Abstract
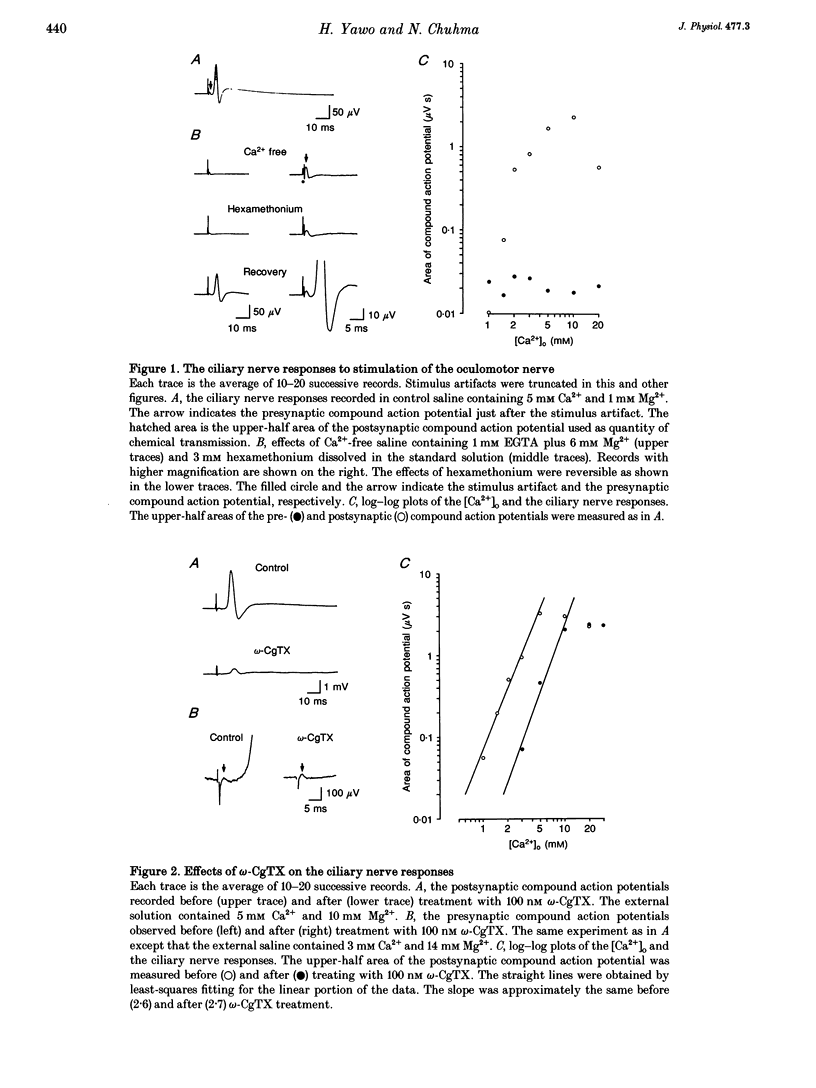
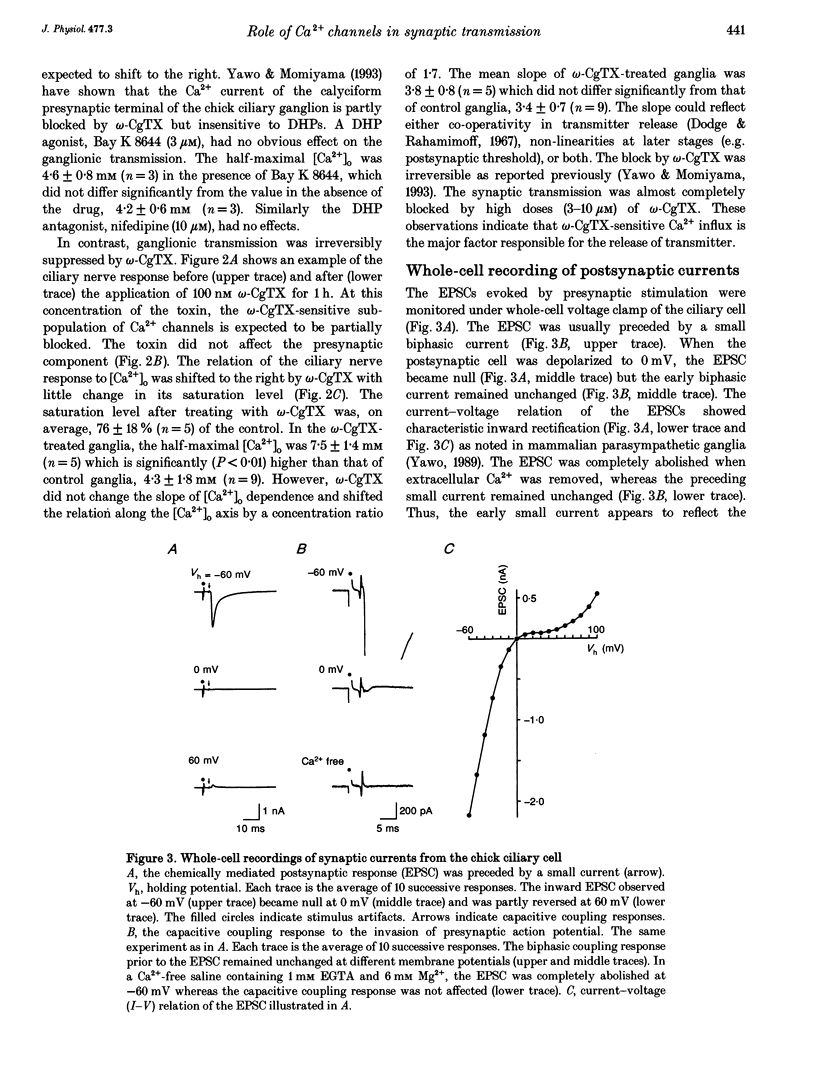
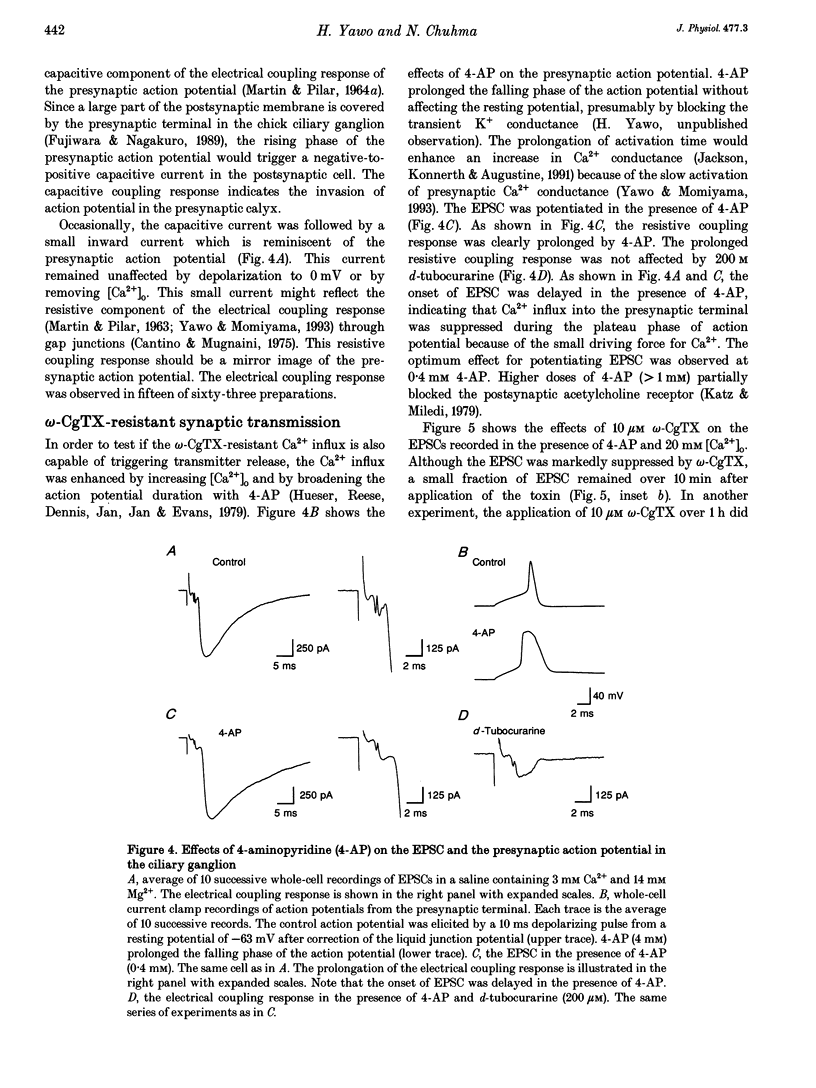
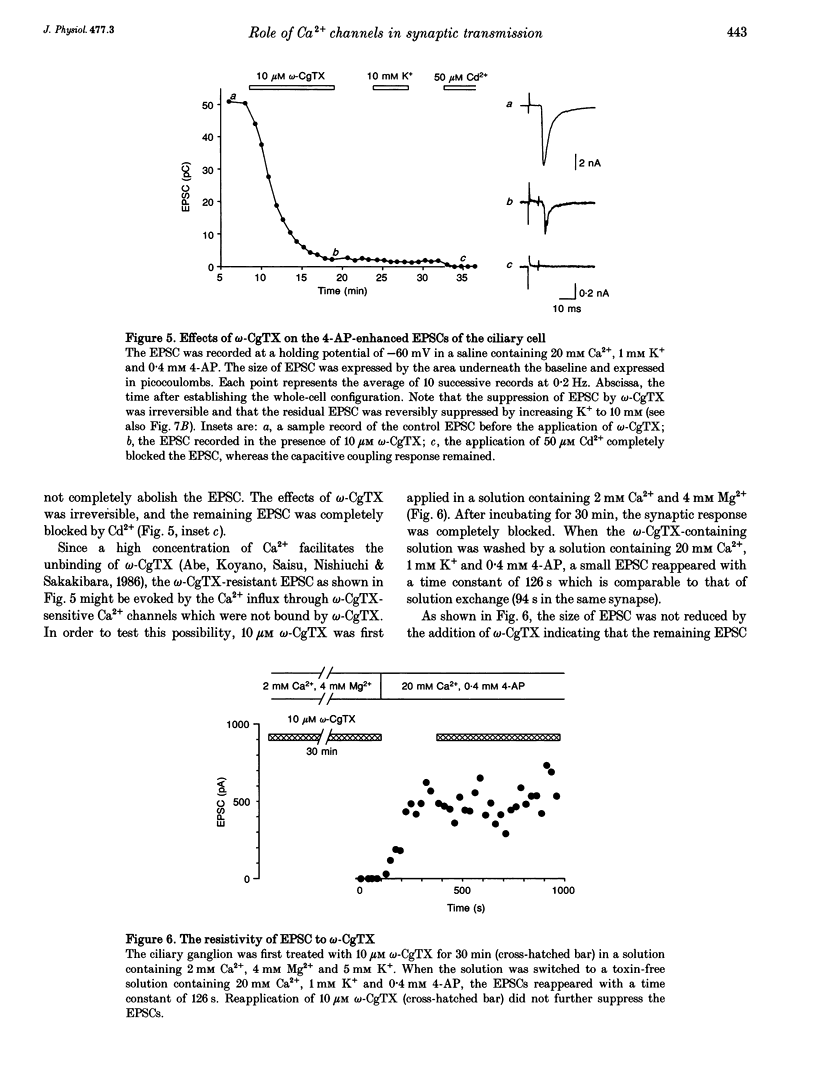
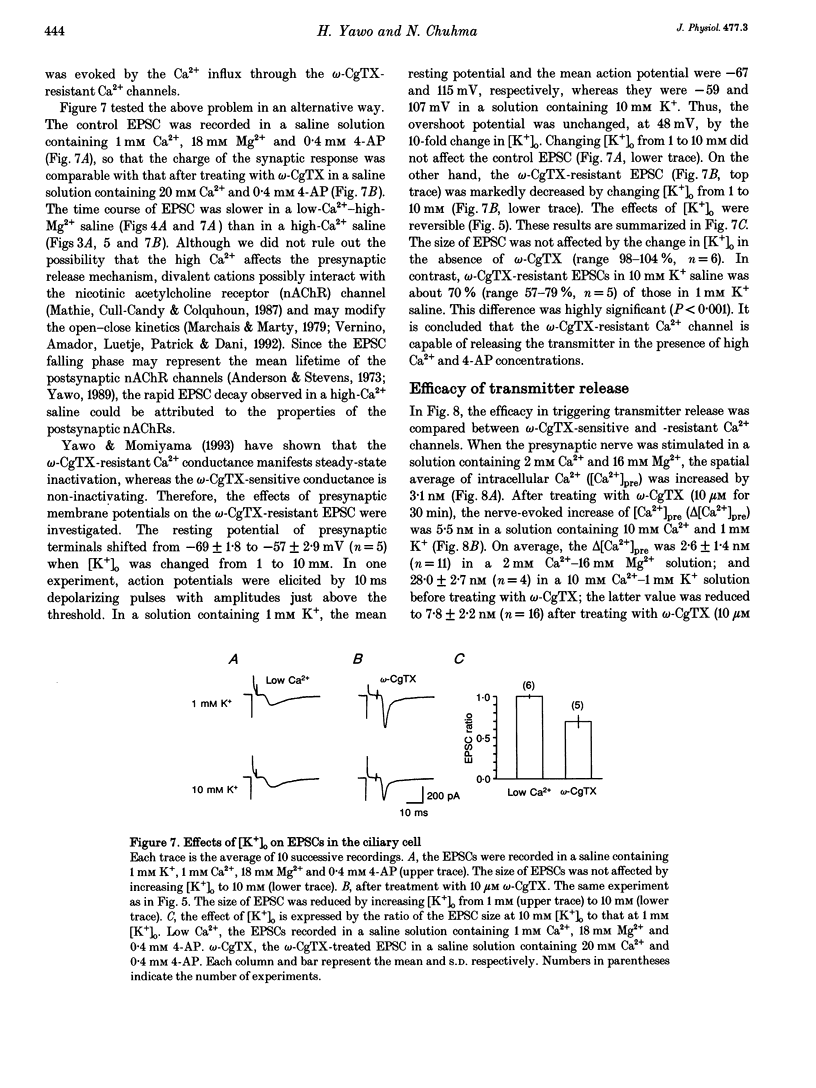
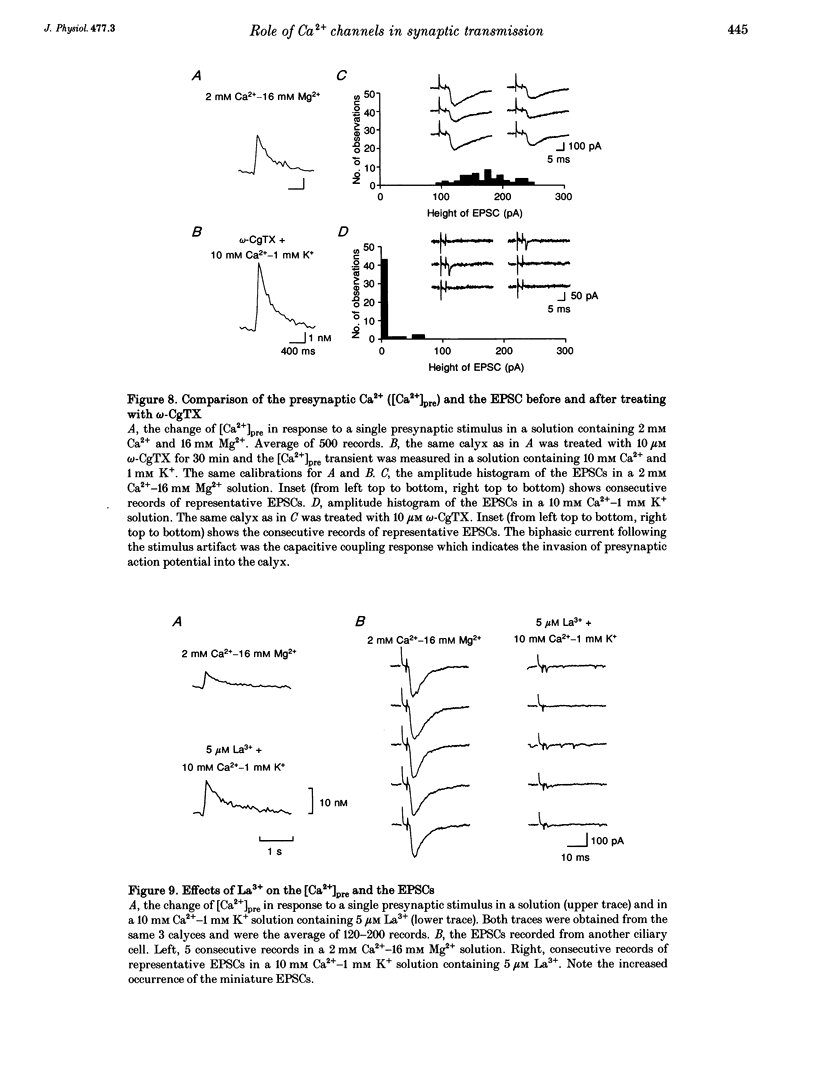
1. Synaptically evoked responses to stimulation of the oculomotor nerve were recorded from the ciliary nerve in chick embryos. The postsynaptic currents in response to presynaptic stimulation (EPSCs) were also recorded under whole-cell voltage clamp of the ciliary cell. 2. The ciliary nerve response was dependent on the extracellular Ca2+ concentration ([Ca2+]o). omega-Conotoxin GVIA (omega-CgTX, 100 nM) increased the [Ca2+]o necessary to evoke the half-maximal response by a factor of 1.7 without changing the slope of [Ca2+]o dependence. Dihydropyridine (DHP) derivatives, nifedipine or Bay K 8644, did not affect the [Ca2+]o sensitivity of ciliary nerve response. 3. The EPSC was usually preceded by the capacitive coupling response of the presynaptic action potential. In some records, the EPSCs were also preceded by the electrical coupling responses which were the mirror images of the presynaptic action potentials. The current-voltage relation of the EPSCs showed inward rectification. 4. The EPSC was potentiated by 4-aminopyridine (4-AP) as a result of prolongation of the falling phase of presynaptic action potential. In the presence of high [Ca2+]o and 4-AP, a small fraction of EPSC was resistant to omega-CgTX. 5. The resting potential of the presynaptic terminal was changed from -69 to -57 mV by increasing [K+]o from 1 to 10 mM. The