Shareable abstract
Find out about some of the diagnostic considerations in the field of rare pleural disease https://bit.ly/3xYefzA
A 78-year-old smoker, with no history of asbestos exposure, but with a history of COPD and arterial hypertension, was admitted to our pulmonology unit for investigation of a refractory pleural effusion. The patient's medical journey dated back to a few months earlier when, following investigations prompted by a car accident, a chest radiograph revealed a mild pleural effusion, which was interpreted as related to trauma (figure 1).
FIGURE 1.
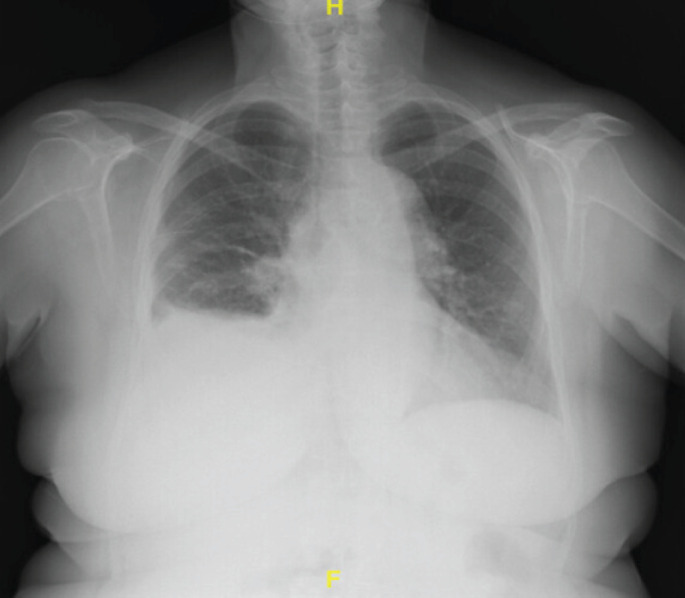
Chest radiograph showing blunting of the right costophrenic angle.
A few weeks after starting corticosteroid therapy prescribed by the emergency physician, the patient underwent a follow-up chest ultrasound at our centre. Chest ultrasound revealed an anechoic effusion, extending at least three intercostal spaces, without signs of visible loculations, organisation, pleural thickening or lung atelectasis.
Task 1
If you were the pulmonologist performing the ultrasound scan after taking the patient's medical history, what would you do?
I would assess the patient at a later point in time (a watch-and-wait approach in the context of post-traumatic pleural effusion).
I would perform a thoracentesis (an interventional approach given the indeterminate nature of the pleural effusion).
I would prescribe a new course of oral corticosteroid therapy.
Answer 1
The correct answer is b. The effusion is of an undetermined nature.
Although a post-traumatic effusion cannot yet be completely ruled out, the reliability of this diagnosis diminishes over time compared with the initial finding. To confirm this, it is necessary to evaluate other factors that are sometimes found in pleural effusions associated with chest trauma [1, 2]. These include ultrasound findings (i.e. internal echogenicity may increase the likelihood of haemothorax identification [3]) and the chemical and physical characteristics of the fluid (i.e. milky appearance to identify chylothorax). Regarding sonographic classification, these types of effusions are usually referred to as “complex” and may be subclassified as homogeneously or heterogeneously echogenic [4]. With the exception of serositis-related pleural effusion, corticosteroids have no indication.
A therapeutic thoracentesis was performed, successfully removing ∼1000 mL of citrine fluid. This procedure also enabled diagnostic tests including cytological and microbiological analyses, both of which were negative. The chemical characteristics identified the fluid as an exudate, according to Light's criteria (increased pleural protein and lactate dehydrogenase levels compared with serum levels).
Before discharge from the pulmonology outpatient clinic, a chest computed tomography (CT) scan with contrast was recommended at 1 month.
