Abstract
Background
The gut microbiota may be involved in neuropathic pain. However, the causal association between gut microbiota and neuropathic pain remains unclear. Whether immune cells and inflammatory factors mediate the pathway from gut microbiota to neuropathic pain has not been elucidated.
Methods
We obtained the summary data of 412 gut microbiota, 731 immune cells, 91 inflammatory factors, and five types of neuropathic pain (drug-induced neuropathy, postherpetic neuralgia, sciatica, trigeminal neuralgia, and unspecified neuralgia) from large-scale genome-wide association study (GWAS) datasets and the FinnGen database. We used bidirectional Mendelian randomization (MR) analysis to explore the causal association between gut microbiota and neuropathic pain. Additionally, we conducted a mediation analysis to identify whether immune cells and inflammatory factors act as mediators within these causal relationships.
Results
Our study revealed 30 causal relationships between 26 gut bacterial taxa and five types of neuropathic pain, including four associated with drug-induced neuropathy, six with postherpetic neuralgia, five with sciatica, eight with trigeminal neuralgia, and seven with unspecified neuralgia. Moreover, we identified 35 gut bacterial pathway abundances causally involved in neuropathic pain. The reverse MR analysis showed no evidence of reverse causality from gut microbiota to neuropathic pain. Mediation analysis demonstrated that the immune cell phenotype “HLA-DR++ monocyte % leukocyte” mediated the causal relationship between p_Proteobacteria and sciatica with a mediation proportion of 36.15% (P = 0.038), whereas “CD11c on CD62L+ myeloid dendritic cell” mediated the causal pathway from assimilatory sulfate reduction to trigeminal neuralgia with a mediation proportion of 27.90% (P = 0.041).
Conclusion
This study identified the causal relationships between several specific gut microbiota and various neuropathic pain subtypes. Additionally, two immune cells may act as potential mediators in the pathways from gut microbiota to neuropathic pain.
Supplementary Information
The online version contains supplementary material available at 10.1186/s10194-024-01906-z.
Keywords: Gut microbiota, Immune cells, Inflammatory factor, Neuropathic pain, Mendelian randomization
Background
Neuropathic pain is a common chronic condition characterized by sustained, irreversible pain caused by a lesion or disease of the somatosensory system involving the peripheral and central neurons [1, 2]. It is caused by various etiologies, including traumatic injuries, infections, diabetes, and exposure to toxins, such as chemotherapy agents [3]. Most patients with neuropathic pain experience either continuous or intermittent spontaneous pain, which may be accompanied by hyperalgesia, allodynia, aftersensations, and referred pain [2]. Neuropathic pain may induce sleep disturbances, fatigue, and emotional disorders, resulting in an imbalance in work, leisure activities, and family relationships [1]. However, the mechanisms underlying neuropathic pain have not been elucidated, resulting in a lack of effective drugs and methods for its treatment.
In humans, the gastrointestinal tract is a vast, populous, and complex microbial ecosystem estimated to contain > 1014 microorganisms, including archaea and eukaryotes, but predominantly bacteria [4, 5]. Substantial evidence strongly demonstrated that neuropathic pain may lead to disturbance of gut microbiota [6], which plays a crucial role in neuropathic pain processes [1]. However, current studies, primarily observational and preclinical, showed inconsistent results. One study suggested that pain was exacerbated after transplanting fecal microbiota from anhedonia-susceptible spared nerve injury (SNI) rats into antibiotic-treated pseudo-germ-free mice, whereas microbiota from resilient SNI rats significantly improved pain [7]. Ma et al. reported that depleting the gut microbiota in mice with various antibiotics prevented or completely suppressed mechanical allodynia and thermal hyperalgesia induced by chronic constriction injury (CCI), oxaliplatin, or streptozotocin [8]. Conversely, an observational study indicated that fluoroquinolone or amoxicillin-clavulanate intake increased the incidence of peripheral neuropathy [9]. Nevertheless, the causal relationship between gut microbiota and neuropathic pain remains unclear.
Interestingly, gut microbiota may regulate immune cells and cytokines, whereas immune cells and inflammation play vital roles in neuropathic pain processes [10–12]. Gut microbiota-derived metabolites, such as short-chain fatty acids (SCFAs), inhibit macrophages activation through negatively regulating the NLRP3 inflammatory signaling pathway [13]. In addition, macrophages contribute to the initiation and maintenance of mechanical hypersensitivity in neuropathic pain [14]. In a recent study, gut microbiota may reduce CCI-induced neuropathic pain by regulating T cells to shift from a pro-inflammatory to an anti-inflammatory profile [15]. Thus, we hypothesized that immune cells and inflammatory factors may be mediators in the relationship between gut microbiota and neuropathic pain.
Mendelian randomization (MR) is a widely accepted method to infer causal relationships between exposures and outcomes by utilizing single nucleotide polymorphisms (SNPs) as instrumental variables (IVs) in genetic studies [16]. Due to the random assignment of SNPs at conception, MR can simulate a randomized controlled experiment, avoiding reverse causality bias and reducing confounding factors in conventional epidemiological and observational studies [17, 18]. We initially conducted a bidirectional two-sample MR analysis to explore the causal effects of gut microbiota on neuropathic pain. Subsequently, we adopted a mediation analysis to determine whether immune cells and inflammatory factors may mediate the causal relationships between gut microbiota and neuropathic pain.
Methods
Study design
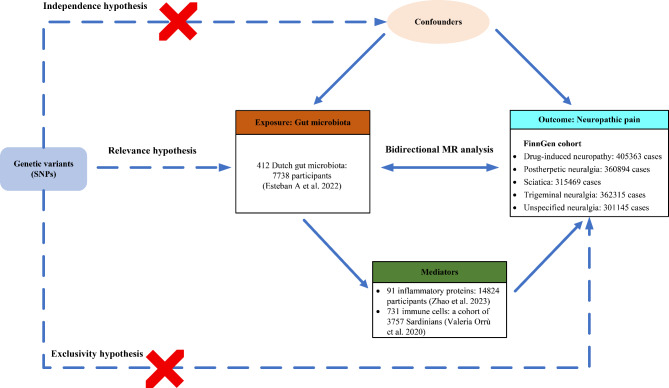
The study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology Mendelian Randomization (STROBE-MR) guidelines [19, 20]. MR is based on three core principles: (1) relevance hypothesis: IVs should be strongly associated with exposure (gut microbiota, immune cells, and inflammatory factors); (2) independence hypothesis: IVs are irrelevant to confounding factors; (3) exclusivity hypothesis: IVs affect outcomes (NP) exclusively through their effect on the exposure [21]. Figure 1 shows the study diagram.
Fig. 1.
Study design overview
Data source
We obtained new data on 412 gut microbiota from the study by Esteban et al., involving 7738 participants and analyzing 207 taxa (including 5 phyla, 10 classes, 13 orders, 26 families, 48 genera, and 105 species) and 205 pathways reflecting microbial composition and activity [22]. We obtained the genome-wide association study (GWAS) summary data on 731 immune cell traits from Orrù et al. [23]. This dataset includes 3757 individuals of European ancestry with no overlapping cohorts. The immune features measured were absolute cell counts (AC, n = 118), median fluorescence intensity for surface antigens (MFI and SAL, n = 389), morphological parameters (MPs, n = 32), and relative cell counts (RCs, n = 192). The MFI, AC, and RC features cover mature stages of various immune cells: B cells, cDCs, T cells, monocytes, myeloid cells, TBNK cells (T cells, B cells, and natural killer cells), and Treg cells. The MP features specifically include cDC and TBNK panels. In addition, the plasma protein quantitative trait loci (pQTL) data of 91 inflammatory factors were obtained from Zhao et al.’s study [24]. Considering the higher prevalence of peripheral neuropathic pain, we obtained the available GWAS summary statistics for the five types of neuropathic pain (drug-induced neuropathy, postherpetic neuralgia, sciatica, trigeminal neuralgia, and unspecified neuralgia) from the FinnGen R10 database (https://r10.finngen.fi/) [25]. Compared with a recent study [26], we used the same version of FinnGen R10 dataset for trigeminal neuralgia, whereas we included more recent data for postherpetic neuralgia and expanded our analysis to cover three additional types: drug-induced neuropathy, sciatica, and unspecified neuralgia. Detailed data source information is provided in Additional file 1: Table S1. No additional ethical review was required for our study because ethical approval for the original GWAS studies was granted.
