Abstract
Lung cancer is one of the leading causes of death worldwide. It can broadly be divided into small cell lung cancer (SCLC) and nonsmall cell lung cancer. There have been many advances over the recent years in both fields. The purpose of this review is to provide a concise summary of SCLC for the general respiratory readership.
Shareable abstract
A comprehensive review of small cell lung cancer and neuroendocrine tumours https://bit.ly/3XteFb8
Introduction
Lung cancer is the second most common cancer worldwide, being the most common in men and the second most common in women. 2.2 million new cases of lung cancer were diagnosed in 2020 alone and resulted in ∼1.8 million deaths worldwide [1, 2]. Smoking remains the main risk factor for the development of lung cancer, with duration of smoking being the strongest determinant for risk [1, 3].
Lung cancer is broadly divided into small cell lung cancer (SCLC) and nonsmall cell lung cancer (NSCLC). SCLC falls in the category of lung neuroendocrine neoplasms (NENs). SCLC is typically detected and diagnosed in the more advanced, non-curative stages [4, 5]. The incidence of SCLC has decreased over the past decade but it remains the most aggressive type of lung cancer, and despite initial significant response to chemoradiotherapy, the 5-year overall survival remains poor [4–6]. The data around other types of NENs are scarcer and will be discussed.
Epidemiology of SCLC and NEN tumors
Every year, there are at least 200 000 fatalities and an estimated 250 000 new cases of SCLC worldwide [7, 8]. SCLC accounts for ∼15% of all lung cancers and is predominantly linked to smoking as a risk factor, with a high incidence among heavy smokers [8]. SCLC is equally prevalent in males and females and age plays a crucial role as its incidence rises with advancing age [9, 10]. Despite advancements in therapeutic approaches, the prognosis remains challenging due to frequent late-stage diagnosis and the unfavourable prognosis of this specific histotype compared to NSCLC [9, 10]. This is discussed in greater detail in the section on “Morphological features”. Other types of NEN are typical and atypical carcinoids, large cell neuroendocrine carcinomas (LCNEC), and carcinomas with neuroendocrine differentiation [11–13]. Atypical carcinoids demonstrate a higher malignant behaviour when compared to typical carcinoids and sometimes denote genomic similarities with LCNEC [14, 15]. LCNEC is a rare and highly aggressive tumour histotype with a poor prognosis [16, 17].
Table 1 shows the incidence (age-adjusted annual incidence rate per 100 000 people) of the various NEN subtypes [17].
TABLE 1.
Incidence (age-adjusted annual incidence rate per 100 000 people) of the various neuroendocrine neoplasm subtypes [17]
| Type | Incidence |
|---|---|
| Typical carcinoid | 0.77 |
| Atypical carcinoid | 0.22 |
| SCNEC | 8.60 |
| LCNEC | 0.41 |
SCNEC: small cell neuroendocrine carcinoma; LCNEC: large cell neuroendocrine carcinoma.
Presenting features specific to SCLC/NEN tumours: paraneoplastic syndromes
For both SCLC and NSCLC, patients are usually diagnosed with the disease when they exhibit symptoms typical of an advanced stage of lung cancer, unless picked up at lung cancer screening or as incidental findings [10]. SCLC commonly exhibits a rapid volume doubling time and so metastasizes early with a propensity for extensive dissemination [11, 18]. Patients frequently manifest symptoms related to advanced disease, such as cough, dyspnoea, chest pain, and systemic symptoms of fatigue, weight loss and anorexia [11]. However, a feature of SCLC/NEN tumours is that they can present with paraneoplastic syndromes due to ectopic hormone production from the neuroendocrine cells [11, 18–20].
Syndrome of inappropriate antidiuretic hormone (SIADH) is the most common paraneoplastic syndrome associated with SCLC and is characterised by hyponatraemia, renal water retention, low plasma osmolality relative to the urine, and high urine sodium levels. 10–45% of patients with SCLC develop SIADH at some point in the course of the disease [18]. Ectopic Cushing syndrome is the second most common paraneoplastic syndrome, occurring in up to 5% of cases, characterised by truncal obesity, moon facies, acne and proximal muscle weakness amongst other signs and symptoms [18].
Neurological paraneoplastic syndromes are thought to occur in up to 9% of SCLC [18]. The presence of anti-Hu antibodies leads to a syndrome comprising of cerebellar degeneration, opsoclonus-myoclonus-ataxia, peripheral nerve palsy and limbic encephalitis; the presence of anti-Yo antibodies leads to cerebellar degeneration; the presence of anti-P/Q type voltage-gated calcium channel antibodies lead to Lambert–Eaton myasthenic syndrome and the presence of anti-Ri antibodies leads to opsoclonus-myoclonus-ataxia syndrome [18]. SCLC can also be associated with an increase in coagulopathies such as Trousseau syndrome, deep venous thrombosis and disseminated intravascular coagulopathy.
Table 2 shows the various paraneoplastic syndromes with the associated antibodies.
TABLE 2.
Paraneoplastic syndromes with their associated antibodies
| Paraneoplastic syndrome | System | Incidence | Causative protein or antibody | Clinical features | Diagnosis |
|---|---|---|---|---|---|
| Hypercalcaemia | Endocrine | 10% | PTHrP binds to PTH receptors in the bone, kidneys and influences Ca–phosphorous regulation | Polydipsia, polyuria, hypertonia, renal failure, vomiting, altered sensorium | Elevated Ca Elevated PTHrP Decreased PTH |
| SIADH | Endocrine | 10–45% | Ectopic ADH secretion by cancer cells which inhibits free water excretion in the distal tubule of the kidney | Nausea, vomiting, cramps, depressed mood, irritability, personality changes (aggression), seizures, stupor or coma | Hyponatraemia; rule out other causes such as drug-induced, excess fluids, low intake of sodium due to cachexia |
| Ectopic Cushing syndrome | Endocrine | 5% | Cancer cells express POMC precursor gene which is translated into a prohormone later cleaved into ACTH | In carcinoids: typical cushingoid features like centripetal fat distribution, proximal myopathy, hypertension In SCLC: less cushingoid features, hyperglycaemia, weight gain due to water retention |
High serum ACTH, hypercortisolism, i.e. increased 24 UFC or salivary cortisol; 1 mg dexamethasone suppression test |
| Carcinoid syndrome | Endocrine | 1–5% | Serotonin release by cancer cells | Acute: prolonged flushing in the upper torso, bronchospasm, diarrhoea Chronic: fibrosis of right heart valves, retroperitoneum Rare: hypotension and cardiac arrest |
24 h urine 5-HIAA Radiolabelled octreotide |
| Lambert–Eaton myasthenic syndrome | Neurological | 3% | Anti-VGCC | Proximal muscle weakness, typically improving with repetitive action | EMG, anti-VGCC antibodies |
| Paraneoplastic cerebellar degeneration | Neurological | <1% | Anti-Yo, anti-Hu, anti-VGCC, anti-Tr, anti-Ri | Rapid onset of truncal and appendicular ataxia, imbalance, dizziness, nausea, diplopia, dysphagia, nystagmus | Paraneoplastic antibody assay, MRI, rapid onset of ataxia |
| Limbic encephalitis | Neurological | <1% | Anti-Hu, anti-Ma2, anti-Ri | Subacute onset of mental status changes, memory deficits, behavioural changes, emotional lability, insomnia, seizures | Clinical symptoms, EEG, MRI, anti-Hu antibodies |
| Paraneoplastic opsoclonus- myoclonus | Neurological | <1% | Anti-Hu, anti-Ri, anti-Ma2, anti-amphiphysin | Large amplitude ocular saccades and other abnormal eye movements alone or in combination with myoclonus; hypotonia, irritability, ataxia and encephalopathy | Physical examination for abnormal eye movement, MRI, anti-Hu antibodies |
| Autonomic neuropathy | Neurological | <1% | Anti-Hu, anti-gAChR | Orthostatic hypotension, arrhythmias, impotence, intestinal pseudo-obstruction | Clinical symptoms, anti-Hu antibodies |
| Hypercoagulability | Vascular | <1% | Unknown | Trousseau syndrome, deep venous thrombosis, disseminated intravascular coagulopathy | Clinical symptoms, coagulation panel, d- dimers |
PTH: parathyroid hormone; PTHrP:- parathyroid hormone-related protein; SIADH: syndrome of inappropriate antidiuretic hormone; ADH: antidiuretic hormone; Ca: calcium; ACTH: adrenocorticotropic hormone; UFC: urine free cortisol; 5-HIAA: 5-hydroxyindoleacetic acid; EMG: electromyography; VGCC: voltage gated calcium channel; EEG: electroencephalogram; MRI: magnetic resonance imaging.
Methods for diagnosis and staging of SCLC/NEN
Accurate histological characterisation of any cancer through biopsy and imaging aids in precise diagnosis and guides tailored therapeutic approaches [12].
