Abstract
Background
Covid-19 healthcare worker testing, isolation and quarantine policies had to balance risks to patients from the virus and from staff absence. The emergence of the Omicron variant led to dangerous levels of key-worker absence globally.
We evaluated whether using two manufacturers’ lateral flow tests (LFTs) concurrently improved SARS-CoV-2 Omicron detection significantly and was acceptable to hospital staff. In a nested study, to understand risks of return to work after a 5-day isolation/quarantine period, we examined virus culture 5–7 days after positive test or significant exposure.
Methods
Fully-vaccinated Liverpool (UK) University Hospitals staff participated (February-May 2022) in a random-order, open-label trial testing whether dual LFTs improved SARS-CoV-2 detection, and whether dual swabbing was acceptable to users. Participants used nose-throat swab Innova and nose-only swab Orient Gene LFTs in daily randomised order for 10 days. A user-experience questionnaire was administered on exit. Selected participants gave swabs for viral culture on days 5–7 after symptom onset or first positive test. Cultures were considered positive if cytopathic effect was apparent or SARS-CoV-2 N gene sub-genomic RNA was detected.
Results
Two hundred and twenty-six individuals reported 1466 pairs of LFT results. Tests disagreed in 127 cases (8.7%). Orient Gene was more likely (78 cf. 49; OR: 2.1, 1.1–4.1; P = 0.03) to be positive. If Innova was swabbed second, it was less likely to agree with a positive Orient Gene result (OR: 2.7, 1.3–5.2; P = 0.005); swabbing first with Innova made no significant difference (OR: 1.1, 0.5–2.3; P = 0.85). Orient Gene positive Innova negative result-pairs became more frequent over time (OR: 1.2, 1.1–1.3; P < 0.001).
Of individuals completing the exit questionnaire, 90.7% reported dual swabbing was easy, 57.1% said it was no barrier to their daily routine and 65.6% preferred dual testing. Respondents had more confidence in dual versus single test results.
Viral cultures from days 5–7 were positive for 6/31 (19.4%, 7.5%-37.5%) and indeterminate for 11/31 (35.5%, 19.2%-54.6%) LFT-positive participants, indicating they were likely still infectious.
Conclusions
Dual brand testing increased LFT detection of SARS-CoV-2 antigen by a small but meaningful margin and was acceptable to hospital workers. Viral cultures demonstrated that policies recommending safe return to work ~ 5 days after Omicron infection/exposure were flawed. Key-workers should be prepared for dynamic self-testing protocols in future pandemics.
Trial registration
https://www.isrctn.com/ISRCTN47058442 (26 January 2022).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-10155-z.
Keywords: Covid-19, SARS-CoV-2, Lateral flow test, Healthcare worker
Background
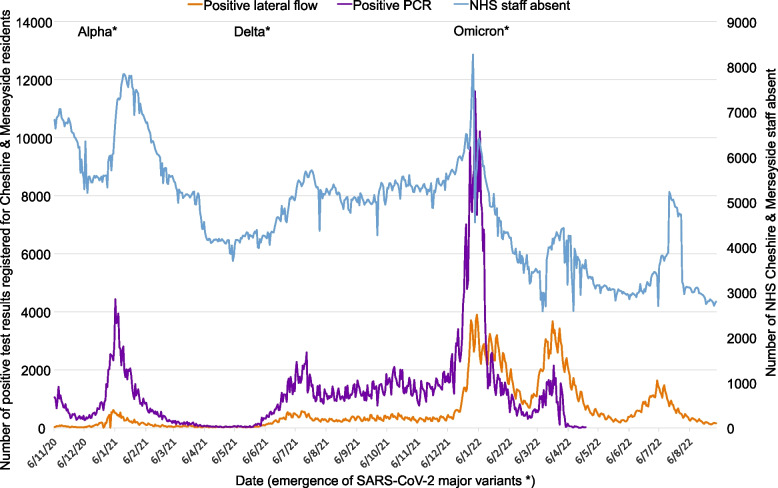
The Covid-19 pandemic stretched health systems worldwide [1, 2]. Healthcare workers suffered high rates of infection and mortality [3–5], and policymakers faced dilemmas in balancing risks. In late 2021, as Omicron hit the UK, hospitalised patients faced risks from having too few staff to care for them (Fig. 1), which potentially posed a greater threat to patient safety and care delivery than Covid-19 itself [6–10]. Omicron’s increased transmissibility and immune evasion demanded a rethink of Covid-19 policies for healthcare workers and the public [10–13].
Fig. 1.
Numbers of NHS staff absent, and numbers of positive PCR and lateral flow test results reported for residents of Cheshire & Merseyside, UK from the start of introduction of lateral flow community testing to the end of the study period
Pre-Omicron, UK healthcare workers required a negative PCR 10 days from exposure to return from quarantine [14, 15]. Waiting (typically 48-h) for PCR results delayed return to work [15], and PCR capacity affected care-service continuity [16, 17]. By December 2021, it was evident that SARS-CoV-2 lateral flow tests (LFTs) were reasonable and affordable indicators of infectiousness [18–20]. LFTs from some manufacturers used nose-only swabbing, others nose-throat swabbing, with nose-only testing assumed to have better compliance. Policymakers were concerned that nose-only swabbing might delay detection of Omicron, which was reportedly shed from the throat ahead of the nose [21] – a concern not addressed by national testing quality assurance programmes [22, 23].
In December 2021 and January 2022, NHS staff testing policies changed to address staff shortages. Based on mathematical modelling, NHS workers were permitted to return from isolation or quarantine: after two consecutive days of negative LFTs beyond 5 days since exposure or first positive test; or if still testing positive, 10 days from symptom onset or first positive test, provided they felt well enough [14, 15, 24]. This guidance was updated on 7th January 2022 to advise local risk assessments for those testing positive on days 10–14 [25].
The modelling of serial negative LFT results to inform return to work was performed by the Scientific Pandemic Influenza Group on Modelling (SPI-M) [26] and UK Health Security Agency (UKHSA) [27] alongside unpublished viral culture studies for the New and Emerging Respiratory Virus Threats Advisory Group (NERVTAG).