Instrumental variable selection
First, we set the significance threshold of P < 5 × 10− 8 to select SNPs as IVs related to 731 immune cells and 91 inflammatory factors. Owing to the limited number of SNPs related to gut microbiota identified under the P < 5 × 10− 8 threshold, a more lenient threshold of P < 1 × 10− 5 was used to select SNPs related to the gut microbiota. Second, a linkage disequilibrium (LD) analysis of SNPs was conducted to ensure independence among SNPs with thresholds of r2 < 0.001 and 10,000 kb distance. Third, we removed palindromic SNPs after harmonization with the outcome’s GWAS summary statistics to ensure consistency between effect alleles and sizes. Fourth, the genetic variance explained (R2) and F-statistic for each SNP were calculated to estimate the strength of the selected SNPs, and SNPs with F-statistic < 10 were removed to reduce weak instrumental bias [27]. We calculated R² as 2 × (1 − MAF) × MAF × beta2 (where MAF is the minor allele frequency and beta is the effect size on the exposure) and F-statistic as F = R² × (N − 2)/(1 − R²), where N is the effective sample size [28].
MR analysis
MR analysis was conducted to evaluate the causal effects of gut microbiota, immune cells, and inflammatory factors on neuropathic pain. To ensure robustness, the inverse variance weighted (IVW) method was used as the primary analysis approach, with MR-Egger, weighted median, weighted mode, debiased IVW, and robust adjusted profile score (RAPS) as supplementary methods. Additionally, the Wald ratio method was applied for exposures that include only one SNP. IVW performs a meta-analysis of Wald ratios from each SNP, ensuring the robustness of the estimates in the absence of pleiotropy [29, 30]. The weighted median method can provide consistent and unbiased causal estimates in the presence of horizontal pleiotropy, even when 50% of the instrumental variables were invalid [31]. The weighted mode method weighs SNP effect estimates by their inverse variance and selects the most common weighted effect as the final causal estimate, ensuring robustness against pleiotropy and invalid SNPs [32]. The MR-Egger method is based on a regression model that allows for pleiotropy and can generate unbiased estimates even with invalid IVs [33]. The debiased IVW method ensures the robustness of the results with the introduction of weak instrument bias [34]. RAPS effectively addresses both systematic and idiosyncratic pleiotropies, ensuring robust inferences for MR analysis with many weak instruments [35]. When the estimates of the IVW method met a threshold of P < 0.05, and the effect directions were consistent across all these methods, the results were considered statistically significant and robust, including them for further analysis.
In the sensitivity analysis, we adopted various approaches to evaluate the robustness of the results. Cochrane’s Q test was performed to detect heterogeneity among the selected SNPs, and P < 0.05 indicated heterogeneity [29]. Moreover, the MR-Egger intercept and MR-PRESSO global test were conducted to assess the potential horizontal pleiotropy [33, 36]. Additionally, MR-PRESSO could identify outliers that may influence horizontal pleiotropy [36]. After pleiotropic SNP removal, a subsequent reanalysis can be conducted to ensure more accurate and robust results. Additionally, a leave-one-out analysis was performed to assess the stability of the causal associations and identify the influence of any single SNP on the results [37].
Mediation analysis
We screened potential mediators in the causal pathway from gut microbiota to neuropathic pain through the following steps: First, we identified gut microbiota, immune cells, and inflammatory factors with significant causal associations with neuropathic pain and showed no heterogeneity or pleiotropy. Second, we explored the causal effects of gut microbiota on immune cells and inflammatory factors based on the same criteria. Third, we retained logically consistent potential mediators based on the directions of the effect values: gut microbiota–neuropathic pain (β), gut microbiota–immune cells and inflammatory factors (β1), and immune cells and inflammatory factors–neuropathic pain (β2). If β was positive, both β1 and β2 are either positive or negative. Conversely, if β was negative, either β1 or β2 should be positive and the other negative. Subsequently, we calculated the mediation effect using the product of coefficients method (β1 × β2) and estimated the proportion of mediation by dividing the mediation effect by the total effect [(β1 × β2) / β]. The delta method was used to estimate the 95% confidence interval (CI) for the mediation effect [38]. A P-value of < 0.05 was indicative of significant mediation effects.
Reverse causality analysis
To clarify reverse causal associations from gut microbiota, immune cells, and inflammatory factors to neuropathic pain, we performed a reverse MR analysis with neuropathic pain as the exposure, and gut microbiota, immune cells, or inflammatory factors as the outcome. IVs were identified as SNPs significantly associated with neuropathic pain (P < 5 × 10–6).
We performed all analyses using R software (V.4.3.0) using the “TwoSampleMR” and “MRPRESSO” packages. We adopted the Bonferroni correction P-value as the threshold for statistical significance, which was 6.84 × 10–5 (0.05/731) and 5.49 × 10–4 (0.05/91) for immune cells and inflammatory factors, respectively. Any P-value of < 0.05 but greater than the Bonferroni correction P-value threshold was considered a suggestive causal association.
Results
IV selection
After LD clumping and harmonization, we identified 3925 SNPs associated with gut microbiota (P < 1 × 10–5) (Additional file 1: Table S2). Subsequently, 2018 and 283 SNPs associated with immune cells and inflammatory factors were selected, respectively (P < 5 × 10–8) (Additional file 1: Table S3 and S4), with F-statistics exceeding 10, indicating a strong representation of neuropathic pain in the MR analysis.