Diagnosing SCLC and NEN involves a multidisciplinary approach using various diagnostic modalities. Initial evaluation often includes imaging studies such as chest radiography, computed tomography (CT) scans, and positron emission tomography scans with 18F-fluorodeoxyglucose (FDG-PET) [12]; these aid in identifying the tumour and assessing its size, location and provide an estimate its metastatic spread.
Diagnostic modalities
For pathological diagnosis, histology is preferred over cytology [5, 12], and sampling remains the diagnostic gold standard, obtained via numerous methods such as bronchoscopy, transthoracic needle aspiration, or surgical resection [21, 22]. Histological examination and immunohistochemical staining confirm neuroendocrine differentiation and subtype classification [23]. Immunohistochemistry plays a crucial role in identifying neuroendocrine markers like chromogranin A (CgA), synaptophysin, and CD56 to distinguish neuroendocrine tumours (NETs) from non-neuroendocrine lung cancers. Molecular testing, including next-generation sequencing, assists in identifying actionable mutations and targeted therapies for specific subtypes, enhancing personalised treatment approaches [23–25]. This is discussed in more detail later.
Staging modalities
The staging of SCLC follows both the American Joint Committee on Cancer (AJCC) TNM (tumour, mode, metastasis) staging system and the older Veterans Administration (VA) scheme [26, 27]. The latter defines an extensive stage (ES) and a limited stage (LS), primarily based on the tumour's anatomical extent. Limited stage SCLC is confined to one hemithorax and can be encompassed within a tolerable radiation field, while extensive stage SCLC extends beyond the confines of a single hemithorax [28]. LS cases may be amenable to curative intent, whereas ES patients have a worse prognosis [29]. The AJCC TNM staging follows the same descriptors for NSCLC [30, 31].
Given the propensity for early metastasis in SCLC, staging assessments are vital. This includes brain imaging with magnetic resonance imaging (MRI) or CT scans to detect central nervous system dissemination, as SCLC presents a tropism for the central nervous system. Evaluating distant metastases may involve bone scans, abdominal imaging and occasionally bone marrow biopsy [10]. Liquid biopsy techniques, analysing circulating tumour cells, cell-free DNA or exosomes, offer minimally invasive methods for tumour detection, monitoring treatment response, and identifying resistance mechanisms [32]. However, their role in diagnosing and staging NENs currently remains investigational [32].
LCNEC cells are avid for FDG-PET due to their high expression of the glucose transporter 1 (GLUT1) and low differentiation. As a result, FDG-PET is a valid imaging assessment that can be used to select patients with early-stage disease for radical treatment. It also has a high sensitivity and specificity [16]. An octreoscan, a type of single-photon emission CT, identifies tumours by radiolabelled targeting (111In-Pentetreotide) of somatostatin receptors. These scans have demonstrated higher sensitivity than FDG-PET in patients with well differentiated NENs, while showing a lower sensitivity in poorly differentiated ones [33]. A PET/CT system with the somatostatin receptor-based PET tracer 1,4,7,10-tetraazacyclododecane-NI,NII,NIII,NIIII-tetraacetic acid (D)-Phe1-thy3-octreotide (68Ga-DOTATOC) has also been used in the evaluation of carcinoids [34].
A panel of biochemical tests is recommended in all patients with NEN and that should include CgA and in patients with potential paraneoplastic syndromes: 24-h urine 5-hydroxyindoleacetic acid, serum cortisol, adrenocorticotropic hormone, 24-h urine free cortisol and serum growth hormone-releasing hormone and insulin-like growth factor 1. Table 3 shows a very simplified summary of the diagnostics.
TABLE 3.
Summary of the diagnostics
| Diagnostic modality | Procedure | Use |
|---|---|---|
| Medical history | Examination by a physician | Identifying typical lung cancer symptoms and risk factors such as smoking history |
| Physical examination | Clinical examination by a physician | Differential diagnosis between infection and oncological disease |
| X-ray | Imaging | Differential diagnosis |
| CT scan | Imaging | Staging: size, tumour location and spread to nearby tissues and organs, identifying metastases (best method of assessing adrenal metastases ) |
| FDG-PET/CT | Imaging | Staging: tumour location, mediastinal lymph node involvement, metastatic spread |
| MRI | Imaging | Staging: brain metastases |
| Bronchoscopy | Biopsy | Cancer tissue sampling |
| Transthoracic needle aspiration | Biopsy | Cancer tissue sampling |
| Surgical resection | Biopsy | Cancer tissue sampling |
| EBUS/EUS | Biopsy | Cancer tissue sampling |
| Mediastinoscopy | Biopsy | Cancer tissue sampling |
| Histology | Pathological analysis | Biological characterisation of lung cancer |
| Immunohistochemistry | Pathological analysis | Subtype classification by expression of specific markers (e.g. expression of neuroendocrine markers synaptophysin, chromogranin A, CD56) |
| Next-generation sequencing | Genetic analysis | Identifying actionable mutations |
CT: computed tomography; FDG-PET: 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography; MRI:- magnetic resonance imaging; EBUS: endobronchial ultrasound; EUS: endoscopic ultrasound.
Morphological features: World Health Organization histological classification, expression of neuroendocrine markers, combined SCLC including LCNECs
Histology, in combination with TNM stage [30, 35], is an important tool for creating therapeutic strategies, as well as a predictor for patient outcomes. The principles of histological classification are the same for all tumours and refer to morphology first, supported by immunohistochemistry (IHC), and then molecular techniques for a more accurate description of specific tumour subtypes.
NENs of the lung are divided into two major biologically and clinically different groups with different molecular features – neuroendocrine tumours and neuroendocrine carcinomas (NECs).
Both NETs and NECs are further subdivided into two subgroups: NETs subdivide into typical (TC) and atypical (AC) carcinoid, whereas NECs are grouped into large cell (LCNEC) and small cell (SCNEC). The current subclassification also recognises two types: pure SCLC/LCNEC and combined SCLC/LCNEC, the latter is determined by the presence of features of a different histological carcinoma, NSCLC, variant or containing at least a 10% large cell carcinoma component. There are also other rare types of thoracic tumours such as mesenchymal tumours, haematolymphoid tumours and tumours of ectopic tissues.
The diagnosis of NENs may be challenging due to the heterogeneous nature of these tumours. Diagnosis is thus based on a typical morphological appearance and a positive reaction in the tumour cells for at least two neuroendocrine markers [36]. CgA and synaptophysin are considered the most sensitive and specific markers of neuroendocrine differentiation that are used in routine diagnostics [37, 38].
CgA is an acidic glycoprotein that is widely expressed by neuroendocrine cells and is one of the most abundant components of secretory granules. When a tumour develops in an endocrine tissue, it becomes the main source of CgA. Measuring the levels of circulating CgA can be useful in diagnosing different types of NENs, because of a high specificity and sensitivity, ranging from 27% to 81% [39].
Synaptophysin is a glycoprotein part of the neuroendocrine secretory granule membrane. It has higher sensitivity but lower specificity than CgA. Both CgA and synaptophysin are appropriate for diagnosis of well-differentiated NENs, but are less helpful when it comes to poorly differentiated NENs.
Other markers have been studied. Neuron-specific enolase (NSE) is used to identify neuroendocrine cells [40]. It is also found in tumours, classified as adenocarcinomas or undifferentiated carcinomas, so its specificity is limited and it is often referred to as “nonspecific enolase” by pathologists [41]. NSE is observed in the majority of NET [42], and because it is a cytosolic marker, it can be detected even in degranulated tumour cells [43]. Therefore, it is considered a useful marker.
CD56, or neural cell adhesion molecule, is also used in the diagnosis of neuroendocrine differentiation. It is a glycoprotein involved in cell binding, migration and differentiation [44]. CD56 mRNA is expressed both in neuroendocrine cells and NENs [45]. It has been shown not to be as specific to NENs in general; however, it is nonetheless useful in diagnosing SCLC [46]. Insulinoma-associated protein 1 (INSM1) is a protein that plays a huge role as a transcription factor in the differentiation of neuroendocrine cells. It is present across various brain regions, as well as in pancreas, adrenal glands, thymus, thyroid and endocrine cells of the gastrointestinal tract [47]. INSM1 has recently emerged as a reliable biomarker for neuroendocrine differentiation in lung neoplasms [48]. Multiple studies demonstrate the high specificity of INSM1 in SCLC, up to 100% [49]. Sensitivities for LCNEC have ranged from 75% [48] to 91.3% [50]. INSM1 has been found to have high sensitivity to carcinoid tumours, ranging from 98% to 100% [51]. Pulmonary NETs, including SCLC, LCNEC, atypical and typical carcinoid tumours, all express INSM1 with high specificity (97%) that is similar to CgA (98%), but greater than CD56 (87%) and synaptophysin (90%).
