Abstract
Aim
This pre-post intervention study aimed to assess the relationship between baseline dietary quality and the efficacy of a dietitian-guided weight reduction program, which has not been thoroughly documented to date.
Methods
Ninety-two consecutive obese or overweight patients visiting a tertiary center clinic for weight reduction were enrolled in this study. Participants received a dietitian-guided weight reduction education program aimed at reducing daily caloric intake by 500 kcal and improving adherence to the Mediterranean diet for 3 months. Baseline dietary quality was assessed using the 14-item Taiwanese Mediterranean Diet Adherence Screener (T-MEDAS), where a higher T-MEDAS score reflects greater adherence to the Mediterranean diet. Additional covariates, including dietary behaviors, lifestyle factors, and comorbidities were also recorded. The primary outcome was the percentage of weight reduction at 3 months, analyzed using restricted cubic spline models and generalized estimating equations (GEE) to account for the correlation between weight change and the baseline T-MEDAS scores.
Results
Thirty-nine participants were excluded due to major illnesses, use of anti-obesity medications, or loss to follow-up. Among the remaining 53 participants (mean age 41.2 ± 12.8 years, 56.6% female), the average weight reduction was 3.9 ± 3.3% from a baseline weight of 98.5 ± 12.8 kg. Participants who did not achieve a weight reduction of more than 5% had higher baseline T-MEDAS scores compared to those who did (5.4 ± 1.7 vs. 4.1 ± 1.8, p = 0.026). A restricted cubic spline model, adjusted for covariates including age, gender, diabetes mellitus (DM), dyslipidemia, and smoking, revealed a significant inverse relationship between higher baseline T-MEDAS scores and weight loss. After controlling for various confounders, GEE analysis demonstrated that higher baseline T-MEDAS scores were significantly associated with less weight loss (beta: -4.1, 95% CI: -5.6 to -2.6, p < 0.001).
Conclusions
Higher baseline dietary quality was associated with reduced effectiveness of a dietitian-guided weight reduction program. This suggests that additional strategies may be required to improve the success of weight loss interventions in individuals with higher baseline dietary quality.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40795-024-00956-5.
Keywords: Dietary quality, Weight reduction program, Dietitian-guided, MEDAS
Introduction
Obesity is a global health crisis significantly impacting individual health and healthcare systems worldwide. Characterized by an excessive accumulation of body fat, it heightens the risk of several health issues, including type 2 diabetes, cardiovascular diseases, certain cancers, and premature death [1]. The World Health Organization has declared obesity a pandemic, highlighting its seriousness and the pressing need for effective management strategies [2]. The complexity of obesity, driven by genetic, environmental, and importantly, behavioral factors, calls for a comprehensive approach to its treatment. Against this backdrop, the development of innovative weight reduction programs becomes crucial in reversing this trend and reducing the associated health risks.
Weight reduction programs have evolved significantly in recent years, adopting a multidisciplinary approach that emphasizes dietary changes and lifestyle modifications, with or without the integration of anti-obesity medications [3, 4]. Dietitian-guided weight reduction continues to be a fundamental aspect of anti-obesity management and has proven effectiveness [4]. This underscores the importance of identifying predictive factors that contribute to its success. Existing evidence indicates several factors linked to greater efficacy in diverse weight reduction programs, such as younger age, female gender, more frequent clinic visits, higher baseline body mass index, and the use of specific medications [5–10]. While these factors contribute valuable insights into weight management outcomes, an overlooked aspect is the impact of baseline dietary quality and lifestyle habits on the success of weight reduction in dietitian-guided interventions.
The Mediterranean diet is extensively studied for its beneficial effects on weight loss and obesity management. Research shows that adherence to this dietary pattern is linked to significant reductions in body weight and obesity risk across different populations, including children, adolescents, and adults [11, 12]. Moreover, the Mediterranean diet has been associated with improved weight loss maintenance [13], making it a valuable dietary intervention for long-term obesity management. Given this evidence, we chose the Mediterranean diet as the healthy eating template for our weight reduction program and evaluated the relationship between baseline adherence to the Mediterranean diet and the effectiveness of a weight reduction program.
Our study aims to fill the gap in literature regarding the influence of baseline dietary quality and lifestyle factors on weight reduction outcomes in a dietitian-guided program, elucidating the relationship between these factors and program efficacy.
