Abstract
Context
Evidence for a beneficial role of vitamin D on blood pressure (BP) outcomes is inconclusive.
Objective
This work aimed to investigate the effect of 2 doses of cholecalciferol (vitamin D3) supplementation coadministered with calcium on systolic blood pressure (SBP) and diastolic blood pressure (DBP).
Methods
Exploratory analyses were conducted from a 1-year, multicenter, double-blind, randomized controlled trial (RCT). Total of 221 ambulatory older overweight individuals received calcium dose and oral vitamin D3, at the equivalent of 600 IU/day or 3750 IU/day.
Results
SBP and DBP decreased significantly in the overall group, and in the high-dose group at 6 and 12 months. Similar trends were observed in the low-dose group, but did not achieve statistical significance. In participants with a body mass index (BMI) greater than 30, SBP decreased significantly in both treatment groups whereas DBP significantly decreased in the high-dose group only. In the subgroups of hypertensive participants (N = 143), there was a decrease in SBP and DBP at 6 and 12 months, with both vitamin D doses and independently of BMI levels. Using multivariate linear mixed models with random effects in the overall group of participants, SBP at 6 and 12 months was significantly predicted by BMI (β = .29; P = .05) and by baseline SBP (β = .16; P < .001), but not by vitamin D treatment dose.
Conclusion
Vitamin D and calcium decrease SBP and DBP in overweight older individuals, but more is not necessarily better. This effect is seen in individuals with BMI greater than 30, in hypertensive patients, and seems to be largely independent of dose.
Keywords: vitamin D, calcium, systolic blood pressure, diastolic blood pressure, randomized controlled trial, overweight, elderly
Vitamin D deficiency is common worldwide, and has been associated with cardiovascular disease, immunological diseases, infections, and cancer [1, 2]. Large cohorts provide epidemiological evidence linking vitamin D deficiency to a higher risk of cardiovascular disorders, including hypertension (HTN) [3-5].
The association between 25 hydroxyvitamin D (25OHD) and blood pressure (BP) appears to be consistent in observational studies, even after adjusting for relevant confounders [6-9]. A meta-analysis of observational studies found that every 16-ng/mL decrease in vitamin D was associated with a 16% higher risk of HTN [6]. A meta-analysis of population genetic studies suggested that polymorphisms related to lower vitamin D status were associated with higher BP [10]. Additionally, low 25OHD levels have been shown to predict future HTN among individuals with normal BP at baseline [3]. However, results of randomized trials have been conflicting, with some suggesting a benefit [6, 11-14]. Although several randomized controlled trials (RCTs) showed null results, they were conducted in younger individuals, free of comorbidities, while most patients with low 25OHD levels are older and have comorbidities including HTN and high BMI [15, 16]. In this study, we capitalize on a completed vitamin D RCT, and conduct post hoc analyses to investigate the effect of high-dose cholecalciferol (vitamin D3) compared to the National Academy of Medicine–recommended dose on BP, and explore modulators of a putative response, in older overweight individuals.
Materials and Methods
Study Design
This study presents post hoc exploratory analyses of a previously completed double-blind RCT conducted at the American University of Beirut Medical Center (AUBMC), St Joseph University Hospital, and Rafic Hariri Governmental University Hospital, comparing supplementation of older individuals with vitamin D at the currently recommended dose by the Institute of Medicine for apparently healthy adults, and a higher dose, that is however still below the upper tolerable level. Recruitment, prescreening, and screening procedures were performed at all centers while enrollment and protocol implementation were exclusively conducted at AUBMC. The trial identifier on ClinicalTrials.gov is NCT01315366.
Study Drug
All participants received 4 tablets of calcium citrate (250 mg) and 125 IU vitamin D3/tab for a total of 1000 mg elemental calcium and 500 IU vitamin D3 daily. Additionally, each individual received 2 pills, identical in shape, color, size, smell, and taste, taken once a week, that consisted of either placebo (low-dose group) or 10 000 IU/tab of vitamin D3 (Euro D) (high-dose group). All tablets were provided by Euro-Pharm, Canada. Based on its certificate of analysis to the Canadian regulatory agencies for all trial lots, the actual average vitamin D content of the calcium citrate/D tablets was 150 IU/pill, and 11 000 IU/pill for the Euro D tablets. Therefore, the total daily intake of vitamin D in the low- and high-dose groups was 600 IU/day and 3750 IU/day, respectively. The study drugs were stored and dispensed to participants in identical boxes at the AUBMC central pharmacy.
The randomization and allocation sequence were implemented by the senior pharmacist at AUBMC, with stratification by center and sex [15]. Allocation was based on a simple randomization approach, concealed, and the entire study team and all participants were blinded to drug assignment until trial and data entry completion.
We conducted this trial in accordance with the ethical principles outlined in the Declaration of Helsinki. The institutional review board at each center approved the protocol, and all participants provided written informed consent. An external data safety monitoring board, (see Acknowledgments), reviewed the final protocol and monitored the trial safety.
Participants
Older (≥65 years), overweight (body mass index [BMI] > 25), and ambulatory individuals with a serum 25OHD between 10 and 30 ng/mL at screening, were recruited through outpatient departments, clinics, and advertisements posted at the 3 major teaching hospitals, as well as health dispensaries of the Ministry of Social Affairs, in the greater Beirut Area.
Because one of the primary end points was insulin resistance [15], exclusion criteria included prediabetes if on oral hypoglycemic drugs, diabetes fasting blood sugar (≥126 mg/dL or glycated hemoglobin A1c ≥ 6.5%), severe chronic diseases, or major organ failure. The latter included severe heart failure (stage III or IV), liver failure and cirrhosis, kidney failure (estimated glomerular filtration rate [GFR] < 30 mL/min), cancer, and autoimmune diseases. Individuals were also excluded if they had conditions or were on medications known to affect bone metabolism, had osteomalacia, a history of kidney stones, fragility fractures, or a 10-year fracture risk for major osteoporotic fractures exceeding 10% based on the fracture risk assessment tool (FRAX) Lebanon risk calculator at study entry using FRAX version 3.08.