Biology and molecular features
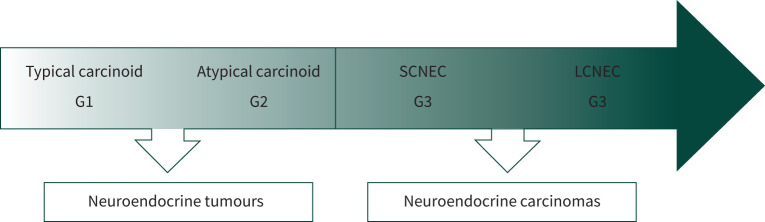
Besides morphological criteria (neuroendocrine morphology, mitotic count per mm2 and necrosis), NENs have a distinct IHC expression of typical neuroendocrine markers, such as synaptophysin and CgA. A significant difference between NETs and NECs lies in their prognosis, closely related to their degree of differentiation: NETs are either well differentiated (G1) for typical carcinoid or moderately differentiated (G2) for atypical carcinoid, whereas SCNEC and LCNEC are both always high-grade carcinomas (G3), with G3 tumours conferring a poorer prognosis (figure 1) [52, 53].
FIGURE 1.
Three-tier spectrum of grade of differentiation within neuroendocrine neoplasms. SCNEC: small cell neuroendocrine carcinoma, LCNEC: large cell neuroendocrine carcinoma.
Still, NETs are distinct and different from NECs as suggested by molecular studies with major differences in IHC and molecular features between the four subgroups. It is considered that pulmonary neuroendocrine cells are the precursor of all NECs (both SCNEC and LCNEC) [54–56]. Similarly, non-G3 NETs (typical and atypical carcinoids) most probably also have a common precursor [57, 58].
Morphological and immunohistochemical features of NEN
Morphological appearance is the first pathological feature, indicating a possible diagnosis of a lung NEN. As neuroendocrine differentiation is typical for NENs, all four subtypes consistently show expression of neuroendocrine biomarkers such as CgA and synaptophysin, as discussed above.
Ki 67 is also a biomarker, largely used in the classification of NENs of other organ origin, e.g. pancreatic, gastrointestinal [35, 59]. It could be a useful biomarker in small samples to distinguish between the two large groups of pulmonary NEN: NETs and NECs [60, 61]. Tumour samples should be submitted to IHC in search of NENs only if neuroendocrine morphology has been first detected. IHC is also helpful in the differential diagnosis to distinguish between NECs and poorly differentiated nonsmall cell carcinomas [62, 63]. They might mimic SCLC and the lack of expression of p40 [64, 65] or high molecular weight cytokeratin, such as CK7, CK8 or CK18 [63, 66], excludes a diagnosis of G3 (basaloid) squamous cell lung carcinoma [63]. The addition of CD56 may also be useful as it is a biomarker of SCLC, but not a marker for neuroendocrine differentiation [67].
Recent publications indicate a new protein – INSM1 as a biomarker [50, 68]. INSM1 is expressed in up to 100% of NETs and over 90% in NECs and is detected in <5% of non-neuroendocrine lung cancers [50]. Not all SCLC expresses neuroendocrine biomarkers and a minority (about 10–15%) lack neuroendocrine markers, identifying a subgroup of SCLC, referred to as variant subtype [69–71]. It is also thyroid transcription factor 1 (TTF1) negative [72]. Abnormal expression of p53, e.g. diffuse staining or lack of expression in all tumour cells, is typically a feature of NECs, but may also rarely be expressed in NETs [58].
IHC expression is important not only as diagnostic marker, but it may also be relevant to therapy. In all NENs, the expression of somatostatin receptors is closely related to the subtype of the lung tumour as well [73]. Expression of other biomarkers such as retinoblastoma [69], delta-like canonical notch ligand 3 [74], mammalian target of rapamycin [75], programmed death-ligand 1 [76] or thymidylate synthase [77, 78] remain emerging targets for the treatment of SCLC and will be discussed in detail below.
Molecular pathology
Lung NENs feature a heterogeneous spectrum of molecular alterations, potentially valid for personalised treatments. NETs and NECS have different molecular profiles.
Neuroendocrine tumours
Non-G3 NETs (atypical and typical carcinoids) probably have a common precursor [57, 58], and due to this they are characterised by similar molecular findings, largely differing from those of NECs. They typically have low tumour mutational burden) [79] and the number of somatic mutations increase with the mitotic count, the proliferation rate, the grade of differentiation and Ki 67 [80]. Enrichment of genes of the mitogen-activated protein kinase pathway, and changes in chromatin remodelling genes such as MEN1 (mutations or low MEN1 expression), AT-rich interaction domain 1A (ARID1A), and eukaryotic translation initiation factor 1A X-linked (EIF1AX), as well as mucin 6 (MUC6), spectrin alpha erythrocytic 1 (SPTA1) and transforming growth factor-β signalling, are one of the most frequent molecular changes in NETs [81]. Signatures with different gene alterations are associated with favourable (low mutational burden) or worse prognosis (RET upregulation, OTP and CD44 downregulation, chromosomal instability) [82–84].
Neuroendocrine carcinomas
NECs are characterised by high tumour mutational burden [80]. Molecular analysis of NECs describes alterations in the SMAD family member 4 (SMAD4 mutations), with mutations in JAK3, NRAS, RB1 and VHL1 in SCLC, and FGFR2 mutations in LCNEC only [85]. Both SCNEC and LCNEC share alterations as RB1, TP53, FGFR1 amplifications and many others (table 4). As a subtype with the highest number of molecular alterations, LCNEC is more frequently characterised by deletions in cyclin-dependent kinase inhibitor 2A (CDKN2A), TTF1 amplifications, and STK11 mutations as in non-neuroendocrine and squamous cell tumours. LCNEC may also carry MEN1 mutations [86, 87].
TABLE 4.
Mutations associated with small cell lung cancer and large cell neuroendocrine carcinoma
| Mutations | SCLC | LCNEC |
|---|---|---|
| TP53 alteration | Present | Present |
| RB1 alteration | Present | Present |
| FGFR1 amplification | Present | Present |
| STK11 mutation | Present | |
| CDKN2A deletion | Present | |
| TTF1 amplification | Present | |
| MEN1 mutation | Present |
SCLC: small cell lung cancer; LCNEC: large cell neuroendocrine carcinoma.
Treatment according to stage
Small cell lung cancer
SCLC is an aggressive cancer with poor prognosis and significant risk of disease progression. The management approach thus requires a proactive multidisciplinary team effort to expedite staging and treatment to improve patient outcomes and survival.
The recommended treatment for SCLC varies according to the disease stage. Most studies used the VA scheme (as mentioned earlier) to categorise patients into ES (stage III–IV) and LS (stage I–III) based on the anatomical extent within the thorax, and performance status of the patient.
Management of LS-SCLC
Both the European Society for Medical Oncology and National Comprehensive Cancer Network clinical practice guidelines recommend a multimodality approach in selected LS disease patients with a curative intent [5, 10]. TNM staging further plays a role for selecting patients with early-stage disease who may be candidates for surgery.
Early-stage SCLC
The role of surgery in LS disease is evolving but remains controversial, owing to the lack of contemporary randomised control data and existing low-quality evidence. Prior to offering surgical treatment to selected patients, pathological mediastinal staging is essential. In selected LS-SCLC patients who present in an early stage (T1–T2N0(−1)M0), surgical resection, preferably lobectomy with mediastinal lymph node dissection or systematic sampling, should be offered [10]. While systematic mediastinal nodal dissection has been long established as the standard of care for nonsmall cell lung carcinoma, the role of systematic nodal dissection in SCLC is still a matter of debate. A recent retrospective study looked at survival and tumour recurrence in patients with cT1–2N0M0 SCLC who underwent curative intent surgery. The group (n=112) that underwent systematic lymph node dissection had better overall survival (OS) (66.4% versus 48.4%) and recurrence-free survival (63.5% versus 37.6%) and lower local recurrence rates (11.6% versus 42.9%) compared with those who underwent sampling (n=35) [88]. These results are promising, however, further prospective large multicentre randomised trials are needed to validate definitive OS and recurrence-free survival benefit, as systematic lymph node dissection does increase operative time and morbidity compared with sampling.
Adjuvant chemotherapy is always indicated and mediastinal radiotherapy is recommended in patients with incomplete resection (R1–2) or in whom locally advanced disease is found (N2/3). A retrospective study of surgical resection in early LS disease, pathological stages I and II, showed improved 5-year survival rates of 64% and 65%, respectively, in the two patient groups. In an analysis of the National Cancer Database of 507 patients with stage I and II SCLC, lobectomy and adjuvant chemotherapy had better OS (median 48.6 months) compared with those receiving concurrent chemoradiotherapy (median OS 28.7 months) [89]. Other treatment options for patients unfit for surgery are fractionated radiotherapy and stereotactic radiotherapy. Further prospective randomised control trials of a single or multimodality approach are needed to inform the most suitable individualised patient care in early-stage SCLC.
