Abstract
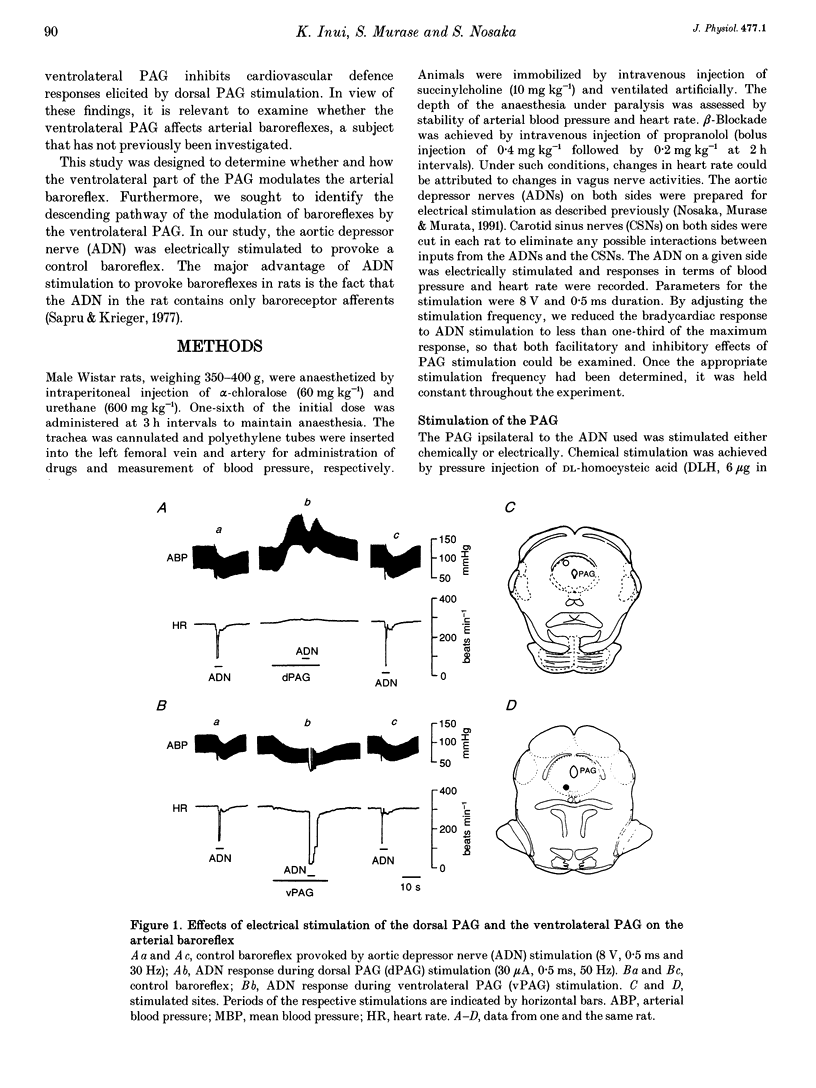
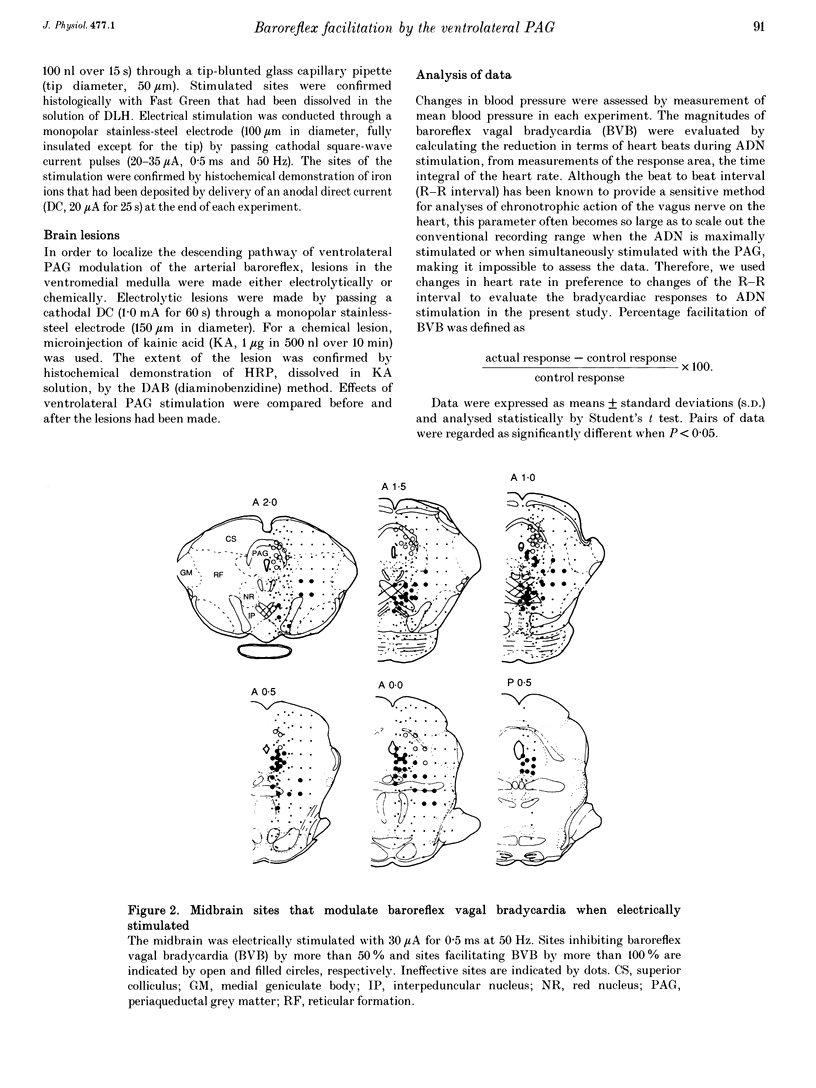
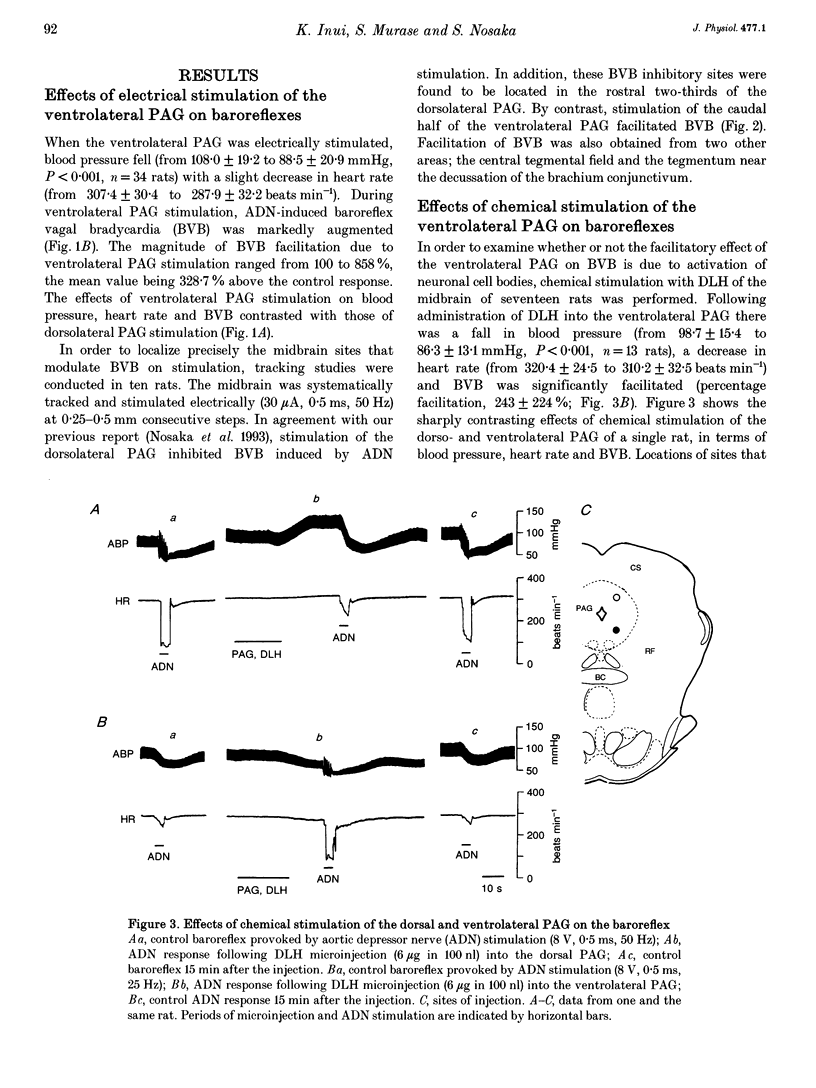
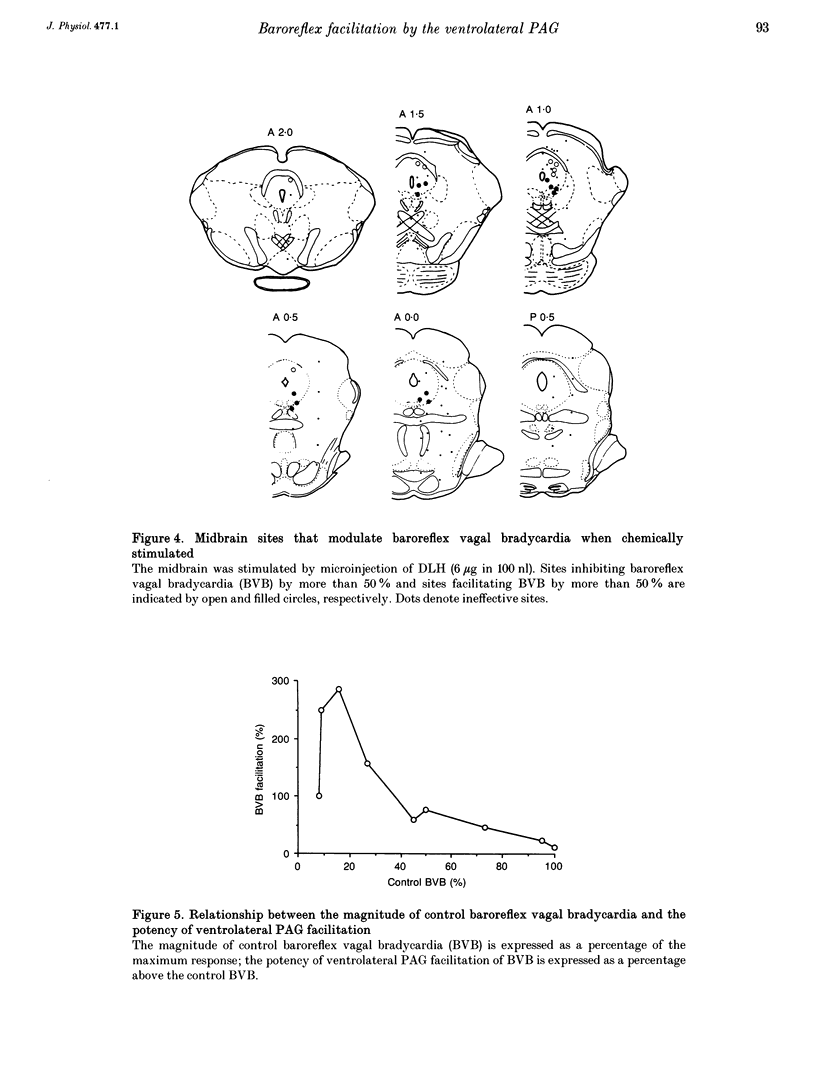
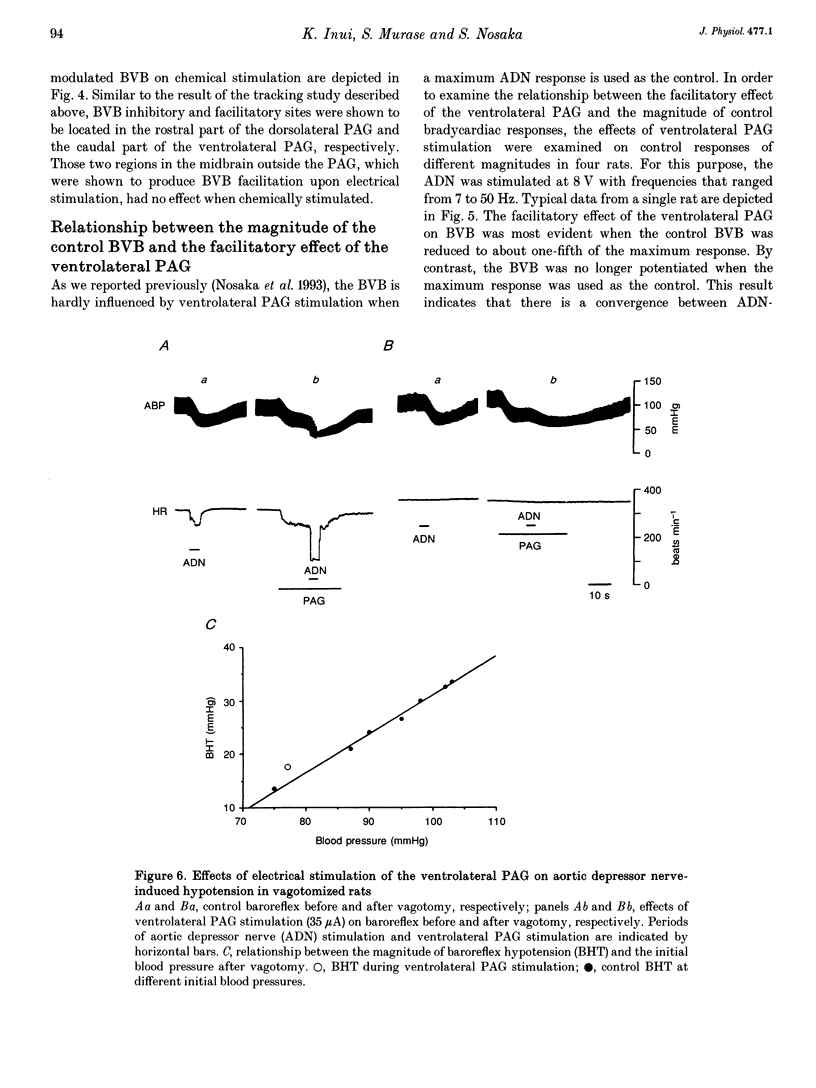
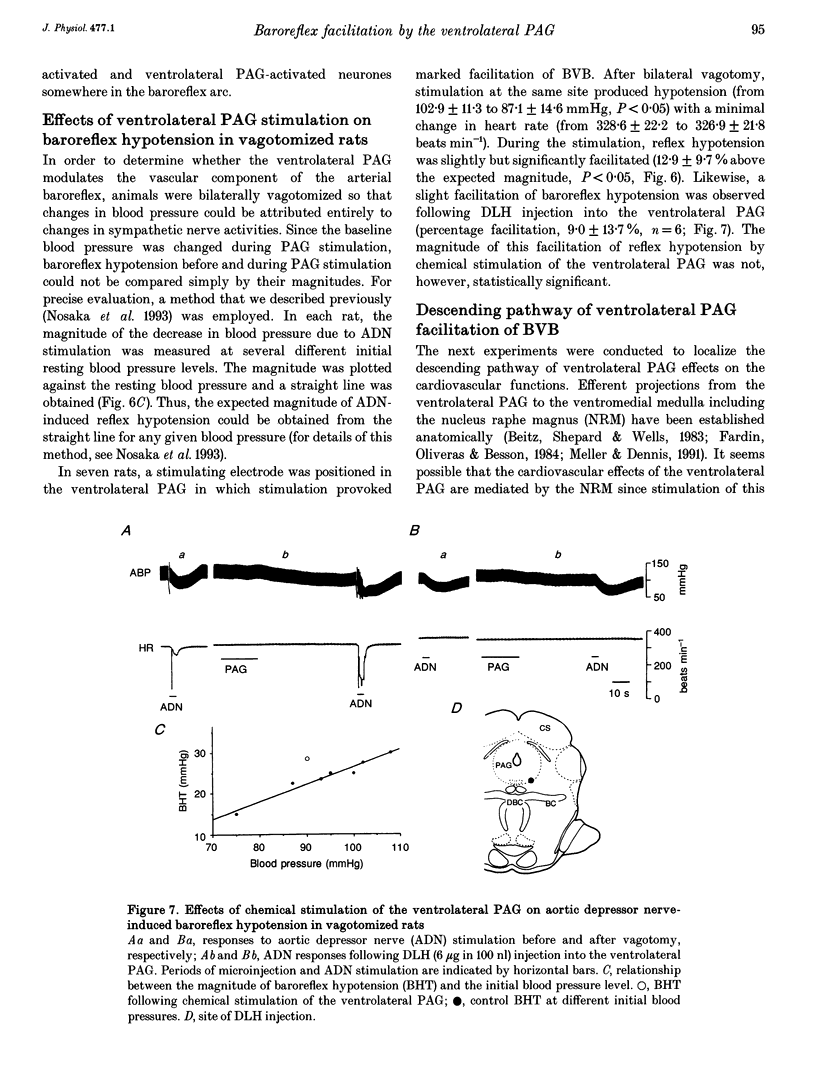
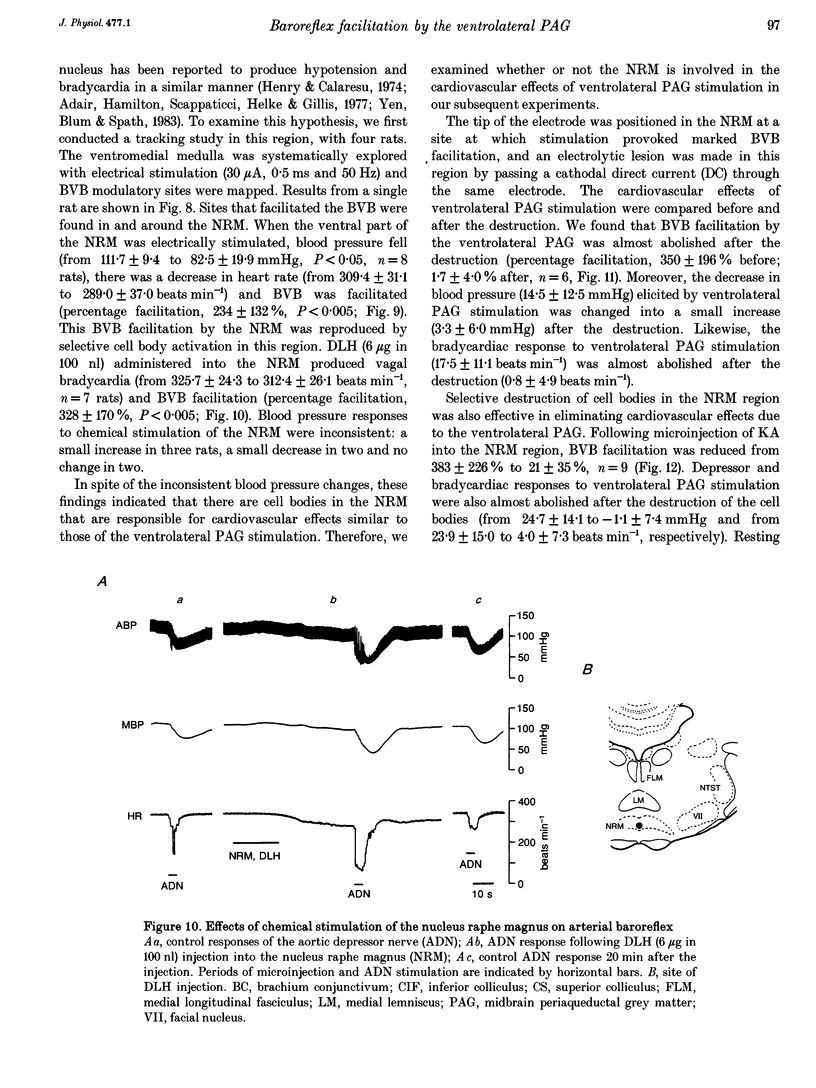
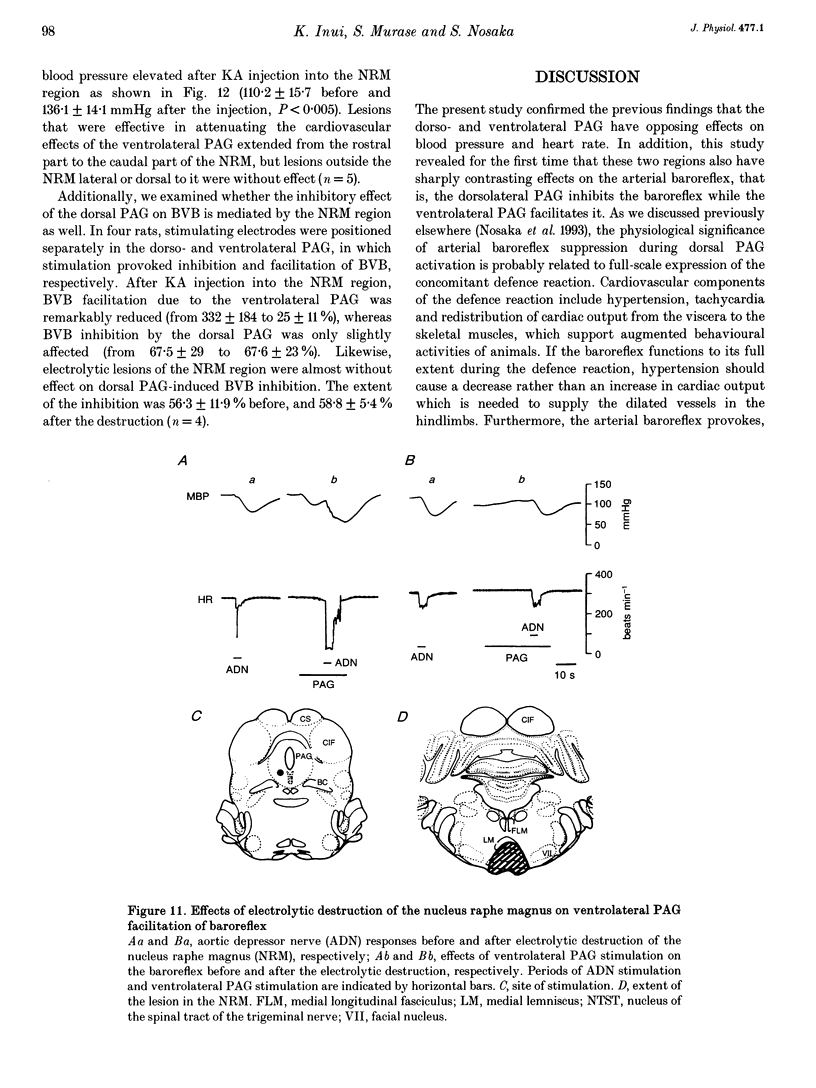
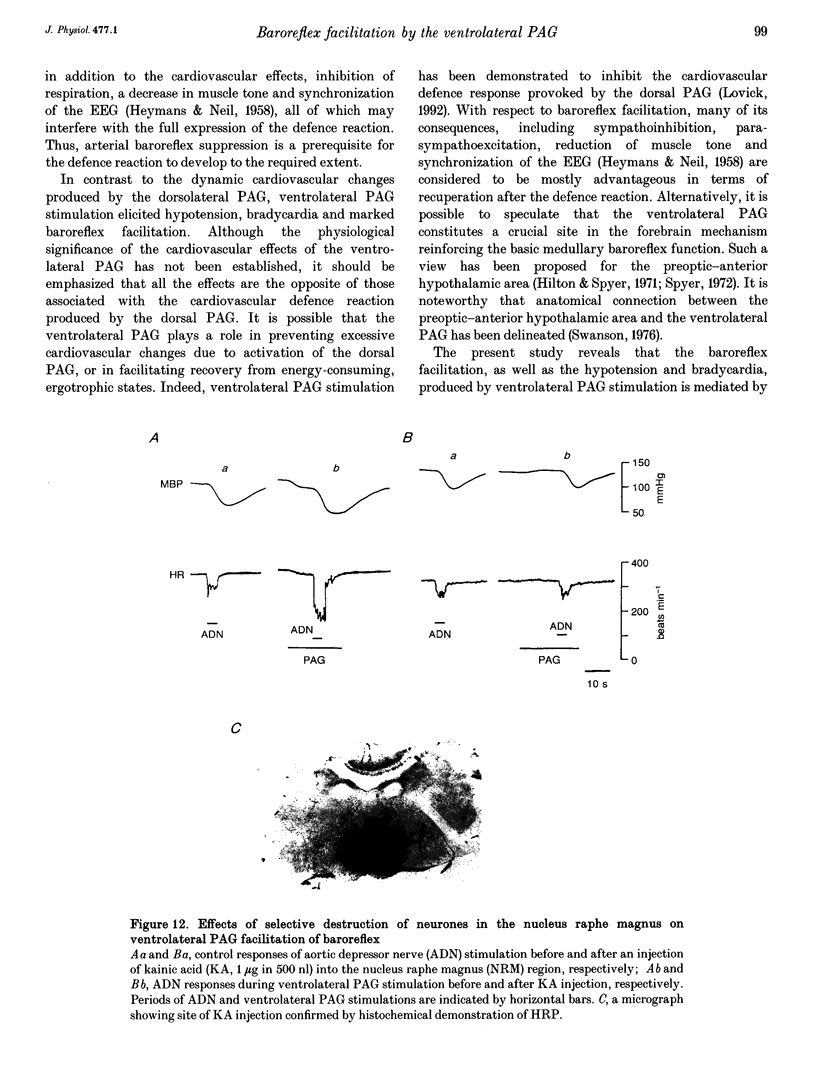
1. The effects of stimulation of the ventrolateral part of the midbrain periaqueductal grey matter (PAG) on the arterial baroreflex were investigated in urethane-chloralose anaesthetized and artificially ventilated rats. 2. Both electrical and chemical stimulation of the ventrolateral PAG provoked hypotension, vagal bradycardia and marked facilitation of baroreflex vagal bradycardia (BVB), which was induced by stimulation of the aortic depressor nerve. The magnitude of ventrolateral PAG facilitation of BVB was 328 +/- 193% (n = 34) for electrical stimulation and 243 +/- 224% (n = 13) for chemical stimulation. Baroreflex hypotension was slightly augmented during either electrical or chemical stimulation of the ventrolateral PAG in vagotomized rats. 3. Stimulation of the nucleus raphe magnus (NRM) also provoked hypotension, vagal bradycardia and facilitation of BVB. The magnitude of BVB facilitation was 234 +/- 132% (n = 