Abstract
A hypertensive response to exercise is a precursor leading to hypertension, which is a major risk factor for the development of heart failure and diastolic dysfunction. Herein, we aimed to assess blood pressure (BP) in patients with a hypertensive response to exercise and different degrees of diastolic dysfunction. Between January 2009 and December 2014, 373 patients with a hypertensive response to exercise (HRE) and echocardiographic data assessing diastolic function were enrolled at the University Hospital of Zurich. ANCOVA was used to assess the changes in BP response during exercise testing in individuals with different degrees of diastolic dysfunction. Normalization of systolic BP was blunted in patients with grade II and III diastolic dysfunction after 3 min of recovery in univariable [β (95%) ‐ 9.2 (‐13.8 to ‐ 4.8) p < .001, ‐16.0 (‐23.0 to 9.0) p < .001, respectively] and adjusted models. In fully adjusted models, when taking maximal effort into account, there were no differences with regard to systolic BP during exercise. Patients without diastolic dysfunction achieved higher heart rates (HRs) [both in absolute terms (p < .001) and as a percentage of the calculated maximum (p = .003)] and greater wattage (p < .001) at maximum exertion. The findings of this cross‐sectional study suggest that exercise capacity is compromised in patients with diastolic dysfunction. A hypertensive response to exercise and the finding of a blunted BP recovery may help identify patients at risk of developing heart failure.
Keywords: diastolic dysfunction, exercise testing, heart failure, hypertensive response to exercise
1. INTRODUCTION
Heart failure (HF) is a syndrome with a plethora of potential causes and is associated with high morbidity and mortality, including hospitalization and high health care cost. Hypertension is the most common cardiovascular disease and the leading risk factor for the onset of HF. 1 Patients with HF and preserved ejection fraction (HFpEF) display an impaired ability of the myocardium to relax 1 and account for at least half of all HF patients. 2 , 3 Additional risk factors for HFpEF include age, female sex, obesity, metabolic syndrome, renal dysfunction, and atrial fibrillation. 3 Due to increasing life expectancy and the growing prevalence of obesity and hypertension, the incidence of HFpEF is rising. 3 , 4 The exact pathogenesis of HFpEF remains unclear, but diastolic dysfunction with impaired left ventricular (LV) relaxation, increased LV chamber stiffness, and increased LV filling pressures play crucial roles. 5 Diagnosis is made by symptoms, clinical signs, elevated natriuretic peptide levels, and echocardiographic assessments. 6
A hypertensive response to exercise (HRE) is a precursor for the development of hypertension, 7 , 8 , 9 , 10 , 11 , 12 the main risk factor for the development of HFpEF. Currently, there is no standardized definition of an HRE. It is usually defined by blood pressure (BP) levels > 210/105 mmHg for men and > 190/105 mmHg for women. 13
Data on the relationship between HRE and the development of HFpEF are scarce. However, we recently showed that increased left atrial volume index (LAVI), elevated E/e’ (as surrogate parameters of increased LV end‐diastolic filling pressure and of diastolic dysfunction), and higher LV mass index are potential independent predictors of HRE in patients without HFpEF. 14 Individuals with an HRE are at an increased risk of developing hypertension, a major risk factor for HFpEF. 7 , 8 , 9 , 10 , 11 , 12 Additionally, an HRE is linked to LV diastolic dysfunction and subsequently to HFpEF. 15 , 16 This implies a continuum of disease from an HRE to overt hypertension and, in the long term, HFpEF. However, the exact behavior of BP during an HRE in patients with different degrees of diastolic dysfunction is unknown. Therefore, in this study, we assessed BP during an exercise test in patients with an HRE and different grades of diastolic dsyfunction focusing not only on the exercise phase, but importantly also on the recovery phase.
2. METHODS
2.1. Study population
The present study was conducted at the Department of Cardiology at the University Hospital of Zurich. We retrospectively analyzed the exercise tests of all patients who underwent any outpatient cardiologic examination from January 2009 to December 2014. Patients with an HRE, defined as a peak systolic BP ≥ 210 mmHg for men and ≥ 190 mmHg for women or a diastolic BP ≥ 105 mmHg for both sexes during exercise, were included. 13 A total of 1004 patients were identified with an HRE. We then extracted further data from electronic medical records and compiled the database with information that had already been collected in the course of the consultation, including sex, age, body height and weight. In addition, body mass index (BMI) was calculated and body surface area was derived by the Mosteller Formula. Further data included characteristics of exercise‐induced hypertension (systolic vs. diastolic), presence of hypertension at rest, presence of cardiovascular or endocrinological disorders, medication taken, resting electrocardiogram (ECG), and ECG changes during exercise. We meticulously reviewed medical histories and diagnostic data to identify and exclude secondary hypertension cases, such as those resulting from endocrine disorders, renal diseases, or medication side effects. The laboratory parameters included creatinine, thyroid‐stimulating hormone, leukocyte and hemoglobin levels, and NT‐proBNP. Data from transthoracic echocardiographic measurements were extracted if the patient was examined within 6 months prior to or after the diagnosis of an HRE. A total of 544 patients were excluded due to incomplete or nonexistent medical data or echocardiographic measurements. Diastolic function was evaluated in 462 patients. After applying the current algorithm for the diagnosis of diastolic dysfunction, 5 a total of 373 patients were included and divided into the following subgroups: “normal diastolic function,” “diastolic dysfunction grade I,” and “diastolic dysfunction grade II and III.” Eighty‐seven patients were excluded because diastolic function could not conclusively be determined. Those patients with an indeterminate classification and without enough parameters to evaluate the diastolic function were excluded (Figure 1). Each study participant provided informed and written consent to the protocol. The study was conducted according to the Declaration of Helsinki and approved by the local ethics committee (Ethics committee of the Canton of Zurich, trial number 2017‐01402).
