P. K. Sodium, potassium, blood pressure, and cardiovascular disease in humans. Curr. Hypertens. Rep.16, 1–8 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Tagawa, K., Tsuru, Y., Yokoi, K., Aonuma, T. & Hashimoto, J. Being overweight worsens the relationship between urinary sodium excretion and albuminuria: the Wakuya study. Eur. J. Clin. Nutr.77, 1044–1050. 10.1038/s41430-023-01327-2 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vedovato, M. et al. Effect of sodium intake on blood pressure and albuminuria in type 2 diabetic patients: the role of insulin resistance. Diabetologia. 47, 300–303. 10.1007/s00125-003-1303-5 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Mozaffarian, D., Appel, L. J. & Van Horn, L. J. C. Components of a cardioprotective diet: new insights. 123, 2870–2891 (2011). [DOI] [PMC free article] [PubMed]
- 10.Folsom, A. R., Parker, E. D. & Harnack, L. J. Degree of concordance with DASH diet guidelines and incidence of hypertension and fatal cardiovascular disease. J. j. o h. 20, 225–232 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Organization, W. H. Guideline: Sodium Intake for Adults and Children (World Health Organization, 2012). [PubMed]
- 12.Organization, W. H. Guideline: Potassium Intake for Adults and Children (World Health Organization, 2012). [PubMed]
- 13.Okayama, A. et al. Dietary sodium-to-potassium ratio as a risk factor for stroke, cardiovascular disease and all-cause mortality in Japan: the NIPPON DATA80 cohort study. BMJ open.6, e011632 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinuta, M. et al. Home blood pressure measurement days and changes in urinary sodium-to-potassium ratio, estimated salt and potassium intakes and blood pressure: 1-year prospective study. Blood Press. Monit.29, 188–194. 10.1097/mbp.0000000000000705 (2024). [DOI] [PubMed] [Google Scholar]
- 15.Xu, C. et al. Association between spot urinary sodium-to-potassium ratio and blood pressure among Chinese adults aged 18–69 years: the SMASH study. Front. Nutr.11, 1383243. 10.3389/fnut.2024.1383243 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baek, S. Y. & Kim, H. K. Association of Dietary Sodium-to-potassium ratio with cardiometabolic risk factors in Korean adults: findings from the Korean National Health and Nutrition Examination Survey. 15, doi: (2023). 10.3390/nu15143134 [DOI] [PMC free article] [PubMed]
- 17.Giles, T. D. & Sander, G. E. Diastolic blood pressure and hypertension phenotypes: the US Food and Drug Administration has it right. J. Clin. Hypertens. (Greenwich Conn). 15, 145–146. 10.1111/jch.12053 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mensah, G. A. & Commentary Hypertension phenotypes: the many faces of a Silent Killer. Ethn. Dis.29, 545–548. 10.18865/ed.29.4.545 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filippini, T. et al. Blood pressure effects of Sodium reduction: dose-response Meta-analysis of experimental studies. Circulation. 143, 1542–1567. 10.1161/circulationaha.120.050371 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filippini, T., Malavolti, M., Whelton, P. K. & Vinceti, M. Sodium intake and risk of hypertension: a systematic review and dose–response meta-analysis of observational cohort studies. Curr. Hypertens. Rep.24, 133–144 (2022). [DOI] [PubMed] [Google Scholar]
- 21.Xu, C., Du, X., Fang, L., Zhong, J. & Lu, F. Association of 24-h urinary sodium excretion with microalbuminuria in a Chinese population. Sci. Rep.13, 1044. 10.1038/s41598-023-27874-z (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cirillo, M. & Cavallo, P. Sodium Intake and Proteinuria/Albuminuria in the Population-Observational. Cross-Sectional Study. 1310.3390/nu13041255 (2021). [DOI] [PMC free article] [PubMed]
- 23.Khaledifar, A., Gharipour, M., Bahonar, A., Sarrafzadegan, N. & Khosravi, A. Association between Salt Intake and Albuminuria in Normotensive and Hypertensive individuals. Int. J. Hypertens.10.1155/2013/523682 (2013). [DOI] [PMC free article] [PubMed]
- 24.Azizi, F. et al. Cardiovascular risk factors in an Iranian urban population: Tehran lipid and glucose study (phase 1). Soz. Praventivmed.47, 408–426 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Azizi, F., Zadeh-Vakili, A. & Takyar, M. Review of Rationale, Design, and initial findings: Tehran lipid and glucose study. Int. J. Endocrinol. Metab.16, e84777. 10.5812/ijem.84777 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahadoran, Z., Mirmiran, P., Shabani, M. & Azizi, F. Higher daily physical activity levels may facilitate pre-diabetes regression to normoglycemia: a longitudinal study among an Iranian population. Prev. Med. Rep.34, 102233. 10.1016/j.pmedr.2023.102233 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tohidi, M. et al. Age- and sex-specific reference values for fasting serum insulin levels and insulin resistance/sensitivity indices in healthy Iranian adults: Tehran lipid and glucose study. Clin. Biochem.47, 432–438. 10.1016/j.clinbiochem.2014.02.007 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Askari, S. et al. Seasonal variations of blood pressure in adults: Tehran lipid and glucose study. Arch. Iran. Med.17, 441–443 (2014). [PubMed] [Google Scholar]
