Abstract
Transfer RNA-derived small RNAs (tsRNAs) are a newly discovered class of small noncoding RNAs (sncRNAs) that include tRNA-derived stress-induced RNAs (tiRNAs) and tRNA-derived fragments (tRFs). Following the development of high-throughput sequencing technology, an increasing number of tsRNAs have been discovered with vital functions in different physiological and pathophysiological processes. Extensive research has revealed that tsRNAs are involved in various diseases, such as cancers, autoimmune illnesses and other diseases. This review focuses on the role and significance of tsRNAs in inflammation, such as the regulation of substances including inflammatory inducers, inflammatory cells and inflammatory factors, which contribute to the pathogenesis of inflammation-related diseases. Moreover, we discuss in-depth the molecular pathogenic mechanisms of tsRNAs in inflammation-related diseases through different signaling pathways and assess their clinical value, providing new perspectives for the exploration of tsRNA functions and inflammation-related diseases.
Keywords: tsRNA, Inflammation, Inflammatory cells, Inflammatory mediators, Signaling pathways
Introduction
Inflammation is an effective defense response of a host against infection and tissue damage that can obstruct the spread of pathogens or promote tissue injury [1]. The typical inflammatory response has four clinical symptoms, redness and swelling with heat and pain, and is composed of four components: inflammatory inducers, the sensors that detect them, the inflammatory mediators induced by the sensors, and the target tissues that are affected by the inflammatory mediators [2]. Since each step involves diverse physiological and pathophysiological phenomena, the underlying mechanism remains to be studied.
Following the development of high-throughput sequencing technology, multiple sncRNAs (such as miRNAs and siRNAs) have been shown to greatly contribute to the mechanisms associated with inflammation and the clinical diagnosis and treatment of inflammation-related diseases. Similarly, as an emerging type of sncRNA, tsRNAs are processed from mature tRNAs or precursor tRNAs. Many experiments have shown that tsRNAs fulfill their unique biological roles through different mechanisms; these findings have led to the discovery of their roles in many diseases, such as cancers, autoimmune diseases, neurological disorders and others [3].
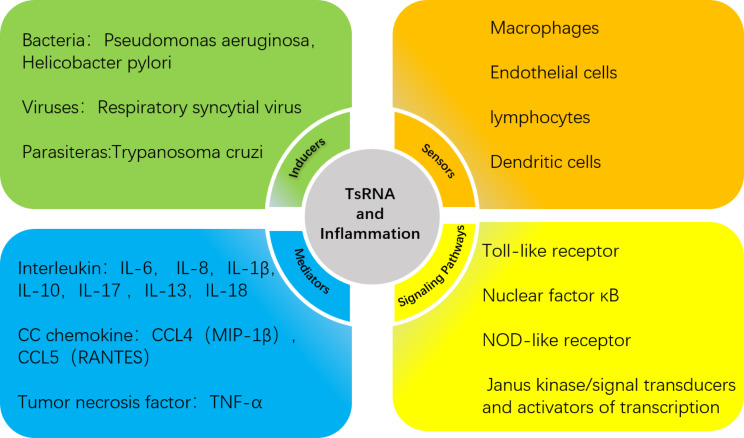
In this paper, we elaborate on the regulatory role of tsRNAs in the inflammatory response in four areas: inflammatory inducers, inflammatory cells, inflammatory mediators and inflammation-related signaling pathways. Moreover, most of the interactions between tsRNAs and these four key elements are interrelated and not independent of one another, implying that the actions of tsRNAs are likely to accompany the inflammatory response at all times. We also summarize the regulatory mechanisms of tsRNAs in the tumor microenvironment (TME) and findings in recent years regarding inflammatory responses in other diseases, which will help us to study the functions of tsRNAs in inflammation and provide new perspectives for exploring the pathogenesis, diagnosis, and even treatment of inflammation-related diseases in the future. Since there is no unified naming system for tsRNAs, to facilitate the understanding and standardization of tsRNA names, in this report we have adopted the tDRnamer system (http://trna.ucsc.edu/tDRnamer/) for uniform naming of the referenced tsRNAs.
TsRNAs and inflammatory inducers
Biogenic factors are regarded as important inflammatory inducers. Moreover, as ancient small RNAs found in all domains of life, tsRNAs have been identified in many species across all three domains of life: Archaea, Bacteria, and some unicellular eukaryotes [4]. The results of many experiments have confirmed that microorganisms such as bacteria, viruses [5], parasites and fungi have specific tsRNAs that play crucial roles in host-microbe interactions [6]. In other words, tsRNAs can participate in host-microbe inflammatory responses. For example, a fragment of a Pseudomonas aeruginosa methionine tRNA, tDR-1:23-fMet-CAT-1 (sRNA52320), is abundant in outer membrane vesicles (OMVs). It not only reduces lipopolysaccharide (LPS)-induced and OMV-induced interleukin (IL)-8 secretion by cultured primary human airway epithelial cells but also attenuates OMV-stimulated secretion of the keratinocyte-derived chemokine(KC) IL-8 homolog and neutrophil infiltration in the mouse lung [7]. Similarly, tDR-1:30-fMet-CAT-1 (sR-2509025) packaged in Helicobacter pylori OMVs can also decrease the secretion of IL-8 induced by LPS or OMVs in AGS cells [8]. Among viruses, after respiratory syncytial virus (RSV) infects the lower respiratory tract, RSV induces airway epithelial cells to produce tDR-1:30-Gly-CCC-2 (tRF5-Gly(CCC)) and tDR-1:30-Lys-CTT-2-M2 (tRF5- Lys(CTT)), which can promote RSV replication and the expression of RSV-induced airway epithelial cell-secreted IL-6, IL-8, macrophage inflammatory factor (MIP)-1β and CCL5, thereby enhancing the inflammatory response [9]. Furthermore, a bioinformatic analysis of modified transcripts of the Trypanosoma cruzi-derived tsRNAThr gene in HeLa cells revealed that CXCL2 has the potential to be a direct target of tsRNAThr. CXCL2, a member of the CXC family of chemokines, is recognized as a key mediator of inflammatory processes [10].