Study Visits and Measurements
Enrolled individuals attended visits every 3 months, during which height, weight, and vital signs were measured, questionnaires administered, study drug bottles returned, and refills provided. Participants were also contacted by phone every 2 weeks to reinforce compliance with study drug. Information on adverse events, intake of medications, and study drug pill counts were obtained at each visit (0, 3, 6 and 12 months). Compliance was measured as a percentage of the full possible dose using pill count ([total number of study drugs pills taken/total number of pills provided for time intervals between study visits] × 100). BP and heart rate were measured in the sitting position after 5 minutes of rest using a SureSigns VS3 monitor (Philips noninvasive BP, including manual, interval, and STAT modes). For participants with a high BP, the measurement was repeated twice with at least 5 minutes separating the serial readings, and the final BP measurement was reported. The HTN categories were defined as follows: HTN: systolic blood pressure (SBP) of 130 mm Hg or greater and/or diastolic blood pressure (DBP) of 80 mm Hg or greater, or use of antihypertensive drugs, including diuretics [16].
Routine chemistries were assayed at 0, 3, 6, and 12 months, depending on the variable. Blood samples were allowed to clot for 30 minutes, centrifuged for 20 minutes, and immediately processed for routine studies, or stored at −20 °C within 2 hours, and then at −80 °C, depending on the assay. Serum 25OHD was run using liquid chromatography–tandem mass spectrometry (ThermoFisher Scientific and Applied Biosystems-MDS Sciex) and assays were performed at the Mayo Clinic Laboratories. Intra-assay coefficients of variation were 3.8%, 2.4%, and 4.7% and interassay coefficients of variation were 6.4%, 6.8%, and 5.0%, at 24, 52, and 140 ng/mL, respectively.
Study Outcomes and Sample Size
The trial had 2 primary outcomes, indices of insulin resistance at 12 months [15], and of bone metabolism at 12 months [17], already reported. The effect of vitamin D supplementation on BP was compared, within and across the 2 treatment doses and, additionally, by HTN status at study entry, by BMI categories, with adjustments for sex and baseline BP, as post hoc exploratory analyses.
We calculated the sample size of the trial based on the primary outcomes of insulin resistance and bone density and a possible 30% dropout rate and a power of 80%, and a statistical significance level of .025 (considering 2 primary outcomes). The sample size needed was 250, and we recruited 257 individuals [15].
Statistical Analyses
We used descriptive statistics, parametric (independent t tests, chi-square test), and analysis of variance (ANOVA) to test for time trends, between and within treatment arms, (repeated measures ANOVA), as indicated. Results are expressed as means ± SD or N (%), for normally distributed variables. Normal distribution was evaluated by visual inspection of histograms and stem leaf plots. Subgroup analyses by potential modulators of BP including BMI and HTN were conducted for the overall group and by treatment dose.
Linear mixed models with random intercepts and unstructured covariance matrix were used to investigate the effect of vitamin D treatment on BP changes while accounting for correlated data at time 0, 6, and 12 months and after adjusting for relevant predictors and modulators, namely, age, sex, BMI, baseline BP, and change in HTN medication administration. Model selection was determined based on the Akaike information and Bayesian information criteria. An identical model was explored substituting vitamin D levels at 6 and 12 months for vitamin D dose group.
IBM SPSS software version 27.0 (SPSS), SigmaPlot 12.0 (Systat Software Inc), and STATA SE version 15.1 were used. P less than .05 was considered statistically significant, and was not adjusted for multiple testing.
Results
Participants and Baseline Characteristics
In total, 257 participants were randomly assigned, 35 individuals (14%) did not complete the study, and no outcome data were available after study discontinuation. One participant did not provide a blood sample for 25OHD analysis and was thus excluded (Supplementary Figure) [18]. Patient characteristics, baseline data, and comorbidities in the remaining 221 participants were similar for the low-dose (n = 111) compared with high-dose (n = 110) vitamin D arms (Table 1; all comparisons nonsignificant). Participants had a mean age of 71.1 (4.7) years and a mean BMI of 30.2 (4.4), and 55% were women. Only 9 engaged in regular physical activity. Mean 25OHD level was 20.4 ng/mL. Overall, 77% had 1 or more comorbidities, 48% of participants were HTN on medication, and 34% (N = 75) of participants had a BP of 130/80 or greater and were not on any treatment at study entry. However, 9 of them were started on treatment after study entry, with 6 in the low-dose arm and 3 in the high-dose arm. None of the baseline characteristics were different between the two vitamin D doses. This includes 25OHD level, mean SBP, and mean DBP (see Table 1).
Table 1.
