Abstract
The 2017 and 2018 wildfire seasons in British Columbia (BC), Canada were unprecedented. Among all the pollutants in wildfire smoke, fine particulate matter (PM2.5) poses the most significant risk to human health. There is limited research on prenatal wildfire smoke exposure and its impacts on infant health. We used a population-based nested case-control design to assess the association between daily PM2.5 exposures during specific developmental windows and the occurrence of otitis media or lower respiratory infections by age 1 year, including infections associated with dispensations of the antibiotic amoxicillin. We observed the strongest association between per 10 μg/m3 increase in PM2.5 exposure and otitis media during the fourth window of eustachian tube development (weeks 19–28) with an OR [95% confidence interval] of 1.31 [1.22, 1.41]. Similarly, the canalicular stage of lower respiratory tract development (weeks 18–27) was associated with the highest odds of lower respiratory infections, with an OR of 1.21 [1.15, 1.28]. Measures to reduce wildfire smoke exposure during pregnancy are warranted.
Keywords: wildfire smoke, PM2.5, prenatal exposure, respiratory infections, infant health
Short abstract
This study suggests that wildfire smoke exposure during specific developmental windows can affect respiratory health in early life. Public health practitioners and healthcare providers should work to protect pregnant people and their children from the detrimental effects of wildfire smoke.
Introduction
Exposure to air pollution during pregnancy has been linked to negative effects in early life, such as cardiovascular and metabolic outcomes, respiratory and allergic responses, and neurodevelopmental impairments.1 Air pollution can directly impact pregnancy through dispersion across the tissue barriers and permeation across the cellular membrane of the placenta.2 Indirectly, oxidative stress and inflammatory reactions in mothers can reduce fetal nutrient and oxygen supply.2,3 Interactions between air pollution and the epigenome can also lead to adverse early life outcomes, though the mechanisms are not completely understood.3
Wildfire smoke is a complex mixture of organic and inorganic gases, and particulate matter. Fine particulate matter measuring less than 2.5 mm in aerodynamic diameter (PM2.5) is generally accepted to be the most health-relevant marker of wildfire smoke. Compared with other PM2.5 sources such as traffic and industry, PM2.5 from wildfires is far more episodic and the magnitudes of the exposure can be very high. Studies have shown that PM2.5 from wildfires may be more toxic4 and harmful to human respiratory health than ambient PM2.5 from other sources.5,6
While there are many studies reporting on the association between prenatal PM2.5 expsoure and health outcomes in early life, they focus mainly on conditions such as asthma and wheezing.7−10 Very few have examined respiratory infections as the primary outcome,11,12 and even fewer have examined wildfire smoke as the dominant source of PM2.5 exposure.13 One recent study in Australia found that a small cohort of infants prenatally exposed to smoke from a coal mine fire had more respiratory symptoms and diagnoses of respiratory tract infections in early life than an unexposed group.14
Acute respiratory infections are the leading cause of morbidity and mortality in early childhood, even in high-income countries.15 According to one study conducted in Canada, respiratory infections can impose substantial strain on acute care infrastructure and the medical system, and the burden is expected to increase with population growth.16 Respiratory infections are also the primary reason for antibiotic use in early life, which can have long-lasting impacts on the infant microbiome and childhood respiratory health.17 The most prevalent infections involving antibiotic treatment are acute otitis media, bronchitis, and upper respiratory tract infections.18
Exposure to wildfire smoke during pregnancy, or certain windows of pregnancy, may increase the risk of infant respiratory infections in early life. It follows that limiting exposure to wildfire smoke during pregnancy could protect infant health. To address this important public health question, our study focuses on the critical stages of gestational development for parts of the upper and lower respiratory tract associated with the two most-diagnosed respiratory infections: otitis media (i.e., middle ear infections) and lower respiratory infections.
Otitis media is an upper respiratory infection that frequently requires extensive pediatric care in the early stages of life. It is characterized by acute or chronic inflammation of the middle ear, resulting from Eustachian tube dysfunction.19,20 Lower respiratory infections include bronchitis, bronchiolitis, and pneumonia, all of which can lead to serious complications. Most lower respiratory infections in children are caused by bacteria, but they can also be caused by viruses and fungi.21 Damage and changes to the upper and lower respiratory tracts during fetal development may lead to higher risk of respiratory diseases, including acute and chronic infections.22−24
Our study aimed to investigate the associations between prenatal PM2.5 exposure and respiratory infections during the first year for infants in utero during two severe (2017 and 2018) and two below-average (2016 and 2019) wildfire seasons using a population-based case-control design. We focused on specific stages of prenatal respiratory tract development and gestational trimesters to identify the periods of highest maternal risk.
Methods
Study Setting and Population
This study is set in the province of British Columbia (BC), on the west coast of Canada. Like other parts of western North America, BC is heavily forested and prone to seasonal wildfires,25 especially from July through September.26 The total area of BC is almost 1 million square kilometres. The 2016 population was approximately 5 million residents, with most living in large urban centers in the southern coastal areas and the rest living in smaller urban, rural, or remote areas throughout the interior and the north (Figure 1A).
Figure 1.
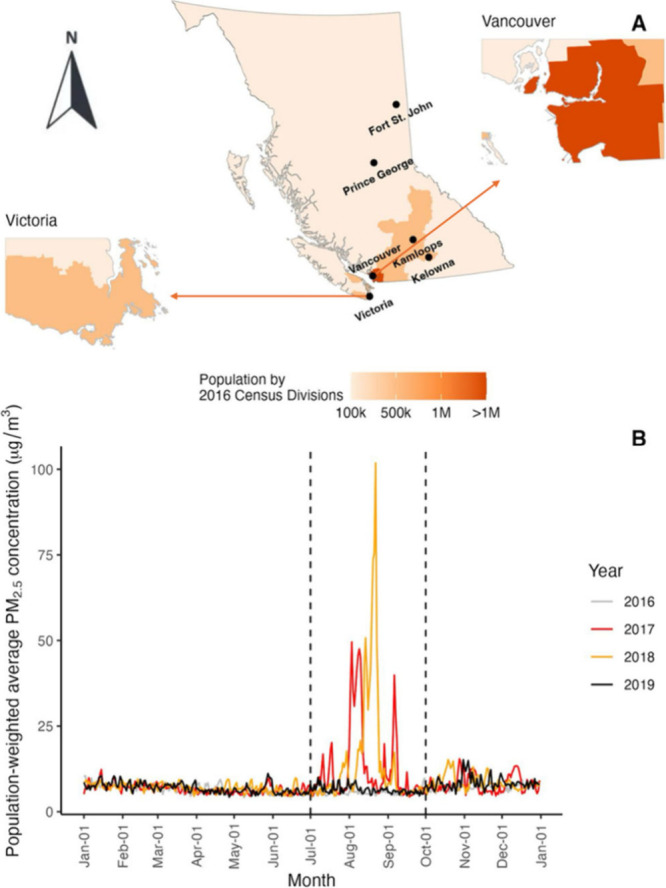
Population of British Columbia by 2016 Census Divisions (panel A) and daily outdoor population-weighted average fine particulate matter (PM2.5) concentrations during the study period (panel B). The dashed lines show the wildfire seasons from July to September.
In this study we focused on the wildfire seasons from 2016 to 2019 because of the contrasts between these four years. The 2017 and 2018 wildfire seasons were both record-setting, with more than 1.2 million hectares burned in 2017 and more than 1.3 million burned in 2018.26,27 In both summers, the smoke impacts were widespread and prolonged (Figure 1B). By contrast, the 2016 and 2019 wildfire seasons were below the 10-year average, with less than 0.13 million hectares burned in both years, and the smoke impacts were limited.27 The study included singleton births for whom the expected 40-week gestational term intersected with any part of the 2016–2019 wildfire seasons (July–September), even if the infant was not carried to term. Given an average gestation period of 38 weeks, this includes most infants born in BC between July 2016 and June 2020.
Health Data Sources
All health data were accessed through the Population Data BC research platform. The population-based cohort was identified using the BC Perinatal Data Registry (BCPDR). The BCPDR compiles extensive data on mothers, fetuses, and newborns, covering 99% of all births in the province.28 Most infants enter the registry at approximately 15 weeks of gestation. The BCPDR provides comprehensive information on delivery and the postpartum period. It also includes many maternal covariates, such as BMI and smoking status, which are described in more detail in the covariates section to follow. For infants, the BCPDR includes birthdate and estimated date of conception (determined by maternal last menstrual period, and/or ultrasound),29 which facilitates precise exposure assessment for critical developmental windows. Finally, the BCPDR includes the residential forward sortation area (FSA) of the mother, which is the first three characters of the 6-digit postal code.