TsRNAs and inflammatory cells
As indispensable components of the inflammatory response, inflammatory cells play complex and diverse roles. For instance, there are neutrophils, major factors in acute inflammation; macrophages, which play various roles in inflammation [11]; and endothelial cells, which rely on angiogenesis to play a special role in inflammation. In summary, all of these cells can be regulated by tsRNAs in related illnesses or inflammatory responses (listed in Table 1).
Table 1.
Studies of correlations between tsRNAs and inflammatory cells
| Inflammatory cell | Uniform name in this paper | Function | Reference |
|---|---|---|---|
| Neutrophils | tDR-1: 23-fMet-CAT-1 | attenuates OMV-stimulated KC IL-8 homolog secretion and neutrophil infiltration in mouse lungs | [7] |
| Macrophages | tsRNA-21,109 | alleviate systemic lupus erythematosus by inhibiting macrophage M1 polarization | [13] |
| tDR-1:34-Gly-CCC-3-G9A | promote the expression of proinflammatory factors and M1 polarization and inhibit the expression of anti-inflammatory factors and M2 polarization | [14] | |
| tDR-55:76-Ile-AAT-1-M4 | modulates M2 macrophage polarization through binding to MIF in colorectal cancer | [31] | |
| tsRNA-14,783 | promotes M2 macrophage polarization in keloid | [15] | |
| Endothelial cells | tDR-T1:T20-Ser-TGA-1-1 | acts as an antiangiogenic factor during ocular angiogenesis via tDR-T1:T20-Ser-TGA-1-1/METTL3/RBPJ-MAML1 signaling | [17] |
| tDR-55:76-Gln-CTG-1-M2 | plays an anti-angiogenic role in choroidal neovascularization. | [18] | |
| tDR-38:71-Arg-CCG-2 | modulates expression of proatherogenic genes in ECs in vitro, including IL-6, IL-1α, ICAM-1, VCAM-1, and MCP-1 | [20] | |
| T cells | tDR-59:76-Leu-TAA-1 | participate in metabolic modulation of IFN-α-induced CD4 + T cell OXPHOS in lupus. | [32] |
| tDR-55:76-Leu-TAA-1 | |||
| tDR-T1:T18-Val-TAC-3-1 | associated with the T-cell activation status in Breast Cancer | [23] | |
| tDR-T1:T34-Thr-CGT-2-1-A15G | |||
| B cells | tDR-55:76-Gly-GCC-2 | differentially expressed in three stages of mature B-cell differentiation and one GC-derived lymphoma cell line, and cloned most frequently in normal GC B cells | [26] |
| tDR-1:33-His-GUG-1-M9 | contributes to cell proliferation in B cells under LA stress. | [28] | |
| Dendritic cells | tDR-1:32-Val-CAC-2 | controls the formation of effector T-cells, activation of regulatory T-cells, and DC maturation | [29] |
Macrophages
Although macrophages can polarize to one of many specific phenotypes, most researchers still use the M1/M2 classification to summarize their characteristics: classically activated macrophages (M1) and alternatively activated macrophages (M2). In the early phase of inflammation, macrophages differentiate into M1 macrophages, which release inflammatory cytokines to exert proinflammatory effects. In the later period of inflammation, M2 macrophages produce anti-inflammatory cytokines to reduce the intensity of the inflammatory response by decreasing reactive oxygen species (ROS) production and neutrophil infiltration [12]. tsRNAs play unexpected roles in regulating macrophage polarization. Dou et al. reported that tsRNA-21109, delivered by mesenchymal stem cell-derived exosomes (MSC-exos), is an effective molecule for inhibiting macrophage M1 polarization. A series of experiments showed that the target genes of tsRNA-21109 were primarily involved in inflammation-related pathways and that tsRNA-21109-derived MSC-exos increased the levels of TNF-α and IL-1β in macrophages [13]. In addition, compared with those in normal muscle tissue, the expression profiles of the tsRNAs were altered in the early stage of cardiotoxin-induced muscle injury, and the overexpressed tsRNAs were found to participate in the early stage of inflammation. Interestingly, several tsRNAs were significantly enriched during the inflammatory response immediately after muscle injury; tDR-1:34-Gly-CCC-3-G9A(5’ tiRNA-Gly‐CCC)had a strong positive association with inflammation. In vitro experiments revealed that tDR-1:34-Gly-CCC-3-G9A stimulated the mRNA expression of proinflammatory cytokines (IL‐1β and IL‐6) and macrophages expressing M1 markers (TNF‐α, CD80, and MCP‐1) and inhibited the mRNA expression of anti‐inflammatory cytokines (IL‐4, IL‐10, and IL‐13) and M2 markers (TGF‐β1 and ARG1) [14]. Furthermore, analysis of the tsRNAs of keloid macrophages revealed that the expression profiles of the tsRNAs differed between M1 and M2 macrophages. Moreover, a cluster heatmap showed that the expression profiles of tRFs could differentiate between M1 and M2 macrophages. More importantly, the expression of tsRNA-14,783, which was upregulated in M2 macrophages, stimulated M2 macrophage polarization [15].
