Abstract
The SCN2A gene encodes the Nav1.2 protein, a voltage-gated sodium channel crucial for initiating and transmitting action potentials in neurons. Dysfunction in Nav1.2, often stemming from genetic mutations in the SCN2A gene, leads to SCN2A-related disorders. Individuals harboring pathogenic SCN2A variants present with severe neurodevelopmental disorders such as epilepsy, autism spectrum disorders, movement disorders, cortical visual impairment, and intellectual disabilities. The FamilieSCN2A Foundation, a 501(c)(3) patient advocacy organization, is dedicated to enhancing the lives of those affected by SCN2A-related disorders. Fueled by a vision of a world with effective treatments and cures for all patients with SCN2A-related disorders, FamilieSCN2A Foundation has charted the course to a cure based on their core values of urgency, integrity, collaboration, and inclusion. Their strategic plan centers on building a comprehensive research-readiness infrastructure that maximizes the probability of bringing curative therapies to SCN2A patients. Appreciating that statistically most drug development initiatives will fail, creating an infrastructure that maximizes the number of drugs in development for SCN2A-related disorders in turn maximizes the net probability of success that FamilieSCN2A Foundation will achieving their vision. Through dynamic initiatives and notable achievements, including raising ~$6 million USD, funding 26 research grants totaling ~$4.7 million USD, and forging strategic partnerships across the SCN2A-related disorder ecosystem the foundation is actively executing its strategic plan. With SCN2A research advancing rapidly and a thriving ecosystem of diverse, engaged stakeholders, FamilieSCN2A Foundation believes the outlook for SCN2A-related disorders is bright.
Keywords: autism, epilepsy, Nav1.2, roadmap, SCN2A
Plain language summary
A patient organization perspective: charting the course to a cure for SCN2A-Related Disorders
Advances in gene discovery for neurodevelopmental disorders have identified SCN2A as a leading cause of early-onset epilepsy, autism spectrum disorder, and intellectual disability. SCN2A encodes a neuronal voltage-gated sodium channel, Nav1.2. Pathogenic genetic variants in SCN2A disrupt the normal flow of sodium ions in the brain and disrupt a neuron’s ability to generate and transmit electrical signals. The spectrum of symptoms and severity in SCN2A-related disorders is broad and can lead to epilepsy, autism, movement disorders, visual impairment, and intellectual disabilities. There is currently no cure for SCN2A-related disorders. The FamilieSCN2A Foundation is a non-profit organization committed to improving the lives of those affected by SCN2A-related disorders and working towards a cure. The foundation focuses on building a research-ready ecosystem to increase the success of new and ongoing drug development efforts. The foundation is actively working towards this goal by building an educated and empowered patient community and raising funds to support basic and translational research projects and natural history. With the field advancing and a growing network of involved supporters, FamilieSCN2A Foundation is optimistic about the future of SCN2A-related disorders.
Introduction to FamilieSCN2A foundation
“Families” is part of our name for a reason
The FamilieSCN2A Foundation is a 501(c)(3) patient advocacy organization dedicated to improving the lives of patients and families affected by SCN2A-related disorders.
FamilieSCN2A Foundation was established in 2015 by a group of caregivers whose children are affected by an SCN2A-related disorder. The name “Families” was chosen by the founders to underscore the significant effect that SCN2A-related disorders have on both the affected individual and their entire family. FamilieSCN2A Foundation is rooted in core values of urgency, integrity, collaboration, and inclusion, and is driven by a vision of a world with effective treatments and cures for all SCN2A-related disorders.
Through the recruitment of a high-impact Board of Directors and the establishment of a strong and committed executive team, FamilieSCN2A Foundation was built on principles of awareness, empowerment, evidence-based research, and equity. These tenants have contributed to the development of a reputation that has attracted key opinion leaders in the field to serve on the organization’s medical and scientific advisory board. The collected efforts by these mission-driven individuals have resulted in FamilieSCN2A Foundation becoming the largest global SCN2A patient advocacy group, currently supporting over 2 000 SCN2A families.
Due to these efforts, the FamilieSCN2A Foundation has become a central liaison for the SCN2A-related disorder ecosystem. With an engaged community, a research and drug development framework, a comprehensive clinical and academic network, and multiple funding mechanisms, FamilieSCN2A Foundation has created an infrastructure that accelerates research, clinical care, drug development, and family empowerment.
Noteworthy FamilieSCN2A Foundation initiatives and achievements include:
Raising ~$6 million USD over the life of the foundation.
Funding 26 research grants totaling ~$4.6 million in awards.
Formation of strategic partnerships in academia, industry, and diagnostics.
Fully funded and executed a SCN2A Clinical Trial Readiness Study (CTRS).
Launched and currently executing an evergreen patient registry and natural history study through National Organization for Rare Disorder’s (NORD) IAMRARE platform.
Facilitated FDA listening sessions raising regulator awareness around SCN2A-related disorders.
Organized the largest annual family and professional SCN2A symposium.
Proclamations for state-wide awareness and recognition of SCN2A-related disorders.
Contributed to policy engagement in support of SCN2A-related disorders.
Offer community support initiatives including patient assistance grants for special equipment, therapy, and travel to conferences.
SCN2A and SCN2A-related disorders
Biology of SCN2A
SCN2A is a gene that encodes the Nav1.2 protein (NaV: voltage-gated sodium channel). This protein is found in neurons and plays a crucial role in initiating and propagating action potentials. 1 These action potentials allow for the rapid transmission of messages throughout the body, enabling movement, sensation, and responses to the environment.
Pathophysiology of SCN2A-related disorders
Symptoms associated with SCN2A-related disorders arise from dysfunction in Nav1.2. 2 This dysfunction typically results from a genetic mutation in the SCN2A gene. Such mutations either alter the function of Nav1.2 or reduce the amount of Nav1.2 present in neurons. There is a robust genotype-phenotype relationship in SCN2A-related disorders,3,4 such that the specific alteration in the SCN2A gene and degree of dysfunction directly influences the nature and degree to which clinical symptoms present in affected individuals.3–6
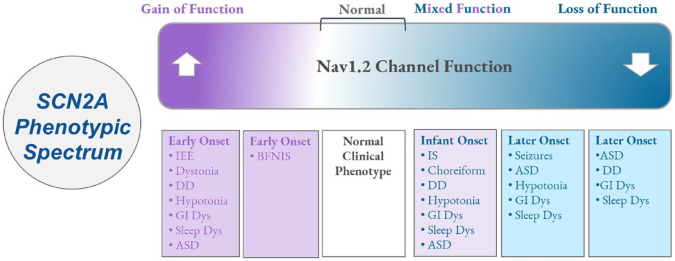
Variations in the SCN2A gene can be conceptualized as a spectrum of functional Nav1.2 phenotypes. On one end are mutations that cause an increase in sodium current compared to the norm (Gain of function mutations; GoF), while on the other end of the spectrum are mutations that entirely block sodium passage through the channel, due either to the absence of the channel or because the mutation completely disrupts the protein’s function (Loss of Function mutation; LoF). 3 Mixed Function represents variants that have mixed electrophysiological functional readouts (some parameters demonstrating more LoF and others more GoF). 6 The nature of the SCN2A gene alteration correlates to the clinical manifestations observed in affected individuals. 3 The relationship is probably more complex but the general distinction in the literature suggests that variants that induce a GoF of the Nav1.2 channel are associated with early-onset (<3 months) epileptic encephalopathy or early-onset self-limited (benign) familial neonatal/infantile epilepsies, whereas variants that cause a LoF of the Nav1.2 channel are associated with later-onset epilepsy or ASD/ID without epilepsy. 6
Individuals may be affected from birth or childhood by a range of neurodevelopmental disorders including epilepsy, autism spectrum disorders, movement disorders, gastrointestinal dysfunction, cortical visual impairment, hypotonia, sleep dysfunction, and intellectual disability (Figure 1). Most, but not all, individuals with mutations in SCN2A will present with epilepsy. Of those with epilepsy, approximately half will present in the neonatal period.5,6 The most commonly reported types of seizures include focal seizures, epileptic spasms, self-limited (benign) familial neonatal/infantile epilepsies, early infantile developmental and epileptic encephalopathy, and later-onset developmental and epileptic encephalopathies.5,6
Figure 1.
