Abstract
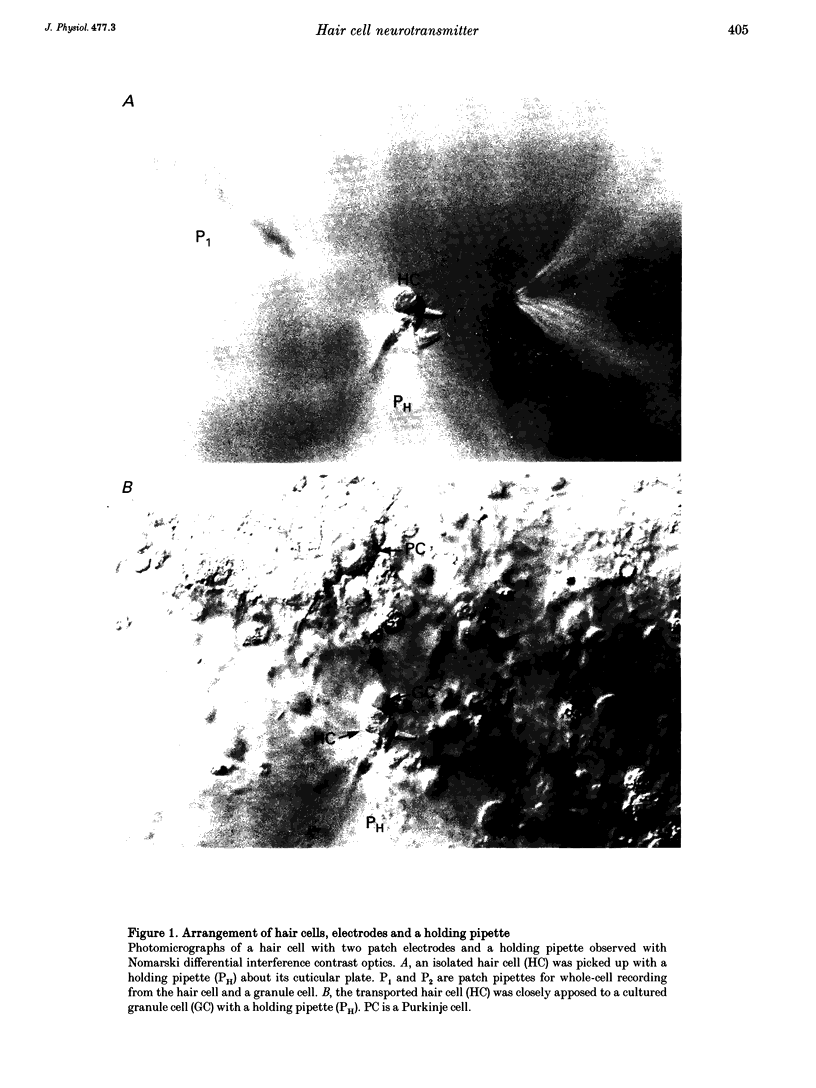
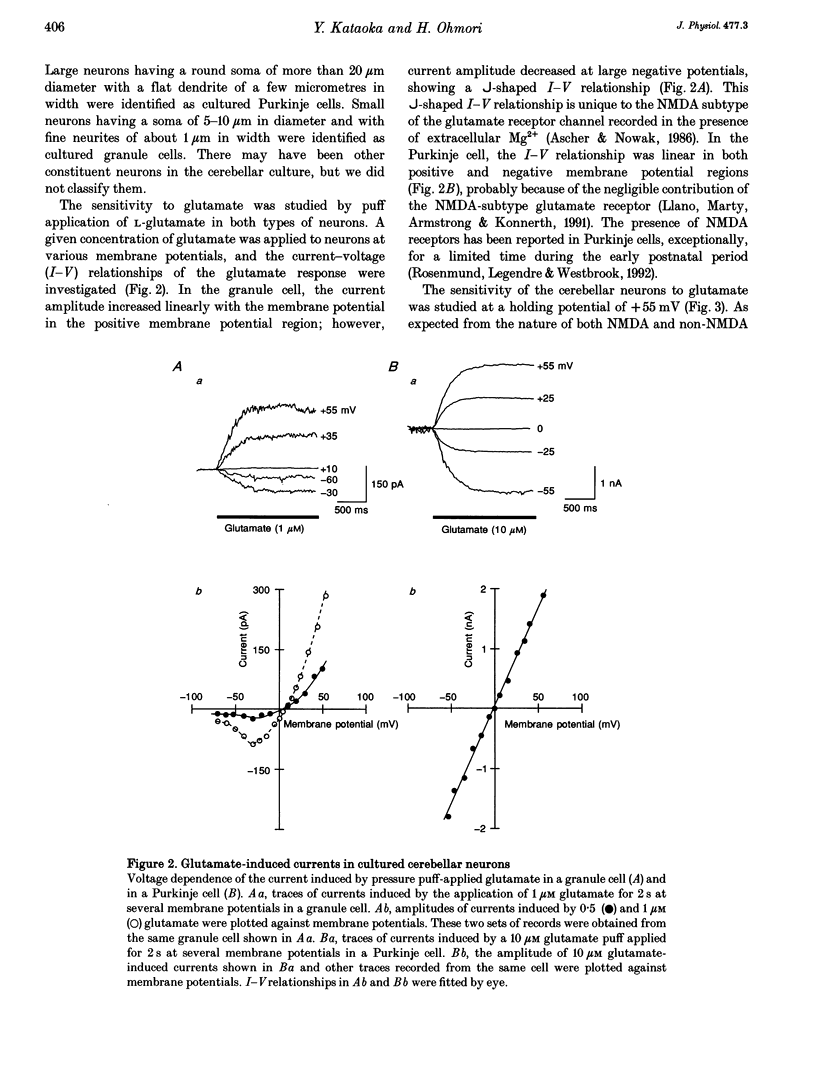
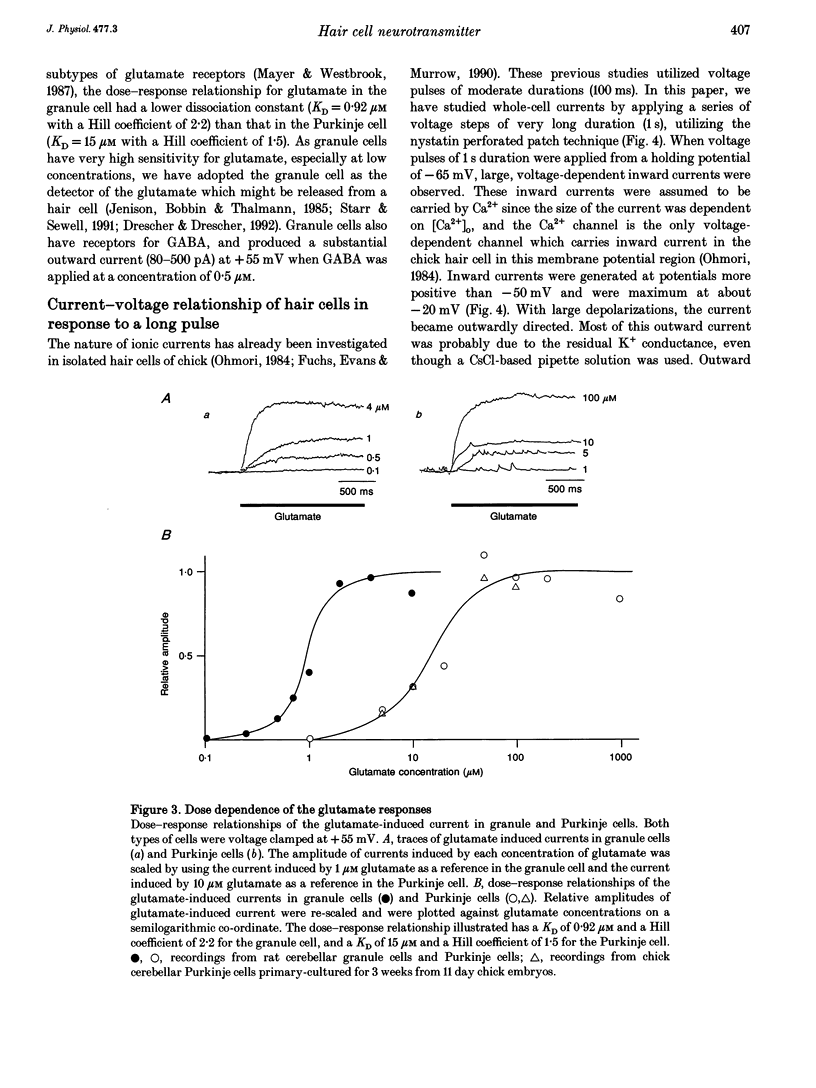
1. Experiments were performed to identify the neurotransmitter released from hair cells of chick cochlea. An isolated hair cell was closely apposed to a cultured granule cell of the rat cerebellum, and both cells were whole-cell voltage clamped by utilizing a nystatin perforated patch technique. 2. Depolarization of hair cells to potentials more positive than -20 mV induced currents in the granule cell in a 10 mM Ca2+ extracellular medium. Amplitudes of induced currents were dependent on the membrane potential of granule cells and showed an outward-going rectification. The induced current in granule cells was reversibly suppressed by a local application of 2-amino-5-phosphonovalerate (APV), which indicates that the current was generated through the activation of an NMDA subtype of the glutamate receptor expressed on the granule cell. 3. The current amplitude of the granule cell was dependent on the size of hair cell depolarization. The size of current induced in a granule cell held at +55 mV was progressively increased with hair cell depolarization from -20 to +10 mV. At more positive potentials, the current amplitude was decreased. This voltage dependence was similar to but did not exactly match that of Ca2+ currents in the hair cell. The granule cell current appeared at more positive membrane potentials than the Ca2+ current in hair cells. 4. When intracellular Ca2+ concentration was increased by UV irradiation of the hair cell loaded with a caged Ca2+ compound, nitr-5, the closely apposed granule cell generated an outward current when voltage clamped at +55 mV. 5. These observations (paragraphs 2-4) imply that the most likely neurotransmitter released from the hair cell at its synapse with the afferent nerve terminal is glutamate.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler E. M., Augustine G. J., Duffy S. N., Charlton M. P. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991 Jun;11(6):1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler R. A., Sheridan C. E., Horn J. W., Wenthold R. J. Immunocytochemical localization of glutamate immunoreactivity in the guinea pig cochlea. Hear Res. 1989 Nov;42(2-3):167–173. doi: 10.1016/0378-5955(89)90142-1. [DOI] [PubMed] [Google Scholar]
- Armstrong D. L., White R. E. An enzymatic mechanism for potassium channel stimulation through pertussis-toxin-sensitive G proteins. Trends Neurosci. 1992 Oct;15(10):403–408. doi: 10.1016/0166-2236(92)90192-b. [DOI] [PubMed] [Google Scholar]
- Ayoub G. S., Korenbrot J. I., Copenhagen D. R. Release of endogenous glutamate from isolated cone photoreceptors of the lizard. Neurosci Res Suppl. 1989;10:S47–S55. doi: 10.1016/0921-8696(89)90008-x. [DOI] [PubMed] [Google Scholar]
- Bobbin R. P., Ceasar G., Fallon M. Potassium induced release of GABA and other substances from the guinea pig cochlea. Hear Res. 1990 Jun;46(1-2):83–93. doi: 10.1016/0378-5955(90)90141-b. [DOI] [PubMed] [Google Scholar]
- Bobbin R. P., Ceasar G. Kynurenic acid and gamma-D-glutamylaminomethylsulfonic acid suppress the compound action potential of the auditory nerve. Hear Res. 1987;25(1):77–81. doi: 10.1016/0378-5955(87)90081-5. [DOI] [PubMed] [Google Scholar]
- Bobbin R. P. Glutamate and aspartate mimic the afferent transmitter in the cochlea. Exp Brain Res. 1979 Jan 15;34(2):389–393. doi: 10.1007/BF00235683. [DOI] [PubMed] [Google Scholar]
- Comis S. D., Leng G. Action of putative neurotransmitters in the guinea pig cochlea. Exp Brain Res. 1979 Jun 1;36(1):119–128. doi: 10.1007/BF00238472. [DOI] [PubMed] [Google Scholar]
- Copenhagen D. R., Jahr C. E. Release of endogenous excitatory amino acids from turtle photoreceptors. Nature. 1989 Oct 12;341(6242):536–539. doi: 10.1038/341536a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Howe J. R., Ogden D. C. Noise and single channels activated by excitatory amino acids in rat cerebellar granule neurones. J Physiol. 