Baseline characteristics of the study participants, overall and by vitamin D dose allocationa
| Vitamin D supplementation | Overall N = 221 | Low dose N = 111 | High dose N = 110 |
|---|---|---|---|
| N (%) or Mean ± SD | |||
| Overall | Low dose | High dose | |
| Sex, female/male N | 122/99 | 59/52 | 63/47 |
| Age, y | 71.1 ± 4.7 | 71.0 ± 4.7 | 71.2 ± 4.8 |
| BMI | 30.2 ± 4.4 | 29.7 ± 4.6 | 30.6 ± 4.4 |
| Current smoker | 53 (24) | 27 (24) | 26 (24) |
| Current alcohol use | 17 (8) | 10 (9) | 7 (6) |
| Comorbidities | N (%) | ||
| Prediabetes | 153 (69) | 75 (67) | 78 (71) |
| Hypertensive (on physical exam)b | 143 (65) | 65 (59) | 78 (71) |
| Dyslipidemia (on baseline testing)c | 142 (64) | 71 (63) | 71 (65) |
| On lipid-lowering drug | 66 (30) | 34 (30) | 32 (29) |
| Participants with comorbiditiesd | 170 (77) | 85 (76) | 85 (77) |
| 1 Comorbidity | 68 (31) | 35 (31) | 33 (30) |
| 2 Comorbidities | 81 (36) | 42 (38) | 39 (35) |
| ≥3 Comorbidities | 21 (9) | 8 (7) | 13 (12) |
| Hypertension status | |||
| Treated with medications for hypertension by self-report | 106 (48) | 52 (46) | 54 (49) |
| −BP ≥130/80, treated | 68 (31) | 29 (26) | 39 (35) |
| −BP < 130/80, treatedb | 38 (17) | 23 (20) | 15 (14) |
| BP ≥ 130/80b, not being treated | 75 (34) | 36 (32) | 39 (36) |
| Hypertension medication type | |||
| ACE inhibitors | 19 (9) | (9) | 11 (10) |
| ARBs | 19 (9) | 8 (9) | 11 (10) |
| β-Blockers | 35 (16) | 14 (16) | 20 (18) |
| Calcium channel blockers | 14 (6) | 10 (6) | 4 (4) |
| Thiazide diuretics | 19 (9) | 7 (9) | 12 (11) |
| SBP, mm Hg | 128.5 ± 16.3 | 127.1 ± 16.2 | 130 ± 16.3 |
| DBP, mm Hg | 74.7 ± 10.6 | 74.2 ± 10.5 | 75.3 ± 10.7 |
| Mean arterial blood pressure, mm Hge | 92.6 ± 11.3 | 91.8 ± 11.4 | 93.5 ± 11.2 |
| Pulse pressure, mm Hgf | 53.8 ± 12.4 | 52.9 ± 11.5 | 54.7 ± 13.2 |
| Mean heart rate, beats/min | 69.3 ± 8.3 | 68.7 ± 8.0 | 69.9 ± 8.5 |
| Serum 25OHD, ng/mL | 20.4 ± 7.4 | 20.0 ± 7.0 | 20.9 ± 8.2 |
| Calcium, mg/dL | 9.5 ± 0.4 | 9.4 ± 0.4 | 9.5 ± 0.4 |
| Creatinine, mg/dL | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 |
| GFRg | 81.3 ± 13.0 | 81.7 ± 85.9 | 80.9 ± 12.8 |
| CKD3ah | 18 (8) | 11 (10) | 7 (6) |
P = independent t test for continuous variables and chi-square for categorical variables between the two doses were not significant for any of the variables listed in the table.
Abbreviations: 25OHD, 25-hydroxyvitamin D; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CKD, chronic kidney disease; DBP, diastolic blood pressure; GFR, glomerular filtration rate; SBP, systolic blood pressure.
a There was no significant difference in any of the baseline characteristics by treatment group.
b Hypertensive was defined as SBP of 130 mm Hg or greater and/or DBP of 80 mm Hg or greater.
c Total cholesterol greater than 130 mg/dL.
d Comorbidities include cardiovascular disease, coronary artery disease, congestive heart failure, hypertension, and hypercholesterolemia.
e Mean arterial pressure was defined as one-third SBP + two-thirds DBP.
f Pulse pressure was defined as the difference between SBP and DBP.
g Estimated with the use of the CKD–Epidemiology Collaboration equation.
h GFR between 45 and 59.
Blood Pressure at 6 and 12 Months and Changes in Blood Pressure at 1 Year
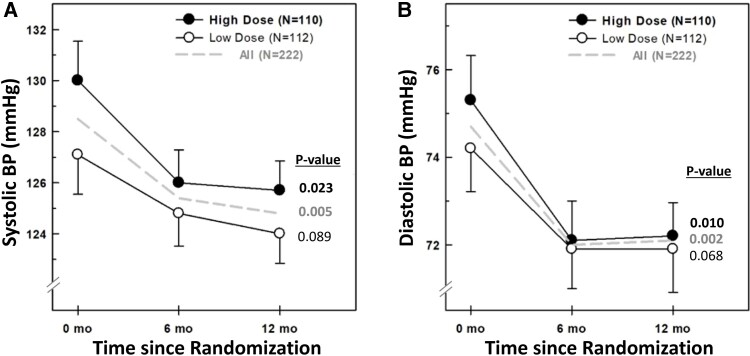
There was a statistically significant decrease in the mean SBP and DBP, in the overall group and high-dose group, by repeated-measures ANOVA (Fig. 1). A similar trend was observed in the low-dose group (see Fig. 1).
Figure 1.
A, Systolic and B, diastolic blood pressure at 0, 6, and 12 months in the overall group (interrupted line), and by vitamin D supplementation (high-dose black circles and low-dose open circles). Numbers expressed as mean ± SEM. P values depicted in the figure are derived from repeated-measures analysis of variance within each group.
Overall, participants experienced a 3.5 (18.7) mm Hg (mean [SD]) reduction in SBP at the 1-year follow-up (P = .005). Individuals in the high-dose arm experienced a 4.2 (19.4) mm Hg reduction (P = .023), while individuals in the low-dose arm had a 2.8 (18.1) mm Hg reduction (P = .089); the difference between the 2 vitamin D arms was 1.4 mm Hg (P = .564).
Similarly, there were modest reductions in DBP 2.8 (12.1) mm Hg (P = .002) in the overall group. The reduction was 3.02 (12.1) mm Hg in the high-dose arm (P = .01) and a 2.6 (12.1) mm Hg reduction in the low-dose arm (P = .089); the differences between the 2 vitamin D arms was 0.3 mm Hg (P = .838).
In addition, 42.5% of participants experienced a decrease in SBP of 4 mm Hg or greater, with 42.3% in the low-dose arm and 42.7% in the high-dose arm. For DBP, 45.7% of participants had a decrease of 2.5 mm Hg or greater, with 46.8% in the low-dose arm and 44.5% in the high-dose arm. Sensitivity analyses were performed to assess the effect of starting BP medication in 9 participants after study entry. There was no change in the overall study results, within the total group and within each treatment arm (using repeated-measures ANOVA), and there was no difference between treatment arms (using linear mixed model analyses) both for SBP and DBP (data not shown).
