Abstract
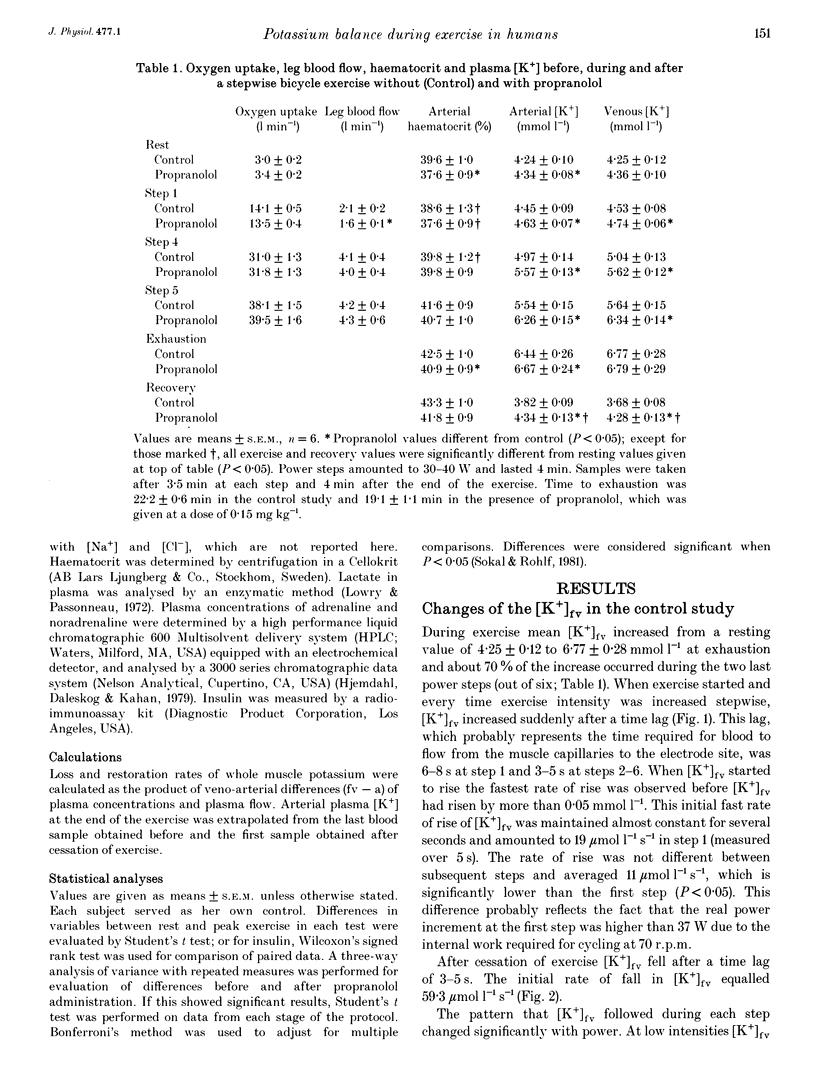
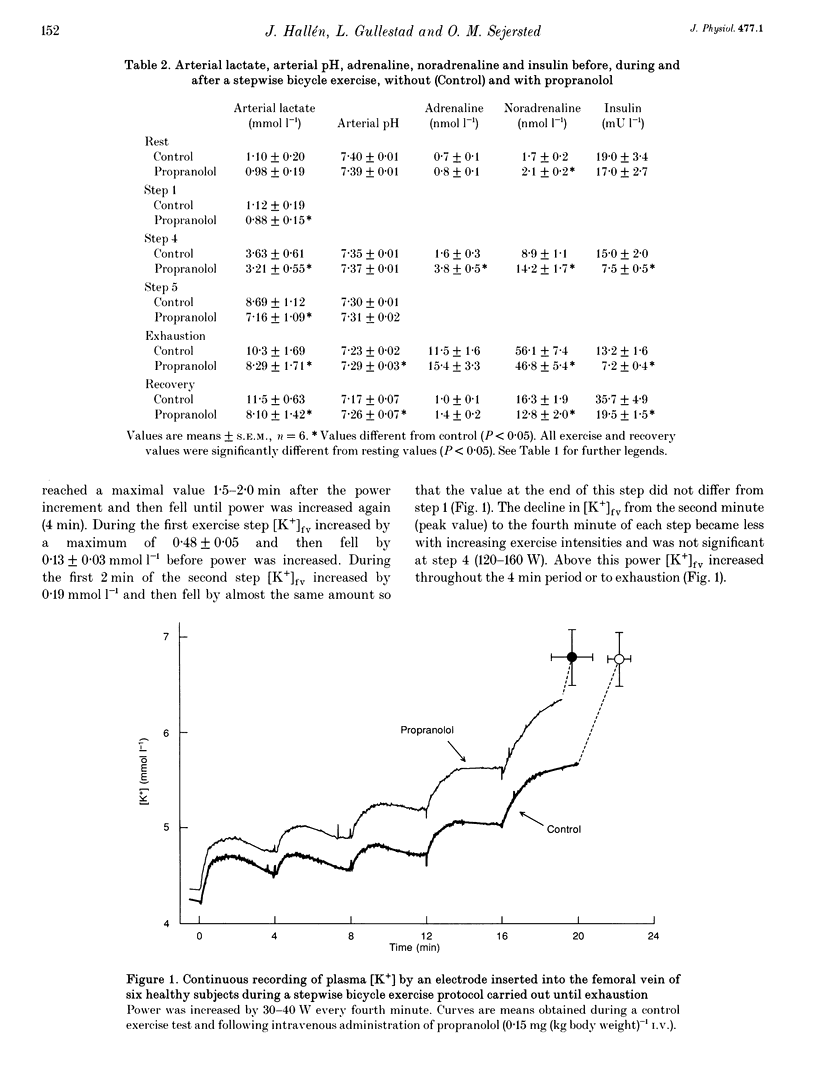
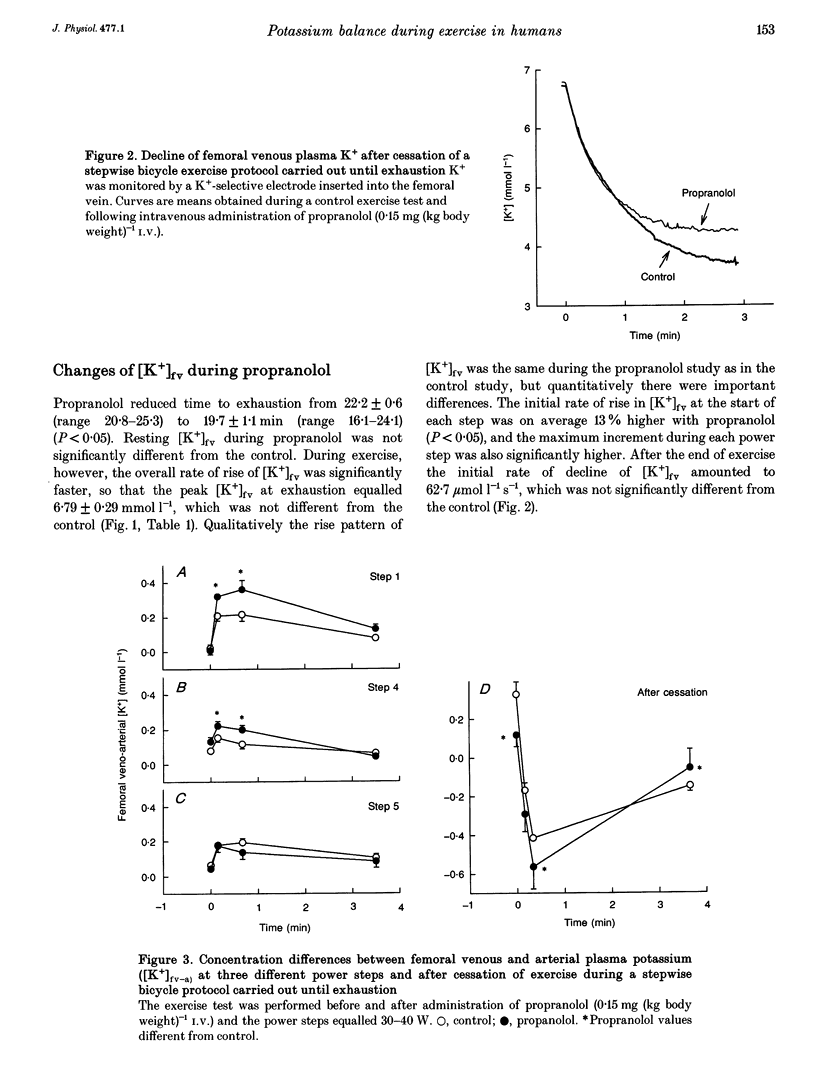
1. K+ efflux rate and control of K+ reuptake rate in exercising muscle cells was examined in six healthy female volunteers. 2. A K(+)-selective electrode in the femoral vein continuously monitored K+ concentration ([K+]fv) during bicycling. Power was increased stepwise 5-6 times by 30-40 W every fourth minute until exhaustion before and after I.V. administration of propranolol. Leg blood flow was measured by bolus injections of Cardiogreen. 3. [K+]fv increased from about 4.3 to 6.8 mmol l-1 at exhaustion both before and after propranolol administration, but after drug infusion endurance was reduced from 22.2 +/- 0.6 to 19.7 +/- 1.1 min, so [K+]fv rose more rapidly. 4. The exercise-induced efflux rate of K+ from the muscle cells was estimated to be about 11 mumol kg-1s-1 at exhaustion both before and after propranolol administration. 5. As an indicator of rate of net loss of K+ from the leg, veno-arterial concentration differences ([K+]fv-a) during first, fourth and fifth power increments were high after 15 and 40 s, but declined toward the end of each power step. Propranolol accentuated [K+]fv-a only after 15 and 40 s of the first and fourth increments. 6. The exercise-induced increase in reuptake rate of K+ in the muscle, estimated at exhaustion, was not significantly changed by propranolol and was about 10 mumol kg-1s-1, corresponding to about 15% of maximum Na(+)-K+ pump capacity in man. 7. Extracellular accumulation and loss of K+ from muscle during bicycle exercise is due to Na(+)-K+ pump lag. The higher [K+]fv during propranolol is mainly due to impaired redistribution outside the exercising muscles. In addition at low powers, beta-adrenoceptor blockade caused a transiently increased net loss due to an accentuated Na(+)-K+ pump lag.