Locally advanced SCLC
The well-established treatment modality in patients with locally advanced SCLC (any T N2–3 M0) is chemoradiotherapy, as surgery is not an option due to nodal involvement. The standard chemotherapy regimen includes cisplatin (or carboplatin for favourable toxicity profile, as it is less emetogenic and nephrotoxic but its dose-limiting toxicity is significant for myelosuppression, particularly thrombocytopenia) plus etoposide. Traditional expert consensus is to prefer a cisplatin-based regimen in LS-SCLC in curative intent settings, while a carboplatin-based regimen is preferred in ES-SCLC in palliative settings for its favourable toxicity profile.
Thoracic radiotherapy plays a significant role in treatment of SCLC (table 5). While the standard of care is concurrent twice daily radiotherapy, for logistical and patient reasons most centres deliver once-daily radiotherapy. The current evidence-based recommendation is to initiate radiotherapy preferably with the first or second cycle of chemotherapy. Four cycles of cisplatin plus etoposide and a course of radiotherapy (45 Gy, given either once or twice daily) beginning with cycle 1 of the chemotherapy resulted in overall 2- and 5-year survival rates of 44% and 23% [91]. A meta-analysis published in 1992 of 13 major trials, comparing chemotherapy alone with chemotherapy combined with radiotherapy for limited SCLC, demonstrated a 14% reduction in the mortality rate (p=0.001) [92]. The more recent CONVERT trial confirmed that survival outcomes did not differ between twice-daily and once-daily concurrent chemoradiotherapy in patients with LS SCLC, and toxicity was similar and lower than expected with both regimens [93]. It is recommended that patients with good performance status (0–1) who respond to the concurrent chemoradiotherapy are offered prophylactic cranial irradiation (PCI) to reduce risk of brain metastases and improve OS [94, 95].
TABLE 5.
Indication and timing of concurrent thoracic radiotherapy and PCI in LS-SCLC
| Thoracic RT in SCLC | LS-SCLC (stage I–III: cT1–4, N0–3, M0) Bulky mediastinal disease Residual mediastinal disease after induction chemotherapy |
Concurrent starting with the first or second cycle of chemotherapy Could consider with third cycle of chemotherapy to achieve tumour response to initial chemotherapy to reduce radiation toxicity |
Twice daily thoracic RT of 45 Gy in 1.5 Gy per fraction over 3 weeks Alternative regimen, daily thoracic RT of 60–70 Gy in 1.8–2.0 Gy per fraction over 5 weeks |
| PCI in LS-SCLC | Recommended for stage II–III LS-SCLC without progressive disease, age <70 years with good performance status (ECOG 0–2) | After the completion of 4–6 cycles of chemotherapy and concurrent thoracic RT | 25 Gy in 10 fractions |
At present, there is no role for adjuvant immune checkpoint inhibitor treatment in locally advanced SCLC. The STIMULI phase 2 study was designed to evaluate efficacy of maintenance nivolumab plus ipilimumab after completion of concurrent chemoradiotherapy and PCI, but was marred by slow accrual and did not meet the primary end-point of progression-free survival [96]. The ongoing phase III ADRIATIC trial of durvalumab±tremelimumab in inoperable LS-SCLC after concurrent chemoradiotherapy may inform the role of immune checkpoint inhibitors in LS-SCLC, but at present the evidence to incorporate this practice is lacking.
Management of ES-SCLC
SCLC is a chemosensitive cancer and usually responds rapidly to treatment with a response rate of up to 80% [97]. The historical mainstay of care has been first-line platinum-based chemotherapy with 4–6 cycles of cisplatin (or carboplatin) plus etoposide, with a median survival of 7–11 months. A systematic review of 36 clinical trials (from 1980 to 1998) of the chemotherapy regimen (cisplatin plus etoposide) showed a survival benefit in favour of the doublet regimen [97]. The other doublet regimens available as first-line treatment includes cisplatin–irinotecan and cisplatin–topotecan. The emergence of immune checkpoint inhibitors, with an increasing pool of evidence of their efficacy, opened the doors to their use as first-line in combination with chemotherapy for ES-SCLC. The IMpower133 study evaluated atezolizumab in combination with carboplatin and etoposide in metastasised SCLC and resulted in an improved 18-month survival rate of 34% compared with 21% for the platinum-doublet alone, with a comparable safety profile and quality of life [98]. The Keynote-604 study demonstrated OS was prolonged in patients receiving pembrolizumab and chemotherapy compared with chemotherapy alone but did not demonstrate an effect on progression-free survival or improvement in quality of life [99]. The CASPIAN trial assessed durvalumab, with or without tremelimumab, to standard platinum plus etoposide chemotherapy in treatment-naive patients with ES-SCLC and showed that addition of durvalumab to chemotherapy significantly improved OS [100]. These studies show adding immune checkpoint inhibitors to standard chemotherapy provides an OS advantage with an acceptable safety profile.
Oral topotecan is the only second-line therapy approved in Europe and has a response rate of <20% with a median survival of 26 weeks compared with a median survival of 14 weeks in patients offered best supportive care only [101]. Lurbinectedin, an alkylating agent granted orphan designation by the European Medicines Agency, showed some promising results in a phase II trial; however, a subsequent ATLANTIS study assessing lurbinectedin and doxorubicin versus topotecan did not improve survival in patients with relapsed SCLC [102].
The role of PCI in ES-SCLC is unclear. In a prospective clinical trial published in 2007, the primary end-point of time to symptomatic brain metastases was lower in the irradiated patient group and the 1-year survival rate was 27% compared with 13% in non-irradiated patient group. A brain CT/MRI was not, however, mandatory before inclusion [103]. Later, a Japanese clinical trial challenged the benefits of PCI as while PCI reduced the incidence of brain metastases, there was no OS or cognitive benefit [104].
Thoracic radiotherapy may be considered after first-line chemotherapy in patients with residual thoracic disease, as it demonstrated improved 2-year OS in the exploratory analysis of the CREST trial [105, 106]. In clinical practice, however, it is frequently reserved for symptom palliation in patients presenting with superior vena cava syndrome and/or central airway compression.
Radiotherapy also plays an important role in the palliation of brain metastases, painful bone lesions and spinal cord compression. There is a lack of evidence to recommend vascular or airway stenting to improve quality of life or OS. Chemotherapy and radiation therapy offer effective palliation of symptoms, but supportive care should be sought with particular emphasis on regular pain and emesis assessment and psychosocial support. Early smoking cessation is associated with improved OS and the implementation of interventions with this aim is recommended (table 6) [107].
TABLE 6.
Treatment in extensive stage small cell lung cancer
| First-line treatment | 4 cycles Carboplatin+etoposide+atezolizumab | Maintenance atezolizumab |
| 4 cycles Platinum+etoposide+durvalumab | Maintenance durvalumab | |
| 4–6 cycles Carboplatin+etoposide | ||
| Carboplatin+oral topotecan | ||
| Cisplatin+irinotecan | ||
| Second-line treatment | Oral or i.v. topotecan |
Carcinoids and other NETs
Surgery is the recommended standard of care for both typical and atypical lung carcinoids even in the presence of N2 disease. The best procedure depends on size, location and previous biopsy assessment but anatomical pulmonary and lymph node resection are preferred [12]. Adjuvant chemotherapy appears to have no benefit and is not routinely recommended but may be considered in fit patients with high risk of relapse (e.g. N2 positive atypical carcinoid). The paucity of clinical trials on metastatic disease is a barrier in the management of these patients, whose main objectives are control of tumour growth and functioning syndromes [12].
Likewise, large clinical trials to define the approach to LCNEC are also lacking and recommendations are largely based on extrapolation from other histological subtypes [108]. Multidisciplinary management of the disease is thus advised. Surgery should be considered first-line in early-stage disease (stage I–III), with lobectomy and pneumonectomy overperforming sub-lobar resection [107–109]. Unlike carcinoids, the existing evidence, though retrospective in nature, supports adjuvant chemotherapy even in localised disease, with cis/carboplatin plus either etoposide or irinotecan [110, 111]. Radiation therapy appears to prolong survival in patients with stage III disease who are not surgical candidates and may be considered first-line therapy in patients with localised disease unfit for surgery, with advantage for stereotactic ablative radiotherapy [111, 112]. A benefit of radiation therapy among patients with stage I–II disease as adjuvant therapy has not, however, been shown. Chemotherapy remains the standard treatment of stage IV disease, with limited evidence supporting the superiority of SCLC regimens (i.e. cis/carboplatin plus etoposide) over NSCLC regimens [113]. While a retrospective study suggests immunotherapy may have a role in treating stage IV disease, further studies are required to both determine the most advantageous regimens as well as which patients will benefit the most [112, 114].