Blood Pressure in the Overall Group by Body Mass Index Category
After stratifying participants by BMI (≤30, >30), SBP decreased significantly in both vitamin D doses groups in individuals with BMI greater than 30 (Table 2); DBP significantly decreased in the high-dose group only (see Table 2). Among individuals with BMI less than or equal to 30, SBP and DBP did not change regardless of dose (see Table 2).
Table 2.
Blood pressure at baseline, 6, and 12 months in the overall group, and by vitamin D treatment allocation, and by body mass index categories
| Baseline | 6 Mo | 12 Mo | |||
|---|---|---|---|---|---|
| Mean ± SD | Pc | ||||
| BMI ≤30 | |||||
| SBP, mm Hg | Overall (N = 122) | 125.2 ± 14.6 | 123.1 ± 12.4 | 124.42 ± 13.4 | NS |
| High dose (N = 54) | 126.8 ± 14.3 | 122.5 ± 13.4 | 125.5 ± 12.9 | NS | |
| Low dose (N = 68) | 124 ± 14.7 | 123.6 ± 11.7 | 123.3 ± 13.7 | NS | |
| DBP, mm Hg | Overall (N = 122) | 72.6 ± 9.8 | 70.4 ± 9.2 | 70.6 ± 9.2 | NS |
| High dose (N = 54) | 73.1 ± 10.4 | 69.8 ± 9.3 | 71.1 ± 7.9 | NS | |
| Low dose (N = 68) | 72.2 ± 9.3 | 70.9 ± 9.2 | 70.3 ± 10.1 | NS | |
| BMI >30 | |||||
| SBP, mm Hg | Overall (N = 99) | 132.5 ± 17.4a | 128.3 ± 14.3b | 125.2 ± 14b | <.0001 |
| High dose (N = 56) | 132.9 ± 17.6a | 129.3 ± 12.6a | 125.8 ± 11.5b,d | .006 | |
| Low dose (N = 43) | 132 ± 17.2a | 127.1 ± 16.4a | 124.5 ± 16.9b,d | .024 | |
| DBP, mm Hg | Overall (N = 99) | 77.3 ± 10.9a | 73.9 ± 9.4a | 73.8 ± 9.1b,e | .010 |
| High dose (N = 56) | 77.3 ± 10.6a | 74.3 ± 9.1a | 73.3 ± 7.9a | .020 | |
| Low dose (N = 43) | 77.3 ± 11.4 | 73.5 ± 9.9 | 74.5 ± 10.5 | NS | |
Abbreviations: ANOVA, analysis of variance; BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure; NS, not significant.
a, b post hoc t test from ANOVA: Values with different letters are statistically significantly different from each other.
c P value from within treatment comparisons, by repeated-measures ANOVA.
d P value at 6 and 12 months equals .055.
e P value at baseline and 12 months is less than .05.
Blood Pressure by Hypertension Medication Use at Entry, and by Body Mass Index Category
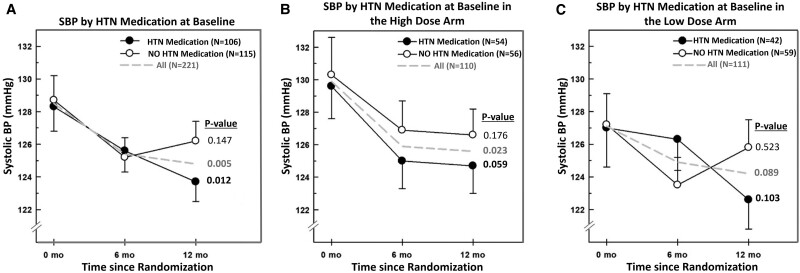
There were 106 participants (48%) who were on anti-HTN medications at baseline. Among those on anti-HTN medication, the SBP and DBP levels decreased significantly over time in the overall group (combining subjects in both vitamin D treatment arms; Supplementary Table S1 [18], Fig. 2A and 2B) [18]. DBP decreased significantly in the low-dose group, whereas SBP changes were of borderline significance in the high-dose group (see Supplementary Table S1) [18]. Participants who were not on HTN medications did not have any changes in their SBP in the overall group, nor at either of the 2 vitamin D treatment doses (see Supplementary Table S1, Fig. 2A-2C) [18]. DBP decreased at 6 and at 12 months in the overall group and the high-dose vitamin D arm (see Supplementary Table S1) [18].
Figure 2.
Systolic blood pressure at 0, 6, and 12 months by the use of hypertension medication at A, baseline, and in the B, high-dose arm and C, low-dose arm. Data depicted for the overall group (interrupted line), and by vitamin D dose (high-dose black circles and low-dose open circles). Numbers expressed as mean ± SEM. P values depicted in the figure are derived from repeated-measures analysis of variance within each group.
Further stratification of the subgroup on anti-HTN medications by BMI category revealed that individuals with a BMI greater than 30 had a statistically significant decrease in SBP in the overall group, and in the 2 vitamin D treatment arms at 6 and 12 months (Supplementary Table S2) [18]. DBP decreased in the overall group and in the low-dose group only. Similar analyses in participants with BMI of 30 or less did not show any changes in SBP nor DBP in any treatment group (see Supplementary Table S2) [18].
The SBP and DBP of individuals who were not on HTN medications did not change by 1 year regardless of dose and BMI (Supplementary Table S3) [18].
Blood Pressure in Participants With Hypertension at Entry by Body Mass Index Category
A total of 143 individuals had a mean SBP of 130 mm Hg or greater or DBP of 80 mm Hg or greater at study entry. There was a consistent and statistically significant decrease in SBP and DBP in the overall group, and within each treatment arm, at 6 and 12 months; differences in BP between 6 and 12 months were not significant (Table 3). This effect was preserved in subgroup analyses in both BMI subcategories (see Table 3).
Table 3.