same procedure decreased the omega-CgTX-resistant EPSC by 30%, whereas the omega-CgTX-untreated EPSC in low-Ca2+ saline was not affected by the change in [K+]o. 6. The nerve-evoked increase in intracellular Ca2+ was recorded from the presynaptic terminal (delta[Ca2+]pre). The delta[Ca2+]pre was larger in a solution containing 10 mM Ca2+ and 1 mM K+ after treating with omega-CgTX than in a solution containing 2 mM Ca2+ and 16 mM Mg2+ before treating with omega-CgTX. The EPSC was, in contrast, smaller in the 10 mM Ca(2+)-1 mM K+ solution after omega-CgTX treatment than in the 2 mM Ca(2+)-16 mM Mg2+ solution before omega-CgTX treatment. 7. Similarly, the EPSC was smaller in the 10 mM Ca(2+)-1 mM K+ solution containing 5 microM La3+ than in the 2 mM Ca(2+)-16 mM Mg2+ solution, whereas the delta [Ca2+]pre was larger in the 10 mM Ca(2+)-1 mM K+ solution containing 5 micrograms La3+ than in the 2 mM Ca(2+)-16 mM Mg2+ solution. 8. It is concluded that the omega-CgTX-sensitive Ca2+ conductance of the presynaptic terminal is the principal source of Ca2+ involved in transmitter release.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Koyano K., Saisu H., Nishiuchi Y., Sakakibara S. Binding of omega-conotoxin to receptor sites associated with the voltage-sensitive calcium channel. Neurosci Lett. 1986 Nov 11;71(2):203–208. doi: 10.1016/0304-3940(86)90559-8. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium action in synaptic transmitter release. Annu Rev Neurosci. 1987;10:633–693. doi: 10.1146/annurev.ne.10.030187.003221. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Ho S. Adenosine modulation of potassium currents in preganglionic nerve terminals of avian ciliary ganglia. Neurosci Lett. 1992 Mar 16;137(1):41–44. doi: 10.1016/0304-3940(92)90293-g. [DOI] [PubMed] [Google Scholar]
