Abstract
Background
Essential amino acid (EAA) and omega-3 fatty acid ingestion independently attenuate leg skeletal muscle disuse atrophy in uninjured persons. However, no data exist regarding the effectiveness of combined EAA and omega-3 fatty acid ingestion to mitigate skeletal muscle disuse atrophy in response to anterior cruciate ligament reconstruction (ACLR) surgery. This pilot trial will explore the feasibility of recruitment and retention of ACLR outpatients from a single center across 18 months to consume either a combination of omega-3 fatty acids and EAAs, or a placebo control, for 4 weeks before and 2 weeks after surgery.
Methods
Thirty adult (≥ 18 years old) ACLR outpatients will be recruited for this single center, double-blind, two-arm randomized controlled feasibility pilot trial. Participants will consume either 5 g⋅day−1 of omega-3 fatty acids (fish oil) and 40 g⋅day−1 of EAAs or 5 g⋅day−1 of a control fatty acid mixture (safflower oil) and 40 g⋅day−1 of non-essential amino acids (NEAAs). Fatty acid supplements will be consumed 4 weeks before and for 2 weeks after ACLR surgery, whereas the EAAs and NEAAs will be consumed 1 week before and for 2 weeks after ACLR surgery. The primary outcomes are feasibility of recruitment and retention, with the goal to recruit 30 outpatients across 18 months and retain 22 participants upon completion of the study protocol following 12 weeks of data collection. These results will be reported using descriptive statistics, along with reasons and timepoints for study dropout. Secondary exploratory outcomes will be reported using inferential statistics for purposes of hypothesis generation and elucidation of mechanistic targets for future work; no inferences to clinical efficacy will be made. These outcomes include integrated rates of skeletal muscle protein synthesis, skeletal muscle protein content and expression of translation factors, skeletal muscle and erythrocyte phospholipid composition, and measures of skeletal muscle mass, strength, and power.
Impact
This work will set the foundation for a future randomized controlled trial powered to detect an effect of EAA + omega-3 fatty acid intake on skeletal muscle size or function in response to ACLR surgery.
Trial registration
ClinicalTrials.gov, NCT06233825. Registered 31 January 2024. https://clinicaltrials.gov/study/NCT06233825?term=NCT06233825&rank=1
Supplementary Information
The online version contains supplementary material available at 10.1186/s40814-024-01561-w.
Keywords: Anterior cruciate ligament, Omega-3 fatty acids, Essential amino acids, Magnetic resonance imaging, Muscle protein synthesis
Background
Over their lifespan, many individuals experience episodes of skeletal muscle disuse due to illness, injury, or elective surgery [1, 2]. Systematic reviews and meta-analyses show skeletal muscle disuse results in loss of skeletal muscle mass (atrophy) [3–5] that precipitates several metabolic effects such as the onset of insulin resistance [6], a reduction in basal metabolic rate [7], and the accrual of body fat [8]. Reduced skeletal muscle mass is also associated with increased hospital length of stay [9, 10], risk of falls [11], hospital readmission, and poor quality of life [12, 13], as well as all-cause mortality [14]. One clinically relevant scenario of skeletal muscle disuse is anterior cruciate ligament reconstruction (ACLR) surgery. Like other surgical populations, ACLR outpatients experience significant loss of skeletal muscle mass and strength [15–17]. Since the early 1980s, incidence rates of ACLR surgery have increased, with ~ 160,000 ACLR surgeries performed per year between Canada and the USA [18]. ACLR surgery affects a broad range of age demographics, with peak ACLR growth rates demonstrating a bimodal distribution between young (< 30 years) and older adults (> 50 years) [19]. Critically, up to half of ACLR outpatients fail to regain complete skeletal muscle function and strength symmetry between the lower limbs at 6 months, 12 months, and even several years after surgery [16, 17]. Failure to fully recover losses of skeletal muscle function and strength in response to ACLR surgery increases risk of re-injury and disability in later life [11–13]. Thus, strategies that mitigate skeletal muscle disuse atrophy in response to, and enhance recovery from, ACLR surgery would have significant clinical impact.
Resistance exercise is one strategy to mitigate skeletal muscle disuse atrophy and is central to many rehabilitation programs following injury. However, for most ACLR outpatients, resistance exercise is neither safe nor feasible in the immediate post-surgical period (< 2 weeks), during which the highest rates of skeletal muscle mass and strength loss occur [4]. In the absence of resistance exercise, nutritional intervention is a strategy to combat skeletal muscle disuse atrophy. In this regard, Dreyer and colleagues reported that 3 weeks of 20 g⋅day−1 essential amino acid (EAA) supplementation for 1 week before and 2 weeks after total knee arthroplasty surgery attenuated skeletal muscle atrophy and enhanced recovery of functional mobility in older adults [20]. Similarly, thrice daily ingestion of an EAA supplement (23.7 g⋅serving−1) fortified with leucine (4 g⋅serving−1) 1 week before and 2 weeks after 1 week of single-leg immobilization attenuated declines in skeletal muscle mass in healthy young men [21].
It is not only EAAs that possess anticatabolic potential. We previously reported that supplementation with 5 g⋅day−1 of omega-3 fatty acids for 2 weeks before and 2 weeks during single-leg immobilization in healthy, young women successfully attenuated declines in quadriceps mass.22 The decline in quadriceps mass was linked to higher integrated (fed + fasted) rates of muscle protein synthesis in the omega-3 fatty acid group compared to control. This finding [22] complements previous work in which supplementation with omega-3 fatty acids potentiated muscle protein synthesis rates in response to a hyperaminoacidemic-hyperinsulinemic clamp, but not fasting rates in healthy younger, middle-aged, and older adults [23, 24]. Interestingly, this same group went on to show that provision of ~ 3.75 g⋅day−1 of omega-3 fatty acids for 6 months improved skeletal muscle strength and power in older women and men [25]. Taken together with the anticatabolic actions of EAAs [20, 21], these data [23, 25] suggest that omega-3 fatty acids and EAAs may act synergistically to enhance muscle anabolism and could be an effective strategy to combat skeletal muscle disuse atrophy following ACLR surgery.
A logical next step would be to perform a randomized controlled trial (RCT) to test whether combined ingestion of EAAs and omega-3 fatty acids protect against the loss, and promote recovery, of skeletal muscle mass and strength in response to ACLR surgery. However, RCTs of this nature are complex, with success dependent on a variety of factors such as effective recruitment and retention of participants as well as execution of proposed methods. It is therefore recommended that researchers perform a pilot study prior to embarking on a large-scale RCT to increase the likelihood of success in the main trial [26, 27]. Pilot trials are an ideal way to gather critical information related to feasibility, participant recruitment, participant retention, and increase the experience of the research team [26]. Additionally, pilot trials enable the collection of site-specific data that can be used to inform statistical power calculations for a larger RCT.
The aim of the present pilot study is to investigate the feasibility of recruitment and retention of ACLR outpatients from a single center across 18 months to consume either a combination of omega-3 fatty acids and EAAs, or a placebo control of safflower oil and NEAAs, for 4 weeks before and 2 weeks after ACLR surgery. Explicit criteria for success are to recruit 30 ACLR outpatients across 18 months and retain 22 ACLR outpatients for 12 weeks of data collection trials. Due to the novelty of the present combined nutritional intervention and population of interest, secondary exploratory analyses will be employed to identify possible mechanisms of interest that will help generate hypotheses and inform the selection of primary outcomes for future clinical trials. These outcomes include integrated rates of skeletal muscle protein synthesis, skeletal muscle protein content and expression of translation factors, skeletal muscle and erythrocyte phospholipid composition, and measures of skeletal muscle mass, strength, and power.