FIGURE 1.
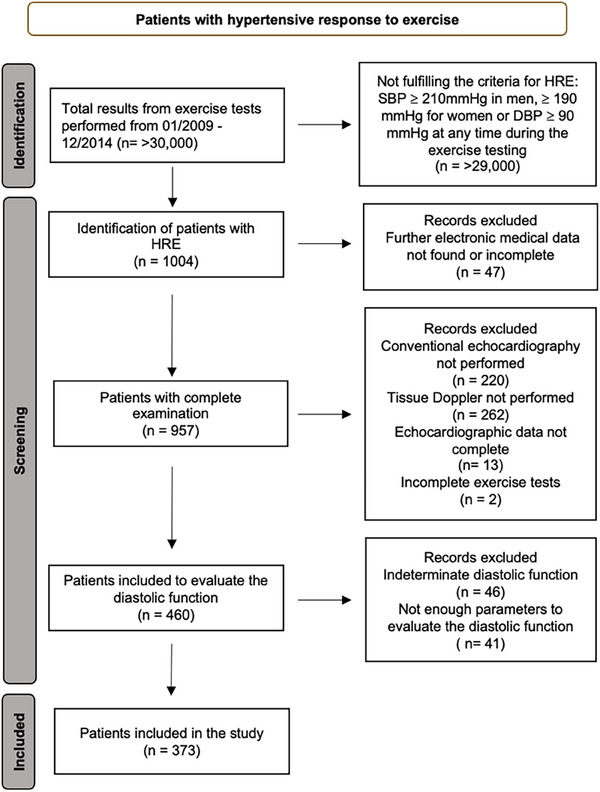
Flow diagram of the study. DBP, diastolic blood pressure; HRE, hypertensive response to exercise; SBP, systolic blood pressure.
2.2. Exercise testing
A maximal exercise stress test was performed on a bicycle ergometer (Ergosana Erg911 plus, Ergoline 900/911) using a ramp protocol with individual adaptation by the attending physician, according to fitness, sex, age, and body height. Patients were encouraged to reach the determined target heart rate (HR) based on their age. ECG and HR were continuously monitored, while both systolic and diastolic BP were non‐invasively assessed using an automated sphygmomanometer at rest and every minute during exercise and throughout the recovery period. For resting BP, we used the BP‐200, and for exercise testing, the BP‐200 Plus (Schiller, 6341 Baar, Switzerland). Both devices are validated for these measurements. The exercise test was terminated in the event of peripheral or general exhaustion, dyspnea, pectanginal discomfort, or other medical reasons. Using standardized estimation of the anticipated wattage, we adopted the ramp protocol starting with 20 W according to the anticipated maximal wattage on the cycle ergometer in order to ensure maximal exhaustion within 8−12 min. The recovery phase lasted 5 min. with an afterload of 20 W for the first minute and no load for the following 4 min.
2.3. Transthoracic echocardiography
Transthoracic echocardiography was performed according to the guidelines of the American Society of Echocardiography and the European Association of Cardiovascular Imaging. 5 Left ventricular M‐mode measurements of wall thickness and end‐diastolic and end‐systolic diameters were used for calculating the fractional area change, relative wall thickness, and left ventricular mass. The left atrial volume was calculated from biplane recordings and divided by the body surface area. Flow velocity over the mitral valve was measured by pulsed‐wave Doppler and the diastolic movements and velocities of the mitral annulus were measured at the medial and lateral corners of the annulus with tissue Doppler imaging and from the apical four‐chamber view using standard methods. The algorithm published in the current guidelines using the following parameters and cutoff values was used to assess diastolic dysfunction: annular e’ velocity: septal e’ < 7 cm/s, lateral e’ < 10 cm/s, average E/e’ ratio > 14, LA volume index (LAVI) > 34 mL/m2, and peak tricuspid regurgitation (TR) velocity > 2.8 m/s. 5
2.4. Statistical analysis
Baseline characteristics were divided into three groups: “normal diastolic function,” “diastolic dysfunction grade I,” and “diastolic dysfunction grade II and III.” Continuous variables are presented as medians and interquartile range (IQRs) or means and standard deviation (SD) and were compared using analysis of variance (ANOVA) or the rank‐based Kruskal–Wallis test as appropriate. Categorical variables are shown as counts and percentages (%) and were compared using the χ2 test.
The ANCOVA was used to assess the BP response during the exercise test between the three groups after correcting for baseline BP during the exercise phase and maximal BP during the recovery phase. The analyses were adjusted for a preselected set of covariates, including sex, age, BMI, coronary artery disease (CAD), any valvular heart disease (VHD), diabetes mellitus type 1 and 2 (DM), obstructive sleep apnea (OSAS), and antihypertensive medication. In Model 1, BP during exercise was corrected for BP at rest, and BP at recovery was corrected for BP at maximum exercise. Model 2 included variables from Model 1 as well as sex (categorical) and age (continuous). Model 3 was additionally corrected for BMI (continuous), CAD (categorial), DM (categorial), HF (categorial), OSAS (categorial), and VHD (categorial). Model 4 was additionally corrected for cardiac medication (categorial), including ACE inhibitors or ARBs, calcium channel blockers diuretics, and beta blockers, as they were not discontinued. The models were applied once for systolic and once for diastolic BP values. Statistical significance was established at two‐tailed p < .05 throughout. All analyses were conducted using IBM SPSS Statistics version 29 (SPSS Inc., Chicago, Illinois, USA).