- Cantino D., Mugnaini E. The structural basis for electrotonic coupling in the avian ciliary ganglion. A study with thin sectioning and freeze-fracturing. J Neurocytol. 1975 Oct;4(5):505–536. doi: 10.1007/BF01351535. [DOI] [PubMed] [Google Scholar]
- Cohen M. W., Jones O. T., Angelides K. J. Distribution of Ca2+ channels on frog motor nerve terminals revealed by fluorescent omega-conotoxin. J Neurosci. 1991 Apr;11(4):1032–1039. doi: 10.1523/JNEUROSCI.11-04-01032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis M. J., Quastel D. M., Saint D. A. Lanthanum as a surrogate for calcium in transmitter release at mouse motor nerve terminals. J Physiol. 1986 Apr;373:243–260. doi: 10.1113/jphysiol.1986.sp016045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayanithi G., Martin-Moutot N., Barlier S., Colin D. A., Kretz-Zaepfel M., Couraud F., Nordmann J. J. The calcium channel antagonist omega-conotoxin inhibits secretion from peptidergic nerve terminals. Biochem Biophys Res Commun. 1988 Oct 14;156(1):255–262. doi: 10.1016/s0006-291x(88)80833-7. [DOI] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Nagakuro C. Three-dimensional structure of the presynaptic nerve ending in the ciliary ganglion of the chick embryo: a scanning electron microscopic study. Neurosci Lett. 1989 Mar 27;98(2):125–128. doi: 10.1016/0304-3940(89)90496-5. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirning L. D., Fox A. P., McCleskey E. W., Olivera B. M., Thayer S. A., Miller R. J., Tsien R. W. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988 Jan 1;239(4835):57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- Holz G. G., 4th, Dunlap K., Kream R. M. Characterization of the electrically evoked release of substance P from dorsal root ganglion neurons: methods and dihydropyridine sensitivity. J Neurosci. 1988 Feb;8(2):463–471. doi: 10.1523/JNEUROSCI.08-02-00463.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Konnerth A., Augustine G. J. Action potential broadening and frequency-dependent facilitation of calcium signals in pituitary nerve terminals. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):380–384. doi: 10.1073/pnas.88.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Estimates of quantal content during 'chemical potentiation' of transmitter release. Proc R Soc Lond B Biol Sci. 1979 Aug 31;205(1160):369–378. doi: 10.1098/rspb.1979.0070. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The release of acetylcholine from nerve endings by graded electric pulses. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):23–38. doi: 10.1098/rspb.1967.0011. [DOI] [PubMed] [Google Scholar]
- Kuno M., Weakly J. N. Quantal components of the inhibitory synaptic potential in spinal mononeurones of the cat. J Physiol. 1972 Jul;224(2):287–303. doi: 10.1113/jphysiol.1972.sp009895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan C. Y., Putney J. W., Jr Uptake and intracellular sequestration of divalent cations in resting and methacholine-stimulated mouse lacrimal acinar cells. Dissociation by Sr2+ and Ba2+ of agonist-stimulated divalent cation entry from the refilling of the agonist-sensitive intracellular pool. J Biol Chem. 1990 Jan 15;265(2):678–684. [PubMed] [Google Scholar]