Methods
Study design and participants
This pre-post intervention study enrolled consecutive patients aged 18 years or older, with a body mass index (BMI) ≥ 25.0 kg/m², who visited a weight reduction clinic at a tertiary center in central Taiwan between May 2020 and October 2021. Patients were excluded if they were pregnant, unable to complete the questionnaires, using anti-obesity medications, had prior bariatric surgery, major illnesses, severe mental disorders, advanced chronic kidney disease, advanced liver cirrhosis, immunodeficiency, or excessive alcohol consumption (> 80 g/day). All participants provided informed consent.This research adopted a scientific methodology, utilizing a study design in accordance with the CONSORT guidelines and the extension to pilot and feasibility trials [14].
Intervention
Participants received a personalized dietitian-guided weight reduction education program, which aimed to reduce daily caloric intake by 500 kcal and improve adherence to the Mediterranean diet for 3 months. The program focused on minimizing the intake of processed foods, specifically targeting items high in added sugars and refined carbohydrates, and encouraging the consumption of whole grains, fresh fruits, vegetables, and other unprocessed foods. The program was personalized, with the dietitian setting specific, attainable goals for weight reduction tailored to each patient’s individual requirements. Participants were encouraged to engage in at least 30 min of moderate-intensity exercise on at least three days per week. Initial consultations with a registered dietitian nutritionist were conducted in person, lasting at least 30 min, followed by weekly sessions, occurred either in person or online during the first month, transitioning to sessions every two weeks thereafter [3].
Data collection and outcomes
Online questionnaires, collected via Google Forms, were completed at baseline to assess dietary quality using a validated Taiwanese version Mediterranean Diet Adherence Screener (T-MEDAS) [15]. The MEDAS score was designed to evaluate adherence to the Mediterranean Diet based on the Prevención con Dieta Mediterránea (PREDIMED) study [16, 17]. Detailed components and calculations of the T-MEDAS score are found in Supplementary Table 1. The cumulative score of the T-MEDAS varied from 0 to 14 points, with higher scores indicating greater adherence to the Mediterranean diet.Other covariates collected at baseline included comorbidities and medications, gathered through patient interviews, and anthropometric measures including body weight, BMI, waist circumference, body fat mass, and skeletal muscle mass, assessed using bioelectrical impedance analysis. Blood tests included fasting glucose, HbA1c, LDL and HDL cholesterol. Dietary behaviors and lifestyle factors were also evaluated through questionnaires administered at baseline via Google Forms. These factors were defined as follows: frequent night meals defined as eating after 9 pm two or more times a week [18], prolonged sedentary time was defined as sitting or reclining for 4 or more hours per day [19]. frequent meals eaten outside defined as having meals outside of home ten or more times a week [20]. Additionally, consuming sweets (e.g., traditional Taiwanese sweets such as pineapple cakes or red bean cakes) three or more times a week was considered frequent sweet consumption [15]. A preference for fried food or other high-fat cooking methods was termed fried food preference [21].Emotional eating was defined as endorsing two or more items in the emotional eating subscale (e.g., eating in response to feeling stressed or eating to cope with negative emotions) [22]. At the end of the 3-month intervention, T-MEDAS, body weight, and BMI were reassessed for outcome analysis.
Statistical analysis
The primary outcome was the percentage of weight reduction at 3 months. Univariate analyses were conducted to compare the baseline T-MEDAS scores and other covariates between participants achieving at least 5% weight reduction and those achieving less than 5%. The Mann-Whitney U test was used to compare the change in T-MEDAS scores between two groups. The relationship between baseline T-MEDAS scores and the percentage of weight loss was further analyzed using restricted cubic spline models and generalized estimating equations (GEE), adjusting for potential confounders.Statistical analyses were performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviation (SD) and compared using Mann-Whitney U test. Categorical variables were expressed as numbers (percentages) and compared using Chi-square tests. A p-value of < 0.05 was considered statistically significant.