3. RESULTS
3.1. Baseline characteristics
Patient characteristics are presented in Table 1. A total of 373 patients were divided into the following subgroups: normal diastolic function (n = 176), diastolic dysfunction grade I (n = 154), and diastolic dysfunction grades II and III (n = 43). Patients with diastolic dysfunction grades I and II/III were significantly older (64 and 66 years, respectively, p < .001) and more likely to have previously diagnosed hypertension (75% and 72% respectively, p < .001) and DM (25% and 23% respectively, p < .001) than patients without diastolic dysfunction (51 years, 45% and 10%, respectively). NT‐pro‐BNP levels significantly increased across groups and were highest in patients with high‐grade diastolic dysfunction (421 pg/mL, p < .001) and lowest in patients without diastolic dysfunction (68 pg/mL). Furthermore, patients with diastolic dysfunction grades II and III were significantly more often treated with diuretics (51%, p < .001), ACE inhibitors or ARBs (67%, p < .001), beta blockers (70%, p < .001), and with calcium channel blockers (42%, p = .001).
TABLE 1.
.Baseline characteristics according to diastolic function.
| No diastolic dysfunction No. = 176 (47%) | Diastolic dysfunction Grade 1 No. = 154 (41%) | Diastolic dysfunction Grade II+III No. = 43 (12%) | p | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Male sex | 100 (56) | 118 (77) | 23 (53) | <.001 |
| Age (years) | 51 ± 14 | 64 ± 12 | 66 ± 16 | <.001 |
| Height (cm) | 170 ± 11 | 172 ± 9 | 168 ± 12 | .171 |
| Weight (kg) | 76 ± 21 | 79 ± 17 | 76 ± 15 | .125 |
| BMI (kg/m2) | 26.0 ± 6.6 | 27.0 ± 5.5 | 26.8 ± 4.6 | .176 |
| Medical history | ||||
| Arterial hypertension | 80 (45) | 115 (75) | 31 (72) | <.001 |
| Coronary artery disease | 0 (0) | 117 (76) | 20 (47) | <.001 |
| Heart failure | 0 (0) | 42 (27) | 7 (16) | <.001 |
| Valvular heart disease | 34 (19) | 30 (19) | 19 (44) | <.001 |
| Psychiatric disease | 23 (13) | 16 (10) | 4 (9) | .654 |
| Type 1&2 diabetes | 17 (10) | 39 (25) | 10 (23) | <.001 |
| Endocrinologic disease | 14 (8) | 11 (5) | 6 (14) | .352 |
| OSAS | 5 (3) | 8 (5) | 1 (2) | .469 |
| Positive family history for HTN | 38 (22) | 20 (13) | 7 (16) | .118 |
| NT‐proBNP (pg/mL) | 68 ± 346 | 152 ± 890 | 421 ± 2756 | <.001 |
| Medication history | ||||
| Beta blockers | 42 (24) | 95 (62) | 30 (70) | <.001 |
| ACE inhibitors /ARBs | 48 (27) | 106 (69) | 29 (67) | <.001 |
| Calcium channel blocker | 29 (16) | 41 (27) | 18 (42) | .001 |
| Diuretics | 28 (16) | 58 (38) | 22 (51) | <.001 |
| Antiplatelet agents | 32 (18) | 123 (80) | 18 (42) | <.001 |
| Oral anticoagulants | 11 (6) | 19 (12) | 14 (33) | <.001 |
| Statins | 27 (15) | 111 (72) | 22 (51) | <.001 |
| Other cardiac medication | 13 (7) | 21 (14) | 8 (19) | .054 |
Data are shown as mean and standard deviation. Categorical data are shown as numbers and percentages. p values are based on Kruskal‐Wallis test or Chi‐square test as appropriate.
Abbreviations: ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; HTN, arterial hypertension; OSAS, obstructive sleep apnea syndrome.
3.2. Transthoracic echocardiographic parameters
Table S1 shows a comparison of transthoracic echocardiographic and exercise test parameters between the groups. According to the definition, patients with diastolic dysfunction grade II had the greatest values for E/e’ (15.7), LAVI (73 mL), TR velocity (2.9 m/s), and LVMMI (108 g/m2, p < .001). The indexed end diastolic volume was similar between the groups and although the LV ejection fraction was significantly lower in patients with diastolic dysfunction [59% in both grade I and II/III (± 8.74, ± 8.40) vs. 61% (± 4.4) in no diastolic dysfunction, p = .003].
3.3. Exercise test parameters
Table 2 shows the mean BP levels and (HR) at different time intervals as well as performance (in wattage) among the three groups. Graphical representations of these data are shown in (Figure 2A, B). The level of performance, measured in wattage as a percentage of the calculated maximum watt, decreased significantly with an increasing degree of diastolic dysfunction [no diastolic dysfunction 94%, (± 25) diastolic dysfunction grad I 83% (± 27), and diastolic dysfunction grades II and III 80% (± 26) p < .001]. We observed a longer exercise duration in patients without diastolic dysfunction (7.5 min) than in those with diastolic dysfunction grade I (6.7 min) or grades II and III (6.4 min, p < .001).
TABLE 2.
Exercise test parameters according to diastolic function.