8) for electrical stimulation and 328 +/- 170% (n = 7) for chemical stimulation. After microinjection of kainic acid into the NRM region, baroreflex facilitation, as well as hypotension and vagal bradycardia, produced by ventrolateral PAG stimulation, was almost abolished. 4. In conclusion, the ventrolateral PAG, besides producing hypotension and bradycardia, facilitates arterial baroreflexes. These effects are exerted via the NRM, sharply contrasting with effects of the dorsal PAG.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair J. R., Hamilton B. L., Scappaticci K. A., Helke C. J., Gillis R. A. Cardiovascular responses to electrical stimulation of the medullary raphe area of the cat. Brain Res. 1977 Jun 3;128(1):141–145. doi: 10.1016/0006-8993(77)90241-4. [DOI] [PubMed] [Google Scholar]
- Bandler R., Carrive P. Integrated defence reaction elicited by excitatory amino acid microinjection in the midbrain periaqueductal grey region of the unrestrained cat. Brain Res. 1988 Jan 26;439(1-2):95–106. doi: 10.1016/0006-8993(88)91465-5. [DOI] [PubMed] [Google Scholar]
- Bandler R., Carrive P., Zhang S. P. Integration of somatic and autonomic reactions within the midbrain periaqueductal grey: viscerotopic, somatotopic and functional organization. Prog Brain Res. 1991;87:269–305. doi: 10.1016/s0079-6123(08)63056-3. [DOI] [PubMed] [Google Scholar]