Quality of life/survivorship
As bronchial carcinoid tumours are often characterised by indolent clinical behaviour, survival is much better compared with SCLC/LCNEC. Most patients treated with surgery will be cured, leading to a 5- and 10-year OS rates of 93% and 82%, respectively. This is slightly worse for those with atypical carcinoids where 5- and 10-year OS rates are 69% and 59%, respectively [115]. For those treated with surgery, advanced age and the extent of lung resection is related to a decline in physical aspects of quality of life [116]. Overall, in long term survival general quality of life is relatively well preserved [117].
However, the survival of patients with SCLC is poor, with a median OS of 2–4 months without treatment. For patients with LS disease, the OS is much better than that for those with ES disease and LS disease patients typically have a median OS of 16–24 months and a 5-year OS rate of 14%, with treatment. For treated ES patients, this declines to a median OS of 6–12 months, whereas long-term survivorship is only 4% [118, 119]. At time of diagnosis, most untreated patients have an impaired quality of life with ES patients being mostly affected [120]. For these patients, the pattern of metastasis is often related to the symptoms patients perceive, reflecting on their quality of life. It is known that bone and brain metastases, the most common sites of metastasis for SCLC, are responsible for more symptoms than other sites [121]. Better quality of life at baseline is a favourable prognostic factor toward survival, whereas patients that respond to treatment report higher scores compared with untreated patients [120, 122]. The addition of immune checkpoint inhibitors to chemotherapy, as introduced in recent years, improved quality of life after initiating treatment, with more pronounced and persistent improvements in those treated with chemo/immunotherapy compared with chemotherapy alone [123]. Observational studies evaluating quality of life in the real world are lacking [120]. PCI reduces the incidence of brain metastases in patients who have shown a response to chemotherapy for their ES-SCLC and might improve OS, however, it comes at the cost of an increased risk of late memory impairment and fatigue [103].
In a large Dutch cohort of 12 796 patients diagnosed with SCLC or LCNEC, patients diagnosed with stage I–II LCNEC do have a better prognosis compared with SCLC (median OS 32.4 versus 17.8 months), whereas this declines at stage III (median OS 12.6 versus 12.2 months). At stage IV, the median survival of LCNEC is worse than SCLC, with a median OS of 4.0 versus 5.3 months [124]. There are no studies reporting on the quality of life for select cohorts of LCNEC patients, but as advanced stage LCNEC resembles SCLC in clinical characteristics, quality of life of these patients will be comparable.
Conclusions
For any cancer, clinicians integrate findings from various diagnostic modalities to create a comprehensive diagnostic profile, determining the tumour's histological subtype, stage and molecular characteristics, which are crucial for devising personalised treatment strategies. Lung NETs and SCLC are no different. Multidisciplinary tumour boards play a pivotal role in refining diagnoses and developing individualised management plans, considering the complex nature of neuroendocrine lung tumours and the evolving landscape of targeted therapies and immunotherapies. Continued research into innovative diagnostic tools and refined molecular profiling holds promise for enhancing early detection and optimising therapeutic interventions for patients with neuroendocrine lung tumours.
Key points
Neuroendocrine tumours, including SCLC, can present with paraneoplastic phenomena.
A multidisciplinary approach is required for staging and deciding on management.
As treatment options improve, long-term survivorship is also increasing.
Footnotes
Author contributions: A. Aujayeb conceptualised the idea, all authors wrote the article, A. Aujayeb revised the article and all authors agreed to the final version of the manuscript.
Conflict of interest: The authors have no conflict of interests.
References
- 1.World Cancer Research Fund International . Lung Cancer Statistics. Date last accessed: 21 January 2024. Date last updated: March 2022. www.wcrf.org/cancer-trends/lung-cancer-statistics
- 2.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 3.Malhotra J, Malvezzi M, Negri E, et al. Risk factors for lung cancer worldwide. Eur Respir J 2016; 48: 889–902. doi: 10.1183/13993003.00359-2016 [DOI] [PubMed] [Google Scholar]
- 4.Kocher F, Hilbe W, Seeber A, et al. Longitudinal analysis of 2293 NSCLC patients: a comprehensive study from the TYROL registry. Lung Cancer 2015; 87: 193–200. doi: 10.1016/j.lungcan.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 5.Dingemans AMC, Fruh M, Ardizzoni A, et al. Small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2021; 32: 839–853. doi: 10.1016/j.annonc.2021.03.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumoulin DW, Aarts MJ, De Ruysscher D, et al. Trends in the epidemiology of small-cell lung cancer: a Dutch nationwide population-based study over 1989–2020. Eur J Cancer 2023; 191: 112985. doi: 10.1016/j.ejca.2023.112985 [DOI] [PubMed] [Google Scholar]
- 7.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 8.Rudin CM, Brambilla E, Faivre-Finn C, et al. Small-cell lung cancer. Nat Rev Dis Primers 2021; 7: 3. doi: 10.1038/s41572-020-00235-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franco F, Carcereny E, Guirado M, et al. Epidemiology, treatment, and survival in small cell lung cancer in Spain: data from the Thoracic Tumor Registry. PLoS One 2021; 16: e0251761. doi: 10.1371/journal.pone.0251761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Small Cell Lung Cancer V.2.2024.
- 11.Caballero Vázquez A, Garcia Flores P, Romero Ortiz A, et al. Small cell lung cancer: recent changes in clinical presentation and prognosis. Clin Respir J 2020; 14: 222–227. doi: 10.1111/crj.13119 [DOI] [PubMed] [Google Scholar]
- 12.Baudin E, Caplin M, Garcia-Carbonero R, et al. Lung and thymic carcinoids: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2021; 32: 439–451. doi: 10.1016/j.annonc.2021.01.003 [DOI] [PubMed] [Google Scholar]
- 13.Fasano M, Della Corte CM, Papaccio F, et al. Pulmonary large-cell neuroendocrine carcinoma. J Thorac Oncol 2015; 10: 1133–1141. doi: 10.1097/JTO.0000000000000589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marty-Ané CH, Costes V, Pujol JL, et al. Carcinoid tumors of the lung: do atypical features require aggressive management? Ann Thorac Surg 1995; 59: 78–83. doi: 10.1016/0003-4975(94)00630-P [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Wang W, Hu Q, et al. Clinic and genetic similarity assessments of atypical carcinoid, neuroendocrine neoplasm with atypical carcinoid morphology and elevated mitotic count and large cell neuroendocrine carcinoma. BMC Cancer 2022; 22: 321. doi: 10.1186/s12885-022-09391-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrini E, Marchese PV, De Biase D, et al. Large cell neuroendocrine carcinoma of the lung: current understanding and challenges. J Clin Med 2022; 11: 1461. doi: 10.3390/jcm11051461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah S, Gosain R, Groman A, et al. Incidence and survival outcomes in patients with lung neuroendocrine neoplasms in the United States. Cancers (Basel) 2021; 13: 1753. doi: 10.3390/cancers13081753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soomro Z, Youssef M, Yust-Katz S, et al. Paraneoplastic syndromes in small cell lung cancer. J Thorac Dis 2020; 12: 6253–6263. doi: 10.21037/jtd.2020.03.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tschernatsch M, Dierkes C, Gerriets T, et al. Paraneoplastic neurological syndromes in patients with carcinoid. Eur J Neurol 2008; 15: 1390–1394. doi: 10.1111/j.1468-1331.2008.02328.x [DOI] [PubMed] [Google Scholar]