| No diastolic dysfunction No. = 176 (47%) | Diastolic dysfunction Grade I No. = 176 (41%) | Diastolic dysfunction Grade II+III No. = 43 (12%) | p | |
|---|---|---|---|---|
| Systolic blood pressure (mmHg) | ||||
| Sys BP at rest | 131 (± 17) | 133 (± 19) | 137 (± 23) | .09 |
| Sys BP at 1 min exercise | 139 (± 20) | 139 (± 22) | 146 (± 27) | .38 |
| Sys BP at 3 min exercise | 158 (± 25) | 161 (± 25) | 163 (± 32) | .84 |
| Sys BP at peak exercise | 204 (± 24) | 201 (± 26) | 193 (± 27) | .12 |
| Sys BP at 1 min recovery | 186 (± 28) | 189 (± 31) | 178 (± 25) | .80 |
| Sys BP at 3 min recovery | 164 (± 25) | 171 (± 26) | 171 (± 28) | .02 |
| Diastolic blood pressure (mmHg) | ||||
| Dia BP at rest | 82 (± 12) | 80 (± 12) | 80 (± 13) | .31 |
| Dia BP at 1 min exercise | 80 (± 12) | 80 (± 15) | 82 (± 15) | .39 |
| Dia BP at 3 min exercise | 84 (± 15) | 84 (± 14) | 82 (± 14) | .14 |
| Dia BP at peak exercise | 100 (± 20) | 100 (± 20) | 100 (± 20) | .20 |
| Dia BP at 1 min recovery | 83 (± 16) | 86 (± 15) | 81 (± 15) | .02 |
| Dia BP at 3 min recovery | 78 (± 11) | 80 (± 14) | 78 (± 14) | .48 |
| Heart rate (bpm) | ||||
| HR rest | 77 (± 14) | 75 (± 12) | 68 (± 11) | .001 |
| HR at 1 min exercise | 93 (± 14) | 88 (± 15) | 83 (± 12) | <.001 |
| HR at 3 min exercise | 107 (± 16) | 102 (± 20) | 99 (± 16) | <.001 |
| Peak HR | 147 (± 38) | 134 (± 61) | 119 (± 47) | <.001 |
| Peak HR predicted of max. (%) | 95 (± 13) | 93 (± 20) | 88 (± 17) | .003 |
| HR at 1 min recovery | 127 (± 22) | 113 (± 20) | 103 (± 19) | <.001 |
| HR at 3 min recovery | 106 (± 18) | 96 (± 20) | 88 (± 20) | <.001 |
| Duration (min) | ||||
| Exercise time | 7.5 (± 2) | 6.7 (± 2) | 6.4 (± 2) | <.001 |
| Recovery time | 5.5 (± 2) | 5.2 (± 2) | 5.2 (± 1) | .90 |
| Performance | ||||
| Max. Watt predicted of max. (%) | 94 (± 25) | 83 (± 27) | 80 (± 26) | <.001 |
Data are shown as mean and standard deviation. The numbers have been rounded. p values are based on Kruskal‐Wallis test.
Abbreviations: BP, blood pressure; bpm, beats per minute; Dia, Diastolic; HR, heart rate; Max, maximal; min, minutes; Sys, Systolic; W, Watt.
FIGURE 2.
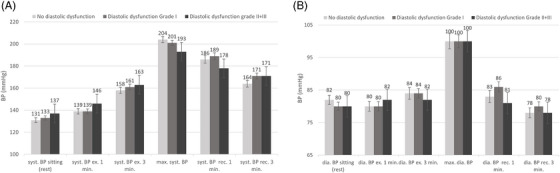
Systolic (A) and diastolic (B) BP during an exercise test according to diastolic dysfunction. Data represent mean with whsikers representing 95% CI. BP, blood pressure; dia, diastolic; ex, exercise; min, minute; rec, recovery; syst, sytolic.
3.4. Relationships of systolic blood pressure during exercise in patients with and without diastolic dysfunction
After adjusting for maximal effort there were no differences regarding systolic BP during exercise (Table 3). After 3 min of recovery, patients with normal diastolic function (Group 0) had a lower systolic BP when compared to patients with diastolic dysfunction grade I (Group 1) or grades II and III (Group 2) [−9.2 (−13.8 to −4.8), p < .001 and −16 (−23.0 to −9.0), p < .001, respectively]. The associations between groups 0 and 2 remained even after adjusting for multiple covariables in various models, as shown in Table 3. When comparing groups 1 and 2, there were marginal differences, although they did not reach statistical significance.
TABLE 3.
Regression analysis for the association of systolic blood pressure at different points during an exercise stress test according to diastolic function.
| Beta (95% CI), p value | ||||||
|---|---|---|---|---|---|---|
| Group | at 1 min exercise | at 3 min exercise | at peak exercise | at 1 min recovery | at 3 min recovery | |
| Model 1 | 0 vs. 1 | 0.9 (−2.7 to 4.5) p = .62 | 0.3 (−4.3 to 4.9) p = .90 | 6.1 (0.8–11.4) p = .02 | −6.5 (−11.3 to −1.7) p = .01 | −9.2 (−13.8 to −4.8) p < .001 |
| 0 vs. 2 | −2.1 (−7.9 to 3.7) p = .48 | 4.1 (−3.2 to 11.3) p = .28 | 15.6 (7.1–24.1) p < .001 | −3.6 (−10.9 – 3.7) p = 0.34 | −16.0 (−23.0 to 9.0) p < .001 | |