We envisage that the data generated from this trial will inform a larger RCT that will examine the effect of the combined EAAs and omega-3 fatty acids on skeletal muscle mass and functional outcomes in response to ACLR. If successful, these findings may inform nutritional strategies to enhance recovery of skeletal muscle mass and function in ACLR outpatients following surgery. The development of viable and accessible strategies to combat skeletal muscle disuse becomes increasingly important as the Canadian health care system shifts its model towards day surgeries and outpatient settings as opposed to in-hospital care, placing increased emphasis on patient-led recovery [28].
Methods
Study setting
This single-site study will be conducted at the Kingston Health Sciences Centre (KHSC) in Kingston, ON, which is home to the Queen’s University School of Medicine, as well as two hospitals (Kingston General Hospital; Hotel Dieu Hospital). Clinicians work between both hospitals, and the orthopedic clinic and facilities are overseen by the same surgeons.
Trial design
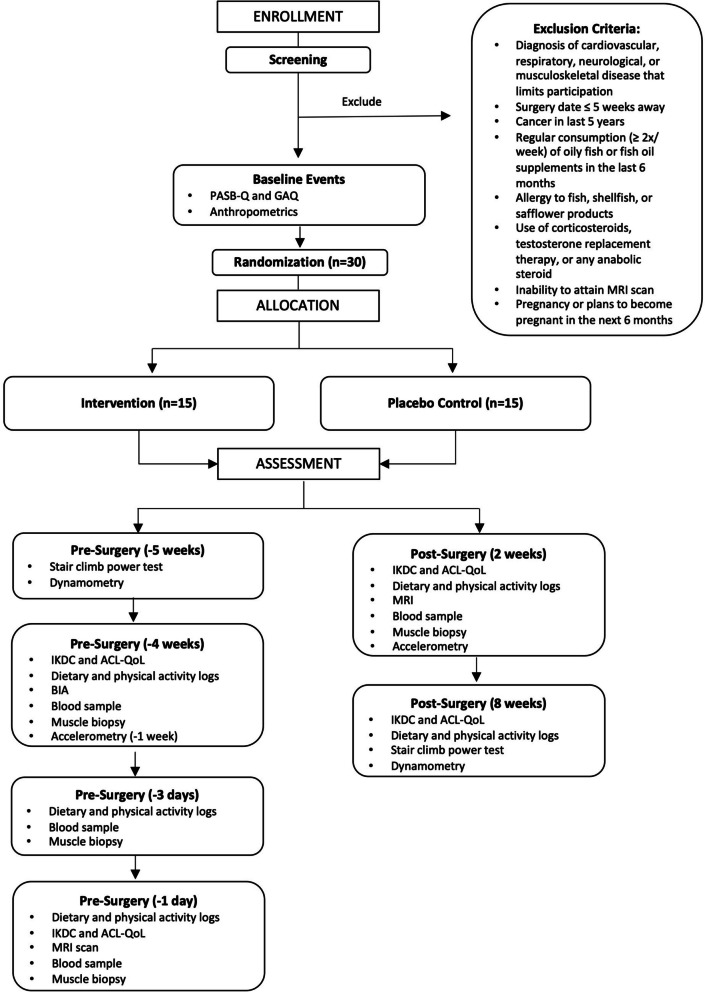
A Consolidated Standards of Reporting Trials (CONSORT) illustrating study timeline can be seen in Fig. 1. This study is a single center, double-blind, two-arm RCT comparing 6 weeks of nutritional intervention to placebo control starting 4 weeks before and continuing 2 weeks after ACLR surgery (Table 1) with a 1:1 allocation ratio (NCT06233825). After baseline assessment, an unblinded researcher at arm’s length of the study will determine group allocation for the patient with a block randomization scheme (n = 6), stratified according to sex. Participants will be allocated to either group “A” or “B,” with conditions concealed from all involved parties by having identical packaging between groups. A sealed copy of the randomization scheme will be kept by the arm’s length researcher, and emergency unblinding will take place immediately in response to serious adverse events, and allocation will be made known to the participant and clinician by said individual.
Fig. 1.
CONSORT flowchart. Intervention is 6 weeks (4 pre-surgery, 2 post) × 5 g⋅day−1 omega-3 FA + 3 weeks (1 pre-surgery, 2 post) × 40 g⋅day−1 EAA. Placebo control is 6 weeks (4 pre-surgery, 2 post) × 5 g⋅day−1 safflower oil + 3 weeks (1 pre-surgery, 2 post) × 40 g⋅day−1 NEAA. ACL, anterior cruciate ligament; ACL-QoL, ACL Quality of Life questionnaire; BIA, bioelectrical impedance analysis; EAA, essential amino acids; GAQ, Get Active Questionnaire; IKDC, International Knee Documentation Committee subjective knee evaluation form; MRI, magnetic resonance imaging; NEAA, non-essential amino acids; PASBQ, Physical Activity Behavior Questionnaire
Table 1.
Schedule of enrollment, assessment, and intervention
| Activity | Staff | Visit | 1 | 2 | 3 | 4 | – | 5 | 6 | 7 | Surgery | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | -6 week | -5 week | -4.5 week | -4 week | -1 week | -4 day | -3 day | -1 day | 0 | 2 week | 8 week | ||
| Recruitment and screening | |||||||||||||
| Outpatient recruitment | Researcher/delegate | x | |||||||||||
| Community recruitment | Researcher | x | |||||||||||
| Exclusion criteria assessment | Researcher | x | |||||||||||
| Informed consent | Researcher | x | |||||||||||
| Assessments | |||||||||||||
| PASB-Q | Researcher | x | |||||||||||
| GAQ | Researcher | x | |||||||||||
| ACL-QoL | Researcher | x | x | x | x | ||||||||
| IKDC | Researcher | x | x | x | x | ||||||||
| Physical activity log | Participant | x | x | x | x | ||||||||
| Dietary log | Participant | x | x | x | x | ||||||||
| Anthropometrics | Researcher | x | x | x | x | x | |||||||
| Thigh circumference | Researcher | x | x | x | x | ||||||||
| BIA | Researcher | x | |||||||||||
| MRI | Technician | x | x | ||||||||||
| Muscle biopsy | Delegate | x | x | x | x | ||||||||
| Venous blood sample | Researcher | x | x | x | x | x | |||||||
| Daily saliva sample | Participant | x | x | x | x | x | |||||||
| Accelerometer | Participant | x | x | x | x | x | x | ||||||
| Stair Climb Power Test | Researcher | x | x | x | |||||||||
| Dynamometry | Researcher | x | x | x | |||||||||
| Adverse event protocol | Researcher | As needed throughout study | |||||||||||
| Interventions | |||||||||||||
| 5 g⋅day−1 capsules (omega-3 fatty acids/safflower oil) | Participant | x | x | x | x | x | x | x | |||||
| 2 × 20 g⋅day−1 amino acid powder (EAA/NEAA) | Participant | x | x | x | x | x | x | ||||||
| D2O ingestion (0.625 mL⋅kg FFM⋅day−1) | Participant | x | x | x | x | x | |||||||
| ACL reconstruction surgery | Orthopedic surgeon | x | |||||||||||
ACL anterior cruciate ligament, ACL-QoL ACL quality of life questionnaire, BIA bioelectrical impedance analysis, D2O deuterium oxide (deuterated water), EAA essential amino acids, FFM fat free mass; GAQ Get Active Questionnaire, IKDC International Knee Documentation Committee subjective knee evaluation form, MRI magnetic resonance imaging, NEAA non-essential amino acids, PASBQ physical activity behaviour questionnaire
The study requires 1 introductory visit (− 6 weeks), 1 familiarization visit (− 5 weeks), 4 “physiological” visits, and 3 “functional” visits. Physiological visits will take place at − 4 weeks, − 3 days, − 1 day, and 2 weeks relative to surgery, and will consist of anthropometrics, body composition analysis, knee and pathology-specific questionnaires, and collection of biological samples such as blood, saliva, and skeletal muscle. The endpoint of 2 weeks was chosen as it captures the period with the highest rates of skeletal muscle mass and strength loss during disuse [3, 4]. Functional visits will take place at approximately − 4.5 weeks before and 8 weeks after surgery, and measure muscle strength and power using clinically applicable tests. A final blood sample and round of questionnaires will also be performed at the latter timepoint. The choice to have 8 weeks as the endpoint for functional outcomes is supported by KHSC ACLR standard care rehabilitation guidelines (Additional file 1) and previous analyses of arthroscopic knee surgery which demonstrated that the greatest slopes of functional recovery occur between the 6–9-week timepoint [29]. Participants will be asked to abstain from strenuous exercise for 48 h, and consumption of alcohol and caffeine for 24 and 12 h, respectively, prior to each visit.