Results
A total of 92 patients were enrolled. After excluding 39 patients (18 due to major illnesses, 5 had concurrent use of anti-obesity medications, 2 had previous bariatric surgery, 4 did not complete the baseline questionnaire, and 10 were lost to follow up), the final analysis included 53 participants. The participants had an average age of 41.2 ± 12.8 years, with females comprising 56% of the participants. The average BMIwas 35.2 ± 6.1 kg/m²and the baseline mean body weight was 98.5 ± 12.8 kg. Finally, the average reduction in body weight at 3 months was 3.9 ± 3.3% and 26.4% of participants achieved a weight reduction of equally to or over 5%. There were no differences in age, gender, or baseline characteristics between these subjects and those 10 participants lost to follow-up (data not shown).
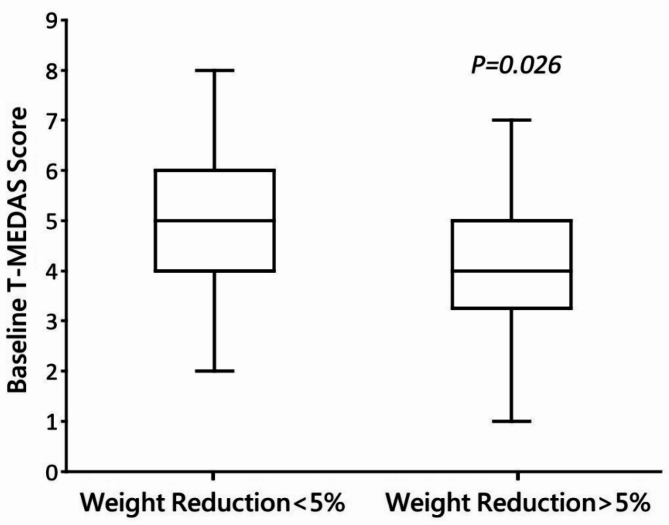
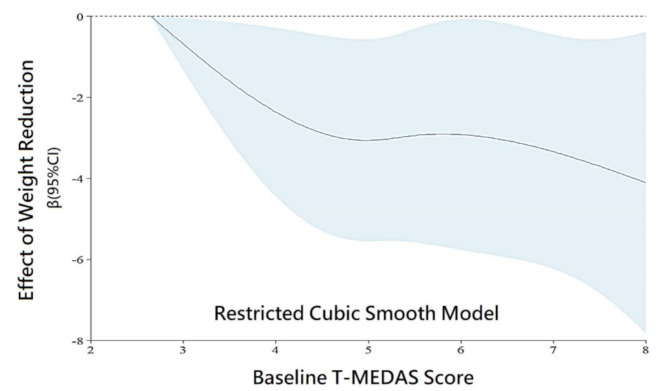
The baseline demographic and lifestyle characteristics are shown in Table 1. Individuals who did not achieve a weight reduction exceeding 5% demonstrated higher baseline T-MEDAS scores (5.4 ± 1.7) compared to those who did (4.1 ± 1.8), and this disparity reached statistical significance (p = 0.026) (Fig. 1). Other demographic and lifestyle factors, including night meals, prolonged sedentary time, and regular exercise showed no significant difference between the two groups. Upon employing a simplified smooth effect using a restricted cubic spline (RCS) model, considering age, gender, DM, dyslipidemia, and current or history of smoking as covariates, we observed a significant correlation (Fig. 2). That is, higher baseline T-MEDAS scores were significantly linked to a reduced effectiveness in weight reduction, with individuals having the lowest baseline T-MEDAS score serving as the reference. By using generalized estimating equations, we found that higher baseline T-MEDAS scores were significantly associated with less weight reduction, as indicated by a negative beta value (beta: -4.1, 95% CI: -5.6 to -2.6, P < 0.001), after adjusting for age, gender, BMI, DM, hypertension, dyslipidemia, current or history of smoking, presence of irritable bowel syndrome, frequent meals outside the home, frequent consumption of sweets, presence of emotional eating, regular exercise, somatic discomfort, and anxiety or depression. In other words, higher baseline T-MEDAS scores were linked to a smaller percentage of weight loss(Table 2).
Table 1.