- Landmesser L., Pilar G. The onset and development of transmission in the chick ciliary ganglion. J Physiol. 1972 May;222(3):691–713. doi: 10.1113/jphysiol.1972.sp009822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos J. R., Nowycky M. C. Two types of calcium channels coexist in peptide-releasing vertebrate nerve terminals. Neuron. 1989 May;2(5):1419–1426. doi: 10.1016/0896-6273(89)90187-6. [DOI] [PubMed] [Google Scholar]
- Lindgren C. A., Moore J. W. Identification of ionic currents at presynaptic nerve endings of the lizard. J Physiol. 1989 Jul;414:201–222. doi: 10.1113/jphysiol.1989.sp017684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. AN ANALYSIS OF ELECTRICAL COUPLING AT SYNAPSES IN THE AVIAN CILIARY GANGLION. J Physiol. 1964 Jun;171:454–475. doi: 10.1113/jphysiol.1964.sp007390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. DUAL MODE OF SYNAPTIC TRANSMISSION IN THE AVIAN CILIARY GANGLION. J Physiol. 1963 Sep;168:443–463. doi: 10.1113/jphysiol.1963.sp007202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. QUANTAL COMPONENTS OF THE SYNAPTIC POTENTIAL IN THE CILIARY GANGLION OF THE CHICK. J Physiol. 1964 Dec;175:1–16. doi: 10.1113/jphysiol.1964.sp007499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchais D., Marty A. Interaction of permeant ions with channels activated by acetylcholine in Aplysia neurones. J Physiol. 