1988 Jun;400:189–222. doi: 10.1113/jphysiol.1988.sp017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drescher M. J., Drescher D. G. Glutamate, of the endogenous primary alpha-amino acids, is specifically released from hair cells by elevated extracellular potassium. J Neurochem. 1992 Jul;59(1):93–98. doi: 10.1111/j.1471-4159.1992.tb08879.x. [DOI] [PubMed] [Google Scholar]
- Eybalin M., Pujol R. A radioautographic study of [3H]L-glutamate and [3H]L-glutamine uptake in the guinea-pig cochlea. Neuroscience. 1983 Aug;9(4):863–871. doi: 10.1016/0306-4522(83)90274-9. [DOI] [PubMed] [Google Scholar]
- Fischer G. Cultivation of mouse cerebellar cells in serum free, hormonally defined media: survival of neurons. Neurosci Lett. 1982 Mar 5;28(3):325–329. doi: 10.1016/0304-3940(82)90079-9. [DOI] [PubMed] [Google Scholar]
- Flock A., Lam D. M. Neurotransmitter synthesis in inner ear and lateral line sense organs. Nature. 1974 May 10;249(453):142–144. doi: 10.1038/249142a0. [DOI] [PubMed] [Google Scholar]
- Fuchs P. A., Evans M. G., Murrow B. W. Calcium currents in hair cells isolated from the cochlea of the chick. J Physiol. 1990 Oct;429:553–568. doi: 10.1113/jphysiol.1990.sp018272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Ishii Y. Neurophysiological studies on hearing in goldfish. J Neurophysiol. 1967 Nov;30(6):1377–1403. doi: 10.1152/jn.1967.30.6.1377. [DOI] [PubMed] [Google Scholar]
- Hirano T., Kubo Y., Wu M. M. Cerebellar granule cells in culture: monosynaptic connections with Purkinje cells and ionic currents. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4957–4961. doi: 10.1073/pnas.83.13.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A. J. Mechanoelectrical transduction by hair cells in the acousticolateralis sensory system. Annu Rev Neurosci. 1983;6:187–215. doi: 10.1146/annurev.ne.06.030183.001155. [DOI] [PubMed] [Google Scholar]