BC has a single-payer healthcare insurance program called the Medical Services Plan (MSP), which covers most people in the province.30 The MSP client roster was used to create a weekly residential history for mothers during their pregnancy and for infants during their first year of life. The residential histories were created using 6-digit postal codes. In urban areas, each 6-digit postal code typically covers one building or one city block, but in rural settings they cover larger areas. In all cases, we used the geographic coordinates of the single link indicator, which indicates the location of most of the population within the postal code.
We used the MSP outpatient physician billings file to identify all infants who were diagnosed with otitis media or a lower respiratory infection by age 1 year. All prescription medications dispensed by community pharmacies in BC are recorded in the PharmaNet database.31 By linking the MSP billings with PharmaNet records for each infant, we were able to identify infections that were associated with a subsequent antibiotic dispensation, as described in further detail in the outcomes section to follow.
Exposure
The Canadian Optimized Statistical Smoke Exposure Model (CanOSSEM) is an empirical framework that estimates daily population exposure to ambient PM2.5 across Canada from 2010 to 2022 at a resolution of 5 km × 5 km for all inhabited areas.32 The model was built using PM2.5 values from over 300 air quality monitoring stations in the National Air Pollution Surveillance (NAPS) program, which encompasses both rural and urban areas. The predictive variables include daily smoke plumes from remote sensing images, measurements of Aerosol Optical Depth, measurements of fire radiative power, and meteorological parameters such as temperature, humidity, and wind speed from satellite products.32 While CanOSSEM is optimized to capture the air quality impacts of wildfires, its daily estimates reflect total PM2.5. CanOSSEM demonstrates excellent performance, with approximately 98% of estimated concentrations within 5 μg/m3 of the observed PM2.5 value for the validation data set.32
Daily estimated PM2.5 concentrations for each 6-digit postal code were derived from CanOSSEM. The estimates were then matched with the residential histories created from the MSP client roster and BCPDR records, resulting in daily maternal (from conception to the birthdate) and infant (from the birthdate to age 1 year) PM2.5 exposure history. The maternal and infant residential histories were created by identifying a continuous set of 6-digit postal codes covering every day of pregnancy or the first year of life. If there were duplicate records for any date, we always chose the 6-digit postal code that matched the 3-digit FSA in the BCPDR for the main analysis. As a sensitivity analysis, we always chose the 6-digit postal code that did not match the 3-digit FSA, which resulted in slightly different exposure estimates.
Outcomes
The cases included any infant who had a diagnosis of otitis media or lower respiratory infection by age 1 year. The controls included all infants who did not have either of these diagnoses by age 1 year. The controls were matched with the cases based on the FSA and epidemiologic week of birth. Diagnoses of otitis media and lower respiratory infections were identified from the MSP outpatient physician billings filed using the International Classification of Diseases, ninth Revision (ICD-9) codes. We used MSP billings for specific codes to identify otitis media (ICD-9 codes 381–382) and lower respiratory infections (ICD-9 codes 466, 480–487). Subsequent dispensations of amoxicillin were identified from PharmaNet. We only included dispensations of amoxicillin because it was the most prescribed antibiotic in the cohort and it is preferred medication for treating infant otitis media and lower respiratory infections in Canada based on clinical guidelines.33,34 We included any amoxicillin dispensation within 7 days of a physician visit for a respiratory infection.
Covariates
Analyses were adjusted for several potentially confounding variables from the BCPDR and MSP, reflecting maternal and infant health. The selection of confounding variables was guided by a Directed Acyclic Graph (DAG) using the disjunctive cause criteria35 (Figure S1). Maternal covariates included: age at delivery; BMI category; maternal smoking status; and neighborhood socioeconomic status (SES) quintile, all of which have been identified as potential confounders in prior research.36−38 Infant variables included indicators for biological sex and type of feeding.37,39,40 Missing values of categorical variables were addressed by including an unknown category. We did not adjust for mediators, including preterm birth and low birthweight, to avoid introducing overadjustment bias.41
Analyses were also adjusted for temperature during pregnancy42 and postnatal exposure to PM2.5,43 which are also associated with an increased risk of respiratory illness. The postnatal PM2.5 exposure for the each case was averaged from birth until the first diagnosis of otitis media or lower respiratory infection. For the controls, postnatal exposure was calculated based on the timing of the first diagnosis observed in each matched case. We used the same temperature data included in CanOSSEM, which were derived from the NASA Modern-Era Retrospective Analysis for Research and Applications, Version 2 (MERRA-2).32
Exclusion Criteria
We excluded infants missing 25% or more days of prenatal PM2.5 or temperature exposure due to incomplete residential histories or missing CanOSSEM estimates. The proportion of missing data was calculated for the whole pregnancy, and for each critical exposure window. Similarly, infants missing more than 25% of postnatal PM2.5 exposure data were excluded. We also excluded infants who could not be matched with the MSP and PharmaNet data sets, and those missing values for maternal age.
Critical Developmental Windows
For otitis media, the critical windows of Eustachian tube development were defined as 0–9 weeks (stage 1); 10–12 weeks (stage 2); 13–18 weeks (stage 3); 19–28 weeks (stage 4); and >28 weeks (stage 5).44 For the lower respiratory tract the developmental windows were defined as 0–7 weeks (embryonic stage); 8–17 weeks (pseudoglandular stage); 18–27 weeks (canalicular stage); 28–36 weeks (saccular stage); and >37 (alveolar stage).45,46 We also examined the effects of each gestational trimester 0–13 weeks (Trimester 1); 14–27 weeks (Trimester 2) ; >27 weeks (Trimester 3).47,48 These critical time windows were identified from the existing literature based on significant physiological changes in both pregnant individuals and their fetuses (Table S1).
Statistical Analysis
We used a population-based nested case-control design to address confounding by seasonality, which is associated with both wildfire smoke exposures (higher in summer months) and respiratory infections (higher in winter months). All infants in the cohort were eligible to be included in the analyses as either cases (i.e., otitis media or lower respiratory infection by age 1 year) or controls (i.e., no infection by age 1 year). We used conditional logistic regression to quantify the association between prenatal PM2.5 and the outcomes, conditioned on the epidemiologic week of birth and FSA. Epidemiologic weeks allow comparison of population health trends between years, with the first epidemiologic week of the year beginning on the first Sunday in January. Some studies suggest conditioning on conception date rather than birthdate to address seasonality,49 and we did this as a sensitivity analysis. We also added an effect modification term to assess differences in the PM2.5 effect estimates between years with severe wildfire seasons (2017 and 2018) and years with below-average wildfire seasons (2016 and 2019).
We used the daily average estimates of PM2.5 in each prenatal exposure window as the main predictor variable. The dependent outcome variables were all otitis media infections, all lower respiratory infections, and the otitis media or lower respiratory infections associated with amoxicillin dispensations. All estimates were adjusted for the covariates described in the previous section (eq 1).
 |
1 |
where “Infection” is a binary indicator of infection status by age 1 year; “PM2.5-window” is the average exposure in the corresponding developmental window or trimester, and separate analyses are executed for each developmental window or trimester; “postnatal PM2.5” is the average exposure from birth to time of infection; “Temp-window” is the average temperature in the corresponding developmental window or trimester; “sex” categorizes the biological sex of the infant as male, female or unknown; “feeding” indicates the type of feeding given to the newborn during the entire hospital stay and at discharge, which includes exclusive breast milk, breast milk and formula, and formula; “age” indicates maternal age at delivery in years; “smoking” categorizes maternal smoking status as never smoker, former smoker, or current smoker; “BMI” indicates whether the mother was underweight (less than 18.5), normal (18.5 to 24.9), overweight (25.0 to 29.9), or obese (30 or greater);50 and “SES” represents maternal neighborhood income quintiles (1 = most deprived to 5 = least deprived) during pregnancy. Finally, “PM2.5-window: Wildfire year” is an interaction term to analyze the effect of “PM2.5” by years with severe wildfire seasons (2017 and 2018) and years with below-average wildfire seasons (2016 and 2019) in each developmental window or trimester. All data preparation and statistical analysis were done using R 4.0.5.51
Ethics Approval
This work received approval from the UBC Research Ethics Board (Certificate number: H20-01077) and Population Data BC (approval number: 20-197).
