Abstract
Fauna inventories reduce biodiversity knowledge gaps by providing comprehensive data on species distribution, richness, and abundance. Furthermore, they identify undocumented species and enhance understanding of ecosystem dynamics and conservation needs. The richness and abundance of amphibian species were studied in two Semideciduous Seasonal Forest areas in the municipalities of Potiraguá (Serra Azul) and Itarantim (Serra do Mandim) in southwestern Bahia, Brazil. Active visual and acoustic surveys were conducted in 24 forest interior transects, two stream transects, and two permanent ponds investigated in the study area. Opportunistic encounters during team movements were also recorded. The richness was 46 amphibian species distributed in 14 families and 26 genera. Approximately half of the species were shared between the two areas, while 11 species were exclusive to Serra Azul and another nine were found only in Serra do Mandim. Cluster analysis for 42 locations in Atlantic Forest, Caatinga, and Cerrado, in a presence/absence matrix with 216 species, revealed that the composition of the amphibians found in Serra do Mandim and Serra Azul is similar to other sampled locations in the northeastern region of Minas Gerais, close to the study site, which are considered transitional between the Atlantic Forest and the Caatinga. Our results demonstrate that the remaining forest fragments in the region, although small and isolated, still sustain a high richness of amphibians with species restricted to the Atlantic Forest and Bahia, such as Bahiusbilineatus, Ololygonstrigilata, Aplastodiscusweygoldti and Vitreoranaeurygnatha, and others considered typical of the Caatinga, such as Leptodactylustroglodytes and Physalaemuscicada. Additionally, we sampled potential new species, filled occurrence gaps, and expanded the geographical range of Pseudisfusca.
Key words: Anura, biodiversity, inventory, species distribution, species richness, transitional forest
Introduction
Amphibians are considered good environmental indicators due to their permeable skin, exposed eggs and embryos, and generally biphasic life cycle, allowing these organisms to respond to disturbances in both terrestrial and aquatic ecosystems (Wells 2007; Da-Silva et al. 2012; Fonte et al. 2019). Additionally, climate change can affect them, and studies demonstrate that some communities in the Neotropical region are already close to their physiological temperature limits (Duarte et al. 2012; Gutiérrez-Pesquera et al. 2016; Carilo-Filho et al. 2021).
The greatest threat to amphibians and fauna in general is habitat loss and fragmentation, which reduces shelter availability, food supply, isolates populations, and affects their genetic variability (Young et al. 2004; Becker et al. 2007). Amphibians stand as the most endangered class of vertebrates, with 40.7% of their species at risk of extinction (Luedtke et al. 2023). Although the proportion of species classified as Data Deficient (DD) has decreased in the most recent Global Amphibian Assessment (from 22.5% to 11.3%), the high number of species still listed as DD poses a challenge for researchers and hinders effective conservation efforts (Hoffmann et al. 2010; Luedtke et al. 2023).
The Atlantic Forest, originally spanning approximately 1.3 million km2, has undergone significant reduction, with estimates suggesting that only between 11.4% and 16% of its original coverage remains (Ribeiro et al. 2009). Nevertheless, the forest remnants still house an exuberant biological diversity, including endemic and threatened species, as well as species with restricted distribution to specific ecosystems (Myers et al. 2000; Haddad et al. 2013; Zappi et al. 2015).
In the southwest of Bahia, the Atlantic Forest is mainly composed of the Seasonal Forest (Deciduous and Semideciduous), which connects to interior forests, such as Caatinga and Cerrado (SOS MATA ATLÂNTICA and INPE 2018). This forest formation has physical and biological characteristics of adjacent regions, allowing faunal elements from other ecosystems to occur in these areas (Willians 1996). Nevertheless, even with the damage caused by human activities, such as pasture creation, logging, and mining (Miles et al. 2006; Silva et al. 2006), few protected areas have been established in the region, such as the REBIO (Biological reserve) Mata Escura and Alto Cariri National Park (ICMBio 2003, 2010) and the RPPN (Private Reserve of Natural Heritage) Mata do Passarinho (ICMBio 2016).
Amphibian surveys in Bahia have revealed significant species richness (e.g., Rojas-Padilla et al. 2020; Protázio et al. 2021; Bastos and Zina 2022), with records of new species (e.g., Vörös et al. 2017; Zucchetti et al. 2023; Santos et al. 2023) and expansions of the geographic distribution (e.g., Dias et al. 2010; Dias et al. 2011; Almeida et al. 2022). Interestingly, a family previously known only from the Amazon was recorded in the state of Bahia through the description of a new species (Caramaschi et al. 2013). Inventories contribute to the knowledge of species richness of a given region, as well as the understanding of the functional structure and population dynamics of amphibians (Droege et al. 1998; Haddad 1998; Camardelli and Napoli 2012). These studies are essential for planning conservation decisions and policies aimed at mitigating anthropogenic effects on species and for the creation of strategic areas for environmental protection (Silvano and Segalla 2005; IUCN 2024). Moreover, they assist in gathering information that enables the reduction of gaps in the distribution and composition of the anurofauna in the country (Rodrigues 2003; Tabarelli and Silva 2003).
Thus, the aim of this study was to conduct an inventory of the amphibians in two remaining Semideciduous Forests in the southwest of Bahia, comparing the amphibian community of these remnants with others from the Atlantic Forest, Caatinga, and Cerrado.
Materials and methods
Study area
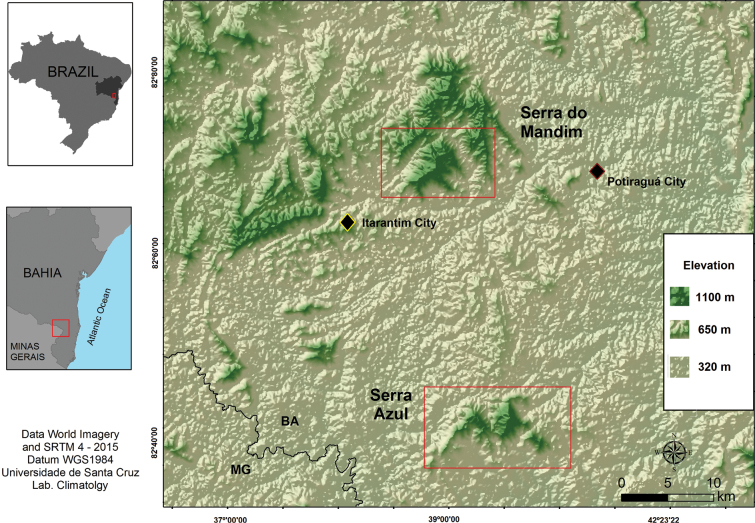
The study was carried out in two fragments of Atlantic Forest in the Southwest region of Bahia: Fugiama farm (15°37'58"S, 39°59'01"W), with approximately 120 hectares of forest, located in the Serra do Mandim, municipality of Itarantim, and Serra Azul farm (15°52'01"S, 39°55'54"W), with about 160 hectares of forest, located in the Serra Azul, municipality of Potiraguá, both located in the state of Bahia (Figs 1, 2). While the mountains themselves reach up to approximately 1100 m in altitude, the areas accessed during the study were at around 800 m in altitude (Table 1).
Figure 1.
Study areas in the southwest region of Bahia, Brazil. Serra do Mandim belongs to the municipality of Itarantim, Bahia and Serra Azul, one of its portions inserted in the municipality of Potiraguá, Bahia.
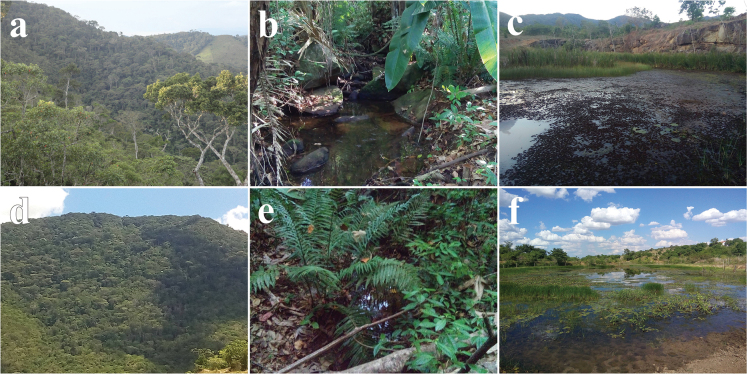
Figure 2.
Study areas in the southwest region of Bahia. Fugiama farm in Serra do Mandim (a, b, c) a semideciduous forest fragment b stream c permanent pond. Serra Azul farm in Serra Azul (d, e, f) d semideciduous forest fragment e stream f permanent pond.
Table 1.
Sampling points of the amphibian survey, coordinates, altitude, and sampling methods in Serra do Mandim, municipality of Itarantim and Serra Azul, municipality of Potiraguá, state of Bahia. TF = Transects in the forest; TS = Transects in the streams; P = permanent ponds.
| Locality | Sampling points | Coordinates (Latitude; Longitude) | Altitude (m) | Sampling method |
|---|---|---|---|---|
| Serra do Mandim-BA | 01 | 15°37'39.2"S, 39°58'41.6"W | 728 m | TF |
| 02 | 15°37'38.9"S, 39°58'39.2"W | 704 m | TF | |
| 03 | 15°37'40.8"S, 39°58'37.5"W | 672 m | TF | |
| 04 | 15°37'47.9"S, 39°58'35.2"W | 584 m | TF | |
| 05 | 15°37'50.4"S, 39°59'02.2"W | 758 m | TF | |
| 06 | 15°37'49.5"S, 39°58'59.9"W | 681 m | TF | |
| 07 | 15°37'47.6"S, 39°58'57.9"W | 635 m | TF | |
| 08 | 15°37'47.8"S, 39°58'50.7"W | 560 m | TF | |
| 09 | 15°37'47.6"S, 39°58'48.9"W | 587 m | TF | |
| 10 | 15°37'47.5"S, 39°58'46.1"W | 574 m | TF | |
| 11 | 15°37'36.9"S, 39°58'43.5"W | 755 m | TF | |
| 12 | 15°37'51.4"S, 39°58'35.9"W | 513 m | TF | |
| 25 | 15°37'54.1"S, 39°58'34.0"W | 485 m | TS | |
| 27 | 15°39'15.4"S, 39°59'00.8"W | 250 m | P | |
| Serra Azul-BA | 13 | 15°52'21.8"S, 39°54'30.6"W | 731 m | TF |
| 14 | 15°52'19.7"S, 39°54'27.1"W | 800 m | TF | |
| 15 | 15°52'22.0"S, 39°54'22.7"W | 739 m | TF | |
| 16 | 15°52'24.7"S, 39°54'22.2"W | 690 m | TF | |
| 17 | 15°52'27.7"S, 39°54'22.5"W | 672 m | TF | |
| 18 | 15°52'26.3"S, 39°54'18.6"W | 780 m | TF | |
| 19 | 15°52'28.1"S, 39°54'10.9"W | 692 m | TF | |
| 20 | 15°52'25.9"S, 39°54'29.3"W | 761 m | TF | |
| 21 | 15°52'29.0"S, 39°54'27.7"W | 650 m | TF | |
| 22 | 15°52'34.5"S, 39°54'22.0"W | 668 m | TF | |
| 23 | 15°52'33.2"S, 39°54'25.0"W | 652 m | TF | |
| 24 | 15°52'29.7"S, 39°54'24.9"W | 648 m | TF | |
| 28 | 15°52'33.7"S, 39°54'24.2"W | 658 m | TS | |
| 26 | 15°51'52.5"S, 39°53'26.1"W | 256 m | P |
The region borders the state of Minas Gerais and is located between two neighboring basins: the Pardo River basin and the Jequitinhonha River basin (IBGE 1997). Both areas are inserted in the Phytogeographic Domain of the Semideciduous Forest, characterized by the presence of climate type Am, with one to three dry months (Köppen 1936) and are ~ 28 km apart from each other. The climatic characteristics of the region encompass humid and sub-humid climates, defined by rainfall between 800 and 1100 mm and thermal averages from 23.5 to 25 °C (IBGE 1997).
The vegetation of the study area is characterized as Semideciduous Forest (SOS MATA ATLÂNTICA and INPE 2018). It occurs latitudinally parallel to the formation zone of the Ombrophilous Forest (coastal), at a distance ranging from 20 km to 140 km from the coast (Mori and Silva 1980; Argôlo 2004).
Sample design
A total of six field campaigns were carried out between December 2014 and March 2016 in Serra do Mandim and Serra Azul. Each expedition lasted approximately seven days, with approximately three or four days dedicated to active search in each mountain range.
A total of 24 transects were used between altitudes of 500–800 meters with a length of 50 meters and a width of 5 meters, spaced 100 meters apart in a forested area, two transects of 120 meters in streams, and two ponds were selected in a lowland area (Table 1). Twelve transects were sampled in forest, one in a stream, and a permanent pond in each of the areas (Figs 1, 2).
The transects in the forest were surveyed for 40 min, totaling 96 h. The streams in the forest were sampled for 90 min each, totaling 18 h. The permanent ponds were sampled for 30 min each, totaling six h. The sampling was carried out by two researchers. The total sampling effort was 44 days of sampling and 240 h/person. Amphibians were sampled using visual and acoustic active search methods (Heyer et al. 1994; Crump and Scott 1994) and by opportunistic encounters (i.e., along roads or trails outside the transects).
All animals were collected with a license from the Brazilian Institute of Environment and Renewable Natural Resources-IBAMA and/or ICMBio (No. 13708), together with the permission of the administrators of the local farms. Some specimens were euthanized through the administration of a lethal dose of 5% xylocaine to the ventral region, followed by fixation in 10% formaldehyde and preservation in 70% alcohol. All specimens were deposited in the Zoology Museum of the State University of Santa Cruz - MZUESC (Appendix 1).
Data analysis
For each species, the total number of individuals observed visually and acoustically was recorded in each sampling unit and environment. To evaluate sample sufficiency, rarefaction curves were constructed based on individuals. Four curves were made for each study area: a general curve considering all individuals sampled in the three standardized methodologies (transect in the forest, transect in the streams and ponds), and three others using individuals collected in each separate methodology. To extrapolate species richness, four non-parametric estimators were used: Chao 2, Jackknife 1, Jackknife 2, and Bootstrap (Magurran 1988; Colwell and Coddington 1994; Toti et al. 2000; Gotelli and Colwell 2001). The analysis was performed using presence/absence data for species during each sampling campaign, with 1000 randomizations.
The species richness recorded in the study area was compared to 42 locations, mostly in the northeast region of Brazil and a smaller number in the northeast region of Minas Gerais, sampled in the Atlantic Forest, Caatinga, and Cerrado (see Table 3). The information extracted from these locations was used to create a binary matrix of presence/absence with 216 amphibian species. The analysis of similarity, considering the specific composition of these areas, was performed using the Jaccard index to calculate dissimilarity and the UPMGA (Unweighted Pair Group Method with Arithmetic) linkage method. Species found in inventories with taxonomic doubts (sp., gr., and aff.) were excluded from the analyses. Subsequently, an ANOSIM (Analysis of Similarities) test was performed considering 9999 permutations, to determine whether the composition of samples recovered in the similarity analysis differs significantly among the groups (Atlantic Forest, Caatinga, and Cerrado). All analyses were conducted using the PAST 4.12 software (Hammer et al. 2001)
Table 3.