| 1 vs. 2 | −3.0 (−8.8 to 2.8) p = .31 | 3.8 (−3.6 to 11.1) p = .32 | 9.5 (1–18) p = .03 | 2.9 (−4.5 to 10.3) p = .44 | −6.7 (−13.8 to 0.3) p = .06 | |
| Model 2 | 0 vs. 1 | 1.7 (−2.3 to 5.7) p = .40 | −0.9 (−5.9 to 4.1) p = .73 | 8.5 (2.6–14.4) p = .01 | −7.6 (−12.8 to −2.3) p = .01 | −9.0 (−14.0 to −4) p < .001 |
| 0 vs. 2 | −0.6 (−6.7 to 5.5) p = .84 | 5.9 (−1.6 to 13.5) p = .12 | 16.3 (7.4–25.3) p < .001 | −3.5 (−11.2 to 4.1) p = .36 | −15.8 (−23.2 to −8.4) p < .001 | |
| 1 vs. 2 | −2.3 (−8.2 to 3.6) p = .434 | 6.8 (−0.5 to 14.1) p = .07 | 7.8 (−0.8 to 16.4) p = .07 | 4.0 (−3.4 to 11.5) p = .29 | −6.8 (−13.9 to 0.3) p = .06 | |
| Model 3 | 0 vs. 1 | −1.0 (−7.1 to 5.1) p = .74 | −4.3 (−12 to 3.4) p = .28 | 3.0 (−6.1 to 12.2) p = .52 | −12.1 (−20.1 to −4.0) p = .003 | −7.4 (−15.2 to 0.4) p = .06 |
| 0 vs. 2 | −3.1 (−9.9 to 3.71) p = .37 | 3.2 (−5.3 to 11.7) p = .46 | 11.8 (1.7–21.9) p = .02 | −7.1 (−15.6 to 1.3) p = .10 | −14.8 (−23.0 to −6.6) p < .001 | |
| 1 vs. 2 | −2.1 (−8.3 to 4.1) p = .50 | 7.5 (−0.1 to 15.2) p = .05 | 8.8 (−0.2 to 17.8) p = .05 | 5.0 (−3.0 to 13.0) p = .22 | −7.4 (−15.0 to 0.3) p = .06 | |
| Model 4 | 0 vs. 1 | −1.1 (−7.3 to 5.2) p = .74 | −3.8 (−11.7 to 4.0) p = .34 | 1.9 (−7.4 to 11.1) p = .69 | −11.3 (−19.5 to −3.1) p = .007 | −8.1 (−16.1 to −0.04) p = .05 |
| 0 vs. 2 | −2.8 (−9.9 to 4.2) p = .43 | 4.0 (−4.8 to 2.8) p = .37 | 10.3 (0.02–20.6) p = .05 | −6.4 (−15.2 to 2.3) p = .15 | −15.5 (−24.0 to −7.0) p < .001 | |
| 1 vs. 2 | −1.8 (−8.1 to 4.5) p = .58 | 7.9 (0.1–15.7) p = .048 | 8.4 (−0.6 to 17.5) p = .07 | −4.9 (−3.2 to 12.9) p = .24 | −7.8 (−16.4 to 0.8) p = .07 | |
| Model 5 | 0 vs. 1 | −0.8 (−7.0 to 5.5) p = .81 | −2.3 (−10.1 to 5.5) p = .56 | 0.5 (−8.6 to 9.6) p = .91 | ||
| 0 vs. 2 | −2.5 (−9.5 to 4.6) p = .49 | 5.7 (−3.0 to 14.4) p = .20 | 8.8 (−1.2 to 18.9) p = .09 | |||
| 1 vs. 2 | −1.7 (−8.0 to 4.6) p = .59 | 8.06 (0.3 – 15.8) p = .04 | 8.3 (−0.5 to 17.2) p = .07 | |||
ANCOVA for the association of systolic blood pressure at different points during exercise testing in patients with and without diastolic dysfunction after correcting for different variables as described in models 1‐4. The different groups were compared with each other.
Group 0: Patients with normal diastolic function;.
Group 1: Patients with diastolic dysfunction grade I.
Group 2: Patients with diastolic dysfunction grade II + III.
Model 1 is corrected for SBP at rest (continuous) during exercise and for peak BP (continuous) during recovery.
Model 2 includes all variables from Model 1 and is additionally corrected for sex (categorical) and age (continuous).
Model 3 includes all variables from Model 2 and is additionally corrected for BMI (continuous), CAD, HF, VHD, DM, OSAS (categorial).
Model 4 includes all variables from Model 3 and is additionally corrected for associated cardiac medication including, diuretics, betablockers, ACE‐Inhibitor or ARB, CCB (categorical).
Model 5 includes all variables from Model 4 and is additionally corrected for percentage of maximal workload (continuous).
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; CCB, calcium channel blocker; DM, diabetes mellitus; HF, heart failure; OSAS, obstructive sleep apnea syndrome; SBP, systolic blood pressure; VHD, valvular heart disease.
3.5. Relationships of diastolic blood pressure during exercise in patients with and without diastolic dysfunction
At 1 min. of recovery, the BP of group 1 was significantly greater than that of group 2 [β (95% CI) 6.7 (2.0–11.4) p = .005]. The relationship remained significant after adjusting for multiple covariables in various models, as shown in Table 4. No further significant differences were observed.
TABLE 4.
Regression analysis for the association of diastolic blood pressure at different points during an exercise stress test according to diastolic function.