Task 2
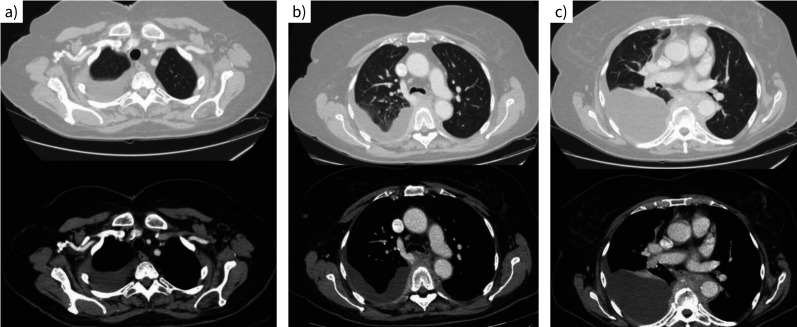
Why is a CT scan with contrast performed? Describe the main findings in figure 2a–c.
FIGURE 2.
Computed tomography scan of the chest with contrast.
FIGURE 2.
Computed tomography scan of the chest with contrast.
Answer 2
The decision to perform a contrast-enhanced CT scan is based on the fact that it greatly improves visualisation, making it easier to identify pleural thickening, plaques and masses, as well as parenchymal lung lesions. In cases of pleural effusion, contrast enhancement can help to determine whether malignant pleural pathology or productive active flogosis (i.e. empyema) is present. The main findings shown in figure 2 are right pleural effusion extending to the apical region (figure 2a) and tissue with a weak post-contrast enhancement noted in the subcarinal-prevertebral region at the level of D4–D10 (figure 2b, c). The pleural effusion involves the lung bases.
This led to the patient's referral to the emergency department through a request for hospitalisation from their general practitioner. Upon triage, the patient was asymptomatic, denied recent fever or night sweats, and had no respiratory symptoms. Routine laboratory blood tests, including inflammatory markers (C-reactive protein), were conducted with negative results. Subsequently, the patient was admitted to the pulmonology unit.
Task 3
How would you proceed with the diagnostic process?
Answer 3
As a completion of the staging process, a total body CT scan and positron emission tomography (PET)-CT could be performed.
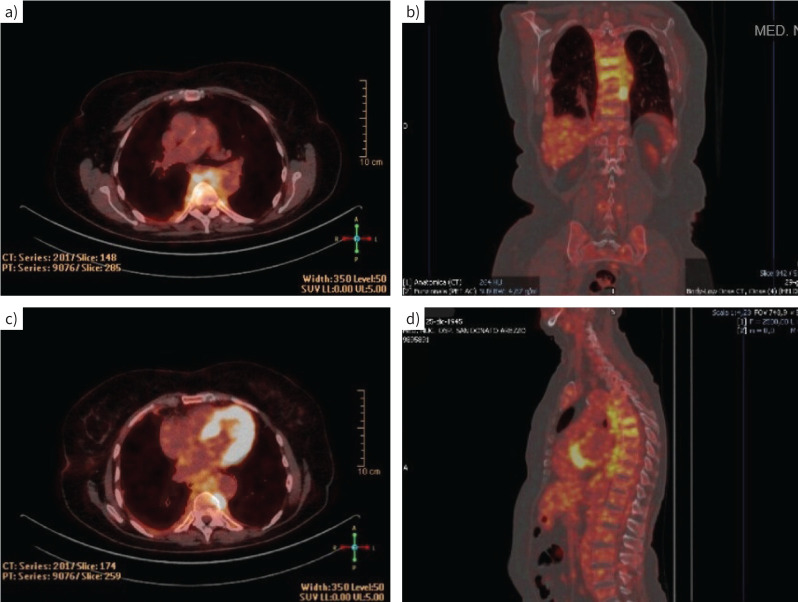
A full-body PET scan was performed to identify other possible sites of disease and to select the most appropriate biopsy test. PET examination revealed increased uptake corresponding to the tissue described in the CT scan in the dorsal paravertebral region between D4 and D11 (figure 3a–d), with a predominant area of marked accumulation on the left side of the D8–D9 passage (figure 3b, c). The total body CT scan and PET-CT did not detect any additional lesions.
FIGURE 3.
Positron emission tomography examination. a–d) Increased uptake corresponding to the tissue in the dorsal paravertebral region between D4 and D11. b, c) Area of marked accumulation on the left side of the D8–D9 passage.