Results and Discussion
Descriptive Summary
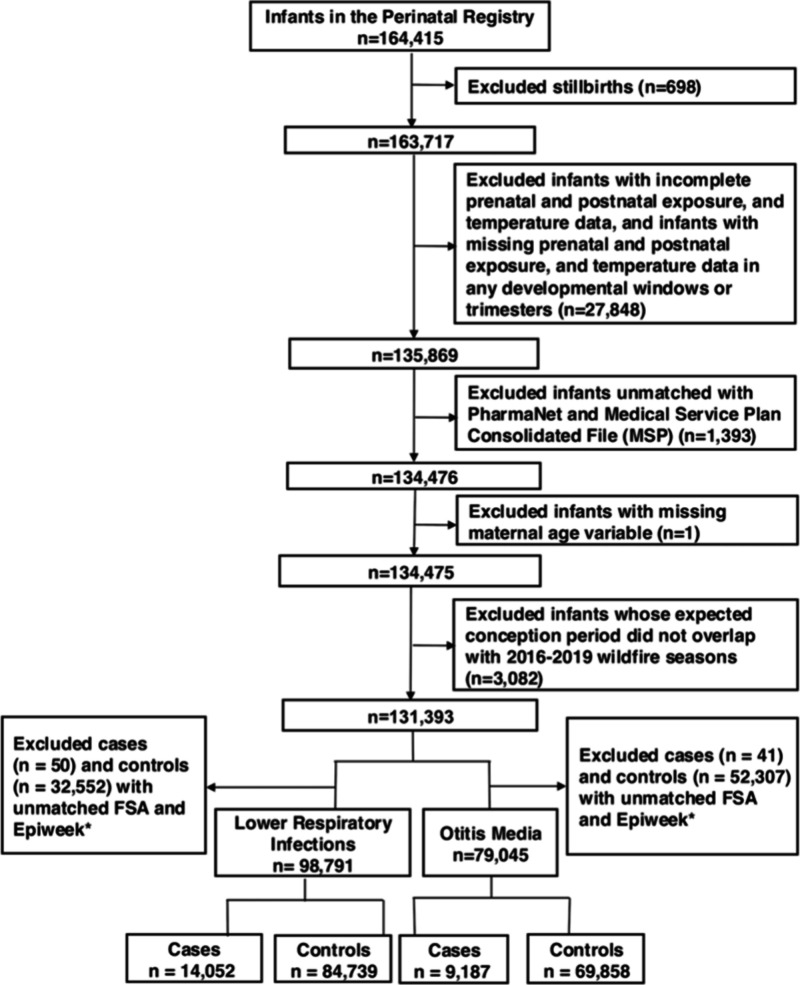
There were 164415 singleton births in BC for whom the expected 40-week gestational window intersected with the wildfire seasons (July–September) of 2016 to 2019. Of these, 131393 were eligible for inclusion in the study and matched to 119103 unique mothers (Figure 2). After matching on FSA and epidemiological week of birth, 79045 were included in the otitis media analyses, and 98791 were included in the lower respiratory infection analyses. Maternal characteristics for these groups (Tables 1 and 2) were calculated based on each pregnancy. The following infants were excluded due to not meeting the eligibility criteria (Figure 2): stillbirths (n = 698); infants missing too much PM2.5 or temperature data (n = 27848); infants that could not be matched to the MSP outpatient physician billings or PharmaNet files (n = 1393); and infants with missing maternal age (n = 1). A further 52348 infants were eligible for otitis media analyses and 32602 were eligible for lower respiratory infection analyses, but excluded because they could not be matched to any case by FSA and epidemiological week of birth.
Figure 2.
Flowchart for the inclusion and exclusion of infants in the population-based cohort covering all the infants prenatally exposed to wildfire seasons from 2016 to 2019. *Epiweek means epidemiologic week of the year, where the first epidemiologic week of the year starts on the first Sunday in January.
Table 1. Characteristics of Otitis Media Cases and Controls and Their Mothersa.
| cases | controls | ||
|---|---|---|---|
| N = 9187b | N = 69858 | p-valuec | |
| mean (SD) of average weekly PM2.5 during pregnancy (μg/m3) | 8.1 (2.1) | 8.0 (2.0) | p < 0.001 |
| mean (SD) of maximum 1-week PM2.5 during pregnancy (μg/m3) | 30.1 (29.5) | 28.0 (28.2) | p < 0.001 |
| mean (SD) PM2.5 in first year of life (μg/m3) | 8.5 (2.3) | 8.3 (2.1) | p < 0.001 |
| mean (SD) temperature during pregnancy (°C) | 8.0 (3.8) | 8.5 (3.7) | p < 0.001 |
| Infant sex (%) | |||
| female | 43.6% | 50.2% | p < 0.001 |
| male | 56.4% | 49.8% | |
| other | 0% | <0.1% | |
| Maternal neighborhood SES quintile (%) | |||
| first (most deprived) | 20.7% | 20.5% | P = 0.201 |
| second | 21.2% | 21.3% | |
| third | 20.5% | 20.7% | |
| fourth | 20.9% | 21.7% | |
| fifth (least deprived) | 15.3% | 14.6% | |
| missing | 1.4% | 1.3% | |
| mean (SD) maternal age at birth (years) | 31.2 (5.2) | 31.8 (5.0) | p < 0.001 |
| Maternal BMI category (%) | |||
| Underweight | 3.3% | 4.1% | p < 0.001 |
| Normal | 41.0% | 45.6% | |
| Overweight | 20.0% | 19.0% | |
| Obese | 15.6% | 12.2% | |
| Missing | 20.3% | 19.1% | |
| Maternal smoking (%) | |||
| current smoker | 6.4% | 4.9% | p < 0.001 |
| former smoker | 8.3% | 7.1% | |
| never smoked | 85.2% | 88.0% | |
| Newborn feeding (%) | |||
| exclusive breast milk | 30.2% | 29.6% | P = 0.007 |
| breast milk and formula | 66.0% | 67.3% | |
| formula | 2.5% | 2.1% | |
| missing | 1.2% | 1.0% |
Subjects were prenatally exposed to wildfire seasons from 2016 to 2019 and matched on geography and epidemiologic week of birth.
6345 infants were dispensed amoxicillin after being diagnosed with otitis media.
A two-sample t test was used to compare continuous variables, a chi-square test was used to compare categorical variables when all expected cell counts were ≥5, and Fisher’s exact test was used when any expected cell counts were <5 for the covariates in cases and controls.
Table 2. Characteristics of Lower Respiratory Infection Cases and Controls and Their Mothersa.
| cases | controls | ||
|---|---|---|---|
| N = 14052b | N = 84739 | p-valuec | |
| mean (SD) of average weekly PM2.5 during pregnancy (μg/m3) | 8.0 (2.1) | 7.9 (2.0) | p < 0.001 |
| mean (SD) of maximum 1-week PM2.5 during pregnancy (μg/m3) | 28.8 (28.8) | 27.9 (27.6) | p < 0.001 |
| mean (SD) PM2.5 in first year of life (μg/m3) | 8.3 (2.6) | 8.3 (2.3) | p = 0.047 |
| mean (SD) temperature during pregnancy (°C) | 8.7 (3.6) | 8.7 (3.5) | p = 0.007 |
| Infant sex (%) | |||
| female | 42.0% | 50.1% | p < 0.001 |
| male | 58.0% | 49.9% | |
| other | 0% | <0.1% | |
| Maternal neighborhood SES quintile (%) | |||
| first (most deprived) | 21.8% | 21.1% | P = 0.082 |
| second | 21.0% | 21.1% | |
| third | 20.1% | 20.8% | |
| fourth | 20.8% | 21.3% | |
| fifth (least deprived) | 14.8% | 14.3% | |
| missing | 1.4% | 1.4% | |
| mean (SD) maternal age at birth (years) | 31.5 (5.2) | 31.9 (5.0) | p < 0.001 |
| Maternal BMI category (%) | |||
| underweight | 3.7% | 4.3% | p < 0.001 |
| normal | 41.1% | 45.9% | |
| overweight | 19.4% | 18.6% | |
| obese | 14.4% | 11.7% | |
| missing | 21.3% | 19.5% | |
| Maternal smoking (%) | |||
| current smoker | 6.6% | 4.5% | p < 0.001 |
| former smoker | 7.9% | 6.9% | |
| never smoked | 85.5% | 88.6% | |
| Newborn feeding (%) | |||
| exclusive breast milk | 31.6% | 29.4% | p < 0.001 |
| breast milk and formula | 64.2% | 67.7% | |
| formula | 3.1% | 1.9% | |
| missing | 1.2% | 0.9% |
Subjects were prenatally exposed to wildfire seasons from 2016 to 2019 and matched on geography and epidemiologic week of birth.
3154 infants were dispensed amoxicillin after being diagnosed with lower respiratory infection.
A two-sample t test was used to compare continuous variables, a χ-square test was used to compare categorical variables when all expected cell counts were ≥5, and Fisher’s exact test was used when any expected cell counts were <5 for the covariates in cases and controls.
There were 9187 cases of otitis media matched with 69858 controls, of which 6345 were associated with amoxicillin dispensations. All otitis media cases were matched to at least one control, with a median of 15 controls and a maximum of 82 controls. There were 14052 cases of lower respiratory infections matched with 84739 controls, of which 3154 were associated with amoxicillin dispensations. All lower respiratory infection cases were matched to at least one control, with a median of 16 and a maximum of 82.