| Beta (95% CI), p value | ||||||
|---|---|---|---|---|---|---|
| Group | at 1 min exercise | at 3 min exercise | at peak exercise | at 1 min recovery | at 3 min recovery | |
| Model 1 | 0 vs. 1 | −0.8 (−3.0 to 1.4) p = .50 | −0.8 (−3.6 to 2.0) p = .56 | −0.5 (−4.5 to 3.5) p = .81 | −3.2 (−6.2 to −0.3) p = .03 | −2.9 (−5.7 to −0.2) p = .04 |
| 0 vs. 2 | −3.4 (−7.0 to 0.1) p = .06 | −0.003 (−4.5 to 4.5) p = .99 | −0.7 (−7.1 to 5.6) p = .82 | 3.5 (−1.1 to −8.1) p = .135 | −1.1 (−5.5 to 3.2) p = .04 | |
| 1 vs. 2 | −2.7 (−6.2 to 0.9) p = .14 | 0.8 (−3.7 to 5.3) p = .72 | −0.3 (−6.7 to 6.2) p = .94 | 6.7 (2.0–11.4) p = .005 | 1.8 (−2.6 to 6.2) p = .42 | |
| Model 2 | 0 vs. 1 | −0.8 (−3.2 to 1.7) p = .55 | −2.4 (−5.5 to 0.7) p = .134 | −1.4 (−5.8 to −3.1) p = .55 | −4.0 (−7.3 to −0.7) p = .02 | −2.9 (−6.0 to 0.2) p = .06 |
| 0 vs. 2 | −2.8 (−6.6 to 0.9) p = .14 | −0.8 (−5.5 to 3.9) p = 0.73 | −2.2 (−9.0 to 4.6) p = .52 | 3.1 (−1.8 to 7.9) p = .21 | −1.4 (−6.0 to 3.2) p = .56 | |
| 1 vs. 2 | −2.1 (−5.7 to −1.5) p = .26 | 1.6 (−3.0 to 6.1) p = .50 | −0.9 (−7.4 to 5.7) p = .79 | 7.0 (2.3–11.7) p = .004 | 1.6 (2.9–6.0) p = .49 | |
| Model 3 | 0 vs. 1 | 0.5 (−3.3 to 4.3) p = .78 | −5.3 (−10.2 to −0.5) p = .03 | −3.6 (−10.7 to 3.4) p = .31 | −4.9 (−10.0 to 0.2) p = .06 | 0.6 (−4.1 to 5.4) p = .79 |
| 0 vs. 2 | −2.0 (−6.2 to 2.3) p = .36 | −2.5 (−7.8 to 2.9) p = .36 | −2.8 (−10.6 to 4.9) p = .47 | 1.9 (−3.4 to 7.2) p = .48 | 0.074 (−4.9 to 5.0) p = .98 | |
| 1 vs. 2 | −2.5 (−6.4 to 1.3) p = .20 | 2.9 (−1.9 to 7.7) p = .24 | 0.8 (6.1–7.7) p = .83 | −6.8 (−11.8 to −1.8) p = .01 | −0.6 (−5.2 to 4.1) p = .814 | |
| Model 4 | 0 vs. 1 | 0.5 (−3.4 to 4.4) p = .80 | −4.7 (−9.6 to 0.3) p = .07 | −3.6 (−10.9 to 3.7) p = .33 | −3.9 (−9.1 to 1.2) p = .13 | 0.6 (−4.3 to 5.4) p = .81 |
| 0 vs. 2 | −2.0 (−6.5 to 2.4) p = .37 | −1.3 (−6.9 to 4.2) p = .64 | −3.1 (−11.2 to 5.0) p = .45 | 3.4 (−2.1 to 8.8) p = .23 | 0.3 (−4.8 to 5.5) p = .91 | |
| 1 vs. 2 | −2.5 (−6.5 to 1.4) p = .20 | 3.3 (−1.6 to 8.2) p = .18 | 0.5 (−6.6 to 7.6) p = .88 | 7.3 (2.2–12.4) p = .01 | −0.3 (−5.0 to 4.5) p = .91 | |
| Model 5 | 0 vs. 1 | 1.0 (−2.9 to 5.0) p = .60 | −8.3 (−10.2 to 1.4) p = .95 | −3.2 (−10.5 to 4.0) p = .38 | ||
| 0 vs. 2 | −1.3 (−5.8 to 3.1) p = .55 | −0.2 (−5.2 to 5.6) p = .95 | −2.6 (−10.7 to 5.5) p = .53 | |||
| 1 vs. 2 | −2.4 (−3.1 to 5.8) p = .55 | 3.6 (−1.1 to 8.4) p = .13 | 0.6 (−6.5 to 7.7) p = .86 | |||
ANCOVA for the association of diastolic blood pressure at different points during exercise testing in patients with and without diastolic dysfunction after correcting for different variables as described in models 1‐4. The different groups were compared with each other.
Group 0: Patients with normal diastolic function.
Group 1: Patients with diastolic dysfunction grade I.
Group 2: Patients with diastolic dysfunction grade II + III.
Model 1 is corrected for DBP at rest (continuous) during exercise and for peak BP (continuous) during recovery.
Model 2 includes all variables from Model 1 and is additionally corrected for sex (categorical) and age (continuous).
Model 3 includes all variables from Model 2 and is additionally corrected for BMI (continuous), CAD, HF, VHD, DM, OSAS (categorial).
Model 4 includes all variables from Model 3 and is additionally corrected for associated cardiac medication including, diuretics, betablockers, ACE‐Inhibitor or ARB, CCB (categorical).
Model 5 includes all variables from Model 4 and is additionally corrected for percentage of maximal workload (continuous).
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; CCB, calcium channel blocker; DM, diabetes mellitus; HF, heart failure; OSAS, obstructive sleep apnea syndrome; SBP, systolic blood pressure; VHD, valvular heart disease.
4. DISCUSSION
In this cross‐sectional study, we showed that an increasing degree of diastolic dysfunction was associated with a blunted recovery response. There were no differences regarding systolic BP during exercise. Furthermore, the exercise capacity, measured in wattage as a percentage of the maximum, decreased with an increasing degree of diastolic dysfunction, indicating a lower level of performance and exercise intolerance. Exercise tolerance can be viewed as a measure of cardiovascular endurance. The most reliable and used indicator is the subjective assessment of exhaustion during an exercise test based on symptoms. This is reflected in the duration of exercise and the maximum wattage achieved. We found that patients with more severe diastolic dysfunction terminated the exercise test earlier and achieved lower wattage than predicted when compared to individuals with diastolic dysfunction grade I and without diastolic dysfunction. The behavior of BP and HR during the recovery period can serve as an additional indicator of exercise tolerance, as demonstrated in our study population.
The exact underlying mechanisms remain unclear. Studies have shown a certain degree of endothelial dysfunction among patients with diastolic dysfunction, such as an impaired flow‐mediated and nitrate‐mediated dilatation in patients with HFpEF. 17 Furthermore, evidence of limitations in chronotropic, contractile, endothelial, and vascular reserves has been observed among patients with HFpEF, resulting in an impaired response to physical exertion and consequently reduced exercise capacity. 18 As suggested by previous studies, the cause of this phenomenon could be a systemic inflammatory state, triggered by comorbidities such as obesity, DM, OSAS, and arterial hypertension. This inflammatory state leads to oxidative stress and microvascular endothelial dysfunction through the remodeling of cardiomyocytes and increased collagen deposition, resulting in a decreased myocardial relaxation, as observed in diastolic dysfunction. 17 , 19 Systemic inflammation is an important factor in the pathophysiology of left ventricular diastolic dysfunction in patients with HFpEF. 17 In our study, patients with diastolic dysfunction were more likely to have DM, arterial hypertension, and additional cardiovascular comorbidities, causative for an inflammatory state.