Blood pressure at baseline, 6, and 12 months in hypertensive individuals at study entry by vitamin D treatment allocation, and body mass index categories
| Baseline | 6 Mo | 12 Mo | |||
|---|---|---|---|---|---|
| Mean ± SD | Pc | ||||
| Overall | |||||
| SBP, mm Hg | Overall (N = 143) | 136.5 ± 14.0a | 127.9 ± 13.8b | 126.5 ± 11.8b | <.001 |
| High dose (N = 78) | 136.5 ± 14.3a | 127.5 ± 13.4b | 126.7 ± 11.5b | <.001 | |
| Low dose (N = 65) | 136.5 ± 13.7a | 128.3 ± 14.4b | 126.3 ± 12.4b | <.001 | |
| DBP, mm Hg | Overall (N = 143) | 79.8 ± 8.2a | 73.8 ± 9.3b | 72.8 ± 8.7b | <.001 |
| High dose (N = 78) | 79.7 ± 8.2a | 73.2 ± 9.5b | 72.8 ± 7.8b | <.001 | |
| Low dose (N = 65) | 79.9 ± 8.3a | 74.7 ± 9.1b | 72.9 ± 9.7b | <.001 | |
| BMI >30 | |||||
| SBP, mm Hg | Overall (N = 71) | 139.3 ± 15.3a | 130.5 ± 14.5b | 126.7 ± 10.7b | <.001 |
| High dose (N = 42) | 139.3 ± 15.4a | 129.7 ± 12.9b | 127.2 ± 10.3b | <.001 | |
| Low dose (N = 29) | 139.3 ± 15.5a | 131.5 ± 16.5b | 125.9 ± 11.4b | <.001 | |
| DBP, mm Hg | Overall (N = 71) | 81.7 ± 8.4a | 74.6 ± 9.9a | 74.1 ± 7.8b | <.001 |
| High dose (N = 42) | 81.6 ± 7.4a | 74.7 ± 9.6b | 73.9 ± 8.1b | <.001 | |
| Low dose (N = 29) | 81.9 ± 9.7a | 74.6 ± 10.5b | 74.1 ± 7.7b | <.001 | |
| BMI ≤30 | |||||
| SBP, mm Hg | Overall (N = 72) | 133.7 ± 12.1a | 125.3 ± 12.7b | 126.4 ± 13.0b | .001 |
| High dose (N = 36) | 133.3 ± 12.4a | 124.9 ± 13.5b | 126.2 ± 12.9b | .036 | |
| Low dose (N = 36) | 134.2 ± 11.8a | 125.6 ± 12.1b | 126.6 ± 13.2b | .012 | |
| DBP, mm Hg | Overall (N = 72) | 77.9 ± 7.7a | 73.1 ± 8.7b | 71.7 ± 9.4b | <.000 |
| High dose (N = 36) | 77.5 ± 8.8a | 71.3 ± 9.2b | 71.5 ± 7.5b | .002 | |
| Low dose (N = 36) | 78.3 ± 6.5a | 74.7 ± 7.9b | 71.9 ± 11.1b | .005 | |
Hypertension is defined as an SBP of 130 mm Hg or greater or DBP of 80 mm Hg or greater.
Abbreviations: ANOVA, analysis of variance; BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure.
a, b post hoc t test from ANOVA: Values with different letters are statistically significantly different from each other.
c P value from within treatment comparisons, by repeated-measures ANOVA.
Adjusted Analyses for Predictors of Systolic Blood Pressure and Diastolic Blood Pressure
The linear mixed model showed that there was no difference in SBP (Table 4, model A) nor DBP changes (see Table 4, model B) between the 2 vitamin D doses, after adjusting for age, sex, BMI, time, change in HTN medication while in trial, and time × treatment BP medications. SBP was associated with BMI and with baseline SBP. DBP was significantly associated with BMI, sex (lower in women), and baseline DBP. Similarly, substituting vitamin D levels at 12 months (continuous variable) for vitamin D treatment arms (low vs high dose) did not change the results, and therefore no associations were observed between vitamin D levels and SBP or DBP (data not shown). Subgroup analyses by 25OHD levels at baseline less than 20 vs 20 ng/mL or greater did not yield any statistically significant results (data not shown).
Table 4.
General linear mixed models with random intercepts for systolic and diastolic blood pressure
| Dependent variable | Predictor | β | P |
|---|---|---|---|
| Overall group using Vitamin D doses groups a | |||
| A, SBP | Vitamin D doses group (reference low) | .689 | .682 |
| Age | .232 | .121 | |
| Sex, reference male | −1.903 | .200 | |
| BMI | .294 | .055 | |
| Baseline SBP | .160 | <.0001 | |
| Change in HTN medication*(reference no medication) | .724 | .564 | |
| Time | −.518 | .724 | |
| Time#Arm interaction | −.197 | .924 | |
| B, DBP | Vitamin D doses group (reference low) | .337 | .762 |
| Age | .142 | .149 | |
| Sex, female vs male | −2.640 | .008 | |
| BMI | .280 | .007 | |
| Baseline DBP | .238 | <.0001 | |
| Change in HTN medication*(reference no medication) | −.411 | .622 | |
| Time | −.429 | .661 | |
| Time#Arm interaction | −.553 | .690 | |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; HTN, hypertension; SBP, systolic blood pressure.
* No change or change in dose or number of hypertension medications.
# Interaction.
a Model was adjusted for vitamin doses group, age, sex, BMI at 6 and 12 months, baseline SBP/DBP, no change or change in dose or number of hypertension medications, time, and included an interaction between vitamin doses group and time.
Discussion
In exploratory analyses of this RCT in older overweight subjects, calcium plus vitamin D3 supplementation reduced BP at 6 and 12 months. In the overall group, the effect estimate at 12 months was −3.5 (±18.7) mm Hg for SBP and −2.8 (±12.1) mm Hg for DBP. There was a decrease both in SBP and DBP in the high-dose arm, and a trend for a decrease in SBP and DBP in the low-dose arm by ANOVA, but no difference was detected by linear mixed method between the 2 doses. This effect was consistently seen in obese participants, both for SBP and DBP in the high-dose arm, and for SBP in the low-dose arm. It was also consistently noted in HTN participants at study entry, both for SBP and DBP, regardless of the dose and BMI levels, and in obese individuals on anti-HTN drugs.