Variations in SCN2A led to a spectrum of clinical phenotypes.
Clinical and functional spectrum of SCN2A-related disorders. Purple indicates more gain of function Nav1.2 properties while blue represents more loss of function, and white/normal represents an SCN2A without mutation/dysfunction. Mixed Function Represents Nav1.2 channels that have some LoF and some GoF electrophysiological parameters. Boxes below include common symptom clustering that occurs within each respective zone of the Nav1.2 functional excitability spectrum.
ASD, autism spectrum disorder; BFNIS, Benign/self-limited Familial Neonatal Infantile Seizures; DD, developmental delay; GI Dys, gastrointestinal dysfunction; IEE, infantile epileptic encephalopathy; IS, infantile spasms; Sleep Dys, sleep dysfunction.
Landscape of current SCN2A research
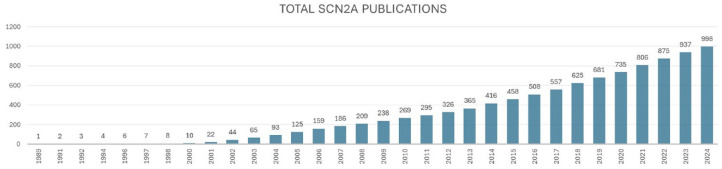
SCN2A has become an area of intense investigation in basic, applied, and clinical research, as well as in the realms of biotech and drug development. Since its discovery, there have been ~1000 publications documented on PubMed dedicated to discussing SCN2A or Nav1.2, with approximately a quarter of these publications emerging within the last 5 years alone (Figure 2).
Figure 2.
Total publications discussing SCN2A or Nav1.2 have increased over time.
Graph shows the number of SCN2A publications on PubMed by year. PubMed search query used was “SCN2A OR NAV1.2” and then manually adjudicated to remove irrelevant publications. Current to August 7, 2024.
Research in the field of SCN2A has been focused on understanding the molecular and clinical consequences of SCN2A mutations. Additionally, researchers have explored potential therapeutic interventions and identified optimal timeframes for such interventions. Specifically, there has been an increased focus on functional characterization of SCN2A variants, genotype-phenotype correlations, development of SCN2A model systems, increasing numbers of natural history and clinical studies, and a focus on precision medicine and therapeutics.
Functional characterization of SCN2A mutations: Evaluation of how specific mutations in SCN2A affect the function of the sodium channel and, consequently, neuronal activity
Functional characterization of SCN2A mutations involves employing a diverse set of methodologies that delve into the intricate details of sodium channel behavior and neuronal activity. Electrophysiological techniques, such as patch-clamp recordings, enable researchers to directly measure the electrical current passing across a cell membrane due to an individual sodium channel. This technique provides valuable insights into the channel’s functional properties, including channel kinetics, voltage sensitivity, and other properties that may contribute to the development of neurological disorders. As therapeutics advance that are specific for either SCN2A loss or gain of function, this work has become critical for patient safety, patient selection, optimal trial design, and maximizing the probability of success of clinical trials. Molecular biology methods, including site-directed mutagenesis, allow for the generation of specific SCN2A mutations to observe their effects in controlled experimental settings. Advanced imaging techniques, such as fluorescence microscopy, aid in visualizing changes in neuronal firing patterns associated with mutated SCN2A channels. Integrating these methodologies allows researchers to construct a comprehensive understanding of the functional consequences of SCN2A mutations, paving the way for targeted therapeutic interventions and advancements in the treatment of neurological disorders.
Genotype-phenotype correlations: Establishing and validating correlations between different SCN2A mutations and clinical manifestations in affected individuals
Understanding how specific mutations relate to the nature and degree of physiologic and neurologic changes in SCN2A-related disorders helps in predicting outcomes, calculating risk for comorbidities, determining appropriate diagnostics, and tailoring treatment approaches.
Genotype-phenotype correlation involves establishing connections between specific genetic variants and the clinical manifestations observed in affected individuals. This complex task integrates genetic analysis and clinical assessments to identify SCN2A variants and then to document the diverse phenotypic changes that correlate with that variant, such as seizure types and cognitive outcomes. 6 Robust statistical analyses and large-scale patient cohorts are essential for validating these correlations, ensuring the reliability of findings. Bioinformatics tools analyze datasets to identify patterns linking distinct SCN2A mutations with specific clinical phenotypes, while longitudinal studies track symptom progression over time. The established genotype-phenotype correlations contribute not only to accurate diagnoses and personalized treatment strategies but also advance our understanding of the molecular basis of neurogenetic disorders, paving the way for precision medicine and targeted therapies tailored to individual genetic profiles. 7
SCN2A model systems: Creation, characterization, and validation of SCN2A model systems
Developing, characterizing, and validating a broad set of preclinical SCN2A model systems is essential for gaining insights into the diverse pathophysiologies associated with the condition and for assessing potential therapeutic strategies. A comprehensive repertoire of preclinical models, spanning cellular, animal, and in silico domains, is instrumental to this endeavor. Cellular models, particularly induced pluripotent stem cells derived from patient cells, allow researchers to study the specific effects of SCN2A mutations in nervous system cells in a controlled laboratory setting, providing valuable insights into cellular disease mechanisms. Animal models, including zebrafish, mice, and rats, offer the opportunity to explore the impact of SCN2A mutations in a more holistic biological context, considering factors such as behavior, learning, and memory, as well as organ function. In silico models, driven by computational approaches, contribute to a deeper understanding of the molecular networks involved, and how those networks may behave if specific molecular interactions are perturbed. The creation, thorough characterization, and validation of these diverse model systems collectively enhance our ability to unravel the complexities of SCN2A-related disorders and pave the way for the development of targeted therapeutic interventions.
Natural history: Research exploring the natural history of SCN2A-related disorders
This involves a comprehensive understanding of the clinical phenotype of the condition and the progression of the condition over time, encompassing the emergence and evolution of symptoms, the variability in clinical presentations, the response to treatments, and the overall trajectory of the disorder. A comprehensive description of a disease’s natural history provides crucial insights into disease progression, informs treatment decisions, and creates benchmarks that can be used in the development of targeted interventions to improve patient outcomes. There is also significant work being done to create and validate endpoints that are both important for patients and caregivers of SCN2A-affected individuals 8 as well as important to demonstrate clinical meaningfulness of changes brought about by investigational therapeutics in clinical trials.
Therapeutic approaches and clinical studies: Development of therapeutic interventions for SCN2A-related disorders
Therapeutic approaches underway include exploring pharmacological agents that can modulate the activity of the sodium channel, 9 genetic medicine approaches,10,11,12,16 and other strategies to mitigate the clinical symptoms produced by SCN2A mutations. 13
The pursuit of therapeutic approaches for SCN2A-related disorders represents a dynamic and evolving field of research. Scientists actively engage in the development and investigation of various potential interventions aimed at addressing the complex challenges posed by SCN2A mutations. This multifaceted endeavor encompasses the exploration of pharmacological agents capable of modulating the activity of the sodium channel, offering opportunities to regulate neuronal function. Additionally, the investigation extends to different genetic medicines, leveraging innovative strategies to correct or counteract the effects induced by specific SCN2A mutations. The diverse range of therapeutic avenues under scrutiny reflects a commitment to uncovering effective treatments tailored to the molecular intricacies of SCN2A-related conditions, marking a crucial step toward improving the prognosis and quality of life for affected individuals.
FamilieSCN2A foundation strategic plan
Our vision and strategy
FamilieSCN2A Foundation’s vision is a world with effective treatments and cures for all SCN2A-related disorders. Our strategic plan executes this vision to create an ecosystem that maximizes the net probability of success for drug development. We appreciate that statistically most drug development initiatives will fail 14 so we hope to raise our net probability of success by maximizing our shots on goal. With this infrastructure in place, it offers a launch pad for the development of therapeutics that provide symptomatic relief and also moonshot cures. This research-readiness infrastructure will support rapid drug development with the aim of growing a pipeline that is as comprehensive and broad as possible.