Participants
Females and males will be considered eligible for this study if they meet the following inclusion criteria: adult (≥ 18 years old), have a clinician-diagnosed ACL injury, and are scheduled for reconstruction surgery in Kingston, ON. Eligible ACLR surgeries include patellar tendon graft, quadriceps tendon graft, or hamstring tendon graft procedures. The Physical Activity Behavior Questionnaire (PASB-Q) will evaluate participant physical activity and sedentary behaviors prior to their surgery. The Canadian Society for Exercise Physiology (CSEP) Get Active Questionnaire (GAQ) will assess participant readiness to become more physically active through participation in the light exercise involved in this study (stair climbing, leg extension). Exclusion criteria include surgery ≤ 5 weeks away from participant enrollment; consumption of fish or fish oil products > 2 × /week in the last 6 months; allergy to fish, shellfish, or safflower products; treatment with anabolic steroids; cancer within the last 5 years; ineligible to undergo a magnetic resonance imaging (MRI) scan; pregnancy or plans to become pregnant within the next 6 months; or a diagnosed cardiovascular, musculoskeletal, or respiratory disease that limits study or exercise participation, as screened by the GAQ.
Intervention
Participants will be randomly allocated to either the nutritional intervention group or a placebo control group. The nutritional intervention group will undergo 6 weeks of daily omega-3 fatty acid consumption (3.75 g eicosapentaenoic acid [EPA] plus 1.25 g docosahexaenoic acid [DHA] via capsules; Wiley’s Finest, OH, USA) starting 4 weeks before ACLR surgery and continuing for 2 weeks after. Additionally, they will begin twice daily EAA consumption (20 g⋅dose−1; powder mixed in 250 mL water; Gruppo Nutrition, Windsor, ON, Canada) 1 week before ACLR surgery and continue through 2 weeks post (Additional files 2 and 3). The placebo control group will follow the same dosing requirements and temporal schedule for capsule and powder supplements; however, they will consume isocaloric safflower oil capsules (Wiley’s Finest, OH, USA) and an isonitrogenous non-essential amino acid blend (NEAA; 20 g⋅dose−1; powder mixed in 250 mL water; Gruppo Nutrition, Windsor, ON, Canada) as opposed to the intervention. Capsules are to be taken with food, and amino acid drinks will be consumed ~ 1 h after waking and ~ 1 h prior to sleeping. The trial includes a home-based supplement schedule and intermittent mandatory laboratory visits for sample and data collection. The former is to maintain pre-surgery and post-surgery care in-line with existing standard care guidelines for ACLR. Participants will complete daily supplement logs to monitor when and how often they consume the provided capsules and powders, and return these logs to researchers at − 1 day and 2 weeks for supplement monitoring. Additionally, participants will return all unused supplements to the research team at 2 weeks. The count of supplements in excess will be used in conjunction with supplement monitoring logs to report adherence. Compliance will be confirmed by means of erythrocyte phospholipid composition, detailed below. We elected to use a 4-week lead-in with omega-3 fatty acid supplementation based on our previous work that prescribed 4 weeks of omega-3 fatty acid ingestion at the approximate dose we propose to use in this investigation and led to a twofold increase in the EPA and DHA content of skeletal muscle in young men at 4 weeks [30].
Recruitment
The goal of the present study and future RCT is to maintain a single center design to minimize possible confounding or effect modification from variation in surgical technique, and afford pre-surgery sample collection in close proximity to operating clinics. Therefore, prospective participants must seek ACLR in Kingston, ON with a KHSC affiliated orthopedic clinic. Recruitment will begin on February 1, 2024. A delegate from KHSC will review chart information of prospective ACLR outpatients from KHSC orthopedic clinics who meet the inclusion criteria, and inform them of the study either in person or by phone call. Prospective participants will also be recruited from the community via flyers posted in local campus buildings, hospitals, rehabilitation clinics, and gyms. Interested individuals will be encouraged to contact the research coordinator to be screened for eligibility via phone call. Eligible individuals will be invited to attend an introductory visit (− 6 weeks) where they will be provided further information about the study, screened for exclusion criteria, formally invited to participate, and given the opportunity to complete the informed consent process. Verbal and written informed consent will be obtained.
Sample size justification
The feasibility success criterion for this pilot study is to recruit 30 ACLR outpatients within 18 months and retain 22 participants at the 8-week visit that marks the final post-surgery trial (12 weeks total data collection). This number of participants is consistent with previous reports in this field [20–22] and will yield more accurate statistical power calculations (using data generated from our own laboratory) to estimate sample sizes required to detect the efficacy of this intervention on identified primary outcomes for future RCTs. We also propose that this sample size will be sufficient for us to build on our existing expertise to improve our understanding of how to successfully execute trials of this nature for future work. Finally, this sample size and timeline is pragmatic based on the available financial resources, personnel, and projected ACLR surgeries in the Kingston, ON, area over the next 18 months.
Questionnaires
Prospective participants will be asked to complete the PASB-Q and GAQ to confirm they are in general good health, save for ACL injury, and are eligible to perform functional tests, as described below. At − 4 weeks, − 1 day, 2 weeks, and 8 weeks timepoints, participants will be asked to complete the International Knee Documentation (IKDC) Committee subjective knee evaluation form [31] and the ACL Quality of Life Questionnaire (ACL-QoL) [32] for patient-reported outcomes that are knee-specific and pathology-specific, respectively. The IKDC subjective knee evaluation form surveys patient-reported symptoms, knee function, and levels of physical activity [31]. The ACL-QoL has five domains that score physical symptoms, occupational concerns, recreational activities, lifestyle, and social and emotional aspects of patient life [32]. Additionally, at the end of the final visit (8 weeks) participants will complete a questionnaire to evaluate the effectiveness of the blinding for supplement group allocation. This questionnaire will ask participants: (i) whether they know what supplement allocation (intervention vs. control) they received, and if yes to this question, (ii) why they think they were allocated to that condition.
Physical activity and dietary reporting
Participants will be asked to complete 3-day dietary and activity logs prior to each physiological visit (− 4 weeks, − 3 days, − 1 day, 2 weeks) to report daily habits. In addition to daily rehabilitation and exercise, activity logs will also self-report use of bracing or crutches and temporal estimations of limb weight bearing. Furthermore, daily average participant step count and non-sedentary time will be recorded during waking hours from a waist-mounted accelerometer (ActiLife 6; Actilife LLC, Pensacola, FL, USA) worn from − 1 week pre-surgery to 2 weeks post-surgery, with all data analyzed by the accompanying software.