Endothelial cells
Endothelial cells (ECs) play important roles in the inflammatory process. In the adaptive immune response, if specialized effector cells are unable to clear antigens, ECs are involved in an additional event associated with chronic inflammation: angiogenesis. The generation of new blood vessels can ensure the continued survival of inflammatory cells within the tissue. Consequently, antiangiogenic factors may reduce novel inflammatory tissue growth and prevent disease progression [16]. Qin et al. reported that tDR-T1:T20-Ser-TGA-1-1 (tRF-1001), which shows downregulated expression in age-related macular degeneration patients, regulates endothelial angiogenic effects via tDR-T1:T20-Ser-TGA-1-1/METTL3/RBPJ-MAML1 signaling and acts as an antiangiogenic factor during ocular angiogenesis [17]. Similarly, tDR-55:76-Gln-CTG-1-M2 (tRF-22-8BWS72092/tRF-22), which shows downregulated expression in the choroids of myopic patients, has antiangiogenic effects by inhibiting choroidal EC proliferation, migration, and tube formation in vitro [18]. Similarly, tsRNAs derived from tRNA-Val-CAC and tRNA-Gly-GCC produced in response to ischemic injury also inhibit the proliferation, migration, and tube formation of ECs, thereby playing a negative role in angiogenesis [19]. Furthermore, in experiments concerning atherosclerosis, one of the high-cholesterol diet-induced intimal tsRNAs, tDR-38:71-Arg-CCG-2 (tsRNA-Arg-CCG), modulated the expression of proatherogenic genes, including IL-6, IL-1α, ICAM-1, VCAM-1, and MCP-1, in ECs in vitro [20].
T-cells
T-cells have a major impact on regulating immune responses in health and disease. As a key event, T-cell differentiation is essential for eliminating intra- and extracellular pathogens and can also lead to inflammation upon dysregulation [21]. It has been demonstrated that the 3′-end of tRNA-AlaUGC (CCACCA sequences) can activate Th1- and toxic T lymphocyte-related immune responses by directly interacting with Toll-like receptors [22]. Additionally, effector CD8 + T-cell activation and its cytotoxic function are known to be positively correlated with improved survival in patients with breast cancer (BC). Previous studies have shown that tDR-T1:T18-Val-TAC-3-1 (ts-34) and tDR-T1:T34-Thr-CGT-2-1-A15G (ts-49) are associated with T-cell activation. Moreover, the effects of tDR-T1:T18-Val-TAC-3-1 and tDR-T1:T34-Thr-CGT-2-1-A15G on the survival of BC patients varied according to T-cell activation status [23]. Furthermore, tsRNAs enriched in extracellular vesicles (EVs) generated by T-cells can inhibit T-cell activation and cytokine production. Conversely, T-cells can utilize the EV-generating pathway to selectively secrete tRFs [24].
B-cells
During chronic inflammation, B-cells contribute to the pathogenesis of inflammatory diseases by acting as specialized antigen-presenting cells, producing cytokines, and through other mechanisms [25]. tRF-3s are the most abundant variety of tsRNAs expressed in mature B-cells. A previous study showed that tDR-55:76-Gly-GCC-2 (CU1276, a representative sequence of the tRF-3 class) is a DICER1-dependent tsRNA expressed in mature B-cells. It is differentially expressed across three stages of mature B-cell differentiation and in a germinal center-derived lymphoma cell line. Conversely, it has the highest cloning frequency in normal germinal center B-cells [26]. Chronic lymphocytic leukemia (CLL) is a heterogeneous disease characterized by CD19+/CD5 + B-cell expansion, and studies of the signatures of tsRNAs in aggressive and indolent CLL have revealed that mature tRFs can be severely dysregulated in CLL [27]. Furthermore, lactate (LA)-induced expression of tDR-1:33-His-GUG-1-M9 (5′-HisGUG half) can impair the cell cycle and proliferation of B-cells, especially EBV-infected B-cell lymphoma cells in microenvironments under LA stress and aid in the growth of B-cell lymphoma [28].
Dendritic cells
Dendritic cells (DCs) are a special type of antigen-presenting cell whose activation can lead to the secretion of proinflammatory mediators and the recruitment of more inflammatory cells to the site of infection. tDR-1:32-Val-CAC-2 (5′-tiRNAVal) has been shown to bind to human Frizzled homolog 3 and mediate the downregulation of a crucial factor of the Wnt signaling pathway, which controls the formation of effector T-cells, activation of regulatory T-cells, and DC maturation [29]. It has also been shown that tsRNAs regulate histone methylation in human monocytes, which can differentiate into DCs when stimulated by a variety of cytokines, including IL-4 [30].
TsRNAs and inflammatory mediators
Inflammatory mediators, including cytokines, growth factors, chemokines, inflammasomes and inflammatory metabolites, have been identified as vital regulators of the initiation and resolution of inflammation [1]. Not only are tsRNAs involved in the regulation of a variety of inflammatory mediators, but inflammatory mediators may also counteract the actions of tsRNAs (listed in Table 2).
Table 2.