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985 Sep;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum H., Nioka S., Johnson R. G., Jr Activation of the Na+, K(+)-ATPase in Narcine brasiliensis. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1247–1251. doi: 10.1073/pnas.87.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T., Everts M. E. Is the Na,K-pump capacity in skeletal muscle inadequate during sustained work? Prog Clin Biol Res. 1988;268B:239–244. [PubMed] [Google Scholar]
- Clausen T., Everts M. E., Kjeldsen K. Quantification of the maximum capacity for active sodium-potassium transport in rat skeletal muscle. J Physiol. 1987 Jul;388:163–181. doi: 10.1113/jphysiol.1987.sp016608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T., Everts M. E. Regulation of the Na,K-pump in skeletal muscle. Kidney Int. 1989 Jan;35(1):1–13. doi: 10.1038/ki.1989.1. [DOI] [PubMed] [Google Scholar]
- Clausen T., Flatman J. A. Beta 2-adrenoceptors mediate the stimulating effect of adrenaline on active electrogenic Na-K-transport in rat soleus muscle. Br J Pharmacol. 1980 Apr;68(4):749–755. doi: 10.1111/j.1476-5381.1980.tb10868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T., Flatman J. A. The effect of catecholamines on Na-K transport and membrane potential in rat soleus muscle. J Physiol. 1977 Sep;270(2):383–414. doi: 10.1113/jphysiol.1977.sp011958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingsen O., Sejersted O. M., Leraand S., Ilebekk A. Catecholamine-induced myocardial potassium uptake mediated by beta 1-adrenoceptors and adenylate cyclase activation in the pig. Circ Res. 1987 Apr;60(4):540–550. doi: 10.1161/01.res.60.4.540. [DOI] [PubMed] [Google Scholar]
- Ellingsen O., Sejersted O. M., Vengen O. A., Ilebekk A. In-vivo quantification of myocardial Na-K pump rate during beta-adrenergic stimulation of intact pig hearts. Acta Physiol Scand. 1989 Apr;135(4):493–503. doi: 10.1111/j.1748-1716.1989.tb08608.x. [DOI] [PubMed] [Google Scholar]