This study was commissioned by the UK Covid-19 Testing Initiatives Evaluation Board (TIEB) to extend its testing quality assurance programme. We investigated whether SARS-CoV-2 antigen detection in daily self-testing was improved by using kits from two manufacturers concurrently; one requiring nose-only and one nose-throat swabbing [22]. Real-world testing sensitivity and NHS staff acceptability were the main outcomes. A nested virus culture study assessed the infectiousness of individuals still testing positive after day-5 since symptom onset or first positive test, as the US policy was to return to work after day-5 without testing. Data from this study informed UK policies via TIEB [28].
Methods
Aim
We aimed to evaluate effectiveness and acceptability of dual versus single brand SARS-CoV-2 antigen lateral flow self-testing among hospital workers, and to determine whether culturable SARS-CoV-2 Omicron was present 5–7 days after a positive test or significant exposure.
Trial design
An open-label, randomised-order trial of using two LFT brands concurrently in daily self-testing with the ‘Test-to-Release’ [29], or Daily Contact Testing design [30–33].
Setting
Participants comprised fully vaccinated [34] NHS workers using Covid-19 staff-testing facilities for contacts or cases at Liverpool University Hospitals NHS Foundation Trust, UK between 7th February and 8th May 2022. Participants entered the study by booking a test routinely on-line where they received study information and consented to participate. Participants were recruited via one of three pathways (Appendix 1) depending on their status at baseline: i) test-negative close contact (reflecting routine daily testing as an alternative to quarantine for an uninfected staff member notified as a close contact of a known case); ii) test-positive symptomatic (reflecting routine isolation of a staff member presenting for testing due to symptoms and testing positive); or iii) test-positive asymptomatic (reflecting routine isolation of a staff member without symptoms testing positive, including via routine daily contact testing in quarantine and additional tests from this study). Data were collected via on-line questionnaires and NHS record linkage.
Intervention
The study used two LFT brands widely available via NHS Test & Trace in February 2022: the nose-only swab Orient Gene and nose-throat swab Innova (Xiamen Biotime Biotechnology) kits. These have similar performance curves versus viral load when compared to PCR results [23].
Participants were asked to take two LFTs daily for 10 days, and on day-1 and day-5 to return swabs for quantitative PCR. Test order was detailed on an information sheet (Appendix 2), with daily LFTs in random order (Innova or Orient Gene first) and PCR on day-1 and day-5. Participants uploaded LFT results via NHS Test & Trace systems – with automated image reading available via the national standard app to ease consistent reporting – participants could override the automated reading: no data were available on overrides but previous studies suggest these were rare [35].
A nested study considered culture of viable virus at days 5–7 from first positive test.
Outcomes
The primary outcome was the discordance of results from concurrent LFTs. Secondary outcomes were participant compliance, and self-reported experience of dual versus single testing from the exit survey detailed in Appendix 3.
Sample size
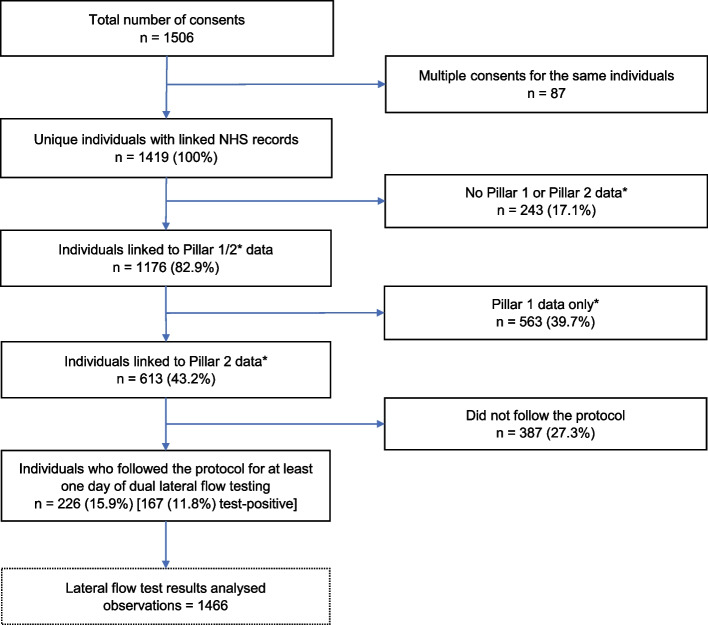
Calculations (see Appendix 4) assumed 18% drop-out and 10% test-positivity. The proportion of consented individuals not returning data was higher than expected (Fig. 2), and test-positivity was > 10%. Power to detect a difference between dual and single testing was the main target and the number of participants testing positive (n = 167) was similar to the number required (n = 164). It was later reported that SARS-CoV-2 LFTs were more sensitive to Omicron than prior variants, with Orient Gene more sensitive than Innova [23].
Fig. 2.
Flow of participants from consent to data analysed. *Pillar 1 comprised PCR tests processed in hospital laboratories. Pillar 2 comprised PCR tests processed in national NHS Test & Trace laboratories and lateral flow test results reported by individuals self-testing and putting results into the national NHS website or app
Viral culture and sequencing to determine lineage
Appendix 5 details viral culture, RNA-extraction, sequencing and bioinformatics methods used to infer the presence of replicable SARS-CoV-2 lineages from swab samples. In brief, Calu3 cells, cultured at 10^5 cells/well in 24 well plates, were inoculated for viral culture, incubated and checked for cytopathic effect (CPE) after three days. If CPE was visible supernatants were sampled for RNA extraction. If no CPE was visible a 2nd passage was performed before supernatant sampling. RNA was extracted from supernatants and used for amplicon sequencing by MinION, using a published method. Fastq reads were analysed using the ARTIC [36] bioinformatic pipeline and lineages were called with Pangolin [37]. LeTRS was used to assess the presence of N gene sub genomic RNA (sgRNA) [38], indicative of active viral transcription.
Statistical methods
Discordance of result-pairs from two LFT brands was analysed with McNemar’s test, including Yang’s adjustment and logistic mixed-effects models to account for test-clustering within individuals over time and in study-day groups [39]. Trends over time in discordance were analysed with a logistic mixed-effects model addressing clustering within individuals with study-day groups disaggregated.
Comparison of users’ confidence in single versus dual testing from questionnaire ordinal score data used a Wilcoxon signed ranks test and exact confidence interval as score distributions were skewed. Confidence intervals for binomial proportions used the Clopper-Pearson method, and for logistic mixed-effects models the Wald method. Analyses were performed using R version 4.3.1. Results were verified independently by two statisticians. Results are presented as main effect with 95% confidence interval.