We identified 13 trials investigating the effect of vitamin D on BP, as primary or secondary outcome. The trials lasted 2 to 12 months, the number of participants varied between 98 and 534 per trial, mean baseline 25OHD varied between 10 and 30 ng/mL, vitamin D doses ranged from 200 to 7000 IU/day, and 7 trials had a placebo control [12-14, 19-28]. Dose-response studies investigating the effect of vitamin D supplementation on BP in older individuals have yielded valuable insights. Sluyter et al [14] studied 517 participants, mean age 65 (8) years, who received 100 000 IU of vitamin D3 monthly or placebo. In participants with vitamin D deficiency, the vitamin D group exhibited reductions in aortic SBP, augmentation index, pulse wave velocity, peak reservoir pressure, and backward pressure amplitude [14]. Abderhalden et al [26] investigated the effects of daily 800 IU or 2000 IU of vitamin D3 in adults, mean age 70.4 (6) years, baseline 25OHD level of 18 ng/mL, for 24 months. Similar to our study, both vitamin D doses reduced BP, a secondary outcome, over 1 and 2 years. The reduction in SBP was 4.22 mm Hg at 1 year and 3.65 mm Hg at 2 years, in the 2000 IU arm [26], decrements comparable to those we reported herein.
Conversely, there was no reduction in BP (as primary outcome) in 159 individuals, with isolated systolic HTN, mean age 76.8 (4.6) years, baseline 25OHD 18 ng/mL, randomly assigned to a single dose of vitamin D dose of 100 000 IU compared to placebo [19]. Null findings were also reported in a study of 305 individuals, mean age 71 (6) years, baseline 25OHD25 ng/mL, randomly assigned to 2000 IU, 4000 IU, or placebo [28]. Similarly, Gepner et al [20] studied 98 postmenopausal women, mean age 61 years, baseline 25OHD 25 ng/mL, duration 6 months, and noted no significant effect of vitamin D supplementation on BP outcomes at doses of 400 IU/day and 2500 IU/day. Pilz et al [21] studied 188 older individuals, aged 61 years, baseline 25OHD 22 ng/mL, with arterial HTN, and showed no significant effects of 2800 IU compared to placebo on 24-hour SBP, over 8 weeks. The lack of significant effects in the aforementioned studies may be explained by the single very high dose [19], small sample size [20], short study duration (8 weeks) [21, 22], and replete vitamin D status of the participants at study entry [20, 21, 28]. A replete 25OHD level also accounts for the null findings on DBP reported in a vitamin D megatrial [29].
Low 25OHD levels at study entry may account for the beneficial effect of vitamin D on BP. Forman and colleagues[24] investigated different daily doses of vitamin D in 283 healthy Black adults, mean age 51 years, median 25OHD level of 15.7 (10.7-23.4) ng/mL, and showed a significant decrease SBP for each additional 1000 IU/day of vitamin D for 3 months. Similarly, Sheikh et al [13] studied 171 individuals, aged 55.8 years, baseline 25OHD 13.8 ng/mL, administered 50 000 IU/week of vitamin D3 for those with vitamin D deficiency (<20 ng/mL), 1000 IU/week of vitamin D3 for those with insufficient levels (20-30 ng/mL), and placebo for the third group, for 2 months. SBP decreased significantly at the first and second month post supplementation (P = .004 and P = .024, respectively). In contrast, null findings were reported in RCTs of younger adults, findings possibly explained by the young age of the participants [23] and replete vitamin D status [22, 25].
Our study findings and synthesis of the literature emphasize the importance of demographic factors (age) and underlying health conditions (prevalent HTN, obesity, and 25OHD levels at study entry) when interpreting the potential effects of vitamin D on BP outcomes [12, 14, 26]. Modulators of BP response to vitamin D reported in RCTs and metanalyses include age, HTN, baseline 25OHD levels, and high BMI as predictors of BP response to vitamin D [12, 26, 30, 31].
Our study has several strengths. These include its randomized, double-blind design, rigorous quality assurance measures, minimizing confounding variables and bias, our systematic dissection of potential modulators through subgroup analyses, and linear mixed models. The duration of the trial, spanning 1 year, provides valuable insights into the longer-term effects of vitamin D supplementation on BP. Furthermore, the use of a multicenter approach enhances the generalizability of our findings to the broader older population with comorbidities and hypovitaminosis D. Our results can also be interpreted as regression to the mean. Without a placebo group, we cannot distinguish these two possibilities. Calcium alone may decrease BP; however, decrements reported in our trial exceed those reported by calcium alone in 2 recent meta-analyses [32, 33]. The efficacy of a combination of calcium and vitamin D on BP is less clear, and certainly less substantial than what we report [34, 35]. Moreover, scrutiny of the evidence for a positive effect of vitamin D (without calcium) from several randomized placebo-controlled trials speak against such possibilities [12-14, 24, 26]. Our study focuses on older individuals, who were sedentary and overweight, many with prediabetes, all conditions known to affect BP, thus limiting the generalizability of our results to other groups. Notably, only 9 participants engaged in regular physical activity, and our study was unable to include physical activity levels in the analysis due to insufficient data, which may have affected our ability to fully capture lifestyle influences on BP outcomes. Other limitations include the exploratory nature of our analyses, and the low power of subgroup analyses. Significant and nonsignificant findings presented are only hypothesis-generating, noting, however, that in vivo and in vitro studies provide biological plausibility for a BP-lowering effect of vitamin D [36, 37], especially in obese, vitamin D–deficient individuals [38].
Our trial and critical synthesis of data from other relevant RCTs suggest a putative beneficial effect of vitamin D in older populations with inadequate vitamin D levels and HTN. Calcium is commonly coadministered with vitamin D in older individuals, without or with osteoporosis drugs. In our study such a combination decreased BP at both doses, but more consistently at the high dose. Age, HTN, high BMI, and possibly dose, appear to be important modulators of such a response. Individual patient-level meta-analyses are needed to validate our findings, and if confirmed, to investigate the optimal dose to be used.