Tactics
Tactically, we are creating a comprehensive infrastructure around the four pillars of research readiness: clinical phenotyping, basic research, translational research, and clinical trial research (Figure 3). With this infrastructure, we can take a proactive approach to identifying or assisting in the identification of, potential therapeutics and partnering with those biotechs that can develop those therapeutics. By positioning SCN2A-related disorders for clinical trial readiness, we can catalyze the launch of multiple development programs for SCN2A-related disorders.
Figure 3.
The FamilieSCN2A Foundation’s Four Pillars of Research-Readiness Strategy: clinical phenotyping, basic research, translational research, and clinical trial research.
Illustration of FamilieSCN2A Foundation’s Four Pillars of Research-Readiness Strategy. Each category described in greater detail in the body of the publication.
Clinical phenotyping
Overview
Clinical phenotyping is the process of meticulously describing, documenting, and publishing SCN2A-related disorders in a scientifically rigorous way to create a comprehensive understanding of the clinical condition. This task aims to create a detailed and nuanced understanding of the disorder from a clinical perspective. Clinicians systematically gather and analyze data related to the varied aspects of SCN2A-related disorders, including, but not limited to, seizure types, cognitive impairments, and developmental outcomes. The information is then documented and published in scientific literature, contributing to a growing body of knowledge that forms the basis for a comprehensive understanding of the clinical spectrum of SCN2A-related conditions. The rigorous nature of clinical phenotyping ensures that the information disseminated is not only accurate but also serves as a foundation for further research, diagnostic refinement, and the development of targeted therapeutic interventions tailored to the specific clinical nuances of SCN2A-related disorders.
FamilieSCN2A efforts into clinical phenotyping research readiness
We are growing a research-readiness infrastructure focused on clinical phenotyping (Figure 4). Fundamentally, clinical phenotyping involves educating and empowering our patient and caregiver community about the significance of documenting the clinical manifestations of SCN2A-related disorders. We encourage our community members to engage with their clinicians, urging them to comprehensively characterize SCN2A-related disorders in the patient’s electronic medical records and advocate for the publication of noteworthy findings in patient case studies. Once published, the foundation disseminates these observations to our stakeholders, including our network of clinical and scientific researchers.
Figure 4.

Clinical phenotyping creates a detailed and nuanced understanding of the disorder from a clinical perspective.
Diagram of FamilieSCN2A Foundation’s Efforts in Clinical Phenotyping described in greater detail in the publication.
In a more systematic approach, the FamilieSCN2A Foundation actively promotes clinical phenotyping by initiating and endorsing opportunities for natural history research. In all the initiatives we support, we make dedicated efforts to ensure that the findings are widely accessible to our patients, their clinical teams, and the broader research and industry communities. Some examples of our initiatives include:
CTRS: The SCN2A Clinical Trials Readiness Study (CTRS) recruited 66 families of children with pathogenic/likely pathogenic SCN2A variants for longitudinal assessment with validated parent-reported instruments, including the Vineland-3, and other measures of key functional abilities. 15 Additionally, each patient enrolled had their variant characterized with electrophysiological techniques for its Nav1.2 functional status. 6 The CTRS was fully funded and executed by the FamilieSCN2A Foundation.
DRAGONFLY Study: The FamilieSCN2A Foundation, in partnership with the NORD, launched the DRAGONFLY Study, a study with global reach to study SCN2A-related disorders. Designed with the input of scientists and patients, this global resource will provide data for researchers to advance drug development and treatment options to help improve SCN2A-related disorder patient care.
Danish Epilepsy Center Grant: a FamilieSCN2A Foundation funded grant intended to establish and maintain a database including clinical, genetic, and epidemiological data of published and unpublished patients with SCN2A-related disorders. This funded grant also intends to perform genetic, protein structure, molecular, and clinical data analysis to study the genotype-phenotype relationships in SCN2A-related disorders.
FamilieSCN2A Foundation also endorses and contributes to external initiatives focused on clinical phenotyping of SCN2A-related disorders. Some ongoing efforts include:
Simons Searchlight: This initiative maintains a continuous SCN2A registry and dataset. Simons Searchlight regularly updates and publishes the Simons Searchlight Registry Report on SCN2A. The FamilieSCN2A Foundation actively supports Simons Searchlight in various ways, including actively promoting participation and providing opportunities for our community to engage in Simons research during our annual FamilieSCN2A Foundation conference.
Citizen Health: Citizen Health is a registry platform that compiles longitudinal health records, organizing them into a disease profile. SCN2A-related disorders have been clinically mapped through this platform. The FamilieSCN2A Foundation supports this work through partnership and promotion within our community, as well as highlighting it during our annual conference.
DAYTool Clinical Picture Marker: This project aimed at capturing the clinical phenotype of various manifestations of SCN2A-related disorders through video documentation aims to facilitate the production, organization, and dissemination of video documentation that can improve the diagnosis and treatment of rare and difficult-to-diagnose disorders. Familie SCN2A Foundation supports these efforts through partnership and promotion within our community, as well as highlighting it during our annual conference.
Basic research
Overview
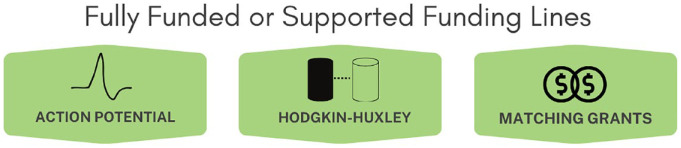
FamilieSCN2A Foundation believes that basic research, or the pursuit of a deeper understanding of the biology, physiology, and mechanism of disease, creates the knowledge base and infrastructure to support translational research and drug development. Furthermore, it establishes a network of expertise that proves invaluable when confronting scientific barriers or challenges. Our academic research-readiness infrastructure encompasses various initiatives, including the facilitation and awarding of multiple funding mechanisms to researchers dedicated to SCN2A-related work (Figure 5). Additionally, we have established multiple platforms fostering scientific and clinical discussions, promoting collaboration, and knowledge exchange within the SCN2A community.
Figure 5.
Funding mechanisms dedicated to SCN2A-related basic research.
Illustration of FamilieSCN2A Foundation’s funding mechanisms, each described in greater detail in the publication.
Action potential grant
Through this investigator-initiated grant program, the FamilieSCN2A Foundation aims to accelerate the development of therapeutic treatments and disease-modifying breakthroughs for those individuals affected by pathogenic alterations in the SCN2A gene. This grant initiative is crafted to support investigations conducted by early investigators, with the potential to lay the groundwork for subsequent grants from various sources including the government, industry, or the FamilieSCN2A Foundation itself.
The FamilieSCN2A Foundation is particularly interested in supporting research that advances our understanding of the cellular, molecular, genetic, and systems-level mechanisms underlying SCN2A-related disorders. Priority is given to innovative projects that hold promise of advancing therapeutic treatments or cures for those with SCN2A-related disorders. This is a 1-year grant program with a maximum award of $100,000. Since 2019, FamilieSCN2A Foundation has granted 10 Action Potential awards, totaling ~$600,000.
Hodgkin-Huxley award
The FamilieSCN2A Foundation Hodgkin-Huxley Award was established to recognize the outstanding contributions of British physiologists Dr. Alan Hodgkin and Dr. Andrew Huxley. Their pioneering research on nerve cells, which elucidated the dynamics of ion movement during an action potential, laid the groundwork for our comprehension of the electrical excitability of neurons and remains a fundamental pillar in the field of neuropsychology. In 1963, they were awarded the Nobel Prize in Physiology or Medicine for their groundbreaking work.
The FamilieSCN2A Foundation is receptive to unsolicited Letters of Inquiry (LOIs) throughout the year and will issue invitations to submit a full application to those LOIs that are best poised to meet the FamiliesSCN2A Foundation’s research objectives on a rolling basis, contingent upon the availability of funds. Since 2023, FamilieSCN2A has granted four Hodgkin-Huxley Awards, amounting to a total of $2.9 million. This award program reflects the foundation’s commitment to advancing research that builds upon the legacy of Hodgkin and Huxley in the realm of neuroscience.
