Abstract
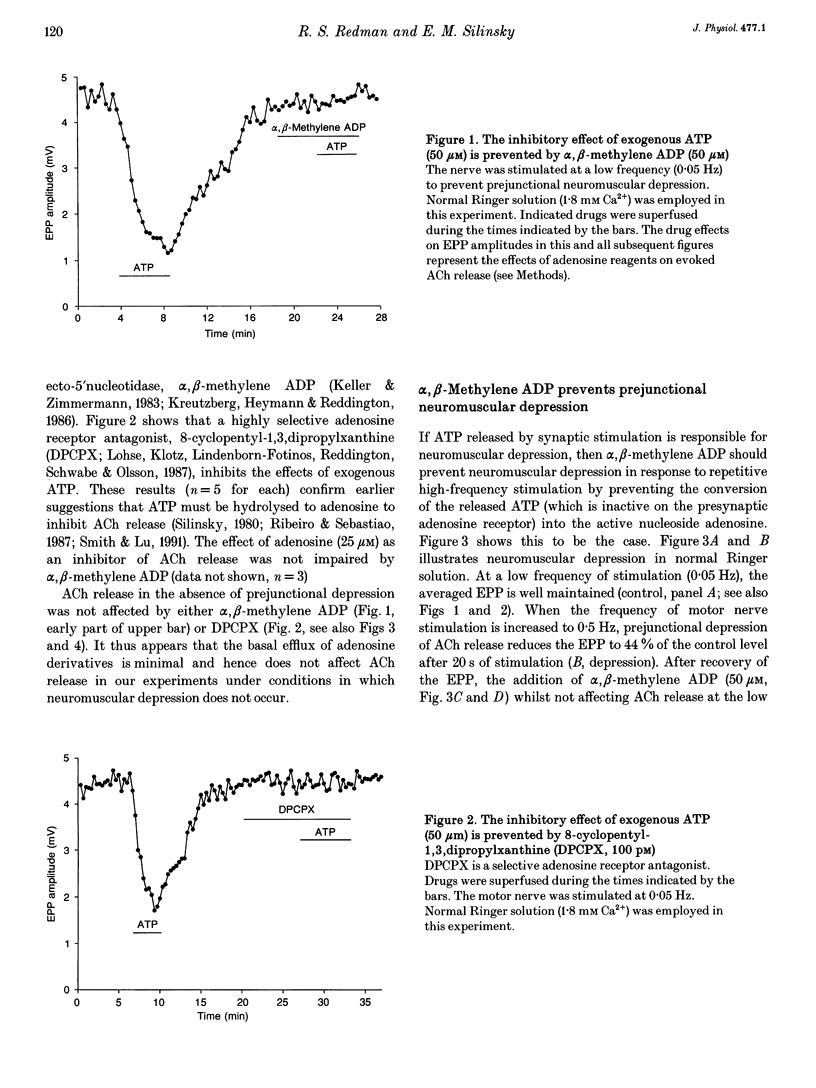
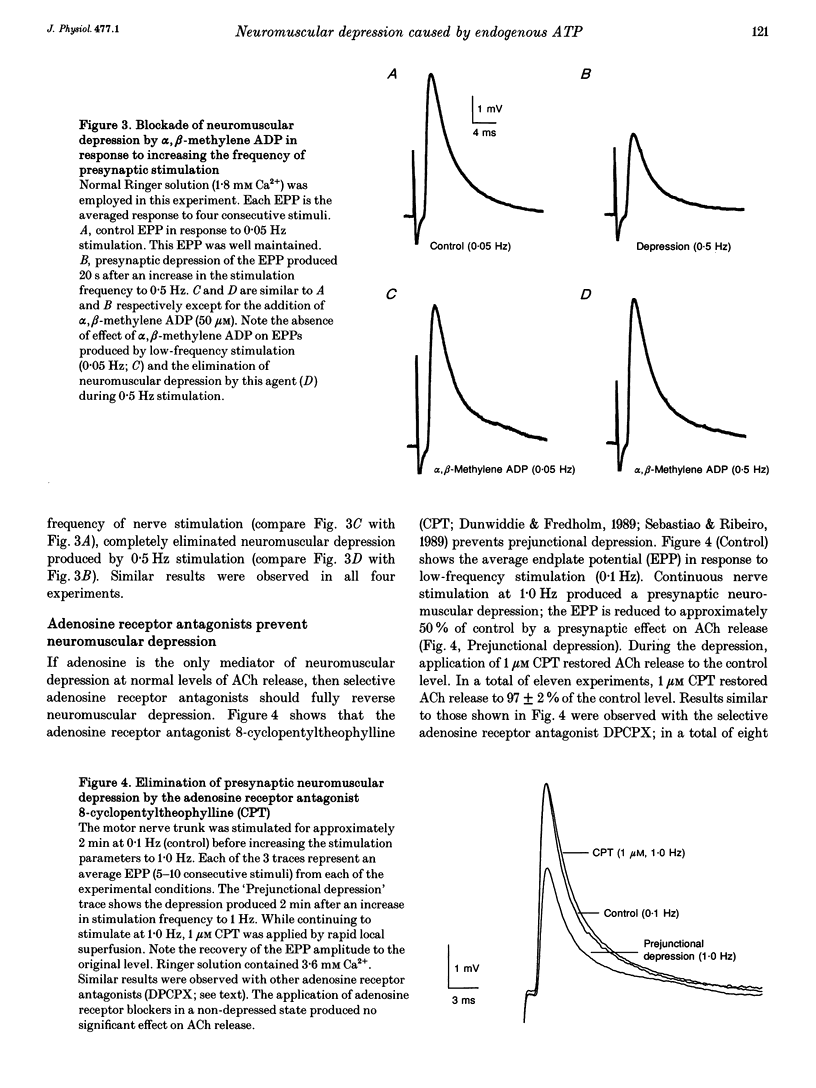
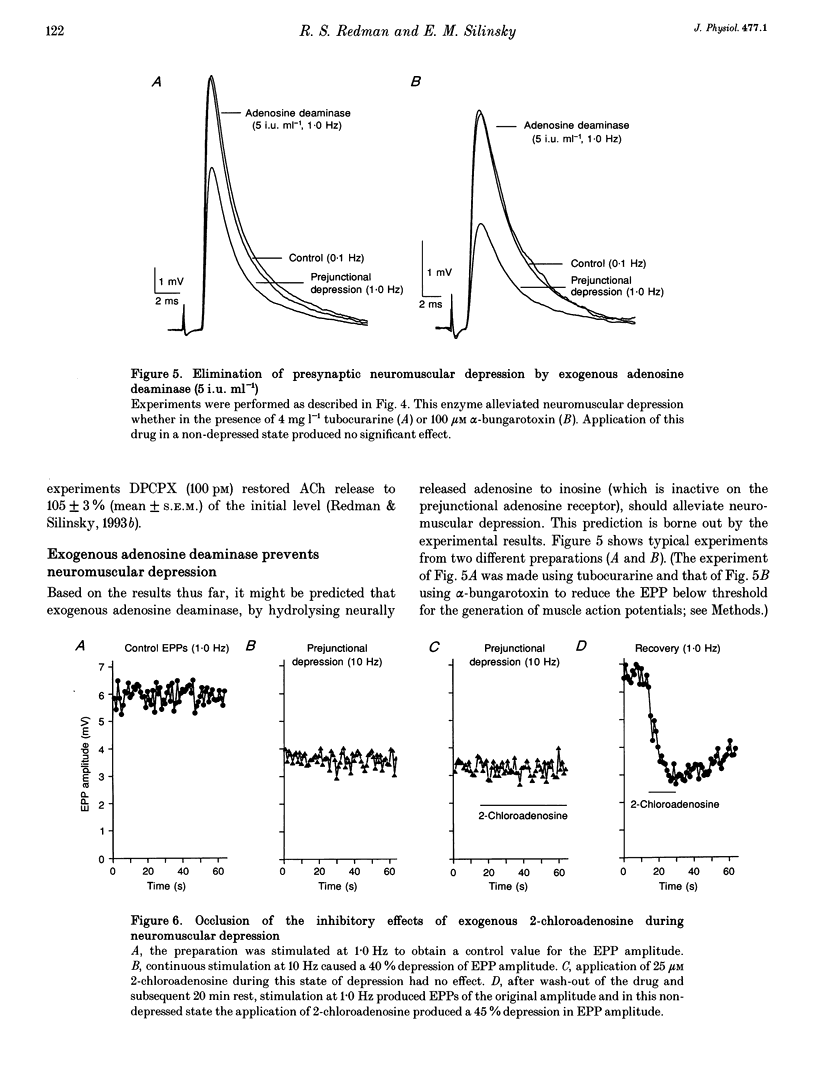
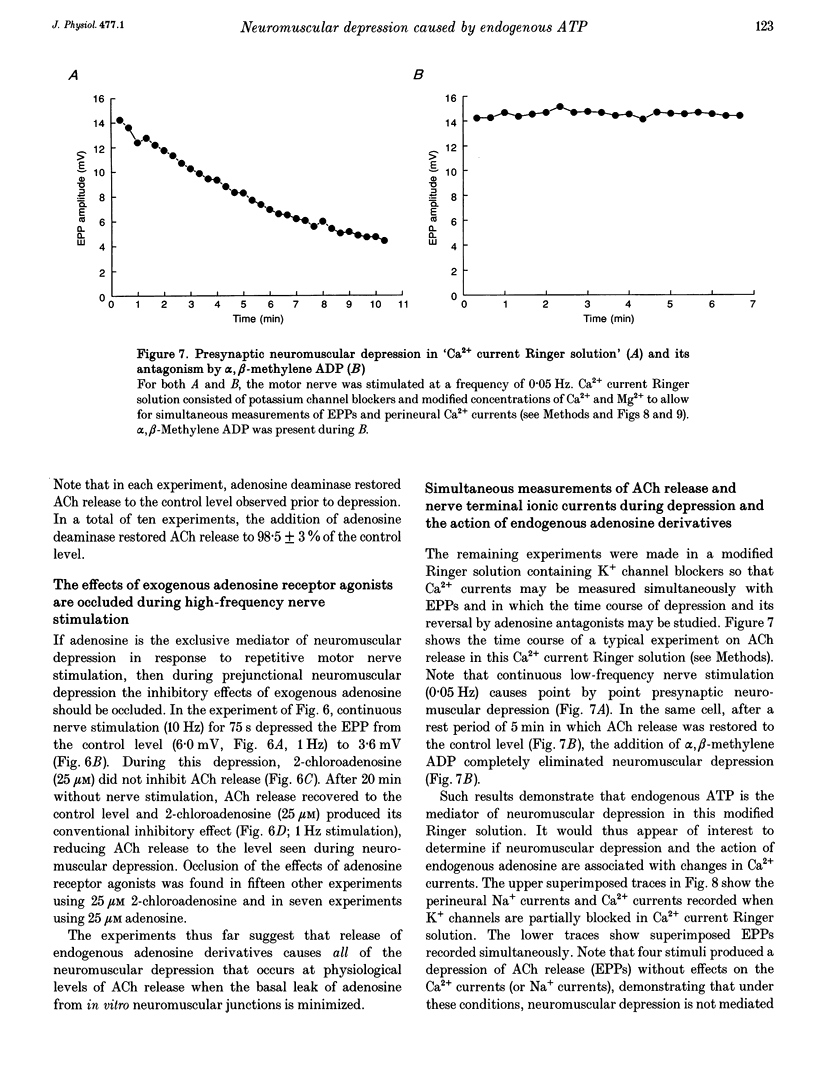
1. The hypothesis that ATP released by presynaptic stimulation is hydrolysed to adenosine and mediates prejunctional neuromuscular depression was tested at vertebrate neuromuscular junctions. Electrophysiological recordings of evoked acetylcholine (ACh) release and perineural ionic currents at motor nerve endings were made using the frog cutaneous pectoris nerve-muscle preparation. Either tubocurarine or alpha-bungarotoxin was used to block muscle contractions. 2. Either alpha,beta-methylene ADP (which inhibits ecto-5'nucleotidases and thus prevents the degradation of ATP to adenosine) or selective adenosine receptor antagonists (8-cyclo-pentyl alkyl xanthines) prevented the inhibitory effects of exogenous ATP on ACh release in response to low-frequency nerve stimulation. These results confirm earlier findings that ATP must be hydrolysed to adenosine to inhibit ACh release. 3. The presence of alpha,beta-methylene ADP completely prevented neuromuscular depression in response to repetitive high-frequency nerve stimulation (0.5-1 Hz). alpha,beta-Methylene ADP had no effect on ACh secretion under conditions where ACh release is well maintained (low-frequency stimulation, 0.05 Hz). 4. Selective adenosine receptor antagonists completely eliminated neuromuscular depression produced by repetitive high-frequency nerve stimulation (1.0 Hz) but had no effect on ACh release at low frequencies of stimulation (0.05 Hz). 5. Exogenous adenosine deaminase (5 i.u. ml-1), which degrades adenosine to its inactive nucleoside inosine, also eliminated neuromuscular depression but had no significant effect on ACh release at frequencies of nerve stimulation too low to produce prejunctional depression. 6. During maximal neuromuscular depression, the effects of exogenous adenosine or 2-chloroadenosine, an adenosine agonist, were occluded. 7. The calcium-sensitive component of perineurial recordings of motor nerve terminal currents did not change during depression or during application of adenosine receptor antagonists and adenosine deaminase, suggesting that neuromuscular depression in this species was not associated with changes in presynaptic Ca2+ currents. 