- Jenison G. L., Bobbin R. P., Thalmann R. Potassium-induced release of endogenous amino acids in the guinea pig cochlea. J Neurochem. 1985 Jun;44(6):1845–1853. doi: 10.1111/j.1471-4159.1985.tb07178.x. [DOI] [PubMed] [Google Scholar]
- Kimitsuki T., Ohmori H. The effect of caged calcium release on the adaptation of the transduction current in chick hair cells. J Physiol. 1992 Dec;458:27–40. doi: 10.1113/jphysiol.1992.sp019404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I., Marty A., Armstrong C. M., Konnerth A. Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J Physiol. 1991 Mar;434:183–213. doi: 10.1113/jphysiol.1991.sp018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López I., Wu J. Y., Meza G. Immunocytochemical evidence for an afferent GABAergic neurotransmission in the guinea pig vestibular system. Brain Res. 1992 Sep 4;589(2):341–348. doi: 10.1016/0006-8993(92)91297-r. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Mroz E. A., Sewell W. F. Pharmacological alterations of the activity of afferent fibers innervating hair cells. Hear Res. 1989 Mar;38(1-2):141–162. doi: 10.1016/0378-5955(89)90136-6. [DOI] [PubMed] [Google Scholar]
- Ohmori H. Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J Physiol. 1985 Feb;359:189–217. doi: 10.1113/jphysiol.1985.sp015581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. Studies of ionic currents in the isolated vestibular hair cell of the chick. J Physiol. 1984 May;350:561–581. doi: 10.1113/jphysiol.1984.sp015218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios J. M., Young W. S., 3rd, Kuhar M. J. Autoradiographic localization of gamma-aminobutyric acid (GABA) receptors in the rat cerebellum. Proc Natl Acad Sci U S A. 1980 Jan;77(1):670–674. doi: 10.1073/pnas.77.1.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel J. L., Ladrech S., Chabert R., Pujol R., Eybalin M. Electrophysiological evidence for the presence of NMDA receptors in the guinea pig cochlea. Hear Res. 1991 Feb;51(2):255–264. doi: 10.1016/0378-5955(91)90042-8. [DOI] [PubMed] [Google Scholar]