Causal effects of gut microbiota, immune cells, and inflammatory factors on neuropathic pain
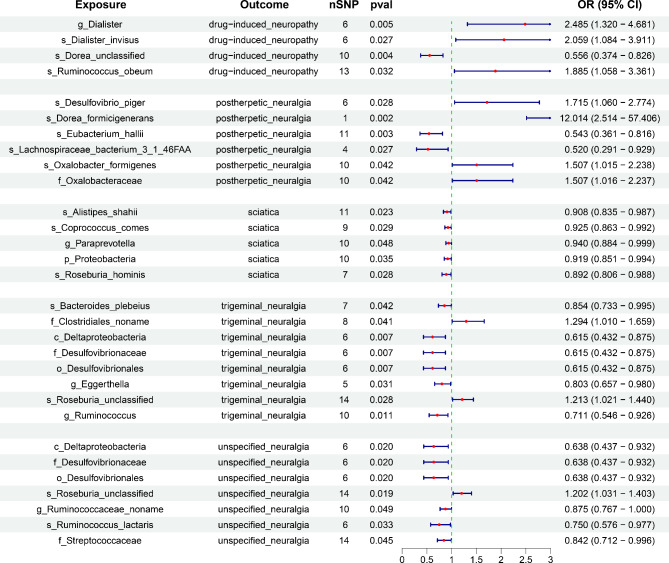
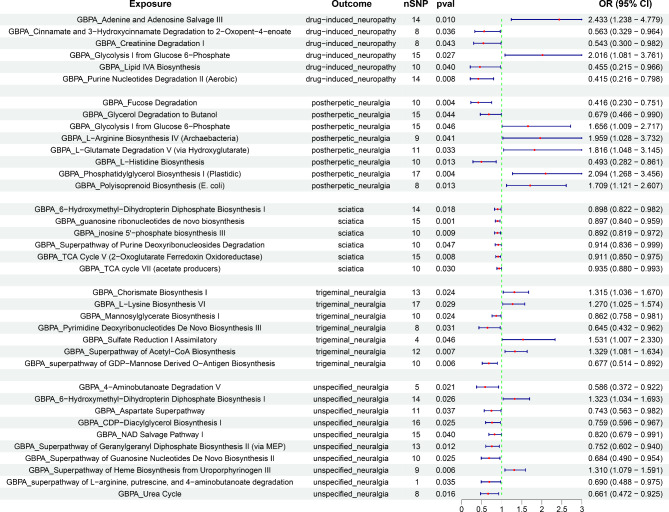
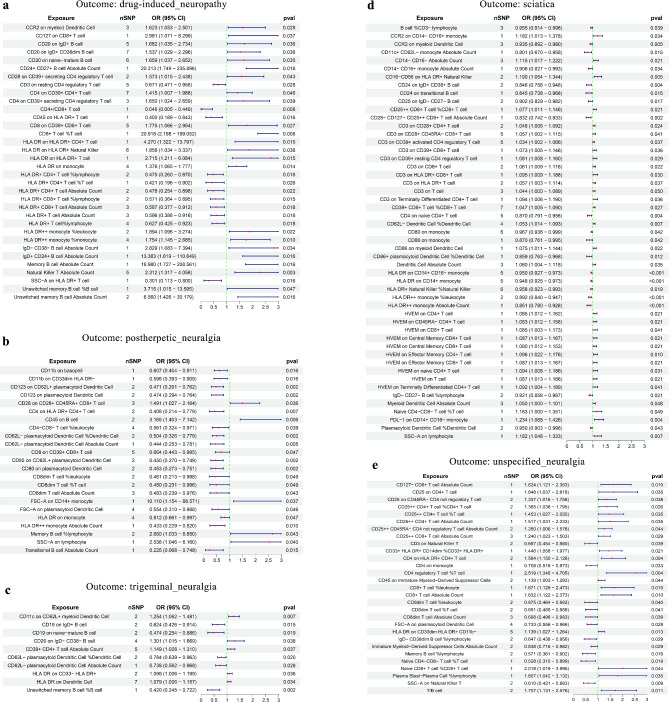
We performed a two-sample MR analysis between gut microbiota and the five neuropathic pain subtypes. Based on IVW method estimates with P < 0.05 and consistent effect directions across all six methods, we finally identified 67 causal associations from gut microbiota to neuropathic pain [26 gut bacterial taxa (1 phylum, 1 class, 1 order, 4 families, 5 genera, and 14 species from p_Actinobacteria, p_Bacteroidetes, p_Firmicutes, and p_Proteobacteria) and 35 gut bacterial pathway abundances (GAPAs)] (Additional file 1: Table S5, Figs. 2 and 3). Subsequently, we identified 147 paired causal associations from immune cells to various forms of neuropathic pain, including 34, 23, 51, 10, and 29 for drug-induced neuropathy, postherpetic neuralgia, sciatica, trigeminal neuralgia, and unspecified neuralgia, respectively (Additional file 1: Table S6, Fig. 4). Additionally, we identified 17 paired causal associations from inflammatory factors to neuropathic pain (2, 1, 3, 4, and 7 for drug-induced neuropathy, postherpetic neuralgia, sciatica, trigeminal neuralgia, and unspecified neuralgia, respectively) (Additional file 1: Table S7, Fig. 5).
Fig. 2.
Mendelian randomization (MR) results of causal associations between gut microbiota and neuropathic pain. OR: odds ratio; CI: confidence interval. The prefix “p_/c_/o_/f_/g_/s_” represents phylum/class/order/family/genus/species, respectively
Fig. 3.
MR results of causal associations between gut bacterial pathway abundances (GBPAs) and neuropathic pain. OR: odds ratio; CI: confidence interval
Fig. 4.
MR results of causal associations between immune cells and neuropathic pain. The results are presented for (a) drug-induced neuropathy, (b) postherpetic neuralgia, (c) trigeminal neuralgia, (d) sciatica, and (e) unspecified neuralgia. OR: odds ratio; CI: confidence interval
Fig. 5.
MR results of causal associations between inflammatory proteins and neuropathic pain
Drug-induced neuropathy
Four gut microbiota (g_Dialister, s_Dialister invisus, s_Dorea unclassified, and s_Ruminococcus obeum) and six gut bacterial pathway abundances (GBPAs) [adenine and adenosine salvage III, cinnamate and 3-hydroxycinnamate degradation to 2-oxopent-4-enoate, creatinine degradation I, glycolysis I from glucose-6-phosphate, lipid IVA biosynthesis, and purine nucleotide degradation II (aerobic)] were causally associated with drug-induced neuropathy. The s_Dorea unclassified (OR = 0.556, 95% CI = 0.374–0.826, P = 0.004) and purine nucleotide degradation II (aerobic) (OR = 0.415, 95% CI = 0.216–0.798, P = 0.008) were most significantly associated with a reduced risk of drug-induced neuropathy. Conversely, g_Dialister (OR = 2.485, 95% CI = 1.320–4.681, P = 0.005) and adenine and adenosine salvage III (OR = 2.433, 95% CI = 1.238–4.779, P = 0.010) were associated with an increased risk of drug-induced neuropathy.
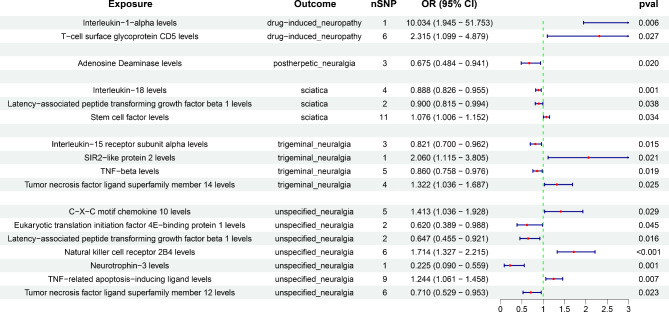
Figure 4a showed that 23 types of immune cells were positively associated with the risk of drug-induced neuropathy, with natural killer T absolute count (OR = 2.312, 95% CI = 1.317–4.058, P = 0.003) having the strongest impact on drug-induced neuropathy. However, 11 types of immune cells were negatively associated with the risk of drug-induced neuropathy, with CD4+/CD8 + T cell (OR = 0.046, 95% CI = 0.005–0.449, P = 0.008) showing the most significant protective effect on drug-induced neuropathy. Moreover, interleukin-1-alpha (OR = 10.034, 95% CI = 1.945–51.753, P = 0.006) and T-cell surface glycoprotein CD5 (OR = 2.315, 95% CI = 1.099–4.879, P = 0.027) were significantly correlated with an increased risk of drug-induced neuropathy (Fig. 5).
Postherpetic neuralgia
Six gut microbiotas and eight GBPAs were causally associated with postherpetic neuralgia (Figs. 2 and 3). The most notable protective factors were s_Eubacterium hallii (OR = 0.543, 95% CI = 0.361–0.816, P = 0.003) and fucose degradation (OR = 0.416, 95% CI = 0.230–0.751, P = 0.004). However, the most significant risk factors were s_Dorea formicigenerans (OR = 12.014, 95% CI = 2.514–57.406, P = 0.002) and phosphatidylglycerol biosynthesis I (plastidic) (OR = 2.094, 95% CI = 1.268–3.456, P = 0.004).