- Beitz A. J., Shepard R. D., Wells W. E. The periaqueductal gray-raphe magnus projection contains somatostatin, neurotensin and serotonin but not cholecystokinin. Brain Res. 1983 Feb 14;261(1):132–137. doi: 10.1016/0006-8993(83)91292-1. [DOI] [PubMed] [Google Scholar]
- Carrive P., Bandler R., Dampney R. A. Somatic and autonomic integration in the midbrain of the unanesthetized decerebrate cat: a distinctive pattern evoked by excitation of neurones in the subtentorial portion of the midbrain periaqueductal grey. Brain Res. 1989 Apr 3;483(2):251–258. doi: 10.1016/0006-8993(89)90169-8. [DOI] [PubMed] [Google Scholar]
- Coote J. H., Hilton S. M., Perez-Gonzalez J. F. Inhibition of the baroreceptor reflex on stimulation in the brain stem defence centre. J Physiol. 1979 Mar;288:549–560. [PMC free article] [PubMed] [Google Scholar]
- Fardin V., Oliveras J. L., Besson J. M. Projections from the periaqueductal gray matter to the B3 cellular area (nucleus raphe magnus and nucleus reticularis paragigantocellularis) as revealed by the retrograde transport of horseradish peroxidase in the rat. J Comp Neurol. 1984 Mar 10;223(4):483–500. doi: 10.1002/cne.902230403. [DOI] [PubMed] [Google Scholar]