Patient and public involvement
UKHSA’s Research Ethics & Governance of Public Health Practice Group (REGG), including lay members, along with TIEB, fed back on drafts of the study protocol as part of the approvals process. Additional public involvement over data governance was provided by Liverpool City Region Civic Data Cooperative.
Results
Main outcomes
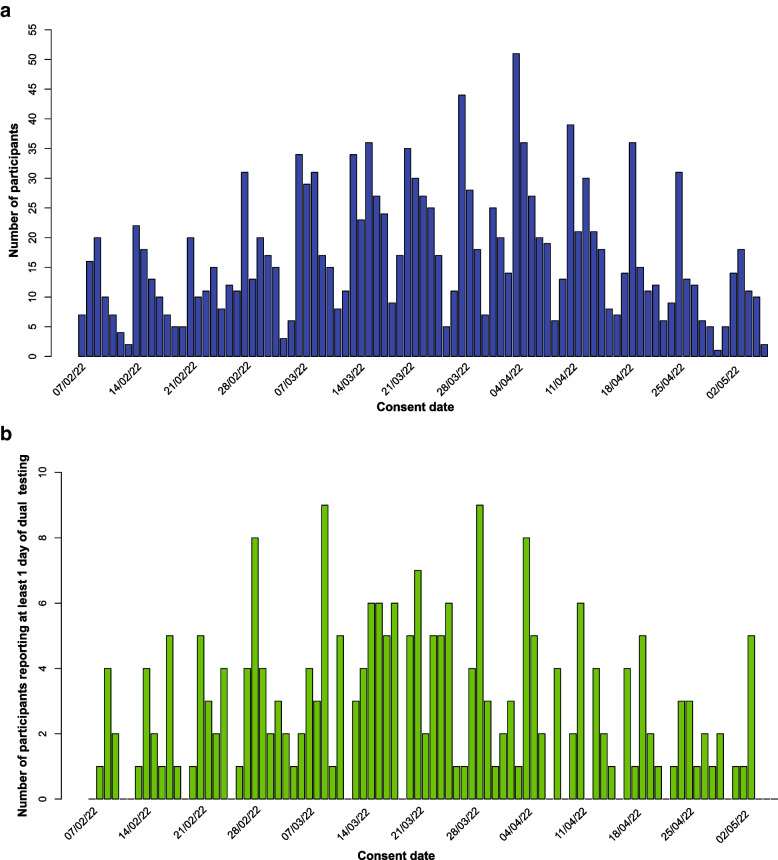
Two hundred and twenty-six participants reported at least one day of dual LFT results between study day-1 and day-10, giving 1466 pairs of tests. Figure 2 shows the flow of participants from consent to analysis, Fig. 3 the recruitment patterns over time.
Fig. 3.
A Number of participants consenting to take part in the study by consent date. B Recruitment pattern over time of 226 participants who completed at least one day of dual lateral flow testing over the study period
The study population comprised 226 participants reporting 1466 pairs of LFT results. 167 (73.9%) of the 226 participants were classified as cases at any point during the study – a) at baseline if they had a positive PCR from the staff testing centre where they consented to participate or reported dual LFT positive results on day 1; and b) if they reported dual LFT positive results on any of days 2–10. PCR results were available for 190 (84.1%) at recruitment/consent and inferred from day 1 LFT results for a further 21 leading to a total of 211 (93.4%) having a definitive infection status at baseline. 46 (20.4%) reported dual LFT results for the full 10 days, where others tended to stop reporting results after turning from positive to negative. Appendix 6 gives further accrual details.
A total of 125 individuals had a positive PCR test at recruitment/consent. In this group, the first reported LFT result pairs were both positive for 116 (92.8%), both negative for 3 (2.4%), Innova positive only for 3 (2.4%) and Orient Gene positive only for 3 (2.4%) – a discrepancy rate (~ detection uplift from dual versus single testing) of 6/125 (4.8%, 1.8%-10.2%). On day 2, 99 were positive on both LFTs (90%), 5 were negative on both (4.5%), 5 were Orient Gene positive only (4.5%), and 1 was Innova positive only (1%) – a discrepancy rate of 6/110 (5.5%, 2.0%-11.5%). Considering two days of consecutive test results, only 2 (1.6%) individuals would have been LFT negative and PCR positive within 48 h.
Of the 1466 pairs of LFT results reported, 127 (8.7%) were discordant (gave different results). For those participants who tested positive at any time point (n = 167, 73.9%), on those days where they tested positive using dual LFT testing (n = 866), the number of pairs where the first recorded test was negative was 46 (5.3%).
Overall, Orient Gene had double the odds of being positive compared to Innova when the two tests disagreed (Table 1). 156 (93.4%; 88.5%-96.7%) suspected infections were detected with Orient Gene compared to 163 (97.6%; 94.0%-99.3%) with Innova. Out of the test-positive cohort of 167, 59 (35.3%) had at least one subsequent period of 2 or more consecutive days of dual negative LFTs. Of these, none had a pair of positive LFT results afterwards.
Table 1.
Lateral flow test results by brand
| Innova | ||||
|---|---|---|---|---|
| Negative | Positive | Total | ||
| Orient Gene | Negative |
596 (40.7%) |
49 (3.3%) |
645 |
| Positive |
78 (5.3%) |
743 (50.7%) |
821 | |
| TOTAL | 674 | 792 |
1466 (100%) |
|
Test pairs with any equivocal result were excluded. McNemar (Yang-adjusted) Chi2 = 4.636, P = 0.03, logistic mixed-effects model for discordant tests odds ratio (OR) = 2.1(1.1–4.1), P = 0.03
When Orient Gene was the first test (Table 2), Orient Gene positive Innova negative was a more likely discordant result than Innova positive Orient Gene negative (OR = 2.7, 1.3–5.2; P = 0.005). No significant difference was observed when Innova was the first test (OR = 1.1, 0.5–2.3; P = 0.85, Table 3). Direct comparison of discordant test pairs shows the odds of an Orient Gene positive with Innova negative discordance was 4.5 times higher when Orient Gene was first versus Innova first (OR = 4.5, 1.1–18.1; P = 0.04).