- 20.Visouli AN, Darwiche K, Kourtoglou GI, et al. Primary lung carcinoid, a rare cause of paraparesis: report of a case and review of the literature. J Thorac Dis 2012; 4: Suppl. 1: 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fantin A, Castaldo N, Tirone C, et al. Endobronchial ultrasound: a pictorial essay. Acta Biomed 2023; 94: e2023113. doi: 10.23750/abm.v94i4.14090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fantin A, Manera M, Patruno V, et al. Endoscopic technologies for peripheral pulmonary lesions: from diagnosis to therapy. Life (Basel) 2023; 13: 254. doi: 10.3390/life13020254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guinee DG, Fishback NF, Koss MN, et al. The spectrum of immunohistochemical staining of small-cell lung carcinoma in specimens from transbronchial and open-lung biopsies. Am J Clin Pathol 1994; 102: 406–414. doi: 10.1093/ajcp/102.4.406 [DOI] [PubMed] [Google Scholar]
- 24.Cainap C, Balacescu O, Cainap SS, et al. Next generation sequencing technology in lung cancer diagnosis. Biology (Basel) 2021; 10: 864. doi: 10.3390/biology10090864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ameri P, Gatto F, Arvigo M, et al. Somatostatin receptor scintigraphy in thoracic diseases. J Endocrinol Invest 2007; 30: 889–902. doi: 10.1007/BF03349233 [DOI] [PubMed] [Google Scholar]
- 26.Jett JR, Schild SE, Kesler KA, et al. Treatment of small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143: 5 Suppl., e400S-e419S. doi: 10.1378/chest.12-2363 [DOI] [PubMed] [Google Scholar]
- 27.Kalemkerian GP, Gadgeel SM. Modern staging of small cell lung cancer. J Natl Compr Canc Netw 2013; 11: 99–104. doi: 10.6004/jnccn.2013.0012 [DOI] [PubMed] [Google Scholar]
- 28.Micke P, Faldum A, Metz T, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer–what limits limited disease? Lung Cancer 2002; 37: 271–276. doi: 10.1016/S0169-5002(02)00072-7 [DOI] [PubMed] [Google Scholar]
- 29.Huang LL, Hu XS, Wang Y, et al. Survival and pretreatment prognostic factors for extensive-stage small cell lung cancer: a comprehensive analysis of 358 patients. Thorac Cancer 2021; 12: 1943–1951. doi: 10.1111/1759-7714.13977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amin MB, Greene FL, Byrd DR, et al. , eds. AJCC Cancer Staging Manual. Cham, Springer, 2016. [Google Scholar]
- 31.Lababede O, Meziane MA. The Eighth Edition of TNM Staging of Lung Cancer: Reference Chart and Diagrams. Oncologist 2018; 23: 844–848. doi: 10.1634/theoncologist.2017-0659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pizzutilo EG, Pedrani M, Amatu A, et al. Liquid biopsy for small cell lung cancer either de novo or transformed: systematic review of different applications and meta-analysis. Cancers (Basel) 2021; 13: 2265. doi: 10.3390/cancers13092265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Squires MH, Volkan Adsay N, Schuster DM, et al. Octreoscan versus FDG-PET for neuroendocrine tumor staging: a biological approach. Ann Surg Oncol 2015; 22; 2295–2301. 10.1245/s10434-015-4471-x [DOI] [PubMed] [Google Scholar]
- 34.Van Binnebeek S, Vanbilloen B, Baete K, et al. Comparison of diagnostic accuracy of (111)In-pentetreotide SPECT and (68)Ga-DOTATOC PET/CT: a lesion-by-lesion analysis in patients with metastatic neuroendocrine tumours. Eur Radiol 2016; 26: 900–909. 10.1007/s00330-015-3882-1 [DOI] [PubMed] [Google Scholar]
- 35.WHO Classification of Tumours Editorial Board . Thoracic Tumours. 5th ed. Lyon, France, International Agency for Research on Cancer, 2021. [Google Scholar]
- 36.Delle Fave G, O'Toole D, Sundin A, et al. Vienna Consensus Conference participants. ENETS consensus guidelines update for gastroduodenal neuroendocrine neoplasms. Neuroendocrinol 2016; 103: 119–124. doi: 10.1159/000443168 [DOI] [PubMed] [Google Scholar]
- 37.Gould VE, Wiedenmann B, Lee I, et al. Synaptophysin expression in neuroendocrine neoplasms as determined by immunocytochemistry. Am J Pathol 1987; 126: 243–257. [PMC free article] [PubMed] [Google Scholar]
- 38.Wiedenmann B, Huttner WB. Synaptophysin and chromogranins/secretogranins—widespread constituents of distinct types of neuroendocrine vesicles and new tools in tumor diagnosis. Virchows Arch B Cell Pathol Incl Mol Pathol 1989; 58: 95–121. doi: 10.1007/BF02890062 [DOI] [PubMed] [Google Scholar]
- 39.Portela-Gomes GM, Hacker GW, Weitgasser R. Neuroendocrine cell markers for pancreatic islets and tumors. Appl Immunohistochem Mol Morphol 2004; 12: 183–192. doi: 10.1097/00129039-200409000-00001 [DOI] [PubMed] [Google Scholar]
- 40.Schmechel D, Marangos PJ, Brightman M. Neurone-specific enolase is a molecular marker for peripheral and central neuroendocrine cells. Nature 1978; 276: 834–836. doi: 10.1038/276834a0 [DOI] [PubMed] [Google Scholar]
- 41.Schmechel DE. Gamma-subunit of the glycolytic enzyme enolase: nonspecific or neuron specific? Lab Invest 1985; 52: 239–242. [PubMed] [Google Scholar]
- 42.Lloyd RV, Mervak T, Schmidt K, et al. Immunohistochemical detection of chromogranin and neuron-specific enolase in pancreatic endocrine neoplasms. Am J Surg Pathol 1984; 8: 607–614. doi: 10.1097/00000478-198408000-00004 [DOI] [PubMed] [Google Scholar]
- 43.Rindi G, Bordi C, Rappel S, et al. Gastric carcinoids and neuroendocrine carcinomas: pathogenesis, pathology, and behavior. World J Surg 1996; 20: 168–172. doi: 10.1007/s002689900026 [DOI] [PubMed] [Google Scholar]
- 44.Langley K, Gratzl M. Neural cell adhesion molecule NCAM in neural and endocrine cells. In:Gratzl M, Langley K, eds. Markers for Neural and Endocrine Cells. Molecular and Cell Biology, Diagnostic Applications. Weinheim, Verlag Chemie; 1991; pp. 133–178. [Google Scholar]
- 45.Jin L, Hemperly JJ, Lloyd RV. Expression of neural cell adhesion molecule in normal and neoplastic human neuroendocrine tissues. Am J Pathol 1991; 138: 961–969. [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng G, Ettinger DS, Maleki Z. Utility of the quantitative Ki-67 proliferation index and CD56 together in the cytologic diagnosis of small cell lung carcinoma and other lung neuroendocrine tumors. Acta Cytol 2013; 57: 281–290. doi: 10.1159/000346394 [DOI] [PubMed] [Google Scholar]
- 47.Lan MS, Breslin MB. Structure, expression, and biological function of INSM1 transcription factor in neuroendocrine differentiation. FASEB J 2009; 23: 2024–2033. doi: 10.1096/fj.08-125971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mukhopadhyay S, Dermawan JK, Lanigan CP, et al. Insulinoma-associated protein 1 (INSM1) is a sensitive and highly specific marker of neuroendocrine differentiation in primary lung neoplasms: An immunohistochemical study of 345 cases, including 292 whole-tissue sections. Mod Pathol 2019; 32: 100–109. doi: 10.1038/s41379-018-0122-7 [DOI] [PubMed] [Google Scholar]
- 49.Doxtader EE, Mukhopadhyay S. Insulinoma-associated protein 1 is a sensitive and specific marker of neuroendocrine lung neoplasms in cytology specimens. Cancer Cytopathol 2018; 126: 243–252. doi: 10.1002/cncy.21972 [DOI] [PubMed] [Google Scholar]
- 50.Rooper LM, Sharma R, Li Q,et al. INSM1 demonstrates superior performance to the individual and combined use of synaptophysin, chromogranin and CD56 for diagnosing neuroendocrine tumors of the thoracic cavity. Am J Surg Pathol 2017; 41: 1561–1569. doi: 10.1097/PAS.0000000000000916 [DOI] [PubMed] [Google Scholar]
- 51.Rosenbaum JN, Guo Z, Baus RM, et al. INSM1: a novel immunohistochemical and molecular marker for neuroendocrine and neuroepithelial neoplasms. Am J Clin Pathol 2015; 144: 579–591. doi: 10.1309/AJCPGZWXXBSNL4VD [DOI] [PubMed] [Google Scholar]
- 52.Zappi A, Persano I, Galvani L, et al. Chemotherapy in well differentiated neuroendocrine tumors (NET) G1, G2, and G3: a narrative review. J Clin Med 2023; 12: 717. 10.3390/jcm12020717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rindi G, Klimstra DS, Abedi-Ardekani B, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol 2018; 31: 1770–1786. doi: 10.1038/s41379-018-0110-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pathology and genetics of tumours of the lung, pleura, thymus and heart. In: Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, eds. World Health Organization classification of tumors. Lyon, IARC Press, 2004. [Google Scholar]