Studies of correlations between tsRNAs and inflammatory mediators
| Uniform name in this paper | Function | Reference |
|---|---|---|
| tDR-1: 23-fMet-CAT-1 | reduces LPS-induced and OMV-induced IL-8 secretion by cultured primary human airway epithelial cells | [7] |
| tDR-1:30-fMet-CAT-1 | decreases LPS- or OMV-induced IL-8 secretion in AGS cells | [8] |
| tDR-1:30-Gly-CCC-2 | promotes RSV replication and the expression of RSV-induced IL-6, IL-8, MIP-1β and CCL5 in airway epithelial cells, enhancing the inflammatory response | [9] |
| tDR-1:30-Lys-CTT-2-M2 | ||
| tDR-56:71-Ala-CGC-1-M4 | suppresses the expression of inflammatory cytokines IL-1β and TNF-α and inhibits apoptosis of NP cells | [33] |
| tDR-60:76-Cys-GCA-2-M7 | inhibits JAK3 expression via AGO/RISC formation in chondrocytes, thereby suppressing downstream target cytokine IL-6 expression and preventing pro-inflammatory signaling, to restore cellular homeostasis | [34] |
| tDR-1:34-Ala-CGC-1-M3-D22GC-A25G-U26C | activates p65 and enhances IL-8 secretion in response to arsenite administration | [37] |
| tDR-1:19-Arg-ACG-1-M2 | increases production of pro-inflammatory cytokines IL-1β and IL-18 | [35] |
| tDR-1:32-Gly-GCC-1 | upregulated in triple-negative breast cancer, mainly involved in the maintenance of stem cell population and cellular response to IL-6 secretion | [38] |
| tDR-1:32-Gly-GCC-1-C31A | ||
| tRF-36 | promotes inflammatory factors TNF-α, IL-6, and IL-1β levels in an AP cell model generated from MCP-83 cells | [39] |
| tRF-47/tRF-47-58ZZJQJYSWRYVMMV5BO | reduces the release of IL-6, TNF-α, IL-10, IL-17 and IL-4 in a model of nonalcoholic steatohepatitis and improves the inflammatory response | [36] |
Pan et al. performed small RNA sequencing of nucleus pulposus (NP) tissues from traumatic lumbar fracture patients, young (IDDY) patients, and old intervertebral disk degeneration (IDDO) patients. The results showed that tDR-56:71-Ala-CGC-1-M4 (tsRNA-04002) was expressed at lower levels in both the IDDY and IDDO groups than in the control group. The overexpression of tDR-56:71-Ala-CGC-1-M4 suppressed the expression of the inflammatory cytokines IL-1β and TNF-α and inhibited the apoptosis of NP cells [33]. As an important player in osteoarthritis pathogenesis, IL-1β can increase the expression level of tDR-60:76-Cys-GCA-2-M7 (tRF-3003a), which is produced from the 3’ end of tRNA-CysGCA, in addition to maintaining its high expression for at least 6 h. This study confirmed that the induction and cleavage of tRNA-CysGCA is an early response to inflammatory cytokine-induced stress [34]. Similarly, tDR-1:19-Arg-ACG-1-M2 (tRF-5014a), which shows significantly upregulated expression in high glucose-stimulated primary cardiomyocytes, increases the production of the proinflammatory cytokines IL-1β and IL-18 and plays a key role in cardiomyocyte injury associated with diabetic cardiomyopathy [35]. Moreover, tectorigenin (TEC), which is abundant in blueberries, induces the secretion of tRF-47 from hepatocytes. tRF-47 reduced the release of IL-6, TNF-α, IL-10, IL-17 and IL-4 in a model of nonalcoholic steatohepatitis and improved the inflammatory response [36].
TsRNAs and inflammation-related signaling pathways
Dysregulation of inflammatory mediators, which act as a bridge between inflammation and chronic disease, is usually caused by dysregulation of signaling pathways, including the nuclear factor κB (NF-κB), Janus kinase/signal transducers and activators of transcription (JAK/STAT), NOD-like receptor (NLR), and Toll-like receptor (TLR) pathways [1].
Numerous experiments have demonstrated that tsRNAs play regulatory roles in inflammation-related signaling pathways or that their production is influenced by inflammation-related signaling pathways. For instance, the expression of 5′-tRNA halves was upregulated by cell surface TLRs in human monocyte-derived macrophages (HMDMs). Additionally, HMDMs selectively package 5′-tRNA halves with extracellular vesicles (EVs) as carriers. More importantly, when tDR-1:34-His-GTG-1 (5′-tRNAHisGUG half) was transfected into HMDMs, the secretion of TNF-α and IL-1β increased, whereas transfection of tDR-1:34-Glu-CTC-1-M2 (5′-tRNAGluCUC half) did not cause an increase. Experiments conducted by Kamlesh et al. demonstrated that the transfer of tDR-1:34-His-GTG-1 to recipient cells can promote cytokine production by stimulating endosomal TLR7 [40]. In addition, the aforementioned tDR-60:76-Cys-GCA-2-M7, which is highly expressed in OA patients, inhibits JAK3 expression via AGO/RISC formation in chondrocytes, thereby suppressing downstream expression of IL-6 and preventing proinflammatory signaling, to restore cellular homeostasis [34]. Liu et al. reported that arsenite-treated airway epithelial cells produce tDR-1:34-Ala-CGC-1-M3-D22GC-A25G-U26C (tRF5-AlaCGC). tDR-1:34-Ala-CGC-1-M3-D22GC-A25G-U26C can not only activate p65 but also enhance the secretion of IL-8 in the arsenite response. p65 is an important transcription factor that belongs to the NF-κB family and is a key factor modulating inflammatory gene expression [37]. Furthermore, Sun et al. identified a novel tsRNA, tDR-59:75-Thr-AGT-1-M2 (tRF3-Thr‐AGT), which showed downregulated expression in the sodium taurocholate (STC)-treated acinar cell line AR42J and in the pancreatic tissues of STC‐induced AP model rats. They also demonstrated that tDR-59:75-Thr-AGT-1-M2 suppressed ZBP1 expression to restrain NLRP3‐mediated pyroptotic cell death and inflammation [41].
In summary, tsRNAs play essential roles in a variety of inflammation-related signaling pathways. However, inflammation-related signaling pathways are complex and diverse, so whether tsRNAs can regulate other proteins involved in inflammation-related signaling pathways remains to be investigated.
TsRNAs and inflammation in diseases
tsRNAs and inflammation in cancers
In some cancers, the inflammatory environment is affected before malignant changes occur. In other cases, an oncogenic change drives an inflammatory microenvironment. Regardless of its origin, “smoldering” inflammation in the TME is not only conducive to the proliferation and survival of malignant cells but also promotes tumor progression. Furthermore, the mediators and cells involved in inflammation are vital constituents of the epithelial TME [42]. Numerous studies have shown the effects of sncRNAs on the TME [43], and it has also been demonstrated that tsRNAs can modulate inflammatory mediators in the TME, thus playing a protumor or tumor suppressing role [44].