The number of amphibian species (S), study duration (SD in months), and region (R) type of different study sites in northeastern Brazil, including the northeastern portion of Minas Gerais. Localities listed as RPPN are Private Natural Heritage Reserves, those labelled as APA are Environmental Protection Areas, EE represents Ecological Stations, and PN denotes National Parks. Region abbreviations include Atlantic Forest (AF), Caatinga (CA), and Cerrado (CE).
| Localities, states of Brazil | S | SD | R | Source |
|---|---|---|---|---|
| RPPN Serra Bonita, BA | 80 | 16 | AF | Dias et al. 2014b |
| RE Michelin, BA | 69 | 30 | AF | Camurugi et al. 2010; Mira-Mendes et al. 2018 |
| APA Lagoa Encantada and River Almada, BA | 59 | 01 | AF | Dias et al. 2014a |
| Serra da Jibóia, BA | 55 | ~ 20 years | AF | Juncá, 2006; Freitas et al. 2018 |
| Serra do Timbó, BA | 55 | 12 | AF | Freitas et al. 2019 |
| PN Serra das Lontras, BA | 49 | 07 | AF | Rojas-Padilla et al. 2020 |
| PN Grande Sertão Veredas, BA/GO/MG | 47 | ~ 11 years | CE | Brandão et al. 2020 |
| Serra Mandim and Serra Azul, BA | 46 | 08 | AF | This study |
| Middle Jequitinhonha River, MG | 46 | 29 | CA/CE | Feio and Caramaschi 1995 |
| Chapada Diamantina, BA | 44 | 06 | CA | Juncá 2005 |
| RPPN Frei Caneca, PE | 42 | 12 | AF | Santos and Santos 2011 |
| Complex Limoeiro, MG | 39 | 03 | AF | Feio et al. 2006b |
| RPPN Estação Veracel, BA | 39 | 01 | AF | Silvano and Pimenta 2003 |
| Complex Nossa Senhora Fatima, MG | 38 | 02 | AF | Feio et al. 2006b |
| Planalto de Ibiapaba, CE | 38 | 24 | AF/CA | Loebmann and Haddad 2010 |
| Tocantins River Basin, MA/TO | 38 | 06 | CE | Brasileiro et al. 2008 |
| Complex Cariri, BA/MG | 36 | 03 | AF | Feio et al. 2006b |
| Morro do Mara, BA | 36 | 15 | AF/CA | Bastos and Zina 2022 |
| EE Serra Geral do Tocantins, TO | 36 | 02 | CE | Valdujo et al. 2011 |
| Guaratinga, BA | 34 | 01 | AF | Silvano and Pimenta 2003 |
| Macaíba, RN | 34 | 14 | AF/CA | Magalhães et al. 2013 |
| Conde, BA | 33 | 04 | AF | Gondim-Silva et al. 2016 |
| São Desiderio, BA | 32 | 02 | CE | Valdujo et al. 2009 |
| Camamu, BA | 32 | 01 | AF | Silvano and Pimenta 2003 |
| Serra do Brejo Novo, BA | 32 | 19 | AF/CA | Lantyer-Silva et al. 2013 |
| PN Chapada Diamantina, BA | 31 | 01 | CA | Magalhães et al. 2015 |
| Cruz das Almas, BA | 31 | 39 | AF | Protázio et al. 2021 |
| Complex Bandeira, BA/MG | 30 | 02 | AF | Feio et al. 2006b |
| Complex Santana, MG | 28 | 02 | AF | Feio et al. 2006b |
| Complex Mumbuca, MG | 27 | 02 | AF | Feio et al. 2006b |
| RPPN Sapiranga, BA | 25 | 05 | AF | Juncá 2006 |
| PN Descobrimento, BA | 25 | 01 | AF | Silvano and Pimenta 2003 |
| Itapebi, BA | 24 | 01 | AF | Silvano and Pimenta 2003 |
| EE Raso da Catarina, BA | 21 | 13 | CA | Garda et al. 2013 |
| PN Catimbau, PE | 21 | 01 | CA | Pedrosa et al. 2014 |
| Curimataú, PB | 21 | 02 | CA | Arzabe et al. 2005 |
| Middle Jaguaribe River, CE | 19 | 01 | CA | Santana et al. 2015 |
| RPPNs in Betânia and Floresta, PE | 19 | 07 | CA | Borges-Nojosa and Santos 2005 |
| Jatobá, PE | 18 | 04 | CA | Silva et al. 2011 |
| RPPN Serra das Almas, CE | 18 | 02 | CA | Borges-Nojosa and Cascon 2005 |
| Paulo Afonso, BA | 17 | 20 | CA | Protázio et al. 2010 |
| Cariri Paraibano, PB | 16 | 23 | CA | Vieira et al. 2007 |
The species were identified based on their original descriptions, redescriptions, or recent taxonomic revisions. In addition to the original descriptions, references consulted for species identification are provided in Table 2. Additionally, the collected material was compared with specimens identified at MZUESC. For nomenclature, we followed Frost (2023), who also maintains an updated database containing all available synonyms for amphibians worldwide. Regarding Adelophryne spp. we follow Lourenço-De-Moraes et al. (2018). The conservation status of the species was classified according to IUCN (2024). Furthermore, we verified which species are endemic of the Atlantic Forest based on Rossa-Feres et al. (2017).
Table 2.
Species of amphibians from Serra do Mandim and Serra Azul, southwest Bahia, Brazil. Legend: SM = Sampling Method (OE = Oportunistic encounters, TF = Transect in the forest, P = Ponds, TS = Transect in the streams). HAB = Habitat (LL = Leaf litter or understory, B = Bromeliads or epiphytes, S = Streams, F = Forest, P = Ponds or open area). N = Number of registered specimens. Additional ID references = Additional references consulted to identify species. # = only acoustic record; † = species only found in the inner forests; * endemic to the Atlantic Rainforest.
| Order/Family/Species | Serra do Mandim | Serra Azul | Additional ID references | ||||
|---|---|---|---|---|---|---|---|
| SM | HAB | N | SM | HAB | N | ||
| ANURA | |||||||
| Brachycephalidae | |||||||
| Ischnocnemaverrucosa (Reinhardt & Lütken, 1862)†* | TF | LL | 02 | TF | LL | 06 | Lynch 1972; Canedo et al 2010; Araújo et al. 2023 |
| Ischnocnema sp. (gr.parva) † | - | - | - | TF | LL | 01 | Heyer et al. 1990; Silva-Soares et al. 2021 |
| Bufonidae | |||||||
| Dendrophryniscusproboscideus (Boulenger, 1882)†* | OE,TF | LL | 08 | - | - | - | Izecksohn 1976; Caramaschi 2012 |
| Rhinellacrucifer (Wied-Neuwied, 1821)* | TF,TS | P,LL | 07 | OE | P,LL | 01 | Baldissera et al. 2004; Oliveira et al. 2014 |
| Rhinelladiptycha (Cope, 1862) | P | P,LL | 02 | P | P,LL | 09 | Stevaux 2002; Lavilla and Brusquetti 2018 |
| Rhinellagranulosa (Spix, 1824) | - | - | - | OE,TF,P | P,LL | 61 | Narvaes and Rodrigues 2009; São Pedro et al. 2011 |
| Craugastoridae | |||||||
| Haddadusbinotatus (Spix, 1824)†* | TF | LL | 51 | OE,TF,TS | LL | 145 | Heyer et al. 1990; Dias et al. 2012 |
| Centrolenidae | |||||||
| Vitreoranaeurygnatha (A. Lutz, 1925)†* | TS | S | 15 | - | - | - | Heyer et al. 1990; Zucchetti et al. 2023 |
| Cycloramphidae | |||||||
| Thoropamiliaris (Spix, 1824)†* | OE,TF,TS | S,LL | 10 | OE,TS | S,LL | 05 | Feio et al. 2006a |
| Eleutherodactylidae | |||||||
| Adelophryne sp.8† | TF | LL | 08 | OE,TF | LL | 27 | Lourenço-de-Moraes et al. 2018 |
| Adelophryne sp.2† | TF,TS | LL | 13 | OE,TF | LL | 29 | Lourenço-de-Moraes et al. 2018 |
| Hemiphractidae | |||||||
| Gastrothecapulchra Caramaschi & Rodrigues, 2007#†* | - | - | - | OE | B | 01 | Duellman 2015 |
| Hylidae | |||||||
| Aplastodiscusweygoldti (Cruz & Peixoto, 1987)†* | TS | S | 06 | TS | S | 08 | Orrico et al. 2006 |
| Boanacrepitans (Wied-Neuwied, 1824) | OE,P | P | 18 | OE,TF,P | P | 21 | Orrico et al. 2017 |
| Boanaexastis (Caramaschi & Rodriguez, 2003)†* | TF | F | 01 | - | - | - | Loebmann et al. 2008 |
| Boanafaber (Wied-Neuwied, 1821)* | OE,TF,P | P,F | 11 | OE,TF | P,F | 06 | Martins and Haddad 1988; Heyer et al. 1990 |
| Dendropsophusbranneri (Cochran, 1948) | OE,P | P | 64 | OE,P | P | 83 | Bastos and Pombal 1996; Nunes et al. 2007; Orrico et al. 2021 |
| Dendropsophuselegans (Wied-Neuwied, 1824)* | OE,P | P | 52 | OE,P | P | 24 | Gomes and Peixoto 1991; Dias et al. 2017a; Pirani et al. 2022 |
| Dendropsophusoliveirai (Bokermann, 1963) | OE,P | P | 60 | OEP | P | 107 | Santana et al. 2011; Orrico et al. 2021 |
| Ololygonstrigilata (Spix, 1824)†* | OE | S | 01 | - | - | - | Pimenta et al. 2007 |
| Phyllodytesmaculosus Cruz, Feio & Cardoso, 2007#†* | - | - | - | TF | B | 01 | Dias et al. 2020 |
| Phyllodytesluteolus (Wied-Neuwied, 1821)* | OE | B | 02 | - | - | - | Bokermann 1966a; Blotto et al. 2021 |
| Pithecopusnordestinus (Caramaschi, 2006) | OE,P | P | 30 | P | P | 13 | Vilaça et al. 2006; Vaz-Silva et al. 2020 |
| Pseudisfusca Garman, 1883* | - | - | - | P | P | 04 | Caramaschi and Cruz 1998; Garda et al. 2010 |
| Scinaxeurydice (Bokermann, 1968)* | OE,TF | P,F | 03 | TF | P,F | 01 | Magrini et al. 2011; Novaes-e-Fagundes et al. 2016; Menezes et al. 2016 |
| Scinaxpachycrus (Miranda-Ribeiro, 1937a) | - | - | - | P | P | 07 | Carneiro et al. 2004; Novaes-e-Fagundes et al. 2016 |
| Scinaxx-signatus (Spix, 1824) | OE,P | P | 07 | OE | P | 01 | Araujo-Vieira et al. 2020a; Novaes-e-Fagundes et al. 2021 |
| Sphaenorhynchusprasinus Bokermann, 1973* | OE,P | P | 16 | OE,P | P | 29 | Araujo-Vieira et al. 2020b |
| Trachycephalusnigromaculatus von Tschudi, 1838* | OE | P,F | 01 | OE | P,F | 04 | Bokermann 1966b |
| Leptodactylidae | |||||||
| Leptodactylusfuscus (Schneider, 1799) | P | P | 58 | OE,P | P | 28 | Heyer 1978; Heyer et al. 1990; De-Sá et al. 2014 |
| Leptodactyluslatrans (Steffen, 1815) | OE,TS,P | P,S | 07 | P | P,S | 06 | Magalhães et al. 2022 |
| Leptodactylusmacrosternum Miranda-Ribeiro, 1926 | TS | P,S | 01 | P | P,S | 01 | Magalhães et al. 2022 |
| Leptodactyluscf.mystaceus (Spix, 1824) | OE | S | 01 | - | - | - | Toledo et al. 2005; De-Sá et al. 2014; Cassini et al. 2013 |
| Leptodactylusmystacinus (Burmeister, 1861) | P | P | 03 | P | P | 04 | Abrunhosa et al. 2001; De-Sá et al. 2014; Cassini et al. 2013 |
| Leptodactylustroglodytes Lutz, 1926 | - | - | - | OE,P | P | 04 | De-Sá et al. 2014 |
| Leptodactylusviridis Jim & Spirandeli-Cruz, 1973* | P | P | 07 | - | - | - | Magalhães et al. 2022 |
| Physalaemuscicada Bokermann, 1966c | - | - | - | OE,P | P | 05 | Nascimento et al. 2005; Hepp and Pombal 2020 |
| Physalaemuscf.erikae Cruz & Pimenta, 2004* | OE,P | P | 18 | OE,P | P | 19 | Nascimento et al. 2005; Hepp and Pombal 2020 |
| Physalaemuskroyeri (Reinhardt & Lütken, 1862) | - | - | - | OE,P | P | 06 | Nascimento et al. 2005; Hepp and Pombal 2020; Braga et al. 2024 |
| Microhylidae | |||||||
| Dermatonotusmuelleri (Boettger, 1885) | - | - | - | OE | P | 02 | Vaz-Silva et al. 2020; Dubeux et al. 2021 |
| Odontophrynidae | |||||||
| Proceratophrysschirchi (Miranda-Ribeiro, 1937b)†* | OE,TF,TS | S,LL | 59 | OE,TS | S,LL | 09 | Izecksohn and Peixoto 1980; Sichieri et al. 2021 |
| Pipidae | |||||||
| Pipacarvalhoi (Miranda-Ribeiro, 1937a) | P | P | 04 | - | - | - | Lima et al. 2020 |
| Strabomantidae | |||||||
| Bahiusbilineatus (Bokermann, 1975) †* | OE,TF | LL | 17 | - | - | - | Dias et al. 2017b |
| Pristimantisvinhai (Bokermann 1975) †* | OE,TF,TS | LL | 276 | OE,TF,TS | LL | 178 | Trevisan et al. 2020 |
| Pristimantis sp. (gr.ramagii)† | - | - | - | OE,TF | LL | 90 | Trevisan et al. 2020 |
| GYMNOPHIONA | |||||||
| Siphonopidae | |||||||
| Siphonopsannulatus (Mikan 1822)† | - | - | - | TF | LL | 01 | Maciel and Hoogmoed 2011 |
Results
A total of 1785 individuals across 46 amphibian species were recorded, encompassing one species of Gymnophiona (Siphonopsannulatus) and 45 anuran species across 14 families (Table 2, Figs 3, 4). The majority of the identified species (n = 24; 53%) are endemic to the Atlantic Rainforest (Rossa-Feres et al. 2017). The Hylidae family was the most representative with 36.9% (n = 17), followed by the Leptodactylidae family, with 21.7% (n = 10) of the amphibians found. The richness identified stands among the highest ever recorded for the northeastern region of Brazil (Table 3). Furthermore, all recognized species identified are listed as Least Concern (LC) on the IUCN Red List (IUCN 2024).