Five types of immune cells were positively associated with the incidence of postherpetic neuralgia, with CD45 on B cell (OR = 3.165, 95% CI = 1.403–7.142, P = 0.006) having the strongest effect on postherpetic neuralgia (Fig. 4b). Conversely, 18 types of immune cells were negatively associated with the incidence of postherpetic neuralgia, with CD62L-plasmacytoid dendritic cell % dendritic cell (OR = 0.504, 95% CI = 0.326–0.779, P = 0.002) having the strongest effect on postherpetic neuralgia. Additionally, adenosine deaminase (OR = 0.675, 95% CI = 0.484–0.941, P = 0.020) acted as a protective factor against postherpetic neuralgia (Fig. 5).
Sciatica
Notably, five gut microbiotas (s_Alistipes shahii, s_Coprococcus comes, g_Paraprevotella, p_Proteobacteria, and s_Roseburia hominis) and six GBPAs [6-hydroxymethyl-dihydropterin diphosphate biosynthesis I, guanosine ribonucleotide de novo biosynthesis, inosine 5’-phosphate biosynthesis III, superpathway of purine deoxyribonucleoside degradation, TCA cycle V(2 − oxoglutarate ferredoxin oxidoreductase), and TCA cycle VII (acetate producers)] were causally associated with decreased sciatica risk, with s_Alistipes shahii (OR = 0.908, 95% CI = 0.835–0.987, P = 0.023) and guanosine ribonucleotide de novo biosynthesis (OR = 0.897, 95% CI = 0.840–0.959, P = 0.001) being the most significant.
Furthermore, 32 and 19 types of immune cells were positively and negatively associated with sciatica risk, respectively (Fig. 4d). Among these, PDL-1 on CD14 + CD16 − monocyte (OR = 1.234, 95% CI = 1.068–1.426, P = 0.004) and HLA DR on CD14 + monocyte (OR = 0.948, 95% CI = 0.925–0.973, P < 0.001) were most significantly associated with increased and decreased sciatica risks, respectively. Moreover, we identified that interleukin-18 (OR = 0.888, 95% CI = 0.826–0.955, P = 0.001) and latency-associated peptide transforming growth factor beta 1 (OR = 0.900, 95% CI = 0.815–0.994, P = 0.038) were significantly associated with reduced sciatica risk (Fig. 5), but stem cell factor (KITLG) (OR = 1.706, 95% CI = 1.006–1.152, P = 0.034) significantly increased sciatica risk.
Trigeminal neuralgia
Eight gut microbiotas and seven GBPAs were associated with trigeminal neuralgia (Figs. 2 and 3). The most notable were c_Deltaproteobacteria, o_Desulfovibrionales, f_Desulfovibrionaceae (OR = 0.615, 95% CI = 0.432–0.875, P = 0.007), and superpathway of GDP-mannose-derived o-antigen biosynthesis (OR = 0.677, 95% CI = 0.514–0.892, P = 0.006). However, s_Roseburia unclassified (OR = 1.213, 95% CI = 1.021–1.440, P = 0.028) and the superpathway of acetyl-CoA biosynthesis (OR = 1.329, 95% CI = 1.081–1.634, P = 0.007) were strongly associated with increased trigeminal neuralgia risk.
Five types of immune cells were positively associated with trigeminal neuralgia risk (Fig. 4c). Notably, CD11c on CD62L + myeloid dendritic cell (OR = 1.254, 95% CI = 1.062–1.481, P = 0.007) showed the strongest effect. Conversely, another five types of immune cells were negatively associated with trigeminal neuralgia risk, with unswitched memory B cell % B cell (OR = 0.420, 95% CI = 0.245–0.722, P = 0.002) being the most significant. Moreover, interleukin-15 receptor subunit alpha (OR = 0.821, 95% CI = 0.700–0.962, P = 0.015) and TNF-beta (OR = 0.860, 95% CI = 0.758 − 0.976, P = 0.019) were significantly correlated with decreased trigeminal neuralgia risk (Fig. 5). However, SIR2-like protein 2 (OR = 2.060, 95% CI = 1.115 − 3.805, P = 0.021) and tumor necrosis factor ligand superfamily member 14 (OR = 1.322, 95% CI = 1.036 − 1.687, P = 0.025) significantly increased trigeminal neuralgia risk (Fig. 5).
Unspecified neuralgia
Seven gut microbiotas were significantly associated with unspecified neuralgia (Figs. 2 and 3). As protective factors, the c_Deltaproteobacteria, o_Desulfovibrionales, and f_Desulfovibrionaceae (OR = 0.638, 95% CI = 0.437–0.932, P = 0.020) showed the strongest associations. However, only s_Roseburia unclassified (OR = 1.202, 95% CI = 1.031–1.403, P = 0.019) was identified as a risk factor. Additionally, 10 GBPAs had causal associations with unspecified neuralgia, with the superpathway of geranylgeranyl diphosphate biosynthesis II (via MEP) (OR = 0.752, 95% CI = 0.602–0.940, P = 0.012) being the most significantly associated with reduced unspecified neuralgia risk. Conversely, the superpathway of heme biosynthesis from uroporphyrinogen III (OR = 1.310, 95% CI = 1.079–1.591, P = 0.006) was the most significantly associated with increased unspecified neuralgia risk.
We identified 18 and 11 types of immune cells that significantly increased and reduced unspecified neuralgia risk, respectively (Fig. 4e). Among these, CD4 regulatory T cell % T cell (OR = 2.519, 95% CI = 1.348–4.705, P = 0.004) was identified as the most notable risk factor, whereas SSC-A on natural killer T (OR = 0.610, 95% CI = 0.421–0.883, P = 0.009) was the most significant protective factor. Furthermore, neurotrophin-3 (OR = 0.225, 95% CI = 0.090–0.559, P = 0.001) was strongly associated with decreased unspecified neuralgia risk. Conversely, the natural killer cell receptor 2B4 (OR = 1.714, 95% CI = 1.327–2.215, P < 0.001) was most significantly associated with increased unspecified neuralgia risk (Fig. 5).
Sensitivity analysis
In sensitivity analysis, Cochran’s Q test indicated no significant evidence of heterogeneity for the associations between gut microbiota and neuropathic pain and between inflammatory factors and neuropathic pain (P > 0.05, Additional file 1: Table S8 and S10). However, heterogeneity was detected for HLA-DR on HLA-DR + natural killer cells, HLA-DR on monocytes, and CD4 on naive CD4 + T cells (P > 0.05, Additional file 1: Table S9). To ensure robust and reliable results, we used the IVW random effects model between the three immune cells and neuropathic pain. MR-Egger intercept and MR-PRESSO global test showed no pleiotropy (P > 0.05, Additional file 1: Table S8–S10). Furthermore, the leave-one-out analysis showed that no individual SNP significantly affected the causal relationship between gut microbiota and neuropathic pain (Additional file 2: Fig. S1–S5). Additionally, beyond the associations between GBPA-sulfate reduction I (assimilatory) and trigeminal neuralgia; g_Paraprevotella and sciatica; g_Ruminococcaceae_noname and unspecified neuralgia; CD8dim T cell % leukocyte, CD8dim T cell % T cell, and memory B cell % lymphocyte and postherpetic neuralgia; and CD3 on T cell and sciatica, all other causal relationships were validated by at least one of the debiased IVW or RAPS methods, confirming the robustness of the results. For significant associations in the forward analysis, the reverse MR analysis showed no obvious causal effect of neuropathic pain on gut microbiotas, immune cells, and inflammatory factors (Additional file 1: Table S11–S13).