8. These results suggest that, under the conditions of these experiments, endogenous ATP, after hydrolysis to adenosine, causes prejunctional neuromuscular depression. This inhibitory effect of endogenous adenosine occurs at a site distal to the locus of Ca2+ entry in the frog.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. J., Harvey A. L. Effects of the potassium channel blocking dendrotoxins on acetylcholine release and motor nerve terminal activity. Br J Pharmacol. 1988 Jan;93(1):215–221. doi: 10.1111/j.1476-5381.1988.tb11424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Karunanithi S., Lavidis N. A. Probabilistic secretion of quanta from nerve terminals in toad (Bufo marinus) muscle modulated by adenosine. J Physiol. 1991 Feb;433:421–434. doi: 10.1113/jphysiol.1991.sp018435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J. Depression of transmitter release at the neuromuscular junction of the frog. J Physiol. 1970 Mar;206(3):629–644. doi: 10.1113/jphysiol.1970.sp009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman W. C., Marshall I. G., Gibb A. J., Harborne A. J. Feedback control of transmitter release at the neuromuscular junction. Trends Pharmacol Sci. 1988 Jan;9(1):16–20. doi: 10.1016/0165-6147(88)90236-2. [DOI] [PubMed] [Google Scholar]
- Christensen B. N., Martin A. R. Estimates of probability of transmitter release at the mammalian neuromuscular junction. J Physiol. 1970 Nov;210(4):933–945. doi: 10.1113/jphysiol.1970.sp009250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie T. V., Fredholm B. B. Adenosine A1 receptors inhibit adenylate cyclase activity and neurotransmitter release and hyperpolarize pyramidal neurons in rat hippocampus. J Pharmacol Exp Ther. 1989 Apr;249(1):31–37. [PubMed] [Google Scholar]