Baseline characteristics of the enrolled patients
| Variables | Total | Weight Loss < 5% |
Weight Loss ≥ 5% |
p value |
|---|---|---|---|---|
| (n = 53) | (n = 39) | (n = 14) | ||
| Age | 41.2 ± 12.8 | 40 ± 13.1 | 44.4 ± 11.9 | 0.279 |
| Female | 30 (56.6%) | 24 (61.5%) | 6 (42.9%) | 0.226 |
| BMI (kg/m2) | 35.2 ± 6.1 | 34.8 ± 6.4 | 36.3 ± 5.4 | 0.443 |
| Weight, kg | 98.5 ± 12.8 | 96.0 ± 23.0 | 105.5 ± 19.0 | 0.174 |
| Waist circumference (cm) | 107.9 ± 12.8 | 107.1 ± 15.0 | 110.8 ± 11.0 | 0.552 |
| Body fat mass (%)a | 41.0 ± 12.8 | 41.2 ± 6.6 | 40.5 ± 7.0 | 0.758 |
| Skeletal muscle mass (kg)a | 32.4 ± 12.8 | 31.4 ± 7.1 | 35.3 ± 7.3 | 0.078 |
| Type 2 diabetes | 13 (24.5%) | 9 (23.1%) | 4 (28.6%) | 0.682 |
| Hypertension | 20 (40.0%) | 11 (30.6%) | 9 (64.3%) | 0.029* |
| Dyslipidemiab | 28 (52.8%) | 23 (59.0%) | 5 (35.7%) | 0.135 |
| Current or former smokers | 12 (23.5%) | 8 (21.6%) | 4 (28.6%) | 0.602 |
| Irritable bowel syndromec | 15 (30.0%) | 10 (27.8%) | 5 (35.7%) | 0.582 |
| Night meals ≥ 2 times per week | 23 (43.4%) | 19 (48.7%) | 4 (28.6%) | 0.192 |
| Meals outside the home ≥ 10 times per week | 23 (43.4%) | 19 (48.7%) | 4 (28.6%) | 0.192 |
| Consumption of sweets ≥ 3 times per week | 26 (50.0%) | 20 (52.6%) | 6 (42.9%) | 0.532 |
| Fried food preference | 29 (58.0%) | 20 (55.6%) | 9 (64.3%) | 0.574 |
| Emotional eating | 20 (43.5%) | 15 (45.5%) | 5 (38.5%) | 0.667 |
| Baseline T-MEDAS Scoref | 5.04 ± 12.8 | 5.4 ± 1.7 | 4.1 ± 1.8 | 0.026* |
| Sedentary time ≥ 4 h per day | 20 (41.7%) | 16 (43.2%) | 4 (36.4%) | 0.685 |
| Exercise ≥ 1.5 h per week | 18 (34.0%) | 11 (28.2%) | 7 (50.0%) | 0.140 |
| Pain/ discomfortd | 20 (40.0%) | 13 (36.1%) | 7 (50.0%) | 0.368 |
| Anxious/ depressedd | 19 (38.0%) | 14 (38.9%) | 5 (35.7%) | 0.840 |
Categorical variables were expressed as numbers (%) and were analyzed using the Chi-square test. Continuous variables were expressed as mean ± standard deviation (SD) and were analyzed using Student’s t-test
a. Measured by bioelectrical impedance analysis
b. Dyslipidemia defined as LDL ≥ 160 mg/dL or HDL < 40 mg/dL for men and < 50 mg/dL for women
c. Rome IV criteria
d. Severity of moderate or severe obtained with questions from the EuroQOL five dimension questionnaire (EQ-5D)
*p < 0.05
Abbreviations: BMI, body-mass index; SD, standard deviation; T-MEDAS, Taiwanese version Mediterranean Diet Adherence Screener
Fig. 1.
Baseline T-MEDAS score of participants with or without 5% weight reduction
Fig. 2.
Significant correlation observed using restricted cubic spline model with age, gender, DM, dyslipidemia, and smoking as covariates
Table 2.
Association between T-MEDAS scores and the percentage of Weight Reduction
| Generalized Estimating Equations | |||
|---|---|---|---|
| Variables | β | 95% CI | P |
| Baseline T-MEDAS | -4.1 | -5.6 to -2.6 | < 0.001 |
Adjusting age, gender, BMI, DM, hypertension, dyslipidemia, current or history of smoking, presence of irritable bowel syndrome, frequent meals outside the home, frequent consumption of sweets, presence of emotional eating, regular exercise, somatic discomfort, and anxiety or depression
Abbreviations: T-MEDAS, Taiwanese version Mediterranean Diet Adherence Screener
After three months, individuals who achieved a weight reduction exceeding 5% showed an average improvement in their T-MEDAS score of 2.22 ± 1.64. In contrast, those who did not achieve this weight reduction recorded an average improvement in T-MEDAS of 1.25 ± 1.39. Despite no statistical significance, there is a trend of association between more dietary score improvement and better weight reduction (p-value = 0.069).