Figure 3.
Amphibians registered in Serra do Mandim and Serra Azul in southwestern Bahia, Brazil. aIschnocnemaverrucosa (MZUESC 15874) bIschnocnema sp. (gr.parva – MZUESC 15896) cDendrophryniscusproboscideus (MZUESC 14688) dRhinellacrucifer (MZUESC 15148) eR.diptycha (MZUESC 15503) fR.granulosa (MZUESC 15055) gHaddadusbinotatus (MZUESC 15646) hVitreoranaeurygnatha (MZUESC 14691) iThoropamiliaris (MZUESC 15782) jAplastodiscusweygoldti (MZUESC 15787) kBoanacrepitans (MZUESC 14675) lB.exastis (MZUESC 15108) mDendropsophusbranneri (MZUESC 14683) nD.elegans (MZUESC 14679) oPhyllodytesluteolus (MZUESC 17501) pOlolygonstrigilata (MZUESC 15001) qPithecopusnordestinus (MZUESC 14682) rPseudisfusca (MZUESC 16528).
Figure 4.
Amphibians registered in Serra do Mandim and Serra Azul in southwestern Bahia, Brazil aScinaxpachycrus (MZUESC 16525) bS.x-signatus (MZUESC 17503) cSphaenorhynchusprasinusdTrachycephalusnigromaculatus (MZUESC 15064) eLeptodactylusmystacinus (MZUESC 16529) fL.troglodytes (MZUESC 15003) gL.viridis (MZUESC 15848) hPhysalaemuscf.erikae (MZUESC 15878) iP.kroyeri (MZUESC 15784) jDermatonotusmuelleri (MZUESC 15070) kProceratophrysschirchi (MZUESC 14689) lPipacarvalhoimPristimantisvinhai (MZUESC 15642) nP. sp (gr.ramagii – MZUESC 16523) oSiphonopsannulatus (MZUESC 15900).
The amphibian richness recorded was similar in the two analyzed areas: Serra do Mandim (n = 34) and Serra Azul (n = 37), with 25 species shared between the areas and the rest divided, with nine exclusive to Serra do Mandim and 11 to Serra Azul (Tables 2, 4). Among the total species recorded in each area, four species (Ololygonstrigilata, Phyllodytesluteolus, Trachycephalusnigromaculatus, and Leptodactyluscf.mystaceus) were sampled exclusively through opportunistic encounters in Serra do Mandim, while another five (Rhinellacrucifer, Gastrothecapulchra, Scinaxx-signatus, Trachycephalusnigromaculatus and Dermatonotusmuelleri) also corresponded to opportunistic encounters in Serra Azul.
Table 4.
Number of species and abundance found in the study area through standardized methodologies and extrapolated richness using richness estimators. TF: Transect in the forest; TS: Transect in the streams and P: Ponds.
| Estimators | Serra Azul | Serra do Mandim | ||||||
|---|---|---|---|---|---|---|---|---|
| TF | TS | P | Total | TF | TS | P | Total | |
| Richness | 13 | 5 | 18 | 32 | 13 | 9 | 15 | 30 |
| Chao 2 | 15.1±2.7 | 5.0±0.2 | 25.8±7.4 | 39.6±6.3 | 15.8±3.5 | 9.8±1.5 | 15.6±1.2 | 35.8±6.0 |
| Jackknife1 | 17.2±2.0 | 5.8±0.8 | 24.7±4.9 | 41.2±4.4 | 17.2±1.5 | 11.6±1.7 | 17.5±1.1 | 35.8±27 |
| Jackknife2 | 18.9 | 5.9 | 28.9 | 45.8 | 19.4 | 12.4 | 17.9 | 39.4 |
| Bootstrap | 14.9 | 5.4 | 20.8 | 36.2 | 14.9 | 10.2 | 16.3 | 32.6 |
| Abundance | 433 | 43 | 392 | 868 | 350 | 106 | 330 | 786 |
In Serra do Mandim most species were found in the monitored pond in the region. Although this was the smallest sampled fragment, some species considered rare and/or with restricted distribution were found only in this location, such as Dendrophryniscusproboscideus, and typical stream species such as Vitreoranaeurygnatha and Ololygonstrigilata.
The anuran richness recorded in Serra Azul was 37 species, and the only species of Gymnophiona recorded in the study was found in this area (Table 2; Fig. 4). Two species were only detected by vocalization, Phyllodytesmaculosus and Gastrothecapulchra. The first vocalized in bromeliads during forest transect sampling, while the latter was registered vocalizing in the canopy of the forest.
A higher number of individuals was recorded in Serra Azul (n = 868) compared to Serra do Mandim (n = 786). The most common species during the study were Pristimantisvinhai (n = 454), Haddadusbinotatus (n = 296), and Dendropsophusoliveirai (n = 167). A total of 131 specimens were collected during opportunistic encounters, 78 at Serra Azul and 53 at Serra do Mandim (Table 2).
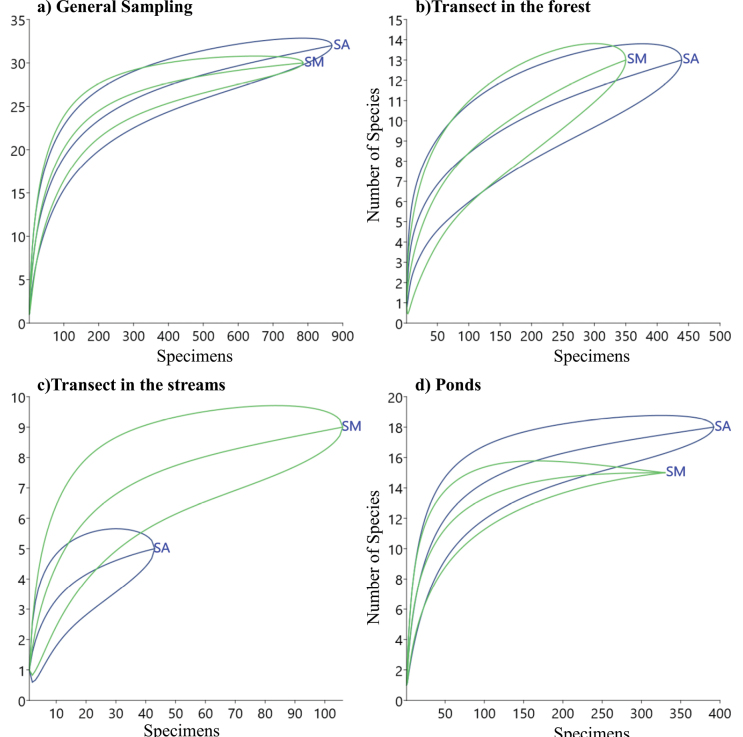
The overall rarefaction curve obtained for each area showed a tendency towards stabilization but did not reach the asymptote (Fig. 5A). The curves made for the different employed methodologies did not show a stabilization trend, except for the species recorded in ponds (Fig. 5D). The richness estimators suggested the occurrence of between 39–46 species in Serra Azul and between 36–39 species in Serra do Mandim (Table 4). Thus, during field activities, between 78.2–94.4% of the estimated richness for each area was sampled. A summary of the richness estimates for each area and the employed methodologies can be found in Table 4.
Figure 5.
Rarefaction curve based on amphibian individuals for the two fragments of Semi-deciduous Seasonal Forest in Serra do Mandim and Serra Azul in southwest Bahia, Brazil a general sampling: Serra do Mandim and Serra Azul (without considering opportunistic records) b transect in the forest c transect in the streams and d ponds. The central line corresponds to the average obtained with 1000 randomizations, and the lines above and below correspond to the 95% confidence interval. The blue line represents Serra Azul (SA), and the green line represents Serra do Mandim (SM).
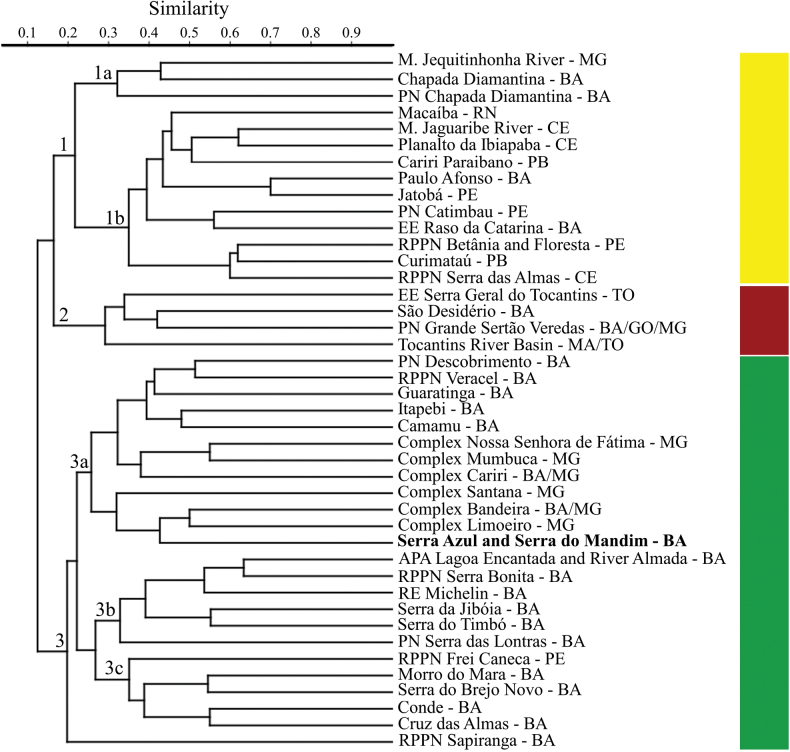
Cluster analysis yielded three main groups, which are mainly based on different regions in which the samplings were carried out (Fig. 6). The ANOSIM test demonstrates significant differences in species composition between the locations sampled in the Caatinga, Cerrado and Atlantic Forest (R = 0.662, P = 0.0001). Group 1 is formed by anuran assemblages sampled mainly in the Caatinga, where two main subdivisions can be highlighted, the first (1a) formed by localities along the middle Jequitinhonha River - MG and two localities in the Chapada Diamantina-BA, the latter hosting several endemic species (e.g., Haddadusaramunha, Leptodactylusoreomantis, Rupiranacardosoi) contributing to the unique anurofauna distinct from other sampled Caatinga locations (1b). Group 2 is formed by the anuran assemblages from the Cerrado.
Figure 6.
Similarity dendrogram for 42 locations, comparing amphibian composition primarily in the northeast region of Brazil. Jaccard index for dissimilarity calculation and UPGMA (Unweighted Pair Group Method with Arithmetic) clustering method (cophenetic correlation coefficient = 0.798). Green: locations sampled in the Atlantic Forest; Red: locations sampled in the Cerrado; Yellow: locations sampled in the Caatinga.
Group 3 is comprised of anuran assemblages within the Atlantic Forest, and three main subdivisions can be highlighted. The first subdivision (3a) encompasses studies conducted in Dense Ombrophilous Forest in the extreme south of Bahia (e.g., RPPN Veracel, National Park of Descobrimento). It also includes transition areas influenced by Seasonal Forests located further inland, such as localities in the northeast of Minas Gerais (e.g., Complex Santana, Bandeira, and Limoeiro), and Serra Azul and Serra do Mandim in Bahia. The second subdivision (3b) formed by areas located in the south of the state of Bahia, primarily sampled in the Dense Ombrophilous Forest, and encompasses locations with the highest species richness in our analysis. Finally, the third subdivision (3c) encompasses transition areas between the Atlantic Forest and Caatinga (Brejo Novo, Morro do Mara), as well as Restinga areas (Conde) and Semideciduous Forest regions (RPPN Frei Caneca and Cruz das Almas).
Taxonomic remarks
Some specimens encountered during field activities posed challenges for identification. Recently, Magalhães et al. (2020) revisited the Leptodactyluslatrans species group, employing multiple lines of evidence (DNA, acoustic, and morphological data), providing morphological diagnoses for the lineages encountered in their study. This facilitated the identification of two species from this group for the study area: L.macrosternum and L.latrans.
Physalaemuserikae was described in 2004 based on morphological and acoustic data, with the type locality in Guaratinga, Bahia (~ 100 km from the study area). This species closely resembles P.kroyeri but differs in having a shorter snout-vent length, a shorter advertisement call duration, and the presence of inguinal glands (Cruz and Pimenta 2004). Although our study area is close to the type locality, most specimens did not exhibit a visible inguinal gland, thus being considered as P.kroyeri. However, we encountered two specimens that exhibited an inguinal gland. For one of these, we recorded the advertisement call, which had a duration varying 0.37–0.46 s (MZUESC 15878). Recently, Hepp and Pombal (2020) reported that the advertisement call duration of P.erikae ranges from 0.478 to 0.566 s (n = 37 calls from four males), and for P.kroyeri it ranges from 0.673 to 0.759 s (n = 190 calls from eight males). Our data fall within the lower range of the reported variation for P.erikae. Braga et al. (2024) analyzed the vocalization of P.kroyeri from Cruz das Almas – Bahia (near the type locality) and found an advertisement call duration of 0.614 to 0.882 s (n = 83 calls from ten males), indicating a longer call duration in this species. However, we emphasize the need for a more comprehensive taxonomic revision of these two species, especially to increase the available data on the morphological and acoustic variation of P.erikae, particularly its acoustic parameters, especially those from its type locality, as well as the necessity of molecular sampling of topotypes of this species for inclusion in a phylogenetic approach.
Four species were not identified at the species level (Adelophryne sp. 2, A. sp. 8, Ischnocnema sp. [gr.parva] and Pristimantis sp. [grramagii]) and are considered candidate species that require further taxonomic investigation (Lourenço-De-Moraes et al. 2018; Trevisan et al. 2020; IR Dias, personal communication). Additionally, three other species (Phyllodytesluteolus, Ischnocnemaverrucosa and Vitreoranaeurygnatha) found in the area exhibit high intraspecific molecular diversity and may represent a similar case to the aforementioned (Blotto et al. 2021; IR Dias, personal communication; Zucchetti and Castroviejo-Fisher 2024).
Discussion
During the present study, 46 amphibian species were recorded in Serra Azul and Serra do Mandim in southwestern Bahia. This diversity is likely to be even greater, as the species accumulation curves have not stabilized. Thus, in these forest remnants, isolated and immersed in a matrix dominated by pastures for livestock, approximately 25% of the species that occur in Bahia can be found (Dias et al. 2014b), showing the importance of maintaining and conserving the fragments of the region. The recorded richness ranks among the top ten highest ever found in the Northeast region of Brazil, with a high proportion of species endemic to the Atlantic Rainforest. However, it is important to note that the sampling effort among the different studied areas is highly unequal, making comparisons difficult. Localities where low sampling effort was employed, such as APA (Environmental Protection Area) Lagoa Encantada and Almada River (1 month) and RPPN Veracel (1 month), have a similar richness to places where sampling effort was much higher, such as the Michelin Ecological Reserve and RPPN Frei Caneca. It is likely that the anuran fauna in these areas where low sampling effort was employed is underestimated.