- Ginsborg B. L., Hirst G. D. The effect of adenosine on the release of the transmitter from the phrenic nerve of the rat. J Physiol. 1972 Aug;224(3):629–645. doi: 10.1113/jphysiol.1972.sp009916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton B. R., Smith D. O. Autoreceptor-mediated purinergic and cholinergic inhibition of motor nerve terminal calcium currents in the rat. J Physiol. 1991 Jan;432:327–341. doi: 10.1113/jphysiol.1991.sp018387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborne A. J., Bowman W. C., Marshall I. G. Effects of tubocurarine on end-plate current rundown and quantal content during rapid nerve stimulation in the snake. Clin Exp Pharmacol Physiol. 1988 Jun;15(6):479–490. doi: 10.1111/j.1440-1681.1988.tb01104.x. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I. Microphysiology of vertebrate neuromuscular transmission. Physiol Rev. 1973 Jul;53(3):674–723. doi: 10.1152/physrev.1973.53.3.674. [DOI] [PubMed] [Google Scholar]
- Keller F., Zimmermann H. Ecto-adenosine triphosphatase activity at the cholinergic nerve endings of the Torpedo electric organ. Life Sci. 1983 Dec 26;33(26):2635–2641. doi: 10.1016/0024-3205(83)90347-8. [DOI] [PubMed] [Google Scholar]
- LILEY A. W., NORTH K. A. An electrical investigation of effects of repetitive stimulation on mammalian neuromuscular junction. J Neurophysiol. 1953 Sep;16(5):509–527. doi: 10.1152/jn.1953.16.5.509. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Klotz K. N., Lindenborn-Fotinos J., Reddington M., Schwabe U., Olsson R. A. 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX)--a selective high affinity antagonist radioligand for A1 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1987 Aug;336(2):204–210. doi: 10.1007/BF00165806. [DOI] [PubMed] [Google Scholar]
- Lopate G., Pestronk A. Autoimmune myasthenia gravis. Hosp Pract (Off Ed) 1993 Jan 15;28(1):109-12, 115-7, 121-2, passim. doi: 10.1080/21548331.1993.11442741. [DOI] [PubMed] [Google Scholar]
- Mallart A. Presynaptic currents in frog motor endings. Pflugers Arch. 1984 Jan;400(1):8–13. doi: 10.1007/BF00670529. [DOI] [PubMed] [Google Scholar]
- Meriney S. D., Grinnell A. D. Endogenous adenosine modulates stimulation-induced depression at the frog neuromuscular junction. J Physiol. 1991 Nov;443:441–455. doi: 10.1113/jphysiol.1991.sp018843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molgó J., del Pozo E., Baños J. E., Angaut-Petit D. Changes of quantal transmitter release caused by gadolinium ions at the frog neuromuscular junction. Br J Pharmacol. 