Discussion
Our study found that among participants in a dietitian-guided weight reduction program, higher baseline dietary quality was associated with a lower percentage of weight reduction. Additionally, it negatively affected the effectiveness of weight reduction in multivariate analysis. For individuals with higher baseline dietary quality, the ability to modify their diet may be limited, potentially reducing the effectiveness of dietary changes.
The average weight loss in our cohort was 3.9 ± 3.3%, slightly surpassing the results of previous studies [23–25].We did not observe a difference in the frequency of visits between those who achieved a 5% weight reduction and those who did not (data not shown). However, comparisons with prior studies suggest that the frequency of dietitian consultations may still significantly influence weight loss outcomes.For instance, Delahanty et al. showed a 2.4% reduction in weight at three months with monthly dietitian visits [23]. Kesman et al. reported a weight reduction of 2.1% after six months, with participants meeting dietitians every two months [24]. Similarly, Niswender et al. demonstrated a weight reduction of 1.24% at six months with monthly consultations [25]. In contrast, our protocol, which involved weekly consultations in the first month and bi-weekly consultations thereafter, appears to have yielded more positive results. From previous studies, the most validated predictors of weight loss success are the frequency of follow-up visits and adherence to the intervention protocol. Therefore, we believe that the positive effects observed in our study, where as many as 26.4% of participants achieved a 5% weight reduction, may be attributed to a higher frequency of visits [5, 9, 10]. Other potential factors which seem irrelevant in our study include a younger population [4] and a lower percentage of individuals with diabetes [8].
The main finding of this study is that lower baseline dietary quality may lead to greater weight reduction, while higher baseline dietary quality is associated with less weight reduction. One possible explanation for the finding that participants with lower baseline dietary quality might observe greater weight loss could be attributed to their greater tendency to improve their dietary components, adopt a healthier diet, and consequently achieve more significant weight loss. Therefore, when conducting dietitian-guided education, focusing on individuals with lower baseline dietary quality could be more beneficial when introducing dietary interventions, like the Mediterranean diet [26].Another possible explanation for our results is that participants with lower baseline dietary quality may have a different baseline gut microbiota, causing them to respond differently to dietary changes. Ben-Yacov et al. highlighted the role of the gut microbiome in dietary intervention responses [27, 28]. Future research should explore the interplay between baseline diet, gut microbiota, and weight loss to refine dietary interventions for weight reduction.
The adoption of the Mediterranean diet in Asian countries faces cultural barriers [29]. For instance, olive oil is not commonly used in Taiwan, where soybean oil is the primary cooking oil, and Taiwanese adults consume high amounts of refined carbohydrates and red meat, while their intake of fruits, vegetables, beans, and nuts is insufficient [30, 31]. Despite these challenges, sequential national surveys have indicated a rising trend in the use of olive oil as the main cooking oil [30], as well as a reduction in the consumption of animal fat and a gradual increase in nut consumption [31].These dietary shifts are promising, as studies conducted in Taiwan have shown that key components of the Mediterranean diet—such as seafood, vegetables, fruits, nuts, and legumes—are inversely associated with health outcomes, including all-cause and cardiovascular disease (CVD) mortalities. In contrast, higher consumption of processed foods, sugary beverages, and red meat has been positively linked to adverse health outcomes [31].Based on this information, there is potential to use the Mediterranean Diet-based approach as a tool for both dietary surveys and education in Taiwan.