1979 Dec;297(0):9–45. doi: 10.1113/jphysiol.1979.sp013025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwitt R., Pilar G., Weakly J. N. Characterization of two ganglion cell populations in avian ciliary ganglia. Brain Res. 1971 Jan 22;25(2):317–334. doi: 10.1016/0006-8993(71)90441-0. [DOI] [PubMed] [Google Scholar]
- Mathie A., Cull-Candy S. G., Colquhoun D. Single-channel and whole-cell currents evoked by acetylcholine in dissociated sympathetic neurons of the rat. Proc R Soc Lond B Biol Sci. 1987 Nov 23;232(1267):239–248. doi: 10.1098/rspb.1987.0072. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., Gray W. R., Zeikus R., McIntosh J. M., Varga J., Rivier J., de Santos V., Cruz L. J. Peptide neurotoxins from fish-hunting cone snails. Science. 1985 Dec 20;230(4732):1338–1343. doi: 10.1126/science.4071055. [DOI] [PubMed] [Google Scholar]
- Perney T. M., Hirning L. D., Leeman S. E., Miller R. J. Multiple calcium channels mediate neurotransmitter release from peripheral neurons. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6656–6659. doi: 10.1073/pnas.83.17.6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Voyvodic J. T., Magrassi L., Yawo H. Nerve terminal remodeling visualized in living mice by repeated examination of the same neuron. Science. 1987 Nov 20;238(4830):1122–1126. doi: 10.1126/science.3685967. [DOI] [PubMed] [Google Scholar]
- Rittenhouse A. R., Zigmond R. E. Omega-conotoxin inhibits the acute activation of tyrosine hydroxylase and the stimulation of norepinephrine release by potassium depolarization of sympathetic nerve endings. J Neurochem. 1991 Feb;56(2):615–622. doi: 10.1111/j.1471-4159.1991.tb08194.x. [DOI] [PubMed] [Google Scholar]