Gastric cancer(GC): A study of GC biomarkers revealed 613 differentially expressed (DE)-tRFs in the TCGA dataset, 19 of which showed upregulated expression and 20 showed downregulated expression. Notably, the downstream targets of the 9 tRFs affected neutrophil activation and degranulation [45]. Moreover, tDR-1:23-Gln-TTG-1 (tRF-24-V29K9UV3IU), which shows significantly downregulated expression in GC tissues compared with adjacent tissues, can target chemokine (CXCR5, CXCR3, CX3CL1, CX3CR1, CXCL9, etc.) signaling pathways [46]. Moreover, a systematic review and meta-analysis of the diagnostic and prognostic values of tsRNAs in GC showed that GC-associated tsRNAs play a role in distinguishing healthy controls from patients [47].
Colorectal cancer (CRC): Compared to healthy adjacent tissues, Lu et al. identified three tsRNAs overexpressed in CRC tissues and plasma exosomes. One of these tsRNAs, tDR-55:76-Ile-AAT-1-M4 (tRF-3022b), regulates the expression of target genes that closely interact with the cytokine-associated tumor microenvironment. In addition, tDR-55:76-Ile-AAT-1-M4 suppressed M2 macrophage polarization by binding to macrophage migration inhibitory factor (MIF) [31]. MIF is a major cytokine produced by both malignant cells and infiltrating leukocytes in some cancers [42].
Pancreatic ductal adenocarcinoma (PDAC): In a study regarding the tumorigenic mechanism of PDAC, Pan et al. identified an inflammatory cytokine–regulated tsRNA, tDR-19:39-Gly-GCC-2-M10 (tRF-21-VBY9PYKHD/tRF-21). As a tumor suppressor in PDAC progression, the production of tDR-19:39-Gly-GCC-2-M10 can be inhibited by IL-6 and leukemia inhibitory factor (LIF). The former is secreted by macrophages and T-cells in the TME, and the latter is derived from tumor cells and other microenvironmental cells, such as pancreatic stellate cells [48].
tsRNAs and inflammation-related diseases
Systemic lupus erythematosus (SLE): Through small RNA sequencing, Geng et al. identified 482 differentially expressed tsRNAs in CD4 + T-cells from patients with SLE and healthy controls. Furthermore, tDR-59:76-Leu-TAA-1 (tRF-3009a) and tDR-55:76-Leu-TAA-1 (tRF-3009b), derived from tRNA-Lue-TAA, were found to be significantly highly expressed in SLE CD4 + T-cells. The expression of tRF-3009 is correlated with disease activity index, lupus nephritis and serum IFN-α levels. Conversely, upon knockdown of tRF-3009, IFN-α-induced ROS production and ATP biogenesis were inhibited in vitro. This indicates that tRF-3009 may act as a metabolic modulator downstream of the type I interferon pathway, which in turn could enhance oxidative phosphorylation in CD4 + T-cells in patients with SLE [32]. Moreover, Zhang et al. reported that tDR-59:76-Ala-AGC-2-M4 (tRF-Ala-AGC-2-M4) was not only more highly expressed in lupus nephritis patients than in healthy controls but was also significantly positively correlated with C-reactive protein and the systemic lupus erythematosus disease activity index 2000. However, tDR-59:76-Ala-AGC-2-M4 expression has little correlation with other indicators (anti-dsDNA, proteinuria, IgG, anti-β2-GP, C3, C4 and eGFR) [49].
Rheumatoid arthritis (RA): A previous study showed that three microbial sRNAs derived from the same tRNA encoding arginine were tsRNAs and had increased expression in RA patients. Two of these sequences were tRFs (tDR-1 and tDR-2), and the third sequence was a 5’ tRNA half (tDR-3). Compared with those in control plasma, the plasma concentrations of tDR-1 and tDR-3 were enriched in RA patients, but not those of inflammatory markers such as ESR, IL-6 and TNF-α. Further, they were inversely associated with the DAS28 score, tender joint count, and swollen joint count [50]. Compared to the correlation between tsRNAs and inflammatory markers in patients with SLE, this finding implies that tsRNAs may have positive or negative correlations with inflammatory markers, but not all tsRNAs are correlated with inflammatory markers.
Acute pancreatitis (AP): In addition to tDR-59:75-Thr-AGT-1-M2 mentioned above, Fan et al. reported that tRF36 was downregulated in the serum of AP patients. Notably, after tRF36 knockdown, the levels of the inflammatory factors TNF-α, IL-6, and IL-1β were reduced in the AP cell model generated from MCP-83 cells. Additionally, cell viability increased while cell death decreased. These results demonstrate that tRF36 can promote cell death to drive AP progression [39].
Spotted fever: Human angiogenin (ANG) is the only angiogenic protein that specifically cleaves tRNA both in vivo and in vitro. In addition to its nuclear role in ECs, ANG is thought to play a novel role in the cytoplasm. In both mouse and human cells infected with spotted fever group Rickettsiae, ANG-induced host cells produced tDR-1:33-Val-AAC-1-M2-D9GU (tRF5-ValGTG) and tDR-1:31-Gly-GCC-2-M3 (tRF5-GlyGCC), which can interact with transcripts associated with endothelial barrier function, the host cell inflammatory response, and autophagy [51].
Other inflammation-related diseases: The pathological changes in chronic kidney disease (CKD) are caused by inflammatory events such as inflammatory cell infiltration, fibroblast activation and proliferation. Khurana et al. observed the differential abundance of exosomal nuclear-encoded tsRNAs in CKD patients compared to healthy controls (HCs). Compared to those in HCs, the abundances of tsRNAs (tRFVal and tRFLeu) in exosomes decreased in CKD patients [52]. In addition, tsRNAs have been found to be closely associated with a wide range of liver diseases [53–55]. 5′ tRNA-halves (5′ tRHs) are particularly abundant in nonmalignant livers. Their abundance is increased in humans and chimpanzees with chronic viral hepatitis and is altered in patients with viral hepatitis-associated cancer [53]. A previous study showed that tsRNAs were the sRNAs with the most significantly altered expression after SARS-CoV-2 infection, and their maximum change in expression exceeded 200-fold. In addition, the expression of these tsRNAs was upregulated in patients infected with SARS-CoV-2 and even more significantly increased in the severe disease group [56].