4.1. Hypertensive response to exercise and heart rate behavior
To increase cardiac output, the SBP increases linearly in response to an augmented load. Consequently, the metabolic needs of the working muscles can be met. Moreover, DBP remains stable or shows a minimal decrease. 20 However, some individuals without previously diagnosed arterial hypertension or cardiovascular disease respond with an exaggerated increase in SBP during exercise. Endothelial dysfunction and arterial stiffness are likely the main underlying mechanisms. 21 , 22 A previous study showed that patients with HFpEF have greater SBP during exercise than individuals without HF. 23 Additionally, exercise intolerance is prevalent in patients with HFpEF and HRE. 15 , 24 An exaggerated increase, also shown in our study population, might be an indicator of impaired vascular relaxation, leading to further cardiovascular complications such as hypertension or HF. 7 , 11 , 25 Multiple studies have documented a greater incidence of cardiovascular events and mortality among individuals with HRE. 26 , 27 , 28
In the healthy population, the HR decreases rapidly after the termination of exercise. HR recovery serves as a reliable indicator of overall fitness. An association between diastolic dysfunction and abnormal HR recovery, indicating exercise intolerance, has been previously shown. 29 Contrary to these results, our study revealed that patients with normal diastolic function had a greater HR during recovery than did those with diastolic dysfunction. However, it must be taken into account that the patients in our study with normal diastolic function may still have had an underlying impaired autonomic function, as evidenced by their exaggerated BP response to exercise. Furthermore, use of beta blockers was less frequent.
4.2. HFpEF and HRE
Until now, only few studies have investigated the effect of HRE on HFpEF, and none have demonstrated a direct link between individuals presenting with HRE and the subsequent development of HFpEF. There exists compelling data on the connection of the continuum of disease. HRE appears to be an independent risk factor for arterial hypertension, while arterial hypertension, in turn, has emerged as one of the primary risk factors for the development of HF with preserved ejection fraction. 14 If a direct association could be shown in further prospective studies, HRE could potentially serve as an early detectable marker to help identify patients at risk of developing HF.
4.3. Limitations
The study included a large, well characterized population of 373 patients who underwent an exercise test with rigorous data collection during the exercise and recovery phase. However, the retrospective single‐center design is a possible limitation. The participants in this study were all patients with an HRE and were not compared to a healthy population. Exercise testing was performed on bicycle ergometry and based on recommendations from 2009. The precision of BP measurements is limited to the 1‐min. measurement interval, ramp protocol, and examination duration. Invasively measured BP would provide more accurate results but is less practicable for an exercise testing. The device we used for measurement, the BP‐200 Plus Schiller, is specifically designed for exercise testing, ensuring a balance between accuracy and practicality. The standardized ramp protocol may not suitably accommodate certain health conditions or disabilities, reducing generalizability to different groups. While this study assessed exercise capacity by measuring wattage as a percentage of the maximum, a likely more meaningful parameter for indicating cardiovascular fitness would be maximal oxygen consumption (VO2 max). As a cross‐sectional study, it is unsuitable for identifying relationships, and consequently, it does not allow for the differentiation between cause and effect.
5. CONCLUSIONS
This study revealed that the SBP remained higher after 3 min. recovery in patients with greater diastolic dysfunction, indicating blunted BP normalization. These findings imply a compromised exercise tolerance in patients with diastolic dysfunction. To assess the exact underlying pathophysiological mechanism and to determine the correlation between an HRE and HFpEF, further follow‐up studies are needed. Monitoring BP responses during exercise, focusing on the recovery time, could serve as an important and accessible diagnostic tool for HF, as well as for assessing the overall cardiovascular health of individuals.
AUTHOR CONTRIBUTIONS
Laura Würzburger made data extraction and interpretation of the data, analysis of the results, drafted and wrote the initial manuscript, and revised the manuscript. Jan Gerrit van der Stouwe contributed to the conceptualization, analysis of the results, and to the writing of the manuscript. Patrick Wiech, Georg Moser, Gloria Petrasch, Victor Schweiger, Philipp Bohm. Valentina A. Rossi, Christian Templin, Stefano Caselli, and Christian M. Schmied revised the manuscript for important intellectual content and approved the final version to be published. David Niederseer contributed to the conceptualization, data analysis, and critically revised the manuscript for important intellectual content, and approved the version to be published. All authors approved the final manuscript as submitted.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to disclose regarding the content of the present manuscript.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
This study was not funded by any entity.
Würzburger L, van der Stouwe JG, Ghidoni C, et al. Blood pressure behavior during exercise in patients with diastolic dysfunction and a hypertensive response to exercise. J Clin Hypertens. 2024;26:1209–1218. 10.1111/jch.14884
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author (DN).