- Robitaille R., Adler E. M., Charlton M. P. Strategic location of calcium channels at transmitter release sites of frog neuromuscular synapses. Neuron. 1990 Dec;5(6):773–779. doi: 10.1016/0896-6273(90)90336-e. [DOI] [PubMed] [Google Scholar]
- Sano K., Enomoto K., Maeno T. Effects of synthetic omega-conotoxin, a new type Ca2+ antagonist, on frog and mouse neuromuscular transmission. Eur J Pharmacol. 1987 Sep 11;141(2):235–241. doi: 10.1016/0014-2999(87)90268-8. [DOI] [PubMed] [Google Scholar]
- Smith S. J., Augustine G. J. Calcium ions, active zones and synaptic transmitter release. Trends Neurosci. 1988 Oct;11(10):458–464. doi: 10.1016/0166-2236(88)90199-3. [DOI] [PubMed] [Google Scholar]
- Stanley E. F., Atrakchi A. H. Calcium currents recorded from a vertebrate presynaptic nerve terminal are resistant to the dihydropyridine nifedipine. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9683–9687. doi: 10.1073/pnas.87.24.9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. F., Goping G. Characterization of a calcium current in a vertebrate cholinergic presynaptic nerve terminal. J Neurosci. 1991 Apr;11(4):985–993. doi: 10.1523/JNEUROSCI.11-04-00985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. F. Single calcium channels on a cholinergic presynaptic nerve terminal. Neuron. 1991 Oct;7(4):585–591. doi: 10.1016/0896-6273(91)90371-6. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Momiyama A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993 Nov 11;366(6451):156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Ellinor P. T., Horne W. A. Molecular diversity of voltage-dependent Ca2+ channels. Trends Pharmacol Sci. 1991 Sep;12(9):349–354. doi: 10.1016/0165-6147(91)90595-j. [DOI] [PubMed] [Google Scholar]