Potential value of tsRNAs in the diagnosis of inflammation-related diseases
Continuous stress responses result in inflammation and disease pathogenesis. Many studies have suggested that the expression levels of tsRNAs are correlated with the degree of tissue injury (such as ischemia‒reperfusion, radiation, and toxic damage). Oxidative stress can mediate a direct conformational change in tRNA structure, subsequently promoting the cleavage of tRNA into tsRNAs. In particular, this process occurs much earlier than DNA damage [57], which means that tsRNAs can potentially be biomarkers for early cell, tissue or organ damage. Furthermore, tsRNAs are present in multiple environments and have multiple associations with tissue states, disease types and personal attributes [58]. Moreover, compared with miRNAs, tsRNAs are stable in circulation and are present in much greater percentages [59]. Therefore, their potential value as noninvasive biomarkers is being actively explored [60].
Conclusion
TsRNAs play regulatory roles in inflammation-related physiological and pathophysiological phenomena. Meantime, discovering the relationships between tsRNAs and inflammatory inducers, inflammatory cells, inflammatory mediators, and inflammation-related signaling pathways will provide evidence for the role of tsRNAs in inflammation-related diseases (illustrated in Fig. 1). Therefore, tsRNAs have potential in the diagnosis, prognosis, and treatment of inflammation-related diseases.
Fig. 1.
TsRNAs are involved in four vital processes of the inflammatory response
Author contributions
Peiru Qiu for the writing, review, and editing; Qi Jiang and Haojun Song revised the manuscript.
Funding
The work was supported by the Medical and Health Research Project of Zhejiang province (No.2022KY1100), the Natural Science Foundation of Ningbo (No. 2023J149), the Basic and Public Research Project of Zhejiang province (No.LBY23H200005). Ningbo Top Medical and Health Research Program (No.2023020612).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
This paper has got consent for publication from all authors listed.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qi Jiang, Email: jiangqigua@163.com.
Haojun Song, Email: 061050225@163.com.
References
- 1.Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–6. [DOI] [PubMed] [Google Scholar]
- 3.M F, J G, M W, J Z, Y C, P J, et al. Emerging roles of tRNA-derived fragments in cancer. Mol Cancer [Internet]. 2023 [cited 2024 Jan 24];22. https://pubmed.ncbi.nlm.nih.gov/36782290/ [DOI] [PMC free article] [PubMed]
- 4.Chen Q, Zhang X, Shi J, Yan M, Zhou T. Origins and evolving functionalities of tRNA-derived small RNAs. Trends Biochem Sci. 2021;46:790–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nunes A, Ribeiro DR, Marques M, Santos MAS, Ribeiro D, Soares AR. Emerging roles of tRNAs in RNA virus infections. Trends Biochem Sci. 2020;45:794–805. [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Stanton BA. Transfer RNA-Derived fragments, the Underappreciated Regulatory small RNAs in Microbial Pathogenesis. Front Microbiol. 2021;12:687632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koeppen K, Hampton TH, Jarek M, Scharfe M, Gerber SA, Mielcarz DW, et al. A novel mechanism of Host-Pathogen Interaction through sRNA in bacterial outer membrane vesicles. PLoS Pathog. 2016;12:e1005672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Zhang Y, Song Z, Li R, Ruan H, Liu Q, et al. sncRNAs packaged by Helicobacter pylori outer membrane vesicles attenuate IL-8 secretion in human cells. Int J Med Microbiol IJMM. 2020;310:151356. [DOI] [PubMed] [Google Scholar]
- 9.Zhou J, Liu S, Chen Y, Fu Y, Silver AJ, Hill MS, et al. Identification of two novel functional tRNA-derived fragments induced in response to respiratory syncytial virus infection. J Gen Virol. 2017;98:1600–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Silva MR, Cabrera-Cabrera F, das Neves RFC, Souto-Padrón T, de Souza W, Cayota A. Gene expression changes induced by Trypanosoma Cruzi shed microvesicles in mammalian host cells: relevance of tRNA-derived halves. BioMed Res Int. 2014;2014:305239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu J, He S, Song Z, Chen S, Lin X, Sun H, et al. Macrophage polarization states in atherosclerosis. Front Immunol. 2023;14:1185587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang Y, Xu X, Xiao L, Wang L, Qiang S. The role of microRNA in the inflammatory response of Wound Healing. Front Immunol. 2022;13:852419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dou R, Zhang X, Xu X, Wang P, Yan B. Mesenchymal stem cell exosomal tsRNA-21109 alleviate systemic lupus erythematosus by inhibiting macrophage M1 polarization. Mol Immunol. 2021;139:106–14. [DOI] [PubMed] [Google Scholar]
- 14.Shen L, Liao T, Chen Q, Lei Y, Wang L, Gu H, et al. tRNA-derived small RNA, 5’tiRNA-Gly-CCC, promotes skeletal muscle regeneration through the inflammatory response. J Cachexia Sarcopenia Muscle. 2023;14:1033–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Hu Z. tRNA derived fragment tsRNA-14783 promotes M2 polarization of macrophages in keloid. Biochem Biophys Res Commun. 2022;636:119–27. [DOI] [PubMed] [Google Scholar]