Anthropometrics and body composition analysis
Participant height, body mass, and thigh circumference will be measured at − 4 weeks, − 1 day, 2 weeks, and 8 weeks. Thigh circumference will be measured at 5 cm, 10 cm, and 15 cm from the proximal knee joint line of both legs [33]. Bioelectrical impedance analysis (BIA; BC 418 Body Composition Analyzer; Tanita, Arlington Heights, IL, USA) will be performed at baseline to estimate participant fat-free mass (FFM). This analysis will inform individual stable isotope tracer dosing protocols that are prescribed using FFM [34]. All measurements will take place in the morning after an overnight fast. Participants will be instructed to consume 500 mL of water ~ 1 h prior to their visit to standardize hydration status.
Quadriceps mass and cross sectional area
Quadriceps muscle mass and cross sectional area (CSA) will be assessed using a 3T MRI Scanner (Siemens MAGNETON Prisma; Siemens Healthcare, Munich, Germany) at the Queen’s University Centre for Neuroscience Studies (Kingston, ON) on day − 1 and 2 weeks of the study. Participants will rest in a supine position on the scanner bed. The bed includes a 32-inch Siemens integrated spine coil (Siemens Healthcare, Munich, Germany) that captures the posterior signal, while 18-inch and 4-inch body coils will be placed in succession over the thighs to capture anterior signal, with the distal end of the second coil covering the patella. Contiguous 3-mm axial MR images will be obtained from both thighs simultaneously from the knee (lateral condyle of the femur) to the hip (greater trochanter of the femur). Fast-recovery fast-spin echo (FRFSE) pulse sequence along with T1 TSE DIXON Scan will be used for decomposition of water and fat to obtain water-only, fat-only, in-phase, and out-of-phase images. The total number of images obtained will range from ~ 120 to 160, depending on thigh length. The top 30% (from the greater trochanter distally) and bottom 20% (from the distal end of the femur proximally) will be excluded so that only the middle 50% region of the assessed area will be used for manual segmentation analsyis [22]. ITK-SNAP software (version 4.0.1) will be used to estimate muscle mass and CSA for each slice. All segmentation analyses will be conducted by the same two investigators and every 10 slices will be blind-checked for quality control by the principal investigator (PI).
Blood samples
Fasted blood samples will be obtained from the antecubital vein during at − 4 weeks, − 3 days, − 1 day, 2 weeks, and 8 weeks. Samples will be centrifuged (4 °C, 2500 g, 5 min), separated into red blood cell (RBC) and plasma fractions, and then stored in the − 80 °C freezer until further analysis. RBC will be used for phospholipid analysis. One plasma aliquot will be analyzed for inflammatory markers including tumor necrosis factor and interleukins by enzyme-linked immunosorbent assay. A second aliquot of plasma will be processed for exploratory proteomic analysis as described below.
Plasma proteomics
Plasma proteome profiling will be performed using the aptamer-based SOMAscan assay (Slow Off-rate Modified Aptamers; SomaLogic, Boulder, CO). Briefly, plasma samples will be incubated with SOMAmer reagents, which are modified nucleic acid sequences designed to bind proteins with high affinity. Protein-SOMAmer complexes are then isolated from unbound reagents with subsequent washing steps. Following liberation of bound proteins, SOMAmers are subsequently hybridized to an array surface. Finally, SOMAmers are quantified based on the fluorescent signal they produce, with the intensity of signal proportional to target protein concentration. To enable normalization within and across plates, samples will be run alongside pooled human calibration and quality control samples. Only samples passing quality control steps will be included in the final analysis.
Muscle samples
Unilateral skeletal muscle biopsies will be obtained in the rested, fasted state from the vastus lateralis at − 4 weeks, − 3 days, − 1 day, and 2 weeks. Biopsies will be collected from the non-surgical leg for the first two samples and the surgical leg for the last two samples. Biopsies will be performed under sterile conditions and collected using a 6-mm Bergstrom needle adapted for manual suction after the administration of local anesthesia (2% lidocaine with epinephrine). Muscle tissue samples (~ 100 mg) will be freed from any visible connective and adipose tissue, segmented, and then rapidly frozen in liquid nitrogen and stored at − 80 °C for later analysis. We recognize that the invasiveness of this procedure may act as a deterrent to potential participants, although it is made explicitly clear during the informed consent process that participants may abstain from any part of the study they decide to do so. In the event participants do not wish to provide a muscle sample, they will be allowed to continue in this study. Willingness to undergo the muscle biopsy procedure will be reported to inform future design.
Blood and skeletal muscle phospholipid analysis
Lipid content of red blood cells and skeletal muscle will be assessed to confirm incorporation of EPA and DHA in phospholipids as a measure of compliance and will be performed as previously described [22]. Briefly, lipids will be extracted from red blood cells and freeze dried skeletal muscle, before being separated and trans-methylated. The resultant fatty methyl esters will be dissolved in hexane and quantification of long-chain fatty acids (in moles) will be deduced by liquid chromatography with comparison to appropriate standard curves. Content of fatty acid species will be calculated as the individual sum relative to the tissue or plasma sample, expressed in nmol·mg−1 per dry tissue or μmol·L−1 concentration in plasma. Those who are in the intervention group who do not exhibit ≥ 1.5-fold increase in omega-3 phospholipid profiles in erythrocytes will be considered non-compliant and their data will be excluded from per protocol analyses [30].
RNA extraction and RT-PCR
Approximately 20 mg of skeletal muscle will be used to isolate RNA using the Trizol phenol–chloroform procedure, as previously described [22]. Reverse transcription will be performed by using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) will serve as a housekeeping gene. Relative amounts of mRNA for transcription factor 4 (ATF4), tumor protein 53 (p53), cyclin-dependent kinase inhibitor 1 (p21), large neutral amino acid transporter small subunit (LAT1), muscle RING-Finger protein-1 (MuRF1), and muscle atrophy F- Box protein (MAFbx) will be calculated using the 2−ΔΔCtmethod [35].
Western blotting
Skeletal muscle samples will be analyzed to target protein content of AFT4, p53, p21, LAT1, focal adhesion kinase (FAK), mechanistic target of rapamycin (mTOR), ribosomal protein S6 kinase 1 (p70S6K1), eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1), and growth differentiation factor 8 (GDF8). Samples will first be homogenized in ice-cold RIPA buffer (Thermo Fisher Scientific, Waltham, MA, USA), and the total protein concentration of the sample will be determined using a colorimetric bicinchoninic acid assay (Thermo Fisher Scientific, Waltham, MA, USA). Equal concentrations of protein (10 μg) from each sample homogenate will be loaded into 4–15% Criterion TGX Stain-Free protein gels (Bio-Rad, Hercules, CA, USA). Each sample will be assayed in duplicate within the same gel. An unstained protein ladder (Bio-Rad) and calibration curve will also be run on each gel such that protein content can be normalized using the calibration curve on each respective gel. Each protein gel will run at 200 V for 45 min prior to transfer to a low-fluorescence PVDF membrane. Successful transfer will be confirmed using UV activation of the gel and membrane pre- and post-transfer (ChemiDoc MP Imaging System; Bio-Rad). Rapid transfer will be performed for all gels using the Transblot Turbo (Bio-Rad), and membranes will be blocked for 2 h in 5% bovine serum albumin. Membranes will be exposed to primary antibodies for 12 h at 4 °C after which they will be washed in Tris-buffered saline and Tween 20 (MilliporeSigma) and incubated in anti-rabbit/anti-mouse IgG conjugates with horseradish peroxidase secondary antibodies (GE Healthcare Life Sciences, Chicago, IL, USA) for 1 h at room temperature. Signals will be detected by using chemiluminescence Super-Signal West Dura Extended Duration Substrate (Thermo Fisher Scientific) on an imaging system (ChemiDoc MP Imaging System; Bio-Rad), and bands will be quantified by using Image Lab 5.2.1 (ChemiDoc MP Imaging System; Bio-Rad). Protein content will normalized using the calibration curve obtained from each gel as we have done previously [22].