- Gallager D. W., Pert A. Afferents to brain stem nuclei (brain stem raphe, nucleus reticularis pontis caudalis and nucleus gigantocellularis) in the rat as demonstrated by microiontophoretically applied horseradish peroxidase. Brain Res. 1978 Apr 14;144(2):257–275. doi: 10.1016/0006-8993(78)90153-1. [DOI] [PubMed] [Google Scholar]
- Gebber G. L., Snyder D. W. Hypothalamic control of baroreceptor reflexes. Am J Physiol. 1970 Jan;218(1):124–131. doi: 10.1152/ajplegacy.1970.218.1.124. [DOI] [PubMed] [Google Scholar]
- Henry J. L., Calaresu F. R. Excitatory and inhibitory inputs from medullary nuclei projecting to spinal cardioacceleratory neurons in the cat. Exp Brain Res. 1974;20(5):485–504. doi: 10.1007/BF00238015. [DOI] [PubMed] [Google Scholar]
- Hilton S. M., Redfern W. S. A search for brain stem cell groups integrating the defence reaction in the rat. J Physiol. 1986 Sep;378:213–228. doi: 10.1113/jphysiol.1986.sp016215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton S. M., Spyer K. M. Participation of the anterior hypothalamus in the baroreceptor reflex. J Physiol. 1971 Oct;218(2):271–293. doi: 10.1113/jphysiol.1971.sp009617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys P. W., Joels N., McAllen R. M. Modification of the reflex response to stimulation of carotid sinus baroreceptors during and following stimulation of the hypothalamic defence area in the cat. J Physiol. 