- 16.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–15. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Q, Ma Y, Zhao Y, Yao M-D, Zhu Y, Zhang Q-Y, et al. tRNA-derived fragment tRF-1001: a novel anti-angiogenic factor in pathological ocular angiogenesis. Mol Ther Nucleic Acids. 2022;30:407–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.C L et al. M L, Y S, X H, R W, Y W,. Targeting choroidal vasculopathy via up-regulation of tRNA-derived fragment tRF-22 expression for controlling progression of myopia. J Transl Med [Internet]. 2023 [cited 2024 Jan 24];21. https://pubmed.ncbi.nlm.nih.gov/37355654/ [DOI] [PMC free article] [PubMed]
- 19.Li Q, Hu B, Hu G-W, Chen C-Y, Niu X, Liu J, et al. tRNA-Derived small non-coding RNAs in response to Ischemia Inhibit Angiogenesis. Sci Rep. 2016;6:20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R H, J S, J L, X L, J W, L Z, et al. PANDORA-Seq unveils the hidden small noncoding RNA landscape in atherosclerosis of LDL receptor-deficient mice. J Lipid Res [Internet]. 2023 [cited 2024 Jan 24];64. https://pubmed.ncbi.nlm.nih.gov/36871792/ [DOI] [PMC free article] [PubMed]
- 21.Goswami R, Awasthi A, Editorial. T cell differentiation and function in tissue inflammation. Front Immunol. 2020;11:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S, Xu Z, Sheng J. tRNA-Derived small RNA: a Novel Regulatory Small non-coding RNA. Genes. 2018;9:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shan N, Li N, Dai Q, Hou L, Yan X, Amei A, et al. Interplay of tRNA-Derived fragments and T cell activation in breast Cancer patient survival. Cancers. 2020;12:2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiou N-T, Kageyama R, Ansel KM. Selective export into Extracellular vesicles and function of tRNA fragments during T cell activation. Cell Rep. 2018;25:3356–e33704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.I T. P M. The role of B cells and their interactions with stromal cells in the context of inflammatory autoimmune diseases. Autoimmun Rev [Internet]. 2022 [cited 2024 Jan 24];21. https://pubmed.ncbi.nlm.nih.gov/35417796/ [DOI] [PubMed]
- 26.Maute RL, Schneider C, Sumazin P, Holmes A, Califano A, Basso K, et al. tRNA-derived microRNA modulates proliferation and the DNA damage response and is down-regulated in B cell lymphoma. Proc Natl Acad Sci U S A. 2013;110:1404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veneziano D, Tomasello L, Balatti V, Palamarchuk A, Rassenti LZ, Kipps TJ, et al. Dysregulation of different classes of tRNA fragments in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2019;116:24252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mo X, Du S, Chen X, Wang Y, Liu X, Zhang C, et al. Lactate induces production of the tRNAHis Half to Promote B-lymphoblastic cell proliferation. Mol Ther J Am Soc Gene Ther. 2020;28:2442–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarais F, Perdomo-Sabogal A, Wimmers K, Ponsuksili S, tiRNAs. Insights into their Biogenesis, functions, and future applications in Livestock Research. Non-Coding RNA. 2022;8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park J, Ahn SH, Shin MG, Kim HK, Chang S. tRNA-Derived small RNAs: novel epigenetic regulators. Cancers. 2020;12:2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu S, Wei X, Tao L, Dong D, Hu W, Zhang Q, et al. A novel tRNA-derived fragment tRF-3022b modulates cell apoptosis and M2 macrophage polarization via binding to cytokines in colorectal cancer. J Hematol OncolJ Hematol Oncol. 2022;15:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.G G, H W, W X, Z L, J C, Z D, et al. tRNA derived fragment (tRF)-3009 participates in modulation of IFN-α-induced CD4 + T cell oxidative phosphorylation in lupus patients. J Transl Med [Internet]. 2021 [cited 2024 Jan 26];19. https://pubmed.ncbi.nlm.nih.gov/34256772/ [DOI] [PMC free article] [PubMed]
- 33.J P, Z L, B S, J X, G D, W X, et al. tsRNA-04002 alleviates intervertebral disk degeneration by targeting PRKCA to inhibit apoptosis of nucleus pulposus cells. J Orthop Surg [Internet]. 2023 [cited 2024 Jan 24];18. https://pubmed.ncbi.nlm.nih.gov/37287061/ [DOI] [PMC free article] [PubMed]
- 34.Ja G, My A, Hc B, Tm H. tRNA-derived fragments (tRFs) regulate post-transcriptional gene expression via AGO-dependent mechanism in IL-1β stimulated chondrocytes. Osteoarthritis Cartilage [Internet]. 2020 [cited 2024 Jan 24];28. https://pubmed.ncbi.nlm.nih.gov/32407895/ [DOI] [PMC free article] [PubMed]
- 35.Y Z, R W, Q Q, J Y, H C, L W. Differentially expressed tRNA-derived fragments and their roles in primary cardiomyocytes stimulated by high glucose. Front Endocrinol [Internet]. 2023 [cited 2024 Jan 24];13. https://pubmed.ncbi.nlm.nih.gov/36714586/ [DOI] [PMC free article] [PubMed]
- 36.Zhu J, Wen Y, Zhang Q, Nie F, Cheng M, Zhao X. The monomer TEC of blueberry improves NASH by augmenting tRF-47-mediated autophagy/pyroptosis signaling pathway. J Transl Med. 2022;20:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu S, Chen Y, Ren Y, Zhou J, Ren J, Lee I, et al. A tRNA-derived RNA fragment plays an important role in the mechanism of Arsenite -induced Cellular responses. Sci Rep. 2018;8:16838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cui Y, Huang Y, Wu X, Zheng M, Xia Y, Fu Z, et al. Hypoxia-induced tRNA-derived fragments, novel regulatory factor for doxorubicin resistance in triple-negative breast cancer. J Cell Physiol. 2019;234:8740–51. [DOI] [PubMed] [Google Scholar]