Table 2.
Lateral flow test results when Orient Gene was recorded first
| Innova | ||||
|---|---|---|---|---|
| Negative | Positive | Total | ||
| Orient Gene | Negative |
307 (40.3%) |
18 (2.4%) |
325 |
| Positive |
47 (6.2%) |
390 (51.2%) |
437 | |
| Total | 354 | 408 | 762 (100%) | |
Test pairs with any equivocal result were excluded. McNemar (Yang-adjusted) Chi2 = 11.668, P = 0.001, logistic mixed-effects model for discordant tests OR = 2.7(1.3–5.2), P = 0.005
Table 3.
Lateral flow test results when Innova was recorded first
| Innova | ||||
|---|---|---|---|---|
| Negative | Positive | Total | ||
| Orient Gene | Negative |
289 (41.1%) |
31 (4.4%) |
320 |
| Positive |
31 (4.4%) |
353 (50.1%) |
384 | |
| TOTAL | 320 | 384 | 704 (100%) | |
Test pairs with any equivocal result were excluded. McNemar (Yang-adjusted) Chi2 < 0.001, P > 0.99, logistic mixed-effects model for discordant tests OR = 1.1(0.5–2.3), P = 0.85
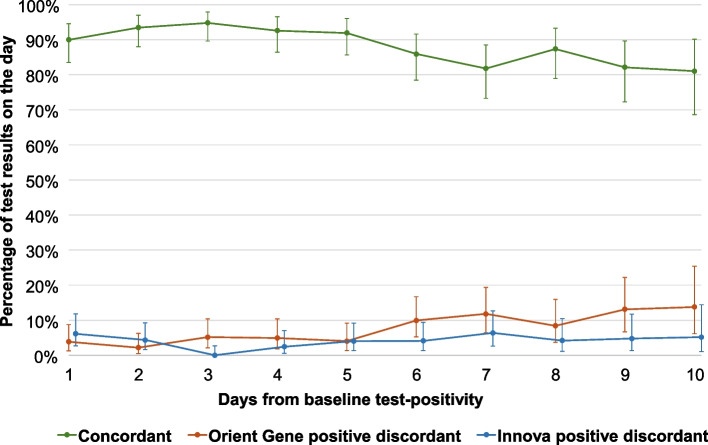
Of the 167 participants who tested PCR or LFT positive at study entry or became LFT test-positive during the study (Table 4), the proportion of Orient Gene positive discordant tests increased significantly over time (OR: 1.2, 1.1–1.3; P < 0.001), and not significantly for Innova (OR: 1.1, 0.97–1.2; P = 0.15). Direct comparison of the two discordant groups using a logistic mixed effects model did not show statistical significance (OR: 1.2, 0.99–1.6; P = 0.07), however, small numbers of discordant groups (Fig. 4) may have limited the power to resolve this effect. For participants who never tested positive, there was very little discordance between test results (Appendix 6: Table A6.6).
Table 4.
Dual lateral flow test (LFT) results by brand and days from baseline (study entry or first positive test) for individuals who tested positive
| Day from baseline test-positivity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dual LFT results | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Concordant |
117 (90.0%) |
129 (93.5%) |
128 (94.8%) |
113 (92.6%) |
114 (91.9%) |
104 (86.0%) |
90 (81.8%) |
83 (87.4%) |
69 (82.1%) |
47 (81.0%) |
| Both positive |
114 (87.7%) |
122 (88.4%) |
123 (91.1%) |
103 (84.4%) |
94 (75.8%) |
73 (60.3%) |
52 (47.3%) |
37 (38.9%) |
20 (23.8%) |
5 (8.6%) |
| Both negative |
3 (2.3%) |
7 (5.1%) |
5 (3.7%) |
10 (8.2%) |
20 (16.1%) |
31 (25.6%) |
38 (34.5%) |
46 (48.4%) |
49 (58.3%) |
42 (72.4%) |
| Discordant |
13 (10.0%) |
9 (6.5%) |
7 (5.2%) |
9 (7.4%) |
10 (8.1%) |
17 (14.0%) |
20 (18.2%) |
12 (12.6%) |
15 (17.9%) |
11 (19.0%) |
| Orient Gene positive |
5 (3.8%) |
3 (2.2%) |
7 (5.2%) |
6 (4.9%) |
5 (4.0%) |
12 (9.9%) |
13 (11.8%) |
8 (8.4%) |
11 (13.1%) |
8 (13.8%) |
| Innova positive |
8 (6.2%) |
6 (4.3%) |
0 (0.0%) |
3 (2.5%) |
5 (4.0%) |
5 (4.1%) |
7 (6.4%) |
4 (4.2%) |
4 (4.8%) |
3 (5.2%) |
| Total | 130 | 138 | 135 | 122 | 124 | 121 | 110 | 95 | 84 | 58 |
Table includes only those participants who tested PCR or LFT positive at study entry or became LFT test-positive throughout the study and counts dual test results they reported on any day. Any LFT pair with any equivocal result was excluded, along with any dual tests prior to the first recorded positive test for each participant
Fig. 4.
Percentage of concordant and discordant lateral flow test result pairs by brand and days from baseline for those testing positive
Viral culture analysis
Viral cultures were analysed for 41 participants (see Appendix 5 for details): 31 continuing LFT positive and 10 reverting negative on days 5–7. 6/31 (19.4%, 7.5%-37.5%) of the continued LFT positives were culture positive, 9/31 (29.0%, 14.2%-48.1%) were indeterminate by cytopathic effect (CPE), none of these had N-sgRNA detected by sequencing. Two additional cultures with no CPE had N-sgRNA detected, both at low level. 2/10 (20.0%, 2.5%-55.6%) of the reverting LFT negatives were indeterminate by CPE with N-sgRNA not detected by sequencing. Two cultures without CPE from LFT negative individuals had N-sgRNA detected at low levels. PCR was carried out on the original swab aliquot: all came back SARS-CoV-2 positive, with S Gene target present in 36 (likely Omicron BA.2) and absent in 5 (likely Omicron BA.1).