1971 Jul;216(2):461–482. doi: 10.1113/jphysiol.1971.sp009535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo P. N., Deuchars J., Spyer K. M. Localization of cardiac vagal preganglionic motoneurones in the rat: immunocytochemical evidence of synaptic inputs containing 5-hydroxytryptamine. J Comp Neurol. 1993 Jan 22;327(4):572–583. doi: 10.1002/cne.903270408. [DOI] [PubMed] [Google Scholar]
- Jones R. O., Kirkman E., Little R. A. The involvement of the midbrain periaqueductal grey in the cardiovascular response to injury in the conscious and anaesthetized rat. Exp Physiol. 1990 Jul;75(4):483–495. doi: 10.1113/expphysiol.1990.sp003425. [DOI] [PubMed] [Google Scholar]
- Kumada M., Nogami K., Sagawa K. Modulation of carotid sinus baroreceptor reflex by sciatic nerve stimulation. Am J Physiol. 1975 May;228(5):1535–1541. doi: 10.1152/ajplegacy.1975.228.5.1535. [DOI] [PubMed] [Google Scholar]
- Lai Y. Y., Siegel J. M. Cardiovascular and muscle tone changes produced by microinjection of cholinergic and glutamatergic agonists in dorsolateral pons and medial medulla. Brain Res. 1990 Apr 23;514(1):27–36. doi: 10.1016/0006-8993(90)90432-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley P. M. Responses of phrenic motoneurones of the cat to stimulation of medullary raphe nuclei. J Physiol. 