Mediation analysis
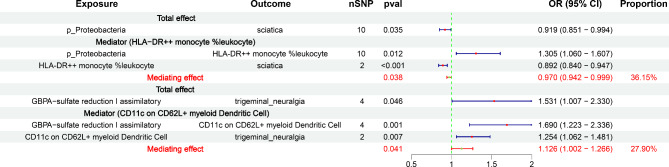
We explored the causal effects of neuropathic pain-related gut microbiota on immune cells and inflammatory factors associated with neuropathic pain (Additional file 1: Table S14). Based on the screening criteria, we preliminarily identified 21 potential gut microbiota–immune cells/inflammatory factor–neuropathic pain pathways (Additional file 1: Table S15). Finally, we identified that HLA-DR + + monocyte % leukocyte mediated the causal pathway between p_Proteobacteria and sciatica (OR = 0.970, 95% CI = 0.942–0.999, P = 0.038), accounting for 36.15% of the total effect. In addition, CD11c on CD62L + myeloid dendritic cells might act as a mediator in the causal association between GBPA-sulfate reduction I (assimilatory) and trigeminal neuralgia (OR = 1.126, 95% CI = 1.002–1.266, P = 0.041), with a mediation proportion of 27.90% (Fig. 6, Additional file 1: Table S16).
Fig. 6.
The results of mediation analysis. OR: odds ratio; CI: confidence interval. The prefix “p_” represents phylum
Discussion
We used a bidirectional two-sample MR analysis to explore the causal relationships between gut microbiota and neuropathic pain, which showed 30 causal associations with genetic predispositions between 26 gut bacterial taxa and five types of neuropathic pain. Additionally, we discovered 37 causal relationships that involved 35 GBPAs associated with neuropathic pain. Some gut microbiota species and GBPAs were risk factors, whereas others were protective factors against various types of neuropathic pain. However, reverse MR analysis did not show significant causal associations from neuropathic pain to gut microbiota. Furthermore, our findings primarily focused on the mediation roles of immune cells in the relationship between gut microbiota and neuropathic pain. Our results indicated that immune cell phenotypes HLA-DR + + monocyte % leukocyte mediated the causal relationship between p_Proteobacteria and sciatica, whereas CD11c on CD62L + myeloid dendritic cells mediated the causal pathway from GBPA-sulfate reduction I (assimilatory) on trigeminal neuralgia.
We identified several bacterial taxa from the phyla Firmicutes and Proteobacteria as protective factors for neuropathic pain. Jiao et al. revealed decreased abundances of f_Lachnospiraceae, g_Dorea, and s_Eubacterium hallii, from the phylum Firmicutes, in patients with postherpetic neuralgia compared with healthy controls [39]. Similarly, the abundances of g_Roseburia and s_Coprococcus_comes were decreased in patients with chronic pain [40, 41]. Additionally, these two taxa had reduced abundances in inflammatory diseases [42–44]. Jalanka-Tuovinen et al. reported a significant decrease in one phylotype within the Ruminococcus lactaris and related species group in patients with abdominal pain [45]. These findings support the hypothesis that these bacterial taxa play a protective role in neuropathic pain. For the phylum Proteobacteria, comprising c_Deltaproteobacteria, o_Desulfovibrionales, and f_Desulfovibrionaceae, a recent study indicated that the abundances of these bacterial taxa were negatively associated with the severity of kidney injury in antineutrophil cytoplasmic antibody-associated vasculitis [46], indicating their protective roles. However, o_Desulfovibrionales and f_Desulfovibrionaceae, which reduce sulfate to H2S, can damage the intestinal barrier, resulting in the production of endotoxins and pro-inflammatory cytokines [47]. This appears contradictory, highlighting the possibility that these bacterial taxa may exert different regulatory roles across different diseases. Further studies are necessary to elucidate the regulatory mechanisms of phylum Proteobacteria and its subordinate bacterial taxa in neuropathic pain.
In terms of risk factors, our study indicated that the high abundances of s_Dialister invisus from the genus Dialister, s_Ruminococcus obeum from the genus Blautia, s_Dorea formicigenerans from the genus Dorea, s_Desulfovibrio piger from the genus Desulfovibrio, and s_Roseburia unclassified from genus Roseburia were associated with an increased neuropathic pain risk. However, direct evidence regarding whether these specific strains influence neuropathic pain is limited. It was reported that the genus Dialister was significantly involved in chronic pain [48]. The relative increases in the ratios of Blautia to Lachnospira might be associated with distal neuropathic pain in HIV patients, suggesting the hypothesis that the genus Blautia could potentially contribute to the risk of neuropathic pain [49]. This hypothesis was further supported by Hadizadeh et al., who found that the abundance of the genus Blautia was increased in patients with abdominal pain [50]. Moreover, previous studies showed that a high abundance of s_Dorea formicigenerans was associated with psoriasis and severe acute pancreatitis [51, 52]. In a recent study, the abundance of the genus Desulfovibrio was reduced in the treatment group of rats with spinal nerve ligation receiving ginger root extract, which alleviated pain [53]. Additionally, Goudman et al. reported an increased abundance of the genus Roseburia in patients with fibromyalgia [54]. These studies provide substantial clues that support our findings that these strains might be associated with increased neuropathic pain risk.
Additionally, our analysis revealed that some gut microbiota metabolic pathways were causally associated with neuropathic pain. Gut microbial metabolites, produced by metabolic pathways, are considered primary mediators through which the gut microbiota impact host physiology. These microbial metabolites play a significant role in regulating various chronic pain conditions such as headache, inflammatory pain, and neuropathic pain [55]. Studies have shown that the mechanisms involved in neuropathic pain are mediated by the activation of certain receptors or ion channels such as Toll-like receptors (TLRs) and transient receptor potential (TRP) channels [1]. Polyunsaturated fatty acids (PUFAs), as endogenous agonists of TRPV4, could stimulate sensory neurons to increase peripheral hypersensitivity, contributing to the development of neuropathic pain [1, 56]. However, acetyl-coenzyme A (CoA) is an essential precursor in de novo biosynthesis of fatty acids [57]. Therefore, we hypothesize that the superpathway of acetyl-CoA biosynthesis may increase the risk of trigeminal neuralgia through a mechanism involving the activation of the TRPV4 channel driven by an increase in PUFAs. In addition, Diogenes et al. reported that lipopolysaccharide (LPS) might contribute to neuropathic pain by triggering TRPV1-mediated capsaicin responses via the TLR4 pathway [58]. Moreover, LPS could induce depolarization and firing of nociceptive neurons by activating the TRPA1 channel in a TLR4-independent manner, thereby leading to neurogenic inflammation and pain [1, 59]. These findings may provide plausible explanations for how s_Desulfovibrio piger, a Gram-negative bacterium that produces LPS, increases the risk of postherpetic neuralgia in our study. However, the exact regulatory mechanisms remain to be further validated.
Acknowledging the close pathological relationships among various neuropathic pain, we further explored the shared gut microbiota and metabolic pathways causally associated with these disorders. Interestingly, our study identified specific species within the genus Roseburia linked to sciatica, trigeminal neuralgia, and unspecified neuralgia, while species from the genus Dorea were associated with drug-induced neuropathy and postherpetic neuralgia. Both genera belong to the family Lachnospiraceae, which has been found to have decreased abundance in chronic pain [41]. This evidence suggests that Lachnospiraceae may play a critical role in shared pathogenesis of various types of neuropathic pain. Additionally, we found that 6-hydroxymethyl dihydropterin diphosphate biosynthesis I was involved in sciatica and unspecified neuralgia, while glycolysis I from glucose-6-phosphate was causally associated with drug-induced neuropathy and postherpetic neuralgia.