In light of these findings and after a multidisciplinary discussion within a thoracic oncology group, the patient underwent medical thoracoscopy which was performed by the pulmonologist team with analgosedation and spontaneous breathing. During the procedure, after extracting 850 mL of serous pleural fluid, there was a notable improvement in lung expansion and nodular lesions in the parietal pleura were discovered (figure 4). Multiple biopsies were conducted at this site.
FIGURE 4.
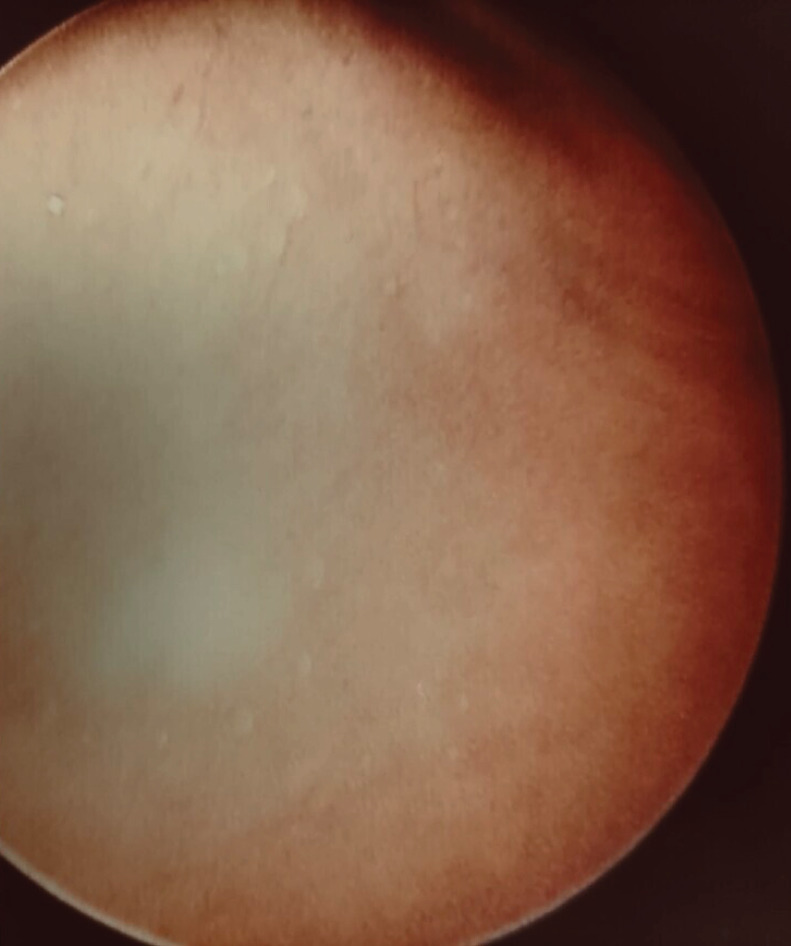
Small white-pale nodular lesions of the parietal pleura.
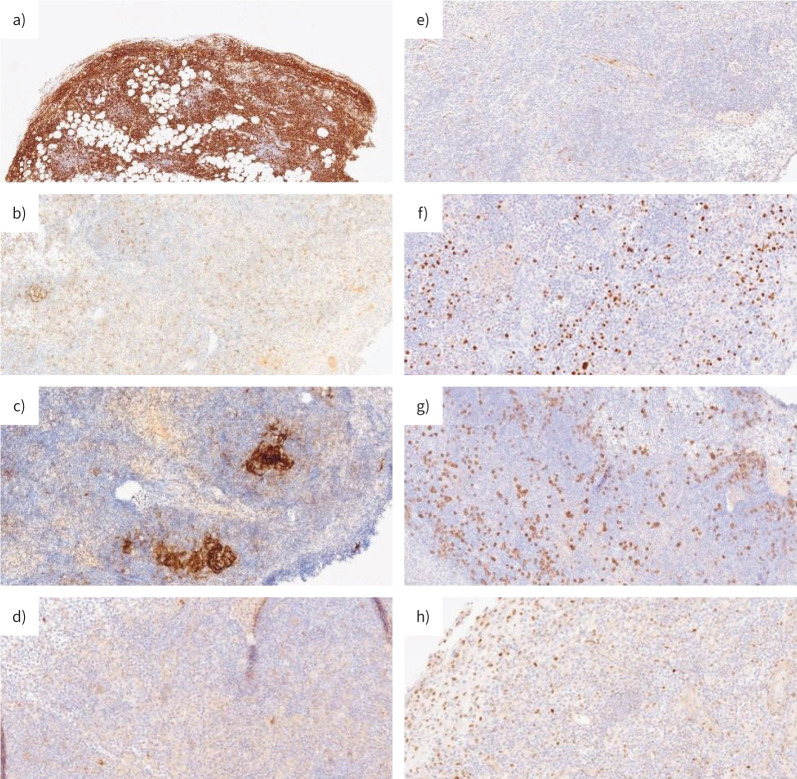
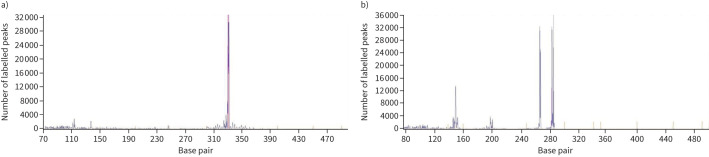
The histopathological examination of pleural specimens documented a significant lymphoid infiltrate of small/medium-sized B cell elements with images of follicular colonisation (CD20+, CD21−, CD23−, Cyclin D−, CD10−, MUM1−, CD138−; proliferative rate 10–20%) (figure 5). In addition, a residual reactive T lymphoid and plasma cellular component was observed. A demonstrable monoclonal rearrangement of immunoglobulin genes was present (figure 6). No positivity for human herpesvirus-8 (HHV-8) or Epstein–Barr virus (EBV) was documented.
FIGURE 5.
Immunohistochemical analysis of the lymphoid component of the lesion. a) Lymphoid cells were CD20 positive. CD20 stain demonstrates B-cell origin of the lymphoid diffuse infiltrate in pleural and subpleural tissue. Lymphoid cells were negative for b) CD21; c) CD10; d) CD23; e) Cyclin D; f) MUM-1; g) CD138; h) BCL-6 protein expression.
FIGURE 6.
GeneScan of immunoglobulin rearrangement in a B lineage. a) Monoclonal FR1–JH rearrangement; b) monoclonal VK–JH rearrangement.
Task 4
What is the most likely diagnosis based on the histological examination?
Primary effusion lymphoma (PEL)
Extranodal marginal zone lymphoma (EMZL)