Orphan disease center sponsored – Million Dollar Bike Ride Pilot Grant
The Orphan Disease Center (ODC) Sponsored Million Dollar Bike Ride Pilot Grant is a collaborative initiative organized by the ODC. It involves teams of cyclists who participate in bike rides to raise funds for partnered organizations and causes. The unique aspect of this initiative is the dollar-for-dollar matching of funds raised by the cyclists with philanthropic donations secured by the ODC. Once the funds are matched, the partnering organization, in this case, the FamilieSCN2A Foundation, opens a Request for Applications (RFA) to the international scientific community. The RFA invites pilot grant proposals for studying the diseases represented in the Million Dollar Bike Ride (MDBR), with a focus on SCN2A-related disorders for the FamilieSCN2A Foundation.
The scientific leadership of the ODC, along with its extramural advisors, reviews the received applications and awards grants to those demonstrating the highest scientific merit and addressing the specific topics outlined in the RFA. Subsequently, the ODC manages the disbursement of funds to the grantees and oversees the progress of scientific research along with monitoring the spending on the awarded grants.
Over the past few years, the FamilieSCN2A Foundation has collaborated with the ODC to support two awards through this innovative and impactful funding mechanism.
American Epilepsy Society sponsored—Junior investigator research award
The American Epilepsy Society (AES) Sponsored Junior Investigator Research Award is designed to provide support for research conducted by recently independent investigators. The primary goal is to facilitate subsequent, longer-term funding from institutions such as the National Institutes of Health (NIH) or other relevant funding sources.
AES is particularly interested in backing research that advances the understanding of basic, translational, and clinical aspects of epilepsy. This includes investigations into disease mechanisms, treatments, epidemiology, behavior, technology development, health services, and outcomes.
The award offers up to $50,000 for 1 year to cover the direct costs of research. Additionally, it includes complimentary membership and registration to the AES Annual Meeting for 1 year. The number of awards granted annually is contingent upon the availability of funds.
The FamilieSCN2A Foundation has been involved in co-funding these awards and may continue to do so in the future. This collaborative effort aligns with the FamilieSCN2A Foundation’s commitment to support research that contributes to the understanding and treatment of SCN2A-related disorders within the broader context of epilepsy research.
Other funding opportunities
The FamilieSCN2A Foundation actively informs its research network about various funding opportunities beyond its own initiatives. These include:
Simons Foundation Autism Research Initiative (SFARI): SFARI issues an open call for scientific proposals aimed at enhancing the understanding, diagnosis, and treatment of autism spectrum disorders. The foundation funds innovative research of the highest quality and relevance.
Charles A. King Trust Postdoctoral Research Fellowship Program: This program offers 2-year grants ranging from $194,100 to $215,000, inclusive of stipend, fringe benefits, and a $25,000 annual flexible expense allowance. The fellowship aims to advance the understanding and improve the treatment of human disease.
Whitehall Foundation Research Grants: Available to established scientists in the United States, these grants are judged based on scientific merit, the innovative aspects of the proposals, and the competence of the applicant. The maximum budget for the 2 and 3-year research grants is $100,000 per year.
By promoting these external funding opportunities, the FamilieSCN2A Foundation contributes to the broader scientific community’s awareness and access to resources, fostering collaboration and advancement in research aimed at understanding and treating SCN2A-related disorders and related conditions.
Created platforms to facilitate scientific discussion around SCN2A
The FamilieSCN2A Foundation, in many ways, acts as the central liaison in the SCN2A network and ecosystem. Being in touch with each level of the ecosystem (patient perspective, basic and translational science, clinical, drug development, policy, regulators, etc.) gives FamilieSCN2A Foundation a unique opportunity to convene all stakeholders to discuss emerging science in constructive and enabling ways. Furthermore, as FamilieSCN2A Foundation hosts many of these forums (Figure 6), the foundation has the ability to ensure the science and topics have the patient voice infused in each project. Examples of such include:
Figure 6.
Depiction of the different forums hosted by the FamilieSCN2A Foundation to facilitate scientific discussions.
Illustration of FamilieSCN2A Foundation’s platforms that facilitate scientific discussion, each described in greater detail in the publication.
International SCN2A family and professional conference
The International SCN2A Family and Professional Conference serves as a unifying platform, bringing together the diverse components of the SCN2A ecosystem, including patients, caregivers, researchers, clinicians, drug developers, investors, regulators, and more. The overarching goal is to facilitate mutual learning and collaboration with a singular focus on enhancing the lives of individuals affected by SCN2A-related disorders. The conference has experienced substantial growth, attracting over 380 participants in 2024.
The conference structure incorporates a dedicated day for a research roundtable, followed by a multi-day general session featuring both live and virtual presentations, informal social gatherings, and opportunities for questions and answers, fostering meaningful interactions between patients and professionals.
While the research roundtable primarily engages professionals, invitations are extended to interested caregivers, recognizing the invaluable contribution of the patient perspective to each discussion. The roundtable commences with a state-of-the-union address, providing an overview of the current state of SCN2A research. This is followed by presentations and discussions covering various key topics carefully selected for their potential to positively impact new treatments and cures for SCN2A-related disorders. The research roundtable also includes a poster presentation segment, culminating in the recognition of excellence through the annual Visionary Award, presented to the best poster of the year.
During the general session of the conference, professional presentations are comprehensive, covering various topics such as current research, updates on drug development and clinical trials, considerations in diagnostics, and the best clinical practices for addressing the diverse phenotypes and symptoms associated with SCN2A-related disorders. This session also features live and recorded presentations by families directly impacted by SCN2A, providing a poignant illustration of the daily challenges associated with the condition.
Throughout the conference, scientists actively engaged in SCN2A research and provided updates to clinicians, therapists, industry groups, and families, sharing both published and unpublished data in their presentations. This collaborative environment allows industry representatives to gain firsthand insights from individuals affected by SCN2A-related disorders and their families, providing a unique perspective that can inform research and development efforts. The emphasis on the exchange of ideas and information among all attendees reflects a commitment to fostering a multidirectional flow of knowledge.
The conference encourages professionals to interact with families outside of a medical setting, recognizing the value of understanding personal stories of diagnosis and treatment. This approach positively contributes to the education of practitioners, enabling them to better manage current and future SCN2A patients, while also motivating researchers to pursue innovative solutions and cures. The emphasis on personal narratives fosters a deeper understanding of the human aspect of SCN2A-related disorders and strengthens the collaborative efforts within the SCN2A community.
SCN2A research collaboration meetings
The SCN2A Research Collaboration Meetings serve as a virtual global gathering point for clinicians, scientists, and industry experts. Uniting their expertise in this setting has the potential to propel collective efforts in advancing SCN2A research. The overarching objective is to expedite research breakthroughs for patients affected by this rare disease by enhancing the quality of research, fostering collaboration among professionals, empowering junior investigators, and minimizing duplicative efforts within our SCN2A ecosystem. These collaborative meetings provide a dedicated forum for sharing knowledge, exchanging ideas, and strategizing on ways to collectively address the challenges associated with SCN2A-related disorders.
HopeRx virtual series
The FamilieSCN2A Foundation HopeRx Virtual Series is an industry-sponsored recurring presentation series featuring distinguished speakers discussing scientific or clinical topics relevant to our SCN2A community. These virtual sessions provide a platform for live question-and-answer interactions between the professional presenters and members of the SCN2A community. The insightful discussions aim to inform and engage the community, fostering a deeper understanding of important subjects related to SCN2A-related disorders.
To ensure accessibility and widespread dissemination of valuable information, the sessions are recorded and made available on the Familie SCN2A Foundation website. This allows individuals who may not have been able to participate in real-time to access and benefit from the shared knowledge and expertise presented in the virtual series. Additionally, it enables families to revisit important topics.