- 29.Bahadoran, Z., Mirmiran, P., Norouzirad, R., Ghasemi, A. & Azizi, F. Monitoring population salt intake using casual urinary sodium: Tehran lipid and glucose study. Nutr. Metabolism. 19, 19. 10.1186/s12986-022-00658-4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaeini, Z. et al. Spot urinary microalbumin concentration, metabolic syndrome and type 2 diabetes: Tehran lipid and glucose study. BMC Endocr. Disorders. 10.1186/s12902-022-00976-x (2022). [DOI] [PMC free article] [PubMed]
- 31.Hosseini-Esfahani, F. et al. Nutrition and Cardio-metabolic risk factors: findings from 20 years of the Tehran lipid and glucose study. Int. J. Endocrinol. Metab.16, e84772. 10.5812/ijem.84772 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jafarnejad, S., Mirzaei, H., Clark, C. C. T., Taghizadeh, M. & Ebrahimzadeh, A. The hypotensive effect of salt substitutes in stage 2 hypertension: a systematic review and meta-analysis. BMC Cardiovasc. Disord.10.1186/s12872-020-01347-x (2020). [DOI] [PMC free article] [PubMed]
- 33.Whelton, P. K. & ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention., Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 71, e127-e248 (2017). 10.1016/j.jacc.2017.11.006 (2018). [DOI] [PubMed]
- 34.Picone, D. et al. (ed, S.) Identifying isolated systolic hypertension from Upper-Arm Cuff blood pressure compared with invasive measurements. Hypertens. (Dallas Tex. : 1979)77 632–639 10.1161/HYPERTENSIONAHA.120.16109 (2021). [DOI] [PubMed] [Google Scholar]
- 35.Li, F. R. et al. Isolated systolic and diastolic hypertension by the 2017 American College of Cardiology/American Heart Association guidelines and risk of cardiovascular disease: a large prospective cohort study. J. Hypertens.39, 1594–1601. 10.1097/hjh.0000000000002805 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Lawton, P. D., Cunningham, J., Hadlow, N., Zhao, Y. & Jose, M. D. Chronic kidney disease in the top end of the Northern Territory of Australia, 2002–2011: a retrospective cohort study using existing laboratory data. BMC Nephrol.16, 168. 10.1186/s12882-015-0166-6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banks, E. & Welsh, J. Comparison of cardiovascular disease risk factors, assessment and management in men and women, including consideration of absolute risk: a nationally representative cross-sectional study. 10, e038761, doi: (2020). 10.1136/bmjopen-2020-038761 [DOI] [PMC free article] [PubMed]
- 38.American Diabetes Association. Classification and diagnosis of diabetes: standards of Medical Care in Diabetes—2021. Diabetes Care. 44, S15–S33. 10.2337/dc21-S002 (2020). [DOI] [PubMed] [Google Scholar]
- 39.Rosenblad, A. A comparison of blood pressure indices as predictors of all-cause mortality among middle-aged men and women during 701,707 person-years of follow-up. J. Hum. Hypertens.32, 660–667. 10.1038/s41371-018-0085-7 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Hadaegh, F., Shafiee, G., Hatami, M. & Azizi, F. Systolic and diastolic blood pressure, mean arterial pressure and pulse pressure for prediction of cardiovascular events and mortality in a Middle Eastern population. Blood Press.21, 12–18. 10.3109/08037051.2011.585808 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Fitzhugh, N., Rasmussen, L. R., Simoni, A. H. & Valentin, J. B. Misuse of multinomial logistic regression in stroke related health research: a systematic review of methodology. Eur. J. Neurosci.58, 3116–3131. 10.1111/ejn.16084 (2023). [DOI] [PubMed] [Google Scholar]
- 42.Bidulka, P. et al. Impact of chronic kidney disease on case ascertainment for hospitalised acute myocardial infarction: an English cohort study. BMJ Open.12, e057909. 10.1136/bmjopen-2021-057909 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alizadeh, Z. et al. Are the determinants of the progression to type 2 diabetes and regression to normoglycemia in the populations with pre-diabetes the same? Front. Endocrinol.13, 1041808. 10.3389/fendo.2022.1041808 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hosmer, D. W., Lemeshow, S. & Cook, E. Applied logistic regression, 2nd edition. (Wiley, 2013).