To better understand the mechanisms by which gut microbiota affect neuropathic pain, we further focus on the pivotal roles of immune cells as mediators. Our study showed that different subtypes of dendritic cells such as myeloid and plasmacytoid dendritic cells, along with various subtypes of T cell, including CD4 + T cells, CD8 + T cells and regulatory T cells (Tregs), were causally associated with neuropathic pain. Dendritic cells recognized invading microbes through TLRs, triggering proinflammatory cytokine production and promoting antigen presentation to T cells, thereby activating adaptive immune responses [60]. Numerous studies have indicated that CD4 + T helper 1 cells might contribute to the development and maintenance of pain, whereas CD8 + T cells and Tregs could alleviate pain [12, 61, 62]. Subsequently, the mediation analysis revealed that the immune cell phenotype HLA-DR + + monocyte % leukocyte mediated the causal effect of p_Proteobacteria on sciatica. The monocyte expression of HLA-DR is crucial for presenting antigens to CD4 + T cells, thereby initiating a specific immune response [63]. Monocytes/macrophages exerted analgesic effects by releasing anti-inflammatory mediators, such as IL-10 and specialized pro-resolving mediators, promoting the resolution of the initial injury [64, 65]. Furthermore, the absence of these cells impeded the resolution of inflammatory pain [66]. These findings supported that p_Proteobacteria might decrease sciatica risk by regulating the anti-inflammatory effects mediated by HLA-DR + + monocytes. Moreover, the immune cell phenotype CD11c on CD62L + myeloid dendritic cells mediated the effect of GBPA-sulfate reduction I (assimilatory) on trigeminal neuralgia. CD11c on CD62L + myeloid dendritic cell represents the expression of myeloid dendritic cells in peripheral blood. Dendritic cells contribute to cancer-associated neuropathic pain by sensitizing nociceptor sensory neurons through paracrine factors [67]. Assimilatory sulfate reduction in bacterial cells could produce cysteine, a precursor to glutathione crucial for maintaining redox homeostasis [68]. Glutathione deficiency inhibited dendritic cell maturation and reduced pro-inflammatory cytokine production [69, 70]. Thus, we deduced that assimilatory sulfate reduction increased trigeminal neuralgia risk by promoting dendritic cell maturation and subsequent pro-inflammatory cytokine production. Although we were unable to determine the mediating effect of certain immune cells and inflammatory factors, our findings offer potential insights into their mediation roles in the pathways from gut microbiota to neuropathic pain, warranting further research.
A recent study by Lan et al. revealed that several immune cells might mediate the effects of gut microbiota on neuropathic pain (trigeminal neuralgia, postherpetic neuralgia, and painful diabetic peripheral neuropathy) [26]. Compared with this recent study, there were some similarities and differences in our analysis. For data sources, we utilized the exact same version of the FinnGen R10 dataset as in Lan et al.’s study in the analysis of trigeminal neuralgia. However, we included a newer dataset for postherpetic neuralgia from FinnGen R10, compared to FinnGen R9 dataset in Lan et al.’s study. Moreover, we expanded our analysis to cover three additional types: drug-induced neuropathy, sciatica, and unspecified neuralgia. These datasets in our study included more samples, providing a more comprehensive and accurate analysis. Regarding methodology, our study has several differences: First, we similarly relaxed the P-value threshold for screening IVs of the gut microbiota from 5 × 10⁻⁸ to 1 × 10⁻⁵ in our study, potentially introducing weak IVs. Lan et al. conducted Bayesian weighted MR analysis as a supplementary analysis, whereas we implemented debiased IVW and RAPS as supplementary methods to reduce weak instrument bias. However, we maintained a strict threshold of P < 5 × 10⁻⁸ for selecting more effective IVs for immune cells and inflammatory factors. Second, we used the F-statistic calculated from R² to provide a more accurate and comprehensive assessment of the genetic variance explained by the IVs, accounting for their correlation and minimizing the impact of multicollinearity. Third, only results with consistent effect directions across all methods were included in our analysis, ensuring the robustness and reliability of our findings. These differences in dataset versions and methodologies might contribute to the discrepancies in the IVs utilized for MR analysis, potentially leading to the differences between our findings and those reported by Lan et al. Furthermore, in addition to determining the mediating role of immune cells in the relationship between gut microbiota and neuropathic pain, we evaluated the potential mediating role of inflammatory factors, thereby enriching our understanding of the underlying mechanisms involved in neuropathic pain.
However, our study has certain limitations. The data in this study primarily come from individuals of European ancestry, potentially limiting the generalizability of our findings. Furthermore, we assumed a linear relationship between exposure and outcome in MR analyses, which may not capture the potentially more complex and nonlinear relationships. Moreover, we ensured consistent effect directions across all methods and applied a strict p-value threshold to select IVs for immune cells and inflammatory factors, enhancing the robustness of our results. However, these rigorous approaches may have inadvertently obscured the observation of some interesting findings. Additionally, due to the lack of individual-level data (such as details on the use of antibiotics, immunosuppressants, or other interventions), we were unable to perform subgroup analysis, potentially introducing some bias into our results. Finally, our results may be at risk for false positives without correction for multiple tests, and this study was intended to generate new hypotheses rather than final definitive conclusions. Thus, our findings should be considered preliminary and warrant further validation in future research.
Conclusion
In this study, we comprehensively explored the relationships between gut microbiota, immune cells, inflammatory factors, and neuropathic pain. There were 67 causal associations with genetic predispositions between gut microbiota (26 gut bacterial taxa and 35 GBPAs) and neuropathic pain. Additionally, immune cells may act as potential mediators in the causal pathways from gut microbiota to neuropathic pain, whereas inflammatory factors seem not to play a mediating role. These findings may provide novel insights into the pathogenesis and treatment strategies for neuropathic pain.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We extend our gratitude to all participants and researchers involved in the included GWAS studies for their invaluable contributions to the GWAS data.
Abbreviations
- SNI
Spared nerve injury
- CCI
Chronic constriction injury
- SCFAs
Short-chain fatty acids
- MR
Mendelian randomization
- SNPs
Single nucleotide polymorphisms
- IVs
Instrumental variables
- STROBE-MR
Strengthening the Reporting of Observational Studies in Epidemiology Mendelian Randomization
- GWAS
Genome-wide association study
- pQTL
Plasma protein quantitative trait loci
- LD
Linkage disequilibrium
- IVW
Inverse variance weighted
- RAPS
Robust adjusted profile score
- GAPAs
Gut bacterial pathway abundances
- TLRs
Toll-like receptors
- TRP
Transient receptor potential
- PUFAs
Polyunsaturated fatty acids
- CoA
Coenzyme A
- LPS
Lipopolysaccharide
- Tregs
Regulatory T cells
Author contributions
H.P. and G.F.Z. jointly conceived and designed the study. H.P. and C.X.L. conducted data analysis and interpretation of results. H.P. and H.J.Z. created and optimized the visualizations. H.P. and G.F.Z. collaboratively wrote, revised, and edited the manuscript. All authors have read and approved the final version.
Funding
This work was in part supported by the following grands from the Natural Science Foundation of Shandong Province (ZR2022MH216 to GFZ) and the National Natural Science Foundation of China (No. 82371279 to GFZ).
Data availability
The GWAS summary statistics in this study are sourced from publicly open access databases including FinnGen (https://www.finngen.fi/en), IEU Open GWAS (https://gwas.mrcieu.ac.uk/), and GWAS Catalog (https://www.ebi.ac.uk/gwas/). Detailed information about the data used in the analyses is provided in the supplementary information files. Statistical code and additional information can be requested from the corresponding author.
Declarations
Ethics approval and consent to participate
Ethical approval was not applicable because this study used data from publicly available databases.