Pyothorax-associated lymphoma (PAL)
Lymphoplasmocytic lymphoma (LPL)
Answer 4
b. The overall findings are consistent with peripheral B cell lymphoma, specifically a marginal zone B cell lymphoma of the pleura.
Task 5
Why perform HHV-8 and EBV testing?
Answer 5
In the presence of a rich lymphocyte infiltrate on histopathological examination it is essential to screen for HHV-8 and EBV, as well as HIV, hepatitis B virus (HBV) and Mycobacterium tuberculosis [5]. This will help in the differential diagnosis of lymphoid proliferations (table 1) [3]. PEL is typically associated with HHV-8 or HIV [6]; PAL is typically associated with EBV or tuberculosis pleuritis/pleural effusion [7]. Moreover, PEL does not always exhibit a distinct mass [8], unlike in our scenario. Lastly, as there was no extra-pleural involvement, this aspect supported the diagnosis of EMZL over LPL.
Finally, analyses of the autoimmune profile and an extensive microbiological panel (including HIV, quantiferon test and serological markers of hepatotropic viruses) were conducted to further investigate and support considerations for the differential diagnosis, with negative results. The patient received conservative management as the mild pleural effusion remained stable over time, thus obviating the need for an indwelling pleural catheter or other interventions.
Discussion
EMZL (also known as mucosa-associated lymphoid tissue (MALT) lymphoma) of the pleura is a very rare type of non-Hodgkin lymphoma described in the literature mostly as case reports. It predominantly affects the elderly and accounts for <1% of such lymphomas [9, 10]. Like other MALT lymphomas, it is believed that lymphomas of primary pleural origin may be triggered by prolonged antigenic stimulation, such as tuberculosis or other causes of chronic pleuritis [11].