Among the species found, three are restricted to the Atlantic Forest of the state of Bahia: Bahiusbilineatus, Physalaemuscf.erikae, Ololygonstrigilata (Juncá and Pimenta 2004; Cruz and Pimenta 2004; Pimenta et al. 2007), and five others have a geographical distribution between southern Bahia and Espirito Santo or northeastern Minas Gerais: Ischnocnemaverrucosa, Aplastodiscusweygoldti, Phyllodytesmaculosus, Dendrophryniscusproboscideus, and Leptodactylusviridis (Caramaschi and Canedo 2006; Cruz et al. 2007; Moura et al. 2009; Lantyer-Silva et al. 2011; Silva et al. 2012).
Some of the species found occur mainly in open areas, such as Rhinelladiptycha and Dermatonotusmuelleri (Andrade and Carnaval 2004; Feio et al. 2006b), while others are considered typical of the Caatinga, such as Leptodactylustroglodytes, Scinaxpachycrus, and Physalaemuscicada (Feio and Caramaschi 1995; Rodrigues 2003; Peixoto and Arzabe 2004; Linares and Mello 2011). The encounter of species restricted to the Atlantic Forest, and others typical of the Caatinga, was expected since the study site is located in a transition area between these two regions. According to the similarity analysis, the composition of the anuran fauna of Serra Azul and Serra do Mandim is similar to other sampled areas in the region in ecotonal areas, which also share faunistic elements of these two morphoclimatic domains (Feio and Caramaschi 1995; Feio et al. 2006b).
Within the state of Bahia, some species found in the study area have records limited to fewer than three occurrence points, such as Trachycephalusnigromaculatus, and Pseudisfusca (Garda et al. 2010; Dias et al. 2010). Pseudisfusca (Fig. 3R), an aquatic species, is found in the states of Bahia, Minas Gerais, and Espírito Santo, Brazil (Garda et al. 2010). In Bahia, the species is known from two municipalities: Guaratinga and Teixeira de Freitas (Garda et al. 2010). The encounter of P.fusca in Serra Azul represents the third record of the species for the state of Bahia, marking an approximately 250 km straight-line increase in its geographical distribution from its type locality (Araçuaí – Minas Gerais). These new records contribute to a better understanding of the distribution patterns of these species in the region, especially for P.fusca, with this new occurrence point now representing the northern limit of the distribution of this species.
Amphibian inventories in southern Bahia have shown high species richness and endemism in the region (e.g., Silvano and Pimenta 2003; Camurugi et al. 2010; Rojas-Padilla et al. 2020; present study), which may be associated with the fact that the central region of the Atlantic Forest is estimated as a zone of climatic stability during the Quaternary glaciations, serving as a large refuge for amphibian species in the Atlantic Forest at the end of the Pleistocene, contributing to the maintenance of regional diversity (Carnaval and Moritz 2008; Carnaval et al. 2009).
Serra do Mandim and Serra Azul still have conserved forest fragments that form a complex network of streams that compose the Jequitinhonha river basins further south (IBGE 1997) and the Rio Pardo basin further north of the region (Pedreira 1999; Cetra et al. 2010). This great abundance of water bodies and the conservation of the study area contributed to the record of typical forest species (e.g., Adelophryne sp. 2, A. sp. 8, Ischnocnemaverrucosa, Pristimantisvinhai, and Gastrothecapulchra) and typical stream species (e.g., Aplastodiscusweygoldti, Ololygonstrigilata, and Vitreoranaeurygnatha). In addition, a high abundance of direct development species using leaf litter (e.g., Pristimantisvinhai, P. sp. [gr.ramagii] and Haddadusbinotatus), including species that are difficult to sample such as Ischnocnemaverrucosa and Dendrophryniscusproboscideus, was found, demonstrating that the sampled forest fragments still have adequate conditions for the maintenance of these populations in the region. Despite this, no protected area has been established to ensure the conservation of these elements of the region’s fauna. Actions are required to secure the preservation of these species.
Our results demonstrate that the remaining forest fragments in the region, although small and isolated, still support a high richness of amphibians with species restricted to the Atlantic Forest and Bahia, such as Bahiusbilineatus and Ololygonstrigilata, and others considered typical of the Caatinga, such as Dermatonotusmuelleri, Leptodactylustroglodytes, and Physalaemuscicada.
Acknowledgments
We thank Cintia de Melo Souto Brige (in memoriam) and family (Fugiama farm) and José Cordeiro de Almeida Filho (in memoriam) and family (Serra Azul farm) for allowing the execution of the study on their properties, and Camila Souza Batista for her support in the field. We thank Kaique Brito Silva for assistance in preparing the map. CASC thanks CAPES-Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and FAPESB-Fundação de Amparo à Pesquisa do Estado da Bahia, for scholarships, as well as the State University of Santa Cruz and the Zoology Graduate Program-PPGZOO for the opportunity and support granted. MS and IRD acknowledge the Brazilian National Council for Scientific and Technological Development (CNPq - PQ 309365/2019-8 and PQ: 315362/2021-9, respectively) for research scholarships. CVM-M acknowledges funding from Universidade Estadual do Maranhão (UEMA) (senior productivity grant 05/2023– PPG/UEMA).
Appendix 1
List of vouchers deposited in the Museu de Zoologia da Universidade Estadual de Santa Cruz-MZUESC.
AMPHIBIANS
BRACHYCEPHALIDAE–Ischnocnemaverrucosa: MZUESC 15874, 15875, 15885, 15886, 15895, 15898, 15899. Ischnocnema sp. (gr.parva): MZUESC 15896. BUFONIDAE–Dendrophryniscusproboscideus: MZUESC 14688, 15126, 15127, 15128, 15798, 15803, 15859, 16532. Rhinellacrucifer: MZUESC 15031, 15148. Rhinellagranulosa: MZUESC 15030, 15055, 15504, 15505, 15506, 15850. Rhinelladiptycha: MZUESC 15503. CRAUGASTORIDAE–Bahiusbilineatus: MZUESC 15095, 15096, 15097, 15794, 15809, 15838, 15842, 15855, 15856, 15860, 15882, 15883. Haddadusbinotatus: MZUESC 15014, 15015, 15032, 15034, 15044, 15047, 15051, 15053, 15054, 15057, 15058, 15104, 15105, 15106, 15107, 15131, 15142, 15465, 15471, 15472, 15473, 15475, 15476, 15478, 15479, 15482, 15526, 15622, 15623, 15634, 15635, 15643, 15644, 15646, 15650, 15651, 15656, 15659, 15660, 15778, 15802. Pristimantisvinhai: MZUESC 15000, 15098–15103, 15109–15112, 15114, 15115, 15120, 15121, 15132, 15133, 15140, 15141, 15143, 15144, 15464, 15481, 15502, 15640, 15642, 15657, 15663, 15664, 15667, 15780, 15785, 15786, 15797, 15799–15801, 15806–15808, 15813–15815, 15837, 15840, 15841, 15843, 15853, 15854, 15857, 15858, 15861, 15879, 15897, 15889. Pristimantis sp. (gr.ramagii): MZUESC 15033, 15474, 15641, 15658, 15662, 15665, 15783, 15849, 15851, 15852, 16523. CENTROLENIDAE–Vitreoranaeurygnatha: MZUESC 14691, 14698, 14699, 14700, 14701, 15810–15812. CYCLORAMPHIDAE–Thoropamiliaris: MZUESC 14674, 15130, 15477, 15782, 15789, 15845, 15846, 15872, 15873, 15880, 15881. ELEUTHERODACTYLIDAE–Adelophryne sp.8: MZUESC 15035, 15036, 15039, 15041, 15043, 15048, 15050, 15052, 15116, 15462, 15466, 15480, 15528–15531, 15625–15627, 15631, 15633, 15637–15639, 15647, 15652, 15661, 15796, 15830, 15834, 15835, 15839, 15862, 16522, 16530. Adelophryne sp.2: MZUESC 15024, 15037, 15038, 15040, 15042, 15045, 15046, 15049, 15090, 15091, 15094, 15113, 15118, 15119, 15123–15125, 15457–15460, 15467–15470, 15524, 15525, 15527, 15533, 15624, 15628, 15629, 15632, 15636, 15645, 15648, 15649, 15655, 15795, 15831, 15832, 15836. HYLIDAE–Aplastodiscusweygoldti: MZUESC 14690, 15787, 15788, 15844, 15887. Boanacrepitans: MZUESC 14673, 14675, 14998, 14999, 15002, 15145, 15486. Boanaexastis: MZUESC 15108. Boanafaber: MZUESC 14676, 15066, 15068, 15147, 15666, 15884. Dendropsophusbranneri: MZUESC 14683, 14684, 15022, 15023, 15509, 15510, 15512, 15818, 15824. Dendropsophuselegans: MZUESC 14678, 14679, 15018, 15019, 15020, 15021, 15138, 15507, 15508, 15820. Dendropsophusoliveirai: MZUESC 14686, 15010, 15011, 15487–15501, 15511, 15791, 15792, 15823, 15825. Ololygonstrigilata: MZUESC 15001. Phyllodytesluteolus: MZUESC 17501, 17502. Pithecopusnordestinus: MZUESC 14680–14682, 14685, 14695, 14997, 15136, 15137, 15826, 15827, 16527. Pseudisfusca: MZUESC 16528. Scinaxeurydice: MZUESC 14672, 14693, 15876, 16531. Scinaxpachycrus: MZUESC 15790, 16525. Scinaxx-signatus: MZUESC 14692, 14694, 15027, 15139, 15892, 15894, 17503. Sphaenorhynchusprasinus: MZUESC 14677, 15004–15009, 15016, 15017, 15135, 15484, 15485, 15793, 15821, 15822. Trachycephalusnigromaculatus: MZUESC 15063, 15064, 15065, 15483, 15888. LEPTODACTYLIDAE–Leptodactyluscf.mystaceus: MZUESC 14696. Leptodactylusfuscus: MZUESC 15012, 15067, 15513–15516. Leptodactyluslatrans: MZUESC 15871, 15893. Leptodactylusmacrosternum: MZUESC 15146, 16524. Leptodactylusmystacinus: MZUESC 16529, 16533. Leptodactylustroglodytes: MZUESC 15003, 15028, 15029. Leptodactylusviridis: MZUESC 15848, 15869, 15870. Physalaemuscicada: MZUESC 15059–15062. Physalaemuscf.erikae: MZUESC 15013, 15878. Physalaemuskroyeri: MZUESC 15517, 15518, 15521, 15523, 15784, 15819, 15877. MICROHYLIDAE – Dermatonotusmuelleri: MZUESC 15069, 15070. ODONTOPHRYNIDAE–Proceratophrysschirchi: MZUESC 14689, 14702–14705, 15071, 15072, 15122, 15134, 15779, 15781, 15804, 15816, 15817, 15833, 15847, 15863–15868. PIPIDAE–Pipacarvalhoi: MZUESC 15129. SIPHONOPIDAE–Siphonopsannulatus: MZUESC 15900.
Citation
Souza-Costa CA, Solé M, Vinicius de Mira-Mendes C, Argôlo AJS, Ribeiro Dias I (2024) Uncovering the rich amphibian fauna of two semideciduous forest fragments in southwestern Bahia, Brazil. ZooKeys 1217: 215–246. https://doi.org/10.3897/zookeys.1217.119844
Additional information
Conflict of interest
The authors have declared that no competing interests exist.
Ethical statement
No ethical statement was reported.
Funding
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior; Fundação de Amparo à Pesquisa do Estado da Bahia.
Author contributions
Conceptualization: CASC, AJSA, CVMM, IRD, MS. Data curation: CASC. Formal analysis: CASC, IRD. Funding acquisition: MS. Investigation: MS, CASC. Methodology: CASC. Project administration: MS, CASC. Supervision: MS, IRD. Validation: CASC. Visualization: CASC. Writing - original draft: MS, AJSA, CVMM, IRD, CASC. Writing - review and editing: AJSA, IRD, MS, CVMM, CASC.
Author ORCIDs
Mirco Solé https://orcid.org/0000-0001-7881-6227
Caio Vinicius de Mira-Mendes https://orcid.org/0000-0002-7707-6439
Iuri Ribeiro Dias https://orcid.org/0000-0002-2825-3494
Data availability
All of the data that support the findings of this study are available in the main text.
References
- Abrunhosa PA, Wogel H, Pombal Jr JP. (2001) Vocalização de quatro espécies de anuros do Estado do Rio de Janeiro, Sudeste do Brasil (Amphibia, Hylidae, Leptodactylidae). Boletim do Museu Nacional do Rio de Janeiro 472: 1–12. [Google Scholar]
- Almeida AP, Bastos DFO, Júnior PBA, Vila Nova MF, Dias IR, Zina J. (2022) New records and geographic distribution map of Proceratophryssanctaritae Cruz & Napoli, 2010 (Anura, Odontophrynidae). Check List 18(6): 1243–1247. 10.15560/18.6.1243 [DOI] [Google Scholar]
- Andrade G, Carnaval AC. (2004) Rhinella jimi. The IUCN Red List of Threatened Species 2004. [accessed on 29 July 2016] 10.2305/IUCN.UK.2004.RLTS.T54674A11184744.en [DOI]
- Araújo AP, Ferreira RB, Canedo C, Zocca C, Lacerda JVA. (2023) After 160 years of ‘silence’: The advertisement call of the frog Ischnocnemaverrucosa. Herpetological Bulletin 163(163): 31–34. 10.33256/hb163.3134 [DOI]
- Araujo-Vieira K, Pombal Jr JP, Caramaschi U, Novaes-e-Fagundes G, Orrico VGD, Faivovich J. (2020a) A neotype for Hylax-signata Spix, 1824 (Amphibia, Anura, Hylidae). Papéis Avulsos de Zoologia 60[e20206056]: 1–30. 10.11606/1807-0205/2020.60.56 [DOI]
- Araujo-Vieira K, Luna MC, Caramaschi U, Haddad CFB. (2020b) A new genus of Lime Treefrogs (Anura: Hylidae: Sphaenorhynchini). Zoologischer Anzeiger 286: 81–89. 10.1016/j.jcz.2020.04.002 [DOI] [Google Scholar]
- Argôlo AJS. (2004) As serpentes dos cacauais do sudeste da Bahia. Editus, Ilhéus, Bahia, Brazil, 259 pp. [Google Scholar]
- Arzabe C, Skuk G, Santana GG, Delfim FR, Lima YCC, Abrantes SHF. (2005) Herpetofauna da área do Curimataú, Paraíba. In: Araujo FS, Rodal MJN, Barbosa MRV (Org) Análise das Variações da Biodiversidade do Bioma Caatinga: suporte a estratégias regionais de conservação. Ministério do Meio Ambiente, Brasília, 259–273.