Acknowledgments
The authors are grateful to the study individuals for their participation. The authors thank Dr Rafic Baddoura and Georges Halabi for making the trial possible. The authors greatly appreciate the time and input of members of the data safety monitoring board, Heike Bischoff-Ferrari, MD, DrPH (University of Zurich, Switzerland), Christopher Gallagher, MD (Creighton University, Omaha, Nebraska, USA), and Reinhold Vieth, PhD, FCACB (Mt Sinai Hospital, Montreal, Canada). The authors thank Dr John Orav (Harvard School of Public Health, Boston, Massachusetts, USA) and Dr Robert Habib (American Society of Thoracic Surgery [STS], Chicago, Illinois, USA) for helpful discussions regarding results interpretation. The authors thank Ms Sawsan Salah for pilot analyses of the manuscript. The authors thank Euro-Pharm Canada for providing the vitamin D/identical placebo tablets and calcium citrate supplements.
Abbreviations
- 25OHD
25-hydroxyvitamin D
- ACE
angiotensin-converting enzyme
- ANOVA
analysis of variance
- ARB
angiotensin receptor blocker
- AUBMC
American University of Beirut Medical Center
- BMI
body mass index
- BP
blood pressure
- CKD
chronic kidney disease
- DBP
diastolic blood pressure
- GFR
glomerular filtration rate
- RCT
randomized controlled trial
- SBP
systolic blood pressure
- vitamin D3
cholecalciferol
Contributor Information
Maya Rahme, Department of Internal Medicine, Division of Endocrinology, Calcium Metabolism and Osteoporosis Program, WHO Collaborating Center for Metabolic Bone Disorders, American University of Beirut Medical Center, Beirut, Lebanon.
Laila Al-Shaar, Department of Public Health Sciences, Penn State University, University Park, PA 16802, USA.
Hani Tamim, Department of Internal Medicine, Clinical Research Institute, American University of Beirut Medical Center, Beirut, Lebanon; College of Medicine, Alfaisal University, Riyadh 11533, Saudi Arabia.
Ghada El-Hajj Fuleihan, Email: gf01@aub.edu.lb, Department of Internal Medicine, Division of Endocrinology, Calcium Metabolism and Osteoporosis Program, WHO Collaborating Center for Metabolic Bone Disorders, American University of Beirut Medical Center, Beirut, Lebanon.
Funding
This work was supported by grants from the American University of Beirut (AUB), St Joseph University, and the Lebanese Council for National Scientific Research. Assays performed outside AUB were made in part possible thanks to institutional grants from the Mayo Clinic, Rochester, Minnesota, USA, and Odense University, Odense, Denmark. Research reported in this publication was in part supported by the Fogarty International Center and Office of Dietary Supplements of the National Institutes of Health (NIH award No. D43 TW009118; principal investigator Ghada El-Hajj Fuleihan). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author Contributions
G. El-Hajj Fuleihan: trial concept and study design, oversight of trial conduct, data analyses, and manuscript write-up; M. Rahme: participant recruitment, trial execution, data entry and analyses, manuscript write-up; L. AL-Shaar: oversight of analyses; H. Tamim: oversight of analyses. All authors reviewed and approved the final version of the manuscript.
Disclosures
All authors state that they have no conflict of interest.
Data Availability
Some or all data sets generated during and/or analyzed during this study are not publicly available but are available from the corresponding author on reasonable request.
Clinical Trial Information
ClinicalTrials.gov identifier number NCT01315366 (registered 2011-02-02).
References
- 1. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266‐281. [DOI] [PubMed] [Google Scholar]
- 2. Bouillon R, Van Schoor NM, Gielen E, et al. Optimal vitamin D status: a critical analysis on the basis of evidence-based medicine. J Clin Endocrinol Metab. 2013;98(8):E1283‐E1304. [DOI] [PubMed] [Google Scholar]
- 3. Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49(5):1063‐1069. [DOI] [PubMed] [Google Scholar]
- 4. Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Joukar F, Naghipour M, Hassanipour S, et al. Association of serum levels of vitamin D with blood pressure status in northern Iranian population: the PERSIAN Guilan Cohort Study (PGCS). Int J Gen Med. 2020;13:99‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burgaz A, Orsini N, Larsson SC, Wolk A. Blood 25-hydroxyvitamin D concentration and hypertension: a meta-analysis. J Hypertens. 2011;29(4):636‐645. [DOI] [PubMed] [Google Scholar]
- 7. Ke L, Mason RS, Kariuki M, Mpofu E, Brock KE. Vitamin D status and hypertension: a review. Integr Blood Press Control. 2015;8:13‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pilz S, Tomaschitz A, Ritz E, Pieber TR. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol. 2009;6(10):621‐630. [DOI] [PubMed] [Google Scholar]
- 9. Jorde R, Figenschau Y, Emaus N, Hutchinson M, Grimnes G. Serum 25-hydroxyvitamin D levels are strongly related to systolic blood pressure but do not predict future hypertension. Hypertension. 2010;55(3):792‐798. [DOI] [PubMed] [Google Scholar]
- 10. Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a Mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719‐729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86(4):1633‐1637. [DOI] [PubMed] [Google Scholar]
- 12. Wood AD, Secombes KR, Thies F, et al. Vitamin D3 supplementation has no effect on conventional cardiovascular risk factors: a parallel-group, double-blind, placebo-controlled RCT. J Clin Endocrinol Metab. 2012;97(10):3557‐3568. [DOI] [PubMed] [Google Scholar]