- Roberts W. M., Jacobs R. A., Hudspeth A. J. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990 Nov;10(11):3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C., Legendre P., Westbrook G. L. Expression of NMDA channels on cerebellar Purkinje cells acutely dissociated from newborn rats. J Neurophysiol. 1992 Nov;68(5):1901–1905. doi: 10.1152/jn.1992.68.5.1901. [DOI] [PubMed] [Google Scholar]
- Ryan A. F., Schwartz I. R. Preferential glutamine uptake by cochlear hair cells: implications for the afferent cochlear transmitter. Brain Res. 1984 Jan 9;290(2):376–379. doi: 10.1016/0006-8993(84)90960-0. [DOI] [PubMed] [Google Scholar]
- Sewell W. F., Norris C. H., Tachibana M., Guth P. S. Detection of an auditory nerve--activating substance. Science. 1978 Nov 24;202(4370):910–912. doi: 10.1126/science.30998. [DOI] [PubMed] [Google Scholar]
- Starr P. A., Sewell W. F. Neurotransmitter release from hair cells and its blockade by glutamate-receptor antagonists. Hear Res. 1991 Mar;52(1):23–41. doi: 10.1016/0378-5955(91)90185-c. [DOI] [PubMed] [Google Scholar]
- Usami S., Igarashi M., Thompson G. C. GABA-like immunoreactivity in the chick basilar papilla and the lagenar macula. Hear Res. 1987;30(1):19–22. doi: 10.1016/0378-5955(87)90178-x. [DOI] [PubMed] [Google Scholar]
- Weber A., Schachner M. Maintenance of immunocytologically identified Purkinje cells from mouse cerebellum in monolayer culture. Brain Res. 1984 Oct 8;311(1):119–130. doi: 10.1016/0006-8993(84)91404-5. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Ohmori H. Voltage-gated and chemically gated ionic channels in the cultured cochlear ganglion neurone of the chick. J Physiol. 1990 Jan;420:185–206. doi: 10.1113/jphysiol.1990.sp017907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M., Ohmori H. Synaptic responses to mechanical stimulation in calyceal and bouton type vestibular afferents studied in an isolated preparation of semicircular canal ampullae of chicken. Exp Brain Res. 1990;80(3):475–488. doi: 10.1007/BF00227989. [DOI] [PubMed] [Google Scholar]



