ABSTRACT
The genomes of three Pseudomonas aeruginosa Phikzvirus bacteriophages isolated in Kenya are described. The genomes of phages vB_PaePAO1-KEN19, vB_Pae3705-KEN49, and vB_Pae10145-KEN51, respectively, had lengths of 278,921, 280,231, and 280,173 bp, with 36.93%, 36.84%, and 36.86% GC content, containing 419, 417, and 417 coding sequences (including seven tRNAs in each genome).
KEYWORDS: bacteriophage, genome, Pseudomonas aeruginosa, Phikzvirus, Kenya, bacteriophage therapy
ANNOUNCEMENT
Rising multidrug-resistant Pseudomonas aeruginosa infections compels the use of phages to augment flagging antibiotics, and several clinical trials have shown efficacy (1–3). We collected virulent P. aeruginosa phages to build durable therapeutic cocktails. The genomes of three unique giant (jumbo) phages isolated in Kenya are described: vB_PaePAO1-KEN19 (KEN19), vB_Pae3705-KEN49 (KEN49), and vB_Pae10145-KEN51 (KEN51).
The phages were isolated in 2021 from sewage samples and runoff in densely populated areas of Nairobi at the geographic coordinates of −1.367662, 36.725424 for KEN19 and 1.258099, 36.863883 for KEN49 and KEN51. Phages were enriched by incubating with P. aeruginosa strains PAO1 (KEN19), MRSN 3705 (KEN49), and ATCC 10145 (KEN51) in Trypticase Soy Broth by shaking at 220 rpm overnight at 37°C. Three rounds of single-plaque isolation ensured purity. Broth-propagated phages at 108 pfu/mL were extracted using QIAamp DNA Mini Kits (Qiagen, Germantown, MD, USA) following DNase + RNase and Proteinase K treatments to yield at least 20 µg/mL. KAPA HyperPlus Kits (Roche Diagnostics, Indianapolis, IN, USA) were used for library preparation. Sequencing was performed on a MiSeq (Illumina, Inc., San Diego, CA, USA) using Reagent Kit v3 (600 cycles, 300-bp reads). The quality of paired-end reads (157,956, 285,978, and 392,269 for KEN19, KEN49, and KEN51, respectively) was evaluated with FastQC (4) version 0.11.9, reads were trimmed by Trimmomatic (5) version 0.39, and genomes were assembled using Unicycler (6) version 0.5.0. Since undetermined by PhageTerm (7) version 1.0.12, termini were manually identified 5,491-bp upstream of the terminase large subunit coding sequence (CDS) start. Phage lifestyle was predicted using BACPHLIP (8) version 0.9.3, and protein CDSs were annotated using the Pharokka pipeline (9–19) version 1.4.0. Amino acid sequence similarity searches used DIAMOND (20, 21) version 2.0.4. Unless otherwise noted, default parameters were used for all analyses.
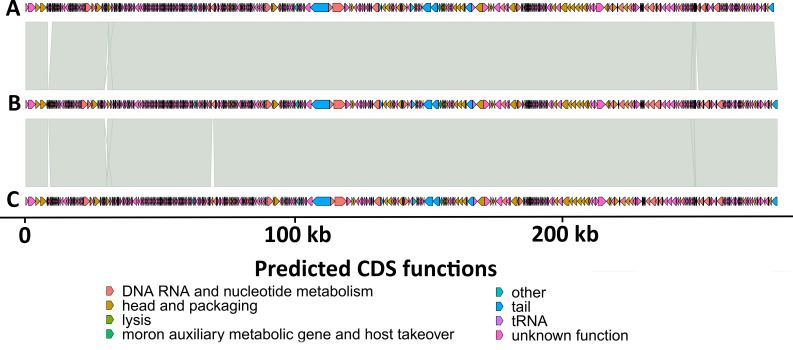
Genome lengths, GC content, and the number of predicted CDSs for KEN19, KEN49, and KEN51 are provided in Table 1. Linear comparison showed high similarity of gene organization (Fig. 1). Each phage encoded seven predicted tRNA genes, postulated to compensate for phage-host codon usage disparity caused by GC content differences (P. aeruginosa host GC%: 65%–67%) (22). They also encoded virion-associated and non-virion RNA polymerases found in other phiKZ-like viruses (23). Using Mash (19) version 2.3 alignment against INPHARED (18) version 1.7, the phages were classified in a group of jumbo phages with myoviral morphology in the genus Phikzvirus that produces a nucleus-like compartment to protect replication by excluding bacterial defense factors (24–26). Phages of this group exhibit lytic activity against up to 50% of a P. aeruginosa diversity panel and are included in commercial therapeutic cocktails in Russia and Georgia (27). There are 20 Phikzvirus phage genomes in the NCBI Nucleotide Database with 98%–99% identity to KEN19, KEN49, and KEN51, indicating these phages belong to the same viral species (28).
TABLE 1.