- 13. Sheikh V, Mozaianimonfared A, Gharakhani M, Poorolajal J, Ph D. Effect of vitamin D supplementation versus placebo on essential hypertension in patients with vitamin D deficiency: a double-blind randomized clinical trial. J Clin Hypertens (Greenwich). 2020;22(10):1867‐1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sluyter JD, Camargo CA Jr, Stewart AW, et al. Effect of monthly, high-dose, long-term vitamin D supplementation on central blood pressure parameters: a randomized controlled trial substudy. J Am Heart Assoc. 2017;6(10):e006802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. El-Hajj Fuleihan G, Baddoura R, Habib RH, et al. Effect of vitamin D replacement on indexes of insulin resistance in overweight elderly individuals: a randomized controlled trial. Am J Clin Nutr. 2016;104(2):315‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127‐e248. [DOI] [PubMed] [Google Scholar]
- 17. Rahme M, Sharara SL, Baddoura R, et al. Impact of calcium and two doses of vitamin D on bone metabolism in the elderly: a randomized controlled trial. J Bone Miner Res. 2017;32(7):1486‐1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rahme M, Al-Shaar L, Tamim H, El-Hajj Fuleihan G. Supplementary data for “Blood Pressure Decreases in Overweight Elderly Individuals on Vitamin D: A Randomized Trial.”. Appendices. Supplementary Materials Tables HTN-BP (figshare.com). doi: 10.6084/m9.figshare.26508091.v2. [DOI] [PMC free article] [PubMed]
- 19. Witham MD, Price RJ, Struthers AD, et al. Cholecalciferol treatment to reduce blood pressure in older patients with isolated systolic hypertension: the VitDISH randomized controlled trial. JAMA Intern Med. 2013;173(18):1672‐1679. [DOI] [PubMed] [Google Scholar]
- 20. Gepner AD, Haller IV, Krueger DC, Korcarz CE, Binkley N, Stein JH. A randomized controlled trial of the effects of vitamin D supplementation on arterial stiffness and aortic blood pressure in Native American women. Atherosclerosis. 2015;240(2):526‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pilz S, Gaksch M, Kienreich K, et al. Effects of vitamin D on blood pressure and cardiovascular risk factors: a randomized controlled trial. Hypertension. 2015;65(6):1195‐1201. [DOI] [PubMed] [Google Scholar]
- 22. Scragg R, Slow S, Stewart AW, et al. Long-term high-dose vitamin D3 supplementation and blood pressure in healthy adults: a randomized controlled trial. Hypertension. 2014;64(4):725‐730. [DOI] [PubMed] [Google Scholar]
- 23. Arora P, Song Y, Dusek J, et al. Vitamin D therapy in individuals with prehypertension or hypertension: the DAYLIGHT trial. Circulation. 2015;131(3):254‐262. [DOI] [PubMed] [Google Scholar]
- 24. Forman JP, Scott JB, Ng K, et al. Effect of vitamin D supplementation on blood pressure in blacks. Hypertension. 2013;61(4):779‐785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jorde R, Sneve M, Torjesen P, Figenschau Y. No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. J Intern Med. 2010;267(5):462‐472. [DOI] [PubMed] [Google Scholar]
- 26. Abderhalden LA, Meyer S, Dawson-Hughes B, et al. Effect of daily 2000 IU versus 800 IU vitamin D on blood pressure among adults age 60 years and older: a randomized clinical trial. Am J Clin Nutr. 2020;112(3):527‐537. [DOI] [PubMed] [Google Scholar]
- 27. Theiler-Schwetz V, Trummer C, Grubler MR, et al. Effects of vitamin D supplementation on 24-hour blood pressure in patients with low 25-hydroxyvitamin D levels: a randomized controlled trial. Nutrients. 2022;14(7):1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tomson J, Hin H, Emberson J, et al. Effects of vitamin D on blood pressure, arterial stiffness, and cardiac function in older people after 1 year: BEST-D (biochemical efficacy and safety trial of vitamin D). J Am Heart Assoc. 2017;6(10):e005707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bischoff-Ferrari HA, Vellas B, Rizzoli R, et al. Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: the DO-HEALTH randomized clinical trial. JAMA. 2020;324(18):1855‐1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. He S, Hao X. The effect of vitamin D3 on blood pressure in people with vitamin D deficiency: a system review and meta-analysis. Medicine (Baltimore). 2019;98(19):e15284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Farapti F, Fadilla C, Yogiswara N, Adriani M. Effects of vitamin D supplementation on 25(OH)D concentrations and blood pressure in the elderly: a systematic review and meta-analysis. F1000Res. 2020;9:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van Mierlo LA, Arends LR, Streppel MT, et al. Blood pressure response to calcium supplementation: a meta-analysis of randomized controlled trials. J Hum Hypertens. 2006;20(8):571‐580. [DOI] [PubMed] [Google Scholar]
- 33. Cormick G, Ciapponi A, Cafferata ML, Cormick MS, Belizán JM. Calcium supplementation for prevention of primary hypertension. Cochrane Database Syst Rev. 2022;1(1):Cd010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu L, Sun D. Effects of calcium plus vitamin D supplementation on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Hum Hypertens. 2017;31(9):547‐554. [DOI] [PubMed] [Google Scholar]
- 35. Morvaridzadeh M, Sepidarkish M, Fazelian S, et al. Effect of calcium and vitamin D co-supplementation on blood pressure: a systematic review and meta-analysis. Clin Ther. 2020;42(3):e45‐e63. [DOI] [PubMed] [Google Scholar]
- 36. Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pál É, Ungvári Z, Benyó Z, Várbíró S. Role of vitamin D deficiency in the pathogenesis of cardiovascular and cerebrovascular diseases. Nutrients. 2023;15(2):334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vaidya A, Forman JP, Williams JS. Vitamin D and the vascular sensitivity to angiotensin II in obese Caucasians with hypertension. J Hum Hypertens. 2011;25(11):672‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Rahme M, Al-Shaar L, Tamim H, El-Hajj Fuleihan G. Supplementary data for “Blood Pressure Decreases in Overweight Elderly Individuals on Vitamin D: A Randomized Trial.”. Appendices. Supplementary Materials Tables HTN-BP (figshare.com). doi: 10.6084/m9.figshare.26508091.v2. [DOI] [PMC free article] [PubMed]
Data Availability Statement
Some or all data sets generated during and/or analyzed during this study are not publicly available but are available from the corresponding author on reasonable request.