1986 Nov;380:349–371. doi: 10.1113/jphysiol.1986.sp016290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovick T. A. Inhibitory modulation of the cardiovascular defence response by the ventrolateral periaqueductal grey matter in rats. Exp Brain Res. 1992;89(1):133–139. doi: 10.1007/BF00229010. [DOI] [PubMed] [Google Scholar]
- Lovick T. A. Integrated activity of cardiovascular and pain regulatory systems: role in adaptive behavioural responses. Prog Neurobiol. 1993 May;40(5):631–644. doi: 10.1016/0301-0082(93)90036-r. [DOI] [PubMed] [Google Scholar]
- Lovick T. A. Serotonergic influence from nucleus raphe obscurus on neurones in the periaqueductal grey matter in the rat. Brain Res. 1993 Mar 19;606(1):92–98. doi: 10.1016/0006-8993(93)91574-c. [DOI] [PubMed] [Google Scholar]
- Meller S. T., Dennis B. J. Efferent projections of the periaqueductal gray in the rabbit. Neuroscience. 1991;40(1):191–216. doi: 10.1016/0306-4522(91)90185-q. [DOI] [PubMed] [Google Scholar]
- Nosaka S., Murase S., Murata K. Arterial baroreflex inhibition by gastric distension in rats: mediation by splanchnic afferents. Am J Physiol. 1991 May;260(5 Pt 2):R985–R994. doi: 10.1152/ajpregu.1991.260.5.R985. [DOI] [PubMed] [Google Scholar]