- 45.Cook, N. R. et al. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the trials of Hypertension Prevention follow-up study. Arch. Intern. Med.169, 32–40. 10.1001/archinternmed.2008.523 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Intersalt. An international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ (Clinical Res. ed.). 297, 319–328. 10.1136/bmj.297.6644.319 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iwahori, T. et al. Estimating 24-h urinary sodium/potassium ratio from casual (‘spot’) urinary sodium/potassium ratio: the INTERSALT Study. Int. J. Epidemiol.46, 1564–1572. 10.1093/ije/dyw287 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Julius, S. Transition from high cardiac output to elevated vascular resistance in hypertension. Am. Heart J.116, 600–606. 10.1016/0002-8703(88)90557-1 (1988). [DOI] [PubMed] [Google Scholar]
- 49.Rassler, B. The renin-angiotensin system in the development of salt-sensitive hypertension in animal models and humans. Pharmaceuticals (Basel Switzerland). 3, 940–960. 10.3390/ph3040940 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aburto, N. J. et al. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. Bmj346 (2013). [DOI] [PMC free article] [PubMed]
- 51.Reducing sodium and increasing potassium may lower risk of cardiovascular disease, https://www.hsph.harvard.edu/news/press-releases/reducing-sodium-and-increasing-potassium-may-lower-risk-of-cardiovascular-disease/ (13 novamber 2021).
- 52.Jayedi, A., Ghomashi, F., Zargar, M. S. & Shab-Bidar, S. Dietary sodium, sodium-to-potassium ratio, and risk of stroke: a systematic review and nonlinear dose-response meta-analysis. Clin. Nutr.38, 1092–1100. 10.1016/j.clnu.2018.05.017 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Averill, M. M. et al. Spot urine sodium-to-potassium ratio is a predictor of stroke. Stroke. 50, 321–327. 10.1161/strokeaha.118.023099 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mirmiran, P. et al. Urinary sodium-to-potassium ratio: a simple and useful indicator of diet quality in population-based studies. 26, 1–8 (2021). [DOI] [PMC free article] [PubMed]
- 55.Mirmiran, P., Bahadoran, Z., Nazeri, P. & Azizi, F. Dietary sodium to potassium ratio and the incidence of hypertension and cardiovascular disease: a population-based longitudinal study. Clin. Experimental Hypertens. (New York N Y : 1993). 40, 772–779. 10.1080/10641963.2018.1431261 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Mosallanezhad, Z., Jalali, M., Bahadoran, Z., Mirmiran, P. & Azizi, F. Dietary sodium to potassium ratio is an independent predictor of cardiovascular events: a longitudinal follow-up study. BMC Public. Health. 23, 705. 10.1186/s12889-023-15618-7 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atasoy, S., Johar, H., Peters, A. & Ladwig, K. H. Association of hypertension cut-off values with 10-year cardiovascular mortality and clinical consequences: a real-world perspective from the prospective MONICA/KORA study. Eur. Heart J.40, 732–738. 10.1093/eurheartj/ehy694 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Kaneko, H. et al. Association of blood pressure classification using the 2017 American College of Cardiology/American Heart Association blood pressure Guideline with Risk of Heart failure and Atrial Fibrillation. Circulation. 143, 2244–2253. 10.1161/CIRCULATIONAHA.120.052624 (2021). [DOI] [PubMed] [Google Scholar]