Significant differences (p-value < 0.05) were observed in the distributions of all covariates and between cases and controls, except for SES (Tables 1 and 2). The largest differences were male infant sex (56.4% cases of otitis media, 49.8% controls of otitis media; 58.0% cases of lower respiratory infections, 49.9% controls of lower respiratory infections) and smoking among mothers (14.7% cases of otitis media, 12% controls of otitis media; 14.5% cases of lower respiratory infections, 11.4% of lower respiratory infections). Small but statistically significant differences between groups were also observed in the PM2.5 and temperature exposures.
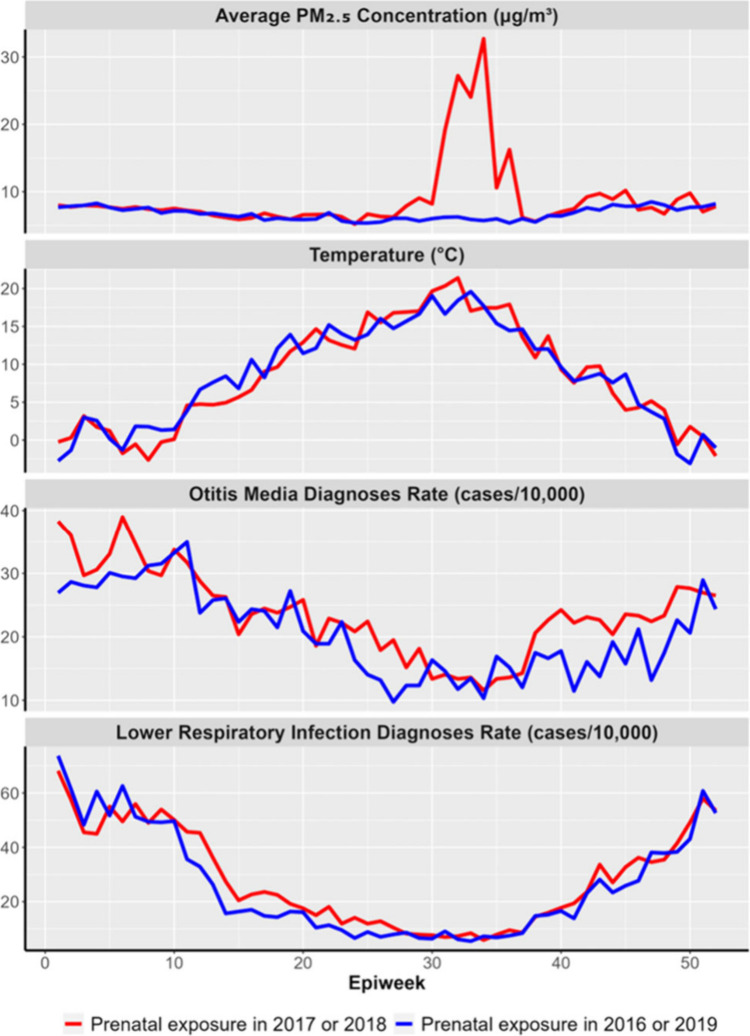
Prenatnal PM2.5 exposure, temperature exposure, and respiratory infections all showed clear seasonal trends by epidemiologic week across the study period. Prenatal PM2.5 and temperature peaked in midsummer, while the number of infections peaked in the winter months. Furthermore, there were clear differences in prenatal PM2.5 exposure and outcomes among the infants prenatally exposed during the severe 2017 and 2018 wildfire seasons compared with those exposed during the below-average 2016 and 2019 seasons (Figure 3).
Figure 3.
Time trends of exposure and outcomes of interests by epidemiologic week (epiweek) for infants prenatally exposed during the severe 2017 and 2018 wildfire seasons (red) and the below-average 2016 and 2019 seasons (blue).
Prenatal Exposure to PM2.5 and Otitis Media
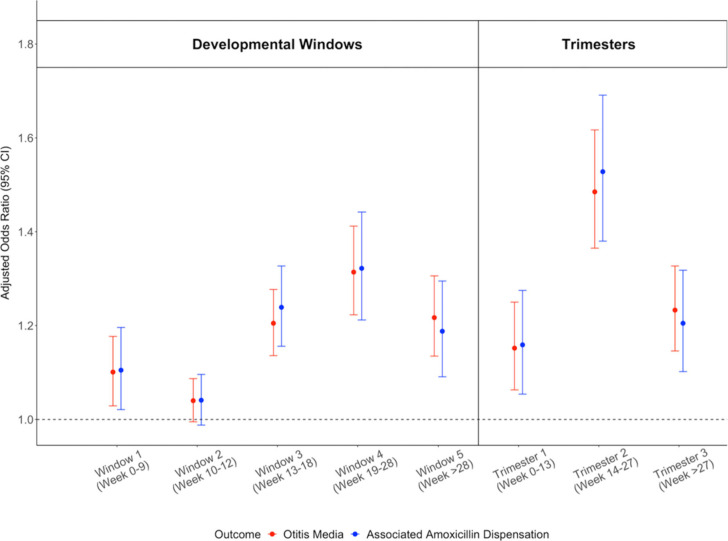
Exposure to PM2.5 in the fourth stage (19–28 weeks) of Eustachian tube development was most strongly associated with increased odds of otitis media diagnosis and associated amoxicillin dispensations. The odds ratio (OR) [95% confidence interval] for a 10 μg/m3 increase in average PM2.5 exposure during the fourth window was 1.31 [1.22, 1.41] for all diagnoses and 1.32 [1.21, 1.44] for those associated with amoxicillin dispensations. The second and fifth windows were also associated with increased odds of otitis media and associated amoxicillin dispensation (Figure 4).
Figure 4.
Adjusted odds ratios for prenatal exposure to PM2.5 and all diagnoses of otitis media in the first year of life (red), as well as otitis media diagnoses with associated amoxicillin dispensations (blue). Plot shows the effects of exposures during critical windows of Eustachian tube development (left) and trimesters (right).
Similarly, the second trimester (14–27 weeks) was most strongly associated with increased odds of otitis media diagnosis and associated amoxicillin dispensations by age 1 year. The OR for a 10 μg/m3 increase in PM2.5 was 1.49 [1.37, 1.62] for otitis media diagnosis and 1.53 [1.38, 1.69] for diagnoses with associated amoxicillin dispensations. Increased odds of otitis media and associated amoxicillin dispensation were also associated with the first and third trimesters (Figure 4). The sensitivity analyses using exposure estimates from alternate residential histories and conditioned on epidemiologic week of conception produced very similar results (Figure S3). The models had a significant interaction (p-value < 0.005) between PM2.5 and year of exposure, with high ORs for all developmental windows in the extreme 2017 and 2018 seasons and protective associations for the below-average 2016 and 2019 seasons (Table 3).
Table 3. Effect of Prenatal PM2.5 Exposure on Otitis Media and Lower Respiratory Infections between Years with Extreme Wildfire Seasons in 2017 and 2018 and Below-Average Wildfire Seasons in 2016 and 2019.