Exit questionnaire survey
Three hundred and eleven participants responded to the exit survey between 10th February and 20th July 2022 (details in Appendix 5). 229 (73.7%) identified as a woman, 77 (24.7%) as a man, 0 as non-binary and 5 (1.58%) preferred not to say. Professions were: 57 (18.5%) doctors; 84 (27.2%) nurses; 78 (25.0%) allied health professionals; 25 (8.2%) clinical support staff; 44 (14.1%) administration/clerical staff; 22 (7.07%) other staff. When asked “how easy was the swabbing process” 154 (49.6%) selected “very easy”, 128 (41.2%) “easy”, 26 (8.4%) “neither easy nor difficult”, 3 (0.9%) “difficult”, and none “very difficult”. When asked “How much of a barrier is having to take a throat as well as a nose swab for your daily rapid test?” 178 (57.1%) selected “not at all”, 80 (25.6%) selected “slight barrier”, 38 (12.3%) selected “somewhat of a barrier”, 12 (3.94%) selected “moderate barrier”, and 3 (1.0%) selected “extreme barrier”. When asked “Could you fit taking two rapid tests into your daily routine within an hour of leaving for work?” 90 (28.9%) respondents selected “definitely”, 58 (18.6%) selected “very probably”, 110 (35.3%) selected “probably”, 44 (14.2%) selected “probably not”, and 9 (2.9%) selected “definitely not”. When asked “If you were asked to continue to do two tests daily instead of one, would you?” 204 (65.6%) selected “yes”, 57 (18.2%) selected “no”, and 50 (16.2%) selected “no preference”. When asked “which mode of testing are you most confident about” and given a rating scale from 1 = “no confidence” to 10 = “full confidence” for “single test” and “double test” the median scores were 8 for single test and 9 for double test – a median difference of 1 (0.5 to 1; P < 0.001) – a small but psychologically significant difference.
Discussion
Main findings
We found that practical combination of LFT brands with different swab types enhanced antigen detection differently at different time points during infection. Combining Orient Gene and Innova LFTs improved SARS-CoV-2 (Omicron BA.1/2) antigen detection by a small amount [4.8% (1.8%-10.2%)] compared to a single test. This marginal effect was relevant for managing safe return to work amid potentially dangerous staff shortages.
Orient Gene was more likely to be the sole positive test – with Orient Gene positive Innova negative results becoming more frequent over successive days. If Innova was swabbed second it was less likely to agree with a positive Orient Gene result; swabbing first with Innova made no significant difference.
Dual testing was largely acceptable to hospital staff, with most reporting dual swabbing manageable. Almost two-thirds preferred to continue dual testing if required and had slightly more confidence in dual versus single testing results. Over half said dual swabbing was no barrier to daily routines. However, the two LFT kits required different swabbing methods and around 40% of staff reported that swabbing both nose and throat was at least a slight barrier.
Almost a fifth of individuals with positive LFTs at days 5–7 had positive culture, indicating they were likely still infectious. We did not identify any convincingly culture positive individuals who had a negative LFT by this time, though we could still detect viral RNA sequences.
Comparison with other studies
Evidence on end-to-end risk mitigation using LFTs in the Covid-19 pandemic is limited, with a few small serial testing and viral culture studies nested within studies comparing LFT and PCR performance [23, 40, 41]. One study found minimal improved sensitivity by using the same manufacturer's LFT in rapid succession [42]. Ours is the only real-world trial, to our knowledge, of combining LFTs to enhance risk-mitigation in balancing risks from Covid-19 versus healthcare staff shortages. The shorter incubation period of Omicron compared with earlier variants challenged previous risk-mitigations [43, 44] and there were concerns over later nasal shedding invalidating nose-only swab LFTs [21], which we showed were mitigated by dual testing.
Viral culture studies with early variants found that people infected with SARS-CoV-2 became infectious 1–2 days before the onset of symptoms and remained infectious until 7 days later [43, 45]. Our data showed a substantial proportion of individuals were potentially infectious beyond this point. Most work on this topic has placed too much emphasis on the median duration of infection at the expense of considering variability and its impact on fixed time-period policies for return from isolation or quarantine.
Strengths and limitations of this study
This is the first study, to our knowledge, to compare dual with single brand LFT self-testing of healthcare workers in managing the risks from Covid-19 versus under-staffed care due to high numbers isolating or quarantined. It was performed when the UK was under pressure from Omicron variants in early 2022. It was thus a realistic test of enhanced risk-mitigation and comprehensively considered LFT sensitivity, participant experience, and security (using viral cultures) of prompt return to work. The 10-day observation period allowed assessment of the contemporary Covid-19 policies for healthcare worker release from isolation and quarantine. The results give insights into the combined performance of two brands of LFT, which cannot be inferred from the usual comparison with concurrent PCR tests [23, 46].
Our study had limitations: slow research approvals meant the peak of the initial Omicron epidemic was missed. Staff burnout and low morale slowed recruitment and prolonged the study duration. National policies for NHS staff testing changed several times during the study [13, 47], and public access to LFTs was reduced on 1st April 2022 [47], potentially also impeding participant uptake. These barriers reflect the real-world challenges of evaluating new risk-mitigations two years into a pandemic and under winter pressures.
Only 16% of consented participants followed the protocol for at least one day – this likely limits the representativeness of the survey results, which also don’t reflect the hospital staff who didn’t wish to participate in the study. Finally, viral culture interpretation was limited – parallel PCR swabbing and sequencing would have given more confidence that SARS-CoV-2 was present and not another virus.
Policy implications
Throughout the Covid-19 pandemic, policies for testing healthcare workers changed many times [10, 13, 16, 48]. In the UK, LFT self-testing became part of life. However, with the rise of Omicron policymakers feared that nose-only LFT swabbing may miss numerous infections. Our study allayed these fears, showing reasonable concordance of the widely available Orient Gene nose-only and Innova nose-throat swab LFTs. Policymakers were therefore right to use all available stocks of LFTs, including those with nose-only swabs, to mitigate elevated risks.
Despite pandemic pressures, study participants reported largely positive experiences of using two LFTs instead of one for daily self-testing. The resulting improvement in detection was small yet meaningful in a universal health system coordinating risk-mitigations system-wide.
Internationally, there was pressure to balance risks from Covid-19 with those from mass absence of key workers. Our viral culture study shows that fixed isolation time policies, such as the US advice to return to work 5 days after testing positive, were flawed [49]. The UK’s ‘test-to-release from isolation’ (after two days of negative LFT results) policy, formed in December 2021, was reasonable given staffing pressures at the time.