- Baldissera Jr FA, Caramaschi U, Haddad CFB. (2004) Review of the Bufocrucifer species group, with descriptions of two new related species (Amphibia, Anura, Bufonidae). Rio de Janeiro. Arquivos do Museu Nacional. Museu Nacional (Brazil) 62(3): 255–282. [Google Scholar]
- Bastos RP, Pombal Jr JP. (1996) A new species of Hyla (Anura: Hylidae) from eastern Brazil. Amphibia-Reptilia 17(4): 325–331. 10.1163/156853896X00054 [DOI] [Google Scholar]
- Bastos DFO, Zina J. (2022) Amphibian fauna in an ecotonal and mountainous area in south-central Bahia State, northeastern Brazil. Herpetology Notes 15: 365–376. [Google Scholar]
- Becker CG, Fonseca CR, Haddad CFB, Batista RF, Prado PI. (2007) Habitat Split and the Global Decline of Amphibians. Science 318(5857): 1775–1777. 10.1126/science.1149374 [DOI] [PubMed] [Google Scholar]
- Blotto BL, Lyra ML, Cardoso MCS, Rodrigues MT, Dias IR, Marciano-Jr E, Vechio FD, Orrico VGD, Brandão RA, Assis CL, Lantyer-Silva ASF, Rutherford MG, Gagliardi-Urrutia G, Solé M, Baldo D, Nunes I, Cajade R, Torres A, Grant T, Jungfer KH, Silva HR, Haddad CFB, Faivovich J. (2021) The phylogeny of the Casque- headed Treefrogs (Hylidae: Hylinae: Lophyohylini). Cladistics 37(1): 36–72. 10.1111/cla.12409 [DOI] [PubMed] [Google Scholar]
- Boettger O. (1885) Liste von Reptilien und Batrachiern aus Paraguay. Zeitschrift für Naturwissenschaften 58: 213–248. [Google Scholar]
- Bokermann WCA. (1963) Nova espécie de Hyla da Bahia, Brasil. Atas da Sociedade de Biologia do Rio de Janeiro 7: 6–8. [Google Scholar]
- Bokermann WCA. (1966c) Notas sôbre três espécies de Physalaemus de Maracás, Bahia (Amphibia, Leptodactylidae). Revista Brasileira de Biologia 26(3): 253–259. [Google Scholar]
- Bokermann WCA. (1966a) O gênero Phyllodytes Wagler, 1830 (Anura, Hylidae). Rio de Janeiro. Anais da Academia Brasileira de Ciências 38(2): 335–344. [Google Scholar]
- Bokermann WCA. (1966b) Una nueva especie de Trachycephalus de Bahia, Brasil (Amphibia, Hylidae). Neotrópica 12(39): 124–210. [Google Scholar]
- Bokermann WCA. (1968) Three new Hyla from the Plateau of Maracás, central Bahia, Brazil. Journal of Herpetology 1(1/4): 25–31. 10.2307/1563259 [DOI]
- Bokermann WCA. (1973) Duas novas espécies de Sphaenorhynchus da Bahia (Anura, Hylidae). Revista Brasileira de Biologia 33: 589–594. [Google Scholar]
- Bokermann WCA. (1975) Três espécies novas de Eleutherodactylus do sudeste da Bahia, Brasil (Anura, Leptodactylidae). Revista Brasileira de Biologia 34(1): 11–18. [Google Scholar]
- Borges-Nojosa DM, Cascon P. (2005) Herpetofauna da área Reserva da Serra das Almas, Ceará. In: Araújo FS, Rodal MJN, Barbosa MRV (Org) Análise das Variações da Biodiversidade do Bioma Caatinga: suporte a estratégias regionais de conservação. Brasília, Ministério do Meio Ambiente, 243–258.
- Borges-Nojosa DM, Santos EM. (2005) Herpetofauna da área de Betânia e Floresta, Pernambuco. In: Araújo FS, Rodal MJN, Barbosa MRV (Org) Análise das Variações da Biodiversidade do Bioma Caatinga: suporte a estratégias regionais de conservação. Brasília, Ministério do Meio Ambiente, 275–289.
- Boulenger GA. (1882) Catalogue of the BatrachiaSalientia s. Ecaudata in the Collection of the British Museum. Second Edition. Taylor and Francis, London, 578 pp. [Google Scholar]
- Braga HSN, Vieira MVSA, Silva TAF, Protázio AS, Protázio AS. (2024) Acoustic partitioning explains the coexistence between two Physalaemus species (Anura, Leptodactylidae) in the Atlantic Forest in Eastern Bahia State, Brazil. Anais da Academia Brasileira de Ciências 95(1): e20211348. 10.1590/0001-3765202320211348 [DOI] [PubMed]
- Brandão RA, Leão PF, Gedraite L. (2020) Anfíbios da Trijunção dos estados da Bahia, Goiás e Minas Gerais e do Parque Nacional Grande Sertão Veredas. In: Brandão RA, Françoso RD, Machado TH, Santos NJ (Org) História Natural do Sertão da Trijunção do Nordeste, Centro–Oeste e Sudeste do Brasil. São Paulo, PerSe 1ª ed.: 127–. $6
- Brasileiro CA, Lucas EM, Oyamaguchi HM, Thomé MTC, Dixo M. (2008) Anurans, Northern Tocantins River Basin, states of Tocantins and Maranhão, Brazil. Check List 4(2): 185–197. 10.15560/4.2.185 [DOI] [Google Scholar]
- Burmeister H. (1861) Reise durch die La Plata-Staaten mit besonderer Rücksicht auf die physische Beschaffenheit und den Culturzustand der Argentinischen Republik. Ausgeführt in den Jahren 1857, 1858, 1859 und 1860. Vol. 2. H. W. Schmidt, Halle, 538 pp. [Google Scholar]
- Camardelli M, Napoli MF. (2012) Amphibian Conservation in the Caatinga Biome and Semiarid Region of Brazil. Herpetologica 68(1): 31–47. 10.1655/HERPETOLOGICA-D-10-00033.1 [DOI] [Google Scholar]
- Camurugi F, Lima TM, Mercês EA, Juncá FA. (2010) Anuros da Reserva Ecológica da Michelin, Município de Igrapiúna, Estado da Bahia, Brasil. Biota Neotropica 10(2): 305–312. 10.1590/S1676-06032010000200032 [DOI] [Google Scholar]
- Canedo CB, Pimenta VS, Leite FSF, Caramaschi U. (2010) New species of Ischnocnema (Anura: Brachycephalidae) from the state of Minas Gerais, southeastern Brazil, with comments on the I.verrucosa species series. Copeia 2010(4): 629–634. 10.1643/CH-09-159 [DOI] [Google Scholar]
- Caramaschi U. (2006) Redefinição do grupo de Phyllomedusahypochondrialis, com redescrição de P.megacephala (Miranda-Ribeiro, 1926), revalidação de P.azurea Cope, 1862 e descrição de uma nova espécie (Amphibia, Anura, Hylidae). Arquivos do Museu Nacional. Museu Nacional 64: 159–179. [Google Scholar]
- Caramaschi U. (2012) A new species of beaked toad, Rhinella (Anura: Bufonidae), from the State of Bahia, Brazil. Zoologia. Sociedade Brasileira de Zoologia. Curitiba 29(4): 343–348. 10.1590/S1984-46702012000400007 [DOI] [Google Scholar]
- Caramaschi U, Canedo C. (2006) Reassessment of the taxonomic status of the genera Ischnocnema Reinhardt and Lütken, 1862 and Oreobates Jiménez-de-la-Espada, 1872 with notes on the synonymy of Leiuperusverrucosus Reinhardt and Lütken, 1862 (Anura: Leptodactylidae). Zootaxa 1116(1): 43–54. 10.11646/zootaxa.1116.1.3 [DOI] [Google Scholar]
- Caramaschi U, Cruz CAG. (1998) Notas taxonômicas sobre Pseudisfusca Garman e P.bolbodactyla A. Lutz, com a descrição de uma nova espécie correlata (Anura, Pseudidae). Revista Brasileira de Zoologia 15(4): 929–944. 10.1590/S0101-81751998000400011 [DOI] [Google Scholar]
- Caramaschi U, Rodrigues MT. (2003) A new large treefrog species, genus Hyla Laurenti, 1768, from southern Bahia, Brazil (Amphibia, Anura, Hylidae). Rio de Janeiro. Arquivos do Museu Nacional. Museu Nacional 61(4): 255–260. [Google Scholar]
- Caramaschi U, Rodrigues MT. (2007) Taxonomic status of the species of Gastrotheca Fitzinger, 1843 (Amphibia, Anura, Amphignathodontidae) of the Atlantic Rain Forest of Eastern Brazil, with description of a new species. Boletim do Museu Nacional, Nova Serie. Zoologia 525: 1–19. [Google Scholar]
- Caramaschi U, Orrico VGD, Faivovich J, Dias IR, Solé M. (2013) A new species of Allophryne (Anura: Allophrynidae) from the Atlantic Rain Forest Biome of eastern Brazil. Herpetologica 69(4): 480–491. 10.1655/HERPETOLOGICA-D-13-00029 [DOI] [Google Scholar]
- Carilo-Filho LM, Carvalho BT, Azevedo BKA, Gutiérrez-Pesquera LM, Mira-Mendes CV, Solé M, Orrico VG. (2021) Natural history predicts patterns of thermal vulnerability in amphibians from the Atlantic Rainforest of Brazil. Ecology and Evolution 11(23): 16462–16472. 10.1002/ece3.7961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnaval AC, Moritz C. (2008) Historical climate modelling predicts patterns of current biodiversity in the Brazilian Atlantic forest. Journal of Biogeography 35(7): 1187–1201. 10.1111/j.1365-2699.2007.01870.x [DOI] [Google Scholar]
- Carnaval AC, Hickerson MJ, Haddad CFB, Rodrigues MT, Moritz C. (2009) Stability Predicts Genetic Diversity in the Brazilian Atlantic Forest Hotspot. Science 323(5915): 785–789. 10.1126/science.1166955 [DOI] [PubMed] [Google Scholar]
- Carneiro MCL, Magalhães PS, Juncá FA. (2004) Descrição do girino e vocalização de Scinaxpachycrus (Miranda-Ribeiro, 1937) (Amphibia, Anura, Hylidae). Rio de Janeiro. Arquivos do Museu Nacional. Museu Nacional 62(3): 241–246. [Google Scholar]
- Cassini CS, Orrico VGD, Dias IR, Solé M, Haddad CFB. (2013) Phenotypic variation of Leptodactyluscupreus Caramaschi, São-Pedro and Feio, 2008 (Anura, Leptodactylidae). Zootaxa 3616(1): 073–084. 10.11646/zootaxa.3616.1.6 [DOI] [PubMed] [Google Scholar]
- Cetra M, Sarmento-Soares LM, Martins-Pinheiro RF. (2010) Peixes de riachos e novas Unidades de Conservação no sul da Bahia. Pan-American Journal of Aquatic Sciences 5(1): 11–21. [Google Scholar]
- Cochran DM. (1948) A new subspecies of tree frog from Pernambuco, Brazil. Journal of the Washington Academy of Sciences 38: 316–318. [Google Scholar]
- Colwell RK, Coddington JA. (1994) Estimating terrestrial biodiversity through extrapolation. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 345(1311): 101–118. 10.1098/rstb.1994.0091 [DOI] [PubMed] [Google Scholar]
- Cope ED. (1862) Catalogues of the reptiles obtained during the explorations of the Parana, Paraguay, Vermejo and Uruguay Rivers, by Capt. Thos. J. Page, U.S.N.; and of those procured by Lieut. N. Michler, U.S. Top. Eng., Commander of the expedition conducting the survey of the Atrato River. Proceedings. Academy of Natural Sciences of Philadelphia 14: 346–35. [Google Scholar]
- Crump ML, Scott Jr NJ. (1994) Visual Encounter Surveys. In: Heyer WR, Donnelly MA, McDiarmid RW, Hayek LC, Foster MS. (Eds) Measuring and monitoring biological diversity: standard methods for amphibians.Smithsonian Institution Press, Washington USA, 84–92.