| extreme seasons (2017 and 2018) | below-average seasons (2016 and 2019) | p-value | |
|---|---|---|---|
| Otitis Media | |||
| Developmental Windows | |||
| window 1 (0–9 weeks) | 1.76 [1.62, 1.90] | 0.56 [0.50, 0.63] | p < 0.001 |
| window 2 (10–12 weeks) | 1.78 [1.65, 1.93] | 0.56 [0.50, 0.61] | p < 0.001 |
| window 3 (13–18 weeks) | 1.75 [1.61, 1.89] | 0.64 [0.57, 0.71] | p < 0.001 |
| window 4 (19–28 weeks) | 1.75 [1.62, 1.90] | 0.65 [0.57, 0.74] | p < 0.001 |
| window 5 (>28 weeks) | 1.82 [1.68, 1.98] | 0.57 [0.50, 0.65] | p < 0.001 |
| Trimesters | |||
| trimester 1 (0–13 weeks) | 1.78 [1.64, 1.93] | 0.54 [0.47, 0.62] | p < 0.001 |
| trimester 2 (14–27 weeks) | 1.72 [1.58, 1.86] | 0.72 [0.63, 0.83] | p < 0.001 |
| trimester 3 (>27 weeks) | 1.83 [1.68, 1.98] | 0.57 [0.50, 0.65] | P = 0.005 |
| Lower respiratory infection | |||
| Developmental Windows | |||
| embryonic stage (0–7 weeks) | 1.33 [1.26, 1.41] | 0.81 [0.74, 0.88] | p < 0.001 |
| pseudoglandular stage (8–17 weeks) | 1.37 [1.29, 1.46] | 0.76 [0.69, 0.84] | p < 0.001 |
| canalicular stage (18–27 weeks) | 1.36 [1.28, 1.45] | 0.81 [0.73, 0.89] | p < 0.001 |
| saccular stage (28–36 weeks) | 1.44 [1.35, 1.53] | 0.68 [0.62, 0.75] | p < 0.001 |
| alveolar stage (>37 weeks) | 1.41 [1.33, 1.50] | 0.70 [0.65, 0.75] | p < 0.001 |
| Trimesters | |||
| trimester 1 (0–13 weeks) | 1.36 [1.28, 1.44] | 0.79 [0.71, 0.88] | p < 0.001 |
| trimester 2 (14–27 weeks) | 1.35 [1.27, 1.44] | 0.84 [0.75, 0.94] | p < 0.001 |
| trimester 3 (>27 weeks) | 1.45 [1.36, 1.55] | 0.66 [0.60, 0.74] | p < 0.001 |
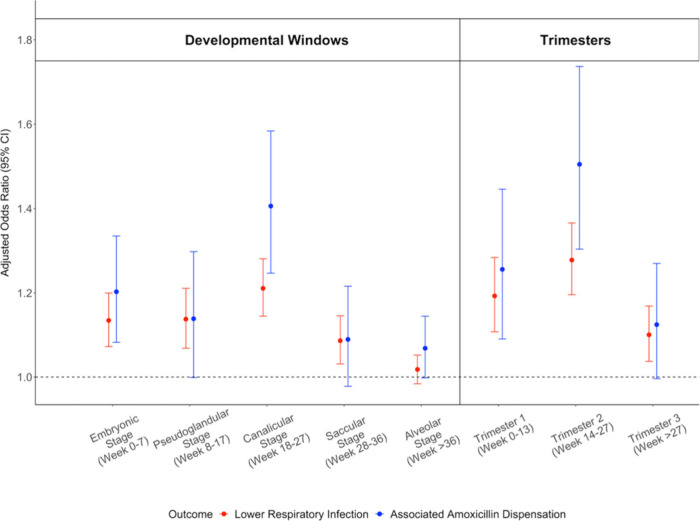
Prenatal Exposure to PM2.5 and Lower Respiratory Infections
Exposure to PM2.5 during the canalicular stage of lower respiratory tract development (18–27 weeks) was most strongly associated with increased odds of lower respiratory infection diagnosis and associated amoxicillin dispensations. The OR for a 10 μg/m3 increase in average PM2.5 exposure was 1.21 [1.15, 1.28] for all diagnoses and 1.41 [1.25,1.58] for those associated with amoxicillin dispensations. The embryonic stage (8–17 weeks) was also associated with increased infections and amoxicillin dispensation (Figure 4). The second trimester (14–27 weeks) was associated with increased odds of lower respiratory infection diagnosis by age 1 year with an OR of 1.28 [1.20, 1.37] and associated amoxicillin dispensation with an OR of 1.51 [1.30, 1.74] (Figure 5). Once again, the sensitivity analyses showed very similar results (Figure S4). Similarly, the models had a significant interaction between PM2.5 and year of exposure (p-value < 0.001), with high ORs in the extreme 2017 and 2018 seasons and protective associations noted in the below-average 2016 and 2019 seasons (Table 3).
Figure 5.
Adjusted odds ratios for prenatal exposure to PM2.5 and all diagnoses of lower respiratory infection in the first year of life (red), as well as lower respiratory infection diagnoses with associated amoxicillin dispensations (blue). Plot shows the effects of exposures during critical windows of lower respiratory tract development (left) and trimesters (right).
Principle Finding
This population-based case-control study examined PM2.5 during critical windows of respiratory prenatal development and associations with respiratory infections by age 1 year. The study included infants whose expected 40-week gestational period intersected with two extreme (2017 and 2018) and two below-average (2016 and 2019) wildfire seasons with different exposure profiles. We found that PM2.5 exposure during the first and second trimesters was associated with increased odds of otitis media and lower respiratory infections, including those treated with amoxicillin. Specifically, exposure during the second trimester showed the strongest associations. Analyses based on critical windows of organ system development may provide more information than those based on trimester when examining the effects of environmental exposures during pregnancy.
Strengths
Our study has several strengths. First, we used the rich, population-based BCPDR to identify most infants in BC who were prenatally exposed to PM2.5 during the 2016–2019 wildfire seasons. Because wildfire smoke was highly variable across these four years, we have excellent exposure contrasts within the cohort. Second, we were able to link BCPDR data with population-based MSP and PharmaNet data to identify all infections and those with associated amoxicillin dispensations. Third, we conditioned the analyses on epidemiologic week of birth and FSA to address confounding by seasonality and geographic variability, which is associated with both the exposure and the outcome. Fourth, we adjusted models for multiple important covariates, including postnatal PM2.5 exposures. The latter was estimated for cases and controls based on the timing of case diagnoses to improve the comparability of postnatal PM2.5 exposure between case and control groups. This approach also provides greater temporal precision, ensuring that we match the times when postnatal PM2.5 is most likely to cause adverse respiratory health outcomes for cases and controls. Lastly, we used a high-quality model optimized for wildfire smoke to estimate daily PM2.5 with high spatial resolution for exposures during pregnancy and the first year of life.
Limitations
Our study also has important limitations. First, CanOSSEM is optimized for wildfire smoke, but it cannot separate total PM2.5 into its component sources. However, wildfire smoke is the primary driver of high PM2.5 exposures in the study area and there are clear differences in exposure between the extreme and below-average years. Second, we used residential history as an indicator for exposure measurements, but we cannot account for mobility or errors in the residential history data, which leads to exposure misclassification and potential attenuation of the true effects. Third, we did not have individual-level SES information, and we used neighborhood-level SES as an approximation. Neighborhood-level SES can even out the distribution of outcomes across income decile, and cause the estimates to be biased toward the null.52 Fourth, we were unable to incorporate some critical covariates identified in literature including maternal stress, maternal atopic status, maternal education, and exposure to environmental tobacco smoke because this information is not reliably captured in the BCPDR.53−56 Finally, all analyses were based on administrative data that are not collected for research purposes. We lacked clinical details of each case, and we assume that physician billings for respiratory infections are a robust indicator of a true infection, especially when followed by an antibiotic dispensation.
Interpretation
Our findings are consistent with Hsu. et al., who reported that the midpregnancy period (16–25 weeks) is a critical window for the relationship between PM2.5 exposure and diagnosis of asthma by the age of six.57 Hazlehurst et al. also reported that higher PM2.5 exposure during the canalicular stage of lower respiratory tract development was linked to an increased risk of asthma.43 Another study identified the pseudoglandular and canalicular stages as the most critical periods for prenatal PM2.5 exposure and the subsequent development of asthma and wheezing after birth.58 The critical developmental windows identified for respiratory outcomes are consistent with the sensitive exposure windows we report.
One other observational study specific to wildfire smoke found that the second and third trimesters were the most sensitive windows for use of upper respiratory medications before age 8 years.13 We also observed the increased risk of otitis media associated with exposure during the third trimester. Another study using a mouse model found that maternal exposure to PM2.5 had a greater impact in the later stages of pregnancy, which could be related to the duration of exposure and accumulation of PM2.5.59
We consistently observed significant positive association during years with severe wildfire seasons (2017 and 2018) and protective effects during years with below-average wildfire seasons (2016 and 2019). We do not have a clear explanation for these observations, but we hypothesize that the overall excellent air quality during below-average wildfire years (most daily PM2.5 concentrations are <10 μg/m3) may contribute. For example, adaptive epigenetic changes resulting from low-dose exposures during pregnancy can trigger hormetic reactions, where mild exposures activate protective mechanisms without causing harm. This process, known as hormesis, has been observed with various environmental stressors such as oxidants, radiation, hypoxia, and stress. Thus, the exposure only poses a risk if it exceeds the capacity of the body’s defenses in terms of amount, intensity, or duration.60 Considering the pro-inflammatory effects of PM2.5 on human tissues, low-dose exposure to PM2.5 on high-quality air days may have induced a hormetic response, potentially enhancing the anti-inflammatory defense mechanisms during periods of occasionally elevated PM2.5 exposure, such as during wildfire events.61
Another study conducted in China found protective effects against congenital respiratory anomalies associated with PM2.5 around 40 to 75 days into pregnancy during the COVID-19 lockdown period, when PM2.5 concentrations decreased by 21.7% compared with the prelockdown period.62 However, there are limited studies on the effects of prenatal exposure to low PM2.5 concentrations (i.e., <10 μg/m3) and respiratory outcomes in early childhood.
Our results suggest that prenatal exposure to PM2.5 may interfere with fetal development. During development of the Eustachian tubes, the fourth stage (19–28 weeks) emerges as a pivotal period characterized by differentiation of certain seromucous glandular extensions within the lumens, which is associated with maturation of submucosal gland.44 The glandular mucus secreted by the submucosal gland aids in the removal of inhaled particles and provides protection to the respiratory airway by combating bacterial threats.63
We hypothesize that prenatal PM2.5 exposure during the fourth stage of Eustachian tube development may be associated with inadequate submucosal gland development. The compromise in the protective functioning of the Eustachian tube potentially increases susceptibility to upper respiratory infections, such as otitis media, during early life. Similarly, the canalicular stage of fetal lower respiratory tract development is associated with the differentiation of type I and type II cells, as well as formation of the alveolar-capillary barrier. Insufficient maturation during the canalicular stage may result in underdeveloped peripheral airways, hindering efficient gas exchange and respiratory balance. This, in turn, can contribute to the proliferation of alveoli populated by inflammatory cells,23,64 which might make infants more prone to lower respiratory infections.