In most cases, pleural involvement is secondary to lymphomas originating primarily from other sites [9]. In some cases, it occurs after some time following the diagnosis of a previous lymphoma. Finally, there is often an association between pleural lymphoma and systemic diseases (autoimmune- or immunodeficiency-related diseases, especially for PEL) [9, 11]. Historically, PAL originated from persistent pyothorax arising from the use of artificial pneumothorax in the treatment of pulmonary tuberculosis or tuberculous pleuritis [12].
This emphasises the importance of a thorough investigation into potential underlying factors that may have contributed to the development of EMZL in this specific patient. However, the examinations performed did not find any type of factor correlated with lymphoproliferative pathology. In particular, there was no evidence of innate or acquired immunosuppression. The negative results of the HIV blood test (both antibody and viral load RNA-PCR) and negative results for HHV-8/EBV in the biopsy samples allowed us to rule out PEL and PAL [6].
The clinical course of pleural MALT lymphoma generally appears to be favourable, similar to other MALT lymphomas [13]. However, the prognosis depends on the degree of differentiation observed in histological examinations and the stage of the disease at the time of diagnosis [10]. Diagnosis can sometimes be delayed due to the lymphoma's slow growth and indolent course [13]. Various treatment approaches have been used in documented cases, including radiotherapy, surgery, and chemotherapy, but it lacks a standard recommended therapy [14].
TABLE 1.
Differential diagnosis of lymphoid proliferations
| Condition | Patient presentation | Radiological features | Microbiological correlation |
|---|---|---|---|
| Primary pleural lymphoma (non-Hodgkin lymphoma) | Patient can suffer from autoimmune diseases or chronic inflammation; in some cases, patients have no history of medical conditions | Pleural nodules, pleural thickenings, mediastinal mass, thoracic lymphadenopathy; pleural effusion can be unilateral (sometimes as chylothorax) | Unclear/none in particular |
| Primary effusion lymphoma (PEL) | Immunocompromised, transplanted or cirrhosis patients | Absence of nodules or masses; pericardial and peritoneal involvement | HHV-8, HIV, HBV, others |
| Pyothorax-associated lymphoma (PAL) | Patients may have history of chronic pleuritis or empyema, pleural TB (especially past artificial pneumothorax therapy) or immunocompromised patients with EBV infection | Pleural mass, invasion of surrounding tissue (ribs), empyema | EBV, Mycobacterium tuberculosis, others |
HHV-8: human herpesvirus-8; HBV: hepatitis B virus; EBV: Epstein–Barr virus; TB: tuberculosis.
Conclusion
In conclusion, the patient's diagnostic journey, initiated by a mild pleural effusion following a car accident, led to the unexpected discovery of a rare condition. Despite the absence of predisposing or risk factors, a comprehensive evaluation, beginning with a chest ultrasound followed by a medical thoracoscopy and finally extensive laboratory tests, led to the diagnosis of primary pleural lymphoma of EMZL type.
Finally, we want to highlight the role of medical thoracoscopy as an effective diagnostic tool in lymphoma especially in view of the possibility of obtaining a high-quality biopsy specimen that, when combined with the pathologist's expertise, greatly aids in achieving a diagnosis [15].
It is essential that every new-onset pleural effusion be thoroughly investigated until the exact cause is identified. Given the many aetiologies of pleural effusions, ranging from benign to malignant conditions, accurate assessment helps to ensure timely initiation of targeted therapies and supportive care strategies. The need to exclude malignancy as the origin of the effusion with medical thoracoscopy emphasises the critical role of diagnostic accuracy of this minimally invasive procedure handled autonomously by a pulmonologist team in optimising patient outcomes [16].
Key points
Primary pleural lymphoma may present with unilateral, anechoic, exudative pleural effusion.
Medical thoracoscopy can lead to the diagnosis of pleural lymphoma.
The differential diagnosis with other conditions, such as PEL and PAL, requires laboratory investigations including autoimmune profiling and microbiological research (HIV, HHV, HBV, interferon-γ release assays).
Footnotes
Consent: Written informed consent was obtained from the patient.
Conflict of interest: The authors have no conflict of interest to declare.