- Cruz CAG, Peixoto OL. (1987) Espécies verdes de Hyla: o complexo “albofrenata” (Amphibia, Anura, Hylidae). Arquivos de Universidade Federal Rural do Rio de Janeiro 8: 59–70. [Google Scholar]
- Cruz CAG, Pimenta BVS. (2004) A new species of Physalaemus Fitzinger, 1826 from Southern Bahia state, Brazil (Anura, Leptodactylidae). Journal of Herpetology 38(4): 480–486. 10.1670/214-02A [DOI] [Google Scholar]
- Cruz CAG, Feio RN, Cardoso MCS. (2007) Description of a new species of Phyllodytes Wagler, 1830 (Anura, Hylidae) from the Atlantic Rain Forest of the states of Minas Gerais and Bahia, Brazil. Arquivos do Museu Nacional. Museu Nacional 64(4): 321–324. [Google Scholar]
- Da-Silva FR, Almeida-Neto M, Prado VHM, Haddad CFB, Rossa-Feres DC. (2012) Humidity levels drive reproductive modes and phylogenetic diversity of amphibians in the Brazilian Atlantic Forest. Journal of Biogeography 39(9): 1720–1732. 10.1111/j.1365-2699.2012.02726.x [DOI] [Google Scholar]
- De-Sá RO, Grant T, Camargo A, Heyer WR, Ponssa ML, Stanley EL. (2014) Systematics of the Neotropical genus Leptodactylus Fitzinger, 1826 (Anura: Leptodactylidae): Phylogeny, the relevance of non-molecular evidence, and species accounts. South American Journal of Herpetology 9(s1): 1–128. 10.2994/SAJH-D-13-00022.1 [DOI]
- Dias IR, Vilaça TRA, Silva JRS, Barbosa RS, Solé M. (2010) Amphibia, Anura, Hylidae, Trachycephalusnigromaculatus Tschudi, 1838: Distribution extension. Check List 6(3): 412–413. 10.15560/6.3.412 [DOI] [Google Scholar]
- Dias IR, Medeiros TT, Solé M, Pimenta BVS. (2011) Amphibia, Anura, Hylidae, Bokermannohylalucianae (Napoli and Pimenta 2003): Distribution extension and geographic distribution map. Check List 7(2): 108–109. 10.15560/7.2.108 [DOI] [Google Scholar]
- Dias IR, Lourenço-de-Moraes R, Solé M. (2012) Description of the advertisement call and morphometry of Haddadusbinotatus (Spix, 1824) from a population from southern Bahia, Brazil. North-Western Journal of Zoology 8(1): 107–111. [Google Scholar]
- Dias IR, Mira-Mendes CV, Solé M. (2014a) Rapid inventory of herpetofauna at the APA (Environmental Protection Area) of the Lagoa Encantada and Rio Almada, Southern Bahia, Brazil. Herpetology Notes 7: 627–637. http://biotaxa.org/hn/article/view/8557/10467 [Google Scholar]
- Dias IR, Medeiros TT, Vila Nova MF, Solé M. (2014b) Amphibians of Serra Bonita, southern Bahia: A new hotpoint within Brazil’s Atlantic Forest hotspot. ZooKeys 449: 105–130. 10.3897/zookeys.449.7494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias IR, Haddad CFB, Argôlo AJS, Orrico VGD. (2017a) The 100th: An appealing new species of Dendropsophus (Amphibia: Anura: Hylidae) from northeastern Brazil. PLoS ONE 12(3): 1–20. 10.1371/journal.pone.0171678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias IR, Mira-Mendes CV, Souza-Costa CA, Juncá FA, Solé M. (2017b) The advertisement call and comments on the distribution of Eleutherodactylusbilineatus Bokermann, 1975, an endemic frog of Bahia State, Brazil (Amphibia, Anura). ZooKeys 677(1): 151–159. 10.3897/zookeys.677.12309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias IR, Novaes-e-Fagundes G, Neto AM, Zina J, Garcia C, Recoder RS, Dal Vechio F, Rodrigues MT, Solé M. (2020) A new large canopy-dwelling species of Phyllodytes Wagler, 1930 (Anura, Hylidae) from the Atlantic Forest of the state of Bahia, northeastern Brazil. PeerJ 8(e8642): 1–27. 10.7717/peerj.8642 [DOI] [PMC free article] [PubMed]
- Droege S, Cyr A, Larivée J. (1998) Checklists: An under-used tool for the inventory and monitoring of plants and animals. Conservation Biology 12(5): 1134–1138. 10.1046/j.1523-1739.1998.96402.x [DOI] [Google Scholar]
- Duarte H, Tejedo M, Katzenberger M, Marangoni F, Baldo D, Beltrán JF, Martí DA, Richter-Boix A, Gonzalez-Voyer A. (2012) Can amphibians take the heat? Vulnerability to climate warming in subtropical and temperate larval amphibian communities. Global Change Biology 18(2): 412–421. 10.1111/j.1365-2486.2011.02518.x [DOI] [Google Scholar]
- Dubeux MJM, Nascimento FAC, Gonçalves U, Mott T. (2021) Identification key for anuran amphibians in a protected area in the northeastern Atlantic Forest. Papéis Avulsos de Zoologia 61[e20216176]: 1–10. 10.11606/1807-0205/2021.61.76 [DOI]
- Duellman WE. (2015) Marsupial Frogs. Gastrotheca & Allied Genera. Johns Hopkins University Press, Baltimore, 432 pp. 10.1353/book.40894 [DOI] [Google Scholar]
- Feio RN, Caramaschi U. (1995) Aspectos Zoogeográficos dos Anfíbios do Médio Rio Jequitinhonha, Nordeste de Minas Gerais, Brasil. Revista Ceres 42(239): 53–61. http://www.ceres.ufv.br/ojs/index.php/ceres/article/view/2238/272 [Google Scholar]
- Feio RN, Napoli MF, Caramaschi U. (2006a) Considerações taxonômicas sobre Thoropamiliaris (Spix, 1824), com revalidação e redescrição de Thoropataophora (Miranda-Ribeiro, 1923) (Amphibia, Anura, Leptodactylidae). Arquivos do Museu Nacional. Museu Nacional 64(1): 41–60. [Google Scholar]
- Feio RN, Nascimento LB, Cruz CAG, Ferreira PL, Pantoja DL. (2006b) Anfíbios das áreas prioritárias dos rios Jequitinhonha e Mucuri. In: Pinto LPS, Bedê L (Org) Biodiversidade e Conservação nos Vales dos Rios Jequitinhonha e Mucuri. Brasília, Ministério do Meio Ambiente 1: 94–119. [Google Scholar]
- Fonte LFM, Mayer M, Lötters S. (2019) Long-distance dispersal in amphibians. Frontiers of Biogeography 11(4): 1–14. 10.21425/F5FBG44577 [DOI] [Google Scholar]
- Freitas MA, Abegg AD, Dias IR, Moraes EPF. (2018) Herpetofauna from Serra da Jibóia, an Atlantic Rainforest remnant in the state of Bahia, northeastern Brazil. Herpetology Notes 11(1): 59–72. https://www.biotaxa.org/hn/article/view/32254/39365 [Google Scholar]
- Freitas MA, Silva TFS, Fonseca PM, Hamdan B, Filadelfo T, Abegg AD. (2019) Herpetofauna of Serra do Timbó, an Atlantic Forest remnant in Bahia State, northeastern Brazil. Herpetology Notes 12: 245–260. https://www.biotaxa.org/hn/article/view/32254/39365 [Google Scholar]
- Frost DR. (2023) Amphibian Species of the World: An Online Reference. Version 6.2. Electronic Database. https://amphibiansoftheworld.amnh.org/
- Garda AA, Santana DJ, São-Pedro VA. (2010) Taxonomic characterization of paradoxical frogs (Anura, Hylidae, Pseudae): geographic distribution, external morphology, and morphometry. Zootaxa 2666(1): 1–28. 10.11646/zootaxa.2666.1.1 [DOI] [Google Scholar]
- Garda AA, Costa TB, Santos-Silva CR, Mesquita DO, Faria RG, Conceição BM, Silva IRS, Ferreira AS, Rocha SM, Palmeira CNS, Rodrigues R, Ferrari SF, Torquato S. (2013) Herpetofauna of protected areas in the Caatinga I: Raso da Catarina Ecological Station (Bahia, Brazil). Check List 9(2): 405–414. 10.15560/9.2.405 [DOI] [Google Scholar]
- Garman S. (1883) A species of Pseudis from the Rio Arassuahy, Brazil. Science Observer. Journal of Science 4: 47.
- Gomes MR, Peixoto OL. (1991) Larvas de Hyla do grupo leucophyllata com a descrição da de H.elegans, 1824 e notas sobre a variação do padrão de colorido do adulto nesta espécie (Anura, Hylidae). Revista Brasileira de Biologia 51(1): 257–262. [Google Scholar]
- Gondim-Silva FAT, Andrade ARS, Abreu RO, Nascimento JS, Corrêa GP, Menezes L, Trevisan CC, Camargo SS, Napoli MF. (2016) Composition and diversity of anurans in the Restinga of the Conde municipality, Northern coast of the state of Bahia, Northeastern Brazil. Biota Neotropica 16(3): 1–16. 10.1590/1676-0611-BN-2016-0157 [DOI] [Google Scholar]
- Gotelli NJ, Colwell RK. (2001) Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters 4(4): 379–391. 10.1046/j.1461-0248.2001.00230.x [DOI] [Google Scholar]
- Gutiérrez-Pesquera LM, Tejedo M, Olalla-Tárraga MÁ, Duarte H, Nicieza A, Solé M. (2016) Testing the climate variability hypothesis in thermal tolerance limits of tropical and temperate tadpoles. Journal of Biogeography 43(6): 1166–1178. 10.1111/jbi.12700 [DOI] [Google Scholar]
- Haddad CFB. (1998) Biodiversidade dos Anfíbios no Estado de São Paulo. In: Joly CA, Bicudo CEM (Org) Biodiversidade do Estado de São Paulo, Brasil: síntese do conhecimento ao final do século XX, 6: vertebrados. São Paulo, Fapesp: 15–26.
- Haddad CFB, Toledo LF, Prado CPA, Loebmann D, Gasparini JL, Sazima I. (2013) Guia de Anfíbios da Mata Atlântica: diversidade e biologia. Anolisbooks, São Paulo, 544 pp. [Google Scholar]
- Hammer Ø, Harper DAT, Ryan PD. (2001) Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica 4(1) [art. 4]: 1–9. [178kb]. http://palaeo-electronica.org/2001_1/past/issue1_01.htm
- Hepp F, Pombal J. (2020) Review of bioacoustical traits in the genus Physalaemus Fitzinger, 1826 (Anura: Leptodactylidae: Leiuperinae). Zootaxa 4725(1): 1–106. 10.11646/zootaxa.4725.1.1 [DOI] [PubMed] [Google Scholar]
- Heyer WR. (1978) Systematics of the fuscus group of the frog genus Leptodactylus (Amphibia, Leptodactylidae). Natural History Museum of Los Angeles County Science Bulletin 29: 1–85. 10.5479/si.00810282.301 [DOI] [Google Scholar]
- Heyer WR, Rand AS, Cruz CAG, Peixoto OL, Nelson CE. (1990) Frogs of Boracéia. Arquivos de Zoologia 31(4): 231–410. 10.11606/issn.2176-7793.v31i4p231-410 [DOI] [Google Scholar]
- Heyer WR, Donnelly MA, McDiarmid RW, Hayek LC, Foster MS. (1994) Measuring and monitoring biological diversity: standard methods for amphibians. Smithsonian Institution Press Washington.
- Hoffmann M, Hilton-Taylor C, Angulo A, Böhm M, Brooks TM, Butchart SH, Carpenter KE, Chanson J, Collen B, Cox NA, Darwall WR, Dulvy NK, Harrison LR, Katariya V, Pollock CM, Quader S, Richman NI, Rodrigues ASL, Tognelli MF, Vié J-C, Aguiar JM, Allen DJ, Allen GR, Amori G, Ananjeva NB, Andreone F, Andrew P, Ortiz ALA, Baillie JEM, Baldi R, Bell BD, Biju SD, Bird JP, Black-Decima P, Blanc JJ, Bolaños F, Bolivar-G W, Burfield IJ, Burton JA, Capper DR, Castro F, Catullo G, Cavanagh RD, Channing A, Chao NL, Chenery AM, Chiozza F, Clausnitzer V, Collar NJ, Collett LC, Collette BB, Fernandez CFC, Craig MT, Crosby MJ, Cumberlidge N, Cuttelod A, Derocher AE, Diesmos AC, Donaldson JS, Duckworth JW, Dutson G, Dutta SK, Emslie RH, Farjon A, Fowler S, Freyhof J, Garshelis DL, Gerlach J, Gower DJ, Grant TD, Hammerson GA, Harris RB, Heaney LR, Hedges SB, Hero J-M, Hughes B, Hussain SA, Icochea M J, Inger RF, Ishii N, Iskandar DT, Jenkins RKB, Kaneko Y, Kottelat M, Kovacs KM, Kuzmin SL, La Marca E, Lamoreux JF, Lau MWN, Lavilla EO, Leus K, Lewison RL, Lichtenstein G, Livingstone SR, Lukoschek V, Mallon DP, McGowan PJK, McIvor A, Moehlman PD, Molur S, Alonso AM, Musick JA, Nowell K, Nussbaum RA, Olech W, Orlov NL, Papenfuss TJ, Parra-Olea G, Perrin WF, Polidoro BA, Pourkazemi M, Racey PA, Ragle JS, Ram M, Rathbun G, Reynolds RP, Rhodin AGJ, Richards SJ, Rodríguez LO, Ron SR, Rondinini C, Rylands AB, Sadovy de Mitcheson Y, Sanciangco JC, Sanders KL, Santos-Barrera G, Schipper J, Self-Sullivan C, Shi Y, Shoemaker A, Short FT, Sillero-Zubiri C, Silvano DL, Smith KG, Smith AT, Snoeks J, Stattersfield AJ, Symes AJ, Taber AB, Talukdar BK, Temple HJ, Timmins R, Tobias JA, Tsytsulina K, Tweddle D, Ubeda C, Valenti SV, Paul van Dijk P, Veiga LM, Veloso A, Wege DC, Wilkinson M, Williamson EA, Xie F, Young BE, Akçakaya HR, Bennun L, Blackburn TM, Boitani L, Dublin HT, da Fonseca GAB, Gascon C, Lacher Jr TE, Mace GM, Mainka SA, McNeely JA, Mittermeier RA, Reid GMG, Rodriguez JP, Rosenberg AA, Samways MJ, Smart J, Stein BA, Stuart SN. (2010) The impact of conservation on the status of the world’s vertebrates. Science 330(6010): 1503–1509. 10.1126/science.1194442 [DOI] [PubMed] [Google Scholar]
- IBGE [Instituto Brasileiro de Geografia e Estatística] (1997) Diagnóstico Ambiental da Bacia do Rio Jequitinhonha: diretrizes gerais para a ordenação territorial. Salvador, Diretoria de Geociências, 1ª Divisão de Geociências do Nordeste-DIGEO 1/NE.1, 64 pp. [Google Scholar]
- ICMBio [Instituto Chico Mendes de Conservação da Biodiversidade] (2003) Decretos MMA s/nº de 05 de Junho de 2003. http://www.icmbio.gov.br/portal/rebio-da-mata escura?highlight=WyJtYXRhIiwiZXNjdXJhIiwibWF0YSBlc2N1cmEiXQ [accessed on 25 March 2018]
- ICMBio [Instituto Chico Mendes de Conservação da Biodiversidade] (2010) Decreto MMA s/nº de 11 de Junho de 2010. http://www.icmbio.gov.br/portal/parna-do-alto-cariri?highlight=WyJjYXJpcmkiXQ [accessed on 25 March 2018]
- ICMBio [Instituto Chico Mendes de Conservação da Biodiversidade] (2016) Portaria MMA nº 110 de 22 de Dezembro de 2016. http://sistemas.icmbio.gov.br/site_media/portarias/2017/01/19/Portaria_RPPN_Mata_do Passarinho_01.pdf [accessed on 25 March 2018]
- IUCN (2024) The IUCN Red List of Threatened Species. Version 2024-1. http://www.iucnredlist.org [accessed on 02 July 2024]
- Izecksohn E. (1976) O status sistemático de Phryniscusproboscideus Boulenger (Amphibia, Anura, Bufonidae). Revista Brasileira de Biologia 36(2): 341–34. [Google Scholar]
- Izecksohn E, Peixoto OL. (1980) Sobre a utilização do nome Stombusprecrenulatus Miranda-Ribeiro, 1937 e a validez da espécie (Amphibia, Anura, Leptodactylidae). Revista Brasileira de Biologia 40(3): 605–609. [Google Scholar]
- Jim J, Spirandeli-Cruz EF. (1973) Uma nova espécie de Leptodactylus do Estado da Bahia, Brasil (Amphibia, Anura). Revista Brasileira de Biologia 39(3): 707–710. [Google Scholar]
- Juncá FA. (2005) Anfíbios e Répteis. In: Juncá FA, Funch L, Rocha W (Org) Biodiversidade e Conservação da Chapada Diamantina. Série Biodiversidade. Brasília, Ministério do Meio Ambiente 13(1): 339–376. [Google Scholar]
- Juncá FA. (2006) Diversidade e uso de hábitat por anfíbios anuros em duas localidades de Mata Atlântica, no norte do Estado da Bahia. Biota Neotropica 6(2): 1–8. 10.1590/S1676-06032006000200018 [DOI] [Google Scholar]
- Juncá FA, Pimenta B. (2004) Ischnocnemabilineta. In: IUCN Red List of Threatened Species (International Union for Conservation of Nature IUCN). 10.2305/IUCN.UK.2004.RLTS.T56462A11468784.en [accessed on 10 August 2022] [DOI]