The speed, convenience, and socialisation of LFT self-testing in the UK allowed enhanced Covid-19 risk-mitigation under pressure from Omicron. A better UK response would have extended testing quality assurance from public health agencies to the NHS. Ideally, more serial samples of daily antigen, nucleic acid and culturable virus testing would have informed policy modelling. Future pandemic preparations globally should consider closer surveillance of serial self-testing to inform evolving risk-mitigations. In addition, dynamic economic evaluations should be built into the monitoring of large-scale pandemic testing programmes.
Conclusion
Policymakers’ fears that nose-only LFT swabbing may miss a substantial proportion of Omicron BA.1/2 infections were allayed by our study of NHS workers in the UK between February and June 2022. Combining the widely available Innova nose-throat and Orient Gene nose-only LFT kits increased Omicron detection and was acceptable to participating hospital staff self-testing in isolation or quarantine. This improvement was small yet meaningful in a universal health system coordinating risk-mitigations system-wide. The US policy of return to work 5 days after testing positive was shown by our viral culture results to be flawed. The speed, convenience, and public socialisation of LFT self-testing in the UK allowed enhanced Covid-19 risk-mitigation during the pressures caused by the Omicron variant. A better UK response would have extended testing quality assurance from public health agencies to the NHS. Future pandemic preparedness may be enhanced by continuous surveillance of serial self-testing, considering end-to-end risk mitigation as well as technology performance.
Supplementary Information
Acknowledgements
We thank all participants and wider staff at Liverpool University Hospitals NHS Trust who supported the study, Cheshire & Merseyside Blood Bikes volunteers who made home-based viral culture swab delivery and collection possible, the UK Testing Initiatives Evaluation Board for their generous support with urgent preparation of the protocol and discussion over interim findings to support policymaking, and UKHSA Research Ethics and Governance Group. Thanks to Kathryn Scott for proofreading this manuscript.
Affirmation
IB affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Dissemination to participants and related patient and public communities
Findings were presented to the UK Testing Initiatives Evaluation Board and associated patient and public involvement groups throughout the study and in final form in September 2022. TN, as infection prevention and control lead and trial site (Liverpool University Hospitals) lead has disseminated results to participants.
Provenance and peer review
The study was commissioned by the UK’s Department of Health and Social Care. The work has been peer reviewed by external reviewers in the UK Testing Initiatives Evaluation Board at each stage, from protocol formation to presentation of findings.
Abbreviations
- Covid-19
Coronavirus disease 2019
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- LFT
Lateral flow test
- CPE
Cytopathic effect
- NHS
UK National Health Service
- TIEB
UK Covid-19 Testing Initiatives Evaluation Board
- UKHSA
UK Health Security Agency
- NERVTAG
New and Emerging Respiratory Virus Threats Advisory Group
- CDC
US Centres for Disease Control and Prevention
Authors’ contributions
I.B., X.Z. and C.C. drafted the manuscript. I.B. drafted the protocol, led the study and the preparation of reports and this paper. C.C. drafted the statistical analysis plan and led data management with G.L. C.C., D.H., G.B., S.D., M.GF. and I.B. ran and corroborated three independent statistical analyses. C.S. led the study management. T.N. ran the trial site. T.F. and S.T. ran the UKHSA operations for the trial. L.T. and C.J. ran the viral culture sub-study. R.PR. and X.D. performed viral sequencing and bioinformatic analyses. M.H. led the exit questionnaire. M.G.S. and T.F. led the policymaker inputs. Each author contributed substantial intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. All authors approved the final version of the manuscript. I.B. is the study guarantor. The corresponding author (I.B.) affirms that all the listed authors meet the authorship criteria and that no others meeting the criteria have been omitted.
Funding
This research was commissioned and funded by the UK Health Security Agency and carried out independently by the University of Liverpool. The work was also supported by the Economic and Social Research Council (grant No ES/L011840/1). IB is supported by the National Institute for Health and Care Research (NIHR) as senior investigator award NIHR205131. IB and XZ are also supported by the NIHR Health Protection Research Unit in Gastrointestinal Infections, a partnership between UKHSA, the University of Liverpool, and the University of Warwick (NIHR200910). MGS and LT are supported by the NIHR Health Protection Unit in Emerging and Zoonotic Infections, a partnership between UKHSA, the University of Liverpool and the University of Oxford (NIHR200907). The NIHR had no role in the study design, data collection and analysis, decision to publish, or preparation of the article. LT, RPR and XD are supported by the U.S. Food and Drug Administration Medical Countermeasures Initiative contract 75F40120C00085. The funders had no role in considering the study design or in the collection, analysis, or interpretation of data, writing of the report, or decision to submit the article for publication. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, NIHR, or Department of Health and Social Care.
Data availability
The study required person identifiable data and the main analyses were conducted on a de-identified extract. The fully anonymised data for reproducing the results are available from https://github.com/iain-buchan/cipha/blob/master/SMART_RR_Anonymised_Data.zip. The study protocol can be downloaded from https://github.com/iain-buchan/cipha/blob/master/SMART_Release_Return.pdf and statistical analysis plan from https://github.com/iain-buchan/cipha/blob/master/SMART_RR_SAP.pdf.
Declarations
Ethics approval and consent to participate
The study protocol was developed with the UK Covid-19 Testing Initiatives Evaluation Board (TIEB) and approved by UK Health Security Agency (UKHSA) Urgent Studies Ethics Committee. TIEB was part of the UK Covid-19 testing initiatives evaluation programme, which included academics and public health professionals independent of this study. The motivation for the study came from a request by Merseyside Resilience Forum to UKHSA and NHS England to vary Covid-19 testing policies in response to dangerous levels of NHS staff absence in December 2021. TIEB signed off the study protocol on 4th January 2022 and UKHSA Research Support and Governance Office approved the study on 25th January 2022 as NR0308. The sponsor code for this study is UoL001685 and trial registration code IRAS ID 311842; https://www.isrctn.com/ISRCTN47058442.