Furthermore, we found that the OR for amoxicillin dispensation associated with otitis media was consistent with the OR for all otitis media diagnoses. In contrast, the OR for amoxicillin dispensation associated with lower respiratory infections was somewhat higher than the OR for all diagnoses. This discrepancy may be attributed to the fact that amoxicillin is generally recommended and commonly prescribed for otitis media.65 In contrast, lower respiratory infections can be classified as either viral or bacterial, with only bacterial lower respiratory infections necessitating amoxicillin treatment.66
In summary, we identified a significant association between prenatal PM2.5 exposure and respiratory infections in infants who were in utero during extreme and below-average wildfire seasons. To mitigate the detrimental impact of wildfire smoke on infant respiratory health, clear communication regarding the risks and protective measures is needed for those who are pregnant. For example, use of indoor air cleaning technologies should be strongly recommended for this population.67 Future studies should focus on the biological mechanisms underlying the effects of prenatal exposure to wildfire smoke, including epigenetics and markers of inflammation. Additionally, extending the follow-up period of the cohort will enable us to examine the longer-term effects associated with prenatal exposure to wildfire smoke, such as development of asthma.
Acknowledgments
This study was funded by a Project Grant from the Canadian Institutes of Health Research (CIHR Grant Number GR015602). Q.L. also received an International Tuition Award, President’s Academic Excellence Initiative PhD Award, and Faculty of Medicine Graduate Award from UBC.
Data Availability Statement
Access to data provided by the Data Stewards is subject to approval but can be requested for research projects through the Data Stewards or their designated service providers. The following data sets were used in this study: (PharmaNet, Medical Services Plan (MSP) Payment Information File, Discharge Abstract Database (Hospital Separations), Consolidation File (MSP Registration & Premium Billing)). You can find further information regarding these data sets by visiting the PopData project webpage at: (https://my.popdata.bc.ca/project_listings/20-197/2021-05-12). All inferences, opinions, and conclusions drawn in this publication are those of the author(s), and do not reflect the opinions or policies of the Data Steward(s).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsestair.4c00213.
Detailed explanations of the rationale behind selecting sensitive developmental windows and the inclusion of confounders, along with thorough details of the sensitivity analyses. Table includes specific time windows critical for significant physiological changes in fetal development. Figures include a causal diagram showing the relationship between prenatal wildfire smoke exposure and respiratory infections, a flowchart outlining the inclusion and exclusion criteria for the cohort with alternative residential histories, and sensitivity analyses using alternate residential histories and the epidemiologic week of conception (PDF)
Author Contributions
Q.L.: Conceptualization; formal analysis; writing–original draft preparation; writing–review and editing. K.W.: Conceptualization; writing–review and editing. S.L.: Conceptualization; writing–review and editing; funding. E.L.: Conceptualization; writing–review and editing; funding. S.W.: Conceptualization; writing–review and editing; funding. S.B.H.: Conceptualization; supervision; writing–original draft preparation; writing–reviewing and editing.
The authors declare no competing financial interest.
Special Issue
Published as part of ACS ES&T Airspecial issue “Wildland Fires: Emissions, Chemistry, Contamination, Climate, and Human Health”.
Supplementary Material
References
- Gheissari R.; Liao J.; Garcia E.; Pavlovic N.; Gilliland F. D.; Xiang A. H.; Chen Z. Health Outcomes in Children Associated with Prenatal and Early-Life Exposures to Air Pollution: A Narrative Review. Toxics Basel 2022, 10 (8), 458. 10.3390/toxics10080458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietti E.; Röösli M.; Frey U.; Latzin P. Air Pollution during Pregnancy and Neonatal Outcome: A Review. J. Aerosol Med. Pulm. Drug Delivery 2013, 26 (1), 9. 10.1089/jamp.2011.0932. [DOI] [PubMed] [Google Scholar]
- Korten I.; Ramsey K.; Latzin P. Air Pollution during Pregnancy and Lung Development in the Child. Paediatr. Respir. Rev. 2017, 21, 38–46. 10.1016/j.prrv.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Bowman W. S.; Schmidt R. J.; Sanghar G. K.; Thompson G. R. III; Ji H.; Zeki A. A.; Haczku A. Air That Once Was Breath” Part 1: Wildfire-Smoke-Induced Mechanisms of Airway Inflammation–“Climate Change, Allergy and Immunology” Special IAAI Article Collection: Collegium Internationale Allergologicum Update 2023. Int. Arch. Allergy Immunol. 2024, 185 (6), 600–616. 10.1159/000536578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera R.; Corringham T.; Gershunov A.; Benmarhnia T. Wildfire Smoke Impacts Respiratory Health More than Fine Particles from Other Sources: Observational Evidence from Southern California. Nat. Commun. 2021, 12 (1), 1493–1498. 10.1038/s41467-021-21708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzi L. M.; Bratt J. M.; Williams K. M.; Last J. A. Why Is Particulate Matter Produced by Wildfires Toxic to Lung Macrophages?. Toxicol. Appl. Pharmacol. 2011, 257 (2), 182–188. 10.1016/j.taap.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y.-H. M; Coull B. A.; Sternthal M. J.; Kloog I.; Schwartz J.; Cohen S.; Wright R. J. Effects of Prenatal Community Violence and Ambient Air Pollution on Childhood Wheeze in an Urban Population. J. Allergy Clin. Immunol. 2014, 133 (3), 713–722.e4. 10.1016/j.jaci.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa M. J.; Just A. C.; Kloog I.; Pantic I.; Schnaas L.; Lee A.; Bose S.; Chiu Y.-H. M.; Hsu H.-H. L.; Coull B.; Schwartz J.; Cohen S.; Téllez Rojo M. M.; Wright R. O.; Wright R. J. Prenatal Particulate Matter Exposure and Wheeze in Mexican Children: Effect Modification by Prenatal Psychosocial Stress. Ann. Allergy. Asthma. Immunol. 2017, 119 (3), 232–237.e1. 10.1016/j.anai.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne E.; Belair M.-A.; Rodriguez Duque D.; Do M. T.; Stieb D. M.; Hystad P.; van Donkelaar A.; Martin R. V.; Crouse D. L.; Crighton E.; Chen H.; Burnett R. T.; Weichenthal S.; Villeneuve P. J.; To T.; Brook J. R.; Johnson M.; Cakmak S.; Yasseen A. S.; Walker M. Effect Modification of Perinatal Exposure to Air Pollution and Childhood Asthma Incidence. Eur. Respir. J. 2018, 51 (3), 1701884–1701884. 10.1183/13993003.01884-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.; Leon Hsu H.-H.; Mathilda Chiu Y.-H.; Bose S.; Rosa M. J.; Kloog I.; Wilson A.; Schwartz J.; Cohen S.; Coull B. A.; Wright R. O.; Wright R. J. Prenatal Fine Particulate Exposure and Early Childhood Asthma: Effect of Maternal Stress and Fetal Sex. J. Allergy Clin. Immunol. 2018, 141 (5), 1880–1886. 10.1016/j.jaci.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen S.; Novack L.; Erez O.; Yitshak-Sade M.; Kloog I.; Shtein A.; Shany E. The Effect of Exposure to Particulate Matter during Pregnancy on Lower Respiratory Tract Infection Hospitalizations during First Year of Life. Environ. Health 2020, 19 (1), 1–90. 10.1186/s12940-020-00645-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. I.; Kim B. J.; Lee S. Y.; Kim H. B.; Lee C. M.; Yu J.; Kang M. J.; Yu H. S.; Lee E.; Jung Y. H.; Kim H. Y.; Seo J. H.; Kwon J. W.; Song D. J.; Jang G.; Kim W. K.; Shim J. Y.; Lee S. Y.; Yang H. J.; Suh D. I.; Hong S. A.; Choi K. Y.; Shin Y. H.; Ahn K.; Kim K. W.; Kim E. J.; Hong S. J. COCOA Study Group. Prenatal Particulate Matter/Tobacco Smoke Increases Infants’ Respiratory Infections: COCOA Study. Allergy Asthma Immunol. Res. 2015, 7 (6), 573–582. 10.4168/aair.2015.7.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra R.; Keeler C.; Staley B. S.; Jardel H. V.; Ward-Caviness C.; Rebuli M. E.; Xi Y.; Rappazzo K.; Hernandez M.; Chelminski A. N.; Jaspers I.; Rappold A. G. Wildfire Smoke Exposure and Early Childhood Respiratory Health: A Study of Prescription Claims Data. Environ. Health 2023, 22 (1), 1–48. 10.1186/s12940-023-00998-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis G. A.; Chappell K.; Williams S.; Melody S. M.; Wheeler A.; Dalton M.; Dharmage S. C.; Zosky G. R.; Johnston F. H. Respiratory and Atopic Conditions in Children Two to Four Years after the 2014 Hazelwood Coalmine Fire. Med. J. Aust. 2020, 213 (6), 269–275. 10.5694/mja2.50719. [DOI] [PubMed] [Google Scholar]