1991 Sep;104(1):133–138. doi: 10.1111/j.1476-5381.1991.tb12397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D., Loctin F., Dunant Y. Inhibition of evoked acetylcholine release: two different mechanisms in the Torpedo electric organ. Eur J Pharmacol. 1987 Jan 13;133(2):225–234. doi: 10.1016/0014-2999(87)90154-3. [DOI] [PubMed] [Google Scholar]
- OTSUKA M., ENDO M., NONOMURA Y. Presynaptic nature of neuromuscular depression. Jpn J Physiol. 1962 Dec 15;12:573–584. doi: 10.2170/jjphysiol.12.573. [DOI] [PubMed] [Google Scholar]
- Redman R. S., Silinsky E. M. A selective adenosine antagonist (8-cyclopentyl-1,3-dipropylxanthine) eliminates both neuromuscular depression and the action of exogenous adenosine by an effect on A1 receptors. Mol Pharmacol. 1993 Oct;44(4):835–840. [PubMed] [Google Scholar]
- Ribeiro J. A., Sebastião A. M. On the role, inactivation and origin of endogenous adenosine at the frog neuromuscular junction. J Physiol. 1987 Mar;384:571–585. doi: 10.1113/jphysiol.1987.sp016470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro J. A., Walker J. The effects of adenosine triphosphate and adenosine diphosphate on transmission at the rat and frog neuromuscular junctions. Br J Pharmacol. 1975 Jun;54(2):213–218. doi: 10.1111/j.1476-5381.1975.tb06931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanziani M., Capogna M., Gähwiler B. H., Thompson S. M. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992 Nov;9(5):919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- Scholz K. P., Miller R. J. Inhibition of quantal transmitter release in the absence of calcium influx by a G protein-linked adenosine receptor at hippocampal synapses. Neuron. 1992 Jun;8(6):1139–1150. doi: 10.1016/0896-6273(92)90134-y. [DOI] [PubMed] [Google Scholar]
- Schweitzer E. Coordinated release of ATP and ACh from cholinergic synaptosomes and its inhibition by calmodulin antagonists. J Neurosci. 1987 Sep;7(9):2948–2956. doi: 10.1523/JNEUROSCI.07-09-02948.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastião A. M., Ribeiro J. A. 1,3,8- and 1,3,7-substituted xanthines: relative potency as adenosine receptor antagonists at the frog neuromuscular junction. Br J Pharmacol. 