References
- 1.de Pereira AEA, Terra RM, Teixeira LR, et al. Recurrent post-traumatic non-eosinophilic pleural effusion: report of three cases. Clinics 2008; 63: 414–415. doi: 10.1590/S1807-59322008000300023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bovy V, De Keukeleire T, Van Schoote E, et al. Post-traumatic pleural effusion: don't forget the chylothorax! Respirol Case Rep 2024; 12: e01274. doi: 10.1002/rcr2.1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012; 38: 577–591. doi: 10.1007/s00134-012-2513-4 [DOI] [PubMed] [Google Scholar]
- 4.Yang PC, Luh KT, Chang DB, et al. Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol 1992; 159: 29–33. doi: 10.2214/ajr.159.1.1609716 [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Farre B, Martinez D, Lopez-Guerra M, et al. HHV8-related lymphoid proliferations: a broad spectrum of lesions from reactive lymphoid hyperplasia to overt lymphoma. Mod Pathol 2017; 30: 745–760. doi: 10.1038/modpathol.2016.233 [DOI] [PubMed] [Google Scholar]
- 6.Shimada K, Hayakawa F, Kiyoi H. Biology and management of primary effusion lymphoma. Blood 2018; 132: 1879–1888. doi: 10.1182/blood-2018-03-791426 [DOI] [PubMed] [Google Scholar]
- 7.Aozasa K, Takakuwa T, Nakatsuka S. Pyothorax-associated lymphoma: a lymphoma developing in chronic inflammation. Adv Anat Pathol 2005; 12: 324–331. doi: 10.1097/01.pap.0000194627.50878.02 [DOI] [PubMed] [Google Scholar]
- 8.Gathers DA, Galloway E, Kelemen K, et al. Primary effusion lymphoma: a clinicopathologic perspective. Cancers 2022; 14: 722. doi: 10.3390/cancers14030722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vega F, Padula A, Valbuena JR, et al. Lymphomas involving the pleura: a clinicopathologic study of 34 cases diagnosed by pleural biopsy. Arch Pathol Lab Med 2006; 130: 1497–1502. doi: 10.5858/2006-130-1497-LITPAC [DOI] [PubMed] [Google Scholar]
- 10.Zucca E, Roggero E, Bertoni F, et al. Primary extranodal non-Hodgkin's lymphomas. Part 2: Head and neck, central nervous system and other less common sites. Ann Oncol 1999; 10: 1023–1033. doi: 10.1023/A:1008313229892 [DOI] [PubMed] [Google Scholar]
- 11.Bende RJ, van Maldegem F, van Noesel CJM. Chronic inflammatory disease, lymphoid tissue neogenesis and extranodal marginal zone B-cell lymphomas. Haematologica 2009; 94: 1109–1123. doi: 10.3324/haematol.2009.005983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aozasa K. Pyothorax-associated lymphoma. J Clin Exp Hematop 2006; 46: 5–10. doi: 10.3960/jslrt.46.5 [DOI] [PubMed] [Google Scholar]
- 13.Motta G, Conticello C, Amato G, et al. Pleuric presentation of extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue: a case report and a review of the literature. Int J Hematol 2010; 92: 369–373. doi: 10.1007/s12185-010-0645-2 [DOI] [PubMed] [Google Scholar]
- 14.Wang YH, Peng L, Jiang JH, et al. Misdiagnosis of primary mucosa-associated lymphoid tissue lymphoma of the pleura: case report and literature review. Transl Cancer Res 2022; 11: 3315–3321. doi: 10.21037/tcr-22-671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Wu YB, Xu LL, et al. Diagnostic value of medical thoracoscopy in malignant pleural effusion induced by non-Hodgkin's lymphoma. Oncol Lett 2017; 14: 8092–8099. doi: 10.3892/ol.2017.7226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allocca V, Guidelli L, Galgano A, et al. Safety and diagnostic yield of medical pleuroscopy (MP) performed under balanced analgosedation by a pneumological team compared to video-assisted thoracic surgery (VATS): a retrospective controlled real-life study (TORAPO). Diagnostics 2024; 14: 569. doi: 10.3390/diagnostics14060569 [DOI] [PMC free article] [PubMed] [Google Scholar]