- Simoes E. A. F.; Cherian T.; Chow J.; Shahid-Salles S. A.; Laxminarayan R.; John T. J.. Acute Respiratory Infections in Children. In Disease Control Priorities in Developing Countries; Jamison D. T., Breman J. G., Measham A. R., Alleyne G., Claeson M., Evans D. B., Jha P., Mills A., Musgrove P., Eds.; World Bank: Washington (DC), 2006. [Google Scholar]
- Santibanez P.; Gooch K.; Vo P.; Lorimer M.; Sandino Y. Acute Care Utilization Due to Hospitalizations for Pediatric Lower Respiratory Tract Infections in British Columbia. Canada. BMC Health Serv. Res. 2012, 12 (1), 451–451. 10.1186/1472-6963-12-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick D. M.; Sbihi H.; Dai D. L. Y.; Al Mamun A.; Rasali D.; Rose C.; Marra F.; Boutin R. C. T.; Petersen C.; Stiemsma L. T.; Winsor G. L.; Brinkman F. S. L.; Kozyrskyj A. L.; Azad M. B.; Becker A. B.; Mandhane P. J.; Moraes T. J.; Sears M. R.; Subbarao P.; Finlay B. B.; Turvey S. E. Decreasing Antibiotic Use, the Gut Microbiota, and Asthma Incidence in Children: Evidence from Population-Based and Prospective Cohort Studies. Lancet Respir. Med. 2020, 8 (11), 1094–1105. 10.1016/S2213-2600(20)30052-7. [DOI] [PubMed] [Google Scholar]
- Sur D. K. C.; Plesa M. L. Antibiotic Use in Acute Upper Respiratory Tract Infections. Am. Fam. Physician 2022, 106 (6), 628–636. [PubMed] [Google Scholar]
- Fireman P. Otitis Media and Eustachian Tube Dysfunction: Connection to Allergic Rhinitis. J. Allergy Clin. Immunol. 1997, 99 (2), s787–s797. 10.1016/S0091-6749(97)70130-1. [DOI] [PubMed] [Google Scholar]
- Mazer B. D.; Leung D. Y. M.; Szefler S. J.; Bonilla F. A.; Akdis C. A.; Sampson H. A. Otitis Media. Pediatric Allergy: Principles and Practice 2016, 219–227.e3. 10.1016/B978-0-323-29875-9.00025-2. [DOI] [Google Scholar]
- Dasaraju P. V.; Liu C.. Infections of the Respiratory System. In Medical Microbiology; Baron S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, 1996. [PubMed] [Google Scholar]
- Biyyam D. R.; Chapman T.; Ferguson M. R.; Deutsch G.; Dighe M. K. Congenital Lung Abnormalities: Embryologic Features, Prenatal Diagnosis, and Postnatal Radiologic-Pathologic Correlation. Radiographics 2010, 30 (6), 1721–1738. 10.1148/rg.306105508. [DOI] [PubMed] [Google Scholar]
- Kotecha S. Lung Growth: Implications for the Newborn Infant. Arch. Dis. Child. - Fetal Neonatal Ed. 2000, 82 (1), F69–F74. 10.1136/fn.82.1.F69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiny Sherlie V.; Varghese A. ENT Changes of Pregnancy and Its Management. Indian J. Otolaryngol. Head Neck Surg. 2014, 66 (Suppl 1), 6–9. 10.1007/s12070-011-0376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Columbia; Ministry of Forests and Range. The State of British Columbia’s Forests 2006.; Ministry of Forests and Range, Victoria, BC, 2007. [Google Scholar]
- BC Wildfire Service. Wildfire Season Summary - Province of British Columbia. https://www2.gov.bc.ca/gov/content/safety/wildfire-status/about-bcws/wildfire-history/wildfire-season-summary (accessed 2022–02–04).
- BC Wildfire Service. Wildfire Averages - Province of British Columbia. https://www2.gov.bc.ca/gov/content/safety/wildfire-status/about-bcws/wildfire-statistics/wildfire-averages (accessed 2022–03–10).
- Perinatal Data Registry. http://www.perinatalservicesbc.ca/health-professionals/data-surveillance/perinatal-data-registry (accessed 2023–12–17).
- BC Perinatal Data Registry | Population Data BC. https://www.popdata.bc.ca/data/health/perinatal (accessed 2024–09–23).
- Ark T. K.; Kesselring S.; Hills B.; McGrail K. M. Population Data BC: Supporting Population Data Science in British Columbia. Int. J. Popul. Data Sci. 2019, 4 (2), 1133. 10.23889/ijpds.v4i2.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Population Data BC. PharmaNet Data Set; 2022. (accessed 2022–07–04).https://www.popdata.bc.ca/data/health/pharmanet.
- Paul N.; Yao J.; McLean K. E.; Stieb D. M.; Henderson S. B. The Canadian Optimized Statistical Smoke Exposure Model (CanOSSEM): A Machine Learning Approach to Estimate National Daily Fine Particulate Matter (PM2.5) Exposure. Sci. Total Environ. 2022, 850, 157956–157956. 10.1016/j.scitotenv.2022.157956. [DOI] [PubMed] [Google Scholar]
- Le Saux N.; Robinson J. Pneumonia in Healthy Canadian Children and Youth: Practice Points for Management. Paediatr. Child Health 2011, 16 (7), 417–420. 10.1093/pch/16.7.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Saux N.; Robinson J. L. Management of Acute Otitis Media in Children Six Months of Age and Older. Paediatr. Child Health 2016, 21 (1), 39–44. 10.1093/pch/21.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele T. J. Principles of Confounder Selection. Eur. J. Epidemiol. 2019, 34 (3), 211–219. 10.1007/s10654-019-00494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harskamp-van Ginkel M. W.; London S. J.; Magnus M. C.; Gademan M. G.; Vrijkotte T. G. A Study on Mediation by Offspring BMI in the Association between Maternal Obesity and Child Respiratory Outcomes in the Amsterdam Born and Their Development Study Cohort. PloS One 2015, 10 (10), e0140641 10.1371/journal.pone.0140641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.; Leon Hsu H.-H.; Mathilda Chiu Y.-H.; Bose S.; Rosa M. J.; Kloog I.; Wilson A.; Schwartz J.; Cohen S.; Coull B. A.; Wright R. O.; Wright R. J. Perinatal Air Pollution Exposure. J. Allergy Clin. Immunol. 2018, 141 (5), 1880–1886. 10.1016/j.jaci.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchin H.; Le Lous M.; Houdouin V. In Utero Exposure to Maternal Smoking: Impact on the Child from Birth to Adulthood - CNGOF-SFT Expert Report and Guidelines for Smoking Management during Pregnancy. Gynecol. Obstet. Fertil. Senol. 2020, 48 (7–8), 567. 10.1016/j.gofs.2020.03.026. [DOI] [PubMed] [Google Scholar]
- Sbihi H.; Tamburic L.; Koehoorn M.; Brauer M. Perinatal Air Pollution Exposure and Development of Asthma from Birth to Age 10 Years. Eur. Respir. J. 2016, 47 (4), 1062–1071. 10.1183/13993003.00746-2015. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Guo Y.; Xiao X.; Bloom M. S.; Qian Z.; Rolling C. A.; Xian H.; Lin S.; Li S.; Chen G.; Jalava P.; Roponen M.; Hirvonen M.-R.; Komppula M.; Leskinen A.; Yim S. H. L.; Chen D.-H.; Ma H.; Zeng X.-W.; Hu L.-W.; Liu K.-K.; Yang B.-Y.; Dong G.-H. Association of Breastfeeding and Air Pollution Exposure With Lung Function in Chinese Children. JAMA Netw. Open 2019, 2 (5), e194186. 10.1001/jamanetworkopen.2019.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananth C. V.; Brandt J. S. A Principled Approach to Mediation Analysis Inperinatal Epidemiology. Am. J. Obstet. Gynecol. 2022, 226 (1), 24–32.e6. 10.1016/j.ajog.2021.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbert A.; Hough I.; Seyve E.; Rolland M.; Quentin J.; Slama R.; Lyon-Caen S.; Kloog I.; Bayat S.; Siroux V.; Lepeule J. Association of Prenatal and Postnatal Exposures to Warm or Cold Air Temperatures With Lung Function in Young Infants. JAMA Netw. Open 2023, 6 (3), e233376–e233376. 10.1001/jamanetworkopen.2023.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlehurst M. F.; Carroll K. N.; Loftus C. T.; Szpiro A. A.; Moore P. E.; Kaufman J. D.; Kirwa K.; LeWinn K. Z.; Bush N. R.; Sathyanarayana S.; Tylavsky F. A.; Barrett E. S.; Nguyen R. H. N.; Karr C. J. Maternal Exposure to PM2.5 during Pregnancy and Asthma Risk in Early Childhood. Environ. Epidemiol. 2021, 5 (2), e130 10.1097/EE9.0000000000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornhoffer J. L.; Leuwer R.; Schwager K.; Wenzel S.. A Practical Guide to the Eustachian Tube; Springer Berlin Heidelberg: Berlin, Heidelberg, 2014. 10.1007/978-3-540-78638-2. [DOI] [Google Scholar]
- McEvoy C. T.; Spindel E. R.Environmental Effects on Lung Morphogenesis and Function:: Tobacco Products, Combustion Products, and Other Sources of Pollution. In Fetal and Neonatal Lung Development: Clinical Correlates and Technologies for the Future; Jobe A. H., Whitsett J. A., Abman S. H., Eds.; Cambridge University Press: Cambridge, 2016; pp 77–93. 10.1017/CBO9781139680349.006. [DOI] [Google Scholar]
- Stocks J.; Hislop A.; Sonnappa S. Early Lung Development: Lifelong Effect on Respiratory Health and Disease. Lancet Respir. Med. 2013, 1 (9), 728–742. 10.1016/S2213-2600(13)70118-8. [DOI] [PubMed] [Google Scholar]
- First, Second & Third Trimester | Pregnancy Milestones and Stages. Maternity. https://maternity.jacksonhealth.org/pregnancy-milestones/first-second-third-trimester/ (accessed 2023–04–29).