Community Town Hall
The FamilieSCN2A Foundation organizes a bi-monthly Community Town Hall, extending an open invitation to the entire SCN2A-related disorder patient and caregiver community. These widely attended town hall sessions serve as forums for unstructured question-and-answer interactions among community members, FamilieSCN2A Foundation team representatives, and invited professional guests. The inclusion of professional speakers provides valuable insights on research topics and important updates related to SCN2A-related disorders.
These regular gatherings offer a platform for the community to engage in safe, honest, and open dialogue with professionals, providing valuable feedback and perspectives that can enhance ongoing research efforts and clinical trials. The Community Town Hall sessions play a crucial role in fostering a sense of connection and collaboration within the SCN2A community, ensuring that information flows freely between professionals and those directly affected by SCN2A-related disorders. These sessions also provide an opportunity for the FamilieSCN2A Foundation and professionals to keep a pulse on important topics within the community.
Translational research
Overview
Translational research, in the context of SCN2A-related disorders, involves actively exploring and advancing the stages of discovery, proof of concept, and optimization of therapeutic interventions. It is a pivotal component within the SCN2A therapeutic pipeline, playing a crucial role in elucidating both the potential and limitations of various approaches aimed at treating these disorders.
The focus of translational research extends to delineating biological targets, understanding the mechanisms for targeting these targets, and identifying the optimal developmental time frame for intervention. This research is instrumental in validating potential methods for assessing treatment efficacy, shedding light on the condition’s aspects that hold promise for viable therapy, and contributing to a comprehensive understanding of the disorder’s complexities.
By bridging the gap between scientific discovery and clinical application, translational research serves as a critical pathway for translating promising scientific insights into tangible therapeutic advancements. This process not only informs the development of potential treatments for SCN2A-related disorders but also contributes to the broader understanding of the disorder’s underlying mechanisms and its clinical implications, which collectively inform trial design.
FamilieSCN2A foundation efforts into translational research readiness
The FamilieSCN2A Foundation is actively involved in supporting translational research readiness through a comprehensive and multifaceted approach (Figure 7). This includes direct funding for a range of translational research projects utilizing the funding instruments mentioned above in academic research. Some specific areas of focus within translational research efforts supported by the foundation include:
Figure 7.
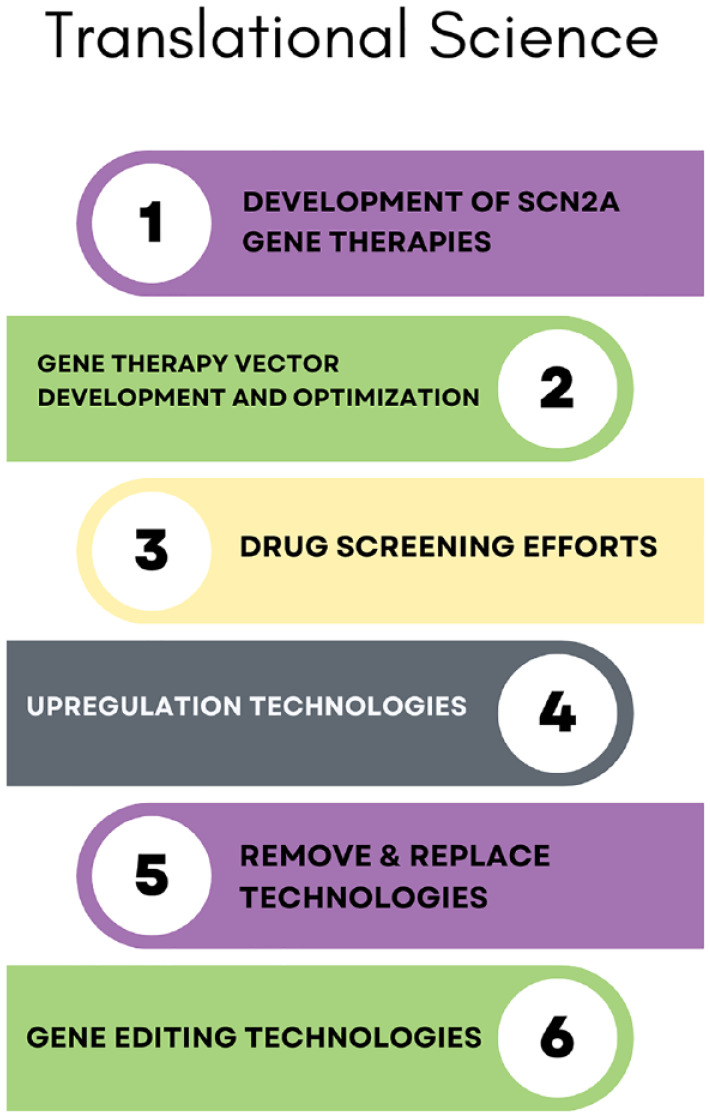
The FamilieSCN2A Foundation comprehensive and multifaceted approach to supporting translational research readiness.
Illustration of FamilieSCN2A Foundation’s muti-faceted approach to supporting translational science, each described in greater detail in the publication.
Development of SCN2A Gene Therapies: Funding initiatives are directed toward projects aiming to develop gene therapies specifically focused on rescuing SCN2A expression or correcting a downstream pathway affected by SCN2A-related disorders.
Gene Therapy Vector Development and Optimization: The FamilieSCN2A Foundation supports research endeavors focused on optimizing gene therapy vectors and their tropisms, a crucial component in the effective delivery of therapeutic interventions. This is particularly important for relatively large genes such as SCN2A, which may lead to limitations in terms of vector transformation efficiency, stability, and the capacity to accommodate additional genetic elements.
Drug screening efforts: Investments are made in projects involving the screening of potential drugs, seeking compounds that show promise for both safety and efficacy, and addressing the manifestations of SCN2A-related disorders.
Upregulation technologies: Research initiatives that explore upregulation technologies as a potential therapeutic strategy for individuals afflicted with loss of function mutations in the SCN2A gene are supported. One copy of a patient’s SCN2A gene becomes compromised in cases of loss of function mutations, thus the proposed upregulation technologies seek to elevate the expression levels of the SCN2A gene, aiming to rectify the dysfunctional sodium channels.
Remove and replace technologies: The FamilieSCN2A Foundation supports efforts in developing technologies that involve the removal and replacement of genetic components. These technologies hold the long-term potential for creating treatments that are not only mutation-agnostic but also independent of the functional status of the pathogenic variant within the SCN2A gene. Thus, these technologies offer promise as a corrective therapy for all SCN2A patients.
Gene editing technologies: The FamilieSCN2A Foundation is actively involved in supporting projects related to gene editing technologies. This includes efforts to advance and optimize technologies that allow for precise editing of the SCN2A gene, potentially correcting genetic abnormalities and offering new avenues for therapeutic intervention.
In addition to these direct funding initiatives, the FamilieSCN2A Foundation is actively involved in advancing preclinical assay development. Preclinical assays to evaluate the therapeutic impact on autism spectrum disorders associated with SCN2A are an area of investigation. These strategic investments ensure that prospective therapeutics are able to undergo rigorous testing for the clinical phenotypes in which the patient community is interested.
Furthermore, the FamilieSCN2A Foundation invests in the creation and characterization of preclinical models, including patient-derived cell lines and animal models. These models serve as valuable tools for subsequent research, offering insights into the biological and behavioral aspects of SCN2A-related disorders and paving the way for more targeted and impactful treatments for individuals affected by SCN2A-associated conditions.
Clinical trial research
Overview
Clinical trial research evaluates whether potential therapeutics are efficacious in a disease state and determines whether they have a clearly favorable risk/benefit safety profile. The vast majority of clinical trial research is performed by biotech and pharmaceutical sponsors but can occasionally also be performed by clinician/institute-sponsored trials and n = 1 studies.