Isotope protocol
Participants will consume stable isotope tracer in the form of deuterium oxide (D2O) to permit measurement of skeletal muscle protein synthesis. D2O loading will take place on day − 4 to rapidly bring participant body water pools to 1–2% isotopic enrichment (2H). Participants will consume 0.625 mL of 70% D2O per kg FFM (~ 30 mL) every 1.5 h over an 10.5-h period [34], for a total of 8 doses. To maintain enrichment across the remaining pre-surgery days and 2-week post-surgery period, participants will sent home with pre-measured daily maintenance doses (0.625 mL of 70% D2O per kg FFM) to consume each morning.
Body water enrichment
To confirm steady state body water enrichment across the isotope protocol, participants will collect daily saliva swabs (~ 2 mL samples) from − 4 days to 2 weeks. Samples will be refrigerated by the participant and returned to the lab at 2 weeks. Samples will be centrifuged (4 °C, 1500 g, 10 min) and diluted with doubly distilled water by a factor of 35. Diluted samples will be analyzed for 2H enrichment by cavity ring-down spectroscopy using a liquid isotope analyzer (L2130-i analyzer; Picarro, Santa Clara, CA, USA) with an automated injection system and micro-combustion module. The water phase of saliva will be injected 6 times, and the average of the last 3 measurements will be used for data analysis. Standards with known 2H content will be measured before and after each participant run to create standard curves to correct for machine drift. The 2H isotopic enrichments for saliva and muscle initially expressed as δ 2H‰ will be converted to mole percent excess (MPE) using standard equations [36].
Muscle protein synthesis
Wet muscle samples will be homogenized in ice cold homogenization buffer (10 μL⋅mg−1 25 mM Tris 0.5% vol:vol Triton X-100 and protease/phosphatase inhibitor cocktail tablets).37 To separate the myofibrillar protein fraction, homogenized sample will be centrifuged at 700 g for 15 min at 4 °C [33]. Supernatant will be removed and centrifuged at 12,000 g for 20 min at 4 °C to pellet mitochondria. Myofibrillar and mitochondrial pellets will be further solubilized, centrifuged, precipitated, and hydrolyzed. Hydrolyzed amino acids will be derivatized as methoxycarbonyl methyl esters (MCME), separated by gas chromatography, pyrolyzed, and then analyzed by isotope ratio mass spectrometry (IRMS; Delta Q, Thermo Scientific, MA, USA) to determine 2H enrichments at respective timepoints for the following equation:
where ΔEAla is the increment in muscle protein–bound 2H-alanine enrichment in MPE between 2 consecutive muscle biopsies [36, 38]. EBW is the mean 2H enrichment (in MPE) in total body water between the sampling timepoints, and t is the tracer incorporation time in days. The calculation of muscle protein synthesis (MPS) rates for pre- and post-surgery will be based on 2H-alanine enrichments from − 3 to − 1 day and − 1 day to 2 weeks, respectively.
Muscle function tests
At − 5 weeks, − 4.5 weeks, and 8 weeks, participants will perform muscle function tests that assess muscle strength—the ability to generate torque—and muscle power—the product of torque and velocity [40]. First, the Stair Climb Power Test (SCPT) will be used. The SCPT is a validated clinical test, requires minimal equipment (tape measure, scale, stairs), can be performed in a clinical, institutional, or community-dwelling setting, and has high and moderate correlations to dynamometer-measured knee extensor strength and power, respectively [39]. Participants will be asked to climb a flight of 10 stairs as fast as possible [37]. Timing starts on the “go” command and ends when the participant has both feet on the top step. This test will be repeated 2 times, with 1 min of rest between attempts. Leg power (watts) will be calculated as force times velocity, with stair height and climb time used to calculate velocity, and body mass and acceleration due to gravity used to calculate force [40]. Next, knee extensor strength will be measured as peak torque using a HUMAC NORM Dynamometer (CSMi Medical Solutions, Stoughton, MA, USA). Participants will be seated in an upright position, and anatomical zero will be set at a knee angle of 0° with full knee extension. Next, the participant will have their knee adjusted to 60° flexion and ankle pad of the stationary arm placed about their lateral malleolus of the fibula [41]. Participants will then be asked to maximally contract their quadriceps by pushing into the ankle pad as hard as possible, maintaining the movement for 5 s to capture maximal voluntary contraction (MVC). For each leg, 1 practice attempt and 3 collection trials will be performed, with 60 s of rest between each MVC. To assess peak isotonic power, knee range of motion will be adjusted to 100° of flexion to full extension, and load will be adjusted to ~ 30% of peak torque recorded during the MVC tests [39]. Participants will perform 2 sets of 10 maximal repetitions, with 2 min of rest between sets [39].
Outcomes
Details of primary and secondary outcomes and their analyses can be found in Table 2. Primary outcomes concern the feasibility of recruitment and retention, and all secondary outcomes are exploratory. Summary data include participant anthropometrics and gender identity, medical chart information such as surgical graft type, surgery duration, tourniquet duration and prescribed medications, muscle biopsy participation, diet and physical activity reporting, accelerometer data, responses to questionnaires, and adverse event reporting, as described below.
Table 2.
Outcomes, measures, and methods of analysis
| Outcome | Measure | Method of analysis |
|---|---|---|
| Primary | ||
| Recruitment | Number of participants in 18 months | Descriptive statistics (mean and standard deviation or count and percent) |
| Retention | Number of participants who complete data collection at 8 weeks | |
| Secondary | ||
| Muscle mass | Change in quadriceps muscle mass and cross sectional area from pre- (− 1 day) to post- (2 weeks) surgery measured by magnetic resonance imaging scan | Mixed effects model (time × group) |
| Muscle protein synthesis | Change in mixed skeletal muscle, myofibrillar, and mitochondrial fractional synthesis rates from pre- (day − 3 to − 1) to post-surgery (day − 1 to 2 weeks) | |
| Skeletal muscle and erythrocyte phospholipid composition | Change in skeletal muscle and erythrocyte phospholipid composition (n-3: n-6) from baseline (− 4 weeks) to post-surgery (2 weeks) | |
| Plasma proteomics | Change in target signal intensity and protein concentration between − 4 weeks, − 1 day, and 2 weeks | |
| Changes in the expression of translational factors related to skeletal muscle protein synthesis | Activation of translational factors involved in skeletal muscle protein synthesis from pre- (− 4 weeks) to post-surgery (2 weeks) | |
| Muscle function | SCPT performance, and dynamometer MVC and peak isotonic power at − 4.5 and 8 weeks | |
Other exploratory outcomes
Permitting availability of extra tissue, expertise, and finances, further exploratory analyses of skeletal muscle mitochondrial function and deuterium incorporation into ligamentous tissue will be performed.