- Pregnancy. ucsfhealth.org. https://www.ucsfhealth.org/Conditions/Pregnancy (accessed 2023–07–17).
- Huang M.; Strickland M. J.; Richards M.; Warren J. L.; Chang H. H.; Darrow L. A. Confounding by Conception Seasonality in Studies of Temperature and Preterm Birth: A Simulation Study. Epidemiol. Camb. Mass 2023, 34 (3), 439–449. 10.1097/EDE.0000000000001588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canada H.Body Mass Index (BMI) Nomogram. https://www.canada.ca/en/health-canada/services/food-nutrition/healthy-eating/healthy-weights/canadian-guidelines-body-weight-classification-adults/body-mass-index-nomogram.html (accessed 2024–09–23).
- R: a language and environment for statistical computing. https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing (accessed 2023–06–20).
- Hanley G. E.; Morgan S. On the Validity of Area-Based Income Measures to Proxy Household Income. BMC Health Serv. Res. 2008, 8 (1), 79–79. 10.1186/1472-6963-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.; Hsu H.-H. L.; Chiu Y.-H. M.; Bose S.; Rosa M. J.; Kloog I.; Wilson A.; Schwartz J.; Cohen S.; Coull B. A.; Wright R. O.; Wright R. J. Prenatal Fine Particulate Exposure and Early Childhood Asthma: Effect of Maternal Stress and Fetal Gender. J. Allergy Clin. Immunol. 2018, 141 (5), 1880–1886. 10.1016/j.jaci.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter C.; Palumbo M. P.; Sauder K. A.; Glueck D. H.; Liu A. H.; Yang I. V.; Ben-Abdallah M.; Fleischer D. M.; Dabelea D. Incidence and Timing of Offspring Asthma, Wheeze, Allergic Rhinitis, Atopic Dermatitis, and Food Allergy and Association with Maternal History of Asthma and Allergic Rhinitis. World Allergy Organ. J. 2021, 14 (3), 100526–100526. 10.1016/j.waojou.2021.100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring U.; Pattenden S.; Slachtova H.; Antova T.; Braun-Fahrlander C.; Fabianova E.; Fletcher T.; Galassi C.; Hoek G.; Kuzmin S. V.; Luttmann-Gibson H.; Moshammer H.; Rudnai P.; Zlotkowska R.; Heinrich J. Parental Education and Children’s Respiratory and Allergic Symptoms in the Pollution and the Young (PATY) Study. Eur. Respir. J. 2006, 27 (1), 95–107. 10.1183/09031936.06.00017205. [DOI] [PubMed] [Google Scholar]
- Vanker A.; Barnett W.; Workman L.; Nduru P. M.; Sly P. D.; Gie R. P.; Zar H. J. Early-Life Exposure to Indoor Air Pollution or Tobacco Smoke and Lower Respiratory Tract Illness and Wheezing in African Infants: A Longitudinal Birth Cohort Study. Lancet Planet. Health 2017, 1 (8), e328–e336. 10.1016/S2542-5196(17)30134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H.-H. L.; Chiu Y.-H. M.; Coull B. A.; Kloog I.; Schwartz J.; Lee A.; Wright R. O.; Wright R. J. Prenatal Particulate Air Pollution and Asthma Onset in Urban Children. Identifying Sensitive Windows and Sex Differences. Am. J. Respir. Crit. Care Med. 2015, 192 (9), 1052–1059. 10.1164/rccm.201504-0658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.; Zhou H.; He G.; Zhu S.; Sun X.; Ye Y.; Chen H.; Xiao J.; Hu J.; Zeng F.; Yang P.; Gao Y.; He Z.; Wang J.; Cao G.; Chen Y.; Feng H.; Ma W.; Liu C.; Liu T.. Effect of Early-Life Exposure to PM2.5 on Childhood Asthma/Wheezing: A Birth Cohort Study. Pediatr. Allergy Immunol. 2022, 33 ( (6), ). 10.1111/pai.13822. [DOI] [PubMed] [Google Scholar]
- Yue H.; Ji X.; Li G.; Hu M.; Sang N. Maternal Exposure to PM 2.5 Affects Fetal Lung Development at Sensitive Windows. Environ. Sci. Technol. 2020, 54 (1), 316–324. 10.1021/acs.est.9b04674. [DOI] [PubMed] [Google Scholar]
- Rossnerova A.; Izzotti A.; Pulliero A.; Bast A.; Rattan S. I. S.; Rossner P. The Molecular Mechanisms of Adaptive Response Related to Environmental Stress. Int. J. Mol. Sci. 2020, 21 (19), 7053. 10.3390/ijms21197053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox L. A. (Tony). Hormesis for Fine Particulate Matter (PM 2.5). Dose-Response 2012, 10 (2), 209–218. 10.2203/dose-response.11-040.Cox. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W.; Xing Y.; Li G.; Du Z.; Yang P.; Wang Q.; Yang X.; Lyu B.; Fa H.; Shi Q.; Xing Q. Windows of Sensitivity for Risk of Adverse Birth Outcomes Related to Gestational PM2.5 Exposure: Evidence from a Natural Experiment. Environ. Pollut. 1987 2024, 347, 123759–123759. 10.1016/j.envpol.2024.123759. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. H.; Wine J. J. Airway Gland Structure and Function. Physiol. Rev. 2015, 95 (4), 1241–1319. 10.1152/physrev.00039.2014. [DOI] [PubMed] [Google Scholar]
- Gurka D. P.; Balk R. A.. 36 - Acute Respiratory Failure. Critical Care Medicine; Elsevier Inc, 2019; pp 591–604.e8. 10.1016/B978-0-323-44676-1.00036-4. [DOI] [Google Scholar]
- Sakulchit T.; Goldman R. D. Antibiotic Therapy for Children with Acute Otitis Media. Can. Fam. Physician 2017, 63 (9), 685–687. [PMC free article] [PubMed] [Google Scholar]
- Feldman C.; Richards G. Appropriate Antibiotic Management of Bacterial Lower Respiratory Tract Infections [Version 1; Peer Review: 2 Approved]. F1000 Res. 2018, 7, 1121. 10.12688/f1000research.14226.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barn P. K.; Elliott C. T.; Allen R. W.; Kosatsky T.; Rideout K.; Henderson S. B. Portable Air Cleaners Should Be at the Forefront of the Public Health Response to Landscape Fire Smoke. Environ. Health 2016, 15 (1), 116–116. 10.1186/s12940-016-0198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Access to data provided by the Data Stewards is subject to approval but can be requested for research projects through the Data Stewards or their designated service providers. The following data sets were used in this study: (PharmaNet, Medical Services Plan (MSP) Payment Information File, Discharge Abstract Database (Hospital Separations), Consolidation File (MSP Registration & Premium Billing)). You can find further information regarding these data sets by visiting the PopData project webpage at: (https://my.popdata.bc.ca/project_listings/20-197/2021-05-12). All inferences, opinions, and conclusions drawn in this publication are those of the author(s), and do not reflect the opinions or policies of the Data Steward(s).