Consent for publication
Not application.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lin B, Wang Y, Zhang P, Yuan Y, Zhang Y, Chen G (2020) Gut microbiota regulates neuropathic pain: potential mechanisms and therapeutic strategy. J Headache Pain 21:103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finnerup NB, Kuner R, Jensen TS (2021) Neuropathic Pain: from mechanisms to treatment. Physiol Rev 101:259–301 [DOI] [PubMed] [Google Scholar]
- 3.Lehmann HC, Wunderlich G, Fink GR, Sommer C (2020) Diagnosis of peripheral neuropathy. Neurol Res Pract 2:20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rinninella E, Cintoni M, Raoul P, Lopetuso LR, Scaldaferri F, Pulcini G, Miggiano GAD, Gasbarrini A, Mele MC (2019) Food Components and Dietary habits: Keys for a healthy gut microbiota composition. Nutrients 11 [DOI] [PMC free article] [PubMed]
- 5.Corriero A, Gadaleta RM, Puntillo F, Inchingolo F, Moschetta A, Brienza N (2022) The central role of the gut in intensive care. Crit Care 26:379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen P, Wang C, Ren YN, Ye ZJ, Jiang C, Wu ZB (2021) Alterations in the gut microbiota and metabolite profiles in the context of neuropathic pain. Mol Brain 14:50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang C, Fang X, Zhan G, Huang N, Li S, Bi J, Jiang R, Yang L, Miao L, Zhu B et al (2019) Key role of gut microbiota in anhedonia-like phenotype in rodents with neuropathic pain. Transl Psychiatry 9:57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma P, Mo R, Liao H, Qiu C, Wu G, Yang C, Zhang Y, Zhao Y, Song XJ (2022) Gut microbiota depletion by antibiotics ameliorates somatic neuropathic pain induced by nerve injury, chemotherapy, and diabetes in mice. J Neuroinflammation 19:169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morales D, Pacurariu A, Slattery J, Pinheiro L, McGettigan P, Kurz X (2019) Association between Peripheral Neuropathy and exposure to oral fluoroquinolone or amoxicillin-clavulanate therapy. JAMA Neurol 76:827–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamada N, Seo SU, Chen GY, Núñez G (2013) Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol 13:321–335 [DOI] [PubMed] [Google Scholar]
- 11.Jiang BC, Liu T, Gao YJ (2020) Chemokines in chronic pain: cellular and molecular mechanisms and therapeutic potential. Pharmacol Ther 212:107581 [DOI] [PubMed] [Google Scholar]
- 12.Bethea JR, Fischer R (2021) Role of Peripheral Immune cells for Development and Recovery of Chronic Pain. Front Immunol 12:641588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao X, Sun S, Zhou Y, Wang H, Yu Y, Hu T, Yao Y, Zhou C (2021) Bacteroides fragilis restricts colitis-associated cancer via negative regulation of the NLRP3 axis. Cancer Lett 523:170–181 [DOI] [PubMed] [Google Scholar]
- 14.Yu X, Liu H, Hamel KA, Morvan MG, Yu S, Leff J, Guan Z, Braz JM, Basbaum AI (2020) Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat Commun 11:264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding W, You Z, Chen Q, Yang L, Doheny J, Zhou X, Li N, Wang S, Hu K, Chen L et al (2021) Gut Microbiota influences Neuropathic Pain through modulating Proinflammatory and anti-inflammatory T cells. Anesth Analg 132:1146–1155 [DOI] [PubMed] [Google Scholar]
- 16.Sekula P, Del Greco MF, Pattaro C, Köttgen A (2016) Mendelian randomization as an Approach to assess causality using Observational Data. J Am Soc Nephrol 27:3253–3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beeghly-Fadiel A, Khankari NK, Delahanty RJ, Shu XO, Lu Y, Schmidt MK, Bolla MK, Michailidou K, Wang Q, Dennis J et al (2020) A mendelian randomization analysis of circulating lipid traits and breast cancer risk. Int J Epidemiol 49:1117–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian D, Zhang L, Zhuang Z, Huang T, Fan D (2022) A two-sample mendelian randomization analysis of modifiable risk factors and intracranial aneurysms. Sci Rep 12:7659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, VanderWeele TJ, Higgins JPT, Timpson NJ, Dimou N et al (2021) Strengthening the reporting of Observational studies in Epidemiology using mendelian randomization: the STROBE-MR Statement. JAMA 326:1614–1621 [DOI] [PubMed] [Google Scholar]
- 20.Skrivankova VW, Richmond RC, Woolf BAR, Davies NM, Swanson SA, VanderWeele TJ, Timpson NJ, Higgins JPT, Dimou N, Langenberg C et al (2021) Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ 375:n2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowden J, Holmes MV (2019) Meta-analysis and mendelian randomization: a review. Res Synth Methods 10:486–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopera-Maya EA, Kurilshikov A, van der Graaf A, Hu S, Andreu-Sánchez S, Chen L, Vila AV, Gacesa R, Sinha T, Collij V et al (2022) Effect of host genetics on the gut microbiome in 7,738 participants of the Dutch Microbiome Project. Nat Genet 54:143–151 [DOI] [PubMed] [Google Scholar]
- 23.Orrù V, Steri M, Sidore C, Marongiu M, Serra V, Olla S, Sole G, Lai S, Dei M, Mulas A et al (2020) Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat Genet 52:1036–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao JH, Stacey D, Eriksson N, Macdonald-Dunlop E, Hedman ÅK, Kalnapenkis A, Enroth S, Cozzetto D, Digby-Bell J, Marten J et al (2023) Genetics of circulating inflammatory proteins identifies drivers of immune-mediated disease risk and therapeutic targets. Nat Immunol 24:1540–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, Reeve MP, Laivuori H, Aavikko M, Kaunisto MA et al (2023) FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 613:508–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lan Z, Wei Y, Yue K, He R, Jiang Z (2024) Genetically predicted immune cells mediate the association between gut microbiota and neuropathy pain. Inflammopharmacology [DOI] [PMC free article] [PubMed]
- 27.Burgess S, Small DS, Thompson SG (2017) A review of instrumental variable estimators for mendelian randomization. Stat Methods Med Res 26:2333–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papadimitriou N, Dimou N, Tsilidis KK, Banbury B, Martin RM, Lewis SJ, Kazmi N, Robinson TM, Albanes D, Aleksandrova K et al (2020) Physical activity and risks of breast and colorectal cancer: a mendelian randomisation analysis. Nat Commun 11:597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowden J, Del Greco MF, Minelli C, Zhao Q, Lawlor DA, Sheehan NA, Thompson J, Davey Smith G (2019) Improving the accuracy of two-sample summary-data mendelian randomization: moving beyond the NOME assumption. Int J Epidemiol 48:728–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgess S, Butterworth A, Thompson SG (2013) Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 37:658–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Liu Z, Choudhury T, Cornelis MC, Liu W (2021) Habitual coffee intake and risk for nonalcoholic fatty liver disease: a two-sample mendelian randomization study. Eur J Nutr 60:1761–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartwig FP, Davey Smith G, Bowden J (2017) Robust inference in summary data mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol 46:1985–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burgess S, Thompson SG (2017) Interpreting findings from mendelian randomization using the MR-Egger method. Eur J Epidemiol 32:377–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye T, Shao J, Kang H (2021) Debiased inverse-variance weighted estimator in two-sample summary-data mendelian randomization. Annals Stat 49:2079–2100 [DOI] [PubMed] [Google Scholar]
- 35.SCORE URAP (2018) Statistical inference in two-sample summary-data mendelian randomization using robust adjusted profile score. arXiv Preprint arXiv :180109652