FamilieSCN2A foundation efforts into clinical trial research readiness
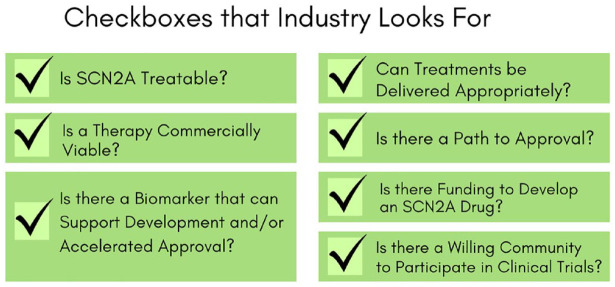
The FamilieSCN2A Foundation actively enhances the preparedness for clinical trial research by strategically positioning SCN2A-related disorders to be appealing to drug developers. Our initiatives toward this goal involves systematically addressing the criteria that the industry seeks when choosing indications (Figure 8). Additionally, we proactively seek interest in SCN2A-related disorders from both drug developers and biotech entities, especially those with promising molecules or innovative platforms. Through these concerted efforts, we aim to create an environment that attracts investment and collaboration, fostering the development of effective therapies for SCN2A-related disorders.
Figure 8.
Diagram of criteria that the FamilieSCN2A Foundation addresses attracts industry investment and collaboration.
Illustration of elements that FamilieSCN2A Foundation believes make SCN2A an attractive target for drug development, each described in greater detail in the publication.
Is SCN2A treatable?
FamilieSCN2A can confidently check the box for treatability of SCN2A-related disorders based on compelling evidence using multiple lines of support, including: (1) Clinical amenability, 6 (2) Proof of concept demonstrated in multiple animal models10–12 and (3) Emerging clinical data investigating novel therapeutics. 16 Studies in several preclinical animal models using multiple therapeutic approaches have demonstrated that the SCN2A condition can be rescued beyond what current therapeutics are able to achieve in humans. Additionally, early clinical trial data with an antisense oligonucleotides (ASO) knockdown approach has yielded very promising results.
Some symptoms (such as epilepsy) associated with SCN2A have been proven, to varying degrees, amenable to therapeutic intervention by commercially approved medicines.6,7 Nevertheless, intractable epilepsy and other clinical and behavioral challenges associated with SCN2A-related disorders are without treatment options, and therefore, significant unmet needs remain.
Can treatments be delivered appropriately?
To check the box on delivery, FamilieSCN2A Foundation has to consider the class of therapy. Different classes of drugs have different pharmacokinetic and pharmacodynamic properties, bioavailability, and delivery profiles when evaluating the potential to be dosed effectively.
Small-molecule drugs have proven to be a very effective class of therapeutics for neurology indications and thus it is widely accepted that small-molecule drugs can cross the blood-brain barrier and be designed for neurological conditions such as SCN2A-related disorders.
In addition, there are multiple subclasses of genetic medicines utilizing different delivery vehicles that have the potential to be therapeutic for SCN2A-related disorders. ASOs can be delivered with or without the aid of a delivery vehicle. There is an ongoing clinical-stage trial in SCN2A-related disorders by Praxis Precision Medicines using non-conjugated ASOs that have promising early data, 16 additionally there are a number of other separate n = 1 trials also ongoing using ASO that are still very early. These results provide early supportive evidence for deliverability for non-conjugated ASOs for SCN2A-related disorders. Conjugates that facilitate delivery of ASOs to brain and neuronal tissues are in development but are currently less validated approaches.
Genetic medications may also be delivered with the aid of viral vectors. AAV9 is generally considered to be the most validated viral vector and has yielded drug approvals in neurology. While AAV9 may prove to have utility for several different genetic approaches to treat SCN2A-related disorders, there are payload limitations that prevent its delivery of full-length SCN2A,20,21 a relatively large gene. FamilieSCN2A Foundation is investing in vector discovery and optimization efforts that have larger capacity limits and appropriate tropisms.
Is a potential therapy commercially viable?
FamilieSCN2A Foundation is confident that there is an attractive market opportunity to justify the development and commercialization risk of therapeutic development. There are an estimated ~16,000 patients with SCN2A Related Disorders in the US and a similar number in the EU5 (UK, France, Germany, Italy, and Spain). Using a multiple of peak sales drug valuation methodology that discounts back to today at a 9% rate, an SCN2A small-molecule that had a broad SCN2A label that reaches near-peak sales in 2033 and has a market penetration of 35% of eligible patients would have an approximate valuation of ~$800M, gene therapy with a label for SCN2A loss of function that reaches near-peak sales in 2035 and market penetration of 20% of eligible patients would have an approximate valuation of ~$2.9B, and an SCN2A genetic medicine for SCN2A gain of function that reaches near-peak sales in 2032 and market penetration of 20% of eligible patients would have an approximate valuation of ~$350M.
Is there a path to approval?
SCN2A is one of the leading monogenic causes of epilepsy 25 and a top monogenic cause of autism.22–24 There are pivotal pathways around seizures that are well validated and have supported the approval of numerous therapeutics. Autism spectrum disorder associated with SCN2A has a less clear path to approval from a pivotal endpoint standpoint. FamilieSCN2A Foundation has carried out meaningful work with our CTRS evaluating and optimizing pre-existing endpoints. Our work has identified clinician-led Vineland-3 Adaptive Behavior Scales (VABS-3) using gross-scale values as an instrument that has minimal floor effects and good resolution of the different clinical phenotypes in SCN2A-related disorders. 15 There are continued efforts to validate this and other endpoints in the context of SCN2A-related disorders by FamilieSCN2A Foundation and numerous other industry groups.
Is there a biomarker that can support development and/or accelerated approval?
Biomarkers are critical indicators for drug development, they can represent early signals of efficacy, or serve as the basis for patient stratification, by identifying best responders to certain therapeutics. Validated biomarkers that are shown to tightly correlate with clinical outcomes may be utilized to support accelerated approval by the FDA. FamilieSCN2A Foundation has supported multiple efforts in SCN2A biomarker development and validation. Two concepts that FamilieSCN2A Foundation has been working diligently to support and appear very promising include electroencephalogram (EEG) characterization17,18 and defining changes in Vestibulo-Occular Reflex (VoR) associated with loss of SCN2A function. 11
Is there funding to develop an SCN2A drug?
Drug development and biotech companies require capital market support to meet the financial needs of developing and commercializing a therapy. While capital markets are influenced by many macro factors and can be volatile, we find it very supportive that in 2023, two of the top three categories of drug approvals were neurology and genetics. Based on recent trends, we believe this is a very supportive financial environment to advance therapeutics for SCN2A-related disorders.
Is there a willing community to participate in clinical trials?
FamilieSCN2A Foundation has worked diligently to ensure the SCN2A community is clinical trial ready. Specifically, we have built our GET Ready plan which consists of Goals of Care, Education & Empowerment, and Trust that we are rolling out to our community. Resources and educational efforts that FamilieSCN2A Foundation has delivered to the SCN2A community include:
FamilieSCN2A foundation’s family and professional conference: Primary themes of the FamilieSCN2A Foundation conference are clinical trial readiness, and patient & caregiver empowerment. Sessions and workshops supporting these themes include education and supportive sessions on goals of care, including mock patient-clinician sessions modeling frequently asked questions and answers giving families the confidence to discuss and advocate for the goals of care that are important to that family. The conference also has key sessions around helping families determine what meaningful change would look like to them. Once patients and caregivers have the thought framework around their goals of care and what is meaningful, it enables more constructive conversations about the rewards and risks of clinical trial participation. There are also educational sessions on how to learn more about both ongoing and upcoming clinical trials through the resources that FamilieSCN2A Foundation has created. Industry groups also give updates and education around different therapeutics currently under investigation for the treatment of SCN2A-related disorders.
Other efforts toward our GET Ready plan are 2×/ month FamilieSCN2A Foundation’s community town halls, HopeRX table talks, newsletters, and communication efforts to the community including many 1 × 1 calls direct to patients and caregivers. Additionally, FamilieSCN2A Foundation’s website www.scn2a.org has multiple layers of resources for the SCN2A community that support clinical trial readiness and community empowerment.
Strategic capital deployment and ROI analysis
Overview
As the list of rare monogenic diseases grows, there is an increasing need for next-generation patient advocacy groups that act as ecosystem makers and enablers. If done right, patient advocacy can rapidly create the infrastructure and culture in the ecosystem to rapidly support the development of highly therapeutic novel treatments and cures that address the unmet needs of the patient community. This is particularly evident with the FDA’s heightened emphasis on patient-focused drug development, clinical outcomes assessments, and patient-centered outcomes. Regulators such as the FDA have even issued guidance that encourages the inclusion of patients, caregivers, and patient advocacy organizations in the drug development process.