Analyses
This protocol was drafted in accordance with the SPIRIT 2013 statement [42], and reporting will be in accordance with the CONSORT extension to randomized pilot and feasibility studies [43]. Outcome assessors will be blinded to group allocation and study timepoints by having biological samples and images coded by a statistician prior to analysis. Once laboratory analysis is completed, coded data will be analyzed and reports of the data and statistical results will be delivered to the research team without deanonymizing subject codes. Descriptive summaries of baseline demographics, anthropometrics, accelerometer activity, surgery details, medical chart reporting, muscle biopsy participation, questionnaire responses, and dietary and physical activity logs will be provided. For primary outcomes, numbers will be presented using descriptive statistics (e.g., mean and standard deviation or count and percent), compared to criteria for success, and reported with reasons and timepoints for study dropout. For all secondary outcomes (biochemical, molecular, and biomechanical data), exploratory inferential statistics will be used to elucidate the potential mechanisms by which the intervention might exert its effects and assist the selection of the future primary outcome(s), but no inferences related to clinical efficacy will be made. Secondary outcomes will be analyzed as intent to treat, and a per protocol sensitivity analyses will be performed for individuals who self-report ≥ 80% supplement adherence, fulfill blood phospholipid compliance criteria as described above, and remain enrolled until the final timepoint for the outcome of interest. Multiple imputation will be performed to address likely missing data and minimize the risk of attrition and sampling biases. Data will be checked for normality using a Shapiro–Wilk test and appropriate transformations will be explored for data that exhibit abnormal distribution. A mixed effects model will be used to test for the within effect of time and between effect of group (intervention vs. control). Secondary outcomes will be presented as mean and standard deviation with confidence intervals and p values. All data will be analyzed with SPSS statistics software (IBM, Armonk, NY, USA). Study sponsors do not have authority over the analysis or interpretation of data.
Mitigation of bias
Identification of potential sources of bias in the present design and strategies to reduce risk of bias are detailed in Table 3.
Table 3.
Potential sources of bias and methods to reduce risk of bias
| Type of bias | Methods to reduce risk of bias |
|---|---|
| Selection bias | A randomization sequence will be generated before data collection using secure web-based software. Randomization will follow a 1:1 allocation ratio. An individual at arm’s length from the study will generate the randomization sequence and stratify according to sex. Delegated personnel in charge of screening and enrolling participants will contact this individual to obtain the group allocation |
| Performance bias | Supplements will be pre-packaged and coded by industrial suppliers. Code details will be provided to the above individual and withheld from other personnel involved in the study. Placebo and experimental supplements will look similar thereby preventing participants and study personnel from determining group assignment |
| Observer bias | While we can blind personnel to group type, the research team cannot be blinded to the timing of the study (i.e., baseline, pre-, post-surgery). In order to reduce the risk of possible observer bias, biological samples and images will be coded by statistician prior to analysis to prevent study personnel analyzing samples/data from known timepoints. Once laboratory analysis is completed, coded data will be analyzed and reports of the data and statistical results (e.g., descriptive summaries, confidence intervals, inferential) will be delivered to the research team without deanonymizing subject codes |
| Attrition bias | Analyses will follow both intention to treat and per protocol analysis (secondary outcomes) and perform a sensitivity analysis using multiple imputation across a range of plausible assumptions. We will record and report the number, timepoint, and reasons for participant dropout or removal |
| Reporting bias | This trial was registered through ClinicalTrials.gov prior to data collection (NCT06233825). This publicly registered information includes a list of our primary and secondary outcomes, a brief description of our study design, and a detailed statistical plan, all of which has been expanded within the present document |
Types of bias were based on Cochrane Collaboration Tool for assessing bias [44]
Adverse events
At the initial study visit (− 6 weeks), participants will be educated on possible types of adverse events and instructed to report all events, as well as injury, illness, or changes in health status, to a member of the research team as they occur. Additionally, a member of the research team will contact participants by phone call or email 48 h after each physical testing visit, and bi-weekly during the 6-week nutritional supplementation period, to inquire about adverse events. Reporting will be unsolicited, and individuals will be asked an open-ended question of whether any events occurred. When an event is disclosed, the researcher will collect information regarding the occurrence, including the severity (mild, moderate, severe), expectedness, relatedness to the nutritional strategy, exercise protocol or other study procedures (i.e., biopsy), actions taken, and the outcome of the adverse event. If a serious adverse event occurs, participants will be instructed to contact their local emergency department and immediately inform the PI. If the event is possibly related to the nutritional supplement, the PI will contact the arm’s length researcher, or in extreme emergency access sealed randomization documents, to disclose participant group allocation to relevant clinicians and research members, in addition to the participant. Serious adverse events will be reported to the Queen’s University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board. Termination of the nutritional supplement or exercise testing will be enforced if a moderate or severe adverse event persists for more than 3 days, or if serious adverse event is determined by the PI to be definitely related or possibly related to either protocol. Serious adverse events, events linked to the nutritional supplement or exercise protocol, and events leading to cessation of either will be described and reported.
Trial steering committee
The PI and research coordinator will be responsible for trial conduct and chairing trial steering committee meetings. These meetings will take place bi-annually and host the PI, research coordinator, lead surgeon, and research delegate. Pertinent study updates including protocol amendments, adverse event reporting, and fulfillment of quartile recruitment and retention targets will be communicated via encrypted email on an as-needed basis to all Co-PIs, delegates, and research assistants. Co-PIs include KHSC lead orthopedic surgeon and specialists in biomechanics, rehabilitation science, proteomics, and clinical trials reporting. There is no data safety monitoring board for this study.
Research ethics and confidentiality
This study has been approved by the Queen’s University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board (HSREB No. 6038901). All participant data will be coded, and participant ID numbers will be assigned and used for all study documents. A linking log will be used to code data collected under participant outpatient medical record numbers. Electronic data will be stored on encrypted computers, and hard copy data will be stored in the locked office of the PI.
Discussion and dissemination
Each year, there are an estimated 160,000 ACLR surgeries performed between Canada and the USA alone [18]. ACLR surgery necessitates nearly all patients to undergo a period of recovery and relative skeletal muscle disuse causing the rapid loss of skeletal muscle mass and function [4]. This loss of skeletal muscle mass and function is linked to multiple negative health outcomes including impaired recovery of skeletal muscle strength and power, and possible development of functional disability and insulin resistance [5–12]. To date, nutritional strategies to combat the loss of skeletal muscle in response to ACLR surgery are lacking. The aim of the proposed pilot trial is to establish the feasibility of performing a large-scale, single center RCT that will test the efficacy of a novel combined nutritional intervention to mitigate the loss, and enhance the recovery of, skeletal muscle mass and function following ACLR surgery in adult women and men. Prospective findings may be extrapolated to inform nutritional guidelines for larger elective surgery cohorts, or advise subsequent work in older populations and more complex models, such as prolonged bed rest.
This study will include several clinically relevant exploratory measures of skeletal muscle mass and function including MRI of skeletal muscle, stair climbing, and maximal isometric and isotonic dynamometry [4, 22, 29]. Moreover, we will record ACLR outpatient willingness for obtaining skeletal muscle biopsies to enable the application of key cellular and molecular techniques that will provide critical insight into the possible mechanisms that cause skeletal muscle loss with disuse [45, 46]. Given the known mechanisms that drive skeletal muscle loss with disuse are predominantly informed by data generated in rodents that have fundamentally different protein turnover rates and metabolism compared to humans [47], such mechanistic data in humans is urgently needed.