- Vernino S., Amador M., Luetje C. W., Patrick J., Dani J. A. Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron. 1992 Jan;8(1):127–134. doi: 10.1016/0896-6273(92)90114-s. [DOI] [PubMed] [Google Scholar]
- Wang X., Treistman S. N., Lemos J. R. Two types of high-threshold calcium currents inhibited by omega-conotoxin in nerve terminals of rat neurohypophysis. J Physiol. 1992 Jan;445:181–199. doi: 10.1113/jphysiol.1992.sp018919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawo H., Chuhma N. Preferential inhibition of omega-conotoxin-sensitive presynaptic Ca2+ channels by adenosine autoreceptors. Nature. 1993 Sep 16;365(6443):256–258. doi: 10.1038/365256a0. [DOI] [PubMed] [Google Scholar]
- Yawo H., Momiyama A. Re-evaluation of calcium currents in pre- and postsynaptic neurones of the chick ciliary ganglion. J Physiol. 1993 Jan;460:153–172. doi: 10.1113/jphysiol.1993.sp019464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawo H. Rectification of synaptic and acetylcholine currents in the mouse submandibular ganglion cells. J Physiol. 1989 Oct;417:307–322. doi: 10.1113/jphysiol.1989.sp017803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikami D., Bagabaldo Z., Olivera B. M. The inhibitory effects of omega-conotoxins on Ca channels and synapses. Ann N Y Acad Sci. 1989;560:230–248. doi: 10.1111/j.1749-6632.1989.tb24100.x. [DOI] [PubMed] [Google Scholar]