Competing interests
All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest and declare: funding from the Department of Health and Social Care, Economic and Social Research Council, and National Institute for Health and Care Research; no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. IB, TF and MGS were members of the UK Covid-19 Testing Initiatives Evaluation Board but did not take part in sessions where this study was adjudicated. The City of Liverpool received a donation from Innova Medical Group towards the foundation of the Pandemic Institute but neither Innova Medical Group or any other commercial entity gave any support to this study or had any participation in it. Lateral Flow Test supply and company interactions was handled independently by the UK Health Security Agency. LT has received consulting fees from MHRA; and from AstraZeneca and Synairgen, paid to the University of Liverpool; speakers’ fees from Eisai Ltd, and support for conference attendance from AstraZeneca.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xingna Zhang and Christopher P. Cheyne joint first authors.
References
- 1.Endacott R, Pattison N, Dall’Ora C, et al. The organisation of nurse staffing in intensive care units: a qualitative study. J Nurs Manag. 2022;30:1283–94. 10.1111/jonm.13611. [DOI] [PubMed] [Google Scholar]
- 2.Richards M, Anderson M, Carter P, et al. The impact of the COVID-19 pandemic on cancer care. Nat Cancer. 2020;1:565–7. 10.1038/s43018-020-0074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Razzak JA, Bhatti JA, Tahir MR, et al. Initial estimates of COVID-19 infections in hospital workers in the United States during the first wave of pandemic. PLoS ONE. 2020;15: e0242589. 10.1371/journal.pone.0242589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mutambudzi M, Niedzwiedz C, Macdonald EB, et al. Occupation and risk of severe COVID-19: prospective cohort study of 120 075 UK Biobank participants. Occup Environ Med. 2021;78:307–14. 10.1136/oemed-2020-106731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhan M, Qin Y, Xue X, et al. Death from Covid-19 of 23 Health Care Workers in China. N Engl J Med. 2020;382:2267–8. 10.1056/NEJMc2005696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makortoff K. Staff shortages spreading to all corners of UK business, survey finds. The Guardian. 2021. https://www.theguardian.com/business/2021/oct/04/staff-shortages-spreading-to-all-corners-of-uk-business-survey-finds.
- 7.Denny-Brown N, Stone D, Hays B, et al. COVID-19 intensifies nursing home workforce challenges. https://aspe.hhs.gov/reports/covid-19-intensifies-nursing-home-workforce-challenges.
- 8.Tyler D, Hunter M, Mulmule N, et al. COVID-19 intensifies home care workforce challenges. https://aspe.hhs.gov/reports/covid-19-intensifies-home-care-workforce-challenges.
- 9.Impact of the COVID-19 pandemic on the hospital and outpatient clinician workforce: challenges and policy responses. ASPE. https://aspe.hhs.gov/reports/covid-19-health-care-workforce. Accessed 11 Apr 2023.
- 10.McCay L. Omicron: Urgent action needed on NHS staffing crisis. BMJ. 2022;376: o18. 10.1136/bmj.o18. [DOI] [PubMed] [Google Scholar]
- 11.Iacobucci G. Covid-19: Advise public to reduce social contacts to keep NHS running, say health leaders. BMJ. 2021;375: n3116. 10.1136/bmj.n3116. [DOI] [PubMed] [Google Scholar]
- 12.Iacobucci G. Covid-19: NHS staff absences rise again as cases increase. BMJ. 2022;376: o737. 10.1136/bmj.o737. [DOI] [PubMed] [Google Scholar]
- 13.Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. The Lancet. 2021;398:2126–8. 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Managing healthcare staff with symptoms of a respiratory infection or a positive COVID-19 test result. GOV.UK. https://www.gov.uk/government/publications/covid-19-managing-healthcare-staff-with-symptoms-of-a-respiratory-infection/managing-healthcare-staff-with-symptoms-of-a-respiratory-infection-or-a-positive-covid-19-test-result. Accessed 14 Apr 2023.
- 15.West A. COVID-19 testing, tracing and isolating strategies in the UK (England). 2023.
- 16.COVID-19: Doubling time of Omicron could make PCR testing ‘meaningless’ - is it time for a rethink? Sky News. https://news.sky.com/story/covid-19-doubling-time-of-omicron-could-make-pcr-testing-meaningless-is-it-time-for-a-rethink-12498526. Accessed 15 Apr 2023.
- 17.Covid-19: High demand blamed for shortage of PCR appointments in England. BBC News. 2021. https://www.bbc.co.uk/news/uk-59651166.
- 18.Peto T, Affron D, Afrough B, et al. COVID-19: Rapid antigen detection for SARS-CoV-2 by lateral flow assay: a national systematic evaluation of sensitivity and specificity for mass-testing. EClinicalMedicine. 2021;36: 100924. 10.1016/j.eclinm.2021.100924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirby JE, Riedel S, Dutta S, et al. Sars-Cov-2 antigen tests predict infectivity based on viral culture: comparison of antigen, PCR viral load, and viral culture testing on a large sample cohort. Clin Microbiol Infect. 2023;29:94–100. 10.1016/j.cmi.2022.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen I, Crozier A, Buchan I, et al. Recalibrating SARS-CoV-2 Antigen Rapid Lateral Flow Test Relative Sensitivity from Validation Studies to Absolute Sensitivity for Indicating Individuals Shedding Transmissible Virus. CLEP. 2021;13:935–40. 10.2147/CLEP.S311977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adamson B, Sikka R, Wyllie AL, et al. Discordant SARS-CoV-2 PCR and rapid antigen test results when infectious: a December 2021 occupational case series. 2022;2022.01.04.22268770.
- 22.COVID-19: striving for quality in the national testing service. GOV.UK. https://www.gov.uk/government/publications/covid-19-striving-for-quality-in-the-national-testing-service. Accessed 18 Sept 2023.
- 23.Eyre DW, Futschik M, Tunkel S, et al. Performance of antigen lateral flow devices in the UK during the alpha, delta, and omicron waves of the SARS-CoV-2 pandemic: a diagnostic and observational study. Lancet Infect Dis. 2023;23:922–32. 10.1016/S1473-3099(23)00129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daily rapid testing for COVID-19 contacts launches this week. GOV.UK. https://www.gov.uk/government/news/daily-rapid-testing-for-covid-19-contacts-launches-this-week. Accessed 14 Apr 2023.
- 25.[Withdrawn] COVID-19: management of staff and exposed patients or residents in health and social care settings. GOV.UK. 2022. https://www.gov.uk/government/publications/covid-19-management-of-exposed-healthcare-workers-and-patients-in-hospital-settings/covid-19-management-of-exposed-healthcare-workers-and-patients-in-hospital-settings. Accessed 17 Apr 2023.