- Nosaka S., Murata K., Inui K., Murase S. Arterial baroreflex inhibition by midbrain periaqueductal grey in anaesthetized rats. Pflugers Arch. 1993 Aug;424(3-4):266–275. doi: 10.1007/BF00384352. [DOI] [PubMed] [Google Scholar]
- Nosaka S., Murata K. Somatosensory inhibition of vagal baroreflex bradycardia: afferent nervous mechanisms. Am J Physiol. 1989 Oct;257(4 Pt 2):R829–R838. doi: 10.1152/ajpregu.1989.257.4.R829. [DOI] [PubMed] [Google Scholar]
- Nosaka S., Nakase N., Murata K. Somatosensory and hypothalamic inhibitions of baroreflex vagal bradycardia in rats. Pflugers Arch. 1989 Apr;413(6):656–666. doi: 10.1007/BF00581817. [DOI] [PubMed] [Google Scholar]
- Prieto G. J., Cannon J. T., Liebeskind J. C. N. raphe magnus lesions disrupt stimulation-produced analgesia from ventral but not dorsal midbrain areas in the rat. Brain Res. 1983 Feb 14;261(1):53–57. doi: 10.1016/0006-8993(83)91282-9. [DOI] [PubMed] [Google Scholar]
- Sakuma Y., Pfaff D. W. Facilitation of female reproductive behavior from mesensephalic central gray in the rat. Am J Physiol. 1979 Nov;237(5):R278–R284. doi: 10.1152/ajpregu.1979.237.5.R278. [DOI] [PubMed] [Google Scholar]
- Sapru H. N., Krieger A. J. Carotid and aortic chemoreceptor function in the rat. J Appl Physiol Respir Environ Exerc Physiol. 1977 Mar;42(3):344–348. doi: 10.1152/jappl.1977.42.3.344. [DOI] [PubMed] [Google Scholar]