REFERENCES
- 1. Gladden JD, Chaanine AH, Redfield MM. Heart failure with preserved ejection fraction. Annu Rev Med. 2018;69:65‐79. [DOI] [PubMed] [Google Scholar]
- 2. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129‐2200. [DOI] [PubMed] [Google Scholar]
- 3. Andersen MJ, Borlaug BA. Heart failure with preserved ejection fraction: current understandings and challenges. Curr Cardiol Rep. 2014;16(7):501. [DOI] [PubMed] [Google Scholar]
- 4. Lam CS, Donal E, Kraigher‐Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13(1):18‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277‐314. [DOI] [PubMed] [Google Scholar]
- 6. Nagueh SF. Heart failure with preserved ejection fraction: insights into diagnosis and pathophysiology. Cardiovasc Res. 2021;117(4):999‐1014. [DOI] [PubMed] [Google Scholar]
- 7. Farah R, Shurtz‐Swirski R, Nicola M. High blood pressure response to stress ergometry could predict future hypertension. Eur J Intern Med. 2009;20(4):366‐368. [DOI] [PubMed] [Google Scholar]
- 8. Yzaguirre I, Grazioli G, Domenech M, et al. Exaggerated blood pressure response to exercise and late‐onset hypertension in young adults. Blood Press Monit. 2017;22(6):339‐344. [DOI] [PubMed] [Google Scholar]
- 9. Sharabi Y, Ben‐Cnaan R, Hanin A, Martonovitch G, Grossman E. The significance of hypertensive response to exercise as a predictor of hypertension and cardiovascular disease. J Hum Hypertens. 2001;15(5):353‐356. [DOI] [PubMed] [Google Scholar]
- 10. Ito K, Iwane M, Miyai N, et al. Exaggerated exercise blood pressure response in middle‐aged men as a predictor of future blood pressure: a 10‐year follow‐up. Clin Exp Hypertens. 2016;38(8):696‐700. [DOI] [PubMed] [Google Scholar]
- 11. Miyai N, Arita M, Morioka I, Miyashita K, Nishio I, Takeda S. Exercise BP response in subjects with high‐normal BP: exaggerated blood pressure response to exercise and risk of future hypertension in subjects with high‐normal blood pressure. J Am Coll Cardiol. 2000;36(5):1626‐1631. [DOI] [PubMed] [Google Scholar]
- 12. Berger A, Grossman E, Katz M, et al. Exercise blood pressure and the risk for future hypertension among normotensive middle‐aged adults. J Am Heart Assoc. 2015;4(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghidoni C, Kruzik M, Rossi VA, Caselli S, Schmied CM. Niederseer D. Definitions for hypertensive response to exercise. Cardiol Rev. 2022;32(3):273‐278. [DOI] [PubMed] [Google Scholar]
- 14. Wiech P, Würzburger L, Rossi VA, Caselli S, Schmied CM, Niederseer D. Hypertensive response to exercise, hypertension and heart failure with preserved ejection fraction (HFpEF)‐a continuum of disease? Wien Klin Wochenschr. 2023;135(24):685‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takamura T, Onishi K, Sugimoto T, et al. Patients with a hypertensive response to exercise have impaired left ventricular diastolic function. Hypertens Res. 2008;31(2):257‐263. [DOI] [PubMed] [Google Scholar]
- 16. Schultz MG, Otahal P, Cleland VJ, Blizzard L, Marwick TH, Sharman JE. Exercise‐induced hypertension, cardiovascular events, and mortality in patients undergoing exercise stress testing: a systematic review and meta‐analysis. Am J Hypertens. 2013;26(3):357‐366. [DOI] [PubMed] [Google Scholar]
- 17. Ambrosino P, Papa A, Buonauro A, et al. Clinical assessment of endothelial function in heart failure with preserved ejection fraction: a meta‐analysis with meta‐regressions. Eur J Clin Invest. 2021;51(8):e13552. [DOI] [PubMed] [Google Scholar]
- 18. Borlaug BA, Olson TP, Lam CS, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56(11):845‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263‐271. [DOI] [PubMed] [Google Scholar]
- 20. Schultz MG, La Gerche A, Sharman JE. Blood pressure response to exercise and cardiovascular disease. Curr Hypertens Rep. 2017;19(11):89. [DOI] [PubMed] [Google Scholar]
- 21. Würzburger L, Wiech P, Rossi VA, et al. Hypertensive response to exercise in athletes: unremarkable finding or relevant marker for future cardiovascular complications? Int J Hypertens. 2022;2022:8476751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim D, Ha JW. Hypertensive response to exercise: mechanisms and clinical implication. Clin Hypertens. 2016;22:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kato S, Onishi K, Yamanaka T, et al. Exaggerated hypertensive response to exercise in patients with diastolic heart failure. Hypertens Res. 2008;31(4):679‐684. [DOI] [PubMed] [Google Scholar]
- 24. Little WC, Kitzman DW, Cheng CP. Diastolic dysfunction as a cause of exercise intolerance. Heart Fail Rev. 2000;5(4):301‐306. [DOI] [PubMed] [Google Scholar]
- 25. SY Jae, Franklin BA, Choo J, Choi YH, Fernhall B. Exaggerated exercise blood pressure response during Treadmill testing as a predictor of future hypertension in men: a longitudinal study. Am J Hypertens. 2015;28(11):1362‐1367. [DOI] [PubMed] [Google Scholar]
- 26. Lewis GD, Gona P, Larson MG, et al. Exercise blood pressure and the risk of incident cardiovascular disease (from the Framingham Heart Study). Am J Cardiol. 2008;101(11):1614‐1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kurl S, Laukkanen JA, Rauramaa R, Lakka TA, Sivenius J, Salonen JT. Systolic blood pressure response to exercise stress test and risk of stroke. Stroke. 2001;32(9):2036‐2041. [DOI] [PubMed] [Google Scholar]
- 28. Laukkanen JA, Kurl S, Rauramaa R, Lakka TA, Venalainen JM, Salonen JT. Systolic blood pressure response to exercise testing is related to the risk of acute myocardial infarction in middle‐aged men. Eur J Cardiovasc Prev Rehabil. 2006;13(3):421‐428. [DOI] [PubMed] [Google Scholar]
- 29. Gharacholou SM, Scott CG, Borlaug BA, et al. Relationship between diastolic function and heart rate recovery after symptom‐limited exercise. J Card Fail. 2012;18(1):34‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author (DN).