- 55.Swarts DR, Ramaekers FC, Speel E-J. Molecular and cellular biology of neuroendocrine lung tumors: evidence for separate biological entities. Biochim Biophys Acta 2012; 1826: 255–271. doi: 10.1016/j.bbcan.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 56.Sutherland KD, Berns A. Cell of origin of lung cancer. Mol Oncol 2010; 4: 397–403. doi: 10.1016/j.molonc.2010.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laddha SV, da Silva EM, Robzyk K, et al. Integrative genomic characterization identifies molecular subtypes of lung carcinoids. Cancer Res 2019; 79: 4339–4347. doi: 10.1158/0008-5472.CAN-19-0214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simbolo M, Mafficini A, Sikora KO, et al. Lung neuroendocrine tumours: deep sequencing of the four World Health Organization histotypes reveals chromatin-remodelling genes as major players and a prognostic role for TERT, RB1, MEN1 and KMT2D. J Pathol 2017; 241: 488–500. doi: 10.1002/path.4853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chai SM, Brown IS, Kumarasinghe MP. Gastroenteropancreatic neuroendocrine neoplasms: selected pathology review and molecular updates. Histopathol 2018; 72: 153–167. doi: 10.1111/his.13367 [DOI] [PubMed] [Google Scholar]
- 60.Aslan DL, Gulbahce HE, Pambuccian SE, et al. Ki-67 immunoreactivity in the differential diagnosis of pulmonary neuroendocrine neoplasms in specimens with extensive crush artifact. Am J Clin Pathol 2005; 123: 874–878. doi: 10.1309/QYV05VGEGKUL2RTT [DOI] [PubMed] [Google Scholar]
- 61.Pelosi G, Rodriguez J, Viale G, et al. Typical and atypical pulmonary carcinoid tumor overdiagnosed as small-cell carcinoma on biopsy specimens: a major pitfall in the management of lung cancer patients. Am J Surg Pathol 2005; 29: 179–187. doi: 10.1097/01.pas.0000149690.75462.29 [DOI] [PubMed] [Google Scholar]
- 62.Yatabe Y, Dacic S, Borczuk AC, et al. Best practices recommendations for diagnostic immunohistochemistry in lung cancer. J Thorac Oncol 2019; 14: 377–407. doi: 10.1016/j.jtho.2018.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thunnissen E, Borczuk AC, Flieder DB,et al. The use of immunohistochemistry improves the diagnosis of small cell lung cancer and its differential diagnosis. An international reproducibility study in a demanding set of cases. J Thor Oncol 2017; 12: 334–346. doi: 10.1016/j.jtho.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 64.Pelosi G, Fabbri A, Bianchi F,et al. DeltaNp63 (p40) and thyroid transcription factor-1 immunoreactivity on small biopsies or cellblocks for typing non-small cell lung cancer: a novel two-hit, sparing-material approach. J Thorac Oncol 2012; 7: 281–290. doi: 10.1097/JTO.0b013e31823815d3 [DOI] [PubMed] [Google Scholar]
- 65.Pelosi G, Rossi G, Cavazza A, et al. DeltaNp63 (p40) distribution inside lung cancer: a driver biomarker approach to tumor characterization. Int J Surg Pathol 2013; 21: 229–239. doi: 10.1177/1066896913476750 [DOI] [PubMed] [Google Scholar]
- 66.Sturm N, Lantuejoul S, Laverriere MH, et al. Thyroid transcription factor 1 and cytokeratins 1, 5, 10, 14 (34βE12) expression in basaloid and large-cell neuroendocrine carcinomas of the lung. Hum Pathol 2001; 32: 918–925. doi: 10.1053/hupa.2001.27110 [DOI] [PubMed] [Google Scholar]
- 67.Kontogianni K, Nicholson AG, Butcher D, et al. CD56: a useful tool for the diagnosis of small cell lung carcinomas on biopsies with extensive crush artefact. J Clin Pathol 2005; 58: 978–980. doi: 10.1136/jcp.2004.023044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fujino K, Yasufuku K, Kudoh S, et al. INSM1 is the best marker for the diagnosis of neuroendocrine tumors: comparison with CGA, SYP and CD56. Int J Clin Exp Pathol 2017; 10: 5393–5405. [Google Scholar]
- 69.Sonkin D, Thomas A, Teicher BA. Are neuroendocrine negative small cell lung cancer and large cell neuroendocrine carcinoma with WT RB1 two faces of the same entity? Lung Cancer Manag 2019; 8: LMT13. doi: 10.2217/lmt-2019-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang W, Girard L, Zhang YA, et al. Small cell lung cancer tumors and preclinical models display heterogeneity of neuroendocrine phenotypes. Transl Lung Cancer Res 2018; 7: 32–49. doi: 10.21037/tlcr.2018.02.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rudin CM, Poirier JT, Byers LA, et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer 2019; 19: 289–297. doi: 10.1038/s41568-019-0133-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Horie M, Miyashita N, Mattsson JSM, et al. An integrative transcriptome analysis reveals a functional role for thyroid transcription factor-1 in small cell lung cancer. J Pathol 2018; 246: 154–165. doi: 10.1002/path.5109 [DOI] [PubMed] [Google Scholar]
- 73.Righi L, Volante M, Tavaglione V, et al. Somatostatin receptor tissue distribution in lung neuroendocrine tumours: a clinicopathologic and immunohistochemical study of 218 ‘clinically aggressive’ cases. Ann Oncol 2010; 21: 548–555. doi: 10.1093/annonc/mdp334 [DOI] [PubMed] [Google Scholar]
- 74.Owen DH, Giffin MJ, Bailis JM, et al. DLL3: an emerging target in small cell lung cancer. J Hematol Oncol 2019; 12: 61. doi: 10.1186/s13045-019-0745-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Righi L, Volante M, Rapa I, et al. Mammalian target of rapamycin signaling activation patterns in neuroendocrine tumors of the lung. Endocr Relat Cancer 2010; 17: 977–987; doi: 10.1677/ERC-10-0157 [DOI] [PubMed] [Google Scholar]
- 76.Tsuruoka K, Horinouchi H, Goto Y, et al. PD-L1 expression in neuroendocrine tumors of the lung. Lung Cancer 2017; 108: 115–120. doi: 10.1016/j.lungcan.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 77.Ibe T, Shimizu K, Nakano T, et al. High-grade neuroendocrine carcinoma of the lung shows increased thymidylate synthase expression compared to other histotypes. J Surg Oncol 2010; 102: 11–17. doi: 10.1002/jso.21576 [DOI] [PubMed] [Google Scholar]
- 78.Metovic J, Barella M, Bianchi F,et al. Morphologic and molecular classification of lung neuroendocrine neoplasms. Virchows Arch 2021; 478: 5–19. doi: 10.1007/s00428-020-03015-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fernandez-Cuesta L, Peifer M, Lu X, et al. Frequent mutations in chromatin-remodelling genes in pulmonary carcinoids. Nat Commun 2014; 5: 3518. doi: 10.1038/ncomms4518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rossi G, Bertero L, Marchio C, et al. Molecular alterations of neuroendocrine tumours of the lung. Histopathol 2018; 72: 142–152. doi: 10.1111/his.13394 [DOI] [PubMed] [Google Scholar]
- 81.Miyanaga A, Masuda M, Motoi N, et al. Whole-exome and RNA sequencing of pulmonary carcinoid reveals chromosomal rearrangements associated with recurrence. Lung Cancer 2020; 145: 85–94. doi: 10.1016/j.lungcan.2020.03.027 [DOI] [PubMed] [Google Scholar]
- 82.Swarts DR, Henfling ME, Van Neste L, et al. CD44 and OTP are strong prognostic markers for pulmonary carcinoids. Clin Cancer Res 2013; 19: 2197–2207. doi: 10.1158/1078-0432.CCR-12-3078 [DOI] [PubMed] [Google Scholar]
- 83.Swarts DR, Van Neste L, Henfling ME, et al. An exploration of pathways involved in lung carcinoid progression using gene expression profiling. Carcinogenesis 2013; 34: 2726–2737. doi: 10.1093/carcin/bgt271 [DOI] [PubMed] [Google Scholar]
- 84.Warth A, Herpel E, Krysa S, et al. Chromosomal instability is more frequent in metastasized than in non-metastasized pulmonary carcinoids but is not a reliable predictor of metastatic potential. Exp Mol Med 2009; 41: 349–353. doi: 10.3858/emm.2009.41.5.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vollbrecht C, Werner R, Walter RF,et al. Mutational analysis of pulmonary tumours with neuroendocrine features using targeted massive parallel sequencing: a comparison of a neglected tumour group. Br J Cancer 2015; 113: 1704–1711. doi: 10.1038/bjc.2015.397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clinical Lung Cancer Genome P, Network Genomic M . A genomics-based classification of human lung tumors. Sci Transl Med 2013; 5: 209ra153. doi: 10.1126/scitranslmed.3006802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rekhtman N, Pietanza MC, Hellmann MD, et al. Next-generation sequencing of pulmonary large cell neuroendocrine carcinoma reveals small cell carcinoma-like and non-small cell carcinoma-like subsets. Clin Cancer Res 2016; 22: 3618–3629. doi: 10.1158/1078-0432.CCR-15-2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cheng, X, Zeng, W, Liu, Y, et al. 2022. Impact of lymph node dissection on survival and tumor recurrence for patients with resected cT1-2N0 small cell lung cancer. Ann Surg Oncol 2022; 29: 7512–7525. doi: 10.1245/s10434-022-12215-7 [DOI] [PubMed] [Google Scholar]