Our study has limitations. The final analysis included only 53 participants after excluding 39 for various reasons, such as major illnesses, concurrent use of anti-obesity medications, and loss to follow-up. This relatively small sample size, along with the absence of a control group, limits the generalizability of the study findings to the broader population. Additionally, the study population is from a tertiary center in central Taiwan, where the average Taiwanese diet includes steamed white rice and white flour-made noodles as the major staples, and pork as the main meat. This dietary pattern may not reflect the dietary habits, cultural influences, and genetic backgrounds of other populations. Furthermore, our population had an average T-MEDAS score of around 5.0, which is lower than the findings from a study conducted in seven European countries around the Mediterranean region, where the average score was 5.4 [32], and another study in Korea, where the average score was 5.8 among participants with steatotic liver disease [33]. This suggests that our participants may benefit more from the Mediterranean diet intervention compared to populations with higher baseline MEDAS scores, where additional efforts might be needed to ensure the diet’s effectiveness for weight management.Moreover, the relatively short three-month duration might not reflect long-term outcomes. We also acknowledge that the T-MEDAS score does not differentiate between fried and non-fried seafood and excludes homemade sweets, which may limit its comprehensiveness.Future studies should investigate the interplay between baseline dietary quality and dietary modifications in individuals undergoing weight reduction or managing weight-related conditions, such as fatty liver disease, within larger-scale longitudinal cohorts to gain a more comprehensive understanding of the outcomes of dietary modification programs.
In conclusion, higher baseline dietary quality reduced the efficacy of dietitian-guided weight reduction programs. For individuals with higher baseline dietary quality, additional strategies should be explored in conjunction with a dietitian-based approach.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank all the participants for their contributions to the research and the Biostatistics Task Force of Taichung Veterans General Hospital for their assistance with the statistical analysis.
Author contributions
Y.C.L. and Y.C.C.; methodology, Y.J.C. and Y.Y.C.; software, H.M.H.; validation, W.H.W.and H.F.L.; formal analysis, Y.J.L., Y.C.P. and T.Y.L.; resources, Y.C.L., S.S.Y., Y.C.C. and S.C.L.; writing—original draft preparation, H.C.L.; writing—review and editing, H.C.L.; supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a grant from Taichung Veterans General Hospital, Taiwan (TCVGH-1123302B).
Data availability
Data available on request from the authors.
Declarations
Competing interests
The authors declare no competing interests.
Institutional review board statement
The study protocol was approved by the ethics committee of Taichung Veterans General Hospital Institutional Review Board (IRB No. CE21192B) and was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines.
Consent to participate
All participants provided written informed consent for the study.
Consent to publication
Not applicable.
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ying-Cheng Lin and Yen-Chien Chen contributed equally to this work.
References
- 1.Boutari C, Mantzoros CS. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metab Clin Exp. 2022;133:155217. 10.1016/j.metabol.2022.155217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obesity. and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. [Accessed 10 March 2024]. 2024.
- 3.Jensen, M. D., Ryan, D. H., Apovian, C. M., Ard, J. D., Comuzzie, A. G., Donato, K.A., Hu, F. B., Hubbard, V. S., Jakicic, J. M., Kushner, R. F., Loria, C. M., Millen,B. E., Nonas, C. A., Pi-Sunyer, F. X., Stevens, J., Stevens, V. J., Wadden, T. A.,Wolfe, B. M., Yanovski, S. Z., American College of Cardiology/American Heart Association Task Force on Practice Guidelines, … Obesity Society (2014). 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Journal of the American College of Cardiology, 63(25 Pt B), 2985–3023. https://doi.org/10.1016/j.jacc.2013.11.004. [DOI] [PubMed]