- Köppen WP. (1936) Das geographische System der Klimate. In: Köppen WG, Geiger RM (Eds) Handbuch der Klimatologie Band I, Teil C. Gebrüder Borntraeger, Berlin, 44 pp. [Google Scholar]
- Lantyer-Silva ASF, Lourenço-De-Moraes R, Siqueira-Jr S, Solé M. (2011) Amphibia, Anura, Bufonidae, Rhinellaboulengeri Chaparro, Pramuk, Gluesenkamp and Frost, 2007: Distribution extension, state of Bahia, Brazil. Check List 7(6): 825–826. 10.15560/7.6.825 [DOI] [Google Scholar]
- Lantyer-Silva ASF, Siqueira-Jr S, Zina J. (2013) Checklist of amphibians in a transitional area between the Caatinga and the Atlantic Forest, central-Southern Bahia, Brazil. Check List 9(4): 725–732. 10.15560/9.4.725 [DOI] [Google Scholar]
- Lavilla EO, Brusquetti F. (2018) On the identity of Bufodiptychus Cope, 1862 (Anura: Bufonidae). Zootaxa 4442(1): 161–170. 10.11646/zootaxa.4442.1.9 [DOI] [PubMed] [Google Scholar]
- Lima LR, Bruschi DP, Nascimento FAC, Araújo PVS, Costa LP, Thomé MTC, Garda AA, Zattera ML, Mott T. (2020) Below the waterline: Cryptic diversity of aquatic pipid frogs (Pipacarvalhoi) unveiled through an integrative taxonomy approach. Systematics and Biodiversity 18(8): 1–13. 10.1080/14772000.2020.1795742 [DOI] [Google Scholar]
- Linares AM, Mello HES. (2011) Physalaemuscicada Bokermann, 1966 (Anura: Leiuperidae): Distribution extension with new South limit and geographic distribution map. Check List 7(6): 859–861. 10.15560/7.6.859 [DOI] [Google Scholar]
- Loebmann D, Haddad CFB. (2010) Amphibians and reptiles from a highly diverse area of the Caatinga domain: Composition and conservation implications. Biota Neotropica 10(3): 227–256. 10.1590/S1676-06032010000300026 [DOI] [Google Scholar]
- Loebmann D, Zina J, Araújo OGS, Toledo LF, Haddad CFB. (2008) Acoustic Repertory of Hypsiboasexastis (Caramaschi and Rodrigues, 2003) (Amphibia, Hylidae). South American Journal of Herpetology 3(2): 96–100. 10.2994/1808-9798(2008)3[96:AROHEC]2.0.CO;2 [DOI]
- Lourenço-De-Moraes R, Dias IR, Mira-Mendes CV, de Oliveira RM, Barth A, Ruas DS, Vences M, Solé M, Bastos RP. (2018) Diversity of miniaturized frogs of the genus Adelophryne (Anura: Eleutherodactylidae): A new species from the Atlantic Forest of northeast Brazil. PLoS ONE 13(9): e0201781. 10.1371/journal.pone.0201781 [DOI] [PMC free article] [PubMed]
- Luedtke JA, Chanson J, Neam K, Hobin L, Maciel AO, Catenazzi A, Borzée A, Hamidy A, Aowphol A, Jean A, Sosa-Bartuano Á, Fong G A, de Silva A, Fouquet A, Angulo A, Kidov AA, Muñoz Saravia A, Diesmos AC, Tominaga A, Shrestha B, Gratwicke B, Tjaturadi B, Martínez Rivera CC, Vásquez Almazán CR, Señaris C, Chandramouli SR, Strüssmann C, Cortez Fernández CF, Azat C, Hoskin CJ, Hilton-Taylor C, Whyte DL, Gower DJ, Olson DH, Cisneros-Heredia DF, Santana DJ, Nagombi E, Najafi-Majd E, Quah ESH, Bolaños F, Xie F, Brusquetti F, Álvarez FS, Andreone F, Glaw F, Castañeda FE, Kraus F, Parra-Olea G, Chaves G, Medina-Rangel GF, González-Durán G, Ortega-Andrade HM, Machado IF, Das I, Dias IR, Urbina-Cardona JN, Crnobrnja-Isailović J, Yang J-H, Jianping J, Wangyal JT, Rowley JJL, Measey J, Vasudevan K, Chan KO, Gururaja KV, Ovaska K, Warr LC, Canseco-Márquez L, Toledo LF, Díaz LM, Khan MMH, Meegaskumbura M, Acevedo ME, Napoli MF, Ponce MA, Vaira M, Lampo M, Yánez-Muñoz MH, Scherz MD, Rödel M-O, Matsui M, Fildor M, Kusrini MD, Ahmed MF, Rais M, Kouamé NGG, García N, Gonwouo NL, Burrowes PA, Imbun PY, Wagner P, Kok PJR, Joglar RL, Auguste RJ, Brandão RA, Ibáñez R, von May R, Hedges SB, Biju SD, Ganesh SR, Wren S, Das S, Flechas SV, Ashpole SL, Robleto-Hernández SJ, Loader SP, Incháustegui SJ, Garg S, Phimmachak S, Richards SJ, Slimani T, Osborne-Naikatini T, Abreu-Jardim TPF, Condez TH, De Carvalho TR, Cutajar TP, Pierson TW, Nguyen TQ, Kaya U, Yuan Z, Long B, Langhammer P, Stuart SN. (2023) Ongoing declines for the world’s amphibians in the face of emerging threats. Nature 622(7982): 308–314. 10.1038/s41586-023-06578-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A. (1925) Batraciens du Brésil. Comptes Rendus et Mémoires Hebdomadaires des Séances de la Société de Biologie et des ses Filiales. Paris 93(2): 137–139. [Google Scholar]
- Lutz A. (1926) Observações sobre batrachios brasileiros/Observations on brazilian batrachians. Memorias do Instituto Oswaldo Cruz 19(2): 139–174. 10.1590/S0074-02761926000200001 [DOI] [Google Scholar]
- Lynch JD. (1972) Generic partitioning of the South American leptodactyloid frog genus Eupsophus Fitzinger, 1843 (sensu lato). Bulletin of the Southern California Academy of Sciences 71: 2–11. [Google Scholar]
- Maciel AO, Hoogmoed MS. (2011) Taxonomy and distribution of caecilian amphibians (Gymnophiona) of Brazilian Amazonia, with a key to their identification. Zootaxa 2984(1): 1–53. 10.11646/zootaxa.2984.1.1 [DOI] [Google Scholar]
- Magalhães FM, Dantas AKBP, Brito MRM, Medeiros PHS, Oliveira AF, Pereira TCSO, Queiroz MHC, Santana DJ, Silva WP, Garda AA. (2013) Anurans from an Atlantic Forest-Caatinga ecotone in Rio Grande do Norte State, Brazil. Herpetology Notes 6: 1–10. [Google Scholar]
- Magalhães FM, Laranjeiras DO, Costa TB, Juncá FA, Mesquita DO, Rohr DL, Silva WP, Vieira GHC, Garda AA. (2015) Herpetofauna of protected areas in the Caatinga IV: Chapada Diamantina National Park, Bahia, Brazil. Herpetology Notes 8: 243–261. http://www.biotaxa.org/hn/article/view/9184/13441 [Google Scholar]
- Magalhães FM, Lyra ML, Carvalho TR, Baldo D, Brusquetti F, Burella P, Colli GR, Gehara MC, Giaretta AA, Haddad CFB, Langone JA, López JA, Napoli MF, Santana DJ, De-Sá RO, Garda AA. (2020) Taxonomic review of South American butter frogs: phylogeny, biogeographic patterns, and species delimitation in the Leptodactyluslatrans species group (Anura: Leptodactylidae). Herpetological Monograph 34(1): 131–177. 10.1655/0733-1347-31.4.131 [DOI] [Google Scholar]
- Magalhães FM, Camurugi F, Lyra ML, Baldo D, Gehara MC, Haddad CFB, Garda AA. (2022) Ecological divergence and synchronous Pleistocene diversification in the widespread South American butter frog complex. Molecular Phylogenetics and Evolution 169(2): 107398. 10.1016/j.ympev.2022.107398 [DOI] [PubMed] [Google Scholar]
- Magrini L, Carvalho-e-Silva SP, Béda AF, Giaretta AA. (2011) Calls of five species of the Scinaxruber (Anura: Hylidae) clade from Brazil with comments on their taxonomy. Zootaxa 3066(1): 37–51. 10.11646/zootaxa.3066.1.3 [DOI] [Google Scholar]
- Magurran AE. (1988) Ecological Diversity and its Measurement. Springer, Dordrecht, 179 pp. 10.1007/978-94-015-7358-0 [DOI] [Google Scholar]
- Martins MR, Haddad CFB. (1988) Vocalizations and reproductive behaviour in the smith frog, Hylafaber Wied (Amphibia: Hylidae). Amphibia-Reptilia 9(1): 49–60. 10.1163/156853888X00206 [DOI] [Google Scholar]
- Menezes L, Canedo C, Batalha-Filho H, Garda AA, Gehara MC, Napoli MF. (2016) Multilocus phylogeography of the treefrog Scinaxeurydice (Anura, Hylidae) reveals a Plio-Pleistocene diversification in the Atlantic Forest. PLoS ONE 11(6): e0154626. 10.1371/journal.pone.0154626 [DOI] [PMC free article] [PubMed]
- Mikan JC. (1822) Delectus Florae et Faunae Brasiliensis. Fasicule 2. Antonii Strauss, Vindobonae, 80 pp. [Google Scholar]
- Miles L, Newton AC, DeFries RS, Ravilious C, May I, Blyth S, Kapos V, Gordon JE. (2006) A global overview of the conservation status of tropical dry forests. Journal of Biogeography 33(3): 491–505. 10.1111/j.1365-2699.2005.01424.x [DOI] [Google Scholar]
- Mira-Mendes CB, Ruas DS, Oliveira RM, Castro IM, Dias IR, Baumgarten JE, Juncá FA, Solé M. (2018) Amphibians of the Reserva Ecológica Michelin: A high diversity site in the lowland Atlantic Forest of southern Bahia, Brazil. ZooKeys 753: 1–21. 10.3897/zookeys.753.21438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Ribeiro A. (1926) Notas para servirem ao estudo dos Gymnobatrachios (Anura) brasileiros. Arquivos do Museu Nacional. Museu Nacional 27: 1–227. [Google Scholar]
- Miranda-Ribeiro A. (1937b) Espécies novas do gênero “Stombus” da série de apêndices oculares reduzidos. O Campo 8(88): 24. [Google Scholar]
- Miranda-Ribeiro A. (1937a) Sobre uma collecção de vertebrados do nordeste brasileiro. Primeira parte: Peixes e batrachios. O Campo 1937: 54–56. [Google Scholar]
- Mori SA, Silva LAM. (1980) O Herbário do Centro de Pesquisas do Cacau em Itabuna, Brasil. Comissão Executiva do Plano da Lavoura Cacaueira. Boletín Técnico 78: 1–31. 10.2307/2806174 [DOI] [Google Scholar]
- Moura MRD, Santana DJ, Ferreira PL, Feio RN. (2009) Amphibia, Anura, Leptodactylidae, Leptodactylusviridis Jim and Spirandeli-Cruz, 1979: Distribution extension, new state record, and geographic distribution map. Check List 5(4): 780–782. 10.15560/5.4.780 [DOI] [Google Scholar]
- Myers N, Mittermeyer RA, Fonseca GAB, Kent J. (2000) Biodiversity hotspots for conservation priorities. Nature 403(6772): 853–858. 10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- Narvaes P, Rodrigues MT. (2009) Taxonomic revision of Rhinellagranulosa species group (Amphibia, Anura, Bufonidae), with a description of a new species. São Paulo. Arquivos de Zoologia 40(1): 1–73. 10.11606/issn.2176-7793.v40i1p1-73 [DOI] [Google Scholar]
- Nascimento LB, Caramaschi U, Cruz CAG. (2005) Taxonomic review of the species group of the genus Physalameus Fitzinger, 1826 with revalidation of the genera Engystomops Jimenez-de-la-Espada, 1872 and Eupemphix Steindachner, 1836 (Amphibia, Anura, Leptodactylidae). Arquivos do Museu Nacional. Museu Nacional 63(2): 297–320. [Google Scholar]
- Novaes-e-Fagundes G, Zina J. (2016) Advertisement call of Scinaxcamposseabrai (Bokermann, 1968) (Anura: Hylidae), with comments on the call of three species of the Scinaxruber clade. Zootaxa 4084(2): 258–266. 10.11646/zootaxa.4084.2.5 [DOI] [PubMed] [Google Scholar]
- Novaes-e-Fagundes G, Araujo-Vieira K, Entiauspe Neto OM, Roberto IJ, Orrico VGD, Solé M, Haddad CFB, Loebmann D. (2021) A new species of Scinax Wagler (Hylidae: Scinaxini) from the tropical forests of northeastern Brazil. Zootaxa 4903(1): 001–041. 10.11646/zootaxa.4903.1.1 [DOI] [PubMed] [Google Scholar]
- Nunes I, Santiago RS, Juncá FA. (2007) Advertisment calls of four hylid frogs from the state of Bahia, northeastern Brazil (Amphibia, Anura, Hylidae). South American Journal of Herpetology 2(2): 89–96. 10.2994/1808-9798(2007)2[89:ACOFHF]2.0.CO;2 [DOI]
- Oliveira RM, Ruas DS, Mira-Mendes CV, Solé M. (2014) Advertisement call of Rhinellacrucifer (Wied-Neuwied, 1821) (Anura: Bufonidae) from southern Bahia, Brazil. Zootaxa 3784(1): 97–98. 10.11646/zootaxa.3784.1.9 [DOI] [PubMed] [Google Scholar]
- Orrico VGD, Carvalho-e-Silva AMPT, Carvalho-e-Silva SP. (2006) Redescription of the advertisement call of Aplastodiscusarildae (Cruz & Peixoto) and description of the call of Aplastodiscusweygoldti (Cruz & Peixoto) with general notes about the genus in Southeastern Brazil (Anura, Hylidae). Revista Brasileira de Zoologia 23(4): 994–1001. 10.1590/S0101-81752006000400003 [DOI] [Google Scholar]
- Orrico VGD, Nunes I, Mattedi C, Fouquet A, Lemos AW, Rivera-Correa M, Lyra ML, Loebmann D, Pimenta BVS, Caramaschi U, Rodrigues MT, Haddad CFB. (2017) Integrative taxonomy supports the existence of two distinct species within Hypsiboascrepitans (Anura: Hylidae). Salamandra (Frankfurt) 53(1): 99–113. [Google Scholar]
- Orrico VGD, Grant T, Faivovich J, Rivera-Correa M, Rada M, Lyra ML, Cassini CS, Valdujo PH, Schargel WE, Machado DJ, Wheeler WC, Barrio-Amorós CL, Loebmann D, Moravec J, Zina J, Solé M, Sturaro MJ, Peloso PLV, Suárez P, Haddad CFB. (2021) The phylogeny of Dendropsophini (Anura: Hylidae: Hylinae). Cladistics 37(1): 73–105. 10.1111/cla.12429 [DOI] [PubMed] [Google Scholar]
- Pedreira AJ. (1999) Evolução sedimentar e tectônica da bacia metassedimentar do Rio Pardo: Uma síntese. Revista Brasileira de Geociencias 29(3): 339–344. 10.25249/0375-7536.199929339344 [DOI] [Google Scholar]
- Pedrosa IMMC, Costa TB, Faria RG, França FGR, Laranjeiras DO, Oliveira TCSP, Palmeira CNS, Torquato S, Mott T, Vieira GHC, Garda AA. (2014) Herpetofauna of protected areas in the Caatinga III: The Catimbau National Park, Pernambuco, Brazil. Biota Neotropica 14(4): 1–12. 10.1590/1676-06032014004614 [DOI] [Google Scholar]
- Peixoto OL, Arzabe C. (2004) Scinaxpachycrus. The IUCN Red List of Threatened Species. 10.2305/IUCN.UK.2004.RLTS.T55984A11391526.en [accessed on 01 March 2016] [DOI]
- Pimenta BVS, Faivovich J, Pombal Jr JP. (2007) On the identity of Hylastrigilata Spix, 1824 (Anura: Hylidae): redescription and neotype designation for a “ghost” taxon. Zootaxa 1441(1): 35–49. 10.11646/zootaxa.1441.1.3 [DOI] [Google Scholar]
- Pirani RM, Tonini JFR, Thomaz AT, Napoli MF, Encarnação LC, Knowles LL, Werneck FP. (2022) Deep genomic divergence and phenotypic admixture of the treefrog Dendropsophuselegans (Hylidae: Amphibia) coincide with riverine boundaries at the Brazilian Atlantic Forest. Frontiers in Ecology and Evolution 10(765977): 1–15. 10.3389/fevo.2022.765977 [DOI] [Google Scholar]
- Protázio AS, Santos-Protázio A, Silva CRS, Ribeiro ES, Nogueira EMS, Moura GJB. (2010) Anurofauna do Município de Paulo Afonso-BA, Bioma Caatinga, Nordeste do Brasil. Revista Nordestina de Zoologia 4(2): 31–38. [Google Scholar]
- Protázio AS, Protázio AS, Silva LS, Conceição LC, Braga HSN, Santos UG, Ribeiro AC, Almeida AC, Gama V, Vieira MVSA, Silva TAF. (2021) Amphibians and reptiles of the Atlantic Forest in Recôncavo Baiano, east Brazil: Cruz das Almas municipality. ZooKeys 1060: 125–153. 10.3897/zookeys.1060.62982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt JT, Lütken CF. (1862) Bidrag til Kundskab om Brasiliens Padder og Krybdyr. Förste Afdeling: Padderne og Öglerne. Videnskabelige Meddelelser fra Dansk Naturhistorisk Forening i Kjøbenhavn, Serie 2 3: 143–242.