FamilieSCN2A Foundation has diligently adapted this model and is working tirelessly to create the infrastructure that supports rapid and sustainable drug development for SCN2A-related disorders. A crucial component of the next-generation patient advocacy model is thoughtful and strategic capital deployment.
FamilieSCN2A foundation capital deployment and return on investment
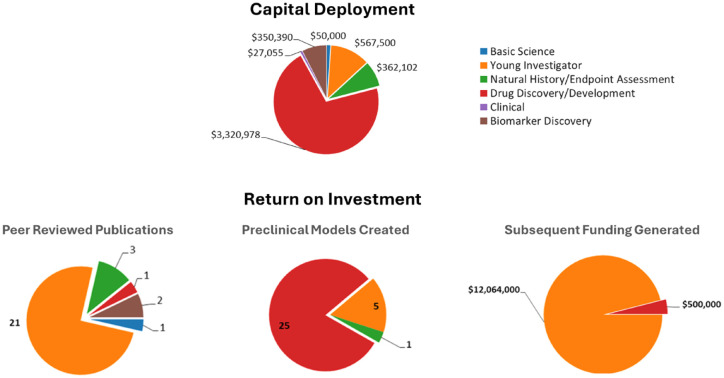
Since 2018, The FamilieSCN2A Foundation has awarded ~$4.6 million in grants, supporting 26 projects aimed at advancing and accelerating research on SCN2A-related disorders (Figure 9). This funding has laid a foundation of knowledge and resources and created a self-perpetuating cycle that is rapidly propelling the FamilieSCN2A Foundation toward its vision of a world with effective treatments and cures for SCN2A-related disorders.
Figure 9.
Strategic capital deployment and ROI analysis.
Capital deployment for the FamilieSCN2A Foundation grants and analysis of some return on investment key performance indicators.
Colors investment legend: Blue: Basic science investments; Orange: Young investigator investments; Green: Natural History/Endpoint Assessment; Red: Drug discovery/Development; Purple: Clinical; Brown: Biomarker Discovery.
Insights gleaned from investments to date
Many of FamilieSCN2A Foundation’s investments are still maturing, and the returns may be unique to our sector or situation. These factors need to be considered alongside any insights gleaned to date. With these caveats in mind, the two most significant trends in our data are:
Investments in early-career researchers continue to yield substantial returns.
Investments in drug discovery generate additional value beyond evaluating the potential of new therapeutics.
There has been a recent shift away from investing in young researchers in favor of direct funding for drug development. While this strategy has proven successful in other areas and should indeed be a part of every portfolio, for SCN2A, FamilieSCN2A’s investments in young researchers have been crucial for building the research ecosystem. These investments have greatly increased research into SCN2A and led to a substantial rise in the overall capital allocated to SCN2A research. We believe this, in turn, boosts the number of researchers in the field, enhances the quality of research, and increases the interest in drug development.
When investing directly in drug discovery and development, the potential rewards come with high risks. Our experience suggests that these high-risk investments also create valuable assets, such as preclinical models, which can further advance basic research and future development. It is crucial for the foundation to secure contractual rights to any models developed during these projects.
Lastly, our ROI analysis is limited in scope. Many investments target key gaps in the field, which can be invaluable to the organization’s mission but may show limited returns in terms of publications, subsequent funding, and preclinical models.
Gaps to a cure
It is reasonable to believe that we are entering a golden age for rare disease treatments and cures. Driven by major advancements in the fields of precision genetic medicine, preclinical model development, artificial intelligence, and diagnostics, the potential for rapid development of transformative medicines has never been higher. With SCN2A research advancing rapidly and a thriving ecosystem of diverse, engaged stakeholders, FamilieSCN2A Foundation believes the outlook for SCN2A-related disorders is bright. However, today there remain friction points to a potential cure that FamilieSCN2A Foundation has identified. These include:
FDA support of clinical trial designs that achieve a broad SCN2A label. There are pivotal regulatory pathways for some SCN2A-related disorder symptoms, such as seizures. However, not all patients consistently present with seizures, and many may never present with seizures in their life, but all still have significant unmet needs.5,6,19 With many root cause therapeutics in development there is a need to continue to develop and validate endpoints that demonstrate a benefit across the entire spectrum of SCN2A-related disorders. Validated endpoints would ensure that broadly effective therapeutics are able to be tested and approved to benefit the greatest number of SCN2A-related disorder patients as possible.
Diagnosis in those without epilepsy. Epilepsy has been a very effective trigger for patients and clinicians to pursue genetic testing. This has greatly increased the total number of SCN2A patients diagnosed and shortened the time to diagnosis. However, many patients with SCN2A-related disorder are believed to never present with seizures5,6,19 and thus, never received an SCN2A genetic diagnosis. As novel and potentially transformative therapeutics continue to mature, it will become increasingly important to find new ways to facilitate genetic testing in potential SCN2A patients.
Consensus on variant characterization interpretations. Patch-clamping provides a wide range of electrophysiological readouts depending on the configuration and application used. Thus there remains a lack of clear consensus in the field on the boundaries that determine functional classification of different SCN2A variants. As therapeutics specific to SCN2A loss of function, mixed function, and gain of function advance, it will become increasingly important to accurately classify variants both for safety and potential efficacy of emerging therapeutics.
Optimal delivery of genetic medicines. Genetic medicines offer real hope for rare monogenic diseases such as SCN2A-related disorders. However, optimal delivery remains one of the major challenges for this therapeutic class. Because SCN2A is a relatively large gene (an SCN2A ORF is ~6 kb) and adeno-associated virus (AAV) vectors are limited by the ~4.5 kb cloning capacity20,21 additional work needs to be done to identify and optimize novel vectors that can deliver larger payloads to the correct regions of the brain and nervous system.
FamilieSCN2A Foundation is strategically positioned, maintaining a vigilant awareness of the ecosystem through active partnerships, relationship-building, research liaison roles, conferences, and peer-reviewed publications. Each avenue provides significant visibility into potential future barriers. By focusing on projects that are topical to our community, maximizing the effort/reward ratio, and continuously lowering barriers we hope to continue to accelerate toward a future with new treatments and cures for SCN2A-related disorders.
Future goals
FamilieSCN2A Foundation is optimistic for a world where transformative therapeutics bring extended or normal lifespans, heightened capacity, and improved quality of life for those with SCN2A-related disorders. Until this future is realized, our near-term goals must seek to realize this vision. FamilieSCN2A believes that its current strategy and tactics provide the highest net probability to ensure this future for all patients with SCN2A-related disorders.
Looking into the future, the FamilieSCN2A Foundation recognizes the importance of strategic planning for success. As our patient community experiences growth in capacity and extended life spans, it becomes imperative to anticipate evolving needs. To effectively support this forthcoming scenario, the foundation will embark on diverse initiatives and educational programs tailored to elevate our community’s needs to the highest achievable standards. This forward-looking approach ensures that FamilieSCN2A is well-prepared to meet the dynamic requirements of its expanding and evolving patient community.
Acknowledgments
None.
Appendix
Abbreviations
501(c)(3) A tax-exempt nonprofit organization designation in the United States.
AES American Epilepsy Society.
ASD Autism Spectrum Disorder.
AAV9 Adeno-Associated Virus Serotype 9.
ASO Antisense Oligonucleotides.
Board of Directors The governing body of the foundation.
Charles A. King Trust A trust providing postdoctoral research fellowships.
CTRS SCN2A Clinical Trial Readiness Study.
FDA U.S. Food and Drug Administration.
Familie SCN2A Foundation The organization dedicated to improving lives affected by SCN2A-related disorders.
GoF Gain of Function mutation.
IAMRARE A platform by NORD for rare disease registries.
ID Intellectual Disability.
MDBR Million Dollar Bike Ride.
NIH National Institutes of Health.
NORD National Organization for Rare Disorders.