- 59.Yano, Y. et al. Association of blood pressure classification in young adults using the 2017 American College of Cardiology/American Heart Association blood pressure Guideline with Cardiovascular events later in Life. Jama. 320, 1774–1782. 10.1001/jama.2018.13551 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bundy, J. D., Mills, K. T. & He, J. Comparison of the 2017 ACC/AHA Hypertension Guideline with earlier guidelines on estimated reductions in Cardiovascular Disease. Curr. Hypertens. Rep.21, 76. 10.1007/s11906-019-0980-5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yilmaz, R. et al. Dietary salt intake is related to inflammation and albuminuria in primary hypertensive patients. Eur. J. Clin. Nutr.66, 1214–1218. 10.1038/ejcn.2012.110 (2012). [DOI] [PubMed] [Google Scholar]
- 62.O’Rourke, M. F. & Adji, A. Guidelines on guidelines: focus on isolated systolic hypertension in youth. J. Hypertens.31, 649–654. 10.1097/HJH.0b013e32835d8230 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Xie, K. et al. The different risk factors for isolated diastolic hypertension and isolated systolic hypertension: a national survey. BMC Public. Health. 10.1186/s12889-021-11686-9 (2021). [DOI] [PMC free article] [PubMed]
- 64.Tsimploulis, A. et al. Systolic-diastolic hypertension versus isolated systolic hypertension and incident heart failure in older adults: insights from the Cardiovascular Health Study. Int. J. Cardiol.235, 11–16. 10.1016/j.ijcard.2017.02.139 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hozawa, A. et al. Prognosis of isolated systolic and isolated diastolic hypertension as assessed by self-measurement of blood pressure at home: the Ohasama study. Arch. Intern. Med.160, 3301–3306. 10.1001/archinte.160.21.3301 (2000). [DOI] [PubMed] [Google Scholar]
- 66.Tanaka, T. et al. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J. Hum. Hypertens.16, 97–103. 10.1038/sj.jhh.1001307 (2002). [DOI] [PubMed] [Google Scholar]
- 67.Koo, H. S. et al. Estimating 24-hour urine sodium level with spot urine sodium and creatinine. J. Korean Med. Sci.29 (Suppl 2), 97–S102. 10.3346/jkms.2014.29.S2.S97 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gumz, M. L. & Rabinowitz, L. Role of circadian rhythms in potassium homeostasis. Semin. Nephrol.33, 229–236. 10.1016/j.semnephrol.2013.04.003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nikolaeva, S. et al. The circadian clock modulates renal sodium handling. J. Am. Soc. Nephrology: JASN. 23, 1019–1026. 10.1681/ASN.2011080842 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ginos, B. N. & Engberink, R. H. Estimation of Sodium and Potassium Intake: current limitations and Future perspectives. Nutrients. 12, 3275 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kogure, M. et al. Consideration of the reference value and number of measurements of the urinary sodium-to-potassium ratio based on the prevalence of untreated home hypertension: TMM Cohort Study. Hypertens. Research: Official J. Japanese Soc. Hypertens.45, 866–875. 10.1038/s41440-021-00843-7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iwahori, T. et al. Six random specimens of daytime casual urine on different days are sufficient to estimate daily sodium/potassium ratio in comparison to 7-day 24-h urine collections. Hypertens. Research: Official J. Japanese Soc. Hypertens.37, 765–771. 10.1038/hr.2014.76 (2014). [DOI] [PubMed] [Google Scholar]
- 73.Derhaschnig, U. et al. Microalbumin measurement alone or calculation of the albumin/creatinine ratio for the screening of hypertension patients? Nephrol. Dialysis Transplantation. 17, 81–85. 10.1093/ndt/17.1.81 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available upon forwarding the request to the corresponding author (z.bahadoran@sbmu.ac.ir) and confirmation of the director of RIES (azizi@sbmu.ac.ir).