- Ribeiro MC, Metzger JP, Martensen AC, Ponzoni FJ, Hirota MM. (2009) The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biological Conservation 142(6): 1141–1153. 10.1016/j.biocon.2009.02.021 [DOI] [Google Scholar]
- Rodrigues MT. (2003) Herpetofauna da Caatinga. In: Leal IR, Tabarelli M, Silva JMC. (Eds) Ecologia e conservação da Caatinga.Editora Universitaria da UFPE, Recife, 181–236.
- Rojas-Padilla O, Menezes VQ, Dias IR, Argôlo AJS, Solé M, Orrico VGD. (2020) Amphibians and reptiles of Parque Nacional da Serra das Lontras: An importante center of endemism within the Atlantic Forest in southern Bahia, Brazil. ZooKeys 1002: 159–185. 10.3897/zookeys.1002.53988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossa-Feres DC, Garey MV, Caramaschi U, Napoli MF, Nomura F, Bispo AA, Brasileiro CA, Thomé MTC, Sawaya RJ, Conte CE, Cruz CAG, Nascimento LB, Gasparini JL, Almeida AP, Haddad CFB. (2017) Anfíbios da Mata Atlântica: Lista de espécies, histórico dos estudos, biologia e conservação. In: Monteiro-Filho ELA, Conte CE. (Eds) Revisões em Zoologia: Mata Atlântica.Editora UFPR, Curitiba, 237–314.
- Santana DJ, Mesquita DO, Garda AA. (2011) Advertisement call of Dendropsophusoliveirai (Anura, Hylidae). Zootaxa 2997(1): 67–68. 10.11646/zootaxa.2997.1.5 [DOI] [Google Scholar]
- Santana DJ, Mângia S, Silveira-Filho RR, Barros LCS, Andrade I, Napoli MF, Juncá FA, Garda AA. (2015) Anurans from the Middle Jaguaribe River Region, Ceará State, Northeastern Brazil. Biota Neotropica 15(3): 1–8. 10.1590/1676-06032015001715 [DOI] [Google Scholar]
- Santos SPL, Santos EM. (2011) Anurofauna da Reserva Particular do Patrimônio Natural Frei Caneca, Município de Jaqueira, Estado de Pernambuco, Brasil. In: Moura GJB, Santos EM, Oliveira MAB, Cabral MCC (Org) Herpetofauna de Pernambuco. Brasília, Ministério do Meio Ambiente: 187–198.
- Santos L, Roseno RS, Solé M, Dias IR. (2023) Another new species (and it’s not over yet) of Phyllodytes Wagler, 1930 (Anura, Hylidae) from the Atlantic Forest of southern Bahia, northeastern Brazil. Zootaxa 5374(4): 519–532. 10.11646/zootaxa.5374.4.4 [DOI] [PubMed] [Google Scholar]
- São Pedro VA, Medeiros PH, Garda AA. (2011) The advertisement call of Rhinellagranulosa (Anura, Bufonidae). Zootaxa 3092(1): 60–62. 10.11646/zootaxa.3092.1.4 [DOI] [Google Scholar]
- Schneider JG. (1799) Historia Amphibiorum Naturalis et Literarariae. Fasciculus Primus. Continens Ranas, Calamitas, Bufones, Salamandras et Hydros in Genera et Species Descriptos Notisque suis Distinctos. Friederici Frommann, Jenae, 264 pp. 10.5962/bhl.title.78757 [DOI] [Google Scholar]
- Sichieri GRF, Cruz CAG, Pimenta BVS, Nunes I. (2021) Advertisement call description of two Proceratophrys species (Anura: Odontophrynidae). Zootaxa 4975(2): 397–400. 10.11646/zootaxa.4975.2.10 [DOI] [PubMed] [Google Scholar]
- Silva JF, Fariñas MR, Felfili JM, Klink CA. (2006) Spatial heterogeneity, land use and conservation in the cerrado region of Brazil. Journal of Biogeography 33(3): 536–548. 10.1111/j.1365-2699.2005.01422.x [DOI] [Google Scholar]
- Silva CRS, Moura GJB, Andrade EVE, Nogueira EMS. (2011) Aspectos Ecológicos da Anurofauna dos municípios de Jatobá-PE e Paulo Afonso-BA, Nordeste do Brasil. In: Moura GJB, Santos EM, Oliveira MAB, Cabral MCC (Org) Herpetofauna de Pernambuco. Brasília, Ministério do Meio Ambiente 1(1): 177–185. [Google Scholar]
- Silva GR, Luna-Dias C, Hepp FSFS, Carvalho-e-Silva AMPT, Carvalho-e-Silva SP. (2012) New record of Aplastodiscusweygoldti (Cruz & Peixoto, 1987) in the municipality of Mimoso do Sul, Espírito Santo State, southeastern Brazil (Anura, Hylidae). Herpetology Notes 5: 371–373. [Google Scholar]
- Silva-Soares T, Ferreira RB, Ornellas IS, Zocca C, Caramaschi U, Cruz CAG. (2021) A new species of Ischnocnema (Anura: Brachycephalidae) from the mountainous region of Atlantic Forest, southeastern Brazil, with a new phylogeny and diagnose for Ischnocnemaparva series. Zootaxa 5082(3): 201–222. 10.11646/zootaxa.5082.3.1 [DOI] [PubMed] [Google Scholar]
- Silvano DL, Pimenta BVS. (2003) Diversidade de anfíbios na Mata Atlântica do Sul da Bahia. In: Prado PI, Landau EC, Moura RT, Pinto LPS, Fonseca GAB, Alger K. (Eds) Corredor de Biodiversidade na Mata Atlântica do Sul da Bahia CD-ROM.IESB/CI/CABS/ UFMG/UNICAMP/ Ilhéus, Bahia, Brazil, 1–22.
- Silvano DL, Segalla MV. (2005) Conservação de anfíbios no Brasil. Megadiversidade 1(1): 79–86. [Google Scholar]
- Sos Mata Atlântica [Fundação Sos Mata Atlântica], INPE [Instituto Nacional de Pesquisas Espaciais] (2018) Atlas dos remanescentes florestais da Mata Atlântica: período 2016–2017. Relatório Técnico, ArcPlan, São Paulo, 63 pp. [Google Scholar]
- Spix JBv. (1824) Animalia nova sive Species novae Testudinum et Ranarum quas in itinere per Brasiliam annis MDCCCXVII–MDCCCXX jussu et auspiciis Maximiliani Josephi I. Bavariae Regis. München, F. S. Hübschmann, ii, 53, 39 plates (numbered 1–17 and 1–22) pp.
- Steffen GA. (1815) De Ranis nonnullis Observationes Anatomicae quas Consensu Gratiosae Facultatis Medicae. Joannis Friderici Starckii, Berlin, 24 pp. [Google Scholar]
- Stevaux MN. (2002) A new species of Bufo Laurenti (Anura, Bufonidae) from northeastern Brazil. Revista Brasileira de Zoologia 19(suppl 1): 235–242. 10.1590/S0101-81752002000500018 [DOI]
- Tabarelli M, Silva JMC. (2003) Áreas e ações prioritárias para a conservação da Caatinga. In: Leal I, Tabarelli M, Silva JMC. (Eds) Ecologia e Conservação da Caatinga.Editora Universitaria da UFPE, Recife, 777–796.
- Toledo LF, Castanho LM, Haddad CFB. (2005) Recognition and distribution of Leptodactylusmystaceus (Anura; Leptodactylidae) in the State of São Paulo, Southeastern Brazil. Biota Neotropica 5(1): 57–62. 10.1590/S1676-06032005000100006 [DOI] [Google Scholar]
- Toti DS, Coyle FA, Miller JA. (2000) A structured inventory of Appalachian grass bald and heath bald spider assemblages and a test of species richness estimator performance. The Journal of Arachnology 28(3): 329–345. 10.1636/0161-8202(2000)028[0329:ASIOAG]2.0.CO;2 [DOI]
- Trevisan CC, Batalha-Filho H, Garda AA, Menezes L, Dias IR, Solé M, Canedo C, Juncá FA, Napoli MF. (2020) Cryptic diversity and ancient diversification in the northern Atlantic Forest Pristimantis (Amphibia, Anura, Craugastoridae). Molecular Phylogenetics and Evolution 148(4): 106811. 10.1016/j.ympev.2020.106811 [DOI] [PubMed] [Google Scholar]
- Valdujo PH, Recoder RS, Vasconcellos MM, Portella AS. (2009) Amphibia, Anura, São Desidério, western Bahia uplands, northeastern Brazil. Check List 5(4): 903–911. 10.15560/5.4.903 [DOI] [Google Scholar]
- Valdujo PH, Camacho A, Recoder RS, Teixeira M Junior, Ghellere JMB, Mott T, Nunes PMS, Nogueira CC, Rodrigues MT. (2011) Anfíbios da Estação Ecológica Serra Geral do Tocantins, região do Jalapão, Estados do Tocantins e Bahia. Biota Neotropica 11(1): 251–261. 10.1590/S1676-06032011000100025 [DOI] [Google Scholar]
- Vaz-Silva W, Maciel NM, Nomura F, Morais AR, Guerra Batista V, Santos DL, Andrade SP, Oliveira AAB, Brandão RA, Bastos RP. (2020) Guia de identificação das espécies de anfíbios (Anura e Gymnophiona) do estado de Goiás e do Distrito Federal, Brasil Central. Sociedade Brasileira de Zoologia, Curitiba, 229 pp. 10.7476/9786587590011 [DOI] [Google Scholar]
- Vieira WLS, Arzabe C, Santana GG. (2007) Composição e distribuição espaço-temporal de anuros no Cariri Paraibano, Nordeste do Brasil. Oecologia Brasiliensis 11(3): 383–396. 10.4257/oeco.2007.1103.08 [DOI] [Google Scholar]
- Vilaça TRA, Silva JES, Solé M. (2006) Vocalization and territorial behaviour of Phyllomedusanordestina Caramaschi, 2006 (Anura: Hylidae) from southern Bahia, Brazil. Journal of Natural History 45(29-30): 1823–1834. 10.1080/00222933.2011.561018 [DOI] [Google Scholar]
- von Tschudi JJ. (1838) Classification der Batrachier mit Berücksichtigung der fossilen Thiere dieser Abtheilung der Reptilien. Petitpierre, Neuchâtel, 99 pp. 10.5962/bhl.title.4883 [DOI] [Google Scholar]
- Vörös J, Dias JR, Solé M. (2017) A new species of Phyllodytes (Anura: Hylidae) from the Atlantic Rainforest of Southern Bahia, Brazil. Zootaxa 4337(4): 584–594. 10.11646/zootaxa.4337.4.9 [DOI] [PubMed] [Google Scholar]
- Wells KD. (2007) The ecology and behavior of amphibians. The University of Chicago Press Books, Chicago, 1400 pp. [Google Scholar]
- Wied-Neuwied MAP. (1821) Reise nach Brasilien in den Jahren 1815 bis 1817. Vol. 2. Henrich Ludwig Brönner, Frankfurt, 345 pp. [Google Scholar]
- Wied-Neuwied MAP. (1824) Abbildungen zur Naturgeschichte Brasiliens. Heft 8. Landes-Industrie-Comptoir, Weimar, 614 pp. [Google Scholar]
- Willians PH. (1996) Mapping variations in strength and breadth of biogeographic transition zones using species turnover. Proceedings. Biological Sciences 263(1370): 579–588. 10.1098/rspb.1996.0087 [DOI] [Google Scholar]
- Young BE, Stuart SN, Chanson JS, Cox NA, Boucher TM. (2004) Joyas que están desapareciendo: El estado de los anfibios en el Nuevo Mundo. NatureServe, Arlington, Virginia, USA, 53 pp. [Google Scholar]
- Zappi DC, Filardi FLR, Leitman P, Souza VC, Walter BMT, Pirani JR, Morim MP, Queiroz LP, Cavalcanti TB, Mansano VF, Forzza RC. (2015) Growing knowledge: An overview of Seed Plant diversity in Brazil. Rodriguésia 66(4): 1085–1113. 10.1590/2175-7860201566411 [DOI] [Google Scholar]
- Zucchetti VM, Castroviejo-Fisher S. (2024) Lost in time: unraveling the identity of Vitreoranaparvula (Boulenger, 1895) (Anura: Centrolenidae). Zootaxa 5415(3): 351–391. 10.11646/zootaxa.5415.3.1 [DOI] [PubMed] [Google Scholar]
- Zucchetti VM, Rojas-Padilla O, Dias IR, Solé M, Orrico VGD, Castroviejo-Fisher S. (2023) An elusive giant: A new species of Vitreorana Guayasamin et al., 2009 (Anura: Centrolenidae) from the northern Atlantic Forest with an osteological description and comments on integumentary spicules. Zootaxa 5249(3): 301–334. 10.11646/zootaxa.5249.3.1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the data that support the findings of this study are available in the main text.