NaV Voltage-gated sodium channel.
Nav1.2 Voltage-gated sodium channel protein encoded by the SCN2A gene.
ODC Orphan Disease Center.
RFA Request for Applications.
ROI Return on Investment.
SCN2A A gene associated with certain genetic disorders.
SFARI Simons Foundation Autism Research Initiative.
VABS-3 Vineland-3 Adaptive Behavior Scales.
VoR Vestibulo-Occular Reflex.
Footnotes
ORCID iD: Shawn M. Egan  https://orcid.org/0009-0006-6422-809X
https://orcid.org/0009-0006-6422-809X
Contributor Information
Leah F. Schust, FamilieSCN2A Foundation 501(c)(3), Gettysburg, PA, USA
Jennifer Burke, FamilieSCN2A Foundation 501(c)(3), Gettysburg, PA, USA.
Christina SanInocencio, FamilieSCN2A Foundation 501(c)(3), Gettysburg, PA, USA.
Brad A. Bryan, FamilieSCN2A Foundation 501(c)(3), Gettysburg, PA, USA
Karen S. Ho, FamilieSCN2A Foundation 501(c)(3), Gettysburg, PA, USA
Shawn M. Egan, FamilieSCN2A Foundation 501(c)(3), 34 Patton Lane, Buffalo, NY 14225, USA.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Leah F. Schust: Conceptualization; Methodology; Project administration; Writing – original draft; Writing – review & editing.
Jennifer Burke: Conceptualization; Writing – original draft; Writing – review & editing.
Christina SanInocencio: Conceptualization; Data curation; Formal analysis; Visualization; Writing – original draft; Writing – review & editing.
Brad A. Bryan: Conceptualization; Methodology; Writing – original draft; Writing – review & editing.
Karen S. Ho: Conceptualization; Writing – original draft; Writing – review & editing.
Shawn M. Egan: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Visualization; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Grassroots funding through FamilieSCN2A Foundation. CZI Rare As One Cycle 2 Grant Recipient which funded organizational capacity.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci 2007; 8: 451–465. [DOI] [PubMed] [Google Scholar]
- 2. Hedrich UBS, Lauxmann S, Lerche H. SCN2A channelopathies: mechanisms and models. Epilepsia 2019; 60(Suppl. 3): S68–S76. [DOI] [PubMed] [Google Scholar]
- 3. Wolff M, Brunklaus A, Zuberi SM. Phenotypic spectrum and genetics of SCN2A-related disorders, treatment options, and outcomes in epilepsy and beyond. Epilepsia 2019; 60(Suppl. 3): S59–S67. [DOI] [PubMed] [Google Scholar]
- 4. Lauxmann S, Verbeek NE, Liu Y, et al. Relationship of electrophysiological dysfunction and clinical severity in SCN2A-related epilepsies. Hum Mutat 2018; 39: 1942–1956. [DOI] [PubMed] [Google Scholar]
- 5. Wolff M, Johannesen KM, Hedrich UBS, et al. Genetic and phenotypic heterogeneity suggest therapeutic implications in SCN2A-related disorders. Brain 2017; 140: 1316–1336. [DOI] [PubMed] [Google Scholar]
- 6. Berg AT, Thompson CH, Myers LS, et al. Expanded clinical phenotype spectrum correlates with variant function in SCN2A-related disorders. Brain 2024; 147(8): 2761–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brunklaus A, Feng T, Brünger T, et al. Gene variant effects across sodium channelopathies predict function and guide precision therapy. Brain 2022; 145(12): 4275–4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berg AT, Kaat AJ, Zelko F, et al. Rare diseases – rare outcomes: Assessing communication abilities for the developmental and epileptic encephalopathies. Epilepsy Behav 2022; 128: 108586. [DOI] [PubMed] [Google Scholar]
- 9. Goodchild SJ, Shuart NG, Williams AD, et al. Molecular pharmacology of selective NaV1.6 and dual NaV1.6/NaV1.2 channel inhibitors that suppress excitatory neuronal activity ex vivo. ACS Chem Neurosci 2024; 15(6): 1169–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen GT, Nair G, Osorio AJ, et al. Enhancer-targeted CRISPR-activation rescues haploinsufficient autism susceptibility genes. bioRxiv [Preprint]. 2024: 2024.03.13.584921. [Google Scholar]
- 11. Wang C, Derderian KD, Hamada E, et al. Impaired cerebellar plasticity hypersensitizes sensory reflexes in SCN2A-associated ASD. Neuron 2024; 112(9): 1444–1455.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li M, Jancovski N, Jafar-Nejad P, et al. Antisense oligonucleotide therapy reduces seizures and extends life span in an SCN2A gain-of-function epilepsy model. J Clin Invest 2021; 131(23): e152079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCluskey KE, Stovell KM, Law K, et al. Autism gene variants disrupt enteric neuron migration and cause gastrointestinal dysmotility. bioRxiv [Preprint] 2024; 2024.05.28.593642. [Google Scholar]
- 14. BIO, QLS Advisors, and Informa UK Ltd. Clinical Development Success Rates and Contributing Factors 2011-2020. BIO, https://go.bio.org/rs/490-EHZ-999/images/ClinicalDevelopmentSuccessRates2011_2020.pdf?_gl=1*oby0rn*_gcl_au*MjAxODMzMTc4Ny4xNzIzMDY0MTEx&_ga=2.136144374.696380129.1723064112-904121653.1712941324 (2021, accessed 2024)
- 15. Egan SM, Evans L, Paltell KC, et al. More than seizures: Expressive communication as a clinical trial outcome for SCN2A developmental and epileptic encephalopathies. In: 2022 AES Annual Meeting Poster Presentation, Nashville, TN. [Google Scholar]
- 16. Praxis Precision Medicine. Corporate Presentation, https://investors.praxismedicines.com/static-files/e25138bd-5451-46c4-91e4-762150f46606 (2024, accessed 2024).
- 17. Fogerson M, Tsitsiklis M, Brimble E, et al. Electroencephalographic insights into variant function and clinical outcomes in SCN2A encephalopathy. medRxiv [Preprint]. 2022. [Google Scholar]
- 18. Hudac CM, Dommer K, Mahony M, et al. Visual and auditory attention in individuals with DYRK1A and SCN2A disruptive variants. Autism Res. Epub ahead of print 30 July 2024. doi: 10.1002/aur.3202. [DOI] [PubMed] [Google Scholar]
- 19. Reynolds C, King MD, Gorman KM. The phenotypic spectrum of SCN2A-related epilepsy. Eur J Paediatr Neurol 2020; 24: 117–122. [DOI] [PubMed] [Google Scholar]
- 20. Samulski RJ, Muzyczka N. AAV-Mediated gene therapy for research and therapeutic purposes. Annu Rev Virol 2014; 1(1): 427–451. [DOI] [PubMed] [Google Scholar]
- 21. Ling Q, Herstine JA, Bradbury A, et al. AAV-based in vivo gene therapy for neurological disorders. Nat Rev Drug Discov 2023; 22(10): 789–806. [DOI] [PubMed] [Google Scholar]
- 22. Olin J. Development of a zinc finger protein transcription factor for repressing SCN2A expression. In: Poster presented at the American Society of Gene & Cell Therapy (ASGCT) Annual Meeting, 2024, https://www.sangamo.com/wp-content/uploads/2024/05/14.-Poster-SGMO_ASGCT-2024_636_Olin_SCN2A_Resized.pdf (2024, accessed 2024). [Google Scholar]
- 23. Fu JM, Satterstrom FK, Peng M, et al. Rare coding variation provides insight into the genetic architecture and phenotypic context of autism. Nat Genet 2022; 54: 1320–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Satterstrom FK, Kosmicki JA, Wang J, et al.; Autism Sequencing Consortium; iPSYCH-Broad Consortium. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell 2020; 180(3): 568–584.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Knowles JK, Helbig I, Metcalf CS, et al. Precision medicine for genetic epilepsy on the horizon: Recent advances, present challenges, and suggestions for continued progress. Epilepsia 2022; 63(10): 2461–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]