- 36.Verbanck M, Chen CY, Neale B, Do R (2018) Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet 50:693–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemani G, Tilling K, Davey Smith G (2017) Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet 13:e1007081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V (2002) A comparison of methods to test mediation and other intervening variable effects. Psychol Methods 7:83–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiao B, Cao X, Zhang C, Zhang W, Yu S, Zhang M, Zhang X (2023) Alterations of the gut microbiota in patients with postherpetic neuralgia. AMB Express 13:108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freidin MB, Stalteri MA, Wells PM, Lachance G, Baleanu AF, Bowyer RCE, Kurilshikov A, Zhernakova A, Steves CJ, Williams FMK (2021) An association between chronic widespread pain and the gut microbiome. Rheumatology (Oxford) 60:3727–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goudman L, Demuyser T, Pilitsis JG, Billot M, Roulaud M, Rigoard P, Moens M (2024) Gut dysbiosis in patients with chronic pain: a systematic review and meta-analysis. Front Immunol 15:1342833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall LJ, Walshaw J, Watson AJ (2014) Gut microbiome in new-onset Crohn’s disease. Gastroenterology 147:932–934 [DOI] [PubMed] [Google Scholar]
- 43.Quiroga R, Nistal E, Estébanez B, Porras D, Juárez-Fernández M, Martínez-Flórez S, García-Mediavilla MV, de Paz JA, González-Gallego J, Sánchez-Campos S, Cuevas MJ (2020) Exercise training modulates the gut microbiota profile and impairs inflammatory signaling pathways in obese children. Exp Mol Med 52:1048–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nie K, Ma K, Luo W, Shen Z, Yang Z, Xiao M, Tong T, Yang Y, Wang X (2021) Roseburia intestinalis: a beneficial gut organism from the discoveries in Genus and Species. Front Cell Infect Microbiol 11:757718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jalanka-Tuovinen J, Salonen A, Nikkilä J, Immonen O, Kekkonen R, Lahti L, Palva A, de Vos WM (2011) Intestinal microbiota in healthy adults: temporal analysis reveals individual and common core and relation to intestinal symptoms. PLoS ONE 6:e23035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu M, Li L, Ren Q, Feng H, Tao S, Cheng L, Ma L, Gou SJ, Fu P (2022) Understanding the gut-kidney Axis in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: an analysis of gut microbiota composition. Front Pharmacol 13:783679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei Y, Lu X, Liu C (2023) Gut microbiota and chronic obstructive pulmonary disease: a mendelian randomization study. Front Microbiol 14:1196751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dekker Nitert M, Mousa A, Barrett HL, Naderpoor N, de Courten B (2020) Altered gut microbiota composition is Associated with Back Pain in overweight and obese individuals. Front Endocrinol (Lausanne) 11:605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ellis RJ, Heaton RK, Gianella S, Rahman G, Knight R (2022) Reduced gut microbiome diversity in people with HIV who have Distal Neuropathic Pain. J Pain 23:318–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hadizadeh F, Bonfiglio F, Belheouane M, Vallier M, Sauer S, Bang C, Bujanda L, Andreasson A, Agreus L, Engstrand L et al (2018) Faecal microbiota composition associates with abdominal pain in the general population. Gut 67:778–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shapiro J, Cohen NA, Shalev V, Uzan A, Koren O, Maharshak N (2019) Psoriatic patients have a distinct structural and functional fecal microbiota compared with controls. J Dermatol 46:595–603 [DOI] [PubMed] [Google Scholar]
- 52.Wang Z, Guo M, Li J, Jiang C, Yang S, Zheng S, Li M, Ai X, Xu X, Zhang W et al (2023) Composition and functional profiles of gut microbiota reflect the treatment stage, severity, and etiology of acute pancreatitis. Microbiol Spectr 11:e0082923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen CL, Wang R, Ji G, Elmassry MM, Zabet-Moghaddam M, Vellers H, Hamood AN, Gong X, Mirzaei P, Sang S, Neugebauer V (2022) Dietary supplementation of gingerols- and shogaols-enriched ginger root extract attenuate pain-associated behaviors while modulating gut microbiota and metabolites in rats with spinal nerve ligation. J Nutr Biochem 100:108904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clos-Garcia M, Andrés-Marin N, Fernández-Eulate G, Abecia L, Lavín JL, van Liempd S, Cabrera D, Royo F, Valero A, Errazquin N et al (2019) Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. EBioMedicine 46:499–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li JS, Su SL, Xu Z, Zhao LH, Fan RY, Guo JM, Qian DW, Duan JA (2022) Potential roles of gut microbiota and microbial metabolites in chronic inflammatory pain and the mechanisms of therapy drugs. Ther Adv Chronic Dis 13:20406223221091177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michalak A, Mosińska P, Fichna J (2016) Polyunsaturated fatty acids and their derivatives: therapeutic value for Inflammatory, Functional Gastrointestinal disorders, and Colorectal Cancer. Front Pharmacol 7:459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiang W, Lv H, Xing F, Sun X, Ma Y, Wu L, Lv G, Zong Q, Wang L, Wu Z et al (2023) Inhibition of ACLY overcomes cancer immunotherapy resistance via polyunsaturated fatty acids peroxidation and cGAS-STING activation. Sci Adv 9:eadi2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diogenes A, Ferraz CC, Akopian AN, Henry MA, Hargreaves KM (2011) LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J Dent Res 90:759–764 [DOI] [PubMed] [Google Scholar]
- 59.Boonen B, Alpizar YA, Meseguer VM, Talavera K (2018) TRP Channels as sensors of bacterial endotoxins. Toxins (Basel) 10 [DOI] [PMC free article] [PubMed]
- 60.Hemmi H, Akira S (2005) TLR signalling and the function of dendritic cells. Chem Immunol Allergy 86:120–135 [DOI] [PubMed] [Google Scholar]
- 61.Laumet G, Edralin JD, Dantzer R, Heijnen CJ, Kavelaars A (2019) Cisplatin educates CD8 + T cells to prevent and resolve chemotherapy-induced peripheral neuropathy in mice. Pain 160:1459–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Austin PJ, Kim CF, Perera CJ, Moalem-Taylor G (2012) Regulatory T cells attenuate neuropathic pain following peripheral nerve injury and experimental autoimmune neuritis. Pain 153:1916–1931 [DOI] [PubMed] [Google Scholar]
- 63.Cheadle WG (1993) The human leukocyte antigens and their relationship to infection. Am J Surg 165:75s–81s [DOI] [PubMed] [Google Scholar]
- 64.Ji RR, Xu ZZ, Gao YJ (2014) Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov 13:533–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji RR (2010) Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med 16:592–597 591p following 597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Willemen HL, Eijkelkamp N, Garza Carbajal A, Wang H, Mack M, Zijlstra J, Heijnen CJ, Kavelaars A (2014) Monocytes/Macrophages control resolution of transient inflammatory pain. J Pain 15:496–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Z, Song K, Zhao W, Zhao Z (2020) Dendritic cells in tumor microenvironment promoted the neuropathic pain via paracrine inflammatory and growth factors. Bioengineered 11:661–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rausch T, Wachter A (2005) Sulfur metabolism: a versatile platform for launching defence operations. Trends Plant Sci 10:503–509 [DOI] [PubMed] [Google Scholar]
- 69.Ghoreschi K, Brück J, Kellerer C, Deng C, Peng H, Rothfuss O, Hussain RZ, Gocke AR, Respa A, Glocova I et al (2011) Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J Exp Med 208:2291–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peterson JD, Herzenberg LA, Vasquez K, Waltenbaugh C (1998) Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci U S A 95:3071–3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The GWAS summary statistics in this study are sourced from publicly open access databases including FinnGen (https://www.finngen.fi/en), IEU Open GWAS (https://gwas.mrcieu.ac.uk/), and GWAS Catalog (https://www.ebi.ac.uk/gwas/). Detailed information about the data used in the analyses is provided in the supplementary information files. Statistical code and additional information can be requested from the corresponding author.