- Everts M. E., Retterstøl K., Clausen T. Effects of adrenaline on excitation-induced stimulation of the sodium-potassium pump in rat skeletal muscle. Acta Physiol Scand. 1988 Oct;134(2):189–198. doi: 10.1111/j.1748-1716.1988.tb08479.x. [DOI] [PubMed] [Google Scholar]
- Fellenius E. Muscle fatigue and beta-blockers--a review. Int J Sports Med. 1983 Feb;4(1):1–8. doi: 10.1055/s-2008-1026008. [DOI] [PubMed] [Google Scholar]
- Gerdle B., Hedberg R., Jonsson B., Fugl-Meyer A. R. Mean power frequency and integrated electromyogram of repeated isokinetic plantar flexions. Acta Physiol Scand. 1987 Jul;130(3):501–506. doi: 10.1111/j.1748-1716.1987.tb08168.x. [DOI] [PubMed] [Google Scholar]
- Gregor R. J., Komi P. V., Järvinen M. Achilles tendon forces during cycling. Int J Sports Med. 1987 Mar;8 (Suppl 1):9–14. doi: 10.1055/s-2008-1025698. [DOI] [PubMed] [Google Scholar]
- Gullestad L., Birkeland K., Nordby G., Larsen S., Kjekshus J. Effects of selective beta 2-adrenoceptor blockade on serum potassium and exercise performance in normal men. Br J Clin Pharmacol. 1991 Aug;32(2):201–207. doi: 10.1111/j.1365-2125.1991.tb03882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullestad L., Dolva L. O., Nordby G., Skaaraas K., Larsen S., Kjekshus J. The importance of potassium and lactate for maximal exercise performance during beta blockade. Scand J Clin Lab Invest. 1989 Oct;49(6):521–528. doi: 10.3109/00365518909089131. [DOI] [PubMed] [Google Scholar]
- Gullestad L., Hallén J., Sejersted O. M. Variable effects of beta-adrenoceptor blockade on muscle blood flow during exercise. Acta Physiol Scand. 1993 Nov;149(3):257–271. doi: 10.1111/j.1748-1716.1993.tb09621.x. [DOI] [PubMed] [Google Scholar]