Genome characteristics of three P. aeruginosa jumbo phages isolated in Kenya
| Phage name | Enrichment strain | Genome length | GC% | No. of CDS | Genus | GenBank accession no. | No. of raw reads | Coverage | SRA accession number |
|---|---|---|---|---|---|---|---|---|---|
| KEN19 | P. aeruginosa PAO1 | 278,921 | 36.93 | 419 | Phikzvirus | PP456878 | 157,956 | 171× | SRX23539368 |
| KEN49 | P. aeruginosa MRSN 3705 | 280,231 | 36.84 | 417 | Phikzvirus | PP456877 | 285,978 | 206× | SRX23539367 |
| KEN51 | P. aeruginosa ATCC 10145 | 280,173 | 36.86 | 417 | Phikzvirus | PP456879 | 392,269 | 206× | SRX23539369 |
Fig 1.
Genome organization of the three Kenyan Phikzvirus phages. (A) KEN19; (B) KEN49; (C) KEN51. This genome comparison was visualized using gggenomes version 1.0.0 (https://thackl.github.io/gggenomes/).
BACPHLIP scored the KEN19, KEN49, and KEN51 genomes at 93%, 85%, and 83%, respectively, compared with a lytic lifestyle threshold of 95% (8). Putative proteins had no homology to those involved in lysogeny or gene transfer, nor to bacterial proteins in antibiotic resistance (14) or virulence (15). KEN19, KEN49, and KEN51 appear to be exclusively lytic and are candidates for therapeutic application.
ACKNOWLEDGMENTS
We thank Yunxiu He for her excellent technical assistance, and Martin Georges and Ivy Mutai for initially isolating the phages at the Walter Reed Army Institute of Research-Africa laboratory at the Kenya Medical Research Institute in Nairobi, Kenya. We also thank Tracey L. Peters for the technical review of the assemblies and manuscript.
This study was supported by the Congressionally Directed Medical Research Program, Peer Reviewed Medical Research Program grant PR182667. The Multidrug Resistant Organism Repository and Surveillance Network (MRSN) at the Walter Reed Army Institute of Research provided the strain P. aeruginosa MRSN 3705 used for phage isolation and the Illumina sequencing.
Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the true views of the Department of the Army or the Department of Defense.
Contributor Information
Mikeljon P. Nikolich, Email: mikeljon.p.nikolich.civ@health.mil.
Catherine Putonti, Loyola University Chicago, Chicago, Illinois, USA.
DATA AVAILABILITY
The KEN19, KEN49, and KEN51 genome BioProject accession number is PRJNA1069762, BioSample accession numbers are SAMN39622049, SAMN39622093, and SAMN39622050, GenBank accession numbers are PP456878, PP456877, and PP456879, and the NCBI Sequence Read Archive accession numbers are SRX23539368, SRX23539367, and SRX23539369, respectively.
REFERENCES
- 1. Qin S, Xiao W, Zhou C, Pu Q, Deng X, Lan L, Liang H, Song X, Wu M. 2022. Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct Target Ther 7:199. doi: 10.1038/s41392-022-01056-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zagaliotis P, Michalik-Provasek J, Gill JJ, Walsh TJ. 2022. Therapeutic bacteriophages for Gram-negative bacterial infections in animals and humans. Pathog Immun 7:1–45. doi: 10.20411/pai.v7i2.516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Santamaría-Corral G, Senhaji-Kacha A, Broncano-Lavado A, Esteban J, García-Quintanilla M. 2023. Bacteriophage-antibiotic combination therapy against Pseudomonas aeruginosa Antibiotics (Basel) 12:1089. doi: 10.3390/antibiotics12071089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andrews S. 2010. FastQC: a quality control tool for high throughput sequence data. Available from: http://www.bioinformatics.babraham.ac.uk/projects/fastqc
- 5. Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wick RR, Judd LM, Gorrie CL, Holt KE. 2017. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLOS Comput Biol 13:e1005595. doi: 10.1371/journal.pcbi.1005595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garneau JR, Depardieu F, Fortier L-C, Bikard D, Monot M. 2017. PhageTerm: a tool for fast and accurate determination of phage termini and packaging mechanism using next-generation sequencing data. Sci Rep 7:8292. doi: 10.1038/s41598-017-07910-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hockenberry AJ, Wilke CO. 2021. BACPHLIP: predicting bacteriophage lifestyle from conserved protein domains. PeerJ 9:e11396. doi: 10.7717/peerj.11396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bouras G, Nepal R, Houtak G, Psaltis AJ, Wormald P-J, Vreugde S. 2023. Pharokka: a fast scalable bacteriophage annotation tool. Bioinformatics 39:btac776. doi: 10.1093/bioinformatics/btac776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Laslett D, Canback B. 2004. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Res 32:11–16. doi: 10.1093/nar/gkh152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bland C, Ramsey TL, Sabree F, Lowe M, Brown K, Kyrpides NC, Hugenholtz P. 2007. CRISPR recognition tool (CRT): a tool for automatic detection of clustered regularly interspaced palindromic repeats. BMC Bioinformatics 8:209. doi: 10.1186/1471-2105-8-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steinegger M, Söding J. 2017. MMseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nat Biotechnol 35:1026–1028. doi: 10.1038/nbt.3988 [DOI] [PubMed] [Google Scholar]