- 89.Wakeam E, Acuna SA, Leighl NB, et al. Surgery versus chemotherapy and radiotherapy for early and locally advanced small cell lung cancer: a propensity-matched analysis of survival. Lung Cancer 2017; 109: 78–88. doi: 10.1016/j.lungcan.2017.04.021 [DOI] [PubMed] [Google Scholar]
- 90.Le Pechoux C, Faivre-Finn C, Ramella S, et al. ESTRO ACROP guidelines for target volume definition in the thoracic radiation treatment of small cell lung cancer. Radiother Oncol 2020; 152: 89–95. doi: 10.1016/j.radonc.2020.07.012 [DOI] [PubMed] [Google Scholar]
- 91.Turrisi AT, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999; 340: 265–271. doi: 10.1056/NEJM199901283400403 [DOI] [PubMed] [Google Scholar]
- 92.Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 1992; 327: 1618–1624. doi: 10.1056/NEJM199212033272302 [DOI] [PubMed] [Google Scholar]
- 93.Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol 2017; 18: 1116–1125. doi: 10.1016/S1470-2045(17)30318-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kristjansen PE, Kristensen CA. The role of prophylactic cranial irradiation in the management of small cell lung cancer. Cancer Treat Rev, 1993; 19: 3–16. doi: 10.1016/0305-7372(93)90023-K [DOI] [PubMed] [Google Scholar]
- 95.Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. N Engl J Med 1999; 341: 476–484. doi: 10.1056/NEJM199908123410703 [DOI] [PubMed] [Google Scholar]
- 96.Peters S, Pujol JL, Dafni U, et al. Consolidation nivolumab and ipilimumab versus observation in limited-disease small-cell lung cancer after chemo-radiotherapy – results from the randomised phase II ETOP/IFCT 4-12 STIMULI trial. Ann Oncol 2022; 33: 67–79. doi: 10.1016/j.annonc.2021.09.011 [DOI] [PubMed] [Google Scholar]
- 97.Mascaux C, Paesmans M, Berghmans T, et al. A systematic review of the role of etoposide and cisplatin in the chemotherapy of small cell lung cancer with methodology assessment and meta-analysis. Lung cancer 2000; 30: 23–36. doi: 10.1016/S0169-5002(00)00127-6 [DOI] [PubMed] [Google Scholar]
- 98.Liu SV, Reck M, Mansfield AS, et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol 2021; 39: 619–630. doi: 10.1200/JCO.20.01055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rudin CM, Awad MM, Navarro A, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol 2020; 38: 2369–2379. doi: 10.1200/JCO.20.00793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. The Lancet 2019; 394: 1929–1939. doi: 10.1016/S0140-6736(19)32222-6 [DOI] [PubMed] [Google Scholar]
- 101.O'Brien MER, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small cell lung cancer. J Clin Oncol 2006; 24: 5441–5447. doi: 10.1200/JCO.2006.06.5821 [DOI] [PubMed] [Google Scholar]
- 102.Aix SP, Ciuleanu TE, Navarro A, et al. Combination lurbinectedin and doxorubicin versus physician's choice of chemotherapy in patients with relapsed small-cell lung cancer (ATLANTIS): a multicentre, randomised, open-label, phase 3 trial. Lancet Respir Med 2023; 11: 74–86. doi: 10.1016/S2213-2600(22)00309-5 [DOI] [PubMed] [Google Scholar]
- 103.Slotman B, Faivre-Finn C, Kramer G. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med 2007; 357: 664–672. doi: 10.1056/NEJMoa071780 [DOI] [PubMed] [Google Scholar]
- 104.Takahashi T, Yamanaka T, Seto T, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2017; 18: 663–671. doi: 10.1016/S1470-2045(17)30230-9 [DOI] [PubMed] [Google Scholar]
- 105.Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet 2015; 385: 36–42. doi: 10.1016/S0140-6736(14)61085-0 [DOI] [PubMed] [Google Scholar]
- 106.Slotman BJ, van Tinteren H, Praag JO, et al. Radiotherapy for extensive stage small-cell lung cancer-Authors’ reply. Lancet 2015; 385: 1292–1293. doi: 10.1016/S0140-6736(15)60679-1 [DOI] [PubMed] [Google Scholar]
- 107.Caini S, Del Riccio M, Vettori V, et al. Quitting smoking at or around diagnosis improves the overall survival of lung cancer patients: a systematic review and meta-analysis. J Thorac Oncol 2022; 17: 623–636. doi: 10.1016/j.jtho.2021.12.005 [DOI] [PubMed] [Google Scholar]
- 108.Glisson BS, Moran CA. Large-cell neuroendocrine carcinoma: controversies in diagnosis and treatment. J Natl Compr Canc Netw 2011; 9: 1122–1129. doi: 10.6004/jnccn.2011.0093 [DOI] [PubMed] [Google Scholar]
- 109.Lutfi W, Schuchert MJ, Dhupar R, et al. Sublobar resection is associated with decreased survival for patients with early stage large-cell neuroendocrine carcinoma of the lung. Interact Cardiovasc Thorac Surg 2019; 29: 517–524. doi: 10.1093/icvts/ivz140 [DOI] [PubMed] [Google Scholar]
- 110.Raman V, Jawitz OK, Yang CJ, et al. Adjuvant therapy for patients with early large cell lung neuroendocrine cancer: a national analysis. Ann Thorac Surg 2019; 108: 377–383. doi: 10.1016/j.athoracsur.2019.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kenmotsu H, Niho S, Tsuboi M, et al. Randomized phase III study of irinotecan plus cisplatin versus etoposide plus cisplatin for completely resected highgrade neuroendocrine carcinoma of the lung: JCOG1205/1206. J Clin Oncol 2020; 38: 4292–4301. doi: 10.1200/JCO.20.01806 [DOI] [PubMed] [Google Scholar]
- 112.Corbett V, Arnold S, Anthony L, et al. Management of large cell neuroendocrine carcinoma. Front Oncol 2021; 11: 653162. doi: 10.3389/fonc.2021.653162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wegner RE, Abel S, Colonias A. Stereotactic ablative body radiotherapy versus conventionally fractionated radiotherapy for early stage large cell neuroendocrine carcinoma of the lung. Lung Cancer Manag 2020; 9: LMT32. doi: 10.2217/lmt-2020-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Komiya T, Ravindra N, Powell E. Role of immunotherapy in stage IV large cell neuroendocrine carcinoma of the lung. Asian Pac J Cancer Prev 2021; 22: 365–370. doi: 10.31557/APJCP.2021.22.2.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Soga J, Yakuwk Y. Bronchopulmonary carcinoids: An analysis of 1,875 reported cases with special reference to a comparison between typical carcinoids and atypical varieties. Ann Thorac Cardiovasc Surg 1999; 5: 211–219. [PubMed] [Google Scholar]
- 116.Möller A, Sartipy U. Predictors of postoperative quality of life after surgery for lung cancer. J Thorac Oncol 2012; 7: 406–411. doi: 10.1097/JTO.0b013e3182398e82 [DOI] [PubMed] [Google Scholar]
- 117.Abdel Jalil R, Abdallah FA, Obeid Z, et al. Maintaining quality of life after major lung resection for carcinoid tumor. J Cardiothorac Surg 2023; 18: 330. doi: 10.1186/s13019-023-02435-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.National Cancer Institute . Small cell lung cancer treatment (PDQ)—health professional version. Date last accessed: 17 January 2024. www.cancer.gov/types/lung/hp/small-cell-lung-treatment-pdq
- 119.National Cancer Institute . SEER*Explorer: An interactive website for SEER cancer statistics. Surveillance Research Program. 2023. Date last accessed: 17 January 2024. https://seer.cancer.gov/statistics-network/explorer/
- 120.Bennett BM, Wells JR, Panter C, et al. The humanistic burden of small cell lung cancer (SCLC): a systematic review of health-related quality of life (HRQoL) literature. Front Pharmacol 2017; 8: 339. doi: 10.3389/fphar.2017.00339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.D'antonio C, Passaro A, Del Signore E, et al. Bone and brain metastasis in lung cancer: recent advances in therapeutic strategies. Ther Adv Med Oncol 2014; 6: 101–114. doi: 10.1177/1758834014521110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reck M, Thatcher N, Smit EF, et al. Baseline quality of life and performance status as prognostic factors in patients with extensive-stage disease small cell lung cancer treated with pemetrexed plus carboplatin vs. etoposide plus carboplatin. Lung Cancer 2012; 78: 276–281. doi: 10.1016/j.lungcan.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 123.Mansfield AS, Każarnowicz A, Karaseva N, et al. Safety and patient-reported outcomes of atezolizumab, carboplatin, and etoposide in extensive-stage small-cell lung cancer (IMpower133): a randomized phase I/III trial. Ann Oncol 2020; 31: 310–317. doi: 10.1016/j.annonc.2019.10.021 [DOI] [PubMed] [Google Scholar]
- 124.Derks JL, Hendriks LE, Buikhuisen WA, et al. Clinical features of large cell neuroendocrine carcinoma: a population-based overview. Eur Respir J 2016; 47: 615–624. doi: 10.1183/13993003.00618-2015 [DOI] [PubMed] [Google Scholar]