- 4.Williams LT, Barnes K, Ball L, Ross LJ, Sladdin I, Mitchell LJ. How effective are dietitians in Weight Management? A systematic review and Meta-analysis of Randomized controlled trials. Healthc (Basel Switzerland). 2019;7(1):20. 10.3390/healthcare7010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weintraub MA, D’Angelo D, Tchang BG, Sahagun AD, Andre C, Aronne LJ, Shukla AP. Five-year weight loss maintenance with obesity pharmacotherapy. J Clin Endocrinol Metab. 2023;108(9):e832–41. 10.1210/clinem/dgad100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Małecki MT, Batterham RL, Sattar N, Levine JA, Rodríguez Á, Bergman BK, Wang H, Ghimpeteanu G, Lee CJ. Predictors of ≥ 15% weight reduction and Associated changes in cardiometabolic risk factors with tirzepatide in adults with type 2 diabetes in SURPASS 1–4. Diabetes Care. 2023;46(12):2292–9. 10.2337/dc23-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huh Y, Kim YS. Predictors for successful weight reduction during treatment with Dapagliflozin among patients with type 2 diabetes mellitus in primary care. BMC Prim care. 2022;23(1):134. 10.1186/s12875-022-01748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachar A, Livshits G, Birk R. Predictors of weight reduction and maintenance in a large cohort of overweight and obese adults in a community setting. Nutr Dietetics: J Dietitians Association Australia. 2018;75(4):390–6. 10.1111/1747-0080.12419. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs S, Radnitz C, Hildebrandt T. Adherence as a predictor of weight loss in a commonly used smartphone application. Obes Res Clin Pract. 2017;11(2):206–14. 10.1016/j.orcp.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Cheng YC, Liu HC, Hsu CY, Lee IT. Duration of treatment in a weight loss program using a Mobile App is Associated with successful weight loss during the COVID-19 pandemic. Diabetes Metabolic Syndrome Obesity: Targets Therapy. 2022;15:1737–47. 10.2147/DMSO.S368608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.López-Gil JF, García-Hermoso A, Sotos-Prieto M, Cavero-Redondo I, Martínez-Vizcaíno V, Kales SN. Mediterranean Diet-based interventions to improve anthropometric and obesity indicators in children and adolescents: a systematic review with Meta-analysis of Randomized controlled trials. Adv Nutr (Bethesda Md). 2023;14(4):858–69. 10.1016/j.advnut.2023.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng X, Zhang W, Wan X, Lv X, Lin P, Si S, Xue F, Wang A, Cao Y. The effects of Mediterranean diet on cardiovascular risk factors, glycemic control and weight loss in patients with type 2 diabetes: a meta-analysis. BMC Nutr. 2024;10(1):59. 10.1186/s40795-024-00836-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poulimeneas D, Anastasiou CA, Santos I, Hill JO, Panagiotakos DB, Yannakoulia M. Exploring the relationship between the Mediterranean diet and weight loss maintenance: the MedWeight study. Br J Nutr. 2020;124(8):874–80. 10.1017/S0007114520001798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lancaster GA, Thabane L. Guidelines for reporting non-randomised pilot and feasibility studies. Pilot Feasibility Stud. 2019;5:114. 10.1186/s40814-019-0499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin YC, Wang WH, Lang HF, Kuo FY, Chong K, Lien HC. Development and validation of a questionnaire to measure adherence to the Mediterranean Diet in Taiwanese adults. Incheon, Korea: Asian Postgraduate Course on Neurogastroenterology and Moility, Paradise City; April, 2022. pp. 1–2.
- 16.Schröder H, Fitó M, Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Lamuela-Raventós R, Ros E, Salaverría I, Fiol M, Lapetra J, Vinyoles E, Gómez-Gracia E, Lahoz C, Serra-Majem L, Pintó X, Ruiz-Gutierrez V, Covas MI. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J Nutr. 2011;141(6):1140–5. 10.3945/jn.110.135566. [DOI] [PubMed] [Google Scholar]
- 17.Estruch R, Ros E, Salas-Salvadó J, Covas I. M, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Fitó M, Gea A, Hernán MA, PREDIMED Study Investigators …. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet supplemented with Extra-virgin Olive oil or nuts. N Engl J Med. 2018;378(25):e34. 10.1056/NEJMoa1800389. [DOI] [PubMed] [Google Scholar]
- 18.Okada C, Imano H, Muraki I, Yamada K, Iso H. The Association of having a late dinner or bedtime snack and skipping breakfast with overweight in Japanese women. J Obes. 2019;2019(2439571). 10.1155/2019/2439571. [DOI] [PMC free article] [PubMed]