Although there are several strengths of our protocol, such as the use of clinically relevant measures of skeletal muscle function [39, 40], MRI-measured skeletal muscle mass [20–22], and skeletal muscle biopsies [23–25], there are some limitations that should be acknowledged. Physical activity and dietary intake will not be controlled in this study. This lack of control could result in participants changing diet and lifestyle practices that confound our secondary outcomes. Due to financial constraints, we are also unable to confirm compliance with the EAA and NEAA supplements by direct means such as spiking the supplements with para-amino-benzoic acid. Instead, we have elected to adopt a returned sachet count and log approach. Should we successfully execute this pilot trial and secure the funds for a larger RCT, physical activity and dietary controls will be implemented throughout, as will direct measures of EAA and NEAA supplementation compliance.
In sum, we envisage that the present work will set the foundation for a future RCT that could have application and direct translatability to nutritional clinical practice and surgical patient care guidelines. Furthermore, we propose that the findings of this study would have broad applicability to other scenarios of surgery-induced skeletal muscle disuse atrophy, such as hip and knee arthroplasty. Primary route to knowledge dissemination will be via the publication of scientific journals, student theses, and abstract presentations at conference meetings. We will also engage with other methods of knowledge transfer such as podcasts and community talks, as well as written and verbal media interviews as we have done previously, to reach a broader audience of participants and clinicians.
Supplementary Information
Supplementary Material 1. Standard care rehabilitation timeline and instructions. Standard care rehabilitation timeline and instructions for anterior cruciate ligament reconstruction outpatients at Kingston Health Sciences Centre.
Supplementary Material 2. Capsule supplement details. Product monographs and analysis details for intervention (omega-3 fatty acids) and placebo control (safflower oil) capsule supplements.
Supplementary Material 3. Powder supplement details. Product monographs and details for intervention (essential amino acid) and placebo control (non-essential amino acid) powder supplements.
Acknowledgements
Thank you to Donald Brien at the Queen's University Centre for Neuroscience Studies who was instrumental in the development of the MRI protocol and collection of subsequent data.
Abbreviations
- ACL
Anterior cruciate ligament
- ACL-QoL
Anterior Cruciate Ligament Quality of Life questionnaire
- ACLR
Anterior cruciate ligament reconstruction
- BIA
Bioelectrical impedance analysis
- CONSORT
Consolidated Standards of Reporting Trials
- CSA
Cross sectional area
- D2O
Deuterium oxide
- DHA
Docosahexaenoic acid
- EAA
Essential amino acids
- EPA
Eicosapentaenoic acid
- FFM
Fat free mass
- FRFSE
Fast-recovery fast-spin echo
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- GAQ
Get Active Questionnaire
- GDF8
Growth differentiation factor 8
- IKDC
International Knee Documentation Committee subjective knee evaluation form
- KHSC
Kingston Health Sciences Centre
- MCID
Minimally clinically important difference
- MCME
Methoxycarbonyl methyl esters
- MPE
Mole percent excess
- MPS
Muscle protein synthesis
- MRI
Magnetic resonance imaging
- MVC
Maximal voluntary contraction
- MyoPS
Myofibrillar protein synthesis
- NEAA
Non-essential amino acids
- PASB-Q
Physical Activity Behavior Questionnaire
- RBC
Red blood cells
- RCT
Randomized control trial
- SCPT
Stair Climb Power Test
- SOMA
Slow Off-rate Modified Aptamers
- SPIRIT
Standard Protocol Items: Recommendations for Interval Trials
Authors’ contributions
DN and CM conceptualized the study. DN, TS, JS, SM, IJ, DB, LG, and CM designed the study. DB is the lead clinician and attending surgeon. DN and OH are responsible for participant recruitment and consent. CM and CS oversee muscle biopsy collection. DN, CP, JS, and CM will collect samples data. DN, JS, IJ, and CM are responsible for data analysis. DN, LG, and CM prepared this manuscript. All authors have read this manuscript and contributed significantly to its revision.
Funding
This project is funded by the Donner Canadian Foundation. This sponsor has no role in study conceptualization, design, data collection, analysis, decision to publish, or preparation of any manuscripts.
Data availability
Data sharing is not applicable to this article as no data sets were generated or analyzed for the current publication. Future data, excluding medical chart information, will be available from the corresponding author on reasonable request after publishing of results.
Declarations
Ethics approval and consent to participate.
This study has been approved by the Queen’s University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board (HSREB No. 6038901). Verbal and written informed consent will be obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–64. [DOI] [PubMed] [Google Scholar]
- 2.English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care. 2010;13(1):34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Preobrazenski N, Janssen I, McGlory C. The effect of single-leg disuse on skeletal muscle strength and size in the non-immobilized leg of uninjured adults: a meta-analysis. J Appl Physiol. 2023;134(6):1359–63. [DOI] [PubMed] [Google Scholar]
- 4.Preobrazenski N, Seigel J, Halliday S, Janssen I, McGlory C. Single-leg disuse decreases skeletal muscle strength, size, and power in uninjured adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2023;14(2):684–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy EJO, Inns TB, Hatt J, Doleman B, Bass JJ, Atherton PJ, et al. The time course of disuse muscle atrophy of the lower limb in health and disease. J Cachexia Sarcopenia Muscle. 2022;13(6):2616–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dirks ML, Wall BT, van de Valk B, Holloway TM, Hollway GP, Chabowski A, et al. One week of bed rest leads to substantial muscle atrophy and induces whole-body insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes. 2016;65(10):2862–75. [DOI] [PubMed] [Google Scholar]
- 7.Haruna Y, Suzuki Y, Kawakubo K, Yanagibori R, Gunji A. Decremental reset in basal metabolism during 20-days bed rest. Acta Physiol Scand Suppl. 1994;616:43–9. [PubMed] [Google Scholar]
- 8.Tzankoff SP, Norris AH. Effect of muscle mass decrease on age-related BMR changes. J Appl Physiol Respir Environ Exerc Physiol. 1977;43(6):1001–6. [DOI] [PubMed] [Google Scholar]
- 9.Kyle UG, Genton L, Pichard C. Hospital length of stay and nutritional status. Curr Opin Clin Nutr Metab Care. 2005;8(4):397–402. [DOI] [PubMed] [Google Scholar]
- 10.Kyle UG, Pirlich M, Lochs H, Schuetz T, Pichard C. Increased length of hospital stay in underweight and overweight participants at hospital admission: a controlled population study. Clin Nutr. 2005;24(1):133–42. [DOI] [PubMed] [Google Scholar]
- 11.Van Ancum JM, Scheerman K, Jonkman NH, Smeenk HE, Kruizinga RC, Meskers CG, et al. Change in muscle strength and muscle mass in older hospitalized participants: a systematic review and meta-analysis. Exp Gerontol. 2017;92:34–41. [DOI] [PubMed] [Google Scholar]
- 12.Huang DD, Ji YB, Zhou DL, Li B, Wang SL, Chen XL, et al. Effect of surgery-induced acute muscle wasting on postoperative outcomes and quality of life. J Surg Res. 2017;218:58–66. [DOI] [PubMed] [Google Scholar]
- 13.van Venrooij LM, Verberne HJ, de Vos R, Borgmeijer-Hoelen MM, van Leeuwen PA, de Mol BA. Postoperative loss of skeletal muscle mass, complications and quality of life in participants undergoing cardiac surgery. Nutrition. 2012;28(1):40–5. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Hai S, Liu Y, Liu Y, Dong B. Skeletal muscle mass as a mortality predictor among nonagenarians and centenarians: a prospective cohort study. Sci Rep. 2019;9(1):2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norte GE, Knaus KR, Kuenze C, Handsfield GG, Meyer CH, Blemker SS, Hart JM. MRI-based assessment of lower-extremity muscle volumes in patients before and after ACL reconstruction. J Sport Rehabil. 2018;27(3):201–12. [DOI] [PubMed] [Google Scholar]