- Hallén J., Sejersted O. M. Intravasal use of pliable K(+)-selective electrodes in the femoral vein of humans during exercise. J Appl Physiol (1985) 1993 Nov;75(5):2318–2325. doi: 10.1152/jappl.1993.75.5.2318. [DOI] [PubMed] [Google Scholar]
- Hjemdahl P., Daleskog M., Kahan T. Determination of plasma catecholamines by high performance liquid chromatography with electrochemical detection: comparison with a radioenzymatic method. Life Sci. 1979 Jul 9;25(2):131–138. doi: 10.1016/0024-3205(79)90384-9. [DOI] [PubMed] [Google Scholar]
- Hjemdahl P. Plasma catecholamines--analytical challenges and physiological limitations. Baillieres Clin Endocrinol Metab. 1993 Apr;7(2):307–353. doi: 10.1016/s0950-351x(05)80179-x. [DOI] [PubMed] [Google Scholar]
- Häggendal J., Hartley L. H., Saltin B. Arterial noradrenaline concentration during exercise in relation to the relative work levels. Scand J Clin Lab Invest. 1970 Dec;26(4):337–342. doi: 10.3109/00365517009046242. [DOI] [PubMed] [Google Scholar]
- Juel C., Bangsbo J., Graham T., Saltin B. Lactate and potassium fluxes from human skeletal muscle during and after intense, dynamic, knee extensor exercise. Acta Physiol Scand. 1990 Oct;140(2):147–159. doi: 10.1111/j.1748-1716.1990.tb08986.x. [DOI] [PubMed] [Google Scholar]
- Langer G. A. The 'sodium pump lag' revisited. J Mol Cell Cardiol. 1983 Oct;15(10):647–651. doi: 10.1016/0022-2828(83)90254-7. [DOI] [PubMed] [Google Scholar]
- Medbø J. I., Sejersted O. M. Plasma potassium changes with high intensity exercise. J Physiol. 1990 Feb;421:105–122. doi: 10.1113/jphysiol.1990.sp017935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nørgaard A., Kjeldsen K., Clausen T. A method for the determination of the total number of 3H-ouabain binding sites in biopsies of human skeletal muscle. Scand J Clin Lab Invest. 1984 Oct;44(6):509–518. doi: 10.3109/00365518409083604. [DOI] [PubMed] [Google Scholar]
- RENKIN E. M. Transport of potassium-42 from blood to tissue in isolated mammalian skeletal muscles. Am J Physiol. 1959 Dec;197:1205–1210. doi: 10.1152/ajplegacy.1959.197.6.1205. [DOI] [PubMed] [Google Scholar]