- 19.Katzmarzyk PT, Lee IM. Sedentary behaviour and life expectancy in the USA: a cause-deleted life table analysis. BMJ open. 2012;2(4):e000828. 10.1136/bmjopen-2012-000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pot P G. K. Sleep and dietary habits in the urban environment: the role of chrono-nutrition. Proc Nutr Soc. 2018;77(3):189–98. 10.1017/S0029665117003974. [DOI] [PubMed] [Google Scholar]
- 21.Miller-Kasprzak E, Musialik K, Kręgielska-Narożna M, Szulińska M, Bogdański P. The relation between Resistin (-420 C/G) single nucleotide variant, Resistin Serum Concentration, Carbohydrate, and Lipid Parameters and Fried Food Taste Preference in patients with Hypertriglyceridemia. Nutrients. 2022;14(23):5092. 10.3390/nu14235092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shriver LH, Dollar JM, Calkins SD, Keane SP, Shanahan L, Wideman L. Emotional eating in adolescence: effects of emotion regulation, Weight Status and negative body image. Nutrients. 2020;13(1):79. 10.3390/nu13010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delahanty LM, Sonnenberg LM, Hayden D, Nathan DM. Clinical and cost outcomes of medical nutrition therapy for hypercholesterolemia: a controlled trial. J Am Diet Assoc. 2001;101(9):1012–23. 10.1016/S0002-8223(01)00250-4. [DOI] [PubMed] [Google Scholar]
- 24.Kesman RL, Ebbert JO, Harris KI, Schroeder DR. Portion control for the treatment of obesity in the primary care setting. BMC Res Notes. 2011;4:346. 10.1186/1756-0500-4-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niswender K, Piletic M, Andersen H, Hiort C, L., Hollander P. Weight change upon once-daily initiation of insulin detemir with or without dietary intervention in overweight or obese insulin-naïve individuals with type 2 diabetes: results from the DIET trial. Diabetes Obes Metab. 2014;16(2):186–92. 10.1111/dom.12218. [DOI] [PubMed] [Google Scholar]
- 26.Staudacher HM, Mahoney S, Canale K, Opie RS, Loughman A, So D, Beswick L, Hair C, Jacka FN. Clinical trial: a Mediterranean diet is feasible and improves gastrointestinal and psychological symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2024;59(4):492–503. 10.1111/apt.17791. [DOI] [PubMed] [Google Scholar]
- 27.Ben-Yacov O, Godneva A, Rein M, Shilo S, Kolobkov D, Koren N, Dolev C, Shmul NT, Wolf T, Kosower BC, Sagiv N, Lotan-Pompan K, Zmora M, Weinberger N, Elinav A, E., Segal E. Personalized postprandial glucose response-targeting Diet Versus Mediterranean Diet for Glycemic Control in Prediabetes. Diabetes Care. 2021;44(9):1980–91. 10.2337/dc21-0162. [DOI] [PubMed] [Google Scholar]
- 28.Ben-Yacov O, Godneva A, Rein M, Shilo S, Lotan-Pompan M, Weinberger A, Segal E. Gut microbiome modulates the effects of a personalised postprandial-targeting (PPT) diet on cardiometabolic markers: a diet intervention in pre-diabetes. Gut. 2023;72(8):1486–96. 10.1136/gutjnl-2022-329201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woodside J, Young IS, McKinley MC. Culturally adapting the Mediterranean Diet pattern - a way of promoting more ‘sustainable’ dietary change? Br J Nutr. 2022;128(4):693–703. 10.1017/S0007114522001945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan WH, Wu HJ, Yeh CJ, Chuang SY, Chang HY, Yeh NH, Hsieh YT. Diet and health trends in Taiwan: comparison of two nutrition and health surveys from 1993–1996 and 2005–2008. Asia Pac J Clin Nutr. 2011;20(2):238–50. [PubMed] [Google Scholar]
- 31.Pan WH, Wu SY, Yeh NH, Hung SY. Healthy Taiwanese eating Approach (TEA) toward total wellbeing and Healthy Longevity. Nutrients. 2022;14(13):2774. 10.3390/nu14132774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García-Conesa MT, Philippou E, Pafilas C, Massaro M, Quarta S, Andrade V, Jorge R, Chervenkov M, Ivanova T, Dimitrova D, Maksimova V, Smilkov K, Ackova DG, Miloseva L, Ruskovska T, Deligiannidou GE, Kontogiorgis CA, Pinto P. Exploring the validity of the 14-Item Mediterranean Diet Adherence Screener (MEDAS): a cross-national study in seven European countries around the Mediterranean Region. Nutrients. 2020;12(10):2960. 10.3390/nu12102960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwon YJ, Choi JE, Hong KW, Lee JW. Interplay of Mediterranean-diet adherence, genetic factors, and metabolic dysfunction-associated steatotic liver disease risk in Korea. J Translational Med. 2024;22(1):591. 10.1186/s12967-024-05408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available on request from the authors.