- 26.Quilty BJ, Pulliam JRC, Pearson CAB. Test to release from isolation after testing positive for SARS-CoV-2. 2022;2022.01.04.21268372.
- 27.Bays D, Whiteley T, Pindar M, et al. Mitigating isolation: The use of rapid antigen testing to reduce the impact of self-isolation periods. 2021;2021.12.23.21268326.
- 28.COVID-19: testing initiative evaluation programme. GOV.UK. 2023. https://www.gov.uk/government/collections/covid-19-testing-initiative-evaluation-programme. Accessed 18 Sept 2023.
- 29.Crozier A, Rajan S, Buchan I, et al. Put to the test: use of rapid testing technologies for covid-19. BMJ. 2021;372: n208. 10.1136/bmj.n208. [DOI] [PubMed] [Google Scholar]
- 30.Young BC, Eyre DW, Kendrick S, et al. Daily testing for contacts of individuals with SARS-CoV-2 infection and attendance and SARS-CoV-2 transmission in English secondary schools and colleges: an open-label, cluster-randomised trial. The Lancet. 2021;398:1217–29. 10.1016/S0140-6736(21)01908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quilty BJ, Clifford S, Hellewell J, et al. Quarantine and testing strategies in contact tracing for SARS-CoV-2: a modelling study. The Lancet Public Health. 2021;6:e175–83. 10.1016/S2468-2667(20)30308-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Love NK, Ready DR, Turner C, et al. Daily use of lateral flow devices by contacts of confirmed COVID-19 cases to enable exemption from isolation compared with standard self-isolation to reduce onward transmission of SARS-CoV-2 in England: a randomised, controlled, non-inferiority trial. Lancet Respir Med. 2022;10:1074–85. 10.1016/S2213-2600(22)00267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.COVID-19: overview of daily contact testing (DCT) trial reports. GOV.UK. https://www.gov.uk/government/publications/covid-19-overview-of-daily-contact-testing-dct-trial-reports. Accessed 18 Sept 2023.
- 34.Government to introduce COVID-19 vaccination as a condition of deployment for all frontline health and social care workers. GOV.UK. https://www.gov.uk/government/news/government-to-introduce-covid-19-vaccination-as-a-condition-of-deployment-for-all-frontline-health-and-social-care-workers. Accessed 8 Sept 2024.
- 35.Beggs AD, Caiado CCS, Branigan M, et al. Machine learning for determining lateral flow device results for testing of SARS-CoV-2 infection in asymptomatic populations. Cell Reports Medicine. 2022;3: 100784. 10.1016/j.xcrm.2022.100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ARTIC. The ARTIC field bioinformatics pipeline. 2023. https://github.com/artic-network/fieldbioinformatics.
- 37.O’Toole Á, Pybus OG, Abram ME, et al. Pango lineage designation and assignment using SARS-CoV-2 spike gene nucleotide sequences. BMC Genomics. 2022;23:121. 10.1186/s12864-022-08358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong X, Penrice-Randal R, Goldswain H, et al. Analysis of SARS-CoV-2 known and novel subgenomic mRNAs in cell culture, animal model, and clinical samples using LeTRS, a bioinformatic tool to identify unique sequence identifiers. Gigascience. 2022;11:giac045. 10.1093/gigascience/giac045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Z, Sun X, Hardin JW. A note on the tests for clustered matched-pair binary data. Biom J. 2010;52:638–52. 10.1002/bimj.201000035. [DOI] [PubMed] [Google Scholar]
- 40.Performance of lateral flow devices during the COVID-19 pandemic. GOV.UK. https://www.gov.uk/government/publications/lateral-flow-device-performance-data/performance-of-lateral-flow-devices-during-the-covid-19-pandemic. Accessed 3 May 2023.
- 41.Chapter 6: testing. GOV.UK. https://www.gov.uk/government/publications/technical-report-on-the-covid-19-pandemic-in-the-uk/chapter-6-testing. Accessed 4 May 2023.
- 42.Futschik ME, Tunkel SA, Turek E, Chapman D, Thorlu-Bangura Z, Kulasegaran-Shylini R, Blandford E, Dodgson A, Klapper PE, Sudhanva M, Crook D, Bell J, Hopkins S, Peto T, Fowler T. Double testing with lateral flow antigen test devices for COVID-19: does a second test in quick succession add value? J Virol Methods. 2024;329:115000. 10.1016/j.jviromet.2024.115000. [DOI] [PubMed]
- 43.Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. The Lancet. 2022;399:437–46. 10.1016/S0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Y, Kang L, Guo Z, et al. Incubation Period of COVID-19 Caused by Unique SARS-CoV-2 Strains: A Systematic Review and Meta-analysis. JAMA Netw Open. 2022;5: e2228008. 10.1001/jamanetworkopen.2022.28008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cevik M, Tate M, Lloyd O, et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. The Lancet Microbe. 2021;2:e13-22. 10.1016/S2666-5247(20)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.UK Health Security Agency. Lateral flow device (LFD) performance data. GOV.UK. 2023. https://www.gov.uk/government/publications/lateral-flow-device-performance-data. Accessed 18 Sept 2023.
- 47.Changes to COVID-19 testing in England from 1 April. GOV.UK. https://www.gov.uk/government/news/changes-to-covid-19-testing-in-england-from-1-april. Accessed 1 May 2023.
- 48.Omicron: Rising numbers of NHS staff off work because of Covid. BBC News. 2021. https://www.bbc.co.uk/news/health-59769251.
- 49.US Centres for Disease Prevention and Control. Interim guidance for managing healthcare personnel with SARS-CoV-2 infection or exposure to SARS-CoV-2. 2021. https://stacks.cdc.gov/view/cdc/112945/cdc_112945_DS1.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study required person identifiable data and the main analyses were conducted on a de-identified extract. The fully anonymised data for reproducing the results are available from https://github.com/iain-buchan/cipha/blob/master/SMART_RR_Anonymised_Data.zip. The study protocol can be downloaded from https://github.com/iain-buchan/cipha/blob/master/SMART_Release_Return.pdf and statistical analysis plan from https://github.com/iain-buchan/cipha/blob/master/SMART_RR_SAP.pdf.