1989 Jan;96(1):211–219. doi: 10.1111/j.1476-5381.1989.tb11802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. Evidence for specific adenosine receptors at cholinergic nerve endings. Br J Pharmacol. 1980;71(1):191–194. doi: 10.1111/j.1476-5381.1980.tb10925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. On the association between transmitter secretion and the release of adenine nucleotides from mammalian motor nerve terminals. J Physiol. 1975 May;247(1):145–162. doi: 10.1113/jphysiol.1975.sp010925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. On the calcium receptor that mediates depolarization-secretion coupling at cholinergic motor nerve terminals. Br J Pharmacol. 1981 Jun;73(2):413–429. doi: 10.1111/j.1476-5381.1981.tb10438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. On the mechanism by which adenosine receptor activation inhibits the release of acetylcholine from motor nerve endings. J Physiol. 1984 Jan;346:243–256. doi: 10.1113/jphysiol.1984.sp015019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M., Solsona C. S. Calcium currents at motor nerve endings: absence of effects of adenosine receptor agonists in the frog. J Physiol. 1992 Nov;457:315–328. doi: 10.1113/jphysiol.1992.sp019380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. The biophysical pharmacology of calcium-dependent acetylcholine secretion. Pharmacol Rev. 1985 Mar;37(1):81–132. [PubMed] [Google Scholar]
- Smith D. O., Lu Z. Adenosine derived from hydrolysis of presynaptically released ATP inhibits neuromuscular transmission in the rat. Neurosci Lett. 1991 Jan 28;122(2):171–173. doi: 10.1016/0304-3940(91)90850-s. [DOI] [PubMed] [Google Scholar]
- Smith D. O. Sources of adenosine released during neuromuscular transmission in the rat. J Physiol. 1991 Jan;432:343–354. doi: 10.1113/jphysiol.1991.sp018388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A. The long-lasting depression in neuromuscular transmission of frog. Jpn J Physiol. 1958 Jun 15;8(2):102–113. doi: 10.2170/jjphysiol.8.102. [DOI] [PubMed] [Google Scholar]
- Yellen G. Single Ca2+-activated nonselective cation channels in neuroblastoma. Nature. 1982 Mar 25;296(5855):357–359. doi: 10.1038/296357a0. [DOI] [PubMed] [Google Scholar]