- 16.Pottkotter KA, Di Stasi SL, Schmitt LC, Magnussen RA, Paterno MV, Flanigan DC, Kaeding CC, Hewett TE. Timeline of gains in quadriceps strength symmetry and patient-reported function early after ACL reconstruction. Intl J Sports Physl Ther. 2020;15(6):995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiemstra LA, Webber SA, MacDonald PB, Kriellaars DJ. Knee strength deficits after hamstring tendon and patellar tendon anterior cruciate ligament reconstruction. Med Sci Sports Exerc. 2000;32(8):1472–9. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, McCammon J, Martin RK, Prior HJ, Leiter J, MacDonald PB. Epidemiological trends of anterior cruciate ligament reconstruction in a Canadian province. Clin J Sports Med. 2020;30(6):e207–13. [DOI] [PubMed] [Google Scholar]
- 19.Paudel YR, Sommerfeldt M, Voaklander D. Increasing incidence of anterior cruciate ligament reconstruction: a 17-year population-based study. KSSTA. 2023;31(1):248–55. [DOI] [PubMed] [Google Scholar]
- 20.Dreyer HC, Strycker LA, Senesac HA, Hocker AD, Smolkowski K, Shah SN, Jewett BA. Essential amino acid supplementation in participants following total knee arthroplasty. J Clin Invest. 2013;123(11):4654–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holloway TM, McGlory C, McKellar S, Morgan A, Hamill M, Afeyan R, et al. A novel amino acid composition ameliorates short-term muscle disuse atrophy in healthy young men. Front Nutr. 2019;6: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGlory C, Gorissen SHM, Kamal M, Bahniwal R, Hector AJ, Baker SK, et al. Omega-3 fatty acid supplementation attenuates skeletal muscle disuse atrophy during two weeks of unilateral leg immobilization in healthy young women. FASEB J. 2019;33(3):4586–97. [DOI] [PubMed] [Google Scholar]
- 23.Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, Mittendorfer B. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia–hyperaminoacidaemia in healthy young and middle-aged men and women. Clin Sci. 2011;121(6):267–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, et al. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr. 2011;93(2):402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith GI, Julliand S, Reeds DN, Sinacore DR, Klein S, Mittendorfer B. Fish oil–derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am J Clin Nutr. 2015;102(1):115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, Robson R, Thabane M, Giangregorio L, Goldsmith CH. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearson N, Naylor PJ, Ashe MC, Fernandez M, Yoong SL, Wolfenden L. Guidance for conducting feasibility and pilot studies for implementation trials. Pilot Feasibility Stud. 2020;6:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Canadian Institute for Health Information. Hip and knee replacements in Canada: CJRR annual report, 2021–2022. Ottawa, ON:CIHI; 2023.
- 29.Kennedy DM, Stratford PW, Hanna SE, Wessel J, Gollish JD. Modeling early recovery of physical function following hip and knee arthroplasty. BMC Musculoskelet Disord. 2006;7(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGlory C, Galloway SD, Hamilton DL, McClintock C, Breen L, Dick JR, et al. Temporal changes in human skeletal muscle and blood lipid composition with fish oil supplementation. PLEFA. 2014;90(6):199–206. [DOI] [PubMed] [Google Scholar]
- 31.Irrgang JJ, Anderson AF, Boland AL, Harner CD, Neyret P, Richmond JC, et al. Responsiveness of the international knee documentation committee subjective knee form. Am J Sports Med. 2006;34(10):1567–73. [DOI] [PubMed] [Google Scholar]
- 32.Mohtadi N. Development and validation of the quality of life outcome measure (questionnaire) for chronic anterior cruciate ligament deficiency. Am J Sports Med. 1998;26(3):350–9. [DOI] [PubMed] [Google Scholar]
- 33.Soderberg GL, Ballantyne BT, Kestel LL. Reliability of lower extremity girth measurements after anterior cruciate ligament reconstruction. Physiother Res Int. 1996;1(1):7–16. [DOI] [PubMed] [Google Scholar]
- 34.Oikawa SY, Kamal MJ, Webb EK, McGlory C, Baker SK, Phillips SM. Whey protein but not collagen peptides stimulate acute and longer-term muscle protein synthesis with and without resistance exercise in healthy older women: a randomized controlled trial. Am J Clin Nutr. 2020;111(3):708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 36.Wilkinson DJ, Franchi MV, Brook MS, Narici MV, Williams JP, Mitchell WK, et al. A validation of the application of D(2)O stable isotope tracer techniques for monitoring day-to-day changes in muscle protein subfraction synthesis in humans. Am J Physiol Endocrinol Metab. 2014;306:E571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burd NA, Andrews RJ, West DW, Little JP, Cochran AJ, Hector AJ, et al. Muscle time under tension during resistance exercise stimulates differential muscle protein sub-fractional synthetic responses in men. J Phsyiol. 2012;590(2):351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacDonald AJ, Small AC, Greig CA, Husi H, Ross JA, Stephens NA, Fearon KC, Preston T. A novel oral tracer procedure for measurement of habitual myofibrillar protein synthesis. Rapid Commun Mass Spectrom. 2013;27(15):1769–77. [DOI] [PubMed] [Google Scholar]
- 39.Sheppard E, Chang K, Cotton J, Gashgarian S, Slack D, Wu K, et al. Functional tests of leg muscle strength and power in adults with cystic fibrosis. Respir Care. 2019;64(1):40–7. [DOI] [PubMed] [Google Scholar]
- 40.Bean JF, Kiely DK, LaRose S, Alian J, Frontera WR. Is stair climb power a clinically relevant measure of leg power impairments in at-risk older adults? Arch Phys Med Rehabil. 2007;88(5):604–9. [DOI] [PubMed] [Google Scholar]
- 41.De Ruiter CJ, Kooistra RD, Paalman MI, De Haan A. Initial phase of maximal voluntary and electrically stimulated knee extension torque development at different knee angles. J Appl Physiol. 2004;97(5):1693–701. [DOI] [PubMed] [Google Scholar]
- 42.Standard Protocol Items: Recommendations for Interventional Trials: the SPIRIT statement for clinical trial protocols. http://www.spirit-statement.org/. Accessed 1 Aug 2023.
- 43.Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, Lancaster GA. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phillips SM, McGlory C. CrossTalk proposal: the dominant mechanism causing disuse muscle atrophy is decreased protein synthesis. J Phys. 2014;592(Pt 24):5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reid MB, Judge AR, Bodine SC. CrossTalk opposing view: the dominant mechanism causing disuse muscle atrophy is proteolysis. J Phys. 2014;592(Pt 24):5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Demetrius L. Of mice and men: when it comes to studying ageing and the means to slow it down, mice are not just small humans. EMBO Rep. 2005;6(S1):S39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1. Standard care rehabilitation timeline and instructions. Standard care rehabilitation timeline and instructions for anterior cruciate ligament reconstruction outpatients at Kingston Health Sciences Centre.
Supplementary Material 2. Capsule supplement details. Product monographs and analysis details for intervention (omega-3 fatty acids) and placebo control (safflower oil) capsule supplements.
Supplementary Material 3. Powder supplement details. Product monographs and details for intervention (essential amino acid) and placebo control (non-essential amino acid) powder supplements.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed for the current publication. Future data, excluding medical chart information, will be available from the corresponding author on reasonable request after publishing of results.