- 13. McNair K, Zhou C, Dinsdale EA, Souza B, Edwards RA. 2019. PHANOTATE: a novel approach to gene identification in phage genomes. Bioinformatics 35:4537–4542. doi: 10.1093/bioinformatics/btz265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alcock BP, Raphenya AR, Lau TTY, Tsang KK, Bouchard M, Edalatmand A, Huynh W, Nguyen A-LV, Cheng AA, Liu S, et al. 2020. CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res 48:D517–D525. doi: 10.1093/nar/gkz935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen L. 2004. VFDB: a reference database for bacterial virulence factors. Nucleic Acids Res 33:D325–D328. doi: 10.1093/nar/gki008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chan PP, Lin BY, Mak AJ, Lowe TM. 2021. tRNAscan-SE 2.0: improved detection and functional classification of transfer RNA genes. Nucleic Acids Res 49:9077–9096. doi: 10.1093/nar/gkab688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Terzian P, Olo Ndela E, Galiez C, Lossouarn J, Pérez Bucio RE, Mom R, Toussaint A, Petit M-A, Enault F. 2021. PHROG: families of prokaryotic virus proteins clustered using remote homology. NAR Genomics Bioinform 3:1–12. doi: 10.1093/nargab/lqab067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cook R, Brown N, Redgwell T, Rihtman B, Barnes M, Clokie M, Stekel DJ, Hobman J, Jones MA, Millard A. 2021. INfrastructure for a PHAge REference Database: identification of large-scale biases in the current collection of cultured phage genomes. PHAGE (New Rochelle) 2:214–223. doi: 10.1089/phage.2021.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ondov BD, Treangen TJ, Melsted P, Mallonee AB, Bergman NH, Koren S, Phillippy AM. 2016. Mash: fast genome and metagenome distance estimation using MinHash. Genome Biol 17:132. doi: 10.1186/s13059-016-0997-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buchfink B, Xie C, Huson DH. 2015. Fast and sensitive protein alignment using DIAMOND. Nat Methods 12:59–60. doi: 10.1038/nmeth.3176 [DOI] [PubMed] [Google Scholar]
- 21. Buchfink B, Reuter K, Drost H-G. 2021. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat Methods 18:366–368. doi: 10.1038/s41592-021-01101-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bailly-Bechet M, Vergassola M, Rocha E. 2007. Causes for the intriguing presence of tRNAs in phages. Genome Res 17:1486–1495. doi: 10.1101/gr.6649807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ceyssens P-J, Minakhin L, Van den Bossche A, Yakunina M, Klimuk E, Blasdel B, De Smet J, Noben J-P, Bläsi U, Severinov K, Lavigne R. 2014. Development of giant bacteriophage ϕKZ is independent of the host transcription apparatus. J Virol 88:10501–10510. doi: 10.1128/JVI.01347-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chaikeeratisak V, Nguyen K, Khanna K, Brilot AF, Erb ML, Coker JKC, Vavilina A, Newton GL, Buschauer R, Pogliano K, Villa E, Agard DA, Pogliano J. 2017. Assembly of a nucleus-like structure during viral replication in bacteria. Science 355:194–197. doi: 10.1126/science.aal2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Danilova YA, Belousova VV, Moiseenko AV, Vishnyakov IE, Yakunina MV, Sokolova OS. 2020. Maturation of pseudo-nucleus compartment in P. aeruginosa, infected with giant phiKZ phage. Viruses 12:1197. doi: 10.3390/v12101197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mendoza SD, Nieweglowska ES, Govindarajan S, Leon LM, Berry JD, Tiwari A, Chaikeeratisak V, Pogliano J, Agard DA, Bondy-Denomy J. 2020. A bacteriophage nucleus-like compartment shields DNA from CRISPR nucleases. Nature New Biol 577:244–248. doi: 10.1038/s41586-019-1786-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krylov V, Bourkaltseva M, Pleteneva E, Shaburova O, Krylov S, Karaulov A, Zhavoronok S, Svitich O, Zverev V. 2021. Phage phiKZ-the first of giants. Viruses 13:149. doi: 10.3390/v13020149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adriaenssens E, Brister JR. 2017. How to name and classify your phage: an informal guide. Viruses 9:70. doi: 10.3390/v9040070 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The KEN19, KEN49, and KEN51 genome BioProject accession number is PRJNA1069762, BioSample accession numbers are SAMN39622049, SAMN39622093, and SAMN39622050, GenBank accession numbers are PP456878, PP456877, and PP456879, and the NCBI Sequence Read Archive accession numbers are SRX23539368, SRX23539367, and SRX23539369, respectively.