- 39.Fan X-R, Huang Y, Su Y, Chen S-J, Zhang Y-L, Huang W-K, et al. Exploring the regulatory mechanism of tRNA-derived fragments 36 in acute pancreatitis based on small RNA sequencing and experiments. World J Gastroenterol. 2023;29:4642–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pawar K, Shigematsu M, Sharbati S, Kirino Y. Infection-induced 5’-half molecules of tRNAHisGUG activate toll-like receptor 7. PLoS Biol. 2020;18:e3000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.B S, Z C, Q C, Y Z, B G. Endogenous tRNA-derived small RNA (tRF3-Thr-AGT) inhibits ZBP1/NLRP3 pathway-mediated cell pyroptosis to attenuate acute pancreatitis (AP). J Cell Mol Med [Internet]. 2021 [cited 2024 Jan 24];25. https://pubmed.ncbi.nlm.nih.gov/34643045/ [DOI] [PMC free article] [PubMed]
- 42.J C, T H. Cancer-related inflammation. J Clin Immunol [Internet]. 2013 [cited 2024 Jan 24];33 Suppl 1. https://pubmed.ncbi.nlm.nih.gov/23225204/ [DOI] [PubMed]
- 43.Yang J, Xu J, Wang W, Zhang B, Yu X, Shi S. Epigenetic regulation in the tumor microenvironment: molecular mechanisms and therapeutic targets. Signal Transduct Target Ther. 2023;8:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang M, Mo Y, Ren D, Liu S, Zeng Z, Xiong W. Transfer RNA-derived small RNAs in tumor microenvironment. Mol Cancer. 2023;22:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maqueda JJ, Santos M, Ferreira M, Marinho S, Rocha S, Rocha M, et al. NGS data repurposing allows detection of tRNA fragments as gastric Cancer biomarkers in patient-derived extracellular vesicles. Int J Mol Sci. 2023;24:8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.X D, X F, X H, S C, W H, J G, et al. Comprehensively Identifying the Key tRNA-Derived Fragments and Investigating Their Function in Gastric Cancer Processes. OncoTargets Ther [Internet]. 2020 [cited 2024 Jan 24];13. https://pubmed.ncbi.nlm.nih.gov/33149609/ [DOI] [PMC free article] [PubMed]
- 47.Gao H, Zhang Q, Wu W, Gu J, Li J. The diagnostic and prognostic value of tsRNAs in gastric cancers: a systematic review and meta-analysis. Expert Rev Mol Diagn. 2023;23:985–97. [DOI] [PubMed] [Google Scholar]
- 48.Zx LPXH, Y L et al. Y, R L, J Z,. Inflammatory cytokine-regulated tRNA-derived fragment tRF-21 suppresses pancreatic ductal adenocarcinoma progression. J Clin Invest [Internet]. 2021 [cited 2024 Jan 24];131. https://pubmed.ncbi.nlm.nih.gov/34779408/ [DOI] [PMC free article] [PubMed]
- 49.X Z PY, D X AK et al. S C, J Z,. Serum tsRNA as a novel molecular diagnostic biomarker for lupus nephritis. Clin Transl Med [Internet]. 2022 [cited 2024 Jan 24];12. https://pubmed.ncbi.nlm.nih.gov/35593207/ [DOI] [PMC free article] [PubMed]
- 50.Ormseth MJ, Wu Q, Zhao S, Allen RM, Solus J, Sheng Q, et al. Circulating microbial small RNAs are altered in patients with rheumatoid arthritis. Ann Rheum Dis. 2020;79:1557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gong B, Lee YS, Lee I, Shelite TR, Kunkeaw N, Xu G, et al. Compartmentalized, functional role of angiogenin during spotted fever group rickettsia-induced endothelial barrier dysfunction: evidence of possible mediation by host tRNA-derived small noncoding RNAs. BMC Infect Dis. 2013;13:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.G RK, M RSSML et al. R, G M,. Identification of urinary exosomal noncoding RNAs as novel biomarkers in chronic kidney disease. RNA N Y N [Internet]. 2017 [cited 2024 Jan 24];23. https://pubmed.ncbi.nlm.nih.gov/27872161/ [DOI] [PMC free article] [PubMed]
- 53.Selitsky SR, Baran-Gale J, Honda M, Yamane D, Masaki T, Fannin EE, et al. Small tRNA-derived RNAs are increased and more abundant than microRNAs in chronic hepatitis B and C. Sci Rep. 2015;5:7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.F Z ZH, Z KJBL et al. W, G Y,. Complement C3 activation regulates the production of tRNA-derived fragments Gly-tRFs and promotes alcohol-induced liver injury and steatosis. Cell Res [Internet]. 2019 [cited 2024 Jan 24];29. https://pubmed.ncbi.nlm.nih.gov/31076642/ [DOI] [PMC free article] [PubMed]
- 55.Zhu J, Cheng M, Zhao X. A tRNA-derived fragment (tRF-3001b) aggravates the development of nonalcoholic fatty liver disease by inhibiting autophagy. Life Sci. 2020;257:118125. [DOI] [PubMed] [Google Scholar]
- 56.Yz XL, Zl W, Jh HXS, Yh W et al. L,. SARS-CoV-2 causes a significant stress response mediated by small RNAs in the blood of COVID-19 patients. Mol Ther Nucleic Acids [Internet]. 2022 [cited 2024 Jan 24];27. https://pubmed.ncbi.nlm.nih.gov/35003892/ [DOI] [PMC free article] [PubMed]
- 57.Chu X, He C, Sang B, Yang C, Yin C, Ji M, et al. Transfer RNAs-derived small RNAs and their application potential in multiple diseases. Front Cell Dev Biol. 2022;10:954431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Magee R, Rigoutsos I. On the expanding roles of tRNA fragments in modulating cell behavior. Nucleic Acids Res. 2020;48:9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.F J, L Y, W W, N Y, S Z, P Y, et al. A novel class of tsRNA signatures as biomarkers for diagnosis and prognosis of pancreatic cancer. Mol Cancer [Internet]. 2021 [cited 2024 Jan 29];20. https://pubmed.ncbi.nlm.nih.gov/34273975/ [DOI] [PMC free article] [PubMed]
- 60.Weng Q, Wang Y, Xie Y, Yu X, Zhang S, Ge J, et al. Extracellular vesicles-associated tRNA-derived fragments (tRFs): biogenesis, biological functions, and their role as potential biomarkers in human diseases. J Mol Med Berl Ger. 2022;100:679–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.