- Shah Y., Dostrovsky J. O. Electrophysiological evidence for a projection of the periaqueductal gray matter to nucleus raphe magnus in cat and rat. Brain Res. 1980 Jul 14;193(2):534–538. doi: 10.1016/0006-8993(80)90183-3. [DOI] [PubMed] [Google Scholar]
- Spyer K. M. Baroreceptor sensitive neurones in the anterior hypothalamus of the cat. J Physiol. 1972 Jul;224(1):245–257. doi: 10.1113/jphysiol.1972.sp009892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L. W. An autoradiographic study of the efferent connections of the preoptic region in the rat. J Comp Neurol. 1976 May 15;167(2):227–256. doi: 10.1002/cne.901670207. [DOI] [PubMed] [Google Scholar]
- Wiklund L., Behzadi G., Kalén P., Headley P. M., Nicolopoulos L. S., Parsons C. G., West D. C. Autoradiographic and electrophysiological evidence for excitatory amino acid transmission in the periaqueductal gray projection to nucleus raphe magnus in the rat. Neurosci Lett. 1988 Nov 11;93(2-3):158–163. doi: 10.1016/0304-3940(88)90074-2. [DOI] [PubMed] [Google Scholar]
- Yen C. T., Blum P. S., Spath J. A., Jr Control of cardiovascular function by electrical stimulation within the medullary raphe region of the cat. Exp Neurol. 1983 Mar;79(3):666–679. doi: 10.1016/0014-4886(83)90031-6. [DOI] [PubMed] [Google Scholar]