- Sahlin K., Broberg S. Release of K+ from muscle during prolonged dynamic exercise. Acta Physiol Scand. 1989 Jun;136(2):293–294. doi: 10.1111/j.1748-1716.1989.tb08666.x. [DOI] [PubMed] [Google Scholar]
- Sejersted O. M., Wasserstrom J. A., Fozzard H. A. Na,K pump stimulation by intracellular Na in isolated, intact sheep cardiac Purkinje fibers. J Gen Physiol. 1988 Mar;91(3):445–466. doi: 10.1085/jgp.91.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan R. M., Renkin E. M. Capillary, interstitial, and cell membrane barriers to blood-tissue transport of potassium and rubidium in mammalian skeletal muscle. Circ Res. 1972 May;30(5):588–607. doi: 10.1161/01.res.30.5.588. [DOI] [PubMed] [Google Scholar]
- Sjøgaard G., Saltin B. Extra- and intracellular water spaces in muscles of man at rest and with dynamic exercise. Am J Physiol. 1982 Sep;243(3):R271–R280. doi: 10.1152/ajpregu.1982.243.3.R271. [DOI] [PubMed] [Google Scholar]
- Thomas C. K., Bigland-Richie B., Johansson R. S. Force-frequency relationships of human thenar motor units. J Neurophysiol. 1991 Jun;65(6):1509–1516. doi: 10.1152/jn.1991.65.6.1509. [DOI] [PubMed] [Google Scholar]
- Vøllestad N. K., Hallén J., Sejersted O. M. Effect of exercise intensity on potassium balance in muscle and blood of man. J Physiol. 1994 Mar 1;475(2):359–368. doi: 10.1113/jphysiol.1994.sp020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODBURY J. W. Interrelationships between ion transport mechanisms and excitatory events. Fed Proc. 1963 Jan-Feb;22:31–35. [PubMed] [Google Scholar]
- Walløe L., Wesche J. Time course and magnitude of blood flow changes in the human quadriceps muscles during and following rhythmic exercise. J Physiol. 1988 Nov;405:257–273. doi: 10.1113/jphysiol.1988.sp017332. [DOI] [